Abstract
The synthesis, phase behavior and semiconductor properties of two novel organosilicon tetramers with dialkyl-substituted [1]benzothieno[3,2-b]benzothiophene (BTBT) moieties, D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct, are described. The synthesis of these molecules was carried out by sequential modification of the BTBT core by carbonyl-containing functional alkyl substituents using the Friedel–Crafts reaction, followed by the reduction in the keto group. The target tetramers, D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct, were obtained by the hydrosilylation reaction between tetraallylsilane and corresponding 1,1,3,3-tetramethyl-1-(ω-(7-alkyl[1]benzothieno[3,2-b]benzothiophen-2-yl)alkyl)disiloxanes. The chemical structure of the compounds obtained was confirmed by NMR 1H-, 13C- and 29Si-spectroscopy, gel permeation chromatography and elemental analysis. Their phase behavior was investigated by differential scanning calorimetry, polarization optical microscopy and X-ray diffraction analysis. It was found that D4-Und-BTBT-Hex shows higher crystallinity at room temperature as compared to D4-Hex-BTBT-Oct, while both molecules possess smectic ordering favorable for active layer formation in organic field-effect transistors (OFETs). The active layers were applied by spin-coating under conditions of a homogeneous thin layer formation with a low content of defects. The devices obtained from D4-Und-BTBT-Hex have demonstrated good semiconductor characteristics in OFETs with a hole mobility up to 3.5 × 10−2 cm2 V−1 s−1, a low threshold voltage and an on/off ratio up to 107.
1. Introduction
In the last decade, there has been significant interest in the development of novel functional materials for various organic electronic devices [1,2,3,4,5]. Organic semiconductors (OSCs) are widely used in organic field-effect transistors (OFETs) [6], organic light-emitting transistors (OLETs) [7], organic electrochemical transistors (OECTs) [8] and the gas and liquid sensors based on them, namely, organic and hybrid photovoltaics [9]. The active components in such devices are based on low-molecular, oligomeric or polymeric organic semiconductors, where the density of states is generally determined by the disordered nature of the molecular solid rather than energy bands [10]. The emergence of a large number of stable organic semiconductors makes it possible to produce various semiconductor devices that can combine all the advantages of organic molecules and conducting systems. The examples of such devices include organic light-emitting diodes (OLEDs) [11], organic photovoltaic cells (OPVs) [12], OFETs [13], chemical and biological sensors, artificial synapses and computing devices [14]. OSCs have a number of advantages, namely the following: the ability to controllably change properties, internal flexibility, relatively low cost and a wide range of applications [15,16,17]. In turn, low-molecular weight and oligomeric OSCs have a number of advantages over the conjugated polymer molecules, such as a strictly defined molecular structure, the absence of defects and reproducibility from batch to batch [18,19]. In this case, oligomeric OSCs are understood as compounds that, unlike classical oligomers, have a strictly defined molecular structure, and, unlike low-molecular weight compounds, cannot be purified by sublimation even in a deep vacuum due to their sufficiently high molecular weight. Their physical and chemical properties can be easily and effectively controlled by modifying functional groups and designing suitable molecular structures. The group of [1]Benzothieno[3,2-b]benzothiophene (BTBT) derivatives is of great interest as low-molecular weight and oligomeric OSCs. Various BTBT derivatives are widely used in different devices (OFETs, OLEDs), due to their high conductivity, good solubility in organic solvents and relatively high stability in air [20]. The chemical properties of BTBT provide the possibility of introducing alkyl, aryl, alkenyl, alkynyl, alkoxyl, carboxyl and other substituents [21,22] into any position of the BTBT annulated core, as well as obtaining symmetrical and asymmetrical derivatives [23,24]. In turn, the functionalization of BTBT derivatives with organosilicon fragments provides flexibility to such compounds while maintaining the semiconductor properties of BTBT. It was previously shown that dimeric organosilicon structures containing alkyl spacers between tetramethyldisiloxane and BTBT units can be used as an active semiconductor layer in OFETs. Ultrathin-layer OFETs manufactured using the Langmuir–Blodgett or Langmuir–Schaeffer technique, based on such dimers, have demonstrated good charge carrier mobilities ranging from 10−4 to 10−1 cm2 V−1 s−1 [25,26,27,28].
In this work, we synthesized and investigated novel tetrameric structures containing four 2,7-dialkyl-substituted BTBT semiconductor moieties with different spacer and terminal alkyl chain lengths attached to a flexible carbosilane–siloxane core (Figure 1). Similar structures using quaterthiophene as a semiconductor moiety have demonstrated good semiconducting properties in OFETs [29] prepared by solution processing. The tetramers with longer oligothiophenes, containing from 5 to 7 conjugated 2,5-thienyl fragments, have shown interesting phase behavior and intramolecular aggregation in solutions [30].
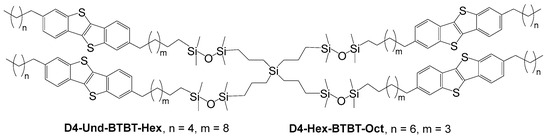
Figure 1.
Chemical structures of flexible tetramers based on four dialkyl-substituted BTBT moieties, with flexible carbosilane–siloxane fragments.
2. Results
2.1. Synthesis
In this work, the synthesis of tetrakis(1-(1,1,3,3-tetramethyl-3-(ω-(7-alkyl-[1]Benzothieno[3,2-b]benzothiophen-2-yl)alkyl)disiloxanyl)propyl)silanes D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct was carried out in order to study their phase behavior and semiconductor properties. The synthesis of the target molecules was carried out by sequential acylation of BTBT according to Friedel–Crafts, followed by a reduction in the keto group [31]. The sequential introduction of alkyl substituents is due to the asymmetry of the molecule—one alkyl was unfunctional to serve as the terminal aliphatic group and the other alkyl contained a bromine-protected double bond as a functionality used for the following reactions. At the second stage, the reaction of debromination of bromine protection was carried out with the formation of a terminal double bond, followed by the addition of tetramethyldisiloxane (TMDS) [25]. At the final stage of the synthesis, the hydrosilylation reaction of the corresponding 1,1,3,3-tetramethyl-1-(6-(ω-alkyl-[1]Benzothieno[3,2-b]benzothiophen-2-yl)alkyl)disiloxanes 7a, b with tetraallylsilane in a ratio of 1:5 in the presence of Karstedt’s (Karstedt’s catalyst: CAS 68478-92-2) catalyst was carried out (Scheme 1).
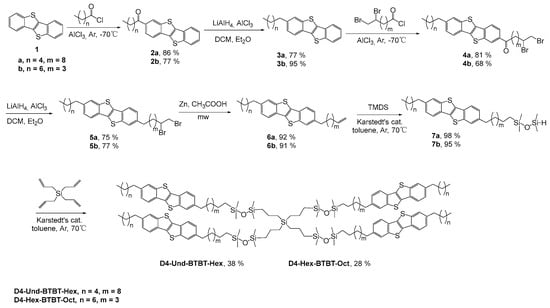
Scheme 1.
Synthesis of compounds D4-Und-BTBT-Hex, D4-Hex-BTBT-Oct.
The reaction progress was monitored by gel-permission chromatography (GPC) and thin-layer chromatography (TLC).
Low yields of reaction products at the last stage are due to the formation of mono-, di- and tri-addition by-products, which is confirmed by GPC (Figure 2). However, the reagent ratio changing has not increased the formation of the target tetra-addition product. Purification of the target compound was carried out by column chromatography, using cyclohexane/toluene 10:1 vol/vol mixture as an eluent.
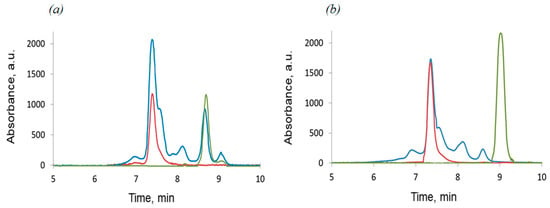
Figure 2.
