On the Role of MoSe2 in Promoting Persulfate Activation by Fe-Based Catalysts: Dual Redox Cycles and Performance and Mechanism of Efficient Phenol Degradation in Water
Abstract
1. Introduction
2. Results and Discussion
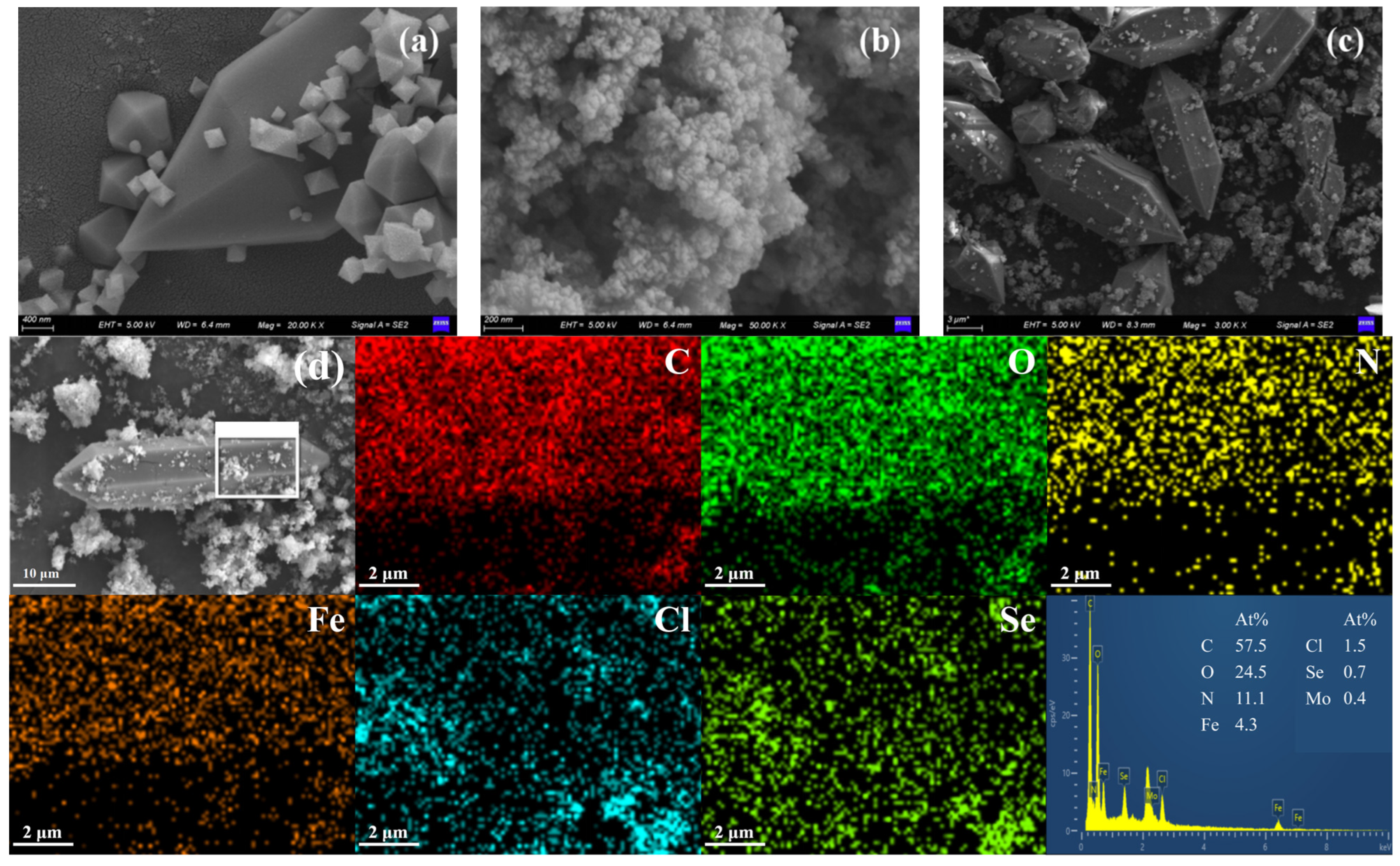
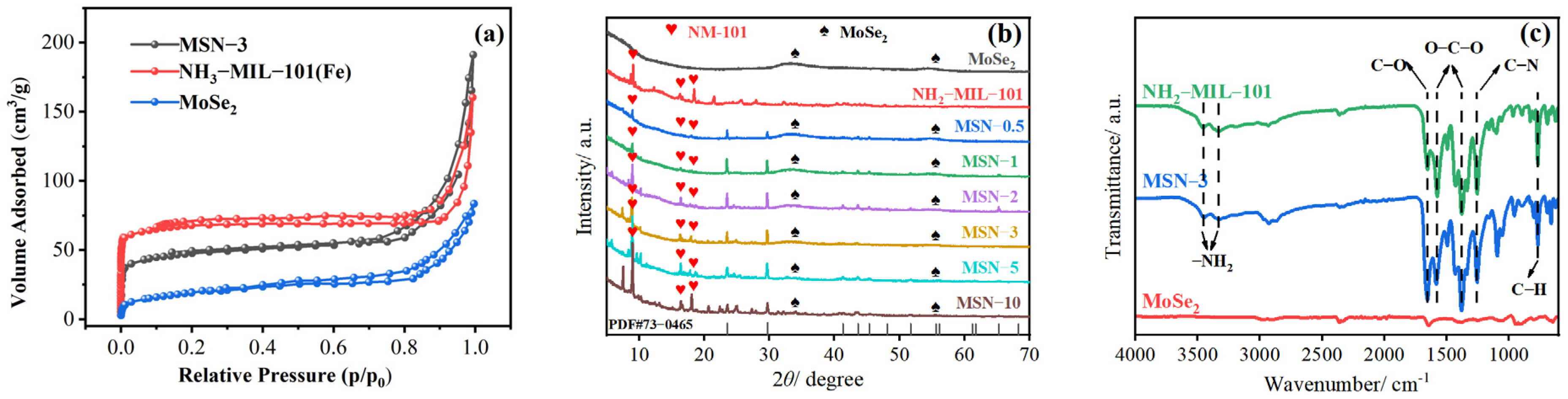
