An Eight-Membered Ring Molecular Framework Based on Carbazole for the Development of Electroluminescent Materials
Abstract
1. Introduction
2. Results and Discussion
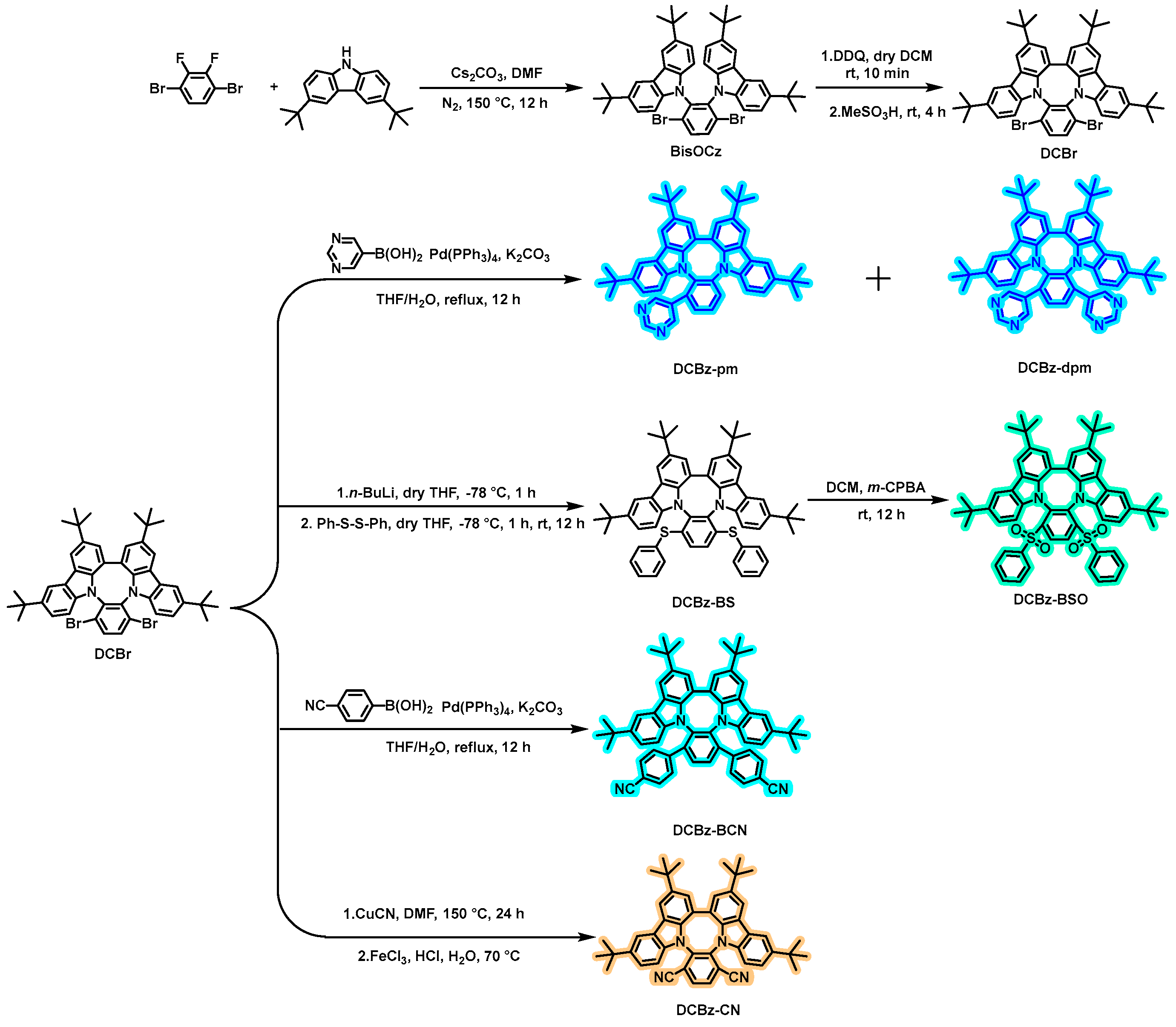
2.1. Synthesis and Characterization
2.2. Thermal and Photophysical Properties
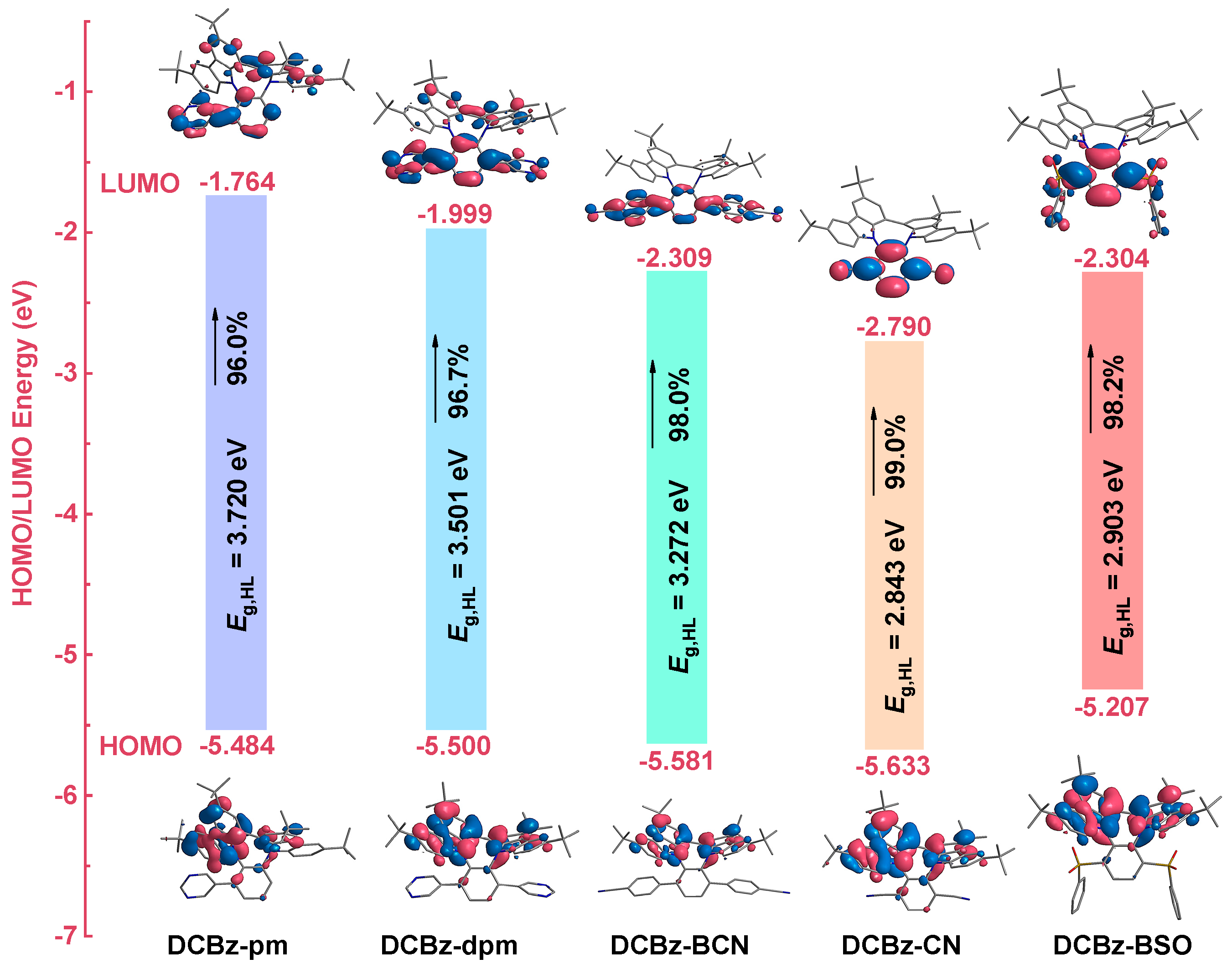
2.3. Theoretical Calculation
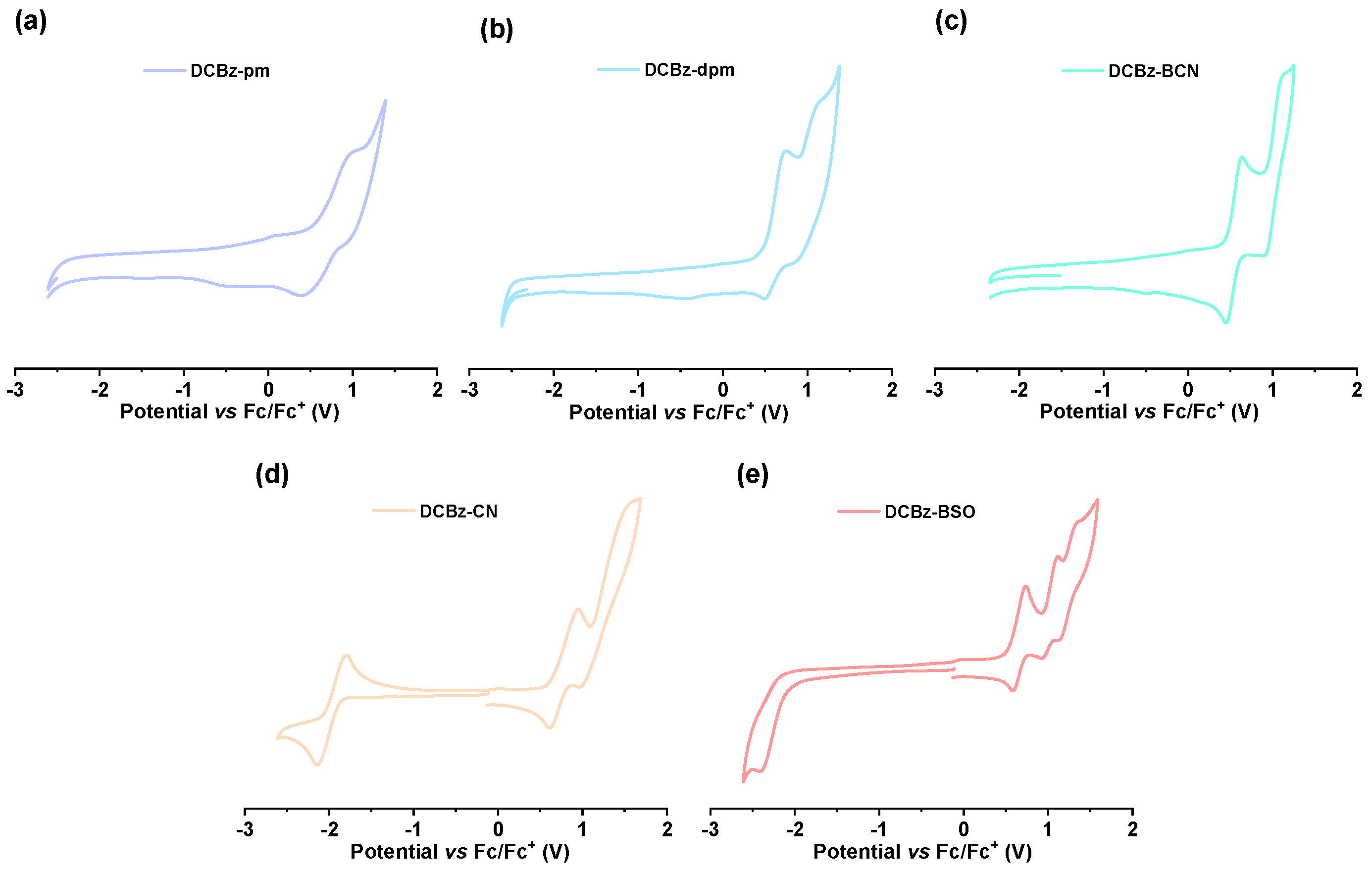
2.4. Electrochemical Properties
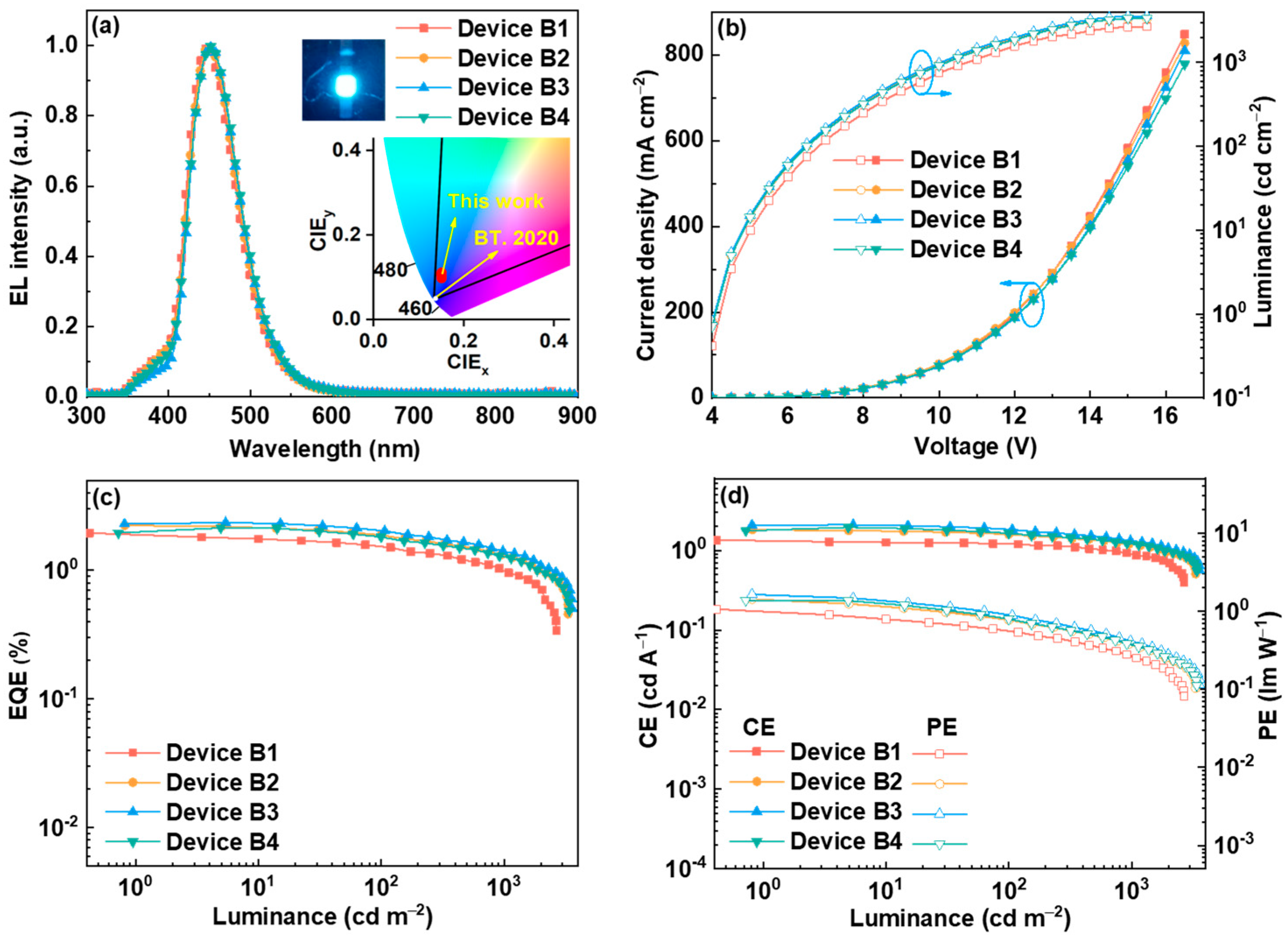
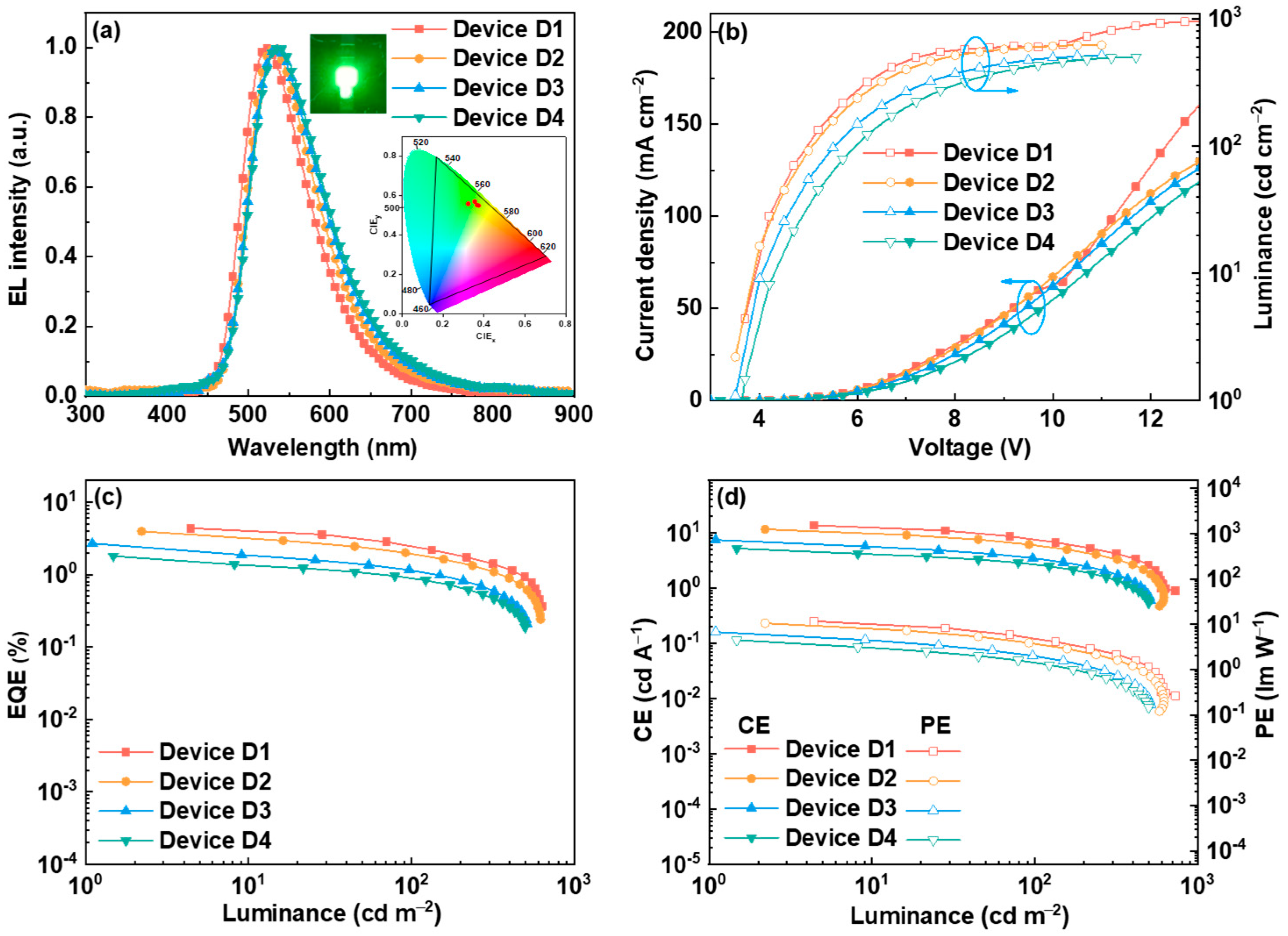
2.5. Electroluminescent Properties
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, N.; Kang, M.J.; Lee, H.J.; Park, J.Y.; Kwak, H.; Park, C.Y.; Ahn, H.J.; Kim, J.Y.; Baek, J.H.; Kim, H.Y.; et al. Enhancing Narrowband Blue TADF OLED Performance with Adamantane Group-Integrated Spatially Hindered 1,3-Bis(N-Carbazolyl)Benzene-Based Host. Adv. Funct. Mater. 2024, 34, 2408491. [Google Scholar] [CrossRef]
- Güven, Z.; Dolati, H.; Wessel, L.; Frank, R. Facile Synthetic Access Towards Sulfur- and Selenium-Functionalized Boron-Based Multiresonance TADF Emitters. Molecules 2024, 29, 5819. [Google Scholar] [CrossRef]
- Wang, W.; Bian, J.; Chen, K.; Li, C.; Long, Y.; Huang, H.; Jiang, L.; Zhao, J.; Liu, S.; Chi, Z.; et al. Achieving Record External Quantum Efficiency of 11.5% in Solution-Processable Deep-Blue Organic Light-Emitting Diodes Utilizing Hot Exciton Mechanism. Angew. Chem. Int. Ed. 2024, 63, e202318782. [Google Scholar] [CrossRef]
- Pidluzhna, A.; Vembris, A.; Grzibovskis, R.; Zommere, M.A.; Bezvikonnyi, O.; Simokaitiene, J.; Baronaite, M.; Volyniuk, D.; Grazulevicius, J.V.; Ali, A.; et al. The Effect of Molecular Structure on the Properties of Fluorene Derivatives for OLED Applications. Molecules 2024, 29, 4918. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, Y.; Tang, Z.; Zhang, B.; Wei, B. Multifunctional Organic Emitters for High-Performance and Low-Cost Organic Light-Emitting Didoes. Chem. Rec. 2019, 19, 1768–1778. [Google Scholar] [CrossRef]