GPC chromatogram monitoring of the synthesis of (a) D4-Und-BTBT-Hex: starting compound 7a (green), reaction mixture (blue), target compound (red); (b) D4-Hex-BTBT-Oct: starting compound 7b (green), reaction mixture (blue), target compound (red) (GPC chromatograms are in Figures S24–S27, ESI).
The compounds obtained were characterized by 1H, 13C and 29Si NMR spectroscopy, mass spectrometry MALDI and elemental analysis (see below in Section 3 as well as spectra Figures S1–S23, Figures S28 and S29, ESI). A distinctive feature of the 1H NMR spectra of both compounds, D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct, is the absence of a signal from the terminal proton at silicon in the region of 4.67 ppm, as well as a doublet in the region of 0.15 ppm, corresponding to six protons of two methyl groups at silicon containing this proton.
2.2. Thermal Properties and Phase Behavior
The thermal and thermo-oxidative stability of the compounds obtained, D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct, were investigated by thermogravimetric analysis (TGA) in the argon atmosphere and in the air, respectively (Figure 3).
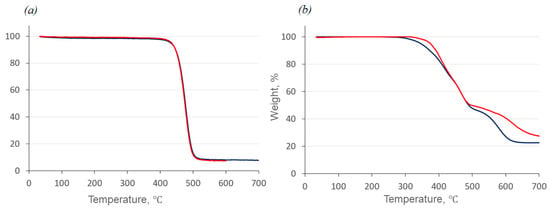
Figure 3.
Results of thermogravimetric analysis of compounds D4-Und-BTBT-Hex (blue curves) and D4-Hex-BTBT-Oct (red curves): (a) in the argon atmosphere; (b) in the air atmosphere.
The results of thermogravimetric analysis have shown that the stabilities of both compounds, D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct, are very close: the temperatures of 5% mass loss are 433–435 °C in the inert atmosphere and 346–375 °C in the air. The lower stability of compound D4-Und-BTBT-Hex in the air can be explained by the presence of longer alkyl substituents in the molecule. TGA performed in air shows a two-stage mass loss, with the first stage representing the loss of alkyl groups and the second stage representing the oxidation of sulfur in BTBT at higher temperature in oxygen [32].
The packing of molecules and their ability to self-organize have a significant effect on the semiconducting properties of the final material [33]. The phase behavior of D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct samples in the bulk was studied by the differential scanning calorimetry (DSC) and polarization optical microscopy (POM) methods.
The DSC scans of the first and second heating of both compounds are shown in Figure 4. The heating of a virgin sample of D4-Und-BTBT-Hex is accompanied by two endothermic peaks. The first one is bimodal with maxima at 73 and 80 °C, while the second one is observed at 134 °C. On the second heating after slow cooling (20 °C min−1), two peaks are also observed at 77 and 127 °C. The bimodality of the first peak is reduced to a low temperature shoulder. The heating of a virgin sample of D4-Hex-BTBT-Oct reveals two endothermic peaks at 31 and 118 °C. However, a low temperature phase is not formed after melt crystallization. The second heating of D4-Hex-BTBT-Oct is accompanied by the jump in heat capacity at −40 °C (ΔCp = 0.29 J g−1 K−1), corresponding to the glass transition of amorphous phase and an endothermic peak at 118 °C. It is assumed that the thermophysical effects at low temperatures correspond to the glass transition and crystallization of flexible alkyl groups [34], while the transitions in the range of 118–127 °C correspond to the disordering of rigid benzothiophene groups and the subsequent isotropization of the material. (Temperatures and enthalpies of transitions are summarized in Table S1, ESI.)
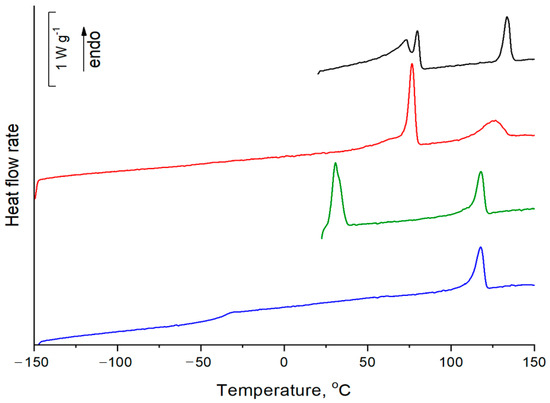
Figure 4.
The DSC curves: D4-Und-BTBT-Hex: the 1st heating—black curve, the 2nd heating—red curve; D4-Hex-BTBT-Oct: the 1st heating—green curve, the 2nd heating—blue curve.
To determine the nature of the high-temperature phases, samples D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct were investigated by POM in crossed polarizers (Figure 5). According to the data obtained, compound D4-Und-BTBT-Hex is characterized by the presence of birefringence in the temperature range from room temperature (ca. 23 °C) until 120 °C, while there were observed texture changes near 70 °C, and further heating led to a transition to an isotropic melt. However, only a transition to an isotropic melt is observed for D4-Hex-BTBT-Oct at the temperature of 118 °C. Both compounds demonstrate the formation of a fan-shaped structure when cooling the samples from the isotropic melts (Figure 5a,c), which is typical for disordered smectic mesophases A or C. However, further cooling of D4-Und-BTBT-Hex below 70 °C is characterized by the braking of the fans, indicating some ordering process (Figure 5b), corresponding to the transition to a crystal or one of the ordered smectic phases (i.e., E, G, F or K). Further cooling the sample of D4-Hex-BTBT-Oct does not lead to any significant changes in the texture (Figure 5d).
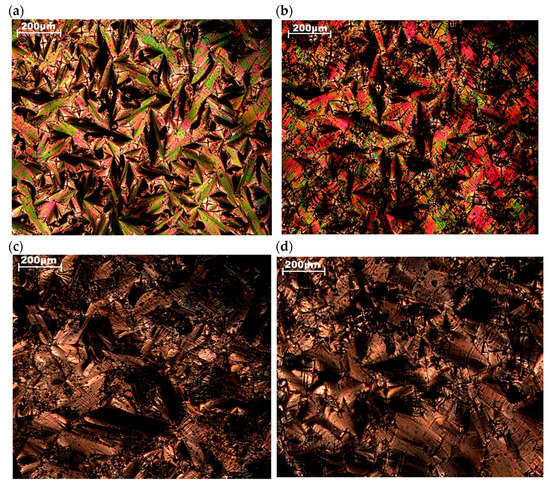
Figure 5.
Micrographs in crossed polarizers during cooling: (a) sample D4-Und-BTBT-Hex at 110 °C, (b) sample D4-Und-BTBT-Hex at 40 °C, (c) sample D4-Hex-BTBT-Oct at 97 °C, (d) sample D4-Hex-BTBT-Oct at 5 °C.
2.3. X-Ray Diffraction
To determine the structure and phase behavior, small- and wide-angle X-ray diffraction analysis was performed during in situ heating (Figure 6). The diffraction pattern of sample D4-Und-BTBT-Hex at room temperature shows a set of reflections that clearly indicate the type of ordering as smectic E (SmE). Wide-angle reflections in the region of q 14–22 nm−1 refer to the orthorhombic lattice of BTBT fragments and are indexed as 110, 020 and 120. The position of the small-angle peak, as well as its orders, allows us to determine a layer period equal to 30.4 Å. The obtained value is less than 35.8 Å reported previously for the dimer of a similar structure with hexyl substituents D2-Und-BTBT-Hex [27], probably due to an increased number of branches. Heating to 80 °C leads to the disappearance of wide-angle reflections and a shift in the small-angle peak that corresponds to the transition to a smectic A (SmA) with a period of 28.7 Å. A decrease in the smectic period with the loss of order is characteristic of most semi-crystalline materials. The transition to an isotropic melt is observed at 118 °C.
