Aqueous Solutions of Oil-Soluble Polyglycerol Esters: Structuring and Emulsifying Abilities
Abstract
1. Introduction
2. Results
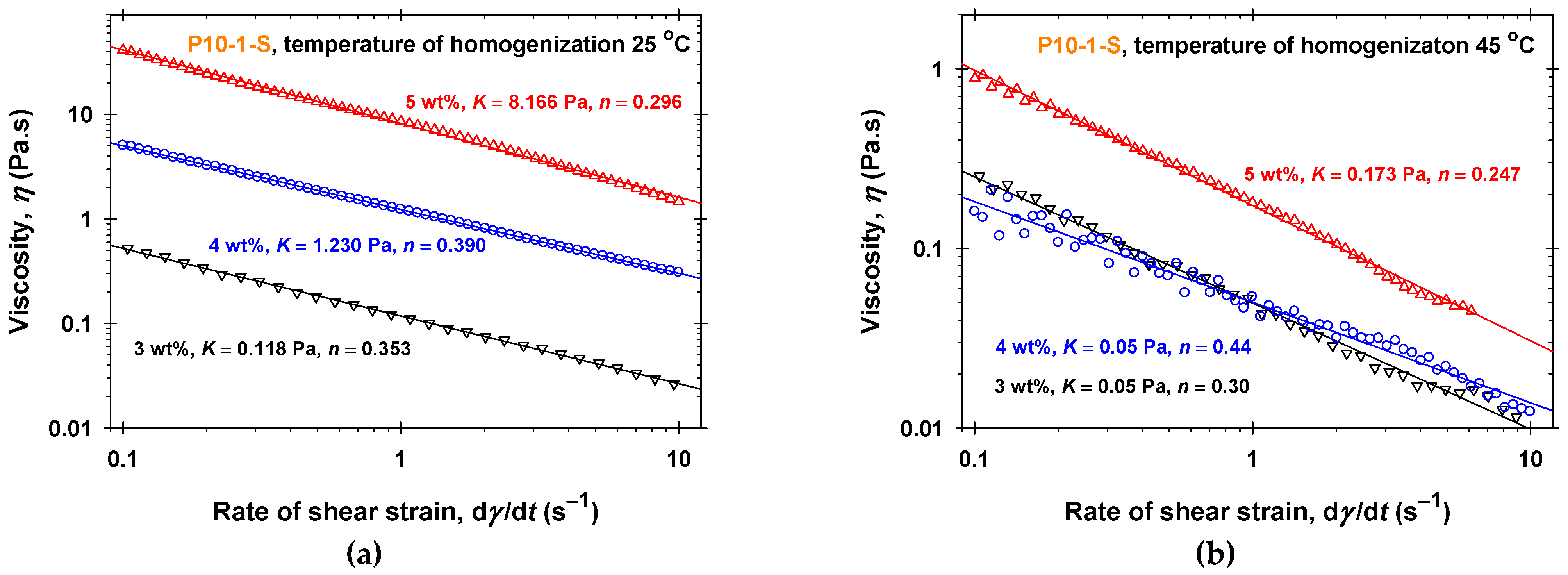
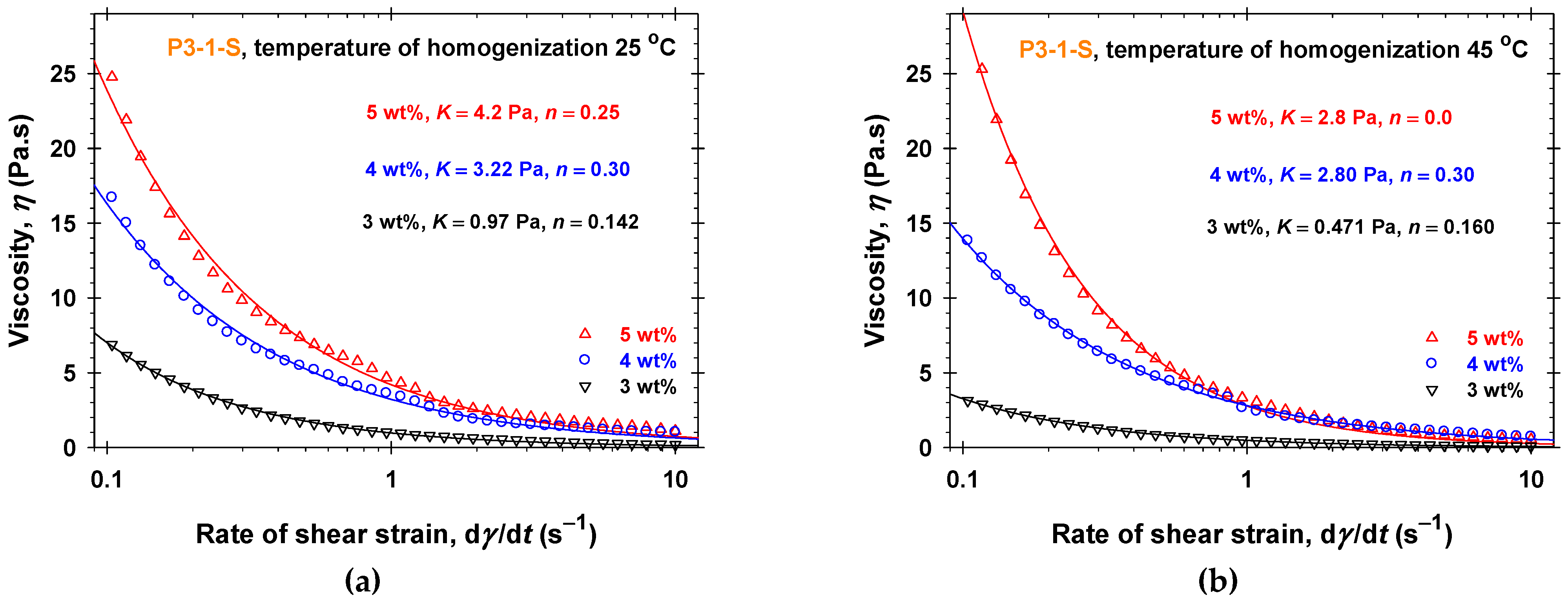
2.1. Rheology in a Steady Shear Regime of PGE Solutions
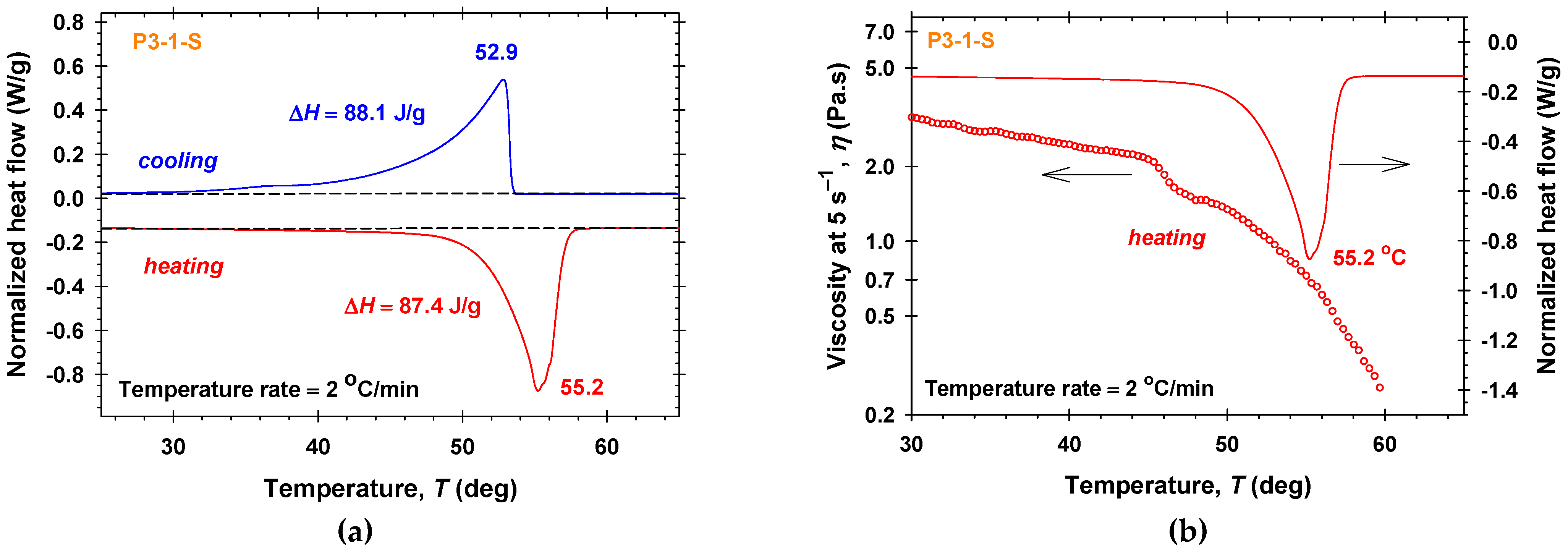
2.2. Differential Scanning Calorimetry Measurements
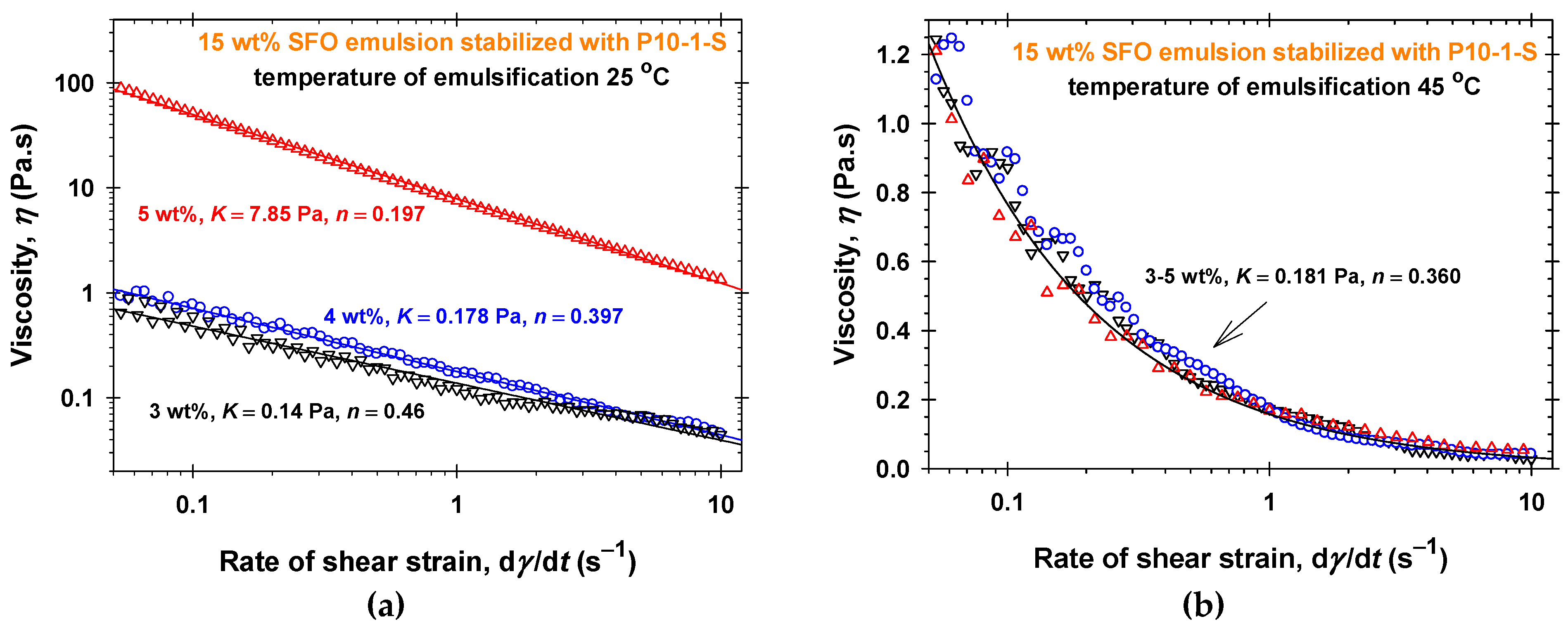
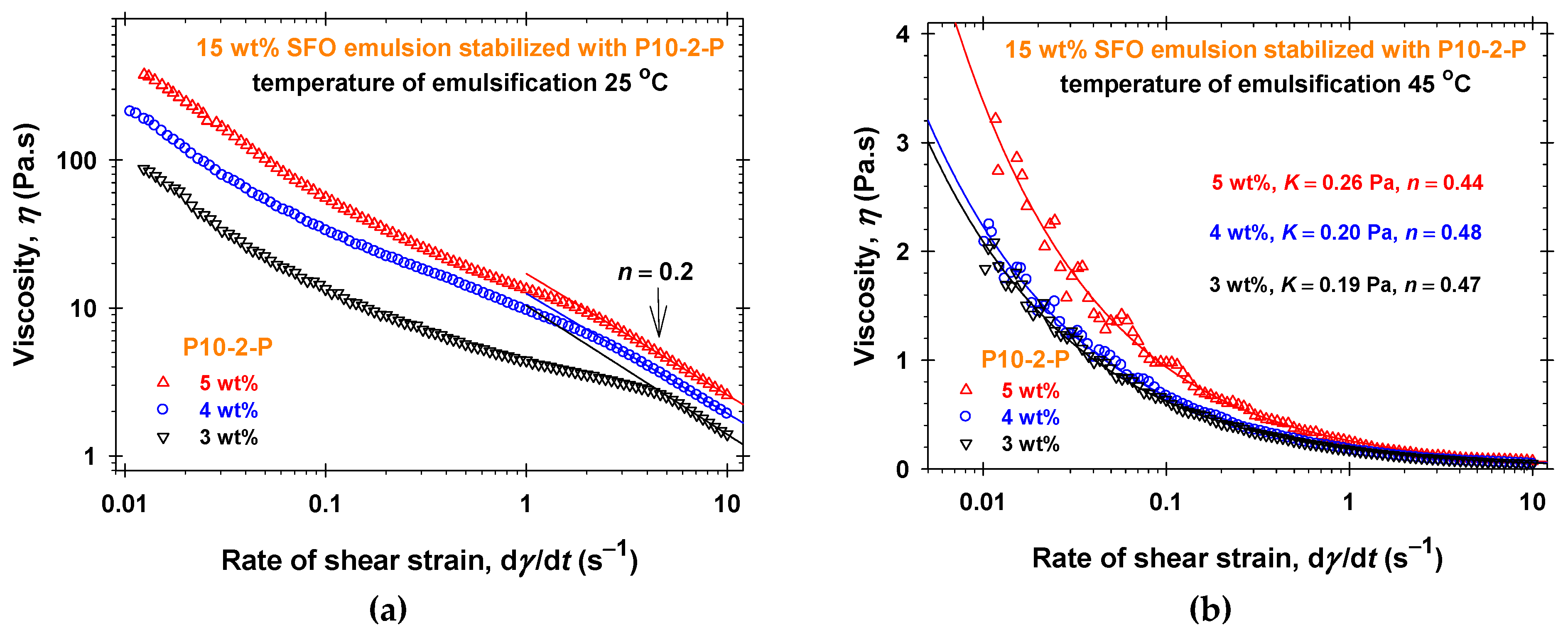
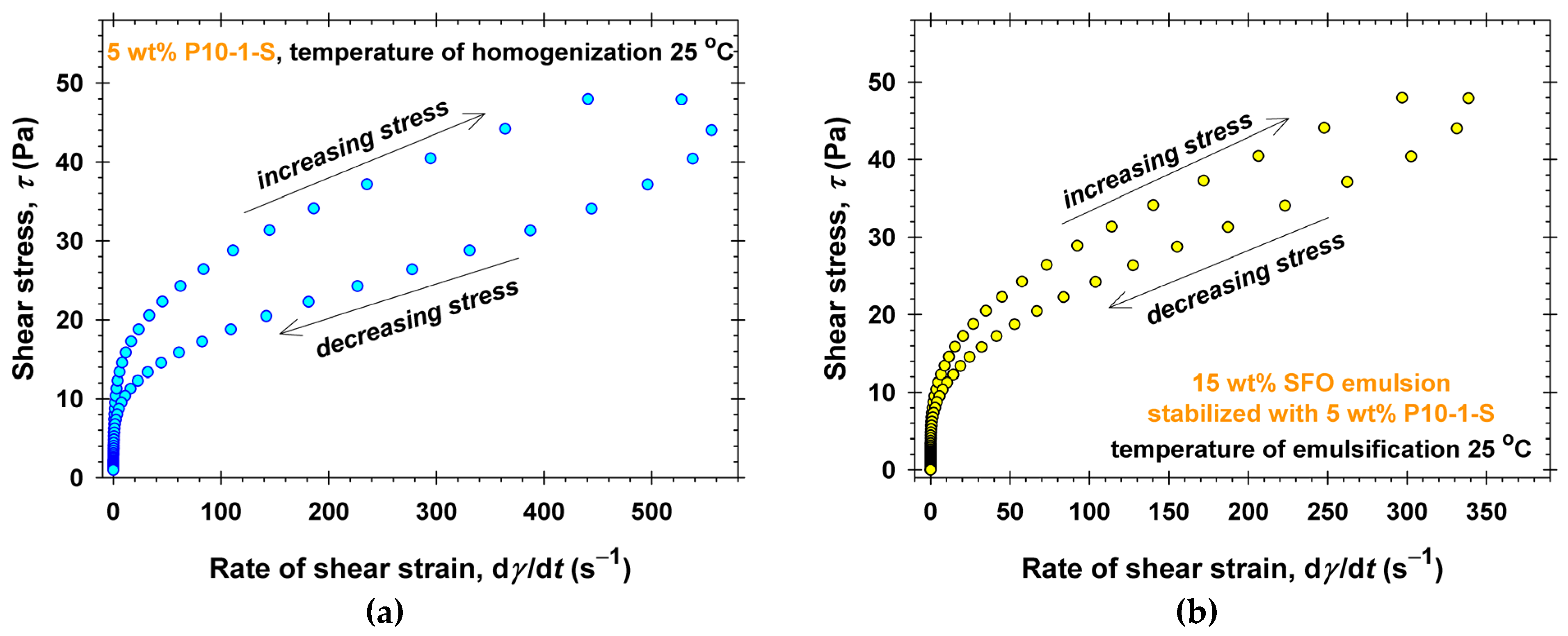
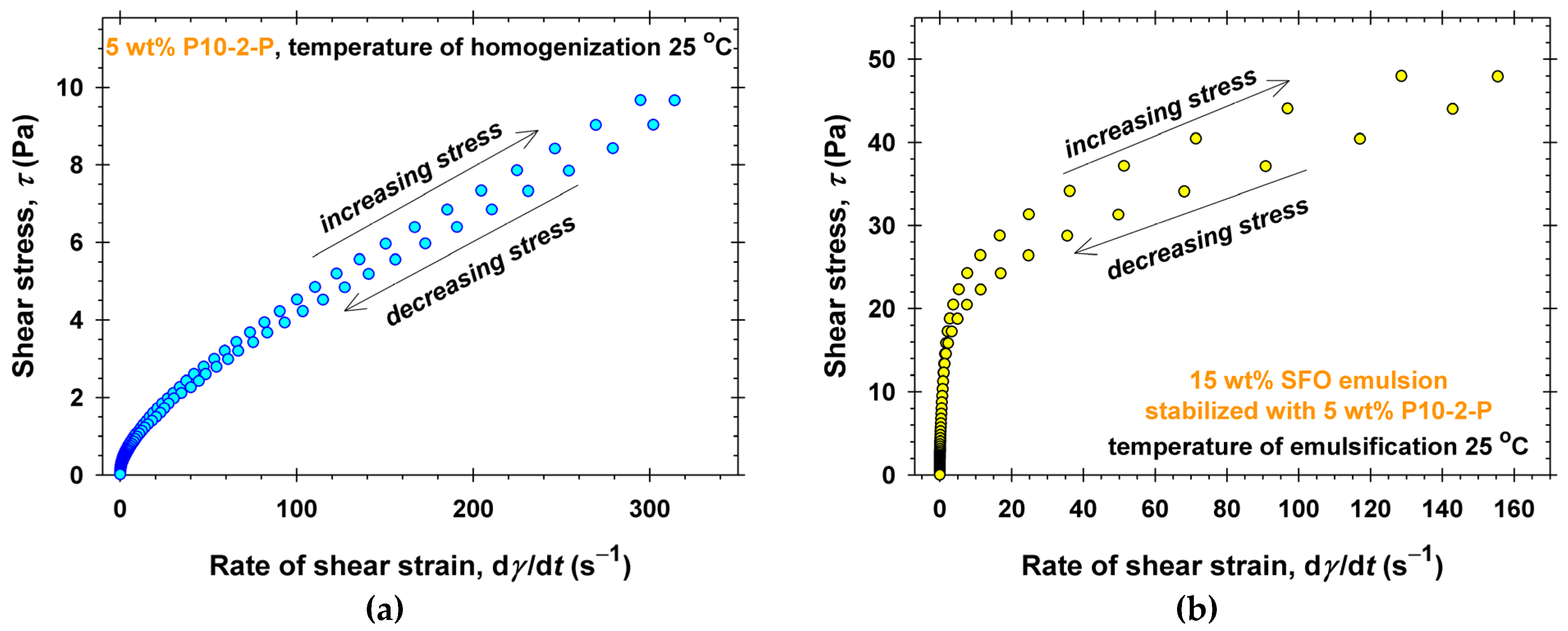
2.3. Emulsifying Ability of PGEs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. PGE Aqueous Solutions and Protocol for Respective Emulsions’ Preparation
4.3. Methods
4.3.1. Rheological Measurements
4.3.2. Differential Scanning Calorimetry (DSC)
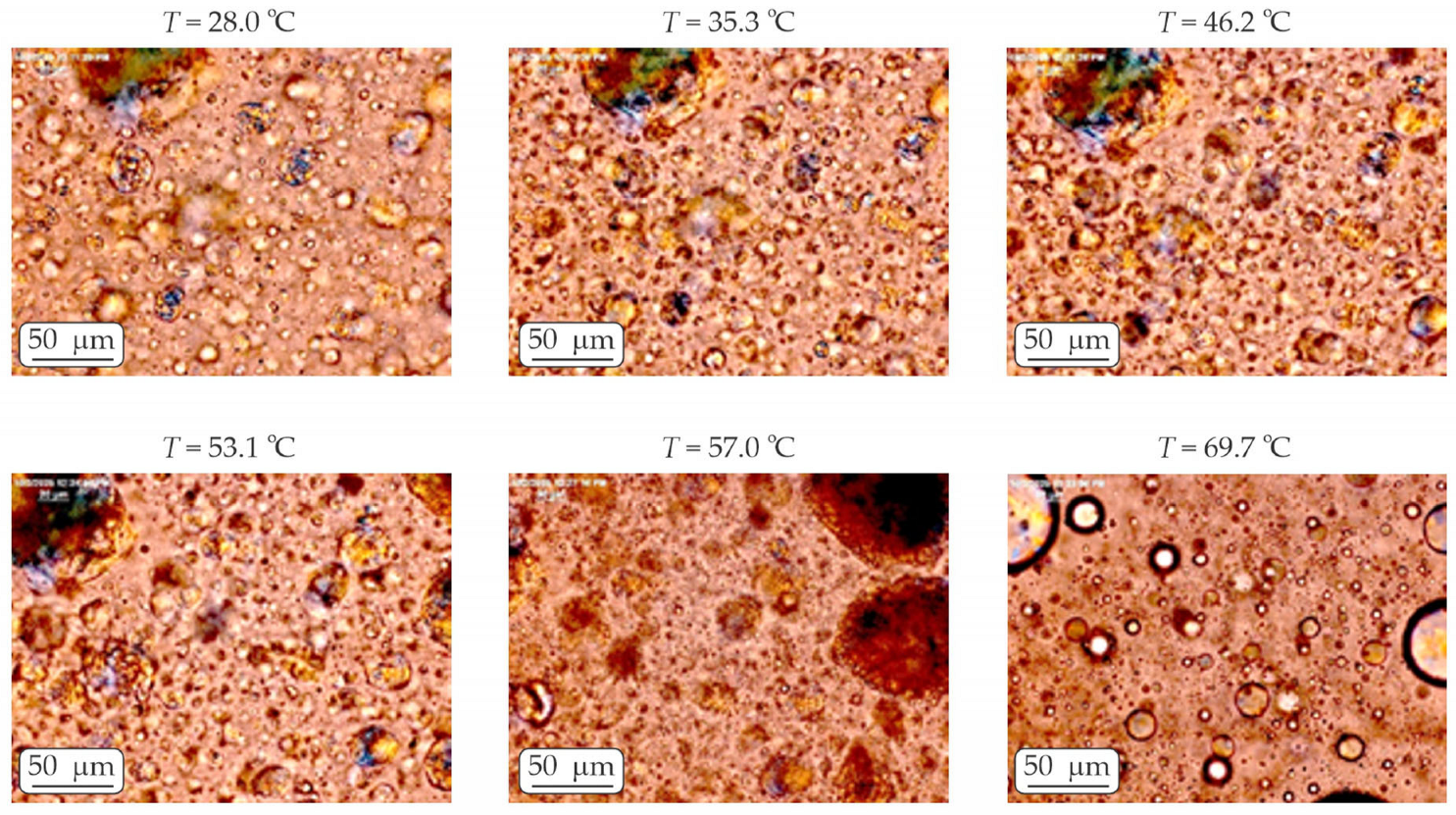
4.3.3. Optical Observations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hemker, W. Associative structures of polyglycerol esters in food emulsions. J. Am. Oil Chem. Soc. 1981, 58, 114–119. [Google Scholar] [CrossRef]
- McIntyre, R.T. Polyglycerol esters. J. Am. Oil Chem. Soc. 1979, 56, 835A–840A. [Google Scholar] [CrossRef]
- Norn, V. Polyglycerol esters. In Emulsifiers in Food Technology; Norn, V., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 181–208. [Google Scholar] [CrossRef]
- Usha, H.S.; Maitra, S. Synthesis characterization and application of polyglycerol esters of fatty acids: Biodegradable surfactants. J. Dispers. Sci. Technol. 2015, 37, 41–47. [Google Scholar] [CrossRef]
- Fan, Z.; Zhao, Y.; Preda, F.; Clacens, J.-M.; Shi, H.; Wang, L.; Feng, X.; De Campo, F. Preparation of bio-based surfactants from glycerol and dodecanol by direct etherification. Green Chem. 2015, 17, 882–892. [Google Scholar] [CrossRef]
- Valerio, O.; Misra, M.; Mohanty, A.K. Poly(glycerol-co-diacids) polyesters: From glycerol biorefinery to sustainable engineering applications. A review. ACS Sustain. Chem. Eng. 2018, 6, 5681–5693. [Google Scholar] [CrossRef]