- Madayanad Suresh, S.; Hall, D.; Beljonne, D.; Olivier, Y.; Zysman Colman, E. Multiresonant Thermally Activated Delayed Fluorescence Emitters Based on Heteroatom-Doped Nanographenes: Recent Advances and Prospects for Organic Light-Emitting Diodes. Adv. Funct. Mater. 2020, 30, 1908677. [Google Scholar] [CrossRef]
- Hamrouni, K.; Spassova, M.; Alshammari, N.A.H.; Hajri, A.K.; Aloui, F. Synthesis of new [6]helicene derivatives for OLED applications. Experimental photophysical and chiroptical properties and theoretical investigation. J. Mol. Struct. 2024, 1311, 138408. [Google Scholar] [CrossRef]
- Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent progress in metal-organic complexes for optoelectronic applications. Chem. Soc. Rev. 2014, 43, 3259–3302. [Google Scholar] [CrossRef]
- Siddiqui, I.; Gautam, P.; Blazevicius, D.; Jayakumar, J.; Lenka, S.; Tavgeniene, D.; Zaleckas, E.; Grigalevicius, S.; Jou, J.H. Bicarbazole-Benzophenone Based Twisted Donor-Acceptor Derivatives as Potential Blue TADF Emitters for OLEDs. Molecules 2024, 29, 1672. [Google Scholar] [CrossRef] [PubMed]
- Beresneviciute, R.; Gautam, P.; Nagar, M.R.; Krucaite, G.; Tavgeniene, D.; Jou, J.H.; Grigalevicius, S. Naphtalimide-Based Bipolar Derivatives Enabling High-Efficiency OLEDs. Molecules 2023, 28, 6027. [Google Scholar] [CrossRef]
- Bauri, J.; Choudhary, R.B.; Mandal, G. Recent advances in efficient emissive materials-based OLED applications: A review. J. Mater. Sci. 2021, 56, 18837–18866. [Google Scholar] [CrossRef]
