Abstract
The addition reactions of water, alcohols, and primary and secondary amines to the 10-acetonitrilium derivative of nido-carborane were studied. Hydrolysis of 10-MeC≡N-7,8-C2B9H11 results in iminol 10-MeC(OH)=HN-7,8-C2B9H11, which, on treatment with a base, gives amide [10-MeC(=O)HN-7,8-C2B9H11]−. The reactions of 10-MeC≡N-7,8-C2B9H11 with alcohols lead to imidates 10-ROC(Me)=HN-7,8-C2B9H11 (R = Me, Et) as mixtures of E- and Z-isomers. In the solid state, 10-MeOC(Me)=HN-7,8-C2B9H11 adopts E-configuration. The reactions of 10-MeC≡N-7,8-C2B9H11 with primary amines result in amidines 10-RNHC(Me)=HN-7,8-C2B9H11 (R = Me, Et) as mixtures of E- and Z-isomers. In the solid state 10-EtNHC(Me)=HN-7,8-C2B9H11 was found to have the Z-configuration, which is stabilized by intramolecular N-H⋯H-B interactions between the NH group originating from the primary amine and the BH group of the carborane cage. These interactions are rather strong (3.7 kcal/mol) and are likely to persist in solution. The reactions of 10-MeC≡N-7,8-C2B9H11 with secondary acyclic (Me2NH, Et2NH) and cyclic (piperidine, morpholine) amines result in amidines 10-R2NC(Me)=HN-7,8-C2B9H11 (R = Me, Et; R2 = N(CH2)5, N(CH2CH2)2O) as single isomers, which, according to single crystal X-ray diffraction data, have the E-configuration.
1. Introduction
Derivatives of polyhedral boron hydrides (boranes, carboranes and metallacarboranes) have found application in a wide variety of fields, from the development of new materials [1,2,3,4,5,6,7,8,9,10] to medicine [11,12,13,14,15,16,17,18,19,20]. This requires the continuous development of new methods for the synthesis of various functional derivatives of polyhedral boron hydrides. As for anionic polyhedral boron hydrides, the most widely used method for their functionalization is based on the nucleophilic opening of their cyclic oxonium derivatives [21,22]. It is especially actively used for the synthesis of boron-containing biologically active compounds [22,23,24,25,26,27,28,29,30]. Another approach that has been in active development recently is based on nucleophilic addition to the activated triple bond of nitrilium derivatives of polyhedral boron hydrides [31]. This approach has been most widely applied for the preparation of derivatives of closo-decaborate [B10H10]2− and closo-dodecaborate [B12H12]2− anions [32,33,34,35,36].
Unlike the closo-decaborate and closo-dodecaborate anions, derivatives of the 7,8-dicarba-nido-undecaborate anion [7,8-C2B9H12]− (nido-carborane) are of interest not only in themselves, but also as ligands in the synthesis of various metallacarboranes [37,38,39,40]. Previously, we have studied the addition reactions of various nucleophiles to propionitrilium [10-EtC≡N-7,8-C2B9H11] [41,42,43] and 3-cyanoethoxypropionitrilium [10-NCCH2CH2O-CH2CH2C≡N-7,8-C2B9H11] [44] derivatives of the 7,8-dicarba-nido-undecaborate anion, whereas the reactions of its simplest acetonitrilium derivative have received little attention. In this contribution, we report the synthesis of iminol, imidates and amidines based on the acetonitrilium derivative of nido-carborane [10-MeC≡N-7,8-C2B9H11].
2. Results and Discussion
The acetonitrilium derivative of nido-carborane [10-MeC≡N-7,8-C2B9H11] (1) was prepared the first time by the reaction of the potassium salt K[7,8-C2B9H12] with acetonitrile in the presence of anhydrous FeCl3 in refluxing benzene. The reaction resulted in a mixture of 9- and 10-isomers, from which the more stable 10-isomer was isolated in 30% yield [45]. Later, [10-MeC≡N-7,8-C2B9H11] was prepared in 81% yield by the reaction of tetramethylammonium (Me4N)[7,8-C2B9H12] with anhydrous AlCl3 in acetonitrile in the presence of acetone [46]. Recently, it was found that the 10-acetonitrilium derivative of nido-carborane can be prepared in 86% yield by the reaction of the potassium salt K[7,8-C2B9H12] with acetonitrile in the presence of HgCl2 in refluxing benzene [41]. In this work, we used the last method to prepare the acetonitrilium derivative of nido-carborane.
Hydrolysis of 1 in boiling acetonitrile results in the corresponding N-protonated iminol 10-MeC(OH)=NH-7,8-C2B9H11 (2), which, on treatment with triethylamine, gives amide (Et3NH)[10-MeC(=O)NH-7,8-C2B9H11] (3) (Scheme 1). It is worth noting that, according to the 1H-1H NOESY NMR spectroscopy data (Figure S7), in solution the iminol 2 adopts mainly the E-configuration.
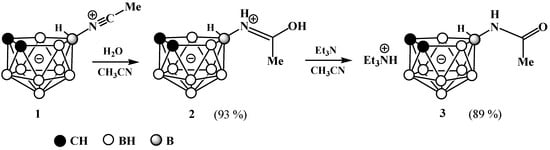
Scheme 1.
Hydrolysis of the acetonitrilium derivative 10-MeC≡N-7,8-C2B9H11 (1).
Reactions of 1 with methanol and ethanol under reflux lead to the corresponding imidates 10-ROC(Me)=NH-7,8-C2B9H11 (R = Me (4) and Et (5)) in the form of mixtures of E- and Z-isomers, which, unlike derivatives based on the propionitrile derivative [41], cannot be separated by column chromatography on silica (Scheme 2). This can probably be explained by a decrease in the isomerization energy of the azomethine fragment when the ethyl group is replaced by a smaller methyl group. In an acetone solution, the ratio of E- and Z-isomers is about 3:1. The assignment of E- and Z-isomers of the synthesized imidates was performed by analogy with the imidates derived from the propionitrile derivative of nido-carborane [41]. In general, in the case of Z-isomers, the 1H NMR spectral patterns are noticeably broader than in the case of E-isomers and the signal of the OR groups in the spectra of E-isomers is low-field shifted by ~0.15 ppm, whereas the signals of the Me groups are high-field shifted by ~0.15 ppm relative to those for Z-isomers. In particular, the singlets of the MeO and Me groups of the E-isomer in the spectrum of 10-MeOC(Me)=NH-7,8-C2B9H11 are located at 4.34 and 2.62 ppm, respectively, while the corresponding signals of the Z-isomer are at 4.17 and 2.76 ppm (Figure S16). Similarly, in the spectrum of 10-EtOC(Me)=NH-7,8-C2B9H11, the signals of the methylene group of the EtO fragment and the amidine methyl group of the E-isomer are located at 4.66 and 2.58 ppm, respectively, while the corresponding signals of the Z-isomer appear at 4.49 and 2.75 ppm (Figure S22). It is worth noting that the differences in the chemical shifts of the signals in the 11B NMR spectra of the E- and Z-isomers of the imidates are insignificant, which leads to an overlapping of the signals with some distortion of their symmetry.
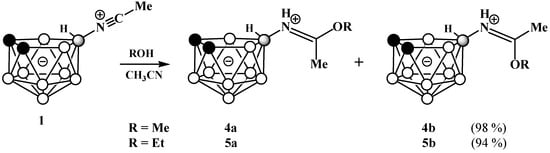
Scheme 2.
Synthesis of imidates 10-MeOC(Me)=NH-7,8-C2B9H11 (4) and 10-EtOC(Me)=HN-7,8-C2B9H11] (5).
The structure of methyl imidate 4 was determined by single crystal X-ray diffraction. In the solid state, 10-MeOC(Me)=NH-7,8-C2B9H11 adopts the E-configuration (Figure 1, Table 1). It should be noted that the E-configuration in the solid state is characteristic of the iminols based on 7,8-dicarba-nido-undecaborate [41,44], closo-decaborate [47,48] and closo-dodecaborate [36] anions.