- Shikhaliev, K.S.; Stolpovskaya, N.V.; Krysin, M.Y.; Zorina, A.V.; Lyapun, D.V.; Zubkov, F.I.; Yankina, K.Y. Production and emulsifying effect of polyglycerol and fatty acid esters with varying degrees of esterification. J. Am. Oil Chem. Soc. 2016, 93, 1429–1440. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Re-evaluation of polyglycerol esters of fatty acids (E 475) as a food additive. EFSA J. 2017, 15, e05089. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF). Follow-up of the re-evaluation of polyglycerol esters of fatty acids (E 475) as a food additive. EFSA J. 2022, 20, e07308. [Google Scholar] [CrossRef]
- Matsumoto, K.; Matsumoto, K. Development of W/O emulsion to form harmless ice slurry to human being. Int. J. Refrig. 2009, 32, 411–420. [Google Scholar] [CrossRef]
- Matsumoto, K.; Shirai, D.; Furudate, Y.; Tsubaki, D.; Kubota, H.; Sekine, K.; Minamiya, K. Active control of supercooling degree using surfactant (in system with solid-liquid interface). Int. J. Refrig. 2015, 58, 199–206. [Google Scholar] [CrossRef]
- Matsumoto, K.; Igarashi, Y.; Shirai, D.; Hayashi, K. Investigation of the influence of surfactant on the degree of supercooling (coexisting system of solid-liquid and gas-liquid interfaces). Int. J. Refrig. 2013, 36, 1302–1309. [Google Scholar] [CrossRef]
- Wang, F.C.; Marangoni, A.G. Advances in the application of food emulsifier α-gel phases: Saturated monoglycerides, polyglycerol fatty acid esters, and their derivatives. J. Colloid Interface Sci. 2016, 483, 394–403. [Google Scholar] [CrossRef]
- Ogawa, A.; Cho, H. Role of food emulsifiers in milk coffee beverages. J. Colloid Interface Sci. 2015, 449, 198–204. [Google Scholar] [CrossRef]
- Hönzke, S.; Gerecke, C.; Elpelt, A.; Zhang, N.; Unbehauen, M.; Kral, V.; Fleige, E.; Paulus, F.; Haag, R.; Schäfer-Korting, M.; et al. Tailored dendritic core-multishell nanocarriers for efficient dermal drug delivery: A systematic top-down approach from synthesis to preclinical testing. J. Control. Release 2016, 242, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Fong, W.-K.; Negrini, R.; Vallooran, J.J.; Mezzenga, R.; Boyd, B.J. Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics. J. Colloid Interface Sci. 2016, 484, 320–339. [Google Scholar] [CrossRef]
