Comparative Analysis of Gold Nanoparticle Synthesis Using PAMAM G2 Dendrimers via Microwave and Sonication Methods for Potential Cancer Theranostic Applications
Abstract
1. Introduction
2. Results
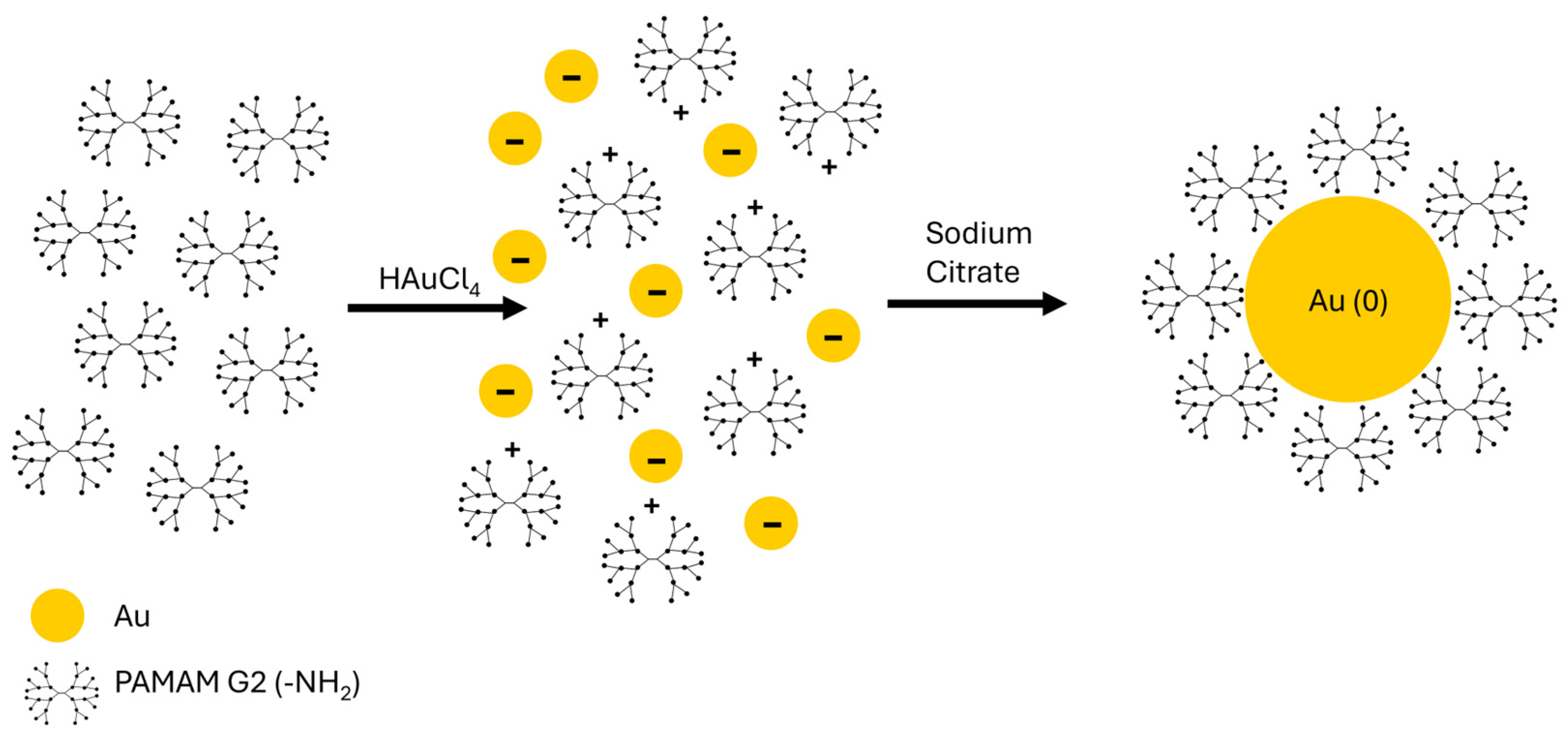
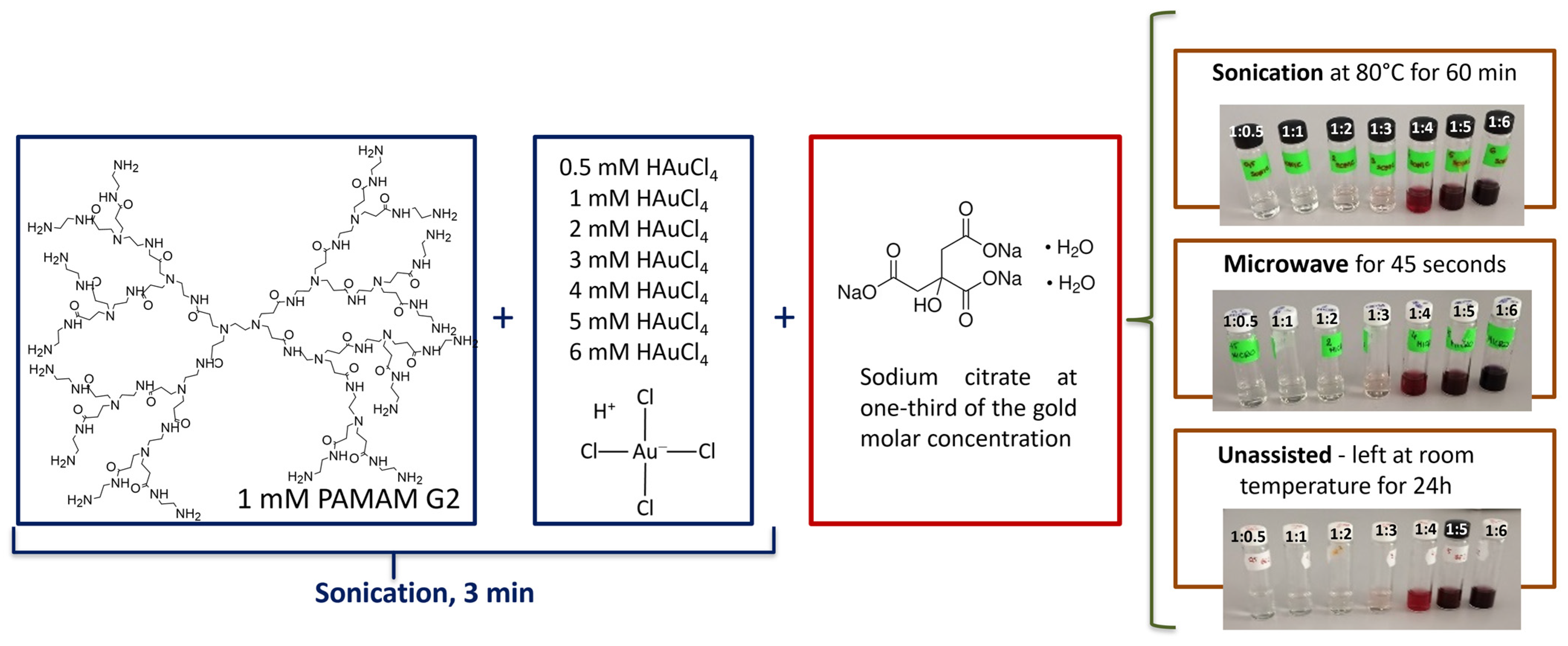
2.1. Synthesis Reaction of AuNPs/PAMAM G2 Complexes