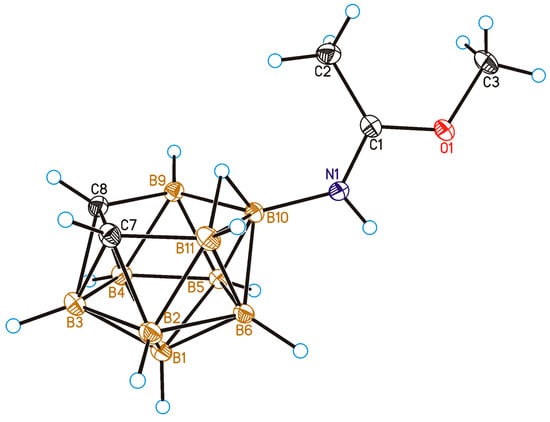
Figure 1.
General view of compound 10-MeOC(Me)=NH-7,8-C2B9H11 (4) showing atomic numbering. Thermal ellipsoids are drawn at 50% probability level.

Table 1.
Selected geometrical parameters of molecules 4, 6, and 7. Comparison of experimental and calculated results for corresponding conformations of substituents.
Reactions of 1 with methylamine and ethylamine give the corresponding amidines 10-RNHC(Me)=NH-7,8-C2B9H11 (R = Me (6) and Et (7)) as mixtures of E- and Z-isomers, which cannot be separated by column chromatography on silica (Scheme 3).
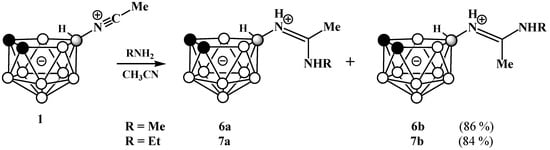
Scheme 3.
Synthesis of amidines 10-MeNHC(Me)=NH-7,8-C2B9H11 (6) and 10-EtNHC(Me)=HN-7,8-C2B9H11] (7).
In an acetone solution, the ratio of E- and Z-isomers is about 2:1. The behavior of the signals of the Me and RNH groups in the 1H NMR spectra of the E- and Z-isomers of amidines shows the same patterns as in the spectra of the imidates, but the difference in chemical shifts in this case is somewhat larger and amounts to ~0.2 ppm. At the same time, significant differences are observed in the 11B NMR spectra of the amidine isomers. A part of the signals is split into two unequal components, the integral ratio of which is almost equal to the ratio of the E- and Z-isomers determined from the 1H NMR spectra. The strongest splitting is observed for the signals corresponding to the boron atoms of the open pentagonal face of nido-carborane B(10) and B(9,11), as well as the antipodal boron atoms B(3) and B(2,4), while the signals of the B(1) and B(5,6) atoms remain unchanged. Such changes in the 11B NMR spectrum pattern may indicate a significant interaction between the substituent and the BH groups of the pentagonal face of nido-carborane, observed for one of the isomers. This is a rather rare case; however, similar intramolecular interactions were observed earlier in the case of 10-dialkylsulfonium derivatives of nido-carborane [49].
Therefore, it was particularly interesting to determine the structure of the obtained N-monoalkylamidine derivatives of nido-carborane. The structures of N-methyl amidine 6 and N-ethyl amidine 7 were determined by single crystal X-ray diffraction (Figure 2).
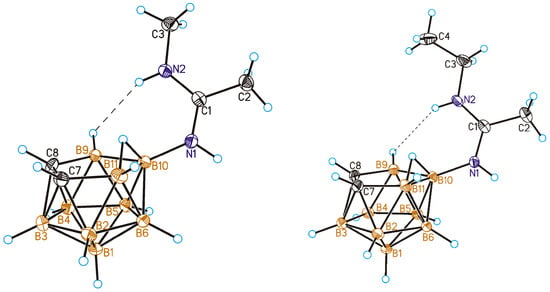
Figure 2.
General view of compounds 10-MeNHC(Me)=NH-7,8-C2B9H11 (6) (left) and 10-EtNHC(Me)=NH-7,8-C2B9H11 (7) (right) showing atomic numbering. The short N(2)-H⋯H-B(9) contacts are shown by dashed lines. Thermal ellipsoids are drawn at 50% probability level.
In the solid state, both compounds adopt the Z-configuration. There are short intramolecular BH⋯HN contacts between the NH group originating from the primary amine and the BH group of the carborane cage (2.24(3) Å for 6, and 2.34(2) Å for 7), which confirms our suggestion. It is worth noting that similar intramolecular BH⋯HN contacts with the Z-configuration of the amidine fragment were found earlier in the solid-state structures of N-monosubstituted amidines based on closo-decaborate [32,34,50,51] and closo-dodecaborate [52,53] anions and cobalt and iron bis(dicarbollides) [54,55].
Both the imidate and amidine substituents in the obtained nido-carborane derivatives adopt a nearly planar geometry, which implies delocalization of the electron density over this fragment. Indeed, when the weak OR donor group is replaced by a stronger NR’R” donor group an elongation of the N(1)=C(1) double bond and a shortening of the bond B(10)-N(1) are observed (Table 1 and Table 2).

Table 2.
Selected geometrical parameters of molecules 8–11. Comparison of experimental and calculated results for corresponding conformations of substituents.
To clarify the role of intramolecular BH⋯HN contacts in the stabilizing the Z-configuration of the amidine fragment in the solid state as well as to obtain further confirmation of existence of intramolecular interactions in solution in the case of N-alkyl-substituted amidine derivatives and their absence in other cases, we have carried out quantum chemical calculations using PBE0 functional and triple-zeta basis sets, which were utilized in our recent studies on carboranes [56,57]. For each molecule, both the E- and Z-conformations were calculated. It appeared that for all five molecules, experimentally observed conformation in their crystals is also more energetically preferred in the isolated state. For compounds 4, 8–11 (see below) no strong intramolecular contacts between substituent and carborane cage are observed. Differences in the energy of the E- and Z-conformations vary in the range of 2–5 kcal/mol. For compounds 6 and 7, somewhat different results are obtained. In the experimentally observed and energetically preferred Z-conformation, relatively strong intramolecular N-H⋯H-B noncovalent contact (4.1 kcal/mol for 6 and 3.7 kcal/mol for 7, Figure 2) is formed while no intramolecular contacts are found in the E-conformation. As a result, the difference between the E- and Z-conformations is more pronounced and corresponds to 6.9 kcal/mol for 6 and 7.3 kcal/mol for 7. This is more than sufficient to form the Z-conformer exclusively in the solid state, whereas in solution, competition for hydrogen bonding with the solvent will lead to an equilibrium mixture of the E- and Z-conformers.
In contrast to primary amines, the reactions with dimethylamine and diethylamine lead to the corresponding amidines 10-R2NC(Me)=NH-7,8-C2B9H11 (R = Me (8) and Et (9)) as single isomers (Scheme 4).
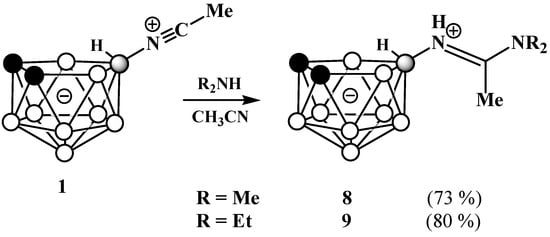
Scheme 4.
Synthesis of amidines 10-Me2NC(Me)=NH-7,8-C2B9H11 (8) and 10-Et2NC(Me)=HN-7,8-C2B9H11] (9).
In the 1H NMR and 13C NMR spectra of amidines 8 and 9, signals of non-equivalent methyl and ethyl groups, respectively, are observed, which indicates the presence of tautomeric equilibrium (Scheme 5).
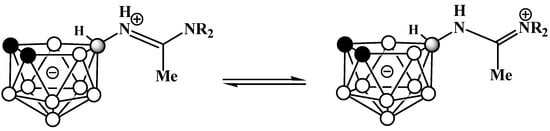
Scheme 5.
Tautomeric equilibrium in amidines 10-Me2NC(Me)=NH-7,8-C2B9H11 and 10-Et2NC(Me)=HN-7,8-C2B9H11].
The structures of amidines 8 and 9 were determined by single crystal X-ray diffraction. Both of them have the E-conformation (Figure 3, Table 2), which is characteristic of other N,N-disubstituted amidines based on 7,8-dicarba-nido-undecaborate [42], closo-decaborate [58] and closo-dodecaborate [36] anions and cobalt bis(dicarbollide) [54].