- Park, S.; Mun, S.; Kim, Y.R. Influences of added surfactants on the water solubility and antibacterial activity of rosemary extract. Food Sci. Biotechnol. 2020, 29, 1373–1380. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of polyglyceryl fatty acid esters as used in cosmetics. Int. J. Toxicol. 2023, 42, 5S–101S. [Google Scholar] [CrossRef] [PubMed]
- Sahle, F.F.; Metz, H.; Wohlrab, J.; Neubert, R.H.H. Polyglycerol fatty acid ester surfactant-based microemulsions for targeted delivery of ceramide AP into the stratum corneum: Formulation, characterization, in vitro release and penetration investigation. Eur. J. Pharm. Biopharm. 2012, 82, 139–150. [Google Scholar] [CrossRef]
- Köpke, D.; Pyo, S.M. Symurban nanocrystals for advanced anti-pollution skincare. Cosmetics 2020, 7, 17. [Google Scholar] [CrossRef]
- Huynh, A.; Garcia, A.G.; Young, L.K.; Szoboszlai, M.; Liberatore, M.W.; Baki, G. Measurements meet perceptions: Rheology-texture-sensory relations when using green, bio-derived emollients in cosmetic emulsions. Int. J. Cosmet. Sci. 2021, 43, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Öhlinger, K.; Meindl, C.; Corzo, C.; Lochmann, D.; Reyer, S.; Salar-Behzadi, S. In vitro toxicity screening of polyglycerol esters of fatty acids as excipients for pulmonary formulations. Toxicol. Appl. Pharmacol. 2020, 386, 114833. [Google Scholar] [CrossRef]
- Hanno, I.; Centini, M.; Anselmi, C.; Bibiani, C. Green cosmetic surfactant from rice: Characterization and application. Cosmetics 2015, 2, 322–341. [Google Scholar] [CrossRef]
- Hamada, H.; Kato, K.; Ito, N.; Takase, Y.; Nanbu, H.; Mishima, S.; Sakaki, H.; Sato, K. Effects of polyglycerol esters of fatty acids and ethylene-vinyl acetate co-polymer on crystallization behavior of biodiesel. Eur. J. Lipid Sci. Technol. 2010, 112, 1323–1330. [Google Scholar] [CrossRef]