2.1. Characterization of the Catalyst
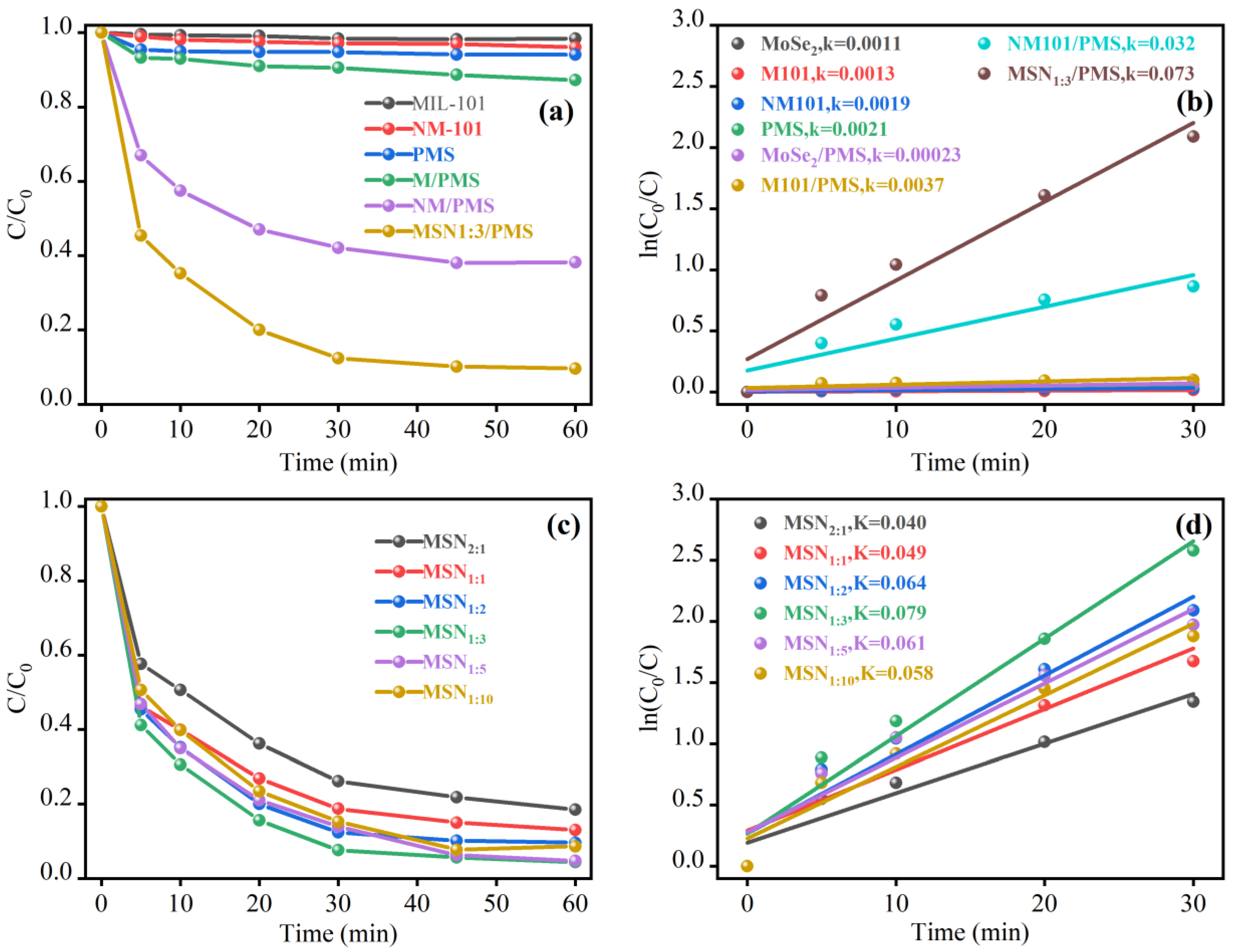
2.2. Effect of MoSe2 on the Degradation Performance of the Catalyst
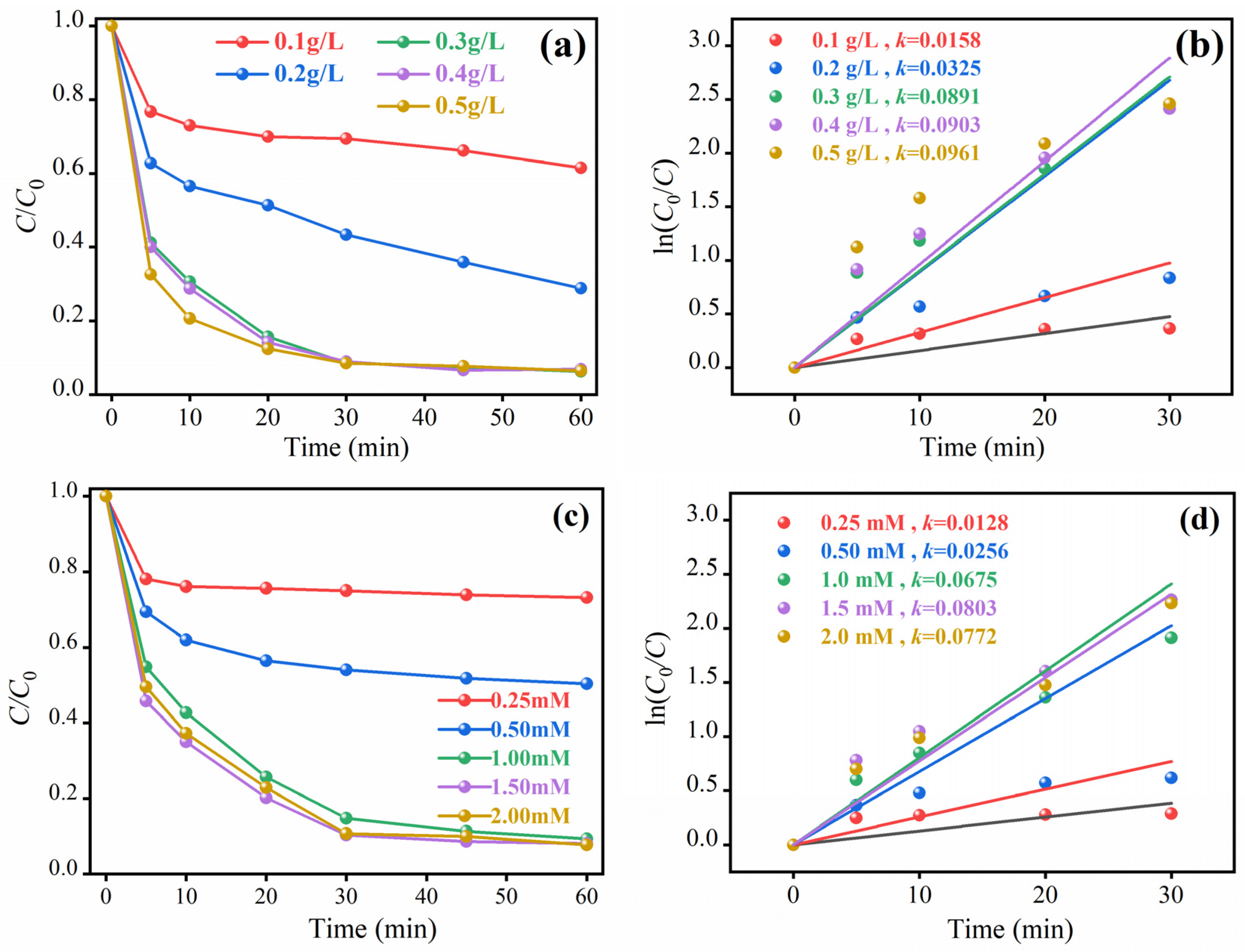
