Abstract
Several polyphenol-rich Terminalia species (Combretaceae) are known to accelerate wound healing. Recently, the Omani medicinal plant Anogeissus dhofarica (now Terminalia dhofarica) was attributed to the genus Terminalia based on phylogenetic studies. Leaves, bark, and extracts of T. dhofarica are traditionally used for various medicinal purposes, including wound treatment and personal hygiene. In the present study, the phytochemical profile of leaves from T. dhofarica was evaluated by ultra-high-performance liquid chromatography coupled with electrospray ionization high-resolution mass spectrometry (UHPLC-ESI-HRMS) and nuclear magnetic resonance (NMR) spectroscopy. Simple phenolics, polyphenolics (e.g., flavonoids and tannins) and their glucosides were characterized as major metabolite classes. In addition, 20 phenolics were isolated and structurally identified. Nine of these compounds were never described before for T. dhofarica. For the first time, we provide complete NMR data for 1-O-galloyl-6-O-p-coumaroyl-d-glucose (1). Biological screening demonstrated moderate efficacy against the Gram-negative bacterium Aliivibrio fischeri, the phytopathogenic fungus Septoria tritici, and the oomycete Phytophthora infestans. In summary, the data expand the knowledge of the phytochemistry of the underexplored species T. dhofarica and underscore its potential for therapeutic applications, particularly in the context of traditional medicine.
1. Introduction
Terminalia dhofarica (A.J.Scott) Gere & Boatwr. (formerly referred to by the homotypic synonym Anogeissus dhofarica A.J.Scott) belongs to the Combretaceae family and is an endemic species of the Dhofar region in Oman and southeastern Yemen, thriving in monsoon ecosystems [1]. Previously integrated in the genus Anogeissus, it was transferred with the whole genus Anogeissus into the genus Terminalia in 2017 [2,3,4], which led to formal taxonomic name changes.
The former genus Anogeissus is primarily distributed across southern Asia, the Arabian Peninsula, and West Africa [5]. Many former Anogeissus species have significant ethnomedicinal uses ranging from gastric disorders, skin diseases, and diabetes to wound healing and coughs [6,7,8,9]. The bioactivity is primarily attributed to their high content of phenolic compounds such as gallic acid, ellagic acid, and their derivatives, as well as flavonoids like quercetin and rutin [10,11].
Traditionally, leaves, bark, and extracts of T. dhofarica are used for various medicinal purposes, including wound treatment and as antiseptic in personal hygiene [8,12]. Previous studies showed that aqueous and alcoholic extracts with a high phenolic content exhibit potent antioxidant activity and display antibacterial and antifungal activities [8,13]. Despite its traditional use and promising activities, the phytochemical and pharmacological profile of T. dhofarica remained underexplored compared to other species of the genus [10]. Recent investigations by Maqsood et al. [14] and Abuarqoub et al. [15] led to a tentative annotation of 28 compounds, predominantly flavonoids and phenolic acids. These results are in line with the observed strong antioxidant and radical scavenging properties. Additionally, the extracts showed potential anticancer, antidiabetic, and anti-inflammatory activities, promoted fibroblast migration and enhanced wound healing, which confirmed its traditional medicinal uses [14,15].
The current study represents the first comprehensive phytochemical characterization of the species. Methanolic crude extracts from leaves were analyzed by UHPLC-ESI-HRMS and NMR for major metabolites and screened for antibacterial and antifungal activity. The identity of constituents was verified by isolation, characterization and complete structure elucidation based on extensive spectroscopic methods.
2. Results and Discussion
2.1. Metabolite Profiling
Dried leaves from T. dhofarica were pulverized and exhaustively extracted with 80% methanol to yield a crude extract. This extract was screened for antibacterial and antifungal activity (Figures S1–S4, see Section 2.4). Furthermore, the metabolite profile was analyzed using UHPLC-ESI-HRMS (Figure 1 and Figure S5, Table 1). An aliquot of the powder was extracted with deuterated methanol and subjected to NMR analysis (Figure 2).
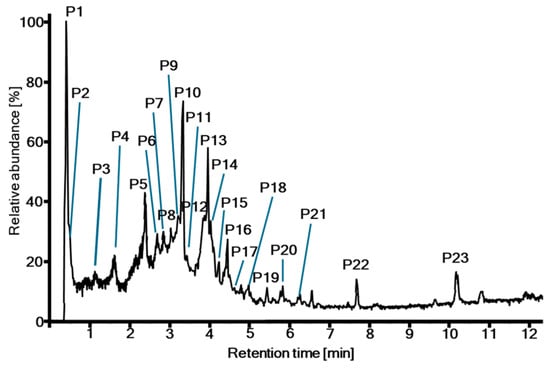
Figure 1.
UHPLC-ESI-HRMS total ion chromatogram of the crude extract from T. dhofarica leaves.

Table 1.
Peak list of the UHPLC-ESI-HRMS analysis of the crude extract from T. dhofarica leaves.

Figure 2.
NMR metabolite profiling (Methanol-d4, 600/150 MHz) of the crude extract from T. dhofarica leaves; (A) 1H NMR; (B) HSQC with annotation of characteristic 1JCH correlations.
The NMR screening of the crude extract revealed a profile consistent with the known characteristics of the genus [10]. Many intense singlet signals appear in the aromatic region of the 1H spectrum, between 6.2 and 7.5 ppm (Figure 2A). The HSQC spectrum associates these signals with a consistent 13C shift of approximately 110 ppm (Figure 2B) in accordance with signals for gallic acid and its derivatives, such as ellagic acid or follow-up tannin structures. In addition to the expected signals for fatty acids, sterols and sugars (Figure 2A), several distinct signals were identified that, according to literature reports, could be attributed to derivatives with a chebulic acid core (Figure 2B). Some compounds contain structural features of both groups, gallic acid derivatives and chebulic acid derivatives, as, e.g., chebulagic acid (18, Figure 3).