- Saitou, K.; Homma, R.; Kudo, N.; Katsuragi, Y.; Sato, K. Retardation of crystallization of diacylglycerol oils using polyglycerol fatty acid esters. J. Am. Oil Chem. Soc. 2014, 91, 711–719. [Google Scholar] [CrossRef]
- Saitou, K.; Taguchi, K.; Homma, R.; Shimizu, M.; Yasunaga, K.; Katsuragi, Y.; Ueno, S.; Sato, K. Retardation mechanism of crystallization of diacylglycerols resulting from the addition of polyglycerol fatty acid esters. Cryst. Growth Des. 2017, 17, 4749–4756. [Google Scholar] [CrossRef]
- Yan, G.; Wang, S.; Li, Y.; Zhang, J.; Ding, H.; Li, Y.; Zhang, L. Effect of different polymerization degrees and fatty acids of polyglycerol esters on the physical properties and whippability of recombined dairy cream. Foods 2023, 12, 22. [Google Scholar] [CrossRef]
- Li, Y.; Liao, T.; Liu, T.; Yan, R.; Sun, Z.; Zhao, M.; Deng, X.; Zhao, Q. The quality of whipped cream: Effect of polyglycerol ester on the crystallization of fat blend and the properties of interface. Food Hydrocoll. 2023, 145, 109145. [Google Scholar] [CrossRef]
- Saw, M.H.; Lim, W.H.; Yeoh, C.B.; Tan, C.P. Effect of storage temperature and duration on rheological and thermal characteristics of superolein oleogels. J. Oil Palm Res. 2023, 35, 256–267. [Google Scholar] [CrossRef]
- Meng, Z.; Guo, Y.; Wang, Y.; Liu, Y. Organogels based on the polyglyceryl fatty acid ester and sunflower oil: Macroscopic property, microstructure, interaction force, and application. LWT–Food Sci. Technol. 2019, 116, 108590. [Google Scholar] [CrossRef]
- Gledovic, A.; Lezaic, A.J.; Nikolic, I.; Tasic-Kostov, M.; Antic-Stankovic, J.; Krstonosic, V.; Randjelovic, D.; Bozic, D.; Ilic, D.; Tamburic, S.; et al. Polyglycerol ester-based low energy nanoemulsions with red raspberry seed oil and fruit extracts: Formulation development toward effective in vitro/in vivo bioperformance. Nanomaterials 2021, 11, 217. [Google Scholar] [CrossRef]