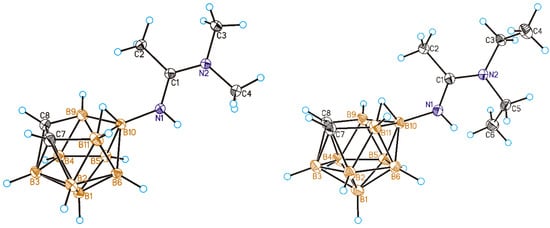
Figure 3.
General view of compounds 10-Me2NC(Me)=NH-7,8-C2B9H11 (8) (left) and 10-Et2NC(Me)=NH-7,8-C2B9H11 (9) (right) showing atomic numbering. Thermal ellipsoids are drawn at 50% probability level.
In a similar way, reactions of the 10-acetonitrilium derivative of nido-carborane 1 with piperidine and morpholine lead to the corresponding cyclic amidines 10-(CH2)5NC(Me)=NH-7,8-C2B9H11 (10) and 10-O(CH2CH2)2NC(Me)=NH-7,8-C2B9H11 (11) (Scheme 6). The structures of amidines 10 and 11 were determined by single crystal X-ray diffraction (Figure 4).
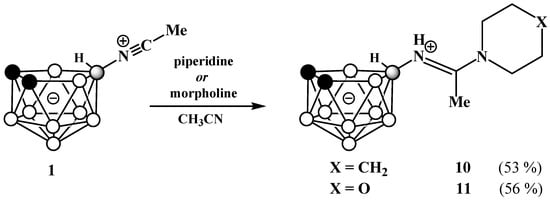
Scheme 6.
Synthesis of cyclic amidines 10-(CH2)5NC(Me)=NH-7,8-C2B9H11 (10) and 10-O(CH2CH2)2NC(Me)=NH-7,8-C2B9H11 (11).
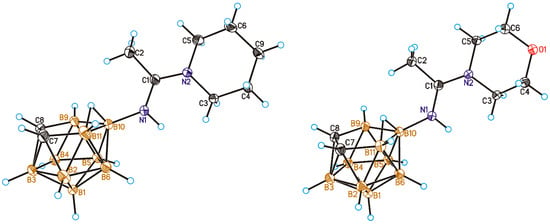
Figure 4.
General view of compounds 10-(CH2)5NC(Me)=NH-7,8-C2B9H11 (10) (left) and 10-O(CH2CH2)2NC(Me)=NH-7,8-C2B9H11 (11) (right) showing atomic numbering. Thermal ellipsoids are drawn at 50% probability level.
It was interesting to compare the bond lengths in central fragments of amidine derivatives of various polyhedral boron hydrides. For this purpose, the structure data for amidine derivatives of nido-carborane, closo-dodecaborate and closo-decaborate anions with methyl group and morpholine fragment [B]-NH=C(Me)N(CH2CH2)2O were analyzed. It was found that, whereas in the nido-carborane derivative the N(1)=C(1) bond is only slightly shorter than the C(1)-N(2) bond, on moving to the closo-dodecaborate and then to the closo-decaborate derivatives a noticeable shortening of the first and lengthening of the second bond is observed (Table 3).

Table 3.
Selected bond lengths in amidine derivatives of polyhedral boron hydrides [B]-NH=C(Me)N(CH2CH2)2O.
The observed regular change in the bond lengths in the amidine fragment is in good agreement with the increase in the electron-donor ability of polyhedral boron hydrides in the series [B10H10]2− > [B12H12]2− > [7,8-C2B9H12]−, previously discovered based on the 1H NMR spectral data of their derivatives [59].
3. Materials and Methods
3.1. General Methods
The acetonitrilium derivative of nido-carborane [10-MeC≡N-7,8-C2B9H11] (1) was synthesized as described in the literature [41]. Acetonitrile was dried using standard procedure [60]. Methanol, ethanol, methylamine, ethylamine, dimethylamine, diethylamine, triethylamine, piperidine and morpholine were commercial analytical grade reagents and used without further treatment. All reactions were carried out in air. The reaction progress was monitored by thin layer chromatography (Merck F254 silica gel on aluminum plates) and visualized using 0.5% PdCl2 in 1% HCl in aq. MeOH (1:10). The NMR spectra at 400.1 MHz (1H), 128.4 MHz (11B) and 100.0 MHz (13C) were recorded with a Varian Inova-400 spectrometer. The signals in the 1H and 13C NMR spectra were referenced to Me4Si, whereas the signals in the 11B NMR spectra were referenced to BF3·Et2O. All spectra were processed in MestRenova version 6.0.2–5475. When processing 11B and 11B{1H} NMR spectra, the baseline alignment was applied to improve the quality of integration. Infrared spectra were recorded on FSM-2201 (INFRASPEC, Saint Petersburg, Russia) instrument. High resolution mass spectra (HRMS) were measured on a Bruker micrOTOF II instrument using electrospray ionization (ESI). The measurements were made in a positive ion mode (interface capillary voltage—4500 V) or in a negative ion mode (3200 V); mass range was calculated from m/z 50 to m/z 3000; external or internal calibration was performed with ESI Tuning Mix, Agilent. A syringe injection was used for solutions in acetonitrile (flow rate 3 mL/min−1). Nitrogen was applied as a dry gas; the interface temperature was set at 180 °C.
3.2. Synthesis of 10-MeC(OH)=NH-7,8-C2B9H11 (2)
Water (2 mL) was added to a solution of the acetonitrilium derivative of nido-carborane 1 (0.20 g, 1.15 mmol) in acetonitrile (5 mL) and the solution was heated under reflux for 3 h. The reaction mixture was cooled to room temperature and evaporated to dryness in vacuo to obtain 0.21 g (93%) of a white crystalline product. 1H NMR (acetone-d6, ppm): δ 10.99 (1H, br s, OH), 8.62 (1H, br s, NH=C), 2.40 (3H, s, CH3), 1.82 (2H, br s, CHcarb), 3.1–−0.1 (8H, br m, BH), −0.45 (1H, br q (1:1:1:1), J = 55 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 176.7 (NH=C), 41.8 (CHcarb), 20.2 (CH3). 11B NMR (acetone-d6, ppm): δ −10.3 (2B, d, J = 132 Hz, B(9,11)), −16.1 (2B, d, J = 133 Hz, B(5,6)), −20.3 (1B, d, J = 144 Hz, B(3)), −22.6 (2B, d, J = 151 Hz, B(2,4)), −25.3 (1B, d, J = 51 Hz, B(10)-N), −39.2 (1B, d, J = 140 Hz, B(1)). IR (film, cm−1): 3345 (ν(O-H)), 3183 (ν(N-H)), 2944 (ν(C-H)), 2536 (ν(B-H)), 1652 (ν(C=N)). HRMS-ESI (m/z): obsd. 191.2030 [M-H]+, calcd. for C4H16B9NO 191.2025 [M-H]+.
3.3. Synthesis of (Et3NH)[10-MeC(=O)NH-7,8-C2B9H11] (3)
Triethylamine (2 mL) was added to a solution of 2 (0.15 g, 0.78 mmol) in acetonitrile (5 mL) and the solution was heated under reflux for 15 min. The reaction mixture was cooled to room temperature and evaporated to dryness in vacuo to obtain 0.20 g (89%) of a yellow crystalline product. 1H NMR (acetone-d6, ppm): δ 6.02 (1H, br s, NH=C), 3.34 (6H, q, J = 7.3 Hz, (CH3CH2)3NH+), 1.89 (3H, s, CH3), 1.70 (2H, br s, CHcarb), 1.39 (1.41 (9H, t, J = 7.3 Hz, (CH3CH2)3NH+), 2.9–−0.1 (8H, br m, BH), −0.48 (1H, br q (1:1:1:1), J = 61 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 173.9 (NH=C), 46.6 ((CH3CH2)3NH+), 40.3 (CHcarb), 23.9 (CH3), 8.5 ((CH3CH2)3NH+). 11B NMR (acetone-d6, ppm): δ −10.2 (2B, d, J = 133 Hz, B(9,11)), −16.1 (2B, d, J = 132 Hz, B(5,6)), −21.3 (1B, d, J = 177 Hz, B(3)), −23.0 (2B, d, J = 143 Hz, B(2,4)), −23.5 (1B, s, B(10)-N), −39.5 (1B, d, J = 140 Hz, B(1)). IR (film, cm−1): 3413 (ν(N–H)), 2999 (ν(C-H)), 2526 (ν(B–H)), 1618 (ν(C=O)). HRMS-ESI (m/z): obsd. 190.2057 [M+H]+, calcd. for C4H15B9NO: 190.2059 [M+H]+.