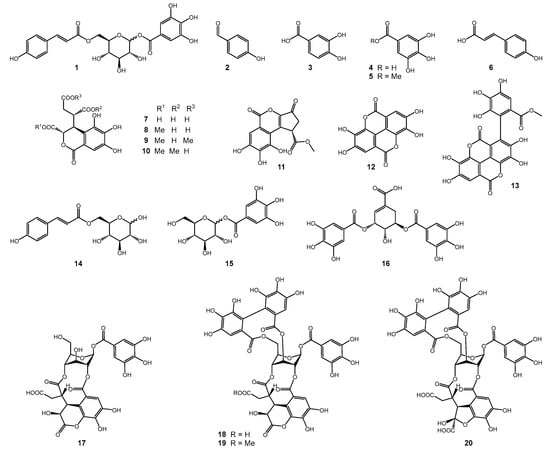
Figure 3.
Structures of compounds isolated from the leaf extract of T. dhofarica.
The presence of gallic acid, chebulic acid and diverse derivatives was further confirmed through UHPLC-ESI-HRMS analysis, which provided a more detailed view of the complexity and diversity of metabolites (Table 1, Figure 1 and Figure S5). A total of 32 metabolites were preliminary annotated from the total ion chromatogram (TIC) of the crude extract, although in some cases, compounds eluted simultaneously. The majority of signals could be attributed to phenolic acids (Table 1, P1 gallic acid + quinic acid; P3 protocatechuic acid; P11 p-coumaric acid), tannins (Table 1, P2; P3 terflavin B, O-galloyl punicalin; P4; P5; P6 brevifolincarboxylate; P7 O-galloyl bis-O-HHDP-glucose; P8; P9; P10; P12; P13; P14, P16, P17, P18 O-methyl ellagic acid, P21, P22), or flavonoids (Table 1, P7 gallocatechin, P15; P18 galloylvitexin isomers; P19; P20), following the classification of compounds by Singh et al. [10]. Consequently, T. dhofarica appears to possess a metabolic profile closely resembling that of its relatives within the genus [10,14,15].
Our data partly overlapped with previous studies on the composition of the crude extract of T. dhofarica. Abuarqoub et al. [15] identified seven phenolic acids by retention times of standard substance only. Two of these acids were also found within this study, both in the LC-HRMS screening and in the isolation approach (gallic acid (4), P1; protocatechuic acid (3), P3). Noteworthy, Abuarqoub et al. identified ortho- and meta- but no para-coumaric acid which, however, is the only coumaric acid derivative found in this study (6, P11). Maqsood et al. [14] annotated 23 compounds by HRMS data from the crude extract. However, their tentative assignments were based on molecular formula calculations with excessively high errors ranging from −299 ppm to +780 ppm [14]. This reduces the reliability of their results significantly since mass accuracy should be lower than ±10 ppm. The authors did not provide MS fragmentation data that could support the annotation. Nevertheless, eight of these compounds were also found in this study. Remarkably, chebulagic acid (18, P13), the main compound from this study, was not described by Maqsood et al., although they annotated chebulic acid (7, P2) on one hand and corillagin (P9) on the other hand which are the two parts of chebulagic acid.
2.2. Isolation and Structure Elucidation
The separation of the crude extract by liquid–liquid partition followed by different chromatographic techniques resulted in the isolation of 20 phenolic compounds (Figure 3). All compounds were identified by extensive spectral analysis (HRMS, NMR) and comparison with previously reported data from the literature [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. A full spectroscopic data set for compounds 2–20 can be found in the Supporting Information. Fifteen of the 20 isolated compounds were never described before for T. dhofarica.
Compound 1 (1-O-galloyl-6-O-p-coumaroyl-d-glucose), a twice modified glucose molecule with gallic acid at position 1 and p-coumaric acid at position 6, was postulated by Mei et al. as a constituent of a Chinese herbal preparation based on extensive HRMS fragmentation analysis only [16]. Here, we provide for the first time complete NMR data for compound 1. Notably, it was obtained as a mixture of α- and β-d-glucoside.
Compound 1 (Figure 4) was isolated as a white solid. The molecular formula was identified as C22H22O12 by its negative ion at m/z 477.1030 [M–H]− (calcd. for 477.1038 C22H21O11−) in the ESI-HRMS spectrum. All NMR data, as well as 2D correlations, are presented in Table 2. The 1H spectra (Figure S6) showed five aromatic signals. The presence of a galloyl group was deduced from HSQC (Figure S9) and HMBC correlations (Figure S10) of the singlet at δH 7.13 (2 H, s, H-2′ + H-6′), resulting in the annotation of the 13C signals δC 110.5, 120.5, 140.7, 146.6 and 166.9 to this substructure. Two pairs of coupling aromatic protons at δH 7.63 and 6.36 (each 1 H, d, J = 15.7 Hz, H-7″ + H-8″) and at δH 7.45 and 6.80 (each 2 H, d, J = 8.5 Hz, H-2″ and H-6″ + H-3″ and H-5″) indicated the presence of a trans configurated double bond and a para-substituted benzene ring, respectively. In combination with the corresponding nine carbons, these signals were attributed to a coumaroyl group. The pattern of aliphatic proton signals, including two anomeric protons at δH 5.66 (0.5 H, d, J = 7.7 Hz, H-1β) and 5.66 (0.5 H, d, J = 3.3 Hz, H-1α), three multiplet signals at δH 3.41–3.46, 3.46–3.52, and 3.65–3.70 and signals of a CH2 group at δH 4.31 (1 H, dd, J = 5.6, 12.2 Hz, H-6b) and δH 4.50 (1 H, dd, J = 2.8, 12.2 Hz, H-6a), suggested the presence of one glucopyranosyl moiety. This was further supported by strong COSY correlations (Figure S8) and the HSQC correlation of the aliphatic proton signals (Table 2). The specific connection pattern of all three moieties was determined by HMBC correlation of the glucose protons H-1 to the galloyl carbon C-7′ and of H-6a and H-6b to the coumaroyl carbon C-9″ (Table 2, Figure S10). Therefore, the compound is identified as 1-O-galloyl-6-O-coumaroyl-d-glucose. In the glucose moiety, α and β configurations appeared in a ratio of 1:1 by comparison of integrals in the 1H spectrum. Structurally, compound 1 is close to fishertannin F (1-O-galloyl-6-O-feruloyl-β-d-glucose) [37], with an additional methoxy group in the cinnamic acid core. Consequently, the NMR data are mainly in accordance with those reported by Zhang et al. [37].

