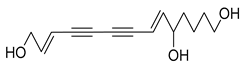Abstract
The Campanula genus (Campanulaceae) comprises nearly 300 herbaceous species widely distributed across the Northern Hemisphere. Beyond their ornamental value, many species have been traditionally employed to treat inflammatory, respiratory, and cardiovascular disorders. Phytochemical studies have revealed a remarkable diversity of bioactive constituents, including phenolic acids, flavonoids, coumarins, acetylenic compounds, triterpenes, and alkaloids. These metabolites exhibit broad pharmacological activities, such as antioxidant, anti-inflammatory, antimicrobial, cytotoxic, and cardioprotective effects. This review provides a comprehensive overview of the isolated compounds from the Campanula species, summarizing their chemical diversity, pharmacological mechanisms, and structure–activity relationships. It also highlights underexplored species and compound classes with potential therapeutic significance. By integrating the phytochemical evidence with pharmacological insights, this work underscores the value of the Campanula species as promising natural resources for future drug discovery and development.
Keywords:
Campanula; phenolic acids; flavonoids; coumarins; acetylenic compounds; triterpenes; alkaloids 1. Introduction
Medicinal plants have been universally used for centuries in various traditional medicine systems, as they contain a wide range of bioactive molecules, such as flavonoids, alkaloids, terpenoids, and essential oils, among others []. These bioactive molecules, extracted from different parts of the plant, often exhibit antioxidant, anti-inflammatory, antifungal, antibacterial, antiproliferative, and other therapeutic effects [,].
Phytochemical screening plays a vital role in the development of both traditional medicine applications and modern pharmaceutical research by validating the efficacy and safety of traditionally used treatments. Furthermore, such screening has led to the discovery of the bioactive molecules applied in agriculture, cosmetics, drugs, and food processing [,,,,].
The genus Campanula, belonging to the Campanulaceae family, comprises approximately 300 herbaceous species, named for the bell-shaped blue flowers characteristic of most species. These species are widely distributed across Asia, the Black Sea, and Mediterranean regions []. Several species have a long history of traditional medicinal use: Campanula glomerata, C. persicifolia, C. rotundifolia, C. bononiensis, C. sibirica, and C. patula are employed in Russian folk medicine for treating epilepsy, nervous disorders, coughs, sore throats, headaches, rheumatism, and inflammation [,]. Similarly, C. medium, C. cervicaria, C. rotundifolia, C. latifolia, and C. trachelium are traditionally used in Italian medicine for comparable purposes [].
From an ethnopharmacological perspective, these traditional uses correlate with the phytochemical composition of the species. The Campanula species are rich in phenolic acids, flavonoids, acetylenic compounds, and triterpenes, which are likely responsible for their reported anti-allergic, antioxidant, spasmolytic, antiviral, and antimicrobial activities []. Furthermore, geographic and taxonomic variations influence the distribution and abundance of these bioactive compounds, providing insight into their species-specific medicinal potential.
From a commercial standpoint, cultivation of the Campanula species is primarily for ornamental purposes []. However, understanding the link between phytochemistry, traditional uses, and geography can inform both conservation strategies and targeted pharmacological research.
The main objective of this study is to provide a comprehensive review of the phytochemistry and pharmacological applications of the Campanula genus. This review aims to support both the preservation of traditional knowledge and modern scientific discovery, serving as a foundation for future investigations into the medicinal potential of the Campanula species.
2. Campanulaceae Family
The Campanulaceae family includes 84 genera and 2400 species of dicotyledonous plants. The species of this family can be found on six continents and many oceanic islands. It is cosmopolitan in distribution, with representatives on six continents and many oceanic islands, and can grow in both cold and dry climatic conditions. The two main subfamilies are Campanuloideae, found mainly in temperate zones of the Old World, and Lobelioideae, distributed in the tropical and subtropical zones of the world.
The species of this family are usually herbaceous perennials, woody or herbaceous lianas that can twine and can be annuals or biennials. They can be described as subshrubs, shrubs, treelets, or pachycaul rosette plants, while the trees can reach up to 15 m tall. Most species are terrestrial, less often epiphytic or aquatic, and can have a milky latex that may be colored. Flowers of the species of this family are actinomorphic or zygomorphic, epigynous, and hermaphrodite. While the leaves are estipulate, alternate, infrequently whorled or opposite, often simple (pinnate when compound), entire, sessile, or petiolate, occasionally sheathed, and serrated in different ways The stems, acaulescent or rhizomatous, can be simple or highly branched woody or herbaceous lianas that can twine [].
The major economic value of the Campanulaceae species is in horticulture. Many species are grown as ornamentals and are commonly available in the gardening trade. The best-known species in this area are perennial bellflowers (e.g., Campanula persicifolia, Campanula carpatica, Campanula glomerata). Other species, such as Lobelia cardinalis, Platycodon grandiflorus, and Trachelium caeruleum, are used for the same purposes. In addition to horticulture, some species are cultivated for their nutritional purposes, such as Campanula rapunculus and Platycodon grandiflorus, that are used as vegetables in Europe and Eastern Asia, respectively. On another hand, several pharmaceutical products are also supplied from species of this family. For example, Lobeline, isolated from Lobelia inflata, is a pyridine alkaloid used in the treatment of asthma, as a respiratory stimulant, and to combat smoking. Moreover, the roots of Codonopsis, mainly C. pilosula, are the source of dangshen used in traditional Chinese medicine. It is a popular, less expensive substitute for ginseng, used as a tonic for fatigue, loss of appetite, and weakness. Studies have shown that the crude extract of this species promotes digestion, strengthens the immune system, dilates peripheral blood vessels, and inhibits the activity of the adrenal cortex; however, the bioactive molecules responsible for these effects are yet to be isolated or identified []. The roots of Platycodon grandiflorus are an important Asian remedy, “jie-geng” or “kikyo”, used as an expectorant and cough suppressant. Studies have shown that it also has analgesic, antipyretic, anti-inflammatory, and antibacterial properties. The active ingredient is a saponin (platycodin or kikyosaponin) []. Many species of the Campanulaceae family have local uses in folk medicine, and particularly among non-industrialized populations, but they have not been studied in detail or exploited commercially due to the complexity of their constituents. For example, Wahlenbergia marginata is used in traditional Chinese medicine in the treatment of coughs, tuberculosis, heart diseases, and as a tonic [].
Bioactive molecules, such as flavonoids [], polyhydroxylated piperidine and pyrrolidine alkaloids [], terpenes, sterols [], and glycosylated polyacetylenes [], have been isolated from various species of the Campanulaceae family.
3. Campanula Genus
The Campanula genus includes 300 herbaceous species. The species of this genus are distributed in the northern hemisphere of the world and especially in mountainous areas. They are present in meadows and subalpine regions; however, many species require full sun for optimal development. The name refers to the blue, bell-shaped flowers of most species. All these species are herbaceous, perennial, biannual, or annual. The corolla can be characterized as campanulate, infundibuliform (funnel-shaped), tubular, cupuliform (bowl-shaped), or rotate, mostly fused and varying in color from blue to violet or white (rarely yellow, red, or pink). The ovary is usually obconical or oblong–obconical with (2–)3–5 locules. Calyx teeth are usually longer than the ovary and they can alternate with appendages. Capsules are pendent or erect, dehiscing by pores or valves or, rarely, indehiscent [,,].
Studies have shown that some Campanula species possess antioxidant, anti-allergic, antiphlogistic, spasmolytic, and antiviral properties [,,].
Some species of the Campanula genus have been used in traditional medicine for the treatment of constipation, laryngitis, tonsillitis, warts, and bronchitis []. For example, Campanula glomerata, C. persicifolia, C. rotundifolia, C. bononiensis, C. sibirica, and C. patula have been used in Russian folk medicine to treat epilepsy, nervous diseases, coughs, sore throats. head, rheumatism, and inflammation [,]
Campanula medium, C. cervicaria, C. rotundifolia, C. latifolia, and C. trachelium are used in Italy for the same purposes [].
In addition, the fresh roots of Campanula sulphurea Boiss. have been chewed for the treatment of lung and heart problems; their infusions have been used for ear inflammations [].
From a commercial point of view, however, the Campanula species are mainly cultivated for ornamental purposes [].
4. Bioactive Compounds Isolated from the Species of the Campanula Genus
Most bioactive molecules isolated from the Campanula genus, between 1970 and 1990, were found in the extracts obtained by extractions carried out by conventional methods using solvents of variable polarities and purifications were carried out by precipitation and/or by polyamide gel chromatography column or by preparative chromatography.
Commonly used solvents for extracting bioactive compounds from the Campanula species include trichloromethane, dichloromethane, ethanol, methanol, water, and sometimes a combination of these solvents. Techniques such as liquid–liquid extraction and Soxhlet extraction have been employed to extract the bioactive compounds from Campanula plants. Compounds have been identified using UV, IR, HPLC, and NMR spectroscopy [,,,,].
Most of the compounds found in the Campanula genus belong to the family of phenolic compounds such as phenolic acids, flavonoids, and coumarins.
4.1. Phenolic Acids Isolated from the Species of the Campanula Genus
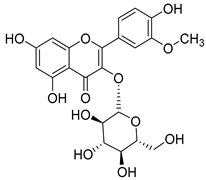
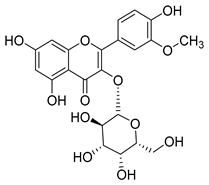
Fourteen compounds belonging to the phenolic acid family have been identified in the Campanula genus (Table 1). Among these, chlorogenic acid (1), isolated from six species of the Campanula genus (C. cephalotes, C. glomerata, C. persicifolia, C. patula, C. rapunculoides L., and C. maleevii), is recognized for its antioxidant, anti-inflammatory, antibacterial, antiviral, hypoglycemic, lipid-lowering, cardioprotective, antimutagenic, anticancer, and immunomodulatory activities [].

Table 1.
Phenolic acids isolated from the species of the Campanula genus.
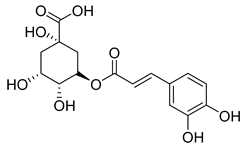
3-p-Coumaroylquinic acid (2), known for its antibacterial and anti-inflammatory properties, has been isolated from four species (C. cephalotes, C. glomerata, C. rapunculoides L., and C. persicifolia).
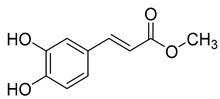
Methyl caffeate (3), found in C. cephalotes, C. glomerata, and C. rapunculoides L., exhibits cytotoxic, antiproliferative, antimicrobial, anti-platelet, and antioxidant activities.
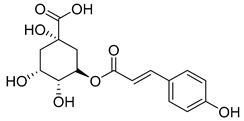
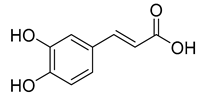
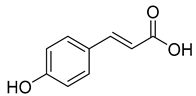
Caffeic acid (4) occurs in C. rotundifolia, C. persicifolia, and C. rapunculoides L., whereas p-coumaric acid (5) is found in C. patula, C. rotundifolia, C. persicifolia, and C. rapunculoides L. Both acids display antioxidant, antibacterial, cytotoxic, anti-inflammatory, and antiproliferative effects.
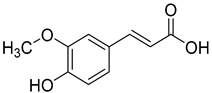
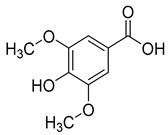
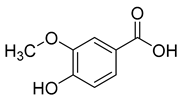
Ferulic acid (6) and syringic acid (7) have been isolated from C. rotundifolia, C. persicifolia, C. persicifolia, and C. patula, respectively. These compounds exhibit a broad spectrum of activities, including antioxidant, anti-inflammatory, cardioprotective, antibacterial, and anticancer effects.
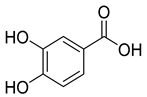
Protocatechuic acid (8), known for its antioxidant, antibacterial, anticancer, antihyperlipidemic, antidiabetic, and anti-inflammatory properties, has been detected in C. persicifolia.
Vanillic acid (9), found in C. persicifolia, C. patula, and C. rotundifolia, possesses antioxidant, anti-inflammatory, immunostimulatory, neuroprotective, hepatoprotective, cardioprotective, and anti-apoptotic activities.