3.4. Synthesis of 10-MeOC(Me)=NH-7,8-C2B9H11 (4)
A solution of the acetonitrilium derivative of nido-carborane 1 (0.15 g, 0.86 mmol) in anhydrous methanol (3 mL) was heated under reflux for 2 h. The reaction mixture was cooled to room temperature and evaporated to dryness in vacuo. The residue was subjected to column chromatography on silica with dichloromethane as the eluent to give a white crystalline product, which was a mixture of 4a and 4b isomers in a ratio of 3.5:1. Yield 0.17 g (98%). 11B NMR (acetone-d6, ppm): δ −10.3 (2B, d, J = 138 Hz, B(9,11), 4a+4b), −16.1 (2B, d, J = 132 Hz, B(5,6), 4a+4b), −20.1 (1B, d, J = 162 Hz, B(3), 4a+4b), −21.2–−24.4 (2.22B, d, J = 153 Hz, B(2,4), 4a+4b, B(10)-N, 4b), −25.5 (0.78B, d, J = 43 Hz, B(10)-N, 4a), −39.1 (1B, d, J = 140 Hz, B(1), 4a+4b). IR (film, cm−1): 3354 (ν(N-H)), 2934 (ν(C-H)), 2551 (ν(B-H)), 1637 (ν(C=N)). HRMS-ESI (m/z): obsd. 224.2608 [M+NH4]+, calcd. for C5H18B9NO 224.2604 [M+NH4]+. 4a: 1H NMR (acetone-d6, ppm): δ 9.04 (1H, br s, NH=C), 4.34 (3H, s, OCH3), 2.62 (3H, s, CH3), 1.86 (2H, br s, CHcarb), 3.0–−0.1 (8H, br m, BH), −0.45 (1H, br q (1:1:1:1), J = 59 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 178.7 (NH=C), 59.0 (OCH3), 41.8 (CHcarb), 17.32 (CH3). 4b: 1H NMR (acetone-d6, ppm): δ 8.50 (1H, br s, NH=C), 4.17 (3H, s, OCH3), 2.76 (3H, s, CH3), 1.94 (2H, s, CHcarb), 3.0–−0.1 (8H, br m, BH), −0.81 (1H, br q (1:1:1:1), J = 51 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 178.7 (NH=C), 57.7 (OCH3), 41.8 (CHcarb), 17.26 (CH3).
3.5. Synthesis of 10-EtOC(Me)=NH-7,8-C2B9H11 (5)
The procedure was similar to that described for the synthesis of compound 4 using the acetonitrilium derivative of nido-carborane 1 (0.15 g, 0.86 mmol) and anhydrous ethanol (3 mL) to give a white crystalline product, which was a mixture of isomers 5a and 5b in a 2.9: 1 ratio. Yield 0.18 g (94%). 11B NMR (acetone-d6, ppm): δ −10.3 (2B, d, J = 140 Hz, B(9,11), 5a+5b), −16.1 (2B, d, J = 133 Hz, B(5,6), 5a+5b), −20.1 (1B, d, J = 162 Hz, B(3), 5a+5b), −21.3–−24.3 (2.26B, d, J = 150 Hz, B(5,6), 5a+5b, B(10)-N, 5b), −25.4 (0.74B, d, J = 45 Hz, B(10)-N, 5a), −39.2 (1B, d, J = 140 Hz, B(1), 5a+5b). IR (film, cm−1): 3329 (ν(N-H)), 2928 (ν(C-H)), 2540 (ν(B-H)), 1637 (ν(C=N)). HRMS-ESI (m/z): obsd. 259.2056 [M+K]+, calcd. for C6H21B9NO: 259.2057 [M + K]+. 5a: 1H NMR (acetone-d6, ppm): δ 8.93 (1H, br s, NH=C), 4.66 (2H, q, J = 7.1 Hz, CH3CH2O), 2.58 (3H, s, CH3), 1.93 (2H, br s, CHcarb), 1.51 (3H, t, J = 7.0 Hz, CH3CH2O) 2.9–−0.1 (8H, br m, BH), −0.40 (1H, br q (1:1:1:1), J = 51 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 177.7 (NH=C), 69.0 (CH3CH2O), 41.7 (CHcarb), 17.6 (CH3CH2O), 13.9 (CH3). 5b: 1H NMR (acetone-d6, ppm): δ 8.40 (1H, br s, NH), 4.49 (2H, q, J = 7.0 Hz, CH3CH2O), 2.75 (3H, s, CH3), 1.93 (2H, br s, CHcarb), 1.38 (3H, t, J = 7.0 Hz, CH3CH2O) 2.9–−0.1 (8H, br m, BH), −0.9 (1H, br q (1:1:1:1), J = 56 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 178.9 (NH=C), 67.6 (CH3CH2O), 41.7 (CHcarb), 17.5 (CH3CH2O), 13.2 (CH3).
3.6. Synthesis of 10-MeNHC(Me)=NH-7,8-C2B9H11 (6)
Methylamine (2 mL) was added to a −30 °C solution of the acetonitrilium derivative of nido-carborane 1 (0.20 g, 1.15 mmol) in acetonitrile (5 mL), the reaction mixture was allowed to warm to room temperature and stirred for 15 min. The reaction mixture was evaporated to dryness in vacuo and the residue was subjected to column chromatography on silica with dichloromethane as the eluent to give a white crystalline product, which was a mixture of 6a and 6b isomers in a ratio of 1.6:1. Yield 0.20 g (86%). 11B NMR (acetone-d6, ppm): δ −10.6 (0.77B, d, J = 139 Hz, B(9,11), 6b), −12.3 (1.23B, d, J = 139 Hz, B(9,11), 6a), −15.2 (2B, d, J = 136 Hz, B(5,6), 6a+6b), −20.0–−23.5 (3.38 B, m, B(2,3,4), 6a+6b + B(10)-N, 6b,), −24.2 (0.62B, s, B(10)-N, 6a), −39.1 (1B, d, J = 141 Hz, B(1), 6a+6b). IR (film, cm−1): 3397 (ν(N-H)), 3344 (ν(N-H)), 2934 (ν(C-H)), 2862 (ν(C-H)), 2532 (ν(B-H)), 1637 (ν(C=N)). HRMS-ESI (m/z): obsd. 223.2776 [M+NH4]+, calcd. for C5H19B9N2: 223.2764 [M+NH4]+. 6a: 1H NMR (acetone-d6, ppm): δ 8.17 (1H, br s, NHCH3), 7.23 (1H, br s, NH=C), 3.17 (3H, s, NHCH3), 2.31 (3H, s, CH3), 1.87 (2H, br s, CHcarb), 2.9–−0.1 (8H, br m, BH), −1.32 (1H, br q (1:1:1:1), J = 52 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 168.7 (NH=C), 42.3 (CHcarb), 29.8 (NHCH3), 17.6 (CH3). 6b: 1H NMR (acetone-d6, ppm): δ 8.38 (1H, br s, NHCH3), 6.22 (1H, br s, NH=C), 2.94 (3H, s, NHCH3), 2.57 (3H, s, CH3), 1.87 (2H, br s, CHcarb), 2.9–−0.1 (8H, br m, BH), −0.99 (1H, br q (1:1:1:1), J = 52 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 153.1 (NH=C), 42.3 (CHcarb), 27.6 (NHCH3), 19.1 (CH3).