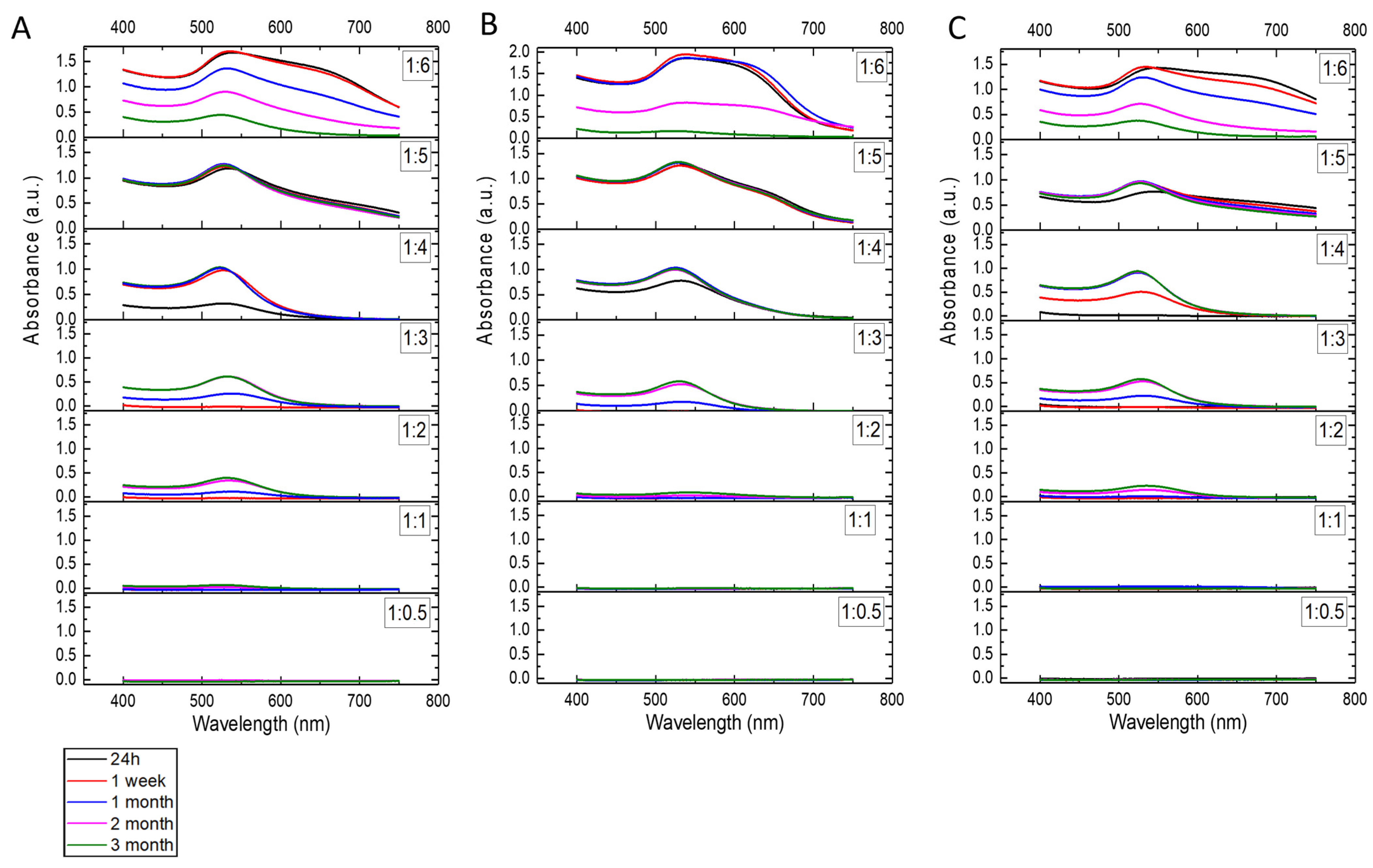
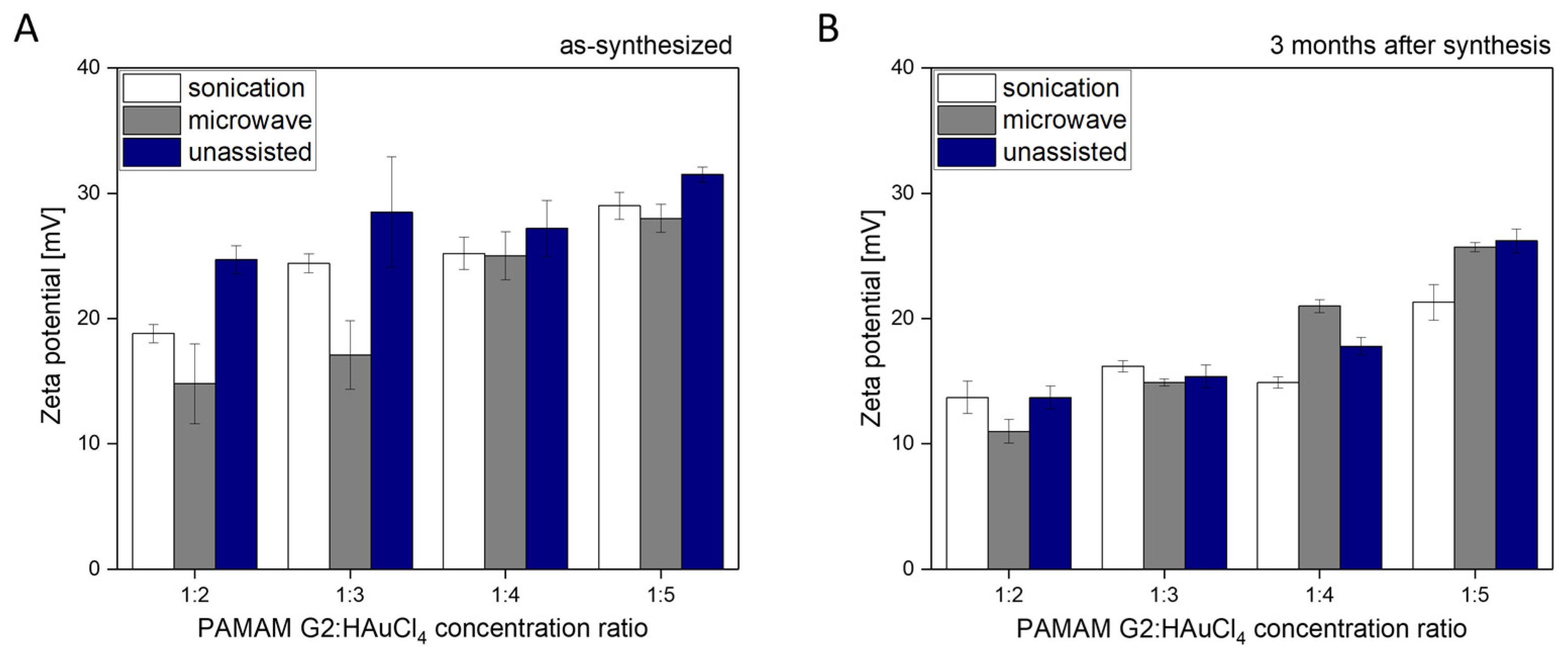
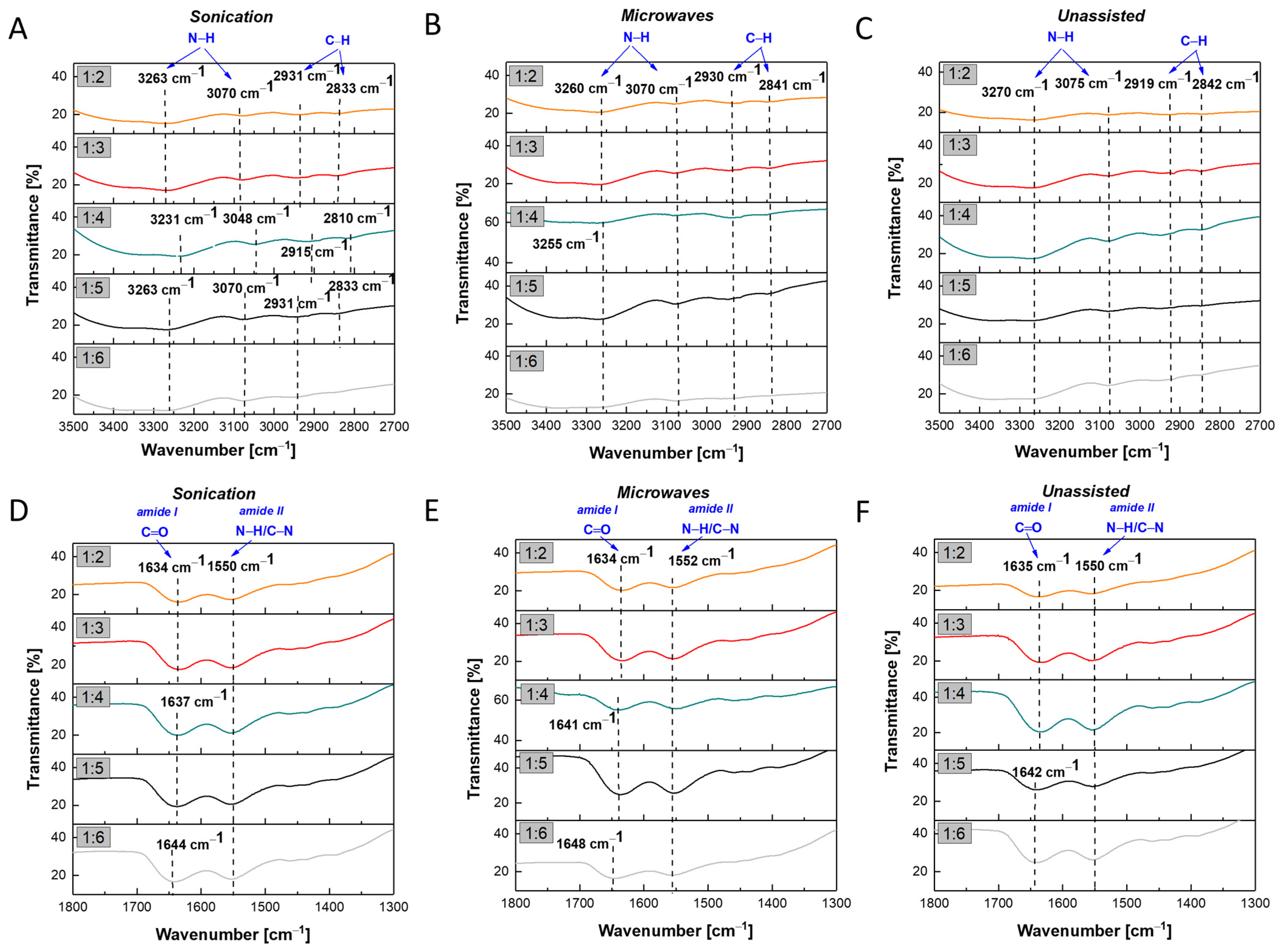
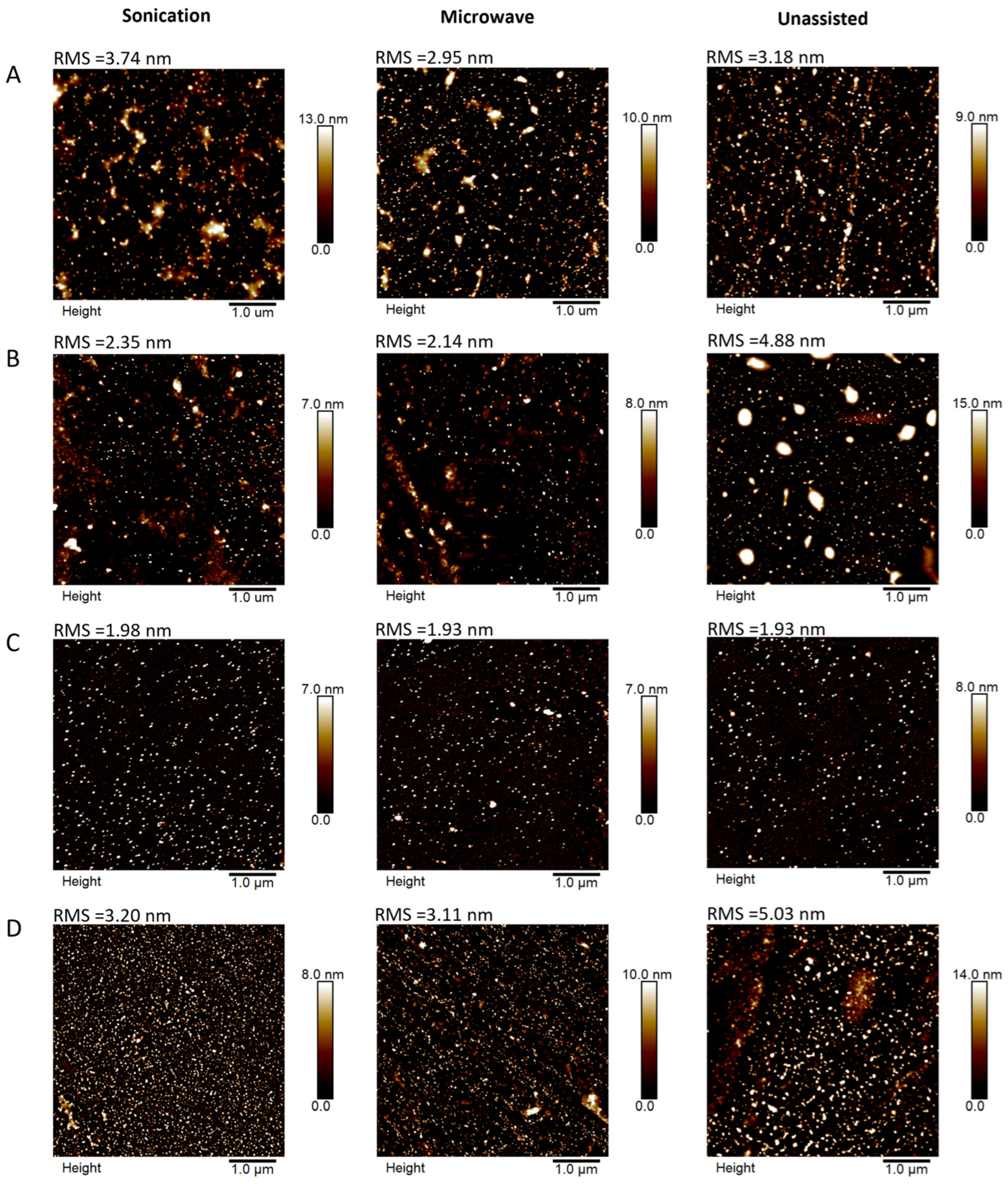
2.2. Physico-Chemical Properties of AuNPs/PAMAM G2
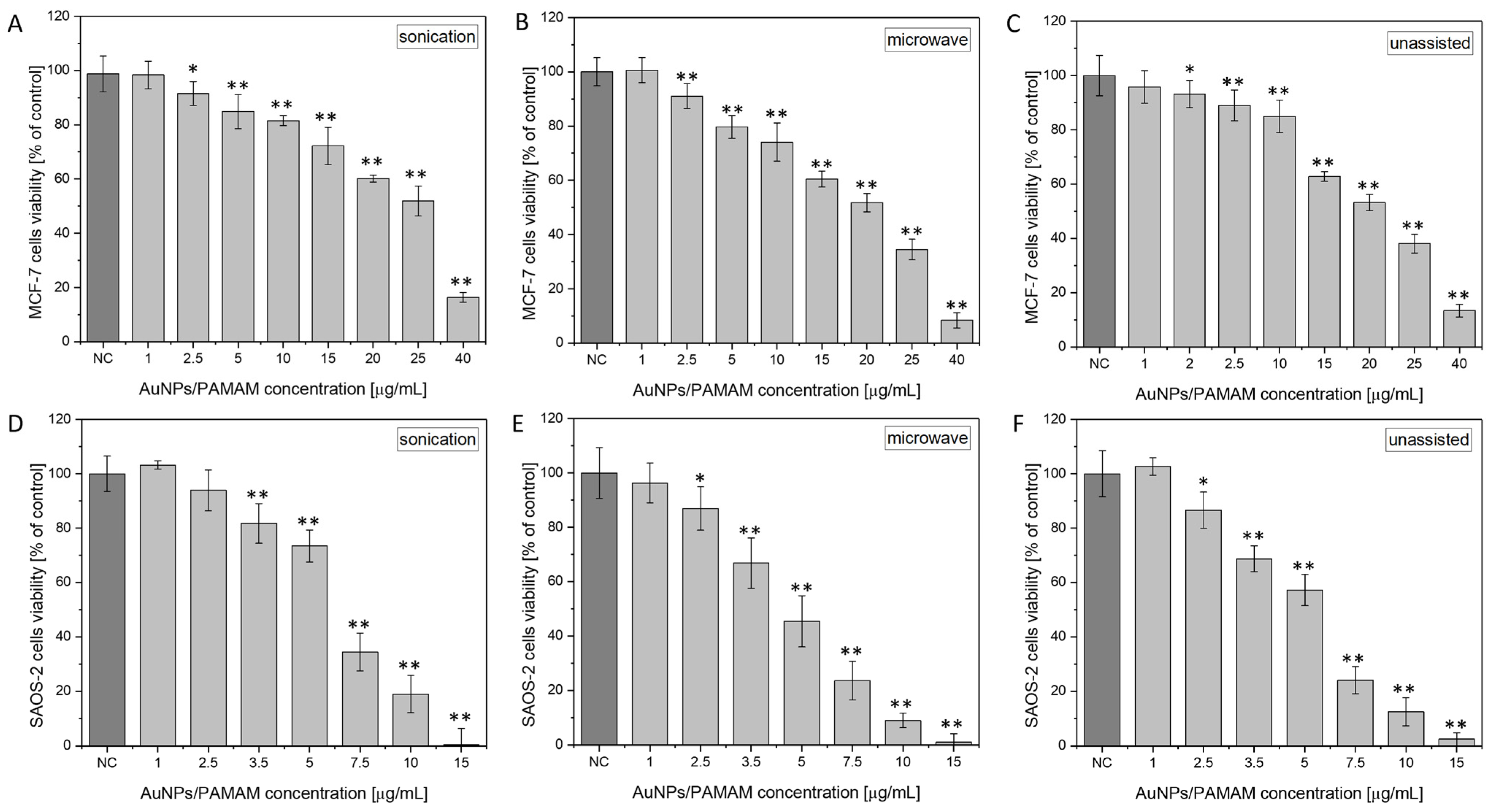
2.3. Investigation of AuNPs/PAMAM G2 Cytotoxicity on MCF-7 and Saos-2 Cell Lines
3. Discussion
4. Materials and Methods
4.1. Synthesis of AuNPs/PAMAM G2
4.2. Ultraviolet-Visible Spectroscopy
4.3. Dynamic Light Scattering Technique and Zeta Potential Measurements
4.4. Atomic Force Microscopy
4.5. Infra-Red Spectroscopy
4.6. Cell Culture
4.7. Cytotoxicity Measurements (XTT Assay)
5. Conclusions
- Three synthesis methods were employed to produce gold nanoparticles stabilized with PAMAM G2 dendrimers. All three types of synthesis methods are repeatable. Both sonication and unassisted methods enable the large-scale production of nanoparticles.
- The obtained nanoparticles synthesized using the second generation of PAMAM dendrimers exhibit long-term colloidal stability for up to 3 months.