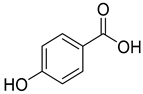
p-Hydroxybenzoic acid (10), isolated from C. persicifolia, C. lactiflora, and C. medium, exhibits antimicrobial, anti-atherogenic, and antioxidant properties.
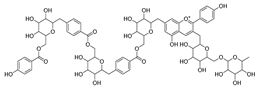
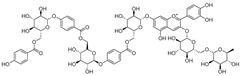
Barbatosides A and B (11, 12), detected in C. barbata, are classified as saponins and display analgesic and anti-inflammatory activities.
Barbatosides C and D (13, 14), also isolated from C. barbata, have not been reported in other Campanula species. These compounds are unique to this species, although their pharmacological properties remain uninvestigated.
Nine of the isolated phenolic acids—chlorogenic acid (1), methyl caffeate (3), caffeic acid (4), p-coumaric acid (5), ferulic acid (6), syringic acid (7), protocatechuic acid (8), vanillic acid (9), and p-hydroxybenzoic acid (10)—demonstrate significant antioxidant properties, which likely contribute to the plants’ defense against oxidative stress. These compounds have been isolated from nine different Campanula species (C. glomerata, C. patula, C. rapunculoides L., C. maleevii, C. persicifolia, C. cephalotes, C. rotundifolia, C. lactiflora, and C. medium).
Table 1 provides a comprehensive summary of all phenolic acids isolated from the Campanula species along with their verified pharmacological activities.
4.1.1. Flavonoids Isolated from the Species of the Campanula Genus
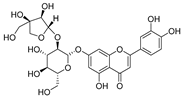
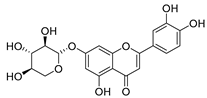
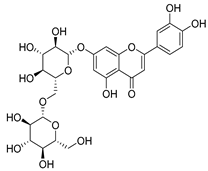
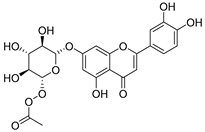
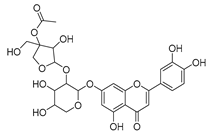
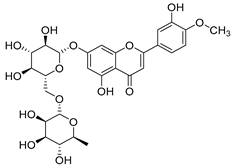
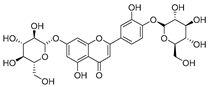
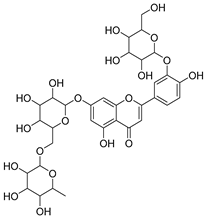
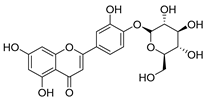
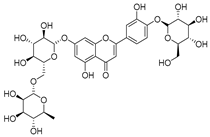
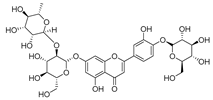
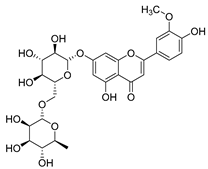
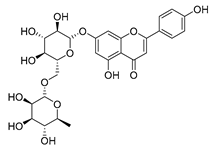
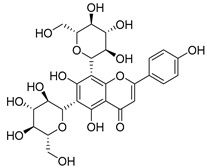
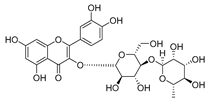
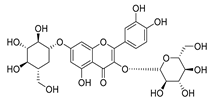
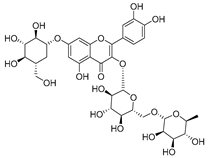
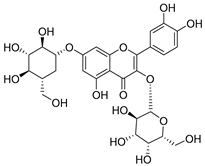
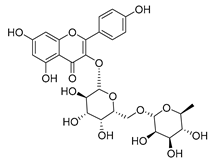
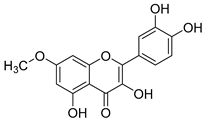
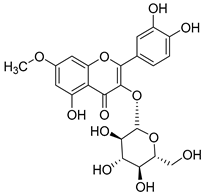
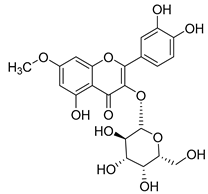
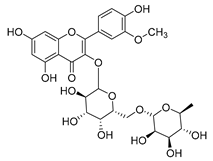
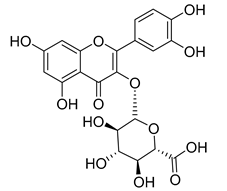
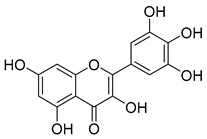
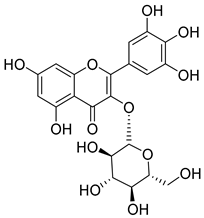
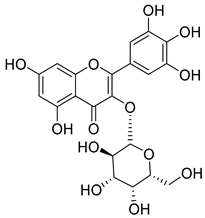
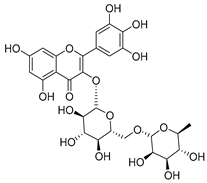
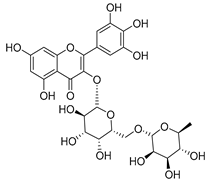
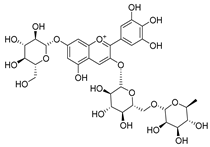
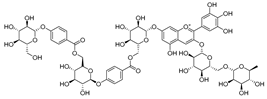
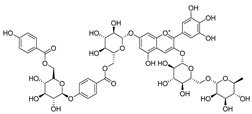
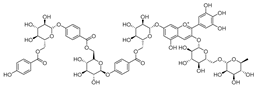
Flavonoids are the most common compounds in plants, and they are known for their wide range of pharmacological and therapeutical effects. Most compounds isolated from the Campanula genus belong to this family. To date, 60 flavonoids have been isolated from 27 species of the Campanula genus (C. persicifolia, C. rotundifolia, C. lactiflora, C. medium, C. ossetica, C. isophylla, C. rotundifolia, C. patula, C. pyramidalis, C. maleevii, C. rapunculoides L., C. glomerata, C. bononiensis L., C. cephalates, C. kolenatiana, C. kemulariae, C. punctata, C. cephalotes, C. barbata, C. alliariifolia, C. biebersteiniana, C. choziatowskyi, C. oblongifolia, C. hypopolia, C. cephalates, C. carpatica, anda C. poscharskyana).
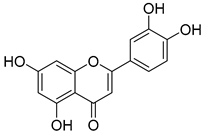
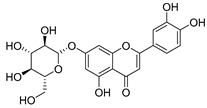
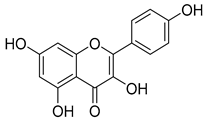
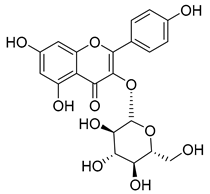
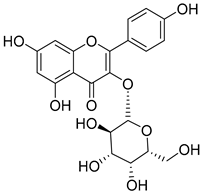
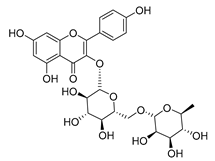
Luteolin (15), along with eleven of its glucosides, have been found in the species of the Campanula genus. Luteolin (15) is a flavone known for its antioxidant, anti-inflammatory, antimicrobial, cancer chemotherapeutic and chemoprotective activities, cardioprotective, antidiabetic, neuroprotective, and anti-allergic activities.
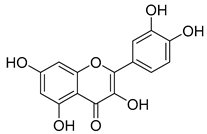
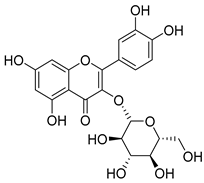
Quercetin (35), a flavonol known for its anti-SARS-CoV-2, antioxidant, anticancer, antiaging, antiviral, and anti-inflammatory activities, has been isolated from one species of the Campanula genus (C. medium). Nine glucoside derivatives of quercetin have been detected in the Campanula genus as well.
Kaempferol (45) has been found in C. medium. Studies have shown that this common flavonol has anti-inflammatory, antioxidant, neuroprotective, cardioprotective, anti-obesity, anti-ulcer, antidiabetic, cosmetic use, anti-osteoporotic, and anticancer properties. Four derivatives of kaempferol have been detected from Campanula genus as well.
Rhamnetin (50) flavonol has been isolated from C. cephalates and C. cephalotes, and it possesses anti-inflammatory, antioxidant, cardioprotective, anti-atherosclerosis, and neuroprotective activities. Eight Rhamnetin glucosides have been found in the species of the Campanula genus as well.
Myricetin (59), along with four derivatives, were isolated from one species of the Campanula genus (C. bononiensis L.). Studies have shown that this flavonol exhibits a wide range of pharmacological activities, such as iron-chelating, antioxidant, anti-inflammatory, anticancer, immunomodulator, antimyocardial damage, antiviral, immune system regulation, oxidative stress reduction, amelioration of cardiac dysfunction, and arrhythmia.
Moreover, eighteen of the flavonoids isolated from the species of the Campanula species have not yet been detected in any other species, and are original for these following species of the Campanula genus:
- Luteolin-7-O-glucosyl(6 → 1)-O-arabinoside (Rotundiside) (18) isolated from C. rotundifolia.
- Patularoside (20), Acetylcynaroside (24), and Campanoside (25) isolated from C. patula.
- Luteolin-7-O-glucoside-4′-O-glucoside (27), Luteolin-7-O-rutinoside-3′-O-glucoside (28), Luteolin-7-O-rutinoside-4′-O-glucoside (30), and Luteolin-7-O-neohesperidoside-4′-O-glucoside (31) isolated from C. persicifolia.
- Quercetin-3-O-rhamnosyl-(1 → 4)-galactoside (39), Isorhamnetin-3-rhamnosyl(1 → 4) glucoside (56) isolated from C. rapunculoides L.
- Quercetin-3-O-rhamnosyl-(1 → 4)-glucoside (41) isolated from C. rapunculoides L. and C. bononiensis L.
- Quercetin-3,7-di-O-glucoside (42) isolated from C. punctata and C. bononiensis L.
- Isorhamnetin-4′-O-(p-hydroxybenzoyl)-3,7-di-O-glucoside (64) isolated from C. lactiflora
- Barbatoflavane (68) isolated from C. barbata.
- Bisdeacylplatyconine (69) isolated from C. isophylla and C. carpatica.
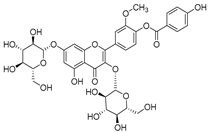
- Campanin (72) isolated from C. medium, C. isophylla and C. carpatica.
- Rubrocampanin (73) and Purprocampanin (74) isolated from C. medium.
A list of flavonoid compounds and flavonoid glycosides isolated from this genus along with their known and proven pharmacological activities (when applicable) are presented in Table 2.

Table 2.
Flavonoids isolated from the species of the Campanula genus.
4.1.2. Coumarins Isolated from the Species of the Campanula Genus
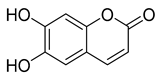
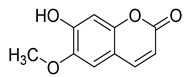
Three coumarins have been isolated from the species of the Campanula genus. Compounds belonging to this family have numerous therapeutic applications, including photochemotherapy, anti-tumor, and anti-HIV treatment. Some are central nervous system stimulants, others are antibacterial, anti-inflammatory, and anticoagulant agents []. Table 3 shows that, among the three isolated coumarins, esculetin (75) has been evaluated for its pharmacological activities, such as antioxidant, anti-tumor, anti-inflammatory, antibacterial, antidiabetic, immunomodulatory, and anti-atherosclerotic activities []. Fraxoside (76) has been detected in C. alliariifolia and C. ochroleuca. A study showed that this coumarin exhibits anticancer activity by providing high binding affinity toward the carbonic anhydrase IX (hCAIX) protein, which is a membrane-spanning metalloenzyme, encoded by the CA9 gene, and can lead to various carcinomas if upregulated [].

Table 3.
Coumarins isolated from the species of the Campanula genus.