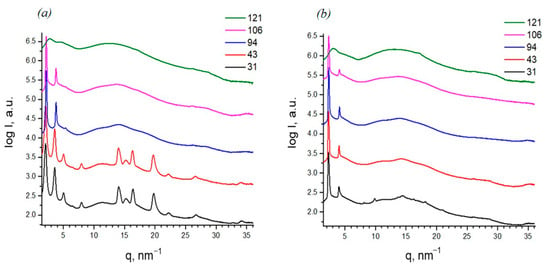
Figure 6.
SWAXS diffraction patterns at elevated temperatures: (a) sample D4-Und-BTBT-Hex; (b) sample D4-Hex-BTBT-Oct. Curves are shown in logarithmic scale and shifted for clarity. (Diffraction patterns in Figure S30, ESI).
For the sample D4-Hex-BTBT-Oct, which has shorter spacers but longer terminal alkyl fragments, an intense small-angle reflection with an interplanar distance of 27.3 Å is also observed. At the same time, in the wide-angle region, reflections similar to those for D4-Und-BTBT-Hex are very weakly expressed, characterized by a large half-width, which indicates a small crystallite size, and finally disappear upon heating to 40 °C, which corresponds to the melting of the crystal lattice of BTBT fragments and the transition to smectic A. In this case, there is no change in the parameter of the liquid crystal packing until the transition to an isotropic melt at 117 °C. The interplanar distance corresponding to the small-angle reflection is 30 Å, which is close to the extended conformation of D4-Und-BTBT-Hex and indicates a structure of smectic ordering of the E type (Figure 7).
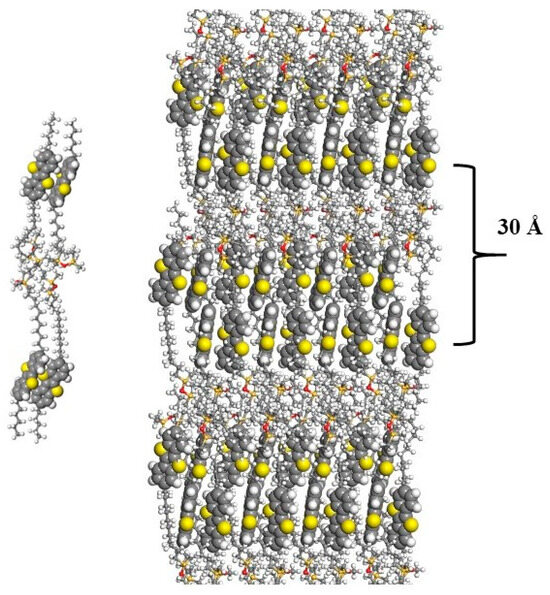
Figure 7.
Molecular model of the compound D4-Und-BTBT-Hex in the extended conformation and in smectic E phase.
Thus, based on the structural data obtained, it can be concluded that the crystal packing is significantly disrupted, with a decrease in the spacer and an increase in the terminal fragments. Probably, the first factor has a determining influence, due to the constraint of free rotation during the ordering of benzothiophene fragments in the lattice, as well as the presence of a well-defined smectic E for the other compounds with BTBT groups and alkyl terminal substituents [35]. At the same time, the temperature stability and the mesophase parameter are preserved when modifying the length of the alkyl fragments.
2.4. Electrical Characteristics
The tetramers obtained, D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct, were tested as active layers for OFETs, since the previously reported siloxane dimers, i.e., D2-Und-BTBT-Hex, D2-Hex-BTBT-Hex and D2-Hept-BTBT-Hex containing both an organosilicon component and a BTBT fragment, have demonstrated good semiconductor characteristics in OFETs [26,28]. The advantages of using organosilicon derivatives of BTBT are their good film forming properties and stability under normal conditions [36].
Thin films of the D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct compounds were obtained on a Si/SiO2 substrate by the spin-coating method. To form an even layer, the two-stage processing method was chosen: 500 rpm for 30 s and 1000 rpm for 90 s [26], varying the solution concentration—1.0 or 2.0 gL−1 (Table 1). The films were obtained for both compounds, D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct (Figures S31–S37, ESI). The films’ thicknesses for both compounds ranged from 6 to 24 nm, which represents a few layers. However, good semiconducting properties were only recorded for the D4-Und-BTBT-Hex compound (Table 1, Figure 8).

Table 1.
Electrical performance data for the OFETs based on different organic semiconductors prepared at different concentrations.
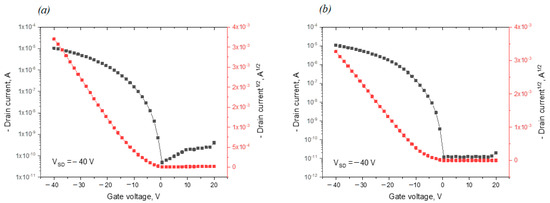
Figure 8.
Transfer characteristics of the best devices for D4-Und-BTBT-Hex coupling in logarithmic coordinates (black) and as a function of the square root of the current (red) for concentrations: (a) 1 g L−1; (b) 2 g L−1. In turn, films based on the D4-Hex-BTBT-Oct compound demonstrated very weak semiconducting properties at high threshold voltages (Table 1). This can be explained by the fact that compound D4-Und-BTBT-Hex has a denser packing due to the longer length of the undecyl spacer between the organosilicon and BTBT fragments, which at room temperature forms an ordered SmE phase, as opposed to compound D4-Hex-BTBT-Oct, which forms a disordered SmA phase only.
The devices fabricated using the D4-Und-BTBT-Hex compound showed reproducible electrical characteristics, with the effective charge carrier mobility of the order of 10−2–10−4 cm2 V−1 s−1, a low threshold voltage and a high on/off ratio of 104−107 (Table 1 and Table S3, ESI). These data are comparable to or even better than the results obtained previously for siloxane dimers D2-Und-BTBT-Hex, D2-Hex-BTBT-Hex and D2-Hept-BTBT-Hex of similar structure under similar preparation conditions [26,28] (Table S3, ESI). Moreover, since a reliability factor rsat [37] for the calculated mobility values is ca. 0.6, the effective mobility values presented in Table 1 are ca. 1.5 times less than the ones measured and reported for the dimers. It is worth noting that, in the case of tetramer D4-Und-BTBT-Hex, changing the solution concentration did not lead to a significant change in its electrical characteristics, unlike the dimer D2-Und-BTBT-Hex. The best characteristics for D4-Und-BTBT-Hex were obtained for batch 2, which showed less dewetting of organic semiconductor film from the substrate (compare Figures S31 and S32, ESI). Similar findings were observed for thin films of D4-Hex-BTBT-Oct, where only batch 2 showed less dewetting of the film with semiconductors characteristics, while batch 1 did not work in the OFETs (compare Figures S33 and S34, ESI). Still further optimization should be possible to reach the mobility values > 0.1 cm2 V−1 s−1, at least for D4-Und-BTBT-Hex, i.e., as had been achieved previously for D2-Und-BTBT-Hex using a PMMA interlayer on the dielectrics, where μmax up to 0.47 cm2 V−1 s−1 was measured [26]. AFM topography images of the thin films surfaces and them profiles for D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct see Figures S35–S37, ESI.