- Among the tested formulations, the colloid PAMAM G2:HAuCl4 ratios of 1:4 were selected for in vitro cytotoxicity evaluation. Nanoparticles synthesized via sonication exhibited the lowest cytotoxicity compared to those produced using microwaves or without assistance.
- These studies provide a foundation for further investigations into the mechanisms underlying the biological activity of PAMAM-stabilized gold nanoparticles and their potential use in cancer treatment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR | attenuated total reflectance |
| AuNPs | Gold nanoparticles |
| CT | Computed Tomography |
| DLS | Dynamic Light Scattering |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EC | effective concentration |
| FBS | Fetal Bovine Serum |
| FT-IR | Fourier transform infrared spectroscopy |
| HAuCl4 | chloroauric acid (hydrogen tetrachloroaurate) |
| MCF-7 | human breast adenocarcinoma cell line |
| NC | negative control |
| PAMAM | polyamidoamine |
| PBS | Phosphate-buffered saline |
| Saos-2 | human osteosarcoma cell line |
| UV-Vis | ultraviolet-visible spectroscopy |
| XTT | 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide |
References
- Choudhary, S.; Kaur, S.D.; Gandhi, H.; Pemmaraju, D.B.; Kapoor, D.N. An updated review on the potential of gold nanoparticles for cancer treatment and detection. Gold Bull. 2025, 58, 4. [Google Scholar] [CrossRef]
- Milivojević, N.; Carvalho, M.R.; Caballero, D.; Radisavljević, S.; Radoićić, M.; Živanović, M.; Oliveira, J.M. Evaluation of Novel Dendrimer–Gold Complex Nanoparticles for Theranostic Application in Oncology. Nanomedicine 2024, 19, 483–497. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Amin, M.C.I.M.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Parthasarathy, N.; Karthikeyan, L.; Fabiola, M.; Vivek, R. Recent progress in multifunctional theranostic inorganic nanomaterials for cancer diagnostic imaging and therapy. Next Nanotechnol. 2025, 8, 100212. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Karsh, E.H.; Taqi, Z.J.; Jabir, M.S. Biocompatibility of gold nanoparticles: In-vitro and In-vivo study. Mater. Today Proc. 2021, 42, 3041–3045. [Google Scholar] [CrossRef]
- Balasubramanian, S.K.; Yang, L.; Yung, L.Y.L.; Ong, C.N.; Ong, W.Y.; Yu, L.E. Characterization, purification, and stability of gold nanoparticles. Biomaterials 2010, 31, 9023–9030. [Google Scholar] [CrossRef]
- Enea, M.; Pereira, E.; Costa, J.; Soares, M.E.; Dias da Silva, D.; Bastos, M.D.L.; Carmo, H.F. Cellular uptake and toxicity of gold nanoparticles on two distinct hepatic cell models. Toxicol. Vitr. 2021, 70, 105046. [Google Scholar] [CrossRef]
- Arcos Rosero, W.A.; Bueno Barbezan, A.; Daruich de Souza, C.; Chuery Martins Rostelato, M.E. Review of Advances in Coating and Functionalization of Gold Nanoparticles: From Theory to Biomedical Application. Pharmaceutics 2024, 16, 255. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, M.; Bin Sabri, A.H.; Holmes, A.; Moss, G.P.J.; Scurr, D. PAMAM Dendrimers as Mediators of Dermal and Transdermal Drug Delivery: A Review. J. Pharm. Pharmacol. 2024, 76, 1284–1300. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, R.; Cao, X.; Shen, M.; Shi, X. Encapsulation of 2-Methoxyestradiol within Multifunctional Poly(amidoamine) Dendrimers for Targeted Cancer Therapy. Biomaterials 2011, 32, 3322–3329. [Google Scholar] [CrossRef]
- Grześkowiak, B.F.; Maziukiewicz, D.; Kozłowska, A.; Kertmen, A.; Coy, E.; Mrówczyński, R. Polyamidoamine Dendrimers Decorated Multifunctional Polydopamine Nanoparticles for Targeted Chemo- and Photothermal Therapy of Liver Cancer Model. Int. J. Mol. Sci. 2021, 22, 738. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, E.R.; Lin, A.Y.; Yan, J.; Luo, L.; Foster, A.E.; Drezek, R.A. Optimization of PAMAM–Gold Nanoparticle Conjugation for Gene Therapy. Biomaterials 2014, 35, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Golshan, M.; Salami-Kalajahi, M.; Mirshekarpour, M.; Roghani-Mamaqani, H.; Mohammadi, M. Synthesis and Characterization of Poly(propylene imine) Dendrimer-Grafted Gold Nanoparticles as Nanocarriers of Doxorubicin. Colloids Surf. B Biointerfaces 2017, 155, 257–265. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Hu, J.; Shao, N.; Wang, F.; Zhang, Q.; Xiao, J.; Cheng, Y. Glutathione-Triggered “Off–On” Release of Anticancer Drugs from Dendrimer-Encapsulated Gold Nanoparticles. J. Am. Chem. Soc. 2013, 135, 9805–9810. [Google Scholar] [CrossRef]
- Yurong Laia, Y.; Chub, X.; Dic, L.; Gaob, W.; Guod, Y.; Liue, X.; Luf, C.; Maog, J.; Shenh, H.; Tangi, H.; et al. Recent advances in the translation of drug metabolism and pharmacokinetics science for drug discovery and development. Acta Pharm. Sin. B 2022, 12, 2751e2777. [Google Scholar] [CrossRef] [PubMed]