- Baldo, M.A.; Thompson, M.E.; Forrest, S.R. High-efficiency fluorescent organic light-emitting devices using a phosphorescent sensitizer. Nature 2000, 403, 750–753. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Z.; Sun, W.; Wu, S.; Liu, J.; Lin, Z.; Jiang, P.; Yu, H.; Zhou, L.; Lu, G. High-efficiency narrowband multi-resonance TADF emitters via the introduction of bulky adamantane units. J. Mater. Chem. C 2024, 12, 6319–6325. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, J.; Guo, J.; Nie, H.; Zhao, Z.; Tang, B.Z. High-Performance Non-doped OLEDs with Nearly 100% Exciton Use and Negligible Efficiency Roll-Off. Angew. Chem. Int. Ed. 2018, 57, 9290–9294. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Xie, F.M.; Li, H.Z.; Zhang, K.; Zou, J.; Li, Y.Q.; Tang, J.X. Bulky neutral group incorporation in Through-Space charge transfer delayed fluorescence emitters for efficient Non-Doped organic Light-Emitting diodes. Chem. Eng. J. 2024, 495, 153511. [Google Scholar] [CrossRef]
- Lee, T.; Jang, J.H.; Nguyen, N.N.T.; Jung, J.; Lee, J.H.; Lee, M.H. Ortho-Carborane Decorated Multi-Resonance TADF Emitters: Preserving Local Excited State and High Efficiency in OLEDs. Adv. Sci. 2024, 11, 2309016. [Google Scholar] [CrossRef]
- Wu, Z.L.; Sun, Y.F.; Lv, X.; Zhang, D.H.; Zhou, L.; Meng, L.; Chen, X.L.; Lu, C.Z. Thermally Activated Delayed Fluorescence Emitters Based on Rigid Lactam Acceptors: Simultaneously Achieving Desirable Emission Efficiency, Horizontal Orientation, and Reverse Intersystem Crossing. Adv. Funct. Mater. 2024, 34, 2314533. [Google Scholar] [CrossRef]
- Cho, Y.J.; Jeon, S.K.; Lee, S.S.; Yu, E.; Lee, J.Y. Donor Interlocked Molecular Design for Fluorescence-like Narrow Emission in Deep Blue Thermally Activated Delayed Fluorescent Emitters. Chem. Mater. 2016, 28, 5400–5405. [Google Scholar] [CrossRef]