Figure 4.
Structure of compound 1 with crucial NMR correlations.

Table 2.
NMR data (Methanol-d4, 600/150 MHz) of compound 1.
Compounds 14 (6-O-trans-p-coumaroyl-d-glucopyranose) and 15 (1-O-galloyl-d-glucose) represent substructures of 1, and both anomeric configurations appeared. This is a well-reported phenomenon for 14 [32].
The anomeric protons of the sugar moieties of compounds 17–20 show unusual chemical shifts and small coupling constants (e.g., 6.35, 1 H, d, J = 2.8 Hz, for H-1 in 17). Usually, β-glucose appears in the energetically favored 4C1 chair conformation. However, in ellagitannins with bridging 2,4-O-chebuloyl substituents, the β-glucose ring is locked into the inverted 1C4 conformation with all ring protons in equatorial instead of axial positions, resulting in small vicinal couplings (<4 Hz) [35].
2.3. Evaluation of Artifacts
Remarkably, some of the isolated compounds exhibited methylations of carboxyl functional groups (5, 8, 9, 10, 11, 13, and 19). However, these compounds were absent in the UHPLC-ESI-HRMS analysis of the crude extract, but their unmethylated form was detected, such as chebulic acid (7) (Table 1, P2), brevifolincarboxylate (Table 1, P6), or flavogallonate (Table 1, P8). Since methanol was used to extract the plant material and as a solvent in multiple purification steps, the mentioned compounds may be artifacts of the isolation process rather than true metabolites of T. dhofarica. To investigate this possibility, a small-scale extraction of leaves was performed with methanol versus ethanol. The analysis of both total ion chromatograms (TICs) revealed all the above-mentioned compounds as probable artifacts. Exemplary, Figure 5 presents the extracted ion chromatogram (XIC) of the methanolic and ethanolic extracts, filtered for pure chebulagic acid and its methylated and ethylated derivatives. The pure compound is present in both extracts, confirming it as a true metabolite. However, the methylated derivative appears only in the methanolic extract, and the ethylated derivative solely in the ethanolic extract, strongly suggesting both are artifacts. This finding is particularly noteworthy, as methylated chebulagic acid was also reported as an artifact in the isolation of Terminalia chebula Retz., another species from the genus [2,38]. In contrast, true methylated metabolites, such as methylated derivatives of ellagic acid (Table 1, P18, P21, and P22), were confirmed by UHPLC-ESI-HRMS analysis in both extracts. Thus, to the best of our knowledge, 15 of 20 isolated compounds are directly of plant origin, and from these, 9 compounds (1; 2; 6; 14–18; 20) are described for the first time within this species.
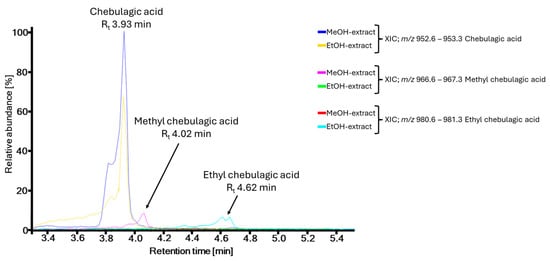
Figure 5.
Test for artifacts on the example of chebulagic acid and methyl chebulagic acid by UHPLC-ESI-HRMS analysis of methanolic and ethanolic crude leaf extracts of T. dhofarica.
2.4. Evaluation of Bioactivity
The methanolic crude extract of leaves was screened for antibacterial and antifungal activity (Table 3, Figures S1–S4). In our investigation, the extract exhibited moderate activity against Gram-negative bacteria (Aliivibrio fischeri at a concentration of 500 µg/mL, Figure S1) but showed no activity against Gram-positive bacteria (Bacillus subtilis). In antifungal assays, the crude extract also exhibited moderate activity against the phytopathogenic fungus Septoria tritici and the oomycete Phytophthora infestans (around 80% of inhibition at 100 and 10 µg/mL, respectively) but did not influence the growth of Botrytis cinerea.

Table 3.
Antifungal (Septoria tritici, Botrytis cinerea), antioomycotic (Phytophthora infestans), and antibacterial (Aliivibrio fisheri) activities of crude extract and isolated compounds from T. dhofarica.
In general, T. dhofarica seems not to deliver consistent effects in antibacterial assays. Maqsood et al. reported no activity against Gram-negative bacteria (E. hormaechei) but observed significant inhibition of Gram-positive bacteria (S. aureus) in a ZOI assay [14]. Conversely, Marwah et al. reported substantial activity against Gram-positive bacteria (S. aureus at 250 µg/mL) and moderate activity against Gram-negative bacteria (P. aeruginosa at 500 µg/mL) [8]. This might be due to differences in assay methods, bacterial strains, or the choice of plant material and extraction conditions (see above for artifact formation). Notably, while both previous studies used mixed plant material for extraction, this analysis focused exclusively on leaves.