- Peng, B.; Xiong, C.-Y.; Huang, Y.; Hu, J.-N.; Zhu, X.-M.; Deng, Z.-Y. Enzymatic synthesis of polyglycerol fatty acid esters and their application as emulsion stabilizers. J. Agric. Food Chem. 2018, 66, 8104–8113. [Google Scholar] [CrossRef]
- Tangsanthatkun, J.; Sonprasert, T.; Sonwai, S. The effect of polyglycerol esters of fatty acids on the crystallization of palm olein. J. Oleo Sci. 2021, 70, 309–319. [Google Scholar] [CrossRef]
- Duerr-Auster, N.; Kohlbrecher, J.; Zuercher, T.; Gunde, R.; Fischer, P.; Windhab, E. Microstructure and stability of a lamellar liquid crystalline and gel phase formed by a polyglycerol ester mixture in dilute aqueous solution. Langmuir 2007, 23, 12827–12834. [Google Scholar] [CrossRef] [PubMed]
- Buitimea-Cantúa, N.E.; Serna-Saldívar, S.O.; Pérez-Carrillo, E.; Jordânia-Silva, T.; Barrera-Arrellano, D.; Buitimea-Cantúa, G.V. Textural and rheological properties of soybean oil organogels structured with polyglycerol and propylene glycol esters during storage. Grasas Aceites 2022, 73, e443. [Google Scholar] [CrossRef]
- Kuwabara, H.; Ogura, T.; Tsuchiya, K.; Akamatsu, M.; Arakawa, K.; Sakai, K.; Sakai, H. Structural analysis of interfacial films of oil/water emulsions prepared by emulsification via lamellar gels. J. Oleo Sci. 2024, 73, 1467–1477. [Google Scholar] [CrossRef]
- Wakisaka, S.; Nishimura, T.; Gohtani, S. O/W nano-emulsion formation using an isothermal low-energy emulsification method in a mixture of polyglycerol polyricinoleate and hexaglycerol monolaurate with glycerol system. J. Oleo Sci. 2015, 64, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Wakisaka, S.; Nakanishi, M.; Gohtani, S. Phase behavior and formation of O/W nano-emulsion in vegetable oil/mixture of polyglycerol polyricinoleate and polyglycerin fatty acid ester/water systems. J. Oleo Sci. 2014, 63, 229–237. [Google Scholar] [CrossRef]