- Khan, A.; Tang, X.; Zhong, C.; Wang, Q.; Yang, S.Y.; Kong, F.C.; Yuan, S.; Sandanayaka, A.S.D.; Adachi, C.; Jiang, Z.Q.; et al. Intramolecular-Locked High Efficiency Ultrapure Violet-Blue (CIE-y < 0.046) Thermally Activated Delayed Fluorescence Emitters Exhibiting Amplified Spontaneous Emission. Adv. Funct. Mater. 2021, 31, 2009488. [Google Scholar]
- Park, H.J.; Han, S.H.; Lee, J.Y.; Han, H.; Kim, E.G. Managing Orientation of Nitrogens in Bipyrimidine-Based Thermally Activated Delayed Fluorescent Emitters To Suppress Nonradiative Mechanisms. Chem. Mater. 2018, 30, 3215–3222. [Google Scholar] [CrossRef]
- Jiang, S.; Yu, Y.; Li, D.; Chen, Z.; He, Y.; Li, M.; Yang, G.X.; Qiu, W.; Yang, Z.; Gan, Y.; et al. Sulfone-Embedded Heterocyclic Narrowband Emitters with Strengthened Molecular Rigidity and Suppressed High-Frequency Vibronic Coupling. Angew. Chem. Int. Ed. 2023, 62, e202218892. [Google Scholar] [CrossRef]
- Zhu, Y.; Qu, C.; Ye, J.; Xu, Y.; Zhang, Z.; Wang, Y. Donor-Acceptor Type of Fused-Ring Thermally Activated Delayed Fluorescence Compounds Constructed through an Oxygen-Containing Six-Membered Ring. ACS Appl. Mater. Interfaces 2022, 14, 47971–47980. [Google Scholar] [CrossRef]
- Huang, Z.; Bin, Z.; Su, R.; Yang, F.; Lan, J.; You, J. Molecular Design of Non-doped OLEDs Based on a Twisted Heptagonal Acceptor: A Delicate Balance between Rigidity and Rotatability. Angew. Chem. Int. Ed. 2020, 59, 9992–9996. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Cheon, H.J.; Woo, S.J.; Kwon, S.K.; Kim, Y.H.; Kim, J.J. Highly Efficient Deep-Blue OLEDs using a TADF Emitter with a Narrow Emission Spectrum and High Horizontal Emitting Dipole Ratio. Adv. Mater. 2020, 32, 2004083. [Google Scholar] [CrossRef]
- Hirai, H.; Nakajima, K.; Nakatsuka, S.; Shiren, K.; Ni, J.; Nomura, S.; Ikuta, T.; Hatakeyama, T. One-Step Borylation of 1,3-Diaryloxybenzenes Towards Efficient Materials for Organic Light-Emitting Diodes. Angew. Chem. Int. Ed. 2015, 54, 13581–13585. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.V.; Lee, H.L.; Kim, I.; Lee, K.H.; Chung, W.J.; Kim, J.; Park, S.; Choi, H.; Son, W.J.; Jeon, S.O.; et al. Purely Spin-Vibronic Coupling Assisted Triplet to Singlet Up-Conversion for Real Deep Blue Organic Light-Emitting Diodes with over 20% Efficiency and y Color Coordinate of 0.05. Adv. Sci. 2021, 8, 1602276. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, Y.; Luo, S.; Gao, Y.; Gao, N.; Wang, K.; Zou, B.; Yang, B.; Ma, Y. Rehybridization of Nitrogen Atom Induced Photoluminescence Enhancement under Pressure Stimulation. Adv. Funct. Mater. 2017, 27, 1602276. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Y.; Li, T.; Zhou, H.; Xie, M.; Chu, L.; Sun, Q.; Zhang, S.T.; Yang, W.; Xue, S. Efficient nondoped Deep-Blue electrofluorescence benefiting from structural hindrance and regular C-H···π stacking. Chem. Eng. J. 2023, 471, 144505. [Google Scholar] [CrossRef]
- Yuan, W.; Hu, W.; Wang, J.; Zhu, M.; Shi, C.; Sun, N.; Tao, Y. Fine Tuning of Donor-Acceptor Structures in Fused-Carbazole Containing Thermally Activated Delayed Fluorescence Materials towards High-Efficiency OLEDs. Chin. J. Chem. 2023, 41, 1829–1835. [Google Scholar] [CrossRef]
- Yuan, W.; Jin, G.; Su, N.; Hu, D.; Shi, W.; Zheng, Y.X.; Tao, Y. The electron inductive effect of dual non-conjugated trifluoromethyl acceptors for highly efficient thermally activated delayed fluorescence OLEDs. Dyes Pigm. 2020, 183, 108705. [Google Scholar] [CrossRef]
- Chang, X.; Lu, K.; Jiang, K.; Ma, B.; Huang, J.; Gan, X.; Liu, Y.; Yu, J.; Zhu, W. Extending rigid electron-deficient skeletons and appending electron-rich units to build high-efficiency red-emitting pyrene-derived TADF materials. J. Mater. Chem. C 2023, 11, 14876–14883. [Google Scholar] [CrossRef]