3.7. Synthesis of 10-EtNHC(Me)=NH-7,8-C2B9H11 (7)
The procedure was similar to that described for the synthesis of compound 6 using the acetonitrilium derivative of nido-carborane (0.20 g, 1.15 mmol) and ethylamine (2 mL) to give a white crystalline product, which was a mixture of 7a and 7b isomers in a ratio of 2.1:1. Yield 0.21 g (84%). 11B NMR (acetone-d6, ppm): δ −10.7 (0.65B, d, J = 138 Hz, B(9,11), 7b), −12.3 (1.35B, d, J = 141 Hz, B(9,11), 7a), −15.3 (2B, d, J = 135 Hz, B(5,6), 7a+7b), −20.0–−23.5 (3.33B, m, B(2,3,4), 7a+7b + B(10)-N, 7b), −24.2 (0.68B, s, B(10)-N, 7a), −39.0 (1B, d, J = 141 Hz, B(1), 7a+7b). IR (film, cm−1): 3394 (ν(N-H)), 2928 (ν(C-H)), 2548 (ν(B-H)), 1642 (ν(C=N)). HRMS-ESI (m/z): obsd. 237.2925 [M+NH4]+, calcd. for C6H21B9N2: 237.2921 [M+NH4]+. 7a: 1H NMR (acetone-d6, ppm): δ 8.14 (1H, br s, NHCH2CH3), 7.23 (1H, br s, NH=C), 3.57 (2H, quint, J = 6.4 Hz, NHCH2CH3), 2.33 (3H, s, CH3), 1.90 (2H, br s, CHcarb), 1.32 (3H, t, J = 7.2 Hz, NHCH2CH3) 2.9–−0.1 (8H, br m, BH), −1.30 (1H, br q (1:1:1:1), J = 50 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 167.1 (NH=C), 42.5 (CHcarb), 38.7 (NHCH2CH3), 17.4 (CH3), 14.2 (NHCH2CH3). 7b: 1H NMR (acetone-d6, ppm): δ 8.24 (1H, br s, NHCH2CH3), 6.22 (1H, br s, NH=C), 3.35 (2H, quint, J = 6.4 Hz, NHCH2CH3), 2.57 (3H, s, CH3), 1.86 (2H, br s, CHcarb), 1.21 (3H, t, J = 7.1 Hz, NHCH2CH3) 2.9–−0.1 (8H, br m, BH), −0.97 (1H, br q (1:1:1:1), J = 50 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 153.3 (NH=C), 42.5 (CHcarb), 36.3 (NHCH2CH3), 19.2 (CH3), 12.3 (NHCH2CH3).
3.8. Synthesis of 10-Me2NC(Me)=NH-7,8-C2B9H11 (8)
The procedure was similar to that described for the synthesis of compound 6 using the acetonitrilium derivative of nido-carborane (0.20 g, 1.15 mmol) and dimethylamine (2 mL) to give white solid 8. Yield 0.18 g (73%). 1H NMR (acetone-d6, ppm): δ 5.89 (1H, br s, NH=C), 3.33 (3H, s, NCH3), 3.14 (3H, s, NCH3), 2.71 (3H, s, CH3), 1.87 (2H, br s, CHcarb), 2.8–−0.1 (8H, br m, BH), −1.03 (1H, br q (1:1:1:1), J = 60 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 167.3 (N=C), 41.8 (CHcarb), 40.6 (NCH3), 37.6 (NCH3),16.3 (CH3). 11B NMR (acetone-d6, ppm): δ −10.8 (2B, d, J = 139 Hz, B(9,11)), −15.1 (2B, d, J = 136 Hz, (B5,6)), −21.1 (1B, d, J = 146 Hz, (B3)), −22.0 (3B, d, J = 124 Hz, (B(2,4) + B(10)-N), −39.0 (1B, d, J = 142 Hz, B(1)). IR (film, cm−1): 3401 (ν(N-H)), 2962 (ν(C-H)), 2837 (ν(C-H)), 2549 (ν(B-H)), 1652 (ν(C=N)). HRMS-ESI (m/z); obsd. 217.2542 [M+H]+, calcd. for C6H21B9N2: 217.2532 [M+H]+.
3.9. Synthesis of 10-Et2NC(Me)=NH-7,8-C2B9H11 (9)
Diethylamine (2 mL) was added to a solution of the acetonitrilium derivative of nido-carborane 1 (0.20 g, 1.15 mmol) in acetonitrile (5 mL) and the reaction mixture was stirred for 15 min. The reaction mixture was evaporated to dryness in vacuo and the residue was subjected to column chromatography on silica with dichloromethane as the eluent to give white product 9. Yield 0.30 g (80%). 1H NMR (acetone-d6, ppm): δ 5.84 (1H, br s, NH=C), 3.63 (2H, q, J = 7.2 Hz, NCH2CH3), 3.56 (2H, q, J = 7.2 Hz, NCH2CH3), 2.72 (3H, s, CH3), 1.88 (2H, br s, CHcarb), 1.28 (3H, t, J = 7.2 Hz, NCH2CH3), 1.15 (3H, t, J = 7.2 Hz, NCH2CH3), 2.8–−0.1 (8H, br m, BH), −1.00 (1H, br q (1:1:1:1), J = 59 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 165.7 (N=C), 46.5 (NCH2CH3), 42.6 (NCH2CH3), 41.9 (CHcarb), 16.2 (CH3) 12.8 (NCH2CH3), 10.9 (NCH2CH3). 11B NMR (acetone-d6, ppm): δ −10.8 (2B, d, J = 138 Hz, B(9,11)), −15.1 (2B, d, J = 136 Hz, B(5,6)), −21.0 (1B, d, J = 142 Hz, B(3)), −22.0 (3B, d, J = 128 Hz, B(2,4) + B(10)-N), −39.1 (1B, d, J = 142 Hz, B(1)). IR (film, cm−1): 3416 (ν(N-H)), 2990 (ν(C-H)), 2529 (ν(B-H)), 1605 (ν(C=N)). HRMS-ESI (m/z): obsd. 245.2839 [M+H]+, calcd. for C8H25B9N2: 245.2845 [M+H]+.
3.10. Synthesis of 10-(CH2)5NC(Me)=NH-7,8-C2B9H11 (10)
The procedure was similar to that described for the synthesis of compound 9 using the acetonitrilium derivative of nido-carborane (0.29 g, 1.67 mmol) and piperidine (2 mL) to give white solid 10. Yield 0.23 g (53%). 1H NMR (acetone-d6, ppm): δ 6.16 (1H, br s, NH=C), 3.70 (2H, m, NCH2CH2CH2), 3.57 (2H, m, NCH2CH2CH2), 2.74 (3H, s, CH3), 1.87 (2H, br s, CHcarb), 1.71 (4H, m, NCH2CH2CH2 + NCH2CH2CH2), 1.64 (2H, m, NCH2CH2CH2), 2.9–−0.1 (8H, br m, BH), −1.02 (1H, br q (1:1:1:1), J = 58 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 165.3 (N=C), 49.5 (NCH2CH2CH2), 45.7 (NCH2CH2CH2), 41.9 (CHcarb), 25.7 (NCH2CH2CH2), 25.0 (NCH2CH2CH2), 23.3 (NCH2CH2CH2), 16.5 (CH3). 11B NMR (acetone-d6, ppm): δ −10.9 (2B, d, J = 137 Hz, B(9,11)), −15.2 (2B, d, J = 136 Hz, B(5,6)), −21.1 (1B, d, J = 136 Hz, B(3)), −21.9 (3B, d, J = 134 Hz, B(2,4) + B(10)-N), −39.1 (1B, d, J = 141 Hz, B(1)). IR (film, cm−1): 3416 (ν(N-H)), 2959 (ν(C-H)), 2514 (ν(B-H)), 1608 (ν(C=N)). HRMS-ESI (m/z): obsd. 257.2846 [M+H]+, calcd. for C9H25B9N2: 257.2845 [M+H]+.