- Saw, W.S.; Anasamy, T.; Do, T.T.A.; Lee, H.B.; Chee, C.F.; Isci, U.; Misran, M.; Dumoulin, F.; Chong, W.Y.; Kiew, L.V.; et al. Nanoscaled PAMAM Dendrimer Spacer Improved the Photothermal–Photodynamic Treatment Efficiency of Photosensitizer-Decorated Confeito-Like Gold Nanoparticles for Cancer Therapy. Macromol. Biosci. 2022, 22, 2200130. [Google Scholar] [CrossRef]
- Kaveh Zenjanab, M.; Samadi Pakchin, P.; Fathi, M.; Dalir Abdolahinia, E.; Adibkia, K. Niosomes Containing Paclitaxel and Gold Nanoparticles with Different Coating Agents for Efficient Chemo/Photothermal Therapy of Breast Cancer. Biomed. Mater. 2024, 19, 035015. [Google Scholar] [CrossRef]
- Xiao, T.; Qin, J.; Peng, C.; Guo, R.; Lu, X.; Shi, X. A Dendrimer-Based Dual Radiodense Element-Containing Nanoplatform for Targeted Enhanced Tumor Computed Tomography Imaging. Langmuir 2020, 36, 3096–3103. [Google Scholar] [CrossRef]
- Xiao, T.; Wen, S.; Wang, H.; Liu, H.; Shen, M.; Zhao, J.; Zhang, G.; Shi, X. Facile Synthesis of Acetylated Dendrimer-Entrapped Gold Nanoparticles with Enhanced Gold Loading for CT Imaging Applications. J. Mater. Chem. B 2013, 1, 2773–2780. [Google Scholar] [CrossRef]
- Alibolandi, M.; Hoseini, F.; Mohammadi, M.; Ramezani, P.; Einafshar, E.; Taghdisi, S.M.; Ramezani, M.; Abnous, K. Curcumin-Entrapped MUC-1 Aptamer Targeted Dendrimer–Gold Hybrid Nanostructure as a Theranostic System for Colon Adenocarcinoma. Int. J. Pharm. 2018, 549, 67–75. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, Z.; Zhang, J.; Peng, C.; Klajnert-Maculewicz, B.; Shen, M.; Shi, X. Zwitterionic Gadolinium(III)-Complexed Dendrimer-Entrapped Gold Nanoparticles for Enhanced Computed Tomography/Magnetic Resonance Imaging of Lung Cancer Metastasis. ACS Appl. Mater. Interfaces 2019, 11, 15212–15221. [Google Scholar] [CrossRef]
- Nguyenova, O.Y.; Hubalek Kalbacova, M.; Dendisova, M.; Sikorova, M.; Jarolimkova, J.; Kolska, Z.; Ulrychova, L.; Weber, J.; Reznickova, A. Stability and Biological Response of PEGylated Gold Nanoparticles. Heliyon 2024, 10, e30601. [Google Scholar] [CrossRef] [PubMed]
- Deol, S.; Weerasuriya, N.; Shon, Y.-S. Stability, Cytotoxicity, and Cell Uptake of Water-Soluble Dendron–Conjugated Gold Nanoparticles with 3, 12, and 17 nm Cores. J. Mater. Chem. B 2015, 3, 6071–6080. [Google Scholar] [CrossRef]
- Dockery, L.T.; Daniel, M.-C. Targeted Doxorubicin-Loaded Dendronized Gold Nanoparticles. Pharmaceutics 2023, 15, 2103. [Google Scholar] [CrossRef]
- Kołodziejczyk, A.M.; Grala, M.; Kołodziejczyk, Ł. Evaluation of PAMAM Dendrimer-Stabilized Gold Nanoparticles: Two-Stage Procedure Synthesis and Toxicity Assessment in MCF-7 Breast Cancer Cells. Molecules 2025, 30, 2024. [Google Scholar] [CrossRef]
- Elancheziyan, M.; Theyagarajan, K.; Saravanakumar, D.; Thenmozhi, K.; Senthilkumar, S. Viologen-Terminated Polyamidoamine (PAMAM) Dendrimer Encapsulated with Gold Nanoparticles for Nonenzymatic Determination of Hydrogen Peroxide. Mater. Today Chem. 2020, 16, 100274. [Google Scholar] [CrossRef]
- Giorgetti, E.; Giusti, A.; Giammanco, F.; Laza, S.; Del Rosso, T.; Dellepiane, G. Photodegradation of PAMAM G5-Stabilized Aqueous Suspensions of Gold Nanoparticles. Appl. Surf. Sci. 2007, 254, 1140–1144. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, Y.; Mao, Y.; Li, Y. Amine-Terminated TEG-Derived PAMAM Dendrimer as Template for Preparation of Gold Nanoparticles in Water. Front. Mater. Sci. China 2010, 4, 95–99. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Byrne, H.J. Polyamidoamine Dendrimer Nanoparticle Cytotoxicity, Oxidative Stress, Caspase Activation, and Inflammatory Response: Experimental Observation and Numerical Simulation. Nanomedicine 2013, 9, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczyk, A.M.; Grala, M.M.; Zimon, A.; Białkowska, K.; Walkowiak, B.; Komorowski, P. Investigation of HUVEC Response to Exposure to PAMAM Dendrimers—Changes in Cell Elasticity and Vesicle Release. Nanotoxicology 2022, 16, 1220–1232. [Google Scholar] [CrossRef] [PubMed]
- Camarada, M.B. Interaction of Gold Nanoparticles with PAMAM Dendrimers: A Molecular Dynamics Study. J. Phys. Chem. A 2017, 121, 8124–8135. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kono, K. Functional dendrimer–gold nanoparticle hybrids for biomedical applications. Polym. Int. 2018, 67, 840–852. [Google Scholar] [CrossRef]
- Camarada, M.B.; Comer, J.; Poblete, H.; Azhagiya Singam, E.R.; Marquez-Miranda, V.; Morales-Verdejo, C.; Gonzalez-Nilo, F.D. Experimental and Computational Characterization of the Interaction between Gold Nanoparticles and Polyamidoamine Dendrimers. Langmuir 2018, 34, 10063–10072. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, X.H.; Wang, Z.Y.; Meng, M.; Li, X.; Ning, Q. Generation 4 Polyamidoamine Dendrimers as a Novel Candidate Nanocarrier for Gene Delivery Agents in Breast Cancer Treatment. Cancer Lett. 2010, 298, 34–49. [Google Scholar] [CrossRef]
- Garcia, M.E.; Baker, L.A.; Crooks, R.M. Preparation and Characterization of Dendrimer–Gold Colloid Nanocomposites. Anal. Chem. 1999, 71, 256–258. [Google Scholar] [CrossRef]