- Li, H.; Yan, H.; Zhang, X.; Shi, K.; Kuang, C.; Zheng, X.; He, Y.; Meng, L.; Xu, H.; Meng, Z.; et al. Highly efficient and stable solution-processed deep-blue OLEDs with LT95 over 50 h at 1000 nit. Chem. Eng. J. 2024, 486, 150142. [Google Scholar] [CrossRef]
- Nakao, K.; Sasabe, H.; Komatsu, R.; Hayasaka, Y.; Ohsawa, T.; Kido, J. Significant Enhancement of Blue OLED Performances through Molecular Engineering of Pyrimidine-Based Emitter. Adv. Opt. Mater. 2017, 5, 1600843. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, J.Y. Engineering of Mixed Host for High External Quantum Efficiency above 25% in Green Thermally Activated Delayed Fluorescence Device. Adv. Funct. Mater. 2014, 24, 3970–3977. [Google Scholar] [CrossRef]
- Jiang, J.; Hu, D.; Hanif, M.; Li, X.; Su, S.; Xie, Z.; Liu, L.; Zhang, S.; Yang, B.; Ma, Y. Twist Angle and Rotation Freedom Effects on Luminescent Donor-Acceptor Materials: Crystal Structures, Photophysical Properties, and OLED Application. Adv. Opt. Mater. 2016, 4, 2109–2118. [Google Scholar] [CrossRef]
- Seino, Y.; Inomata, S.; Sasabe, H.; Pu, Y.J.; Kido, J. High-Performance Green OLEDs Using Thermally Activated Delayed Fluorescence with a Power Efficiency of over 100 lm W−1. Adv. Mater. 2016, 28, 2638–2643. [Google Scholar] [CrossRef]
- Li, Y.; Chen, T.; Huang, M.; Gu, Y.; Gong, S.; Xie, G.; Yang, C. Tuning the twist angle of thermally activated delayed fluorescence molecules via a dendronization strategy: High-efficiency solution-processed non-doped OLEDs. J. Mater. Chem. C 2017, 5, 3480–3487. [Google Scholar] [CrossRef]
- Li, C.; Nobuyasu, R.S.; Wang, Y.; Dias, F.B.; Ren, Z.; Bryce, M.R.; Yan, S. Solution-Processable Thermally Activated Delayed Fluorescence White OLEDs Based on Dual-Emission Polymers with Tunable Emission Colors and Aggregation-Enhanced Emission Properties. Adv. Opt. Mater. 2017, 5, 1700435. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, C.; Su, L.; Wang, D.; Jiang, Y.; Tsuboi, T.; Zhang, Q. Efficient Intramolecular Charge-Transfer Fluorophores Based on Substituted Triphenylphosphine Donors. Angew. Chem. Int. Ed. 2021, 60, 15049–15053. [Google Scholar] [CrossRef]