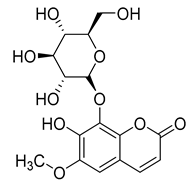
Fraxetol (77) has been isolated from two species of the Campanula genus (C. alliariifolia and C. ochroleuca). This coumarin has been detected in other species such as Fraxinus excelsior L. [], Pentalinon andrieuxii [], Stewartia pseudocamellia [], and Xanthoceras sorbifolium [] as well; however, no study has been realized yet to detect its biological activities.
4.1.3. Acetylenic Compounds Isolated from the Species of the Campanula Genus
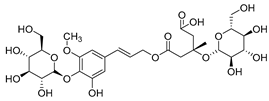
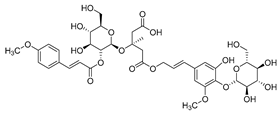
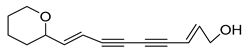
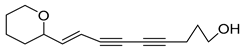
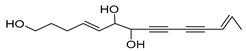
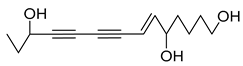
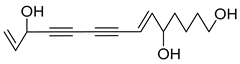
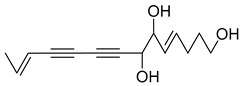
Nine acetylenic compounds have been isolated from six species of the Campanula genus. Among these compounds, lobetyol (80), isolated from two species of this genus, is known for its antioxidant, antiviral, anti-inflammatory, and cell proliferation inhibitory capacities [,]. While lobetyolin (81), isolated from five species of this genus, is known for its anticancer, antioxidant, cardioprotective, and anti-inflammatory activities [].
Among the isolated acetylenic compounds, six were original for the species of the Campanula genus:
- -
- 9-(tetrahydropyran-2-yl)-nona-trans,trans-2,8-diene-4,6-diynol (78) and 9-(tetrahydropyran-2-yl)-trans-nona-8-ène-4,6-diynol (79) isolated from C. glomerata;
- -
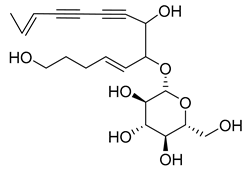
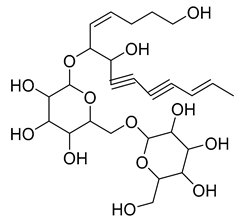
- tetradeca-6-ene-8,10-diyne-1,5,12-triol (83), tetradeca-6,13-diene-8,10-diyne-1,5,12-triol (84), and tetradeca-6,12-diene-8,10-diyne-1,5,14-triol (85) detected in C. medium and C. pyramidalis;
- -
- tetradeca-4,12-diene-8,10-diyne-1,6,7-triol (86) found in C. pyramidalis.
However, despite their detection, research into their potential pharmacological properties is yet to be conducted.
Moreover, no study has been conducted yet to reveal the pharmacological effects of lobetyolinin (82), even though it has been isolated from C. glomerata and other species, such as Lobelia chinensis [] and Radix Codonopsis [].
A list of acetylenic compounds isolated from this genus along with their known and proven pharmacological activities (when applicable) is presented in Table 4.

Table 4.
Acetylenic compounds isolated from the species of the Campanula genus.
4.1.4. Triterpenes Isolated from the Species of the Campanula Genus
In addition, twelve compounds from the triterpene family have been isolated from eight species of the Campanula genus.
β-sitosterol (87), a common triterpene usually found in plants and known for a wide range of pharmacological activities, such as antimicrobial, anti-inflammatory, anticancer, antifertility, angiogenic, antioxidant, immunomodulatory, antidiabetic, and antinociceptive activities, has been isolated from four species of the Campanula genus (C. cephalotes, C. punctata, C. dasyantha, and C. istriaca Feer).
Daucosterol (88), found in C. cephalotes, C. dasyantha, C. langsdorffiana, and C. lactiflora, exhibits antioxidant, antidiabetic, hypolipidemic, anti-inflammatory, immunomodulatory, neuroprotective, and anticancer activities.
Stigmasterol (89) and ursolic acid (90) were isolated, respectively, from C. cephalotes, C. istriaca Feer, C. persicifolia, C. rotundifolia, and C. patula. Studies have revealed a wide range of biological activities of those two compounds, such as antibacterial, antioxidant, anti-inflammatory, anti-cardiovascular, and anticancer activities.
Oleanolic acid (91) has been detected in C. langsdorffiana, while β-amyrin acetate (92) and Urs-20(30)-en-3-β-ol (93) have been detected in C. patula. These acids have showed anti-inflammatory, antinociceptive, hepatoprotective, anti-allergic, and many other pharmacological properties (Table 5).
3-β-acetoxylup-20(30)-en-29-al (94), 3-β-acetoxylup-20(29)-ene (95), 3-acetylptiloepoxide (96), and Lactifloroside A (97), isolated from C. lactiflora, have not yet been isolated from any other species, but no research has been realized yet to detect their pharmacological properties. While Lactifloroside B (98), detected in C. lactiflora, was isolated from only one other species (Phyteuma japonicum), and it possesses anti-inflammatory activity [].
A list of these triterpenes isolated from this genus along with their known and proven pharmacological activities is presented in Table 5.

Table 5.
Triterpenes isolated from the species of the Campanula genus.
Table 5.
Triterpenes isolated from the species of the Campanula genus.
| No. | Name | Structure | Species | Known Pharmacological Activities |
|---|---|---|---|---|
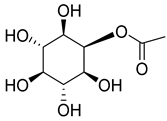
| 87 | β-sitosterol | 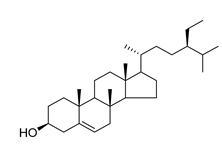 | C. punctata [] | Antimicrobial, anti-inflammatory, anticancer, antifertility, angiogenic, antioxidant, immunomodulatory, antidiabetic, and antinociceptive [] |
| C. cephalotes [] | ||||
| C. dasyantha [] | ||||
| C. istriaca Feer [] | ||||
| 88 | Daucosterol | 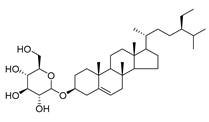 | C. punctata [] | Antioxidant, antidiabetic, hypolipidemic, anti-inflammatory, immunomodulatory, neuroprotective, and anticancer [] |
| C. cephalotes [] | ||||
| C. dasyantha [] | ||||
| C. langsdorffiana [] | ||||
| C. lactiflora [] | ||||
| 89 | Stigmasterol | 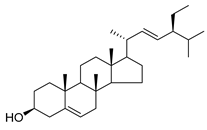 | C. cephalotes [] | Anticancer, anti-osteoarthritis, anti-inflammatory, antidiabetic, immunomodulatory, antiparasitic, antifungal, antibacterial, antioxidant, and neuroprotective [] |
| C. istriaca Feer [] | ||||
| 90 | Ursolic acid | 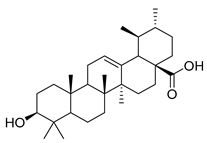 | C. persicifolia [] | Antimicrobial, anti-HIV, anti-HCV, antimalarial, anticancer agent; protective effect on lungs, kidneys, liver, and brain, anti-inflammatory, anabolic effects on skeletal muscles, and anti-osteoporosis [] |
| C. rotundifolia [] | ||||
| C. patula [] | ||||
| C. istriaca Feer [] | ||||
| 91 | Oleanolic acid | 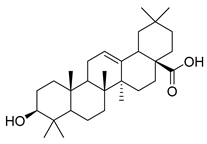 | C. langsdorffiana [] | Antidiabetic, antiviral, anti-HIV, antibacterial, antifungal, anticarcinogenic, anti-inflammatory, hepatoprotective, gastroprotective, hypolipidemic and anti-atherosclerotic activities, and anticancer [] |
| 92 | β-amyrin acetate | 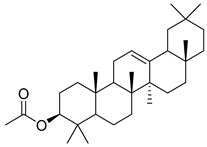 | C. patula [] | Anti-inflammatory [], antinociceptive, hepatoprotective, and anti-allergic [] |
| 93 | Urs-20(30)-en-3-β-ol | 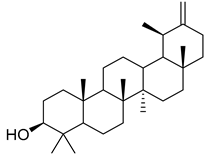 | C. patula [] | Anti-inflammatory, anti-oxidative, anti-carcinogenic, antiviral, and anti-allergic [] |
| 94 | 3-β-acetoxylup-20(30)-en-29-al | 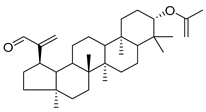 | C. lactiflora [] | - |
| 95 | 3-β-acetoxylup-20(29)-ene | 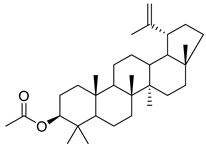 | C. lactiflora [] | - |
| 96 | 3-acetylptiloepoxide | 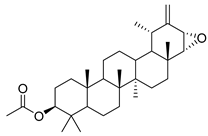 | C. lactiflora [] | - |
| 97 | Lactifloroside A | 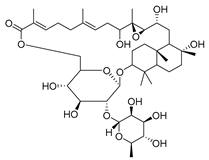 | C. lactiflora [] | - |
| 98 | Lactifloroside B | 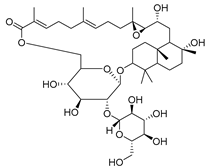 | C. lactiflora [] | Anti-inflammatory [] |
4.1.5. Alkaloids Isolated from the Species of the Campanula Genus
Two alkaloids, (−)-Lobeline (99) and Campedin (100), have been isolated from the genus Campanula and specifically from the species C. medium. Among them, (−)-Lobeline (99) is used in the treatment of chronic bronchitis and asthmatic bronchitis, and it also inhibits dopamine uptake and promotes dopamine release from presynaptic storage vesicles []. Campedin (100) has only been detected in the Campanula genus, and no study has been conducted yet on its biological activities (Table 6).

Table 6.
Alkaloids isolated from the species of the Campanula genus.
Table 6.
Alkaloids isolated from the species of the Campanula genus.
| No. | Name | Structure | Species | Known Pharmacological Activities |
|---|---|---|---|---|
| 99 | (−)-Lobeline | 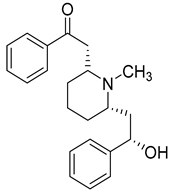 | C. medium [] | Treatment of chronic bronchitis and asthmatic bronchitis, inhibits dopamine uptake, and promotes dopamine release from presynaptic storage vesicles [] |
| 100 | Campedin | 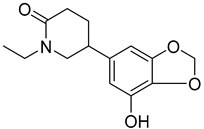 | C. medium [] | - |
4.1.6. Various Compounds Isolated from the Species of the Campanula Genus
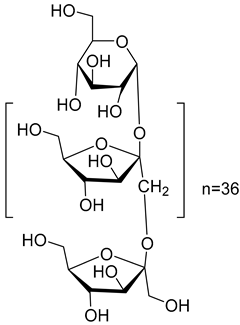
Finally, five various compounds have been characterized from the species of the Campanula genus. Among them, inulin (101) has been isolated from C. rapunculoides L. Studies have shown that this compound has many biological activities, such as prebiotic effect, antioxidant, regulates sugar/lipid metabolism, promotes mineral absorption, reduces the risk of colon cancer, reduces the risk of obesity, regulates the immune system, antiviral, and anti-inflammatory.
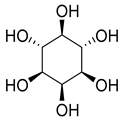
While myo-inositol (102), known as a promotor of female fertility, mediator of cellular energetic metabolism, follicle maturation with menstrual cycle progression, and cellular motility, was detected in five species of the Campanula genus (C persicifolia, C. elatior, C. taurica, C. hohenackeri, and C. cephalotes).
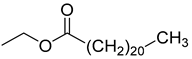
Bis(2-ethylhexyl)adipate (105), isolated from C. lactiflora, has been identified as a toxic and irritant compound. However, this compound is original for the Campanula genus along with two other compounds, ethyl docosanoate (104) and 2-acetyl-myo-inositol (103), which have been respectively isolated from C. lactiflora, C. rapunculoides, C. trachelium, C. pyramidalis, C. persicifolia, C. muralis, C. leutweinii, C. carpatica, and C. cephalotes. Nevertheless, no study has been conducted yet to reveal the pharmacological effects of these two compounds (Table 7).