- Villanueva-Flores, F.; Castro-Lugo, A.; Ramírez, O.T.; Palomares, L.A. Understanding cellular interactions with nanomaterials: Towards a rational design of medical nanodevices. Nanotechnology 2020, 31, 132002. [Google Scholar] [CrossRef]
- Aieneravaie, M.; Ho, J.Q.; Arabi, L.; Kim, A.; Liang, S.; Jones, S.; Javdani, N.; Georgala, P.; Sepand, M.R.; Rafat, M.; et al. Cell–Nanoparticle Interactions. In Nanomedicine for Ischemic Cardiomyopathy; Mahmoudi, M., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 9, pp. 125–142. [Google Scholar] [CrossRef]
- de Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Svenson, S.; Chauhan, A.S. Dendrimers for enhanced drug solubilization. Nanomedicine 2008, 3, 679–702. [Google Scholar] [CrossRef]
- Avila-Salas, F.; González, R.I.; Ríos, P.L.; Araya-Durán, I.; Camarada, M.B. Effect of the Generation of PAMAM Dendrimers on the Stabilization of Gold Nanoparticles. J. Chem. Inf. Model. 2020, 60, 2966–2976. [Google Scholar] [CrossRef]
- Kim, Y.G.; Oh, S.K.; Crooks, R.M. Preparation and Characterization of 1-2 Nm Dendrimer-Encapsulated Gold Nanoparticles 395 Having Very Narrow Size Distributions. Chem. Mater. 2004, 16, 167–172. [Google Scholar] [CrossRef]
- Szekeres, G.P.; Kneipp, J. SERS Probing of Proteins in Gold Nanoparticle Agglomerates. Front. Chem. 2019, 7, 30. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Römer, I.; Wang, Z.W.; Merrifield, R.C.; Palmer, R.E.; Lead, J. High-Resolution STEM-EELS Study of Silver Nanoparticles Exposed to Light and Humic Substances. Environ. Sci. Technol. 2016, 50, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Zuber, A.; Purdey, M.; Schartner, E.; Forbes, C.; van der Hoek, B.; Giles, D.; Abell, A.; Monro, T.; Ebendorff-Heidepriem, H. Detection of Gold Nanoparticles with Different Sizes Using Absorption and Fluorescence-Based Methods. Sens. Actuators B Chem. 2016, 227, 117–127. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Ahmad, T.; Moniruzzaman, M.; Bhattacharjee, S.; Abdullah, B. Size and stability modulation of ionic liquid functionalized gold nanoparticles synthesized using Elaeis guineensis (oil palm) kernel extract. Arab. J. Chem. 2020, 13, 75–85. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, T.; Jiang, H.; Li, Y.; Jiang, T.; Zhang, J.; Wang, S. Incorporation of Carvedilol into PAMAM-Functionalized MWNTs as a Sustained Drug Delivery System for Enhanced Dissolution and Drug-Loading Capacity. Asian J. Pharm. Sci. 2013, 8, 278–286. [Google Scholar] [CrossRef]
- Behera, M.; Ram, S. Inquiring the Mechanism of Formation, Encapsulation, and Stabilization of Gold Nanoparticles by Poly(vinyl pyrrolidone) Molecules in 1-Butanol. Appl. Nanosci. 2014, 4, 247–254. [Google Scholar] [CrossRef]
- Rozenberg, M.; Shoham, G.; Reva, I.; Fausto, R. A Correlation between the Proton Stretching Vibration Red Shift and the Hydrogen Bond Length in Polycrystalline Amino Acids and Peptides. Phys. Chem. Chem. Phys. 2005, 7, 2376–2383. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G. Development of Noncytotoxic Chitosan–Gold Nanocomposites as Efficient Antibacterial Materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–10999. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Miranda, O.R.; Thompson, M.A.; Pabelick, C.M.; Bhattacharya, R.; Robertson, J.D.; Rotello, V.M.; Prakash, Y.S.; Mukherjee, P. Effect of Nanoparticle Surface Charge at the Plasma Membrane and Beyond. Nano Lett. 2010, 10, 2543–2548. [Google Scholar] [CrossRef]
- Yue, Z.G.; Wei, W.; Lv, P.P.; Yue, H.; Wang, L.Y.; Su, Z.G.; Ma, G.H. Surface Charge Affects Cellular Uptake and Intracellular Trafficking of Chitosan-Based Nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.P.; Ma, B.Y.; Wei, X.W.; Qian, Z.Y. The In Vitro and In Vivo Toxicity of Gold Nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of Gold Nanoparticles Functionalized with Cationic and Anionic Side Chains. Bioconjug. Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef]
- Patra, H.K.; Banerjee, S.; Chaudhuri, U.; Lahiri, P.; Dasgupta, A.K. Cell Selective Response to Gold Nanoparticles. Nanomedicine 2007, 3, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, H.; Chen, Z.; Zheng, Y. Penetration of Lipid Membranes by Gold Nanoparticles: Insights into Cellular Uptake, Cytotoxicity, and Their Relationship. ACS Nano 2010, 4, 5421–5429. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Nanoparticle–Cell Interactions: Molecular Structure of the Protein Corona and Cellular Outcomes. Acc. Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Younis, M.A.; Tawfeek, H.M.; Abdellatif, A.A.H.; Abdel-Aleem, J.A.; Harashima, H. Clinical translation of nanomedicines: Challenges, opportunities, and keys. Adv. Drug Deliv. Rev. 2022, 181, 114083. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Fortune, A.; Aime, A.; Raymond, D.; Kumar, S. Nanotechnology in medicine: A double-edged sword for health outcomes. Health Nanotechnol. 2025, 1, 9. [Google Scholar] [CrossRef]