- Dahl, V.; Friedrich, A.; Meyer, J.; Venzmer, J.; Belkoura, L.; Strey, R.; Mayer, C.; Michel, R.; Gradzielski, M. Structural analysis of a modern o/w-emulsion stabilized by a polyglycerol ester emulsifier and consistency enhancers. Colloids Interfaces 2018, 2, 3. [Google Scholar] [CrossRef]
- Gupta, M.; Hooghten, R.V.; Fischer, P.; Gunes, D.Z.; Vermant, J. Limiting coalescence by interfacial rheology: Over-compressed polyglycerol ester layers. Rheol. Acta 2016, 55, 537–546. [Google Scholar] [CrossRef]
- Hashizaki, K.; Imai, M.; Yako, S.; Tsusaka, H.; Sakanishi, Y.; Saito, Y.; Fujii, M. Highly viscoelastic reverse wormlike micellar systems from a mixture of lecithin, polyglycerol fatty acid monoesters, and an oil. J. Oleo Sci. 2017, 66, 997–1007. [Google Scholar] [CrossRef]
- Iwase, H.; Kobayashi, J.; Kasama, Y.; Fujii, W.; Nanbu, H. Structural analysis of polyglycerol fatty acid ester-coenzyme Q10 aggregates in solution. Food Res. Int. 2024, 175, 113741. [Google Scholar] [CrossRef]
- Shi, L.; Cheng, Y.; Jia, C.; Lin, H.; Zhang, W.; He, J. Stable complex of sodium caseinate and hexaglycerol monooleate with improved oil-in-water emulsion stability and curcumin encapsulation. Food Biophys. 2024, 19, 321–333. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Ciacciarelli, A.; Quassinti, L.; Bramucci, M.; Miano, A.; Casettari, L.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Properties and stability of nanoemulsions: How relevant is the type of surfactant? J. Drug Deliv. Sci. Technol. 2020, 58, 101772. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kimura, Z.; Misono, T.; Tsuchiya, K.; Sakai, K.; Abe, M.; Sakai, H. Preparation and properties of nonionic vesicles prepared with polyglycerol fatty acid esters using the supercritical carbon dioxide reverse phase evaporation method. J. Oleo Sci. 2016, 65, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Uchino, T.; Fujimori, S.; Hatta, I.; Miyazaki, Y.; Kamiya, D.; Fujino, H.; Suzuki, R.; Kirishita, Y.; Eda, T.; Murashima, K.; et al. Development of novel polyglycerol fatty acid ester-based nanoparticles for the dermal delivery of tocopherol acetate. Int. J. Pharm. 2021, 592, 120004. [Google Scholar] [CrossRef]