3. Materials and Methods
3.1. Materials
Commercially available reagents were used in this work: octanoyl chloride (Sigma-Aldrich, Burlington, MA, USA, 99%), hexanoyl chloride (Sigma-Aldrich, 97%), tetraallylsilane (Sigma-Aldrich, 97%), aluminum chloride (Sigma-Aldrich, 99%), zinc powder (Sigma-Aldrich, 99%), lithium aluminum hydride (Thermo Scientific, Waltham, MA, USA, 98%), glacial acetic acid (JSC “Base No. 1 of Chemical Reactants” Moscow, Russia), Karstedt catalyst (“abcr”, Karlsruhe, Germany, 3–3.5% Pt), 1,1,3,3-tetramethyldisiloxane (Sigma-Aldrich, 97%) and anhydrous sodium sulfate (JSC “Lenreaktiv”, Saint-Petersburg, Russia, chemically pure). Dry toluene (10 ppm) (JSC Vekton, Moscow, Russia, analytical grade), dry dichloromethane (20 ppm) (JSC Vekton, analytical grade), dry tetrahydrofuran (20 ppm) (ECOS-1, Moscow, Russia, chemically pure), cyclohexane (ECOS-1, analytical grade), diethyl ether (JSC Vekton, analytical grade), acetone (ECOS-1, analytical grade) and isopropanol (Reaktiv-express, Moscow, Russia, analytical grade) were used as solvents. All solvents were dried using standard methods; water content was monitored using a K. Fischer 831 KF Coulometer titrator (Metrohm, Herisau, Switzerland). Ultrapure deionized water with a resistance of 18 MΩ*cm was obtained using an Aquilon D-301 deionizer (Podolsk, Russia).
3.2. NMR-Spectroscopy
1H NMR spectra were recorded on a Bruker WP-250 SY spectrometer (Billerica, MA, USA) at a frequency of 250.13 MHz using the CDCl3 signal (7.25 ppm) as an internal standard. 13C and 29Si NMR spectra were recorded on a Bruker Avance II 300 spectrometer at a frequency of 75 MHz.
For 1H NMR spectroscopy, 2% solutions of the analyzed substances were prepared, for 13C and 29Si NMR spectroscopy—5% solutions, the results were processed on a computer using special ACDLabs 12.0 software.
3.3. Thermal Analysis
Thermogravimetric analysis of the samples was carried out in a dynamic mode in the range from 20 to 700 °C using the STA JUPITER 443 F3 NETZSCH system (Selb, Germany) with an accuracy of sample weight determination up to 1 mg. The heating rate was 10 deg/min in an atmosphere of air and argon.
DSC of the samples was carried out using a Perkin-Elmer DSC7 (Shelton, CT, USA) differential scanning calorimeter in a helium flow (30 mL min−1). Sample weight was about 10 mg. Weighing was performed using a Perkin-Elmer AD-4 Autobalance with an accuracy of 10−5 g. The samples were heated with a heating rate of 20 deg min−1, then cooled with the same rate and heated again.
3.4. X-Ray Diffraction Analysis
Small-angle and wide-angle X-ray diffraction analysis (XRD) of the samples was performed at the BioMUR station of the Kurchatov synchrotron (Moscow, Russia). The radiation source was a 1.7 T bending magnet with an energy of 8 keV (1.435 Å) with a resolution of dE/E 10−3 and a photon flux of 109. The beam size on the sample was 0.5 × 0.3 mm2, and a Dectris Pilatus 1 M two-dimensional detector (DECTRIS Ltd., Baden-Daettwil, Switzerland) was used to record diffraction patterns. The sample-detector distance was 150 mm, and background scattering was subtracted before subsequent processing. Silver behenate and NaC (Na2Ca3Al2F14) were used as calibration standards at small and large angles. The range of backscattering vector q was 0.8–33 nm−1. A Linkam THMS 600 heating stage (Linkam Scientific Instruments Ltd., Redhill, Surrey, UK) with a heating rate of 7 deg min−1 was used to heat the sample in situ. The exposure time was 23 s; the samples were placed in Hilgenberg X-ray capillaries with a diameter of 2 mm and a wall thickness of 0.01 mm (Hilgenberg GmbH, Malsfeld, Germany). The open software packages Fit2D 18.0 (ESRF, Grenoble, France) and ImageJ 1.52u (NIH, Washington, D.C.,USA) were used to process the obtained scattering patterns.
3.5. Chromatographic Methods
Analysis by GPC was carried out on a chromatographic system: Shimadzu (Kyoto, Japan) with a RID10AVP refractometer, SPD-M10AVP diode array, 300 mm long and 7.8 mm in diameter (300 × 7.8 mm2) columns (Phenomenex, Torrance, CA, USA) filled with the Phenogel sorbent (Phenomenex, USA), pore size 500 Å, thermostatting temperature—40 °C ± 0.1 °C, eluent—tetrahydrofuran (THF). The system consisted of a STAYER series 2 high-pressure pump (Aquilon, Russia), a SmartlineRI 2300 refractometric detector (KNAUER, Berlin, Germany) and a JETSTREAM 2 PLUS column thermostat (KNAUER, Germany). Thermostat temperature 40 °C ± 0.1 °C, eluent—THF, flow rate—1.0 mL/min, columns 300 mm long and 7.8 mm in diameter (300 × 7.8 mm2) filled with Phenogel sorbent (Phenomenex, USA), particle size—5 μm, pore size 104 Å. The analysis results were processed using the MultiChrom 1.6 GPC program (Ampersend, Krasnogorsk, Russia) using polystyrene standards.
For preparative separation of the mixture, a PuriFlash XS 520Plus chromatograph (Interchim, Montleçon, France) with a PF-XS520Plus 200–400 nm UV detector, a panVap-6 four-channel air pump and PF-50SIHP-00120 columns filled with Silica Inorganic Sorbent (Sigma-Aldrich, USA), pore size 50 μm were used.
3.6. Mass-Spectrometry MALDI
Analysis by MALDI was carried out on mass spectrometer “Autobio Autof MS 1600” (Autobio Diagnostics Co., Zhengzhou, China). Samples were dissolved in toluene (for spectroscopy “Component reactive”, Mocsow, Russia) to a concentration of 12 mg/mL. DCTB (trans-2-[3-(4-tert-butylphenyl)-2-propenylidene]malononitrile with a concentration of 20 mg/mL in propanol) was used as a matrix. The probe (volume 3 μL) was applied layer by layer and dried at 25 °C. Positive ions were recorded in a linear mode in the range of 200–4000 Da. External calibration was performed using polyethylene glycol standards in the range of 480–3860 Da.
3.7. Laboratory Equipment for Synthesis
Microwave organic synthesis system “CEM Discovery” (Matthews, NC, USA) was used to synthesize compounds 6a, b at 100 °C and 50 W power for 15 min.
3.8. Synthesis Methods
Compounds 1–7 were obtained according to the literature methods: compound 1 [38]; compounds 2–7 [25,32].
5,6-dibromo-1-(7-octyl[1]Benzothieno[3,2-b]benzothiophen-2-yl)hexan-1-one (4b).
The yield was 5.01 g (68%). 1H NMR spectrum (CDCl3) δ ppm: 8.53 (d, J = 1.22 Hz, 1H), 8.02–8.08 (m, 1H), 7.73–7.93 (m, 3H), 7.27–7.34 (m, 1H), 4.16–4.29 (m, 1H), 3.84–3.93 (m, 1H), 3.62–3.73 (m, 1H), 3.09–3.19 (m, 2H), 2.76 (t, J = 7.78 Hz, 2H), 1.88–2.37 (m, 4H), 1.68 (d, J = 7.02 Hz, 2H), 1.22–1.42 (m, 10H), 0.83–0.93 (m, 3H). 13C NMR spectrum (CDCl3) δ ppm: 198.10, 151.12, 146.23, 139.72, 137.28, 136.32, 133.74, 124.82, 123.12, 122.54, 120.22, 53.96, 42.07, 37.21, 36.71, 32.26, 29.62, 29.58, 22.76, 20.18, 13.23. Found, %: C 55.14; H 5.28; S 10.58. Calculated, %: C 55.27; H 5.30; S 10.54.