Based on the biological effects of the crude extract, all isolated compounds, including the potential artifacts, were screened for biological activity against the Gram-negative bacterium A. fischeri, as well as the phytopathogenic fungi B. cinerea, S. tritici and the oomycete P. infestans (Table 3). In contrast to expectations, none of the isolated compounds exhibited inhibitory effects on A. fischeri, although the crude extract displayed moderate activity (Figure S1). This suggests that the active compound was either not isolated or modified (e.g., by methylation) or that synergistic effects were required, which may have been lost during the separation of synergistic partners.
Several compounds demonstrated some level of antifungal activity against S. tritici and P. infestans (up to 90% inhibition at 100 µM, Figures S2–S4). The most remarkable effect can be reported for the artifact 6′-O-methyl-chebulagic acid (19), which showed 82% inhibition against P. infestans at a concentration of 10 µM (Figure S2). In general, the methylated artifacts were most active against this oomycete. Thus, the reported antifungal properties of T. dhofarica may be attributed to the combined effects of various active polyphenolic compounds with rather nonspecific activity. This is common for mixtures of plant phenolics. It is in alignment with the use of crude mixtures in external (or intestinal) applications, as the tanning and gluing effect underlying these compounds on microorganisms is not systemic.
3. Materials and Methods
3.1. Materials and Chemicals
TLC plates: silica gel 60 normal phase (SG60), silica gel 60 reversed phase 18 F254 (Merck, Darmstadt, Germany) or silica gel 60 reversed phase 2 UV254 (Macherey-Nagel, Düren, Germany); Column materials: Lichroprep RP 18 40–63 µm (Merck, Darmstadt; Germany), Sephadex LH 20 (GE Healthcare, Uppsala, Sweden), Sephadex G10 (Pharmacia, Uppsala, Sweden); Solvents: ultrapure water (ThermoScientific Barnstead GenPure Pro, Langenselbold, Germany), methanol, ethyl acetate (technical grade solvents distilled prior use), n-heptane (Roth, Karlruhe, Germany), acetonitrile for LC-MS Chromasolv, formic acid for mass spectrometry (Honeywell Fluka, Seelze, Germany); DMSO (Duchefa Biochemie, Haarlem, The Netherlands); NMR solvents: methanol-d4, DMSO-d6 (Deutero, Kastellaun, Germany); Chemicals: vanillin (Tokyo Chemicals, Tokyo, Japan), chloramphenicol (Roth, Karlsruhe, Germany), 2-aminoethyl diphenylborinate, epoxiconazole and terbinafine (Sigma-Aldrich, Darmstadt, Germany).
3.2. Analytical Instruments and General Procedures
Thin layer chromatography (TLC) analyses were performed using different solvent systems, as indicated in Section 3.4. To visualize the compound spots, long-wavelength UV light (366 nm), short-wavelength UV light (254 nm) and spraying with vanillin–H2SO4 reagent, followed by heating or spraying with natural product spray reagent (1 g 2-aminoethyl diphenylborinate/200 mL methanol) were applied.
Low-resolution ESI-MS spectra were performed on a Sciex API-3200 instrument (Applied Biosystems, Concord, ON, Canada) combined with an HTC-XT autosampler (CTC Analytics, Zwingen, Switzerland).
The semi-preparative HPLC was performed on a Shimadzu prominence system (Kyoto, Japan), which consists of an SPD-M20A diode array detector, an FRC-10A fraction collector, a CBM-20A communications bus module, a DGU-20A5R degassing unit, an LC-20AT liquid chromatograph, and a SIL-20A HT autosampler.
The UHPLC-ESI-HRMS spectra were acquired using a TripleTOF (time of flight) 6600-1 mass spectrometer (Sciex, Darmstadt, Germany) combined with an ACQUITY UPLC I-Class UHPLC System (Waters GmbH, Eschborn, Germany) as described by Kappen et al. [39] with minor modifications. For separation, a Waters Acquity UPLC® BEH C18 column (1.7 µm, 130 Å, 50 × 2.1 mm I.D., Waters GmbH, Eschborn, Germany) was used. Data acquisition was performed in MS1-TOF mode in a mass range of m/z 65 to 1250 with an accumulation time of 75 ms and in MS2-TOF mode in the m/z range of 50–1000 with an accumulation time of 20 ms.
1H and 13C NMR spectra were recorded on an Agilent DD2 400 NMR spectrometer (Santa Clara, CA, USA) at 399.917 and 100.570 MHz, respectively. Chemical shifts are reported relative to TMS (1H NMR) or peaks of solvent. For samples with low concentration, 1D 1H and 13C NMR spectra and 2D spectra (HSQC, HMBC, COSY, TOCSY, NOESY) were recorded on a Bruker Avance Neo 500 NMR spectrometer (Billerica, MA, USA) at 500.234 and 125.797 MHz, respectively, using a 5 mm prodigy probe with the TopSpin 4.0.7 spectrometer software or on an Agilent VNMRS 600 MHz NMR spectrometer equipped with 5 mm inverse detection cryoprobe, using standard CHEMPACK 8.1 pulse sequences implemented in Varian VNMRJ 4.2 spectrometer software.
3.3. Plant Material
Leaves of Terminalia dhofarica (A.J.Scott) Gere & Boatwr. (synonym Anogeissus dhofarica A.J.Scott) were collected in autumn 2019 and 2020 in Wadi Nahiz, which is located on the northern side of Salalah City, Dhofar, Sultanate of Oman. The leaves were shadow-dried at room temperature, pulverized, and stored at room temperature. A voucher (ADA/11/2020) was deposited in the herbarium of the Natural & Medical Sciences Research Center, University of Nizwa, Oman.