2.3. The Effect of Different Influencing Factors on the Degradation of Phenol by MSN-3
2.3.1. Effect of Catalyst Dosage
2.3.2. Oxidant Dosage
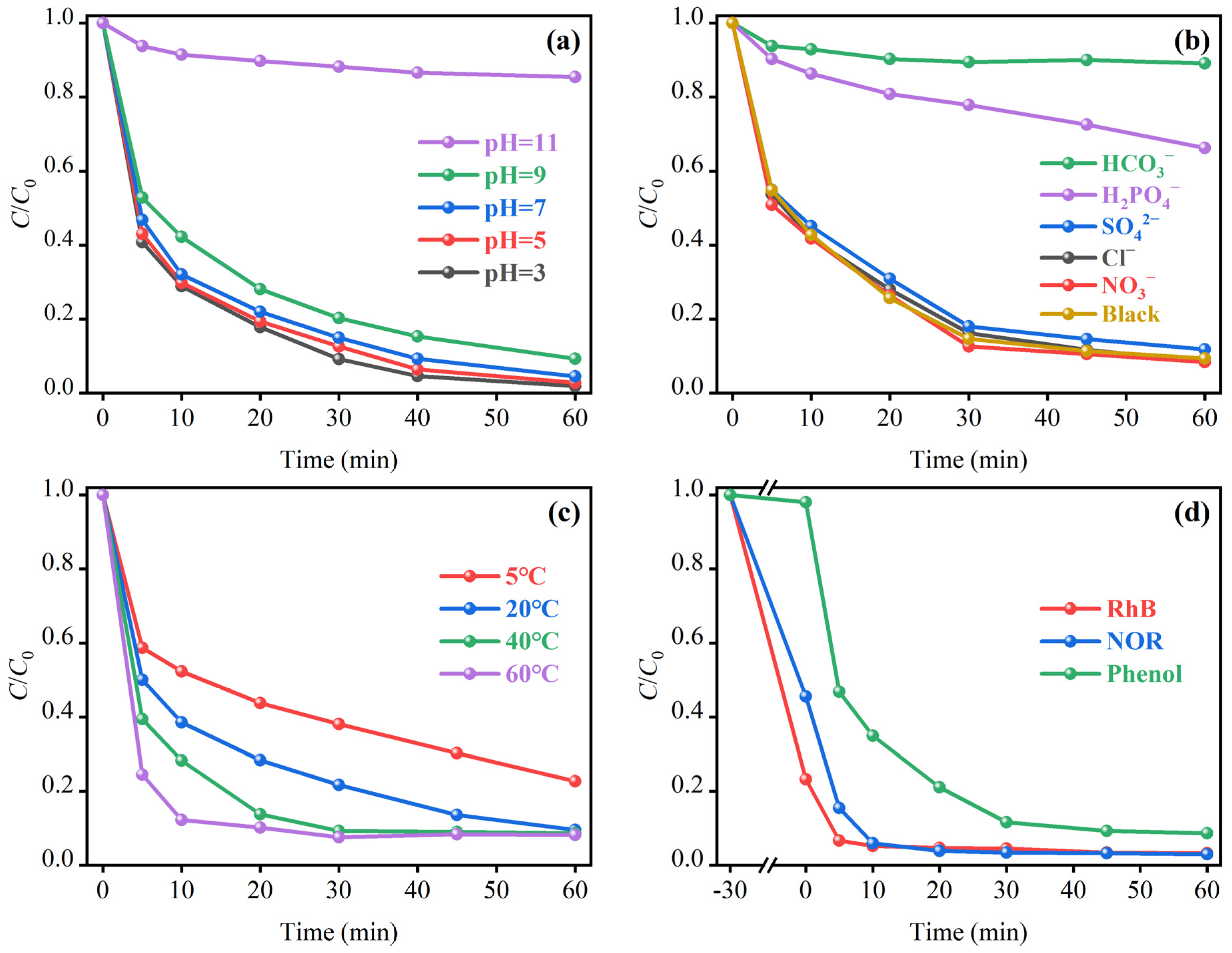
2.3.3. Effect of pH
2.3.4. Effects of Inorganic Anions
2.3.5. Effect of Temperature
2.3.6. Material Suitability