Table 7.
Various compounds isolated from the species of the Campanula genus.
4.2. Mechanistic Overview of Reported Bioactivities
The Campanula genus is a rich source of structurally diverse bioactive compounds. Understanding their mechanisms of action is essential for further pharmacological development and potential therapeutic applications. Compounds isolated from the Campanula species exhibit a wide range of biological activities, including antioxidant, anti-inflammatory, antimicrobial, and anticancer effects. Elucidating their molecular mechanisms can provide valuable insights for integrating these compounds into modern medicine.
4.2.1. Antioxidant Effects
Numerous compounds isolated from the Campanula species display potent antioxidant activity, including chlorogenic acid (1), methyl caffeate (3), caffeic acid (4), p-coumaric acid (5), ferulic acid (6), syringic acid (7), protocatechuic acid (8), vanillic acid (9), p-hydroxybenzoic acid (10), luteolin (15), luteolin-7-O-glucoside (16), luteolin-7-O-rutinoside (17), luteolin-7-O-primeveroside (cesioside) (19), graveobioside A (luteolin-7-O-glucopyranosido (2 → 1)-O-apio(D or L)furanoside (21), diosmin (26), chrysoeriol-7-O-rutinoside (32), vicinin-2 (34), quercetin (35), isoquercitrin (36), quercetin-3-O-galactoside (hyperoside) (37), quercetin-3-O-rutinoside (rutin) (38), kaempferol (45), kaempferol-3-O-glucoside (astragalin) (46), kaempferol-3-O-galactoside (trifolin) (47), rhamnetin (50), isorhamnetin-3-O-rutinoside (55), isorhamnetin-3-O-robinobioside (57), myricetin (59), myricetin-3-O-glucoside (60), myricetin-3-o-galactoside (61), cyanidin-3-O-glucoside (65), esculetin (75), lobetyol (80), lobetyolin (81), β-sitosterol (87), daucosterol (88), stigmasterol (89), and inulin (101).
The antioxidant activity of these compounds is mediated via multiple mechanisms:
- Scavenging reactive oxygen species (ROS): Donating electrons or hydrogen atoms to neutralize hydroxyl radicals (•OH), superoxide anions (O2•−), and peroxyl radicals (ROO•), thereby preventing oxidative damage to lipids, proteins, and DNA []
- Inhibition of lipid peroxidation: Preventing the formation of malondialdehyde and other toxic lipid peroxidation products, protecting cellular membranes [].
- Modulation of endogenous antioxidant enzymes: Upregulating superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) to enhance intrinsic cellular defense against oxidative stress [].
- Metal ion chelation: Binding iron and copper ions to reduce metal-catalyzed ROS generation [].
- Regulation of gene expression: Upregulating antioxidant enzyme genes and downregulating pro-inflammatory genes, further mitigating oxidative damage [].
4.2.2. Anti-Inflammatory Effect
Compounds with demonstrated anti-inflammatory activity include chlorogenic acid (1), 3-p-coumaroylquinic acid (2), p-coumaric acid (5), ferulic acid (6), syringic acid (7), protocatechuic acid (8), vanillic acid (9), barbatoside A (11), barbatoside B (12), luteolin (15), luteolin-7-O-glucoside (16), luteolin-7-O-primeveroside (Cesioside) (19), diosmin (26), vicinin-2 (34), quercetin (35), kaempferol (45), kaempferol-3-O-glucoside (Astragalin) (46), kaempferol-3-O-rutinoside (48), rhamnetin (50), isorhamnetin-3-O-galactoside (54), myricetin (59), myricetin-3-O-galactoside (61), cyanidin-3-O-glucoside (65), esculetin (75), lobetyol (80), lobetyolin (81), β-sitosterol (87), daucosterol (88), stigmasterol (89), ursolic acid (90), oleanolic acid (91), β-amyrin acetate (92), urs-20(30)-en-3-β-ol (93), lactifloroside B (98), and inulin (101).
The anti-inflammatory mechanisms include the following:
- Inhibition of pro-inflammatory mediators: Suppressing TNF-α, IL-1β, IL-6, IL-8, NO, and PGE2, which play central roles in the initiation and progression of inflammation [].
- NF-κB pathway modulation: Preventing NF-κB activation reduces transcription of pro-inflammatory genes, including iNOS, COX, and cytokines [].
- Inhibition of pro-inflammatory enzymes: Downregulation of cyclooxygenase (COX) and lipoxygenase (LOX) activities decreases mediator production [].
- MAPK pathway regulation: Attenuation of ERK, JNK, and p38 kinase activation diminishes inflammatory signaling [].
4.2.3. Antimicrobial and Antibacterial Effects
Bioactive compounds exhibiting antimicrobial activity include chlorogenic acid (1), 3-p-coumaroylquinic acid (2), methyl caffeate (3), caffeic acid (4), ferulic acid (6), syringic acid (7), protocatechuic acid (8), p-hydroxybenzoic acid (10), luteolin (15), patuloside (22), diosmin (26), quercetin-3-O-rutinoside (Rutin) (38), esculetin (75),β-sitosterol (87), stigmasterol (89), oleanolic acid (91), and ursolic acid (90).
Mechanisms of antimicrobial action include the following:
- Disruption of microbial membranes and efflux systems.
- Inhibition of DNA and RNA synthesis and function.
- Interference with intermediary metabolism.
- Induction of cytoplasmic coagulation.
- Quorum sensing inhibition, which prevents microbial communication and biofilm formation [].
4.2.4. Antiproliferative and Cytotoxic Effects
Compounds showing antiproliferative activities include methyl caffeate (3), p-coumaric acid (5), luteolin-7-O-glucoside (16), kaempferol (45), isorhamnetin-3-O-rutinoside (55), and lobetyol (80).
Compounds with cytotoxic potential include chlorogenic acid (1), methyl caffeate (3), caffeic acid (4), syringic acid (7), protocatechuic acid (8), luteolin (15), luteolin-7-O-glucoside (16), patuloside (22), diosmin (26), chrysoeriol-7-O-rutinoside (32), apigenin-7-O-rutinoside (33), vicinin-2 (34), quercetin (35), isoquercitrin (36), quercetin-3-O-galactoside (hyperoside) (37), kaempferol (45), kaempferol-3-O-glucoside (astragalin) (46), myricetin (59), cyanidin-3-O-glucoside (65), delphinidin-3-glucoside (67), fraxoside (76), lobetyolin (81), β-sitosterol (87), daucosterol (88), stigmasterol (89), ursolic acid (90), oleanolic acid (91), and inulin (101).
Mechanisms of antiproliferative/cytotoxic effects include the following:
- Induction of apoptosis: Activation of caspase-3 and -9, along with modulation of Bcl-2 family proteins, triggers programmed cell death [].
- Cell cycle arrest: Halting progression at G0/G1 or S phases allows DNA repair or promotes cancer cell death [,].
- Inhibition of angiogenesis: Downregulation of VEGF and other pro-angiogenic factors reduces tumor vascularization and growth [].
- Anti-inflammatory synergy: Modulation of NF-κB and MAPK pathways contributes to suppression of cell proliferation and tumor progression [].
5. Discussion
The Campanula genus is a rich source of diverse bioactive secondary metabolites, including flavonoids, phenolic acids, coumarins, triterpenes, and acetylenic compounds, while only two alkaloids have been reported to date. These compounds contribute to a wide range of biological activities, such as antioxidant, anti-inflammatory, antimicrobial, and antiproliferative/cytotoxic effects.
5.1. Critical Evaluation of Bioactivities
Antioxidant activity is one of the most consistently reported effects in the Campanula species, supported by multiple in vitro studies across various species and compounds (e.g., chlorogenic acid, quercetin, and kaempferol). Mechanisms include ROS scavenging, metal ion chelation, inhibition of lipid peroxidation, and modulation of endogenous antioxidant enzymes (SOD, CAT, GPx), providing a robust foundation for further in vivo validation.
Anti-inflammatory effects are also well-supported, particularly for compounds such as chlorogenic acid, luteolin derivatives, quercetin, kaempferol, and saponins (Barbatosides A–B). These compounds modulate NF-κB and MAPK signaling, inhibit pro-inflammatory cytokines (TNF-α, IL-1β, IL-6), and suppress enzymes such as COX and LOX, demonstrating reproducible pharmacological potential.
Antimicrobial and antibacterial activities have been demonstrated for several phenolic acids, flavonoids, and triterpenes (e.g., chlorogenic acid, caffeic acid, oleanolic acid, and ursolic acid). Mechanisms include membrane disruption, inhibition of DNA/RNA synthesis, interference with metabolism, and disruption of quorum sensing. These activities are reasonably well supported in vitro, but in vivo and clinical validation remain limited.
Antiproliferative and cytotoxic effects have been reported for multiple compounds (e.g., methyl caffeate, luteolin-7-O-glucoside, kaempferol, quercetin derivatives, myricetin, lobetyol, and ursolic and oleanolic acids). Mechanisms involve apoptosis induction, cell cycle arrest, and angiogenesis inhibition (VEGF downregulation). While these findings are promising, most evidence comes from in vitro assays, and further mechanistic and in vivo studies are required before drawing definitive conclusions.
5.2. Gaps and Limitations in the Literature
Despite significant advances, several gaps remain:
- Many Campanula species remain uninvestigated, leaving potential bioactive compounds undiscovered.
- Some isolated compounds, particularly Barbatosides C–D and rare triterpenes, lack pharmacological evaluation.
- Mechanistic studies are limited, especially for cardiovascular, cytotoxic, and neuroprotective effects.
- Dose–response relationships, long-term safety, and in vivo validations are often missing, limiting translation to clinical relevance.
5.3. Future Perspectives
Addressing these gaps requires the following:
- Targeted phytochemical screening guided by observed bioactivities.
- In vivo and mechanistic studies to validate preliminary in vitro results.
- Exploration of understudied species for novel bioactive compounds.
- Integration of modern pharmacological and molecular tools to elucidate mechanisms and therapeutic potential.
6. Materials and Methods
All ethnobotanical, phytochemical, and pharmacological information on the Campanula species was collected from online journals, books, and other scholarly sources published between 1951 and 2023. The following electronic databases were searched: Google, Google Scholar, ScienceDirect, PubMed, ResearchGate, and other relevant online collections.
The following keywords were used in various combinations:
- Campanulaceae, Campanula, Campanula species.
- Names of individual bioactive compounds described in this manuscript.
- Terms related to pharmacological activities of these compounds.
Inclusion criteria is as follows:
- Publications providing phytochemical, pharmacological, or ethnobotanical data on the Campanula species or its bioactive compounds.
- Articles published in English or with a reliable English translation.
Exclusion criteria is as follows:
- Studies not directly related to the Campanula species or its chemical constituents.
- Publications lacking sufficient experimental or observational data.
Data handling and synthesis are as follows:
- Duplicate records were manually removed.
- Relevant data were extracted and summarized for narrative synthesis.
- Quality assessment of included studies was not formally performed, as this is a narrative review.
PRISMA-style narrative for transparency is as follows:
- Total records identified through database searching: ~2450
- Records after duplicates removed: ~2180
- Records screened for relevance: ~2180
- Records excluded due to non-relevance: ~1640
- Full-text articles assessed for eligibility: ~540
- Studies included in the narrative synthesis: ~480
7. Conclusions
The present review demonstrates that the Campanula genus is a rich source of diverse bioactive secondary metabolites, including flavonoids, phenolic acids, coumarins, triterpenes, and acetylenic compounds, while only two alkaloids have been reported to date.
Evidence for antioxidant and anti-inflammatory activities is particularly robust, supported by multiple studies across different species and experimental models. By contrast, activities such as cytotoxic, antiproliferative, cardiovascular, and other therapeutic effects remain preliminary or speculative, often based solely on in vitro assays, and require further validation.
Despite progress, a significant number of the Campanula species remain understudied, and many isolated compounds lack detailed pharmacological evaluation or mechanistic understanding. Additional gaps include limited in vivo studies, dose–response data, and long-term safety assessments.