3.4. Isolation
Dried pulverized leaves (300 g) from T. dhofarica were exhaustively extracted with 80% aq. methanol to produce 92 g of dried crude extract after evaporation of the solvent. An aliquot of the crude extract (30.8 g) was successively partitioned by liquid–liquid extraction between water (700 mL) and n-heptane (2 × 250 mL), followed by ethyl acetate (6 × 300 mL). This resulted in three fractions: n-heptane (1.6 g), ethyl acetate (4.2 g), and water (20.8 g).
The ethyl acetate fraction was submitted to an RP18 column (l: 34 cm, d: 3.5 cm) and eluted with a mixture of methanol and water (1:1, v/v), which yielded three fractions (A1–A3), based on the TLC profile (RP18, MeOH/H2O, 1:1, v/v) of which A1 (Rf 0.95–0.59) and A2 (Rf 0.59–0.38) were further purified. A3 was identified as ellagic acid (12, 547.4 mg, Rf = 0.36 in MeOH/H2O (1:1, v/v) on RP18.
A1 was submitted to a Sephadex G10 column (l: 120 cm, d: 3.5 cm) with a mixture of methanol and water (1:4, v/v), yielding seven fractions (B1–B7), based on the TLC profile (RP18, MeOH/H2O, 2:3, v/v), of which B2 (Rf 0.98–0.88), B5 (Rf 0.79–0.62), and B6 (Rf 0.62–0.31) were further purified. B3 (Rf 0.83) was identified as gallic acid (4), 153.8 mg, Rf = 0.83 in MeOH/H2O (2:3, v/v) on RP18.
B2 was purified by preparative reversed-phase HPLC (Agilent-Zorbax Eclipse-XDB C18, 5 µm, 9.4 mm × 250 mm) using a water + 0.1% formic acid (A) and methanol + 0.1% formic acid (B) gradient system (0−3.0 min, 5% B; 3.0–43.0 min, 5–25% B) and a flow rate of 1.50 mL/min at 25 °C to yield chebulic acid (7) (4.6 mg, Rt = 8.80 min), 1-O-galloyl-d-glucose (15) (2.5 mg, Rt = 12.48 min), protocatechuic acid (3) (3.5 mg, Rt = 26.71 min), 12-O-methyl chebulic acid (8) (8.9 mg, Rt = 28.93 min), 11,12-O-dimethyl chebulic acid (9) (4.1 mg, Rt = 38.07 min), and 12,13-O-dimethyl chebulic acid (10) (6.5 mg, Rt = 41.40 min).
B5 was submitted to an RP18 column (l: 36 cm, d: 3.5 cm) with a gradient of methanol and water (500 mL, 1:4, v/v; 500 mL, 1:2, v/v; 500 mL, 2:3, v/v), yielding nine fractions (C1–C9), based on the TLC profile (RP18, MeOH/H2O, 1:1, v/v), of which C4 (Rf 0.72) and C7 (Rf 0.70–0.54) were further purified.
C4 was submitted to a Sephadex LH20 column (l: 60 cm, d: 2.5 cm) with methanol yielding eight fractions (D1–D8), of which D3 was identified as chebulagic acid (18) (16.5 mg, Rf = 0.57 in MeOH/H2O (2:3, v/v) on RP18.
C7 was purified by preparative reversed-phase HPLC (YMC-Triart C18, 5 µm, 10 mm × 150 mm) using a water + 0.1% formic acid (A) and methanol + 0.1% formic acid (B) gradient system (0–2.5 min, 35% B; 2.5–22.5 min, 35–50% B; 22.5–27.5 min, 50–60% B) and a flow rate of 2.64 mL/min at 25 °C to yield 7″-O-methyl flavogallonate (13) (2.1 mg, Rt = 11.50 min), and 11-O-methyl brevifolincarboxylate (11) (1.5 mg, Rt = 13.80 min).
B6 was submitted to an RP18 column (l: 36 cm, d: 3.5 cm) and eluted with a 10% step gradient of methanol and water with 0.1% TFA (10–40% MeOH, each 250 mL; 50–90% MeOH, each 200 mL; 100% MeOH, 400 mL) which yielded thirteen fractions (E1–E13), based on the TLC profile (RP18, MeOH/H2O, 2:3, v/v) of which E8 (Rf 0.63–0.25) was further purified.
E8 was purified by preparative reversed-phase HPLC (Agilent-Zorbax Eclipse-XDB C18, 5 µm, 9.4 mm × 250 mm) using a water + 0.1% formic acid (A) and methanol + 0.1% formic acid (B) gradient system (0–6.0 min, 25% B; 6.0–36.0 min, 25–45% B; 36.0–37.0 min, 45–100%) and a flow rate of 3.3 mL/min at 25 °C to yield phyllanembilinin C (20) (1.2 mg, Rt = 11.62 min), chebulanin (17) (2.4 mg, Rt = 15.92 min), 3,5-di-O-galloylshikimic acid (16) (1.4 mg, Rt = 18.38 min), and 6′-O-methyl-chebulagic acid (20) (4.8 mg, Rt = 21.74 min).
A2 was submitted to a Sephadex LH20 column (l: 75 cm, d: 2.5 cm) with methanol, yielding five fractions (F1–F5), based on the TLC profile (RP18, MeOH/H2O, 1:1, v/v), of which F1 (Rf 0.62), F2 (Rf 0.55), and F4 (Rf 0.36–0.24) were further purified. F3 (Rf 0.53) was identified as 7-O-methyl gallic acid (5) (15.2 mg, Rf = 0.53 in MeOH/H2O (1:1, v/v) on RP18).
F1 was purified by preparative reversed-phase HPLC (YMC-ODS-A C18, 12 µm, 10.0 mm × 150 mm) using a water + 0.1% formic acid (A) and methanol + 0.1% formic acid (B) gradient system (0–2.5 min, 30% B; 2.5–17.5 min, 30–45% B) and a flow rate of 3.6 mL/min at 25 °C to yield trans-p-coumaric acid (6) (3.3 mg, Rt = 8.41 min).