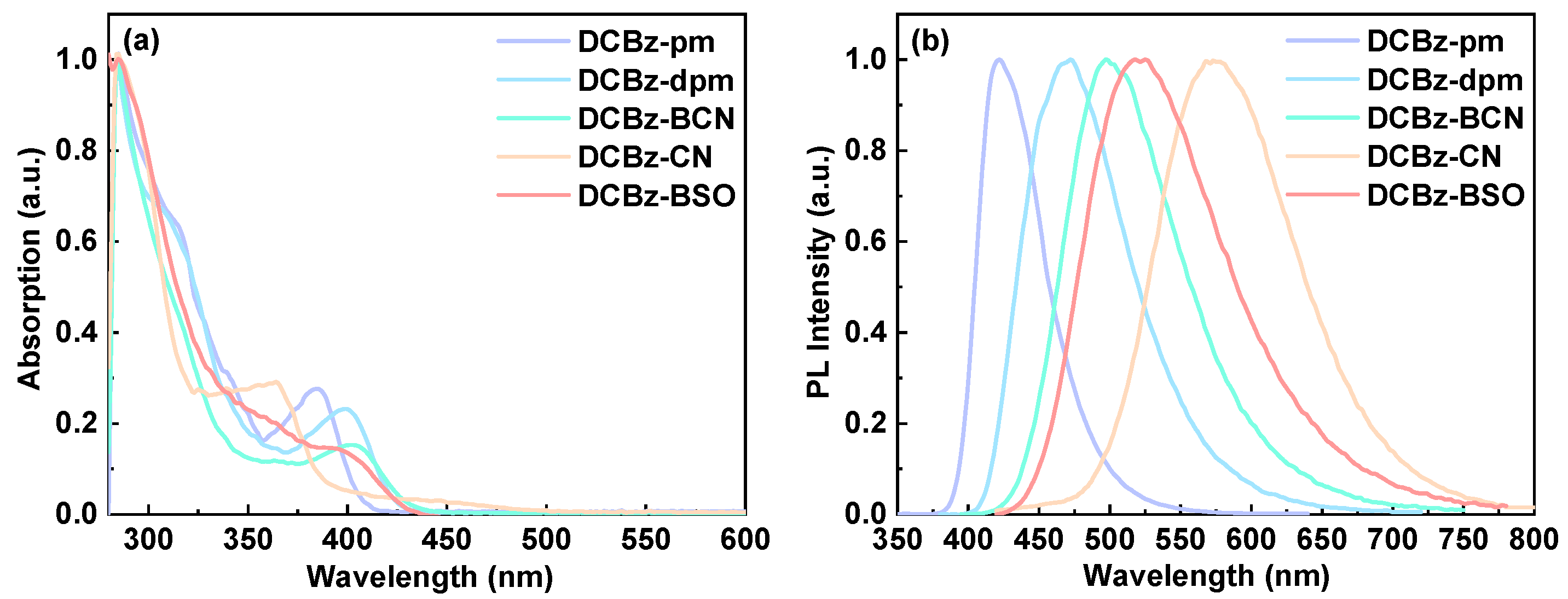




| Compound | λabs (nm) a | λem (nm) a | τ (ns) a | PLQY b | Td (°C) |
|---|---|---|---|---|---|
| DCBz-pm | 285(4.36), 313(4.16), 385(3.78) | 418 | 4.59 | 0.45 | 355 |
| DCBz-dpm | 284(4.43), 308(4.38), 398(3.91) | 468 | 12.30 | 0.49 | 380 |
| DCBz-BCN | 285(4.68), 315(4.34), 402(3.88) | 497 | 14.37 | 0.48 | 385 |
| DCBz-CN | 285(4.41), 364(3.89) | 573 | 7.47 | 0.69 | 356 |
| DCBz-BSO | 285(4.42), 397(3.57) | 520 | 15.00 | 0.36 | 160 |
| Compound | Eox a (V) | Ered a (V) | EHOMO b (eV) | ELUMO (eV) | Eg(CV) (eV) |
|---|---|---|---|---|---|
| DCBz-pm | 0.75 | / | −5.44 | −2.37 d | 3.07 c |
| DCBz-dpm | 0.81 | / | −5.40 | −2.46 d | 2.94 c |
| DCBz-BCN | 0.65 | / | −5.31 | −2.41 d | 2.90 c |
| DCBz-CN | 0.89 | −1.86 | −5.58 | −2.83 e | 2.75 f |
| DCBz-BSO | 0.77 | −2.31 | −5.46 | −2.38 e | 3.08 f |
| Device (Doping Level) | Von (V) | L (cd m−2) | EQE (%) | CE (cd A−1) | PE (lm W−1) | λEL (nm) | CIE (x, y) |
|---|---|---|---|---|---|---|---|
| A1 (5.0 wt%) | 4.5 | 2508 | 2.05 | 0.71 | 0.44 | 423 | (0.158, 0.046) |
| A2 (10.0 wt%) | 4.5 | 2446 | 2.35 | 0.81 | 0.57 | 424 | (0.160, 0.046) |
| A3 (15.0 wt%) | 4.5 | 2557 | 2.35 | 0.81 | 0.57 | 425 | (0.158, 0.046) |
| A4 (20.0 wt%) | 4.5 | 2720 | 2.52 | 0.98 | 0.70 | 426 | (0.157, 0.047) |
| B1 (5.0 wt%) | 4.5 | 2679 | 1.80 | 1.28 | 1.06 | 446 | (0.153, 0.100) |
| B2 (10.0 wt%) | 4.0 | 3330 | 2.23 | 1.82 | 1.43 | 450 | (0.151, 0.103) |
| B3 (15.0 wt%) | 4.0 | 3576 | 2.35 | 2.10 | 1.64 | 453 | (0.150, 0.108) |
| B4 (20.0 wt%) | 4.0 | 3400 | 2.13 | 1.95 | 1.38 | 454 | (0.152, 0.115) |