2-(5,6-dibromohexyl)-7-octyl[1]Benzothieno[3,2-b]benzothiophene (5b).
The yield was 3.76 g (77%). 1H NMR spectrum (CDCl3) δ ppm: 7.69–7.80 (m, 4H), 7.23–7.28 (m, 2H), 4.10–4.24 (m, 1H), 3.85 (dd, J1 = 10.38 Hz, J2 = 4.27 Hz, 1H), 3.56–3.68 (m, 1H), 2.70–2.84 (m, 4H), 2.13–2.29 (m, 1H), 1.61–1.93 (m, 7H), 1.23–1.42 (m, 10H), 0.83–0.93 (m, 3H). 13C NMR spectrum (CDCl3) δ ppm: 141.23, 138.46, 136.82, 124.72, 123.91, 122.42, 52.36, 38.48, 37.51, 35.86, 31.98, 31.26, 31.02, 29.60, 29.48, 25.41, 22.12, 13.26. Found, %: C 56.62; H 5.81; S 10.74. Calculated, %: C 56.57; H 5.76; S 10.79.
2-(hex-5-en-1-yl)-7-octyl[1]Benzothieno[3,2-b]benzothiophene (6b).
The yield was 2.46 g (91%). 1H NMR spectrum (CDCl3) δ ppm: 7.67–7.80 (m, 4H), 7.23–7.28 (m, 2H), 5.81 (ddt, J1 = 17.01, J2 = 10.30, J3 = 6.49 Hz, 1H), 4.91–5.07 (m, 2H), 2.75 (td, J1 = 7.55, J2 = 3.81 Hz, 4H), 2.04–2.18 (m, 2H), 1.62–1.79 (m, 4H), 1.42–1.58 (m, 3H), 1.23–1.40 (m, 10H), 0.82–0.92 (m, 3H). 13C NMR spectrum (CDCl3) δ ppm: 141.12, 139.46, 137.13, 136.82, 123.12, 122.43, 122.01, 116.03, 35.86, 34.13, 29.48, 28.92, 28.18, 22.12, 13.23. Found, %: C 77.52; H 7.79; S 14.69. Calculated, %: C 77.37; H 7.88; S 14.75.
1,1,3,3-tetramethyl-1-(6-(7-octyl[1]Benzothieno[3,2-b]benzothiophen-2-yl)hexyl)disiloxane (7b).
The yield was 2.99 g (95%). 1H NMR spectrum (CDCl3) δ ppm: 7.67–7.80 (m, 4H), 7.25–7.31 (m, 2H), 4.63–4.71 (m, 1H), 2.75 (t, J = 7.63 Hz, 4H), 1.66–1.71 (m, 4H), 1.22–1.40 (m, 16H), 0.82–0.93 (m, 3H), 0.54 (d, J = 8.85 Hz, 2H), 0.15 (d, J = 2.75 Hz, 6H), 0.02–0.09 (m, 6H). 13C NMR spectrum (CDCl3) δ ppm: 139.73, 137.29, 136.32, 123.12, 123.05, 122.52, 36.71, 35.86, 31.28, 29.62, 22.76, 19.83, 13.23, 5.96, 2.12. Found, %: C 67.52; H 8.36; S 11.21. Calculated, %: C 67.55; H 8.50; S 11.27.
General procedure for the synthesis of compounds D4-Und-BTBT-Hex, D4-Hex-BTBT-Oct.
A single-neck flask (10 mL), a reflux condenser and an argon inlet/outlet valve were dried at 150 °C for 1 h. The reaction apparatus was assembled and cooled in an argon stream. Tetraallylsilane (1 equiv.) and a solution of compound 7 (5 equiv.) in a minimum volume of dry toluene (11 ppm) were added to the reaction flask. Then, 30 mol% of Karstedt’s catalyst was added to the reaction flask and stirred while heating at 70 °C for 6 h. The reaction was monitored by GPC and TLC (eluent cyclohexane/toluene 3%). The resulting reaction mixture was evaporated on a vacuum rotary evaporator. Purification was carried out by column chromatography on silica gel, eluent cyclohexane/toluene 10%.
Tetrakis(3-(1,1,3,3-tetramethyl-1-(11-(7-hexyl[1]Benzothieno[3,2-b]benzothiophen-2-yl)undecyl)disiloxanyl)propyl)silane (D4-Und-BTBT-Hex).
From 0.81 g (1.33 mmol) of compound 7a, 0.053 g (0.27 mmol) of tetraallylsilane, 65 μL (0.12 mmol) of Karstedt catalyst and 5 mL of toluene, 0.28 g (38%, purity 97%) of substance D4-Und-BTBT-Hex was obtained as a white powder. 1H NMR spectrum (CDCl3) δ ppm: 7.64–7.79 (m, 16H), 7.21–7.30 (m, 8H), 2.68–2.81 (m, 16H), 1.67 (d, J = 7.15 Hz, 16H), 1.30 (d, J = 19.07 Hz, 96H), 0.84–0.95 (m, 12H), 0.46–0.66 (m, 24H), 0.04 (s, 48H). 13C NMR spectrum (CDCl3) δ ppm: 142.35, 140.00, 132.49, 131.14, 125.77, 123.26, 121.01, 36.10, 33.51, 31.72, 31.67, 29.75, 29.66, 29.57, 29.45, 29.37, 28.98, 23.42, 23.32, 22.60, 18.43, 17.98, 17.19, 14.11, 0.51, 0.43. 29Si NMR spectrum (CDCl3) δ ppm: 7.30, 6.72. Found, %: C 69.28; H 9.04; S 9.65. Calculated, %: C 69.24; H 9.02; S 9.73. MALDI (m/z): found [M − H+]:2632.3052; calculated [M − H+]: 2632.3874.
Tetrakis(3-(1,1,3,3-tetramethyl-1-(6-(7-octyl[1]Benzothieno[3,2-b]benzothiophen-2-yl)hexyl)disiloxanyl)propyl)silane (D4-Hex-BTBT-Oct).
From 1.20 g (2.11 mmol) of compound 7b, 0.081 g (0.42 mmol) of tetraallylsilane, 100 μL (0.18 mmol) of Karstedt’s catalyst and 5 mL of toluene, 0.24 g (28%, purity 99%) of substance D4-Hex-BTBT-Oct was obtained as a white powder. 1H NMR spectrum (CDCl3) δ ppm: 7.63–7.76 (m, 16H), 7.22 (m, 8H), 2.72 (m, 16H), 1.61–1.70 (m, 16H), 1.30 (d, J = 17.70 Hz, 72H), 0.83–0.93 (m, 12H), 0.44–0.63 (m, 24H), 0.02–0.08 (m, 48H). 13C NMR spectrum (CDCl3) δ ppm: 142.12, 140.03, 132.54, 131.24, 125.79, 123.37, 121.06, 35.98, 33.61, 31.92, 31.57, 29.78, 29.56, 29.44, 22.71, 22.43, 20.02, 19.19, 13.21, 0.54, 0.40. 29Si NMR spectrum (CDCl3) δ ppm: 7.31, 6.72. Found, %: C 68.09; H 8.76; S 10.42. Calculated, %: C 68.12; H 8.66; S 10.39. MALDI (m/z): found [M + H+]:2466.3345; calculated [M + H+]: 2466.2153.
3.9. OFET Architecture
OFETs were fabricated on 20 mm × 15 mm Si/SiO2 substrates pre-washed in piranha, acetone and isopropanol. Highly doped silicon wafers with thermally grown 300 nm thick silicon dioxide layers were used as the gate electrode and dielectric (dielectric capacitance 11.5 nFcm−2). The bottom electrodes “source” and “drain” were fabricated by thermal evaporation (Au, 40 nm) on the substrates through shadow masks with gold electrodes in the bottom contact geometry, and had the following geometric dimensions: channel length L = 30 µm and channel width W = 1000 µm (W/L ratio = 33).