F2 was purified by preparative reversed-phase HPLC (Merck-LiChrospher C18, 5 µm, 10.0 mm × 250 mm) using a water (A) and acetonitrile (B) gradient system (0–3.5 min, 12% B; 3.5–18.5 min, 12–20% B) and a flow rate of 4.00 mL/min at 25 °C to yield p-hydroxybenzaldehyde (2) (2.9 mg, Rt = 15.89 min).
F4 was purified by analytical reversed-phase HPLC (YMC-ODS-A C18, 12 µm, 10.0 mm × 150 mm) using a water (A) and methanol (B) gradient system (0–2.5 min, 20% B; 2.5–20.0 min, 20–38% B) and a flow rate of 4.80 mL/min at 25 °C to yield 6-O-trans-p-coumaroyl-β-d-glucopyranose (14) (0.9 mg, Rt = 9.45 min), and 1-O-galloyl-6-O-trans-p-coumaroyl-β-d-glucopyranose (1) (0.6 mg, Rt = 19.97 min).
3.5. Biological Assays
Antibacterial assays: The crude extract and compounds were evaluated against the Gram-negative Aliivibrio fischeri (DSM507) using bioluminescence, following the method as outlined by Ware et al. (2023) [40], and against the Gram-positive Bacillus subtilis 168 (DSM 10) using absorption measurements as described by Kappen et al. [39]. In both assays, the synthetic bacteriostatic antibiotic chloramphenicol (100 µM) was applied as a positive control to achieve complete bacterial growth inhibition (100%). The results (mean ± standard deviation, n = 6) are presented as relative values (percent inhibition) compared to the negative control (bacterial growth without test compound). Negative values indicate an increase in bacterial growth.
Antifungal assays: The antifungal activity was tested in triplicate on the phytopathogenic ascomycetes Botrytis cinerea Pers. and Septoria tritici Desm. and the oomycete Phytophthora infestans (Mont.) de Bary according to protocols from the Fungicide Resistance Action Committee (FRAC) with minor modifications as described by Ware et al. [40]. The commercially used fungicides, epoxiconazole and terbinafine (Sigma-Aldrich, Darmstadt, Germany), served as positive control.
4. Conclusions
In conclusion, the Omani medicinal plant Terminalia dhofarica was found to be very rich in phenolic acids, tannins, and flavonoids, as well as their glucosides, as major compound classes. This is consistent with other species in the genus. A total of 20 compounds were isolated, including the first full characterization of compound 1 with a complete set of NMR data to unequivocally determine its structure. However, a critical examination of the data revealed that seven compounds isolated are likely artifacts of the isolation process due to the methylation of carboxyl groups. Therefore, 13 compounds remain with true plant origins, of which 9 were described for the first time within this species. All metabolites detected or isolated represent phenols or polyphenols. This compound class is known for its wound-healing effects based on anti-inflammatory, antimicrobial, and antioxidant properties [41]. The polyphenol content of T. dhofarica is also in line with the moderate antibacterial and antifungal effects observed within this study. In summary, our data corroborate the reported non-systemic use of T. dhofarica extracts and underline the traditional application of the species in wound treatment and as antiseptics.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30040952/s1, Figure S1: Antibacterial assay against Gram-negative A. fischeri; Figure S2: Antifungal assay against P. infestans; Figure S3: Antifungal assay against S. tritici; Figure S4: Antifungal assay against B. cinerea; Figure S5: TWC and TIC obtained by UHPLC-ESI-HRMS of the crude extract from T. dhofarica; Figure S6: 1H spectrum of compound 1; Figure S7: 13C spectrum of compound 1; Figure S8: COSY spectrum of compound 1; Figure S9: HSQC spectrum of compound 1; Figure S10: HMBC spectrum of compound 1; Full spectroscopic data set of compounds 1–20.
Author Contributions
Conceptualization, L.A.W. and L.R.; methodology, J.K.; validation, J.K. and K.F.; formal analysis, J.K.; investigation, J.K.; resources, L.A.W. and L.R.; data curation, J.K. and K.F.; writing—original draft preparation, J.K.; writing—review and editing, K.F., L.R. and L.A.W.; visualization, J.K. and K.F.; supervision, K.F. and L.A.W.; project administration, L.A.W.; funding acquisition, L.A.W. All authors have read and agreed to the published version of the manuscript.
Funding
Jonas Kappen and Katrin Franke were funded within the frame of the projects ProCognito by EFRE and the state Saxony-Anhalt (ZS/2018/11/95581) and HyperSpace (BMBF, 031B1448A).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and the Supplementary Material. The raw data supporting the conclusions of this article will be made available by the corresponding authors upon request. All primary data and reference compounds are stored at the IPB primary data storage for 10+ years and in the compound depository to the extent available or stable.
Acknowledgments
The authors would like to thank M. Brode (IPB Halle) for the performance of antibacterial and antifungal bioassays. We are grateful to P. Stark, N. Hünecke, and G. Hahn (IPB Halle) for NMR measurements, as well as to A. Laub, A. Soboleva, M. Hempel, and E. Kysil (IPB Halle) for HRMS measurements.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kürschner, H.; Hein, P.; Kilian, N.; Hubaishan, M.A. The Hybantho durae-Anogeissetum dhofaricae ass. Nova-phytosociology, structure and ecology of an endemic South Arabian forest community. Phytocoenologia 2004, 34, 569–612. [Google Scholar] [CrossRef]
- Maurin, O.; Gere, J.; van der Bank, M.; Boatwright, J.S. The inclusion of Anogeissus, Buchenavia and Pteleopsis in Terminalia (Combretaceae: Terminaliinae). Bot. J. Linn. 2017, 184, 312–325. [Google Scholar] [CrossRef]
- IPNI. Anogeissus dhofarica. Available online: https://www.ipni.org/n/169727-1 (accessed on 8 November 2024).