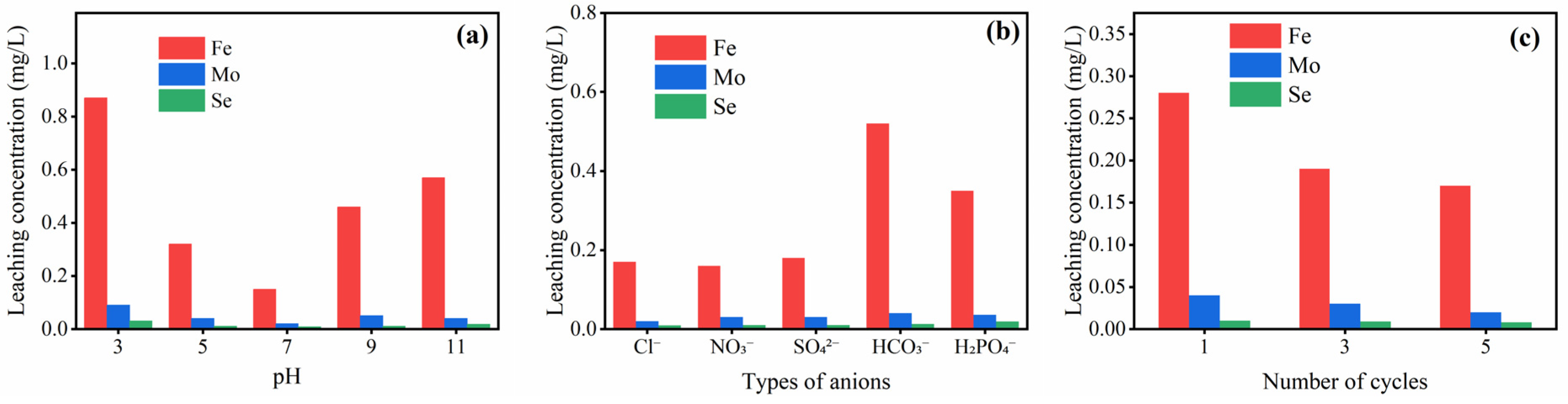
2.4. Study on the Cycling Stability of the Catalyst
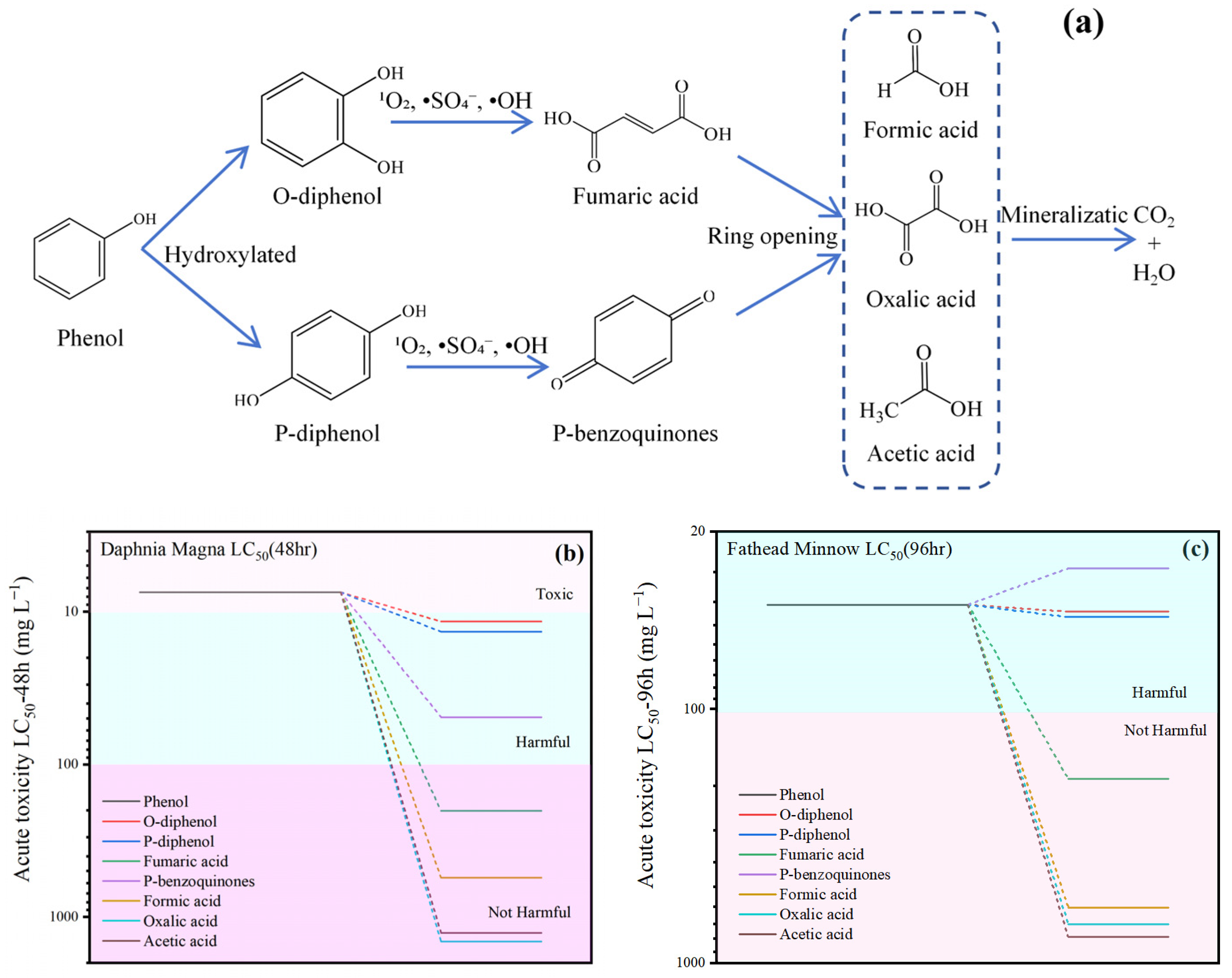