3.11. Synthesis of 10-O(CH2CH2)2NC(Me)=NH-7,8-C2B9H11 (11)
The procedure was similar to that described for the synthesis of compound 9 using the acetonitrilium derivative of nido-carborane (0.27 g, 1.56 mmol) and morpholine (2 mL) to give white solid 11. Yield 0.23 g (56%). 1H NMR (acetone-d6, ppm): δ 6.33 (1H, br s, NH=C), 3.78 (4H, m, OCH2), 3.73 (2H, m, NCH2), 3.64 (2H, m, NCH2), 2.77 (3H, s, CH3), 1.88 (2H, br s, CHcarb), 3.0–−0.1 (8H, br m, BH), −1.00 (1H, br q (1:1:1:1), J = 59 Hz, BHB). 13C NMR (acetone-d6, ppm): δ 166.6 (N=C), 65.8 (OCH2), 65.2 (OCH2), 48.2 (NCH2), 44.7 (NCH2), 42.0 (CHcarb), 16.3 (CH3). 11B NMR (acetone-d6, ppm): δ −10.9 (2B, d, J = 142 Hz, B(9,11)), −15.3 (2B, d, J = 136 Hz, B(5,6)), −20.9 (1B, d, J = 152 Hz, B(3)), −22.0 (3B, d, J = 130 Hz, B(2,4) + B(10)-N), −39.0 (1B, d, J = 142 Hz, B(1)). IR (film, cm−1): 3374 (ν(N-H)), 2934 (ν(C-H)), 2517 (ν(B-H)), 1608 (ν(C=N)). HRMS-ESI (m/z): obsd. 259.2646 [M+H]+, calcd. for C8H23B9N2O: 259.2638 [M+H]+.
3.12. Single Crystal X-Ray Diffraction Study
Single crystal X-ray diffraction experiments for compounds 4 and 6–11 were carried out using a SMART APEX2 CCD diffractometer (λ(Mo-Kα) = 0.71073 Å, graphite monochromator, ω-scans) at 100 K. Collected data were processed by the SAINT and SADABS programs incorporated into the APEX2 program package [61]. The structures were solved by the direct methods and refined by the full-matrix least-squares procedure against F2 in anisotropic approximation. The refinement was carried out with the SHELXTL program [62]. The details of data collection and crystal structure refinement are summarized in Tables S1 and S2 along with CCDC numbers which contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.
3.13. Quantum Chemical Calculations
All quantum chemical calculations were carried out with the Gaussian 09 program [63]. The PBE0 functional with the triple zeta basis set was found to be reliable for the optimization of molecular geometries of different families of compounds and calculation of noncovalent intra- and intermolecular interactions [49,56,64,65] and was adopted throughout this study.
All calculated molecular conformers were fully optimized and converged to the true energy minima. Theoretical electron density was treated within the AIM approach [66] using the AIMAll program package [67]. For the estimation of energy of noncovalent atom–atom contact, we used the E = 1/2V(r) formula [68,69], in which V(r) is the potential energy density at the bond critical point between interacting atoms. It has frequently been shown that this approach demonstrates realistic energetic characteristics for noncovalent interactions [49,56,64,65,70].
4. Conclusions
The addition reactions of various nucleophiles to the 10-acetonitrilium derivative of nido-carborane were studied. Hydrolysis of 10-MeC≡N-7,8-C2B9H11 leads to the N-protonated iminol 10-MeC(OH)=HN-7,8-C2B9H11, which, on treatment with a base, gives amide [10-MeC(=O)HN-7,8-C2B9H11]−. The reactions of 10-MeC≡N-7,8-C2B9H11 with alcohols result in imidates 10-ROC(Me)=HN-7,8-C2B9H11 (R = Me, Et) as mixtures of E- and Z-isomers. In the solid state, 10-MeOC(Me)=HN-7,8-C2B9H11 was found to adopt the E-conformation. The reactions of 10-MeC≡N-7,8-C2B9H11 with primary amines give amidines 10-RNHC(Me)=HN-7,8-C2B9H11 (R = Me, Et) as mixtures of E- and Z-isomers. The single crystal X-ray diffraction study revealed that in the solid state 10-EtNHC(Me)=HN-7,8-C2B9H11 and 10-EtNHC(Me)=HN-7,8-C2B9H11 have the Z-conformation, which is stabilized by intramolecular N-H⋯H-B interactions between the NH group originating from the primary amine and the BH group of the carborane cage. These interactions are rather strong (3.7–4.1 kcal/mol) and are likely to persist in solution. The reactions of 10-MeC≡N-7,8-C2B9H11 with secondary acyclic (Me2NH, Et2NH) and cyclic (piperidine, morpholine) amines result in amidines 10-R2NC(Me)=HN-7,8-C2B9H11 (R = Me, Et; R2 = N(CH2)5, N(CH2CH2)2O) as single isomers, which according to single crystal X-ray diffraction data have the E-conformation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30040828/s1, Figures S1–S66: NMR spectra of compounds 2–11; Table S1: Crystallographic data for compounds 4, 6, and 7; Table S2: Crystallographic data for compounds 8–11.
Author Contributions
Synthesis, K.R.P., E.V.B. and M.Y.S.; NMR spectroscopy, S.A.A. and M.Y.S.; single crystal X-ray diffraction, K.Y.S.; quantum-chemical calculations, K.Y.S.; manuscript preparation, I.B.S., K.Y.S., E.V.B., S.A.A. and M.Y.S.; supervision, I.B.S.; project administration, V.I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (075-00276-25-00).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Crystallographic data for the structures of 10-MeOC(Me)=NH-7,8-C2B9H11 (4), 10-MeNHC(Me)=NH-7,8-C2B9H11 (6), 10-EtNHC(Me)=NH-7,8-C2B9H11 (7), 10-Me2NC(Me)=NH-7,8-C2B9H11 (8), 10-Et2NC(Me)=NH-7,8-C2B9H11 (9), 10-(CH2)5NC(Me)=NH-7,8-C2B9H11 (10), and 10-O(CH2CH2)2NC(Me)=NH-7,8-C2B9H11 (11) were deposited in the Cambridge Crystallographic Data Centre as supplementary publications CCDC 2413512 (for 4), 2422305 (for 6), 2413510 (for 7), 2413511 (for 8), 2413509 (for 9), 2422304 (for 10), and 2413508 (for 11). The Supplementary Materials contain NMR spectra for compounds 2–11.