A new chemotaxonomic perspective highlights compound class enrichment in specific species and identifies taxa that may harbor novel bioactive metabolites. This insight can guide targeted phytochemical screening and prioritize species for future research.
In conclusion, a systematic exploration of the phytochemistry, pharmacology, and chemotaxonomy of the Campanula species could reveal novel bioactive molecules, strengthen mechanistic understanding, and support the development of new therapeutic agents, while addressing current knowledge gaps.
Author Contributions
J.A.: Writing—review and editing, Writing—original draft, Validation, Software, Methodology, Investigation, Data curation, Conceptualization, Supervision, Resources, Formal analysis; A.A.R.: Writing—review and editing, Writing—original draft, Validation, Software, Methodology, Investigation, Data curation, Conceptualization, Supervision, Resources, Formal analysis; C.A.-B.: Writing—review and editing, Writing—original draft, Validation, Software, Methodology, Investigation, Data curation, Conceptualization, Supervision, Resources, Formal analysis; M.T.R.: Writing—review and editing, Writing—original draft, Validation, Software, Methodology, Investigation, Data curation, Conceptualization, Supervision, Resources, Formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alhage, J.; Elbitar, H. In Vitro Screening for Antioxidant and Antimicrobial Properties of Three Lebanese Medicinal Plants Crude Extracts. Pharmacogn. Res. 2019, 11, 127–133. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Harvey, A.L. Natural Products in Drug Discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.S.; Mohammadzadeh, V.; Yazdi, M.E.T.; Barani, M.; Rahdar, A.; Kyzas, G.Z. Plant-Based Gums and Mucilages Applications in Pharmacology and Nanomedicine: A Review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.U.; Arshad, M.; Mukhtar, K.; Nabi, B.G.; Goksen, G.; Starowicz, M.; Nawaz, A.; Ahmad, I.; Walayat, N.; Manzoor, M.F. Natural Plant Extracts: An Update about Novel Spraying as an Alternative of Chemical Pesticides to Extend the Postharvest Shelf Life of Fruits and Vegetables. Molecules 2022, 27, 5152. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef]
- Abilkassymova, A.; Turgumbayeva, A.; Sarsenova, L.; Tastambek, K.; Altynbay, N.; Ziyaeva, G.; Blatov, R.; Altynbayeva, G.; Bekesheva, K.; Abdieva, G. Exploring Four Atraphaxis Species: Traditional Medicinal Uses, Phytochemistry, and Pharmacological Activities. Molecules 2024, 29, 910. [Google Scholar] [CrossRef]
- Alhage, J.; Elbitar, H.; Taha, S.; Benvegnu, T. In Vitro Assessment of Antioxidant, Antimicrobial, Cytotoxic, Anti-inflammatory, and Antidiabetic Activities of Campanula retrorsa Crude Extracts. Pharmacogn. Res. 2018, 10, 397–403. [Google Scholar] [CrossRef]
- Barnaulov, O.D.; Limarenko, A.Y.; Teslov, L.S. Antispasmodic Properties of Preparations from Some Species of Campanulaceae. Rastit. Resur. 1983, 19, 20–27. [Google Scholar]
- Barnaulov, O.D.; Manicheva, O.A.; Teslov, L.S. Comparative Evaluation of the Effect of Preparations from Plants of Campanulaceae Juess Family on Gastric Alteration. CABI 1984, 18, 853–857. [Google Scholar]
- Crook, H.C. Campanulas: Their Cultivation and Classification; Country Life: London, UK, 1951. [Google Scholar]
- Assiri, A.M.A.; Ramadan, M.F.; Alshmy, H.; Morsel, J.-T. Bioactive Compounds and Antiradical Potential of Campanula medium Lipids. Chem. Nat. Compd. 2014, 50, 1088–1091. [Google Scholar] [CrossRef]
- Brandt, K.; Ishimaru, K. Campanula (Bellflower) Species: In Vitro Culture, Micropropagation, and the Production of Anthocyanins, Polyacetylenes, and Other Secondary Metabolites. In Medicinal and Aromatic Plants X; Bajaj, Y.P.S., Ed.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1998; Volume 41, pp. 45–66. ISBN 978-3-642-63748-3. [Google Scholar]
- Lammers, T.G. Campanulaceae: Campanulaceae Jussieu, Gen. Pl. 163 (1789), Nom. Cons. In Flowering Plants Eudicots; Kadereit, J.W., Jeffrey, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 26–56. ISBN 978-3-540-31050-1. [Google Scholar]
- Saeki, T.; Nikaido, T. Evaluations of Saponin Properties of HPLC Analysis of Platycodon grandiflorum A. DC. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2003, 123, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.G.; Tan, R.X.; Fuzzati, N.; Li, Q.S.; Wolfender, J.-L.; Hostettmann, K. Wahlenbergioside, a Phenylpropanoid Glucoside from Wahlenbergia marginata. Phytochemistry 1997, 45, 411–415. [Google Scholar] [CrossRef]
- Dzhumyrko, S.F. Flavonoids from Species of the Genus Campanula. Chem. Nat. Compd. 1985, 21, 531–532. [Google Scholar] [CrossRef]
- Asano, N.; Nishida, M.; Miyauchi, M.; Ikeda, K.; Yamamoto, M.; Kizu, H.; Kameda, Y.; Watson, A.A.; Nash, R.J.; Fleet, G.W. Polyhydroxylated Pyrrolidine and Piperidine Alkaloids from Adenophora triphylla Var. Japonica (Campanulaceae). Phytochemistry 2000, 53, 379–382. [Google Scholar] [CrossRef]
- Hou, Z.-F.; Tu, Y.-Q.; Li, Y. Two New Steroids from Adenophora stenanthina Subsp. Xifengensis. Die Pharm. 2002, 57, 209–211. [Google Scholar]
- Tanaka, N.; Matsuura, E.; Terahara, N.; Ishimaru, K. Secondary Metabolites in Transformed Root Cultures of Campanula glomerata. J. Plant Physiol. 1999, 155, 251–254. [Google Scholar] [CrossRef]
- Fedorov, A.A.; Kovanda, M. Plantaginaceae to Compositae (and Rubiaceae). Flora Eur. 1976, 4, 74–93. [Google Scholar]
- Liveri, E.; Crowl, A.A.; Cellinese, N. Past, Present, and Future of Campanula (Campanulaceae) Systematics–A Review. Bot. Chron. 2019, 22, 209–222. [Google Scholar]
- Okinaka, Y.; Shimada, Y.; Nakano-Shimada, R.; Ohbayashi, M.; Kiyokawa, S.; Kikuchi, Y. Selective Accumulation of Delphinidin Derivatives in Tobacco Using a Putative Flavonoid 3′, 5′-Hydroxylase cDNA from Campanula medium. Biosci. Biotechnol. Biochem. 2003, 67, 161–165. [Google Scholar] [CrossRef]
- Morton, J.F. Major Medicinal Plants: Botany, Culture, and Uses; Charles C Thomas Publisher: Springfield, IL, USA, 1977. [Google Scholar]
- Rameau, J.-C.; Mansion, D.; Dumé, G. French Forest Flora; Institut pour le Développement Forestier: Paris, France, 1989. [Google Scholar]
- Jaradat, N.A.; Abualhasan, M. Comparison in Vitro of Antioxidant Activity between Fifteen Campanula Species (Bellflower) from Palestinian flora. Pharmacogn. J. 2015, 7, 276–279. [Google Scholar] [CrossRef]
- Yayli, N.; Yildirim, N.; Usta, A.; Özkurt, S.; Akgün, V. Chemical Constituents of Campanula lactiflora. Turk. J. Chem. 2003, 27, 749–756. [Google Scholar]
- Teslov, L.S.; Koretskaya, L.N.; Tsareva, G.I. Phenolic Compounds of Campanula rotundifolia and C. persicifolia. Khimiya Prir. Soedin. 1983, 19, 387. [Google Scholar] [CrossRef]
- Dzhumyrko, S.F. Coumarins of Species of the genus Campanula. Chem. Nat. Compd. 1984, 20, 616–617. [Google Scholar] [CrossRef]
- Cuendet, M.; Potterat, O.; Hostettmann, K. Flavonoids and Phenylpropanoid Derivatives from Campanula barbata. Phytochemistry 2001, 56, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Xiang, L. Pharmacological Action and Potential Targets of Chlorogenic Acid. In Advances in Pharmacology; Du, G., Ed.; Pharmacological Advances in Natural Product Drug Discovery; Academic Press: Cambridge, MA, USA, 2020; Volume 87, pp. 71–88. [Google Scholar]
- Teslov, L.S.; Geras’kina, S.S. Phytochemical and Pharmacological Study of Preparations from Campanula cephalates and Campanula glomerata; Tomsk State University: Tomsk, Russia, 1975. [Google Scholar]
- Teslov, L.S. Phenolic Compounds of Campanula patula. In Khimiya Prirodnykh Soedinenii; 1981; pp. 719–720. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19810390903 (accessed on 14 November 2025).