3.10. Surface Preparation
Before deposition of the semiconductor layer, the substrates were plasma-treated using a low-pressure plasma system Diener Electronic (Diener Electronic GmbH, Ebhausen, Germany) at a frequency of 40 kHz and a power of 200 W. The contacts were modified in a solution of 2,3,4,5,6-pentafluorobenzenethiol (PFBT) (Sigma Aldrich Co., USA), according to the literature method [39].
3.11. Application Method
The spin-coating method was chosen as a method for producing the active semiconductor layer of OFETs using a WS-650MZ-8NPP/UD3 setup (Laurell Technologies Corporation, Lansdale, PA, USA). The deposition solution was prepared by dissolving the tetramers in toluene at a concentration of 1.0 and 2.0 g L−1. The active layer was deposited under the following conditions: step 1—acceleration 500 rpm, rotation speed 750 rpm, rotation time 30 s; step 2—1000 rpm, rotation time 90 s. Dropping volume—50 μL.
3.12. Film Characterization
The morphology of thin films was studied using an NT-MDT atomic-force microscope (SOLVER NEXT, Zelenograd, Russia) in tapping mode under ambient conditions using NT-MDT HA-FM silicon probes with a resonance frequency of 77 kHz. Optical micrographs were obtained on an Axioscop A40Pol polarization optical microscope (Carl Zeiss, Oberkochen, Germany).
3.13. Electrical Measurements
The current-voltage characteristics were measured in air on a probe station (ProbeStation 100, Printeltech, Moscow, Russia) using a Keithley 2634B source-measure unit (Tektronix, Beaverton, OR, USA). To avoid current leakage, the perimeter of the active zone of the device was carefully scratched. The charge carrier mobility and threshold voltages were estimated according to the Shockley model [40]. Since nonlinearities were observed in the OFET characteristics, the effective mobility μeff. was calculated based on the reliability factor rsat introduced by V. Podzorov et al. [37] and described in [39].
4. Conclusions
In this work, two novel organosilicon tetramers with dialkyl-substituted [1]Benzothieno[3,2-b]benzothiophene moieties, D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct, were synthesized and investigated. The target tetramers were obtained by the hydrosilylation reaction between tetraallylsilane and 1,1,3,3-tetramethyl-1-(ω-(7-alkyl[1]Benzothieno[3,2-b]benzothiophen-2-yl)alkyl)disiloxanes in 28–38% yields. The chemical structure of the compounds obtained was confirmed by 1H, 13C and 29Si NMR spectroscopy, gel permeation chromatography and elemental analysis. The phase behavior of these compounds was studied by DSC, POM and XRD. It was shown that, in compound D4-Und-BTBT-Hex, there are two phase transitions which correspond to phase transitions from smectic E to smectic A with subsequent isotropization. In turn, for compound D4-Hex-BTBT-Oct, wide-angle reflections are weakly expressed, characterized by a large half-width, which indicates a small crystallite size and, up to 40 °C, a phase transition to smectic A occurs with subsequent isotropization at 118 °C. A significant disruption of the crystal packing in the obtained tetramers occurs, with a decrease in the spacer length and an increase in the terminal fragments. Temperature stability and mesophase parameters are maintained, with a change in the length of the alkyl moieties. The investigation of tetrameric structures D4-Und-BTBT-Hex and D4-Hex-BTBT-Oct as an active layer in OFETs showed that the tetramer with the longer undecyl spacer length and the shorter hexyl terminal groups D4-Und-BTBT-Hex has demonstrated good electrical properties, with a maximum charge mobility of up to 3.5 × 10−2 cm2 V−1 s−1, a low threshold voltage and an on/off ratio up to 107. This is comparable or even better for the semiconductor properties of the similar disiloxane dimer D2-Und-BTBT-Hex, measured previously under similar conditions. However, a tetramer with a shorter hexyl spacer and even longer alkyl end groups, D4-Hex-BTBT-Oct, exhibited weak semiconducting properties in OFETs, consistent with DSC and X-ray diffraction data, indicating its lower order at room temperature. These results pave the way for the development of more advanced soluble organic semiconductor solutions with pronounced semiconducting properties.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30234639/s1, Figure S1: 1H NMR spectra of compound 1; Figure S2: 1H NMR spectra of compound 2a; Figure S3: 1H NMR spectra of compound 2b; Figure S4: 1H NMR spectra of compound 3a; Figure S5: 1H NMR spectra of compound 3b; Figure S6: 1H NMR spectra of compound 4a; Figure S7: 1H NMR spectra of compound 4b; Figure S8: 13C NMR spectra of compound 4b; Figure S9: 1H NMR spectra of compound 5a; Figure S10: 1H NMR spectra of compound 5b; Figure S11: 13C NMR spectra of compound 5b; Figure S12: 1H NMR spectra of compound 6a; Figure S13: 1H NMR spectra of compound 6b; Figure S14: 13C NMR spectra of compound 6b; Figure S15: 1H NMR spectra of compound 7a; Figure S16: 1H NMR spectra of compound 7b; Figure S17: 13C NMR spectra of compound 7b; Figure S18: 1H NMR spectra of compound D4-Und-BTBT-Hex; Figure S19: 13C NMR spectra of compound D4-Und-BTBT-Hex; Figure S20: 29Si NMR spectra of compound D4-Und-BTBT-Hex; Figure S21: 1H NMR spectra of compound D4-Hex-BTBT-Oct; Figure S22: 13C NMR spectra of compound D4-Hex-BTBT-Oct; Figure S23: 29Si NMR spectra of compound D4-Hex-BTBT-Oct; Figure S24: GPC curve of compound 7a; Figure S25: GPC curve of compound D4-Und-BTBT-Hex; Figure S26: GPC curve of compound 7b; Figure S27: GPC curve of compound D4-Hex-BTBT-Oct; Figure S28: Mass spectrum of the compound D4-Und-BTBT-Hex; Figure S29: Mass spectrum of the compound D4-Hex-BTBT-Oct; Table S1: Temperatures and enthalpies of phase transitions; Figure S30: Diffraction patterns during heating in situ in an X-ray beam at a rate of 6 °C/min: (a) sample D4-Und-BTBT-Hex; (b) sample D4-Hex-BTBT-Oct; Table S2: Original data for electrical characteristics of the devices (batch 2); Table S3: Electrical performance dataset for BTBT-based OFETs fabricated under different conditions applied by spin-coating method; Figure S31: Optical micrographs of D4-Und-BTBT-Hex thin films obtained from the solutions with a concentration of 1.0 g L−1 (a–c) and with a concentration of 2.0 g L−1 (d–f) in cross polarizers (a,d), in dark field (b,e) and in bright field (c,f); batch 2; Figure S32: Optical micrographs of D4-Und-BTBT-Hex thin films obtained from the solutions with a concentration of 1.0 g L−1 (a–c) and with a concentration of 2.0 g L−1 (d–f) in cross polarizers (a,d), in dark field (b,e) and in bright field (c,f), batch 1; Figure S33: Optical micrographs of D4-Hex-BTBT-Oct thin films obtained from the solutions with a concentration of 1.0 g L−1 (g–i) and with a concentration of 2.0 g L−1 (j–l) in cross polarizers (g,j), in dark field (h,k) and in bright field (i,l), batch 2; Figure S34: Optical micrographs of D4-Hex-BTBT-Oct thin films obtained from the solutions with a concentration of 1.0 g L−1 (g–i) and with a concentration of 2.0 g L−1 (j–l) in cross polarizers (g,j), in dark field (h,k) and in bright field (i,l), batch 1; Figure S35: AFM topography images of D4-Und-BTBT-Hex thin films surface obtained from the solutions with a concentration of 1.0 g L−1 (a) and with a concentration of 2.0 g L−1 (b) and corresponding cross-sections along horizontal gray lines in each image (c,d) (batch 2); Figure S36: AFM topography images of D4-Hex-BTBT-Oct thin films surface obtained from the solutions with a concentration of 1.0 g L−1 (a) and with a concentration of 2.0 g L−1 (b) and corresponding cross-sections along horizontal gray lines in each image (c,d) (batch 2); Figure S37: Thin film surface profiles of the AFM topography image fragments of D4-Und-BTBT-Hex (concentration of 1.0 g L−1) (a); of D4-Hex-BTBT-Oct (concentration of 1.0 g L−1) (b) (batch 2).