- IPNI. Terminalia dhofarica. Available online: https://www.ipni.org/n/77164562-1 (accessed on 8 November 2024).
- Scott, A.J. A revision of Anogeissus (Combretaceae). Kew Bull. 1979, 33, 555. [Google Scholar] [CrossRef]
- Jain, A.; Katewa, S.S.; Galav, P.K.; Sharma, P. Medicinal plant diversity of Sitamata wildlife sanctuary, Rajasthan, India. J. Ethnopharmacol. 2005, 102, 143–157. [Google Scholar] [CrossRef]
- Meena, K.L.; Yadav, B.L. Studies on ethnomedicinal plants conserved by Garasia tribes of Sirohi district, Rajasthan, India. Indian J. Nat. Prod. Resour. 2010, 1, 500–506. [Google Scholar]
- Marwah, R.G.; Fatope, M.O.; Mahrooqi, R.A.; Varma, G.B.; Abadi, H.A.; Al-Burtamani, S.K.S. Antioxidant capacity of some edible and wound healing plants in Oman. Food Chem. 2007, 101, 465–470. [Google Scholar] [CrossRef]
- Manosroi, J.; Moses, Z.Z.; Manosroi, W.; Manosroi, A. Hypoglycemic activity of Thai medicinal plants selected from the Thai/Lanna Medicinal Recipe Database MANOSROI II. J. Ethnopharmacol. 2011, 138, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Baghel, U.S.; Gautam, A.; Baghel, D.S.; Yadav, D.; Malik, J.; Yadav, R. The genus Anogeissus: A review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 194, 30–56. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, A.; Latifou, L.; Dodehe, Y.; Cyril, A.; Catherine, V.-S. In vitro antiplasmodial and antileishmanial activities of flavonoids from Anogeissus leiocarpus (Combretaceae). Int. J. Pharm. Sci. Rev. Res. 2011, 11, 1–6. [Google Scholar]
- Miller, A.G.; Morris, M. Plants of Dhofar: The Southern Region of Oman; Traditional, Economic and Medicinal Uses; The Office of The Adviser for Conservation of The Environment: Muscat, Oman, 1988; ISBN 0715708082. [Google Scholar]
- Al-Noumani, A.J.; Al-Qasmi, M.Z.J.; Al-Shabibi, A.S.A.; Al-Mashani, S.A.I.; Said, S.A. Antimicrobial, antioxidant and cytotoxic activities of Anogeissus dhofarica from Oman. Int. J. Recent Adv. Pharm. Res. 2013, 3, 35–38. [Google Scholar]
- Maqsood, R.; Khan, F.; Ullah, S.; Khan, A.; Al-Jahdhami, H.; Hussain, J.; Weli, M.; Maqsood, D.; Rahman, S.M.; Hussain, A.; et al. Evaluation of antiproliferative, antimicrobial, antioxidant, antidiabetic and phytochemical analysis of Anogeissus dhofarica A. J. Scott. Antibiotics 2023, 12, 354. [Google Scholar] [CrossRef]
- Abuarqoub, D.; Aburayyan, W.; Rashan, L.; Dayyih, W.A.; Al-Matubsi, H.Y. Phytochemical analysis and in vitro investigation of wound healing, cytotoxicity, and inflammatory response in potentially active extracts of Anogeissus dhofarica. J. Appl. Pharm. Sci. 2024, 14, 152–162. [Google Scholar] [CrossRef]
- Mei, Y.; Hu, Y.; Tao, X.; Shang, J.; Qian, M.; Suo, F.; Li, J.; Cao, L.; Wang, Z.; Xiao, W. Chemical profiling of Shen-Wu-Yi-Shen tablets using UPLC-Q-TOF-MS/MS and its quality evaluation based on UPLC-DAD combined with multivariate statistical analysis. J. Chromatogr. Sci. 2024, 62, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Kuivila, H.G.; Nahabedian, K.V. Electrophilic displacement reactions. X. General acid catalysis in the protodeboronation of areneboronic acids 1–3. J. Am. Chem. Soc. 1961, 83, 2159–2163. [Google Scholar] [CrossRef]
- Yan, P.; Zeng, R.; Bao, B.; Yang, X.-M.; Zhu, L.; Pan, B.; Niu, S.-L.; Qi, X.-W.; Li, Y.-L.; Ouyang, Q. Red light-induced highly efficient aerobic oxidation of organoboron compounds using spinach as a photocatalyst. Green Chem. 2022, 24, 9263–9268. [Google Scholar] [CrossRef]
- Meng, Q.; Li, G.; Luo, B.; Wang, L.; Lu, Y.; Liu, W. Screening and isolation of natural antioxidants from Ziziphora clinopodioides Lam. with high performance liquid chromatography coupled to a post-column Ce(IV) reduction capacity assay. RSC Adv. 2016, 6, 62378–62384. [Google Scholar] [CrossRef]
- Zaher, A.M.; Anwar, W.S.; Makboul, M.A.; Abdel-Rahman, I.A.M. Potent anticancer activity of (Z)-3-hexenyl-β-D-glucopyranoside in pancreatic cancer cells. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 2311–2320. [Google Scholar] [CrossRef]
- Xu, K.-Z.; Xiang, S.-L.; Wang, Y.-J.; Wang, B.; Jia, A.-Q. Methyl gallate isolated from partridge tea (Mallotus oblongifolius (Miq.) Müll.Arg.) inhibits the biofilms and virulence factors of Burkholderia thailandensis. J. Ethnopharmacol. 2024, 320, 117422. [Google Scholar] [CrossRef] [PubMed]
- Terfassi, S.; Dauvergne, X.; Cérantola, S.; Lemoine, C.; Bensouici, C.; Fadila, B.; Christian, M.; Marchioni, E.; Benayache, S. First report on phytochemical investigation, antioxidant and antidiabetic activities of Helianthemum getulum. Nat. Prod. Res. 2022, 36, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-R.; Kuo, Y.-H.; Ho, Y.-L.; Wang, C.-Y.; Yang, C.-S.; Lin, C.-W.; Chang, Y.-S. Studies on cytotoxic constituents from the leaves of Elaeagnus oldhamii Maxim. in non-small cell lung cancer A549 cells. Molecules 2014, 19, 9515–9534. [Google Scholar] [CrossRef]