- Teslov, L.S. Phenolic Compounds of the Above-Ground Part of Campanula rapunculoides L. Rastit. Resur. 1996, 32, 87–92. [Google Scholar]
- Teslov, L.S.; Podushkin, V.Y. Flavonoids of Campanula maleevii. Chem. Nat. Compd. 1988, 24, 255. [Google Scholar] [CrossRef]
- Bouymajane, A.; Filali, F.R.; Moujane, S.; Majdoub, Y.O.E.; Otzen, P.; Channaoui, S.; Ed-Dra, A.; Bouddine, T.; Sellam, K.; Boughrous, A.A.; et al. Phenolic Compound, Antioxidant, Antibacterial, and In Silico Studies of Extracts from the Aerial Parts of Lactuca saligna L. Molecules 2024, 29, 596. Molecules 2024, 29, 596. [Google Scholar] [CrossRef]
- Ghouti, D.; Rached, W.; Abdallah, M.; Pires, T.C.S.P.; Calhelha, R.C.; Alves, M.J.; Abderrahmane, L.H.; Barros, L.; Ferreira, I.C.F.R. Phenolic Profile and in Vitro Bioactive Potential of Saharan Juniperus phoenicea L.; Cotula cinerea (Del) Growing in Algeria. Food Funct. 2018, 9, 4664–4672. [Google Scholar] [CrossRef]
- Balachandran, C.; Emi, N.; Arun, Y.; Yamamoto, Y.; Ahilan, B.; Sangeetha, B.; Duraipandiyan, V.; Inaguma, Y.; Okamoto, A.; Ignacimuthu, S.; et al. In Vitro Anticancer Activity of Methyl Caffeate Isolated from Solanum Torvum Swartz. Fruit. Chem. -Biol. Interact. 2015, 242, 81–90. [Google Scholar] [CrossRef]
- Balachandran, C.; Duraipandiyan, V.; Al-Dhabi, N.A.; Balakrishna, K.; Kalia, N.P.; Rajput, V.S.; Khan, I.A.; Ignacimuthu, S. Antimicrobial and Antimycobacterial Activities of Methyl Caffeate Isolated from Solanum torvum Swartz. Fruit. Indian. J. Microbiol. 2012, 52, 676–681. [Google Scholar] [CrossRef]
- Pyo, M.K.; Lee, Y.; Yun-Choi, H.S. Anti-Platelet Effect of the Constituents Isolated from the Barks and Fruits of Magnolia obovata. Arch. Pharm. Res. 2002, 25, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids Protect Neuronal Cells from Oxidative Stress by Three Distinct Mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant Activity of Caffeic Acid (3,4-Dihydroxycinnamic Acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Campos, F.M.; Couto, J.A.; Hogg, T.A. Influence of Phenolic Acids on Growth and Inactivation of Oenococcus oeni and Lactobacillus hilgardii. J. Appl. Microbiol. 2003, 94, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Michaluart, P.; Masferrer, J.L.; Carothers, A.M.; Subbaramaiah, K.; Zweifel, B.S.; Koboldt, C.; Mestre, J.R.; Grunberger, D.; Sacks, P.G.; Tanabe, T.; et al. Inhibitory Effects of Caffeic Acid Phenethyl Ester on the Activity and Expression of Cyclooxygenase-2 in Human Oral Epithelial Cells and in a Rat Model of Inflammation1. Cancer Res. 1999, 59, 2347–2352. [Google Scholar]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic Acid: A Review of Its Potential Use in Medications and Cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Zang, L.-Y.; Cosma, G.; Gardner, H.; Shi, X.; Castranova, V.; Vallyathan, V. Effect of Antioxidant Protection by P-Coumaric Acid on Low-Density Lipoprotein Cholesterol Oxidation. Am. J. Physiol. -Cell Physiol. 2000, 279, C954–C960. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and Anti-Inflammatory Effect of p-Coumaric Acid, a Common Dietary Polyphenol on Experimental Inflammation in Rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Janicke, B.; Hegardt, C.; Krogh, M.; Önning, G.; Åkesson, B.; Cirenajwis, H.M.; Oredsson, S.M. The Antiproliferative Effect of Dietary Fiber Phenolic Compounds Ferulic Acid and p-Coumaric Acid on the Cell Cycle of Caco-2 Cells. Nutr. Cancer 2011, 63, 611–622. [Google Scholar] [CrossRef]
- Boo, Y.C. P-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on Its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef]
- Choi, R.; Kim, B.H.; Naowaboot, J.; Lee, M.Y.; Hyun, M.R.; Cho, E.J.; Lee, E.S.; Lee, E.Y.; Yang, Y.C.; Chung, C.H. Effects of Ferulic Acid on Diabetic Nephropathy in a Rat Model of Type 2 Diabetes. Exp. Mol. Med. 2011, 43, 676–683. [Google Scholar] [CrossRef]
- Jayamani, J.; Naisini, A.; Madhan, B.; Shanmugam, G. Ferulic Acid, a Natural Phenolic Compound, as a Potential Inhibitor for Collagen Fibril Formation and Its Propagation. Int. J. Biol. Macromol. 2018, 113, 277–284. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, Z.; Li, J.; Shi, Y.; Jin, N.; Zou, W.; Gao, Q.; Wang, W.; Liu, F. Ferulic Acid Inhibits Bovine Endometrial Epithelial Cells against LPS-Induced Inflammation via Suppressing NK-κB and MAPK Pathway. Res. Vet. Sci. 2019, 126, 164–169. [Google Scholar] [CrossRef]
- Hao, P.-Q.; Zhang, X.-Y.; Guo, H.; Yang, Y.; An, S.; Liu, Y.; Guo, X.-X.; Xu, T.-R.; Hao, Q. Research progress on pathophysiological function of SOS1 protein. Sheng Li Xue Bao 2018, 70, 565–570. [Google Scholar] [PubMed]
- Choi, J.-H.; Park, J.-K.; Kim, K.-M.; Lee, H.-J.; Kim, S. In Vitro and in Vivo Antithrombotic and Cytotoxicity Effects of Ferulic Acid. J. Biochem. Mol. Toxicol. 2018, 32, e22004. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Liu, L.; Liu, Y.; Wang, X. Ferulic Acid Inactivates Shigella flexneri through Cell Membrane Destruction, Biofilm Retardation, and Altered Gene Expression. J. Agric. Food Chem. 2020, 68, 7121–7131. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.; Guo, S.; Luan, F.; Liu, R.; Zeng, N. Ferulic Acid: A Review of Its Pharmacology, Pharmacokinetics and Derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic Acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tiwari, N.; Vyas, M.; Khurana, N.; Muthuraman, A.; Utreja, P. An Overview of Therapeutic Effects of Vanillic Acid. Plant Arch. 2020, 20, 3053–3059. [Google Scholar]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A Comprehensive Review on Biological Activities of P-Hydroxy Benzoic Acid and Its Derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Terahara, N.; Toki, K.; Saito, N.; Honda, T.; Isono, T.; Furumoto, H.; Kontani, Y. Structures of Campanin and Rubrocampanin, Two Novel Acylated Anthocyanins with p-Hydroxybenzoic Acid from the Flowers of Bellflower, Campanula medium L. J. Chem. Soc. Perkin Trans. 1 1990, 1, 3327–3332. [Google Scholar] [CrossRef]
- Cordell, G.A.; Lyon, R.L.; Fong, H.H.; Benoit, P.S.; Farnsworth, N.R. Biological and Phytochemical Investigations of Dianthus barbatus Cv. “China Doll” (Caryophyllaceae). Lloydia 1977, 40, 361–363. [Google Scholar]
- Teslov, L.S.; Teslov, S.V. Cynaroside and Luteolin from Campanula persicifolia and C. Rotundifolia. Chem. Nat. Compd. 1972, 8, 117. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Dzhumyrko, S. Flavonoids of campanula genus plants. Khimiya Prir. Soedin. 1973, 273–274. [Google Scholar] [CrossRef]
- Nung, V.N.; Bezanger-Beauquesne, L.; Torck, M. Characterization of Flavonoids. Plant Med. Phytother. 1971, 5, 177–187. [Google Scholar]
- Dzhumyrko, S.F.; Oganesyan, E.T.; Shinkarenko, A.L. Luteolin 7-Glucoside from Campanula lactiflora. Chem. Nat. Compd. 1969, 5, 365–366. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef]
- Ho, H.; Chen, P.; Lo, Y.; Lin, C.; Chuang, Y.; Hsieh, M.; Chen, M. Luteolin-7-O-glucoside Inhibits Cell Proliferation and Modulates Apoptosis through the AKT Signaling Pathway in Human Nasopharyngeal Carcinoma. Environ. Toxicol. 2021, 36, 2013–2024. [Google Scholar] [CrossRef]
- Justesen, H.; Andersen, A.S.; Brandt, K. Accumulation of Anthocyanins and Flavones during Bud and Flower Development in Campanula isophylla Moretti. Ann. Bot. 1997, 79, 355–360. [Google Scholar] [CrossRef]
- Teslov, L.S.; Koretskaya, L.N. Flavonoids of Campanula persicifolia. I. Chem. Nat. Compd. 1983, 19, 749–750. [Google Scholar] [CrossRef]
- Kim, N.M.; Kim, J.; Chung, H.Y.; Choi, J.S. Isolation of Luteolin 7-O-Rutinoside and Esculetin with Potential Antioxidant Activity from the Aerial Parts of Artemisia montana. Arch. Pharm. Res. 2000, 23, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Orhan, F.; Barış, Ö.; Yanmış, D.; Bal, T.; Güvenalp, Z.; Güllüce, M. Isolation of Some Luteolin Derivatives from Mentha longifolia (L.) Hudson Subsp. Longifolia and determination of their genotoxic potencies. Food Chem. 2012, 135, 764–769. [Google Scholar] [PubMed]
- Teslov, L.S. Flavonoids of Campanula rotundifolia. Khimiya Prir. Soedin. 1980, 414–415. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19810303246 (accessed on 14 November 2025).
- Teslov, L.S. Campanula rotundiflora Flavonoids. II. Khim. Prir. Soedin. 1981, 4, 520–521. [Google Scholar]
- Odontuya, G.; Hoult, J.R.S.; Houghton, P.J. Structure-Activity Relationship for Antiinflammatory Effect of Luteolin and Its Derived Glycosides. Phytother. Res. 2005, 19, 782–786. [Google Scholar] [CrossRef]
- Teslov, L.S. Flavonoids of Campanula patula. Chem. Nat. Compd. 1977, 13, 106–107. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, J.; Tu, Q.; Yan, F.; Hu, Z.; Zhang, Y.; Song, C. Antioxidant Capacities and Enzymatic Inhibitory Effects of Different Solvent Fractions and Major Flavones from Celery Seeds Produced in Different Geographic Areas in China. Antioxidants 2022, 11, 1542. [Google Scholar] [CrossRef]
- Teslov, L.S.; Blinova, K.F. A New Flavone Glycoside from Campanula patula. Chem. Nat. Compd. 1974, 10, 93. [Google Scholar] [CrossRef]
- Oyinloye, B.E.; Agbebi, E.A.; Agboola, O.E.; Ubah, C.S.; Owolabi, O.V.; Aruleba, R.T.; Onikanni, S.A.; Ejeje, J.N.; Ajiboye, B.O.; Omotuyi, O.I. Skin Anti-Aging Potentials of Phytochemicals from Peperomia pellucida against Selected Metalloproteinase Targets: An In Silico Approach. Cosmetics 2023, 10, 151. [Google Scholar] [CrossRef]
- Kartika, I.; Insanu, M.; Safitri, D.; Putri, C.A.; Adnyana, I.K. New Update: Traditional Uses, Phytochemical, Pharmacological and Toxicity Review of Peperomia pellucida (L.) Kunth. Pharmacologyonline 2016, 2, 30–43. [Google Scholar]
- Teslov, L.S.; Zapesochnaya, G.G. Acetylcynaroside, a New Acylated Flavonoid from Campanula patula. Chem. Nat. Compd. 1976, 12, 228–229. [Google Scholar] [CrossRef]
- Belenovskaya, L.M.; Markova, L.P.; Kapranova, G.I. Structure of Campanoside. Chem. Nat. Compd. 1980, 6, 835–836. [Google Scholar]
- Teslov, L.S.; Zapesochnaya, G.G. Diosmin from Campanula patula. Chem. Nat. Compd. 1978, 14, 225. [Google Scholar] [CrossRef]
- Huwait, E.; Mobashir, M. Potential and Therapeutic Roles of Diosmin in Human Diseases. Biomedicines 2022, 10, 1076. [Google Scholar] [CrossRef]
- Teslov, L.S. Flavone Glycosides from Campanula persicifolia. III. Chem. Nat. Compd. 1986, 22, 730. [Google Scholar] [CrossRef]
- Plouvier, V. Coumarin heterosides (fraxoside and isofraxoside) and flavones in some Campanulaceae and Caprifoliaceae. Comptes Rendus Hebd. Des Seances De L Acad. Des Sci. Ser. D 1970, 270, 1526. [Google Scholar]
- Teslov, L.S. Flavonoids and Other Natural Compounds from Campanula persicifolia. IV. Chem. Nat. Compd. 1988, 24, 507–508. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, P.-G.; Liang, W.-Q.; Hu, Y.-J.; Xu, P.; Zhou, J.; Pu, J.-B.; Zhang, H.-J. Luteolin-4′-O-Glucoside and Its Aglycone, Two Major Flavones of Gnaphalium affine D. Don, Resist Hyperuricemia and Acute Gouty Arthritis Activity in Animal Models. Phytomedicine 2018, 41, 54–61. [Google Scholar] [CrossRef]
- Teslov, L.S. Luteolin Triosides from Campanula persicifolia. II. Chem. Nat. Compd. 1984, 20, 749–750. [Google Scholar] [CrossRef]
- Aboulaghras, S.; Sahib, N.; Bakrim, S.; Benali, T.; Charfi, S.; Guaouguaou, F.-E.; Omari, N.E.; Gallo, M.; Montesano, D.; Zengin, G. Health Benefits and Pharmacological Aspects of Chrysoeriol. Pharmaceuticals 2022, 15, 973. [Google Scholar] [CrossRef] [PubMed]
- Ngaffo, C.M.; Tchangna, R.S.; Mbaveng, A.T.; Kamga, J.; Harvey, F.M.; Ngadjui, B.T.; Bochet, C.G.; Kuete, V. Botanicals from the Leaves of Acacia sieberiana Had Better Cytotoxic Effects than Isolated Phytochemicals towards MDR Cancer Cells Lines. Heliyon 2020, 6, e05412. [Google Scholar] [CrossRef] [PubMed]
- Gulluce, M.; Orhan, F.; Adiguzel, A.; Bal, T.; Guvenalp, Z.; Dermirezer, L.O. Determination of Antimutagenic Properties of Apigenin-7-O-Rutinoside, a Flavonoid Isolated from Mentha longifolia (L.) Huds. Ssp. Longifolia with Yeast DEL Assay. Toxicol. Ind. Health 2013, 29, 534–540. [Google Scholar] [PubMed]
- Liu, L.-Z.; Fang, J.; Zhou, Q.; Hu, X.; Shi, X.; Jiang, B.-H. Apigenin Inhibits Expression of Vascular Endothelial Growth Factor and Angiogenesis in Human Lung Cancer Cells: Implication of Chemoprevention of Lung Cancer. Mol. Pharmacol. 2005, 68, 635–643. [Google Scholar] [CrossRef]
- Islam, M.N.; Ishita, I.J.; Jung, H.A.; Choi, J.S. Vicenin 2 Isolated from Artemisia capillaris Exhibited Potent Anti-Glycation Properties. Food Chem. Toxicol. 2014, 69, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Y.; Yao, L.; Gu, W.; Zhao, S.; Shen, Z.; Lin, Z.; Liu, W.; Yan, T. Pharmacological Activity of Quercetin: An Updated Review. Evid. -Based Complement. Altern. Med. 2022, 2022, 3997190. [Google Scholar] [CrossRef]
- Teslov, L.S. Flavonol glycosides of Campanula persicifolia. Khimiya Prir. Soedin. 1990, 271–272. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19910302072 (accessed on 14 November 2025).