2.5. Analysis of Intermediates and Toxicity
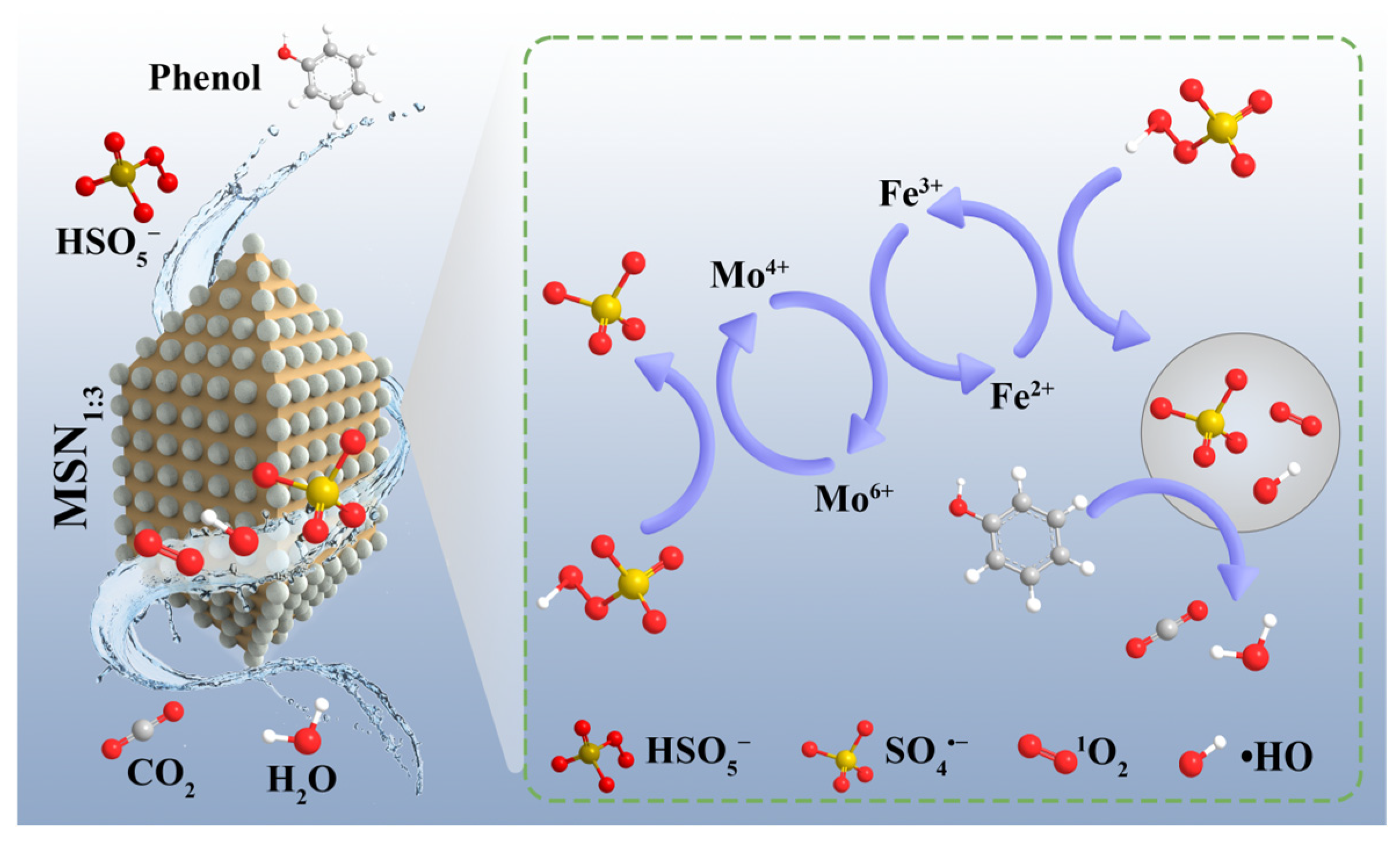
2.6. Identification of Active Free Radicals and Catalytic Mechanism
3. Experimental Section