- Lee, H.-S.; Jung, S.-H.; Yun, B.-S.; Lee, K.-W. Isolation of chebulic acid from Terminalia chebula Retz. and its antioxidant effect in isolated rat hepatocytes. Arch. Toxicol. 2007, 81, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, H.W.; Yang, H.; Sung, S.H. Hydrolyzable tannins from the fruits of Terminalia chebula Retz and their α-glucosidase inhibitory activities. Phytochemistry 2017, 137, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-Y.; Lan, Q.; He, S.; Su, B.-J.; Wang, Y.-Q.; Liao, H.-B.; Wang, H.-S.; Liang, D. Chebulic acid derivatives from Balakata baccata and their antineuroinflammatory and antioxidant activities. Bioorg. Chem. 2021, 116, 105332. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yang, L.; Zhai, L.; Huang, Y.; Chen, F.; Duan, W.; Yang, J. Methyl brevifolincarboxylate, a novel influenza virus PB2 inhibitor from Canarium album (Lour.) Raeusch. Chem. Biol. Drug Des. 2020, 96, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-N.; Su, B.-J.; Wang, Y.-Q.; Liao, H.-B.; Chen, Z.-F.; Liang, D. Isolation, absolute configuration, and biological activities of chebulic acid and brevifolincarboxylic acid derivatives from Euphorbia hirta. J. Nat. Prod. 2020, 83, 985–995. [Google Scholar] [CrossRef]
- Navarro, F.; Hamri, S.; Reches, R.; Viñas, M.; Jahani, D.; Ginard, J.; Vilardell, J.; Abián, O.; Pujol, M.D. Convenient synthesis of ellagic acid from methyl gallate and SARS-CoV-2 3CLpro antiviral activity. Synthesis 2023, 55, 657–662. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, D.; Bisht, R.; Savita, K.; Singh, K.; Rani, P.; Chanda, D.; Dev, K. Psiguanol, a novel α-pyrone derivative from Psidium guajava leaves and vasorelaxant activity in rat aorta cells through intracellular cGMP-dependent opening of calcium-activated potassium channels. Nat. Prod. Res. 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.S.A.; El-Toumy, S.A.A.; Moharram, F.A.; Shalaby, N.M.M.; Ahmed, A.A.E. Pharmacologically active ellagitannins from Terminalia myriocarpa. Planta Med. 2002, 68, 523–527. [Google Scholar] [CrossRef]
- Shimomura, H.; Sashida, Y.; Adachi, T. Phenylpropanoid glucose esters from Prunus buergeriana. Phytochemistry 1988, 27, 641–644. [Google Scholar] [CrossRef]
- Ni, J.-C.; Shi, J.-T.; Tan, Q.-W.; Chen, Q.-J. Phenylpropionamides, piperidine, and phenolic derivatives from the fruit of Ailanthus altissima. Molecules 2017, 22, 2107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-J.; Wu, M.-D.; Khamthong, N.; Tseng, M. Polar metabolites from the actinobacterium Isoptericola chiayiensis isolated from mangrove soil. Chem. Nat. Compd. 2021, 57, 1134–1136. [Google Scholar] [CrossRef]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Abe, T.; Tanaka, T.; Yang, C.R.; Kouno, I. Phyllanemblinins A–F, new ellagitannins from Phyllanthus emblica. J. Nat. Prod. 2001, 64, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.-N.; Guo, L.-B.; He, J.; Liu, P.-H.; Tian, H.-Y.; Zhang, W.-K.; Xu, J.-K. Diverse gallotannins with α-glucosidase and α-amylase inhibitory activity from the roots of Euphorbia fischeriana Steud. Phytochemistry 2022, 202, 113304. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yang, H.; Kim, H.W.; Sung, S.H. New polyhydroxytriterpenoid derivatives from fruits of Terminalia chebula Retz. and their α-glucosidase and α-amylase inhibitory activity. Bioorg. Med. Chem. Lett. 2017, 27, 34–39. [Google Scholar] [CrossRef]
- Kappen, J.; Manurung, J.; Fuchs, T.; Vemulapalli, S.P.B.; Schmitz, L.M.; Frolov, A.; Agusta, A.; Muellner-Riehl, A.N.; Griesinger, C.; Franke, K.; et al. Challenging structure elucidation of lumnitzeralactone, an ellagic acid derivative from the mangrove Lumnitzera racemosa. Mar. Drugs 2023, 21, 242. [Google Scholar] [CrossRef] [PubMed]
- Ware, I.; Franke, K.; Dube, M.; El Enshasy, H.A.; Wessjohann, L.A. Characterization and bioactive potential of secondary metabolites isolated from Piper sarmentosum Roxb. Int. J. Mol. Sci. 2023, 24, 1328. [Google Scholar] [CrossRef]
- Utpal, B.K.; Sutradhar, B.; Zehravi, M.; Sweilam, S.H.; Panigraphy, U.P.; Urs, D.; Fatima, A.F.; Nallasivan, P.K.; Chabra, G.S.; Sayeed, M.; et al. Polyphenols in wound healing: Unlocking prospects with clinical applications. Naunyn Schmiedeberg’s Arch. Pharmacol. 2024, 1–27. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).