- Grala, M.; Kołodziejczyk, A.M.; Białkowska, K.; Walkowiak, B.; Komorowski, P. Assessment of the Influence of Gold Nanoparticles Stabilized with PAMAM Dendrimers on HUVEC Barrier Cells. Micron 2023, 168, 103430. [Google Scholar] [CrossRef]
- Li, Q.; Yu, C.; Gao, R.; Xia, C.; Yuan, G.; Li, Y.; Zhao, Y.; Chen, Q.; He, J. A novel DNA biosensor integrated with Polypyrrole/streptavidin and Au-PAMAM-CP bionanocomposite probes to detect the rs4839469 locus of the vangl1 gene for dysontogenesis prediction. Biosens. Bioelectron. 2016, 15, 674–681. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, J.; Ma, H.; Jiang, Y.; Huang, C.; Han, E. Optimized dendrimer-encapsulated gold nanoparticles and enhanced carbon nanotube nanoprobes for amplified electrochemical immunoassay of E. coli in dairy product based on enzymatically induced deposition of polyaniline. Biosens. Bioelectron. 2016, 80, 666–673. [Google Scholar] [CrossRef] [PubMed]

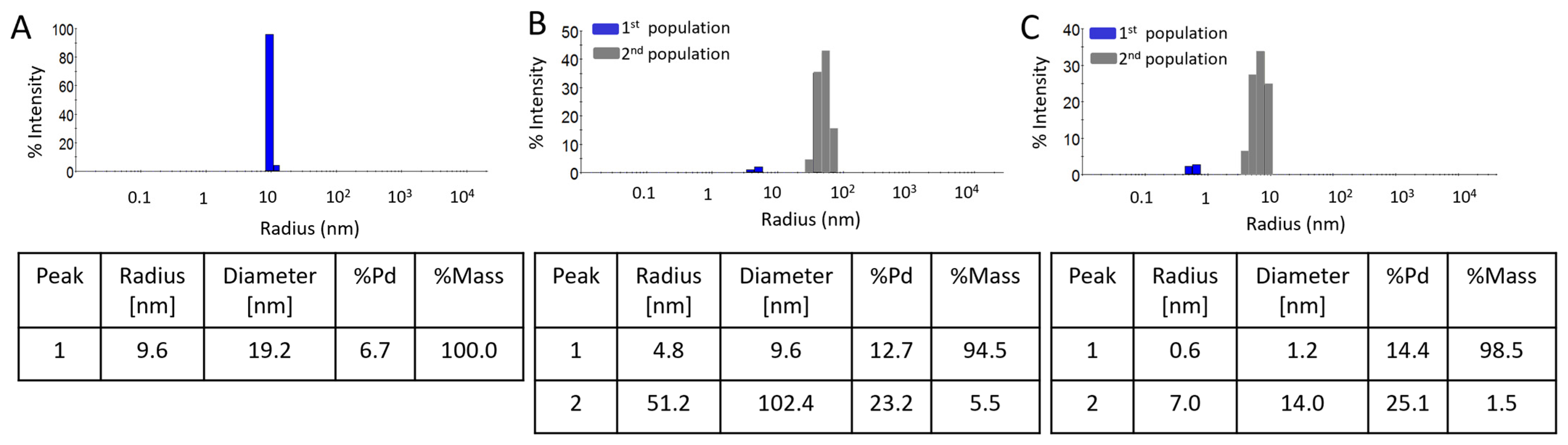
| Sample–Sonication | Population | Hydrodynamic Diameter During Time [nm] (%Mass) | |||
|---|---|---|---|---|---|
| 24 h | 1 Week | 1 Month | 3 Months | ||
| 1:2 | Peak 1 | 3.6 (99.7%) | 2.6 (98.8%) | 2.6 (98.9%) | 2.0 (98.8%) |
| Peak 2 | 42.8 (0.3%) | 48.6 (1.2%) | 26.0 (1.1%) | 24.6 (1.2%) | |
| 1:3 | Peak 1 | 7.6 (97.2%) | 5.2 (99.5%) | 4.8 (98.3%) | 3.6 (98.6%) |
| Peak 2 | 33.0 (2.7%) | 42.2 (0.5%) | 32.4 (1.7%) | 26.0 (1.4%) | |
| Peak 3 | 82.2 (0.2%) | - | - | - | |
| 1:4 | Peak 1 | 19.2 (100%) | 20.2 (100%) | 18.8 (100%) | 18.4 (100%) |
| 1:5 | Peak 1 | 2.6 (93.0%) | 3.6 (86.3%) | 4.8 (79.4%) | 6.4 (99.2%) |
| Peak 2 | 19.0 (6.4%) | 16.2 (12.8%) | 13.6 (18.7%) | - | |
| Peak 3 | 128.2 (0.6%) | 111.6 (0.9%) | 102.2 (93.9%) | 77.6 (0.8%) | |
| Sample–Microwave | Population | Hydrodynamic Diameter During Time [nm] (%Mass) | |||
|---|---|---|---|---|---|
| 24 h | 1 Week | 1 Month | 3 Months | ||
| 1:2 | Peak 1 | immeasurable | immeasurable | 1.8 (99.3%) | 30.2 (100%) |
| Peak 2 | 20.1 (0.7%) | - | |||
| 1:3 | Peak 1 | immeasurable | 1.2 (98.6%) | - | - |
| Peak 2 | 14.4 (1.4%) | 20.4 (100%) | 24.2 (100%) | ||
| 1:4 | Peak 1 | 9.6 (94.5%) | 7.0 (98.7%) | 6.6 (99.0%) | 8.6 (97.9%) |
| Peak 2 | 102.4 (5.5%) | 103 (1.3%) | 104.4 (1.0%) | 114.6 (2.1%) | |
| 1:5 | Peak 1 | 5.2 (96.7%) | 4.4 (93.0%) | 10.0 (81.8%) | 4.2 (97.6%) |
| Peak 2 | 34.2 (0.8%) | 38.2 (3.2%) | 30.4 (2.1%) | 17.8 (1.7%) | |
| Peak 3 | 157.4 (2.5%) | 173.2 (3.7%) | 175.4 (16.2%) | 152.4 (0.8%) | |
| Sample–Unassisted | Population | Hydrodynamic Diameter During Time [nm] (%Mass) | |||
|---|---|---|---|---|---|
| 24 h | 1 Week | 1 Month | 3 Months | ||
| 1:2 | Peak 1 | immeasurable | immeasurable | 20.2 (100%) | 27.2 (100%) |
| 1:3 | Peak 1 | immeasurable | 15.2 (99.9%) | 21.0 (100%) | 22.8 (100%) |
| Peak 2 | 213.4 (0.1%) | - | - | ||
| 1:4 | Peak 1 | 1.2 (98.5%) | - | - | - |
| Peak 2 | 14.0 (1.5%) | 15.6 (100%) | 17.2 (100%) | 16.6 (100%) | |
| 1:5 | Peak 1 | 7.8 (92.5%) | 7.6 (92.4%) | 9.0 (91.2%) | 9.2 (94.4%) |
| Peak 2 | - | 21.6 (4.7%) | 40.2 (2.8%) | - | |
| Peak 3 | 163.4 (7.5%) | 150.0 (2.9%) | 150.8 (6.0%) | 143.6 (5.6%) | |
| Sonication [µg/mL] | Microwave [µg/mL] | Unassisted [µg/mL] | |
|---|---|---|---|
| EC10 | 3.2 ± 0.7 | 3.3 ± 0.7 | 5.5 ± 0.7 |
| EC25 | 13.1± 0.3 | 9.3 ± 0.3 | 11.7 ± 0.4 |
| EC50 | 25.4 ± 0.4 | 20 ± 0.7 | 22.2 ± 0.2 |
| Sonication [µg/mL] | Microwave [µg/mL] | Unassisted [µg/mL] | |
|---|---|---|---|
| EC10 | 2.3 ± 0.2 | 1.4 ± 0.1 | 1.7 ± 0.3 |
| EC25 | 3.9 ± 0.2 | 2.9 ± 0.1 | 3.2 ± 0.2 |
| EC50 | 6.6 ± 0.1 | 5.4 ± 0.1 | 5.7 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grala, M.; Karwowski, B.; Kołodziejczyk, A.M. Comparative Analysis of Gold Nanoparticle Synthesis Using PAMAM G2 Dendrimers via Microwave and Sonication Methods for Potential Cancer Theranostic Applications. Molecules 2025, 30, 4509. https://doi.org/10.3390/molecules30234509
Grala M, Karwowski B, Kołodziejczyk AM. Comparative Analysis of Gold Nanoparticle Synthesis Using PAMAM G2 Dendrimers via Microwave and Sonication Methods for Potential Cancer Theranostic Applications. Molecules. 2025; 30(23):4509. https://doi.org/10.3390/molecules30234509
Chicago/Turabian StyleGrala, Magdalena, Bolesław Karwowski, and Agnieszka Maria Kołodziejczyk. 2025. "Comparative Analysis of Gold Nanoparticle Synthesis Using PAMAM G2 Dendrimers via Microwave and Sonication Methods for Potential Cancer Theranostic Applications" Molecules 30, no. 23: 4509. https://doi.org/10.3390/molecules30234509
APA StyleGrala, M., Karwowski, B., & Kołodziejczyk, A. M. (2025). Comparative Analysis of Gold Nanoparticle Synthesis Using PAMAM G2 Dendrimers via Microwave and Sonication Methods for Potential Cancer Theranostic Applications. Molecules, 30(23), 4509. https://doi.org/10.3390/molecules30234509