- Shahzadi, I.; Fürst, A.; Knoll, P.; Bernkop-Schnürch, A. Nanostructured lipid carriers (NLCs) for oral peptide drug delivery: About the impact of surface decoration. Pharmaceutics 2021, 13, 1312. [Google Scholar] [CrossRef]
- Motoyama, T.; Katsuumi, Y.; Sasakura, H.; Nakamura, T.; Suzuki, H.; Tsuchiya, K.; Akamatsu, M.; Sakai, K.; Sakai, H. Preparation of highly stable oil-in-water emulsions with high ethanol content using polyglycerol monofatty acid esters as emulsifiers. J. Oleo Sci. 2022, 71, 829–837. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, G. Recent advances in the properties and applications of polyglycerol fatty acid esters. Polymers 2025, 17, 879. [Google Scholar] [CrossRef] [PubMed]
- Stanimirova, R.D.; Danov, K.D.; Georgiev, M.T.; Petkov, J.T. Colloid, interface, and foam properties of water-soluble polyglycerol esters solutions. J. Colloid Interface Sci. 2025, 677, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Piñón-Balderrama, C.I.; Martínez, I.C.; Saavedra-Leos, M.Z. Application of differential scanning calorimetry (DSC) and modulated differential scanning calorimetry (MDSC) in food and drug industries. Polymers 2020, 12, 5. [Google Scholar] [CrossRef]
- Cholakova, D.; Denkov, N. Polymorphic phase transitions in triglycerides and their mixtures studied by SAXS/WAXS techniques: In bulk and in emulsions. Adv. Colloid Interface Sci. 2024, 323, 103071. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Lee, Y.-Y.; Lu, Y.; Wang, Y.; Zhang, Z. Internal factors affecting the crystallization of the lipid system: Triacylglycerol structure, composition, and minor components. Molecules 2024, 29, 1847. [Google Scholar] [CrossRef]
- Yavrukova, V.I.; Radulova, G.M.; Danov, K.D.; Kralchevsky, P.A.; Xu, H.; Ung, Y.W.; Petkov, J.T. Rheology of mixed solutions of sulfonated methyl esters and betaine in relation to the growth of giant micelles and shampoo applications. Adv. Colloid Interface Sci. 2020, 275, 102062. [Google Scholar] [CrossRef]