Author Contributions
Conceptualization, S.A.P.; methodology, S.A.P., S.N.C. and O.V.B.; formal analysis, I.O.G., E.A.Z., A.I.B., A.V.B., Y.O.T. and S.A.P.; investigation, I.O.G., E.A.Z., A.I.B., Y.O.T. and A.V.B.; resources, O.V.B., S.N.C. and S.A.P.; data curation, O.V.B., S.N.C. and S.A.P.; writing—original draft preparation, I.O.G., E.A.Z., A.I.B., A.V.B., Y.O.T., O.V.B., S.N.C. and S.A.P.; writing—review and editing, I.O.G., A.I.B., A.V.B., Y.O.T. and S.A.P.; visualization, I.O.G., E.A.Z., A.I.B., A.V.B. and Y.O.T.; supervision, O.V.B., S.N.C. and S.A.P.; project administration, O.V.B. and S.A.P.; funding acquisition, S.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 19-73-30028.
Data Availability Statement
Supplementary data associated with this article can be found in the online version at [reference to Supplementary Information of this article].
Acknowledgments
NMR and MALDI spectra were recorded on the equipment of the Center for Collective Use “Polymer Research Center” with the support of the Ministry of Science and Higher Education of the Russian Federation (topic FFSM-2024-0003).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BTBT | [1]Benzothieno[3,2-b]benzothiophene |
| NMR | Nuclear magnetic resonance |
| OFETs | Organic field-effect transistors |
| OSC | Organic semiconductor |
| OLET | Organic light-emitting transistor |
| OECT | Organic electrochemical transistor |
| OLED | Organic light-emitting diode |
| OPVs | Organic photovoltaics |
| TMDS | 1,1,3,3-Tetramethyldisiloxane |
| GPC | Gel permeation chromatography |
| TLC | Thin-layer chromatography |
| TGA | Thermogravimetric analysis |
| POM | Polarization optical microscopy |
| DSC | Differential scanning calorimetry |
| XRD | X-ray diffraction analysis |
| AFM | Atomic-force microscope |
References
- Kang, S.-H.; Lee, S.M.; Lee, K.C.; Yang, C. Viable Regiochemical Control in Semiconducting Polymers for Field-Effect Transistors: From High-Mobility Enhancement toward High Deformability. ACS Appl. Mater. Interfaces 2025, 17, 44030–44052. [Google Scholar] [CrossRef]
- Friederich, P.; Fediai, A.; Kaiser, S.; Konrad, M.; Jung, N.; Wenzel, W. Toward Design of Novel Materials for Organic Electronics. Adv. Mater. 2019, 31, 1808256. [Google Scholar] [CrossRef] [PubMed]
- Umoren, S.A.; Solomon, M.M. Protective polymeric films for industrial substrates: A critical review on past and recent applications with conducting polymers and polymer composites/nanocomposites. Prog. Mater. Sci. 2019, 104, 380–450. [Google Scholar] [CrossRef]
- Abroshan, H.; Kwak, H.S.; An, Y.; Brown, C.; Chandrasekaran, A.; Winget, P.; Halls, M.D. Active Learning Accelerates Design and Optimization of Hole-Transporting Materials for Organic Electronics. Front. Chem. 2022, 17, 800371. [Google Scholar] [CrossRef]
- Desu, M.; Sharma, S.; Cheng, K.-H.; Wang, Y.-H.; Nagamatsu, S.; Chen, J.-C.; Pandey, S.S. Controlling the molecular orientation of a novel diketopyrrolopyrrole-based organic conjugated polymer for enhancing the performance of organic field-effect transistors. Org. Electron. 2023, 113, 106691. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, C.; Huang, J. Organic Field-Effect Transistor-Based Sensors: Recent Progress, Challenges and Future Outlook. J. Mater. Chem. C 2025, 13, 8354–8424. [Google Scholar] [CrossRef]
- Qin, Z.; Gao, C.; Li, H.; Dong, H.; Hu, W. Organic Light-Emitting Transistors Entering a New Development Stage. Adv. Mater. 2021, 33, 2007149. [Google Scholar] [CrossRef]
- Alsufyani, M.; Rashid, R.B.; Giovannitti, A.; Inal, S.; Nielsen, C.B. The Effect of Organic Semiconductor Electron Affinity on Preventing Parasitic Oxidation Reactions Limiting Performance of N-Type Organic Electrochemical Transistors. Adv. Mater. 2024, 36, 2403265. [Google Scholar] [CrossRef]
- Mullen, K.; Scherf, U. Conjugated Polymers: Where We Come From, Where We Stand, and Where We Might Go. Macromol. Chem. Phys. 2023, 224, 2200337. [Google Scholar] [CrossRef]
- Zarrabi, N.; Sandberg, O.J.; Meredith, P.; Armin, A. Subgap Absorption in Organic Semiconductors. Phys. Chem. Lett. 2023, 14, 3174–3185. [Google Scholar] [CrossRef]
- Wong, M.Y.; Zysman-Colman, E. Purely Organic Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, H.; Feng, K.; Guo, X. Polymer Acceptors for High-Performance All-Polymer Solar Cells. Chem. Eur. J. 2022, 28, e202200222. [Google Scholar] [CrossRef]
- Mei, J.; Diao, Y.; Appleton, A.L.; Fang, L.; Bao, Z. Integrated Materials Design of Organic Semiconductors for Field-Effect Transistors. J. Am. Chem. Soc. 2013, 135, 6724–6746. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, S.; Huang, J. OFET chemical sensors: Chemical sensors based on ultrathin organic field-effect transistors. Polym. Int. 2021, 70, 414–425. [Google Scholar] [CrossRef]
- Hu, P.; He, X.; Jiang, H. Greater than 10 cm2V−1s−1: A breakthrough of organic semiconductors for field-effect transistors. InfoMat 2021, 3, 613–630. [Google Scholar] [CrossRef]
- Zhang, L.; Hasan, M.M.; Tang, Y.; Khan, A.R.; Yan, H.T.; Sun, Y.X.; Zhang, J.; Zhu, J.; Zhang, Y.; Lu, Y. 2D organic single crystals: Synthesis, novel physics, high-performance optoelectronic devices and integration. Mater. Today 2021, 50, 422–475. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, S.; Zhang, X.; Hu, W. Patterning organic semiconductor crystals for optoelectronics. Appl. Phys. Lett. 2021, 119, 040501. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, G. Multicomponent Blend Systems Used in Organic Field-Effect Transistors: Charge Transport Properties, Large-Area Preparation, and Functional Devices. Chem. Mater. 2021, 33, 2229–2257. [Google Scholar] [CrossRef]
- Kousseff, C.J.; Halaksa, R.; Parr, Z.S.; Nielsen, C.B. Mixed Ionic and Electronic Conduction in Small-Molecule Semiconductors. Chem. Rev. 2021, 122, 4397–4419. [Google Scholar] [CrossRef]