Acknowledgments
The NMR spectra were obtained using equipment from the Center for Molecular Structure Studies at A.N. Nesmeyanov Institute of Organoelement Compounds, operating with financial support from the Ministry of Science and Higher Education of the Russian Federation.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wee, K.-R.; Cho, Y.-J.; Jeong, S.; Kwon, S.; Lee, J.-D.; Suh, I.-H.; Kang, S.O. Carborane-based optoelectronically active organic molecules: Wide band gap host materials for blue phosphorescence. J. Am. Chem. Soc. 2012, 134, 17982–17990. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, H.; Zhao, Q. Carboranes as a tool to tune phosphorescence. Chem. Eur. J. 2016, 22, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Planas, J.G.; Teixidor, F.; Viñas, C. N,O-Type carborane-based materials. Crystals 2016, 6, 50. [Google Scholar] [CrossRef]
- Li, Z.; Ma, C.; Wen, Y.; Wei, Z.; Xing, X.; Chu, J.; Yu, C.; Wang, K.; Wang, Z.-K. Highly conductive dodecaborate/MXene composites for high performance supercapacitors. Nano Res. 2020, 13, 196–202. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Timofeev, S.V.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B. Bis(dicarbollide) complexes of transition metals as a platform for molecular switches. Study of complexation of 8,8′-bis(methylsulfanyl) derivatives of cobalt and iron bis(dicarbollides). Molecules 2020, 25, 5745. [Google Scholar] [CrossRef] [PubMed]
- Ochi, J.; Tanaka, K.; Chujo, Y. Recent progress in the development of solid-state luminescent o-carboranes with stimuli responsivity. Angew. Chem. Int. Ed. 2020, 59, 9841–9855. [Google Scholar] [CrossRef]
- Li, S.; Qiu, P.; Kang, J.-X.; Shi, Z.; Zhang, Y.; Ma, Y.; Chen, X. Halogenated sodium/lithium monocarba-closo-decaborates: Syntheses, characterization, and solid-state ionic conductivity. Mater. Chem. Front. 2021, 5, 8037–8046. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, T.; Duttwyler, S.; Zhang, Y. Supramolecular Cu(II)–dipyridyl frameworks featuring weakly coordinating dodecaborate dianions for selective gas separation. CrystEngComm 2021, 23, 282–291. [Google Scholar] [CrossRef]
- Zhang, X.; Rendina, L.M.; Müllner, M. Carborane-containing polymers: Synthesis, properties, and applications. ACS Polym. Au 2024, 4, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lin, X.; Huang, J.; Zhang, L. Recent advances in carborane-based crystalline porous materials. Molecules 2024, 29, 3916. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Sjöberg, S. Polyhedral boron compounds as potential linkers for attachment of radiohalogens to targeting proteins and peptides. A review. Collect. Czech. Chem. Commun. 2002, 67, 913–935. [Google Scholar] [CrossRef]
- Řezáčová, P.; Pokorná, J.; Brynda, J.; Kožíšek, M.; Cígler, P.; Lepšík, M.; Fanfrlík, J.; Řezáč, J.; Grantz Šašková, K.; Sieglová, I.; et al. Design of HIV protease inhibitors based on inorganic polyhedral metallacarboranes. J. Med. Chem. 2009, 52, 7132–7141. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as pharmacophores: Properties, synthesis, and application strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef] [PubMed]
- Leśnikowski, Z.J. Challenges and opportunities for the application of boron clusters in drug design. J. Med. Chem. 2016, 59, 7738–7758. [Google Scholar] [CrossRef]
- Hu, K.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.-R. Boron agents for neutron capture therapy. Coord. Chem. Rev. 2020, 405, 213139. [Google Scholar] [CrossRef]
- Kugler, M.; Nekvinda, J.; Holub, J.; El Anwar, S.; Das, W.; Šícha, V.; Pospíšilová, K.; Fábry, M.; Král, V.; Brynda, J.; et al. Inhibitors of CA IX enzyme based on polyhedral boron compounds. ChemBioChem 2021, 22, 2741–2761. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.; Uchman, M. Boron cluster compounds as new chemical leads for antimicrobial therapy. Coord. Chem. Rev. 2021, 431, 213684. [Google Scholar] [CrossRef]
- Oloo, S.O.; Smith, K.M.; Vicente, M.G.H. Multi-functional boron-delivery agents for boron neutron capture therapy of cancers. Cancers 2023, 15, 3277. [Google Scholar] [CrossRef]
- Cebula, J.; Fink, K.; Boratyński, J.; Goszczyński, T.M. Supramolecular chemistry of anionic boron clusters and its applications in biology. Coord. Chem. Rev. 2023, 477, 214940. [Google Scholar] [CrossRef]
- Gos, M.; Cebula, J.; Goszczyński, T.M. Metallacarboranes in medicinal chemistry: Current advances and future perspectives. J. Med. Chem. 2024, 67, 8481–8501. [Google Scholar] [CrossRef]
- Semioshkin, A.A.; Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives of polyhedral boron hydrides and their synthetic applications. Dalton Trans. 2008, 977–992. [Google Scholar] [CrossRef]
- Druzina, A.A.; Shmalko, A.V.; Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives of cobalt and iron bis(dicarbollides) and their use in organic synthesis. Russ. Chem. Rev. 2021, 90, 785–830. [Google Scholar] [CrossRef]
- Tsurubuchi, T.; Shirakawa, M.; Kurosawa, W.; Matsumoto, K.; Ubagai, R.; Umishio, H.; Suga, Y.; Yamazaki, J.; Arakawa, A.; Maruyama, Y.; et al. Evaluation of a novel boron-containing α-D-mannopyranoside for BNCT. Cells 2020, 9, 1277. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Sivaev, I.B.; Dubey, R.D.; Semioshkin, A.; Shmal’ko, A.V.; Kosenko, I.D.; Lebedeva, K.V.; Mandal, S.; Sreejyothi, P.; Sarkar, A.; et al. Boron-containing lipids and liposomes: New conjugates of cholesterol with polyhedral boron hydrides. Chem. Eur. J. 2020, 26, 13832–13841. [Google Scholar] [CrossRef]
- Nakagawa, F.; Kawashima, H.; Morita, T.; Nakamura, H. Water-soluble closo-docecaborate-containing pteroyl derivatives targeting folate receptor-positive tumors for boron neutron capture therapy. Cells 2020, 9, 1615. [Google Scholar] [CrossRef]
- Novopashina, D.S.; Vorobyeva, M.A.; Lomzov, A.A.; Silnikov, V.N.; Venyaminova, A.G. Terminal mono- and bis-conjugates of oligonucleotides with closo-dodecaborate: Synthesis and physico-chemical properties. Int. J. Mol. Sci. 2021, 22, 182. [Google Scholar] [CrossRef] [PubMed]
- Cebula, J.; Fink, K.; Goldeman, W.; Szermer-Olearnik, B.; Nasulewicz-Goldeman, A.; Psurski, M.; Cuprych, M.; Kędziora, A.; Dudek, B.; Bugla-Płoskońska, G.; et al. Structural patterns enhancing the antibacterial activity of metallacarborane-based antibiotics. J. Med. Chem. 2023, 66, 14948–14962. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Kashiwagi, H.; Morita, T.; Fukuo, Y.; Okada, S.; Miura, K.; Matsumoto, Y.; Sugawara, Y.; Enomoto, T.; Suzuki, M.; et al. Efficient neutron capture therapy of glioblastoma with pteroyl-closo-dodecaborate-conjugated 4-(p-iodophenyl)butyric acid (PBC-IP). J. Control. Release 2023, 360, 249–259. [Google Scholar] [CrossRef]
- Garaev, T.M.; Yudin, I.I.; Breslav, N.V.; Grebennikova, T.V.; Matveev, E.Y.; Eshtukova-Shcheglova, E.A.; Avdeeva, V.V.; Zhizhin, K.Y.; Kuznetsov, N.T. In vitro study of antiviral properties of compounds based on 1,4-dioxane derivative of closo-decaborate anion with amino acid ester residues against influenza virus A/IIV-Orenburg/83/2012(H1N1)pdm09. Molecules 2024, 29, 5886. [Google Scholar] [CrossRef]
- Nishimura, K.; Tanaka, S.; Miura, K.; Okada, S.; Suzuki, M.; Nakamura, H. A Water-soluble small molecule boron carrier targeting biotin receptors for neutron capture therapy. ACS Omega, 2024; in press. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Sivaev, I.B.; Bregadze, V.I. Nitrilium derivatives of polyhedral boron compounds (boranes, carboranes, metallocarboranes): Synthesis and reactivity. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 983–988. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Klyukin, I.N.; Zhdanov, A.P.; Grigor’ev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Synthesis of substituted derivatives of closo-decaborate anion with a peptide bond: The way towards designing biologically active boron-containing compounds. Russ. J. Inorg. Chem. 2019, 64, 1499–1506. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Nucleophilic addition of amino acid esters to nitrilium derivatives of closo-decaborate anion. Mendeleev Commun. 2021, 31, 201–203. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Selivanov, N.A.; Bykov, A.Y.; Klyukin, I.N.; Kubasov, A.S.; Zhdanov, A.P.; Zhizhin, K.Y.; Kuznetsov, N.T. New method for synthesis of N-borylated amino acids based on closo-decaborate and closo-dodecaborate anions. Russ. J. Inorg. Chem. 2022, 67, 1776–1784. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Selivanov, N.A.; Bykov, A.Y.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Karpechenko, N.Y.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Primary amine nucleophilic addition to nitrilium closo-dodecaborate [B12H11NCCH3]−: A Simple and effective route to the new BNCT drug design. Int. J. Mol. Sci. 2021, 22, 13391. [Google Scholar] [CrossRef]