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, Toxicology, and Metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Teslov, L.S. Flavonol Glycosides of the Above-Ground Part of Campanula bononiensis L. Rastit. Resur. 1997, 33, 68–72. [Google Scholar]
- Hashiba, K.; Iwashina, T.; Matsumoto, S. Variation in the Quality and Quantity of Flavonoids in the Leaves of Coastal and Inland Campanula punctata. Biochem. Syst. Ecol. 2006, 34, 854–861. [Google Scholar] [CrossRef]
- Teslov, L.S.; Blinova, K.F. Flavonol Glycosides of Campanula cephalotes. Chem. Nat. Compd. 1973, 9, 411. [Google Scholar] [CrossRef]
- Dumlu, M.U.; Gurkan, E.; Tuzlaci, E. Chemical Composition and Antioxidant Activity of Campanula alliariifolia. Nat. Prod. Res. 2008, 22, 477–482. [Google Scholar] [CrossRef]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A Review of Its Structure, Synthesis, Pharmacology, Pharmacokinetics and Toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef]
- Wu, L.; Yang, X.; Huang, Z.; Liu, H.; Wu, G. In Vivo and in Vitro Antiviral Activity of Hyperoside Extracted from Abelmoschus manihot (L) Medik. Acta Pharmacol. Sin. 2007, 28, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Dzhumyrko, S.F.; Shinkarenko, A.L. Hyperoside from Campanula Biebersteiniana. Chem. Nat. Compd. 1972, 8, 118. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Teslov, L.S.; Blinova, K.F. Flavonolic Biosides of Campanula glomerata from the Altai Mountains. Rastit. Resur. 1974, 10, 371–375. [Google Scholar]
- Dzhumyrko, S.F. Rutin from Campanula oblongifolia. Chem. Nat. Compd. 1970, 6, 655. [Google Scholar] [CrossRef]
- Silva dos Santos, J.; Goncalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Recent Studies on Kaempferol and Its Biological and Pharmacological Activities. EXCLI J. 2020, 19, 627–634. [Google Scholar] [PubMed]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A Bioactive Phytochemical with Potential Therapeutic Activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef] [PubMed]
- Dzhumyrko, S.F. Glycosides of Kaempferol from Campanula hypopolia. Chem. Nat. Compd. 1974, 10, 262. [Google Scholar] [CrossRef]
- Sanz, M.J.; Ferrandiz, M.L.; Cejudo, M.; Terencio, M.C.; Gil, B.; Bustos, G.; Ubeda, A.; Gunasegaran, R.; Alcaraz, M.J. Influence of a Series of Natural Flavonoids on Free Radical Generating Systems and Oxidative Stress. Xenobiotica 1994, 24, 689–699. [Google Scholar] [CrossRef]
- Dehaghani, Z.A.; Asghari, G.; Dinani, M.S. Isolation and Identification of Nicotiflorin and Narcissin from the Aerial Parts of Peucedanum aucheri Boiss. J. Agric. Sci. Technol. A 2017, 7, 45–51. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Huleihel, M.; Zaccai, M.; Ben-Shabat, S. Potent Antiviral Flavone Glycosides from Ficus benjamina Leaves. Fitoterapia 2012, 83, 362–367. [Google Scholar] [CrossRef]
- Novo Belchor, M.; Hessel Gaeta, H.; Fabri Bittencourt Rodrigues, C.; Ramos da Cruz Costa, C.; de Oliveira Toyama, D.; Domingues Passero, L.F.; Dalastra Laurenti, M.; Hikari Toyama, M. Evaluation of Rhamnetin as an Inhibitor of the Pharmacological Effect of Secretory Phospholipase A2. Molecules 2017, 22, 1441. [Google Scholar] [CrossRef]
- Park, E.-S.; Kang, J.C.; Jang, Y.C.; Park, J.S.; Jang, S.Y.; Kim, D.-E.; Kim, B.; Shin, H.-S. Cardioprotective Effects of Rhamnetin in H9c2 Cardiomyoblast Cells under H2O2-Induced Apoptosis. J. Ethnopharmacol. 2014, 153, 552–560. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Li, W. Rhamnetin Ameliorates Macrophage-Mediated Inflammation and pro-Atherosclerosis Pathways in Apolipoprotein E-Deficient Mice. J. Physiol. Pharmacol. 2021, 72, 10–26402. [Google Scholar] [CrossRef]
- Teslov, L.S.; Blinova, K.F. Flavonoids from Campanula cephalotes. Chem. Nat. Compd. 1972, 8, 387. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.Y.; Lee, S.; Kang, K.S. In Vitro Studies to Assess the α-Glucosidase Inhibitory Activity and Insulin Secretion Effect of Isorhamnetin 3-o-Glucoside and Quercetin 3-o-Glucoside Isolated from Salicornia herbacea. Processes 2021, 9, 483. [Google Scholar] [CrossRef]
- Ku, S.-K.; Kim, T.H.; Lee, S.; Kim, S.M.; Bae, J.-S. Antithrombotic and Profibrinolytic Activities of Isorhamnetin-3-O-Galactoside and Hyperoside. Food Chem. Toxicol. 2013, 53, 197–204. [Google Scholar] [CrossRef]
- Kim, T.H.; Ku, S.; Bae, J. Anti-inflammatory Activities of Isorhamnetin-3-O-galactoside against HMGB1-induced Inflammatory Responses in Both HUVECs and CLP-induced Septic Mice. J. Cell. Biochem. 2013, 114, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.H.; Chevalot, I.; Harscoat-Schiavo, C.; Paris, C.; Fick, M.; Humeau, C. Biological Activities of Flavonoids from Nitraria retusa (Forssk.) Asch.; their acylated derivatives. Food Chem. 2011, 124, 486–494. [Google Scholar] [CrossRef]
- Boubaker, J.; Sghaier, M.B.; Skandrani, I.; Ghedira, K.; Chekir-Ghedira, L. Isorhamnetin 3-O-Robinobioside from Nitraria retusa Leaves Enhance Antioxidant and Antigenotoxic Activity in Human Chronic Myelogenous Leukemia Cell Line K562. BMC Complement. Altern. Med. 2012, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Teslov, L.S.; Blinova, K.F. Phenolic Compounds of Campanula glomerata. Chem. Nat. Compd. 1974, 10, 392. [Google Scholar] [CrossRef]
- Teslov, L.S. Comparative Study of the Flavonoid Composition of Some Species of the Genus Campanula L. of the Series Rapunculoideae charadze from the Section Campanula. Rastit. Resur. 2000, 36, 3–17. [Google Scholar]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A Review of the Most Recent Research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Wu, C.; He, L.; Zhang, Y.; You, C.; Li, X.; Jiang, P.; Wang, F. Separation of Flavonoids with Significant Biological Activity from Acacia mearnsii Leaves. RSC Adv. 2023, 13, 9119–9127. [Google Scholar] [CrossRef]
- De Oliveira Azevedo, A.; Campos, J.J.; De Souza, G.G.; De Carvalho Veloso, C.; Duarte, I.D.G.; Braga, F.C.; De Castro Perez, A. Antinociceptive and Anti-Inflammatory Effects of Myricetin 3-O-β-Galactoside Isolated from Davilla elliptica: Involvement of the Nitrergic System. J. Nat. Med. 2015, 69, 487–493. [Google Scholar] [CrossRef]
- Hayder, N.; Bouhlel, I.; Skandrani, I.; Kadri, M.; Steiman, R.; Guiraud, P.; Mariotte, A.-M.; Ghedira, K.; Dijoux-Franca, M.-G.; Chekir-Ghedira, L. In Vitro Antioxidant and Antigenotoxic Potentials of Myricetin-3-o-Galactoside and Myricetin-3-o-Rhamnoside from Myrtus communis: Modulation of Expression of Genes Involved in Cell Defence System Using cDNA Microarray. Toxicol. Vitr. 2008, 22, 567–581. [Google Scholar] [CrossRef]
- Strugala, P.; Loi, S.; Bażanów, B.; Kuropka, P.; Kucharska, A.Z.; Wloch, A.; Gabrielska, J. A Comprehensive Study on the Biological Activity of Elderberry Extract and Cyanidin 3-O-Glucoside and Their Interactions with Membranes and Human Serum Albumin. Molecules 2018, 23, 2566. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-Glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed]
- Akkarachiyasit, S.; Yibchok-Anun, S.; Wacharasindhu, S.; Adisakwattana, S. In Vitro Inhibitory Effects of Cyandin-3-Rutinoside on Pancreatic α-Amylase and Its Combined Effect with Acarbose. Molecules 2011, 16, 2075–2083. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Z.; Reheman, A.; Jin, J.W.; Li, C.; Wang, Y.; Andrews, M.C.; Chen, P.; Zhu, G.; Ling, W. Plant Food Delphinidin-3-Glucoside Significantly Inhibits Platelet Activation and Thrombosis: Novel Protective Roles against Cardiovascular Diseases. PLoS ONE 2012, 7, e37323. [Google Scholar] [CrossRef]
- Sharma, A.; Choi, H.-K.; Kim, Y.-K.; Lee, H.-J. Delphinidin and Its Glycosides’ War on Cancer: Preclinical Perspectives. Int. J. Mol. Sci. 2021, 22, 11500. [Google Scholar] [CrossRef]
- Brandt, K.; Kondo, T.; Aoki, H.; Goto, T. Structure and Biosynthesis of Anthocyanins in Flowers of Campanula. Phytochemistry 1993, 33, 209–212. [Google Scholar] [CrossRef]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.F. A Review of Coumarin Derivatives in Pharmacotherapy of Breast Cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Cai, B. Pharmacological Activities of Esculin and Esculetin: A Review. Medicine 2023, 102, e35306. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Rahman, E.; Nain, Z.; Billah, M.; Karmakar, S.; Mohanto, S.C.; Paul, G.K.; Amin, A.; Acharjee, U.K.; Saleh, A. Computational Discovery of Plant-Based Inhibitors against Human Carbonic Anhydrase IX and Molecular Dynamics Simulation. J. Biomol. Struct. Dyn. 2021, 39, 2754–2770. [Google Scholar] [CrossRef]
- Ti, M.G.; Lupuleasa, D.; Mogo, G.D. Histo-Anatomical Researches on the Leaf of Fraxinus excelsior L. Species. Curr. Health Sci. J. 2010, 36, 232–234. [Google Scholar]
- Pan, L.; Lezama-Davila, C.M.; Isaac-Marquez, A.P.; Fuchs, J.R.; Satoskar, A.R.; Kinghorn, A.D. Compounds with Antileishmanial Activity Isolated from the Stems of Pentalinon andrieuxii. Planta Med. 2012, 78, PI239. [Google Scholar] [CrossRef]
- Seong, Z.K.; Won, Y.M.; Kim, J.L.; Song, H.H.; Kim, D.Y.; Oh, S.R.; Cho, H.W.; Cho, J.H.; Lee, H.K. Two New Phenylacylphenol Types from the Twigs of Stewartia pseudocamellia. Planta Med. 2014, 80, PD38. [Google Scholar] [CrossRef]