- Kim, Y.; Yun, C.; Yun, S.; Ho, D.; Earmme, T.; Kim, C.; Seo, S.Y. Modification of alkyl side chain on thiophene-containing benzothieno[3,2-b]benzothiophene-based organic semiconductors for organic field-effect transistors. Synth. Met. 2022, 291, 117173. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, L. Comprehensive Study on the Mobility Anisotropy of Benzothieno[3,2-b][1]benzothiophenes: Toward Isotropic Charge-Transport Properties. J. Phys. Chem. C 2023, 127, 13327–13337. [Google Scholar] [CrossRef]
- Mori, T.; Nishimura, T.; Yamamoto, T.; Doi, I.; Miyazaki, E.; Osaka, I.; Takimiya, K. Consecutive Thiophene-Annulation Approach to π-Extended Thienoacene-Based Organic Semiconductors with [1]Benzothieno[3,2-b][1]benzothiophene (BTBT) Substructure. J. Am. Chem. Soc. 2013, 135, 13900–13913. [Google Scholar] [CrossRef]
- Wawrzinek, R.; Sobus, J.; Chaudhry, M.U.; Ahmad, V.; Grosjean, A.; Clegg, J.K.; Namdas, E.B.; Lo, S.-C. Mobility Evaluation of [1]Benzothieno[3,2-b][1]benzothiophene Derivatives: Limitation and Impact on Charge Transport. ACS Appl. Mater. Interfaces 2019, 11, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Monobe, H.; An, L.; Hu, P.; Wang, B.-Q.; Zhao, K.-Q.; Shimizu, Y. Charge transport property of asymmetric Alkyl-BTBT LC semiconductor possessing a fluorophenyl group. Mol. Cryst. Liq. Cryst. 2017, 647, 119–126. [Google Scholar] [CrossRef]
- Borshchev, O.V.; Sizov, A.S.; Agina, E.V.; Bessonov, A.A.; Ponomarenko, S.A. Synthesis of organosilicon derivatives of [1]benzothieno[3,2-b][1]-benzothiophene for efficient monolayer Langmuir–Blodgett organic field effect transistors. Chem. Commun. 2017, 53, 885–888. [Google Scholar] [CrossRef]
- Trul, A.A.; Sizov, A.S.; Chekusova, V.P.; Borshchev, O.V.; Agina, E.V.; Shcherbina, M.A.; Bakirov, A.V.; Chvalun, S.N.; Ponomarenko, S.A. Organosilicon dimer of BTBT as a perspective semiconductor material for toxic gas detection with monolayer organic field-effect transistors. J. Mater. Chem. C. 2018, 6, 9649–9659. [Google Scholar] [CrossRef]
- Polinskaya, M.S.; Trul, A.A.; Borshchev, O.V.; Skorotetcky, M.S.; Gaidarzhi, V.P.; Toirov, S.K.; Anisimov, D.S.; Bakirov, A.V.; Chvalun, S.N.; Agina, E.V.; et al. The influence of terminal alkyl groups on the structure, and electrical and sensing properties of thin films of self-assembling organosilicon derivatives of benzothieno[3,2-b][1]benzothiophene. J. Mater. Chem. C 2023, 11, 1937–1948. [Google Scholar] [CrossRef]
- Trul, A.A.; Chekusova, V.P.; Anisimov, D.S.; Borshchev, O.V.; Polinskaya, M.S.; Agina, E.V.; Ponomarenko, S.A. Operationally Stable Ultrathin Organic Field Effect Transistors Based on Siloxane Dimers of Benzothieno[3,2-b][1]Benzothiophene Suitable for Ethanethiol Detection. Adv. Electron. Mater. 2022, 8, 2101039. [Google Scholar] [CrossRef]
- Ponomarenko, S.A.; Tatarinova, E.A.; Muzafarov, A.M.; Kirchmeyer, S.; Brassat, L.; Mourran, A.; Moeller, M.; Setayesh, S.; Leeuw, D. Star-Shaped Oligothiophenes for Solution-Processible Organic Electronics: Flexible Aliphatic Spacers Approach. Chem. Mater. 2006, 18, 4101–4108. [Google Scholar] [CrossRef]
- Polinskaya, M.S.; Luponosov, Y.N.; Borshchev, O.V.; Gülcher, J.; Ziener, U.; Mourran, A.; Wang, J.; Buzin, M.I.; Muzafarov, A.M.; Ponomarenko, S.A. Synthesis and Aggregation Behavior of Novel Linear and Branched Oligothiophene-Containing Organosilicon Multipods. Eur. J. Org. Chem. 2022, 2022, e202101495. [Google Scholar] [CrossRef]
- Gudkova, I.O.; Sorokina, E.A.; Zaborin, E.A.; Polinskaya, M.S.; Borshchev, O.V.; Ponomarenko, S.A. Peculiar Features of the Reduction of Keto Group in the Synthesis of Mono- and Dialkyl-Substituted Benzo[b]benzo[4,5]thieno[2,3-d]thiophene. Russ. J. Org. Chem. 2024, 60, 1074–1085. [Google Scholar] [CrossRef]
- Borrallo-Aniceto, M.C.; Pintado-Sierra, M.; Valverde-González, A.; Díaz, U.; Sánchez, F.; Maya, E.M.; Iglesias, M. Unveiling the potential of a covalent triazine framework based on [1]benzothieno[3,2-b][1]benzothiophene (DPhBTBT-CTF) as a metal-free heterogeneous photocatalyst. Green Chem. 2024, 26, 1975–1983. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Liquid Crystals: Versatile Self-Organized Smart Soft Materials. Chem. Rev. 2022, 122, 48874926. [Google Scholar] [CrossRef]
- Cholakova, D.; Denkov, N. Rotator phases in alkane systems: In bulk, surface layers and micro/nano-confinements. Adv. Colloid Interface Sci. 2019, 269, 7–42. [Google Scholar] [CrossRef]
- Zaborin, E.A.; Borshchev, O.V.; Skorotetskii, M.S.; Gorodov, V.V.; Bakirov, A.V.; Polinskaya, M.S.; Chvalun, S.N.; Ponomarenko, S.A. Synthesis and Thermal and Phase Behavior of Polysiloxanes with Grafted Dialkyl-Substituted [1]Benzothieno[3,2-b][1]benzothiophene Groups. Polym. Sci. Ser. B 2022, 64, 841–854. [Google Scholar] [CrossRef]
- Shaposhnik, P.A.; Trul, A.A.; Poimanova, E.Y.; Sorokina, E.A.; Borschev, O.V.; Agina, E.V.; Ponomarenko, S.A. BTBT-based organic semiconducting materials for EGOFETs with prolonged shelf-life stability. Org. Electron. 2024, 129, 107047. [Google Scholar] [CrossRef]
- Choi, H.; Cho, K.; Frisbie, C.; Sirringhaus, H.; Podzorov, V. Critical assessment of charge mobility extraction in FETs. Nat. Mater. 2018, 17, 2–7. [Google Scholar] [CrossRef]
- Saito, M.; Osaka, I.; Miyazaki, E.; Takimiya, K.; Kuwabara, H.; Ikeda, M. One-step synthesis of [1]benzothieno[3,2-b][1]benzothiophene from o-chlorobenzaldehyde. Tetrahedron Lett. 2011, 52, 285–288. [Google Scholar] [CrossRef]
- Levkov, L.L.; Surin, N.M.; Borshchev, O.V.; Titova, Y.O.; Dubinets, N.O.; Svidchenko, E.A.; Shaposhnik, P.A.; Trul, A.A.; Umarov, A.Z.; Anokhin, D.V.; et al. Three Isomeric Dioctyl Derivatives of 2,7-Dithienyl[1]benzo-thieno[3,2-b][1]benzothiophene: Synthesis, Optical, Thermal, and Semiconductor Properties. Materials 2025, 18, 743. [Google Scholar] [CrossRef] [PubMed]
- Kalb, W.L.; Batlogg, B. Calculating the trap density of states in organic field-effect transistors from experiment: A comparison of different methods. Phys. Rev. B 2010, 81, 035327. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).