- Laskova, J.; Ananiev, I.; Kosenko, I.; Serdyukov, A.; Stogniy, M.; Sivaev, I.; Grin, M.; Semioshkin, A.; Bregadze, V.I. Nucleophilic addition reactions to nitrilium derivatives [B12H11NCCH3]− and [B12H11NCCH2CH3]−. Synthesis and structures of closo-dodecaborate-based iminols, amides and amidines. Dalton Trans. 2022, 51, 3051–3059. [Google Scholar] [CrossRef]
- Grimes, R.N. Metallacarboranes in the new millennium. Coord. Chem. Rev. 2000, 200–202, 773–811. [Google Scholar] [CrossRef]
- Satapathy, R.; Dash, B.D.; Maguire, J.A.; Hosmane, N.S. Advances in the metallacarborane chemistry of f-block elements. Dalton Trans. 2010, 39, 6613–6625. [Google Scholar] [CrossRef] [PubMed]
- Grimes, R.N. Carboranes, 2nd ed.; Academic Press: London, UK, 2016; pp. 711–903. [Google Scholar] [CrossRef]
- Kar, S.; Pradhan, A.N.; Ghosh, G. Polyhedral metallaboranes and metallacarboranes. In Comprehensive Organometallic Chemistry IV; Elsevier: Amsterdam, The The Netherlands, 2022; Volume 9, pp. 263–369. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Anisimov, A.A.; Sivaev, I.B.; Bregadze, V.I. Nucleophilic addition reactions to the ethylnitrilium derivative of nido-carborane 10-EtC≡N-7,8-C2B9H11. New J. Chem. 2018, 42, 17958–17967. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Anisimov, A.A.; Godovikov, I.A.; Sivaev, I.B.; Bregadze, V.I. Synthesis of novel carboranyl amidines. J. Organomet. Chem. 2020, 909, 121111. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Markov, V.Y.; Sivaev, I.B. Synthesis and crystal structures of nickel(II) and palladium(II) complexes with o-carboranyl amidine ligands. Dalton Trans. 2021, 50, 4967–4975. [Google Scholar] [CrossRef] [PubMed]
- Stogniy, M.Y.; Erokhina, S.A.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. 10-NCCH2CH2OCH2CH2C≡N-7,8-C2B9H11: Synthesis and reactions with various nucleophiles. Polyhedron 2019, 174, 114170. [Google Scholar] [CrossRef]
- Young, D.C.; Howe, D.V.; Hawthorne, M.F. Ligand derivatives of (3)-1,2-dicarbadodecahydroundecaborate(-1). J. Am. Chem. Soc. 1969, 91, 859–862. [Google Scholar] [CrossRef]
- Frank, R.; Auer, H.; Hey-Hawkins, E. Functionalisation of the nido-dicarbaborate anion nido-7,8-C2B9H12− by hydride abstraction. J. Organomet. Chem. 2013, 747, 217–224. [Google Scholar] [CrossRef]
- Zhdanov, A.P.; Lisovsky, M.V.; Goeva, L.V.; Razgonyaeva, G.A.; Polyakova, I.N.; Zhizhin, K.Y.; Kuznetsov, N.T. Nucleophilic addition of alcohols to the C-N multiple bonds of the nitrilium substituent in the anion [2-B10H9(N≡CMe)]−. Russ. Chem. Bull. 2009, 58, 1694–1700. [Google Scholar] [CrossRef]
- Zhdanov, A.P.; Klyukin, I.N.; Bykov, A.Y.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Nucleophilic addition of alcohols to anionic [2-B10H9NCR]− (R = Et, t-Bu): An approach to producing new borylated imidates. Polyhedron 2017, 123, 176–183. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Bregadze, V.I. Synthesis of 10-methylsulfide and 10-alkylmethylsulfonium nido-carborane derivatives: B–H···π interactions between the B–H–B hydrogen atom and alkyne group in 10-RC≡CCH2S(Me)-7,8-C2B9H11. Eur. J. Inorg. Chem. 2017, 2017, 4436–4443. [Google Scholar] [CrossRef]
- Zhdanov, A.O.; Polyakova, I.N.; Razgonyaeva, G.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Reactions of nucleophilic addition of primary amines to the nitrilium derivative of the closo-decaborate anion [2-B10H9(N≡CCH3)]−. Russ. J. Inorg. Chem. 2011, 56, 847–855. [Google Scholar] [CrossRef]
- Voinova, V.V.; Klyukin, I.N.; Zhdanov, A.P.; Grigor’ev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Synthesis of new boron-containing ligands and their hafnium(IV) complexes. Russ. J. Inorg. Chem. 2020, 65, 839–845. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Wang, T.; Liu, J.; Spingler, B.; Duttwyler, S. Synthesis and structural characterization of amidine, amide, urea and isocyanate derivatives of the amino-closo-dodecaborate anion [B12H11NH3]−. Molecules 2018, 23, 3137. [Google Scholar] [CrossRef]
- Ryabchikova, M.N.; Nelyubin, A.V.; Smirnova, A.V.; Finogenova, Y.A.; Skribitsky, V.A.; Shpakova, K.E.; Kubasov, A.S.; Zhdanov, A.P.; Lipengolts, A.A.; Grigorieva, E.Y.; et al. Preparation of closo-dodecaborate anion conjugate with ethyl glycinate and study of its biodistribution in melanoma model B16F10. Russ. J. Inorg. Chem. 2024, 69. [Google Scholar] [CrossRef]
- Šícha, V.; Plešek, J.; Kvíčalová, M.; Císařová, I.; Grüner, B. Boron(8) substituted nitrilium and ammonium derivatives, versatile cobalt bis(1,2-dicarbollide) building blocks for synthetic purposes. Dalton Trans. 2009, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Stogniy, M.Y.; Anufriev, S.A.; Bogdanova, E.V.; Gorodetskaya, N.A.; Anisimov, A.A.; Suponitsky, K.Y.; Grishin, I.D.; Sivaev, I.B. Charge-compensated nido-carborane derivatives in the synthesis of iron(II) bis(dicarbollide) complexes. Dalton Trans. 2024, 53, 3363–3376. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Erokhina, S.A.; Suponitsky, K.Y.; Godovikov, I.A.; Filippov, O.A.; Fabrizi de Biani, F.; Corsini, M.; Chizhov, A.O.; Sivaev, I.B. Methylsulfanyl-stabilized rotamers of cobalt bis(dicarbollide). Eur. J. Inorg. Chem. 2017, 2027, 4444–4451. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Bregadze, V.I. Practical synthesis of 9-methylthio-7,8-nido-carborane [9-MeS-7,8-C2B9H11]−. Some evidences of BH···X hydride-halogen bonds in 9- XCH2(Me)S-7,8-C2B9H11 (X = Cl, Br, I). J. Organomet. Chem. 2017, 849–850, 315–323. [Google Scholar] [CrossRef]
- Zhdanov, A.P.; Nelyubin, A.V.; Klyukin, I.N.; Selivanov, N.A.; Bortnikov, E.O.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Nucleophilic addition reaction of secondary amines to acetonitrilium closo-decaborate [2-B10H9NCCH3]−. Russ. J. Inorg. Chem. 2019, 64, 841–846. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Prikaznov, A.V.; Anufriev, S.A. On relative electronic effects of polyhedral boron hydrides. J. Organomet. Chem. 2013, 747, 254–256. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals; Butterworth Heinemann: Burlington, VT, USA, 2009. [Google Scholar]
- Bruker AXS Inc. APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Kudin, K.N., Jr.; Burant, J.C.; Millam, J.M.; et al. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, UK, 2004. [Google Scholar]
- Suponitsky, K.Y.; Burakov, N.I.; Kanibolotsky, A.L.; Mikhailov, V.A. Multiple noncovalent bonding in halogen complexes with oxygen organics. I. Tertiary amides. J. Phys. Chem. A 2016, 120, 4179–4190. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Aleksandrova, N.S.; Semyakin, S.S.; Suponitsky, K.Y.; Lempert, D.B. Synthesis and characterization of 3-(5-(fluorodinitromethyl)-1H-1,2,4-triazol-3-yl)-4-nitrofurazan: A novel promising energetic component of boron-based fuels for rocket ramjet engines. Chem. Asian J. 2019, 14, 4255–4261. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Keith, T.A. AIMAll, version 15.05.18; TK Gristmill Software: Overland Park, KS, USA, 2015.
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Rozas, I.; Elguero, J.; Molins, E. About the evaluation of the local kinetic, potential and total energy densities in closed-shell interactions. Chem. Phys. Lett. 2001, 336, 457–461. [Google Scholar] [CrossRef]
- Lyssenko, K.A. Analysis of supramolecular architectures: Beyond molecular packing diagrams. Mendeleev Commun. 2012, 22, 1–7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).