- Danov, K.D.; Stanimirova, R.D.; Kralchevsky, P.A.; Marinova, K.G.; Alexandrov, N.A.; Stoyanov, S.D.; Blijdenstein, T.B.J.; Pelan, E.G. Capillary meniscus dynamometry—Method for determining the surface tension of drops and bubbles with isotropic and anisotropic surface stress distributions. J. Colloid Interface Sci. 2015, 440, 168–178. [Google Scholar] [CrossRef]
- Danov, K.D.; Stanimirova, R.D.; Kralchevsky, P.A.; Marinova, K.G.; Stoyanov, S.D.; Blijdenstein, T.B.J.; Cox, A.R.; Pelan, E.G. Adhesion of bubbles and drops to solid surfaces, and anisotropic surface tension studied by capillary meniscus dynamometry. Adv. Colloid Interface Sci. 2016, 233, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.A. A Handbook of Elementary Rheology; Institute of Non-Newtonian Fluid Mechanics: Wales, UK; University of Wales: Wales, UK, 2000. [Google Scholar]
- Barnes, H. Thixotropy—A review. J. Non-Newton. Fluid Mech. 1997, 70, 1–33. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanimirova, R.; Georgiev, M.; Danov, K.; Petkov, J. Aqueous Solutions of Oil-Soluble Polyglycerol Esters: Structuring and Emulsifying Abilities. Molecules 2025, 30, 4507. https://doi.org/10.3390/molecules30234507
Stanimirova R, Georgiev M, Danov K, Petkov J. Aqueous Solutions of Oil-Soluble Polyglycerol Esters: Structuring and Emulsifying Abilities. Molecules. 2025; 30(23):4507. https://doi.org/10.3390/molecules30234507
Chicago/Turabian StyleStanimirova, Rumyana, Mihail Georgiev, Krassimir Danov, and Jordan Petkov. 2025. "Aqueous Solutions of Oil-Soluble Polyglycerol Esters: Structuring and Emulsifying Abilities" Molecules 30, no. 23: 4507. https://doi.org/10.3390/molecules30234507
APA StyleStanimirova, R., Georgiev, M., Danov, K., & Petkov, J. (2025). Aqueous Solutions of Oil-Soluble Polyglycerol Esters: Structuring and Emulsifying Abilities. Molecules, 30(23), 4507. https://doi.org/10.3390/molecules30234507