| Device (Doping Level) | Von (V) | L (cd m−2) | EQE (%) | CE (cd A−1) | PE (lm W−1) | λEL (nm) | CIE (x, y) |
|---|---|---|---|---|---|---|---|
| C1 (5.0 wt%) | 3.5 | 1387 | 2.31 | 4.00 | 3.10 | 472 | (0.163, 0.250) |
| C2 (10.0 wt%) | 3.5 | 2530 | 2.74 | 5.14 | 4.62 | 479 | (0.164, 0.271) |
| C3 (15.0 wt%) | 3.5 | 2543 | 2.61 | 5.03 | 4.52 | 480 | (0.166, 0.285) |
| C4 (20.0 wt%) | 3.5 | 2549 | 2.01 | 4.44 | 4.00 | 480 | (0.168, 0.296) |
| D1 (5.0 wt%) | 3.7 | 951 | 4.36 | 13.83 | 11.74 | 520 | (0.323, 0.558) |
| D2 (10.0 wt%) | 3.5 | 621 | 3.96 | 11.75 | 10.55 | 530 | (0.355, 0.566) |
| D3 (15.0 wt%) | 3.5 | 513 | 2.69 | 7.52 | 6.76 | 533 | (0.367, 0.561) |
| D4 (20.0 wt%) | 3.7 | 500 | 1.79 | 5.26 | 4.47 | 534 | (0.374, 0.561) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, A.; Xu, S.; Du, X.; Zhu, C.; Li, S.; Yang, X.; Zhou, G.; Sun, Y. An Eight-Membered Ring Molecular Framework Based on Carbazole for the Development of Electroluminescent Materials. Molecules 2025, 30, 716. https://doi.org/10.3390/molecules30030716
Yan A, Xu S, Du X, Zhu C, Li S, Yang X, Zhou G, Sun Y. An Eight-Membered Ring Molecular Framework Based on Carbazole for the Development of Electroluminescent Materials. Molecules. 2025; 30(3):716. https://doi.org/10.3390/molecules30030716
Chicago/Turabian StyleYan, An, Shipan Xu, Xuyang Du, Chengyun Zhu, Shengli Li, Xiaolong Yang, Guijiang Zhou, and Yuanhui Sun. 2025. "An Eight-Membered Ring Molecular Framework Based on Carbazole for the Development of Electroluminescent Materials" Molecules 30, no. 3: 716. https://doi.org/10.3390/molecules30030716
APA StyleYan, A., Xu, S., Du, X., Zhu, C., Li, S., Yang, X., Zhou, G., & Sun, Y. (2025). An Eight-Membered Ring Molecular Framework Based on Carbazole for the Development of Electroluminescent Materials. Molecules, 30(3), 716. https://doi.org/10.3390/molecules30030716