- Kang, Y.-X.; Zhang, H.-C.; Wang, P.; Liu, J.-J.; Ma, Y.-M. Chemical Constituents of the Leaves from Xanthoceras sorbifolia. Chem. Nat. Compd. 2012, 48, 875–876. [Google Scholar] [CrossRef]
- Shen, J.; Lu, X.; Du, W.; Zhou, J.; Qiu, H.; Chen, J.; Shen, X.; Zhong, M. Lobetyol Activate MAPK Pathways Associated with G1/S Cell Cycle Arrest and Apoptosis in MKN45 Cells in Vitro and in Vivo. Biomed. Pharmacother. 2016, 81, 120–127. [Google Scholar] [CrossRef]
- Bailly, C. Anticancer Properties of Lobetyolin, an Essential Component of Radix codonopsis (Dangshen). Nat. Prod. Bioprospect. 2021, 11, 143–153. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Huang, Y.; Zhao, C.; Cheung, H.-Y. Chemical Profiling of Lobelia chinensis with High-Performance Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry (HPLC/Q-TOF MS) Reveals Absence of Lobeline in the Herb. Molecules 2018, 23, 3258. [Google Scholar] [CrossRef]
- Bentley, R.K.; Jenkins, J.K.; Jones, E.R.H.; Thaller, V. Natural Acetylenes. Part XXIX. Polyacetylenes from the Campanulaceae Plant Family. Tetrahydropyranyl Polyacetylenic Alcohols from the Clustered Bellflower (Campanula glomerata L.). J. Chem. Soc. C 1969, 830–832. [Google Scholar] [CrossRef]
- Ishimaru, K.; Ando, M.; Yamakawa, T. Polyacetylene Production in Transformed Root Cultures of Campanula lactiflora. Nat. Med. 1998, 52, 448–451. [Google Scholar]
- Tada, H.; Nakashima, T.; Kunitake, H.; Mori, K.; Tanaka, M.; Ishimaru, K. Polyacetylenes in Hairy Root Cultures of Campanula medium L. J. Plant Physiol. 1996, 147, 617–619. [Google Scholar] [CrossRef]
- Badanyan, S.O.; Bentley, R.K.; Jenkins, J.K.; Jones, E.R.; Thaller, V. Natural Acetylenes. Part XXXVII. Polyacetylenes from the Campanulaceae Plant Family. Tetrahydropyranyl and Open Chain C 14 Polyacetylenic Alcohols from Campanula pyramidalis L.; Campanula medium L. J. Chem. Soc. Perkin Trans. 1 1973, 145–147. [Google Scholar] [CrossRef]
- Kim, C.S.; Kwon, O.W.; Kim, S.Y.; Kim, K.H.; Lee, K.R. A New Cyclic Triterpene Saponin from Phyteuma japonicum. Heterocycles 2014, 89, 1913–1922. [Google Scholar]
- Gorovoi, P.G.; Ponomarchuk, G.I.; Strigina, L.I. A Chemotaxonomic Study on Russian Far-Eastern Campanulaceae. Phytochemistry 1971, 10, 2419–2423. [Google Scholar] [CrossRef]
- Ambavade, S.D.; Misar, A.V.; Ambavade, P.D. Pharmacological, Nutritional, and Analytical Aspects of β-Sitosterol: A Review. Orient. Pharm. Exp. Med. 2014, 14, 193–211. [Google Scholar] [CrossRef]
- Stanic, G.; Petricic, J.; Sugar, I.; Todoric, A.; Blazevic, N. Triterpenic Acid and Sterols from Campanula istriaca Feer. Acta Pharm. Jugosl. 1989, 39, 81–83. [Google Scholar]
- El Omari, N.; Jaouadi, I.; Lahyaoui, M.; Benali, T.; Taha, D.; Bakrim, S.; El Menyiy, N.; El Kamari, F.; Zengin, G.; Bangar, S.P. Natural Sources, Pharmacological Properties, and Health Benefits of Daucosterol: Versatility of Actions. Appl. Sci. 2022, 12, 5779. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Teslov, L.S. Triterpene Compounds of Campanula patula. Chem. Nat. Compd. 1979, 15, 510. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Okoye, N.N.; Ajaghaku, D.L.; Okeke, H.N.; Ilodigwe, E.E.; Nworu, C.S.; Okoye, F.B.C. Beta-Amyrin and Alpha-Amyrin Acetate Isolated from the Stem Bark of Alstonia boonei Display Profound Anti-Inflammatory Activity. Pharm. Biol. 2014, 52, 1478–1486. [Google Scholar] [CrossRef]
- Soldi, C.; Pizzolatti, M.G.; Luiz, A.P.; Marcon, R.; Meotti, F.C.; Mioto, L.A.; Santos, A.R. Synthetic Derivatives of the α-and β-Amyrin Triterpenes and Their Antinociceptive Properties. Bioorganic Med. Chem. 2008, 16, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Teslov, L.S. Triterpenoids from Campanula patula. Chem. Nat. Compd. 1984, 20, 635. [Google Scholar] [CrossRef]
- Jiao, F.; Tan, Z.; Yu, Z.; Zhou, B.; Meng, L.; Shi, X. The Phytochemical and Pharmacological Profile of Taraxasterol. Front. Pharmacol. 2022, 13, 927365. [Google Scholar] [CrossRef]
- Yayli, N.; Yildirim, N.; Doğan, N.; Usta, A.; Altun, L. Triterpenes from Campanula lactiflora: Note. J. Asian Nat. Prod. Res. 2005, 7, 771–775. [Google Scholar] [CrossRef]
- Yayli, N.; Usta, A.; Yaşar, A.; Üçüncü, O.; Güleç, C.; Küçükislamoğlu, M. Cyclic Triterpenoid Saponins from Campanula lactiflora. Turk. J. Chem. 2006, 30, 21–28. [Google Scholar]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of Plant Alkaloids on Human Health: A Review of Biological Activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Döpke, W.; Fritsch, G. Alkaloid Content of Campanula medium. Die Pharm. 1970, 25, 128. [Google Scholar]
- Vergauwen, R.; Van den Ende, W.; Van Laere, A. The Role of Fructan in Flowering of Campanula rapunculoides. J. Exp. Bot. 2000, 51, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Guo, H.; Liang, Y.; Zhou, C.; Liu, Z.; Li, K.; Niu, F.; Zhai, X.; Wang, L. The Physiological Functions and Pharmaceutical Applications of Inulin: A Review. Carbohydr. Polym. 2020, 246, 116589. [Google Scholar] [CrossRef]
- Gambioli, R.; Forte, G.; Buzzaccarini, G.; Unfer, V.; Laganà, A.S. Myo-Inositol as a Key Supporter of Fertility and Physiological Gestation. Pharmaceuticals 2021, 14, 504. [Google Scholar] [CrossRef]
- Dzhumyrko, S.F.; Shinkarenko, A.L. Mesoinositol from Some Species of the Genus Campanula. Chem. Nat. Compd. 1972, 8, 374–375. [Google Scholar] [CrossRef]
- Teslov, L.S.; Blinova, K.F. Cyclitols from Campanula cephalotes. Chem. Nat. Compd. 1972, 8, 640–641. [Google Scholar] [CrossRef]
- Plouvier, V. Presence of Myoinositol Monoacetate in Campanula. Detection of L-Inositol Scylliton, Mannitol and Sorbitol in Some Botanical Groups. Comptes Rendus Hebd. Des Seances De L’academie Des sciences. Ser. D Sci. Nat. 1970, 270, 560–563. [Google Scholar]
- Sheikh, I.A.; Beg, M.A. Structural Characterization of Potential Endocrine Disrupting Activity of Alternate Plasticizers Di-(2-Ethylhexyl) Adipate (DEHA), Acetyl Tributyl Citrate (ATBC) and 2, 2, 4-Trimethyl 1, 3-Pentanediol Diisobutyrate (TPIB) with Human Sex Hormone-Binding Globulin. Reprod. Toxicol. 2019, 83, 46–53. [Google Scholar]
- Farah, A.; de Paulis, T.; Moreira, D.P.; Trugo, L.C.; Martin, P.R. Chlorogenic Acids and Lactones in Regular and Water-Decaffeinated Arabica Coffees. J. Agric. Food Chem. 2006, 54, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Huang, Y.; Zhu, Q.-F.; Song, M.; Xiong, S.; Manyande, A.; Du, H. The Mechanism of Chlorogenic Acid Inhibits Lipid Oxidation: An Investigation Using Multi-Spectroscopic Methods and Molecular Docking. Food Chem. 2020, 333, 127528. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Kono, Y.; Kashine, S.; Yoneyama, T.; Sakamoto, Y.; Matsui, Y.; Shibata, H. Iron Chelation by Chlorogenic Acid as a Natural Antioxidant. Biosci. Biotechnol. Biochem. 1998, 62, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Pluemsamran, T.; Onkoksoong, T.; Panich, U. Caffeic Acid and Ferulic Acid Inhibit UVA-Induced Matrix Metalloproteinase-1 through Regulation of Antioxidant Defense System in Keratinocyte HaCaT Cells. Photochem. Photobiol. 2012, 88, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic Acid: A Review on Its Mechanisms of Anti-Inflammation, Disease Treatment, and Related Delivery Systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef]
- Vitiello, M.; Galdiero, M.; Finamore, E.; Galdiero, S.; Galdiero, M. NF-κB as a Potential Therapeutic Target in Microbial Diseases. Mol. BioSyst. 2012, 8, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Roschek, B.; Fink, R.C.; Li, D.; McMichael, M.; Tower, C.M.; Smith, R.D.; Alberte, R.S. Pro-Inflammatory Enzymes, Cyclooxygenase 1, Cyclooxygenase 2, and 5-Lipooxygenase, Inhibited by Stabilized Rice Bran Extracts. J. Med. Food 2009, 12, 615–623. [Google Scholar] [CrossRef]
- Radulović, N.; Blagojevic, P.; Stojanović-Radić, Z.; Stojanovic, N. Antimicrobial Plant Metabolites: Structural Diversity and Mechanism of Action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-S.; Kuo, C.-L.; Wang, J.-P.; Cheng, J.-S.; Huang, Z.-W.; Chen, C.-F. Growth Inhibitory and Apoptosis Inducing Effect of Perilla Frutescens Extract on Human Hepatoma HepG2 Cells. J. Ethnopharmacol. 2007, 112, 557–567. [Google Scholar] [CrossRef]
- Bailly, F.; Toillon, R.-A.; Tomavo, O.; Jouy, N.; Hondermarck, H.; Cotelle, P. Antiproliferative and Apoptotic Effects of the Oxidative Dimerization Product of Methyl Caffeate on Human Breast Cancer Cells. Bioorganic Med. Chem. Lett. 2013, 23, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Vijayakurup, V.; Carmela, S.; Carmelo, D.; Corrado, T.; Srinivas, P.; Gopala, S. Phenethyl Caffeate Benzo[Kl]Xanthene Lignan with DNA Interacting Properties Induces DNA Damage and Apoptosis in Colon Cancer Cells. Life Sci. 2012, 91, 1336–1344. [Google Scholar] [CrossRef]
- Pan, C.-H.; Lin, W.-H.; Chien, Y.-C.; Liu, F.-C.; Sheu, M.-J.; Kuo, Y.-H.; Wu, C.-H. K20E, an Oxidative-Coupling Compound of Methyl Caffeate, Exhibits Anti-Angiogenic Activities through down-Regulations of VEGF and VEGF Receptor-2. Toxicol. Appl. Pharmacol. 2015, 282, 215–226. [Google Scholar] [CrossRef]
- Jantas, D.; Chwastek, J.; Malarz, J.; Stojakowska, A.; Lasoń, W. Neuroprotective Effects of Methyl Caffeate against Hydrogen Peroxide-Induced Cell Damage: Involvement of Caspase 3 and Cathepsin D Inhibition. Biomolecules 2020, 10, 1530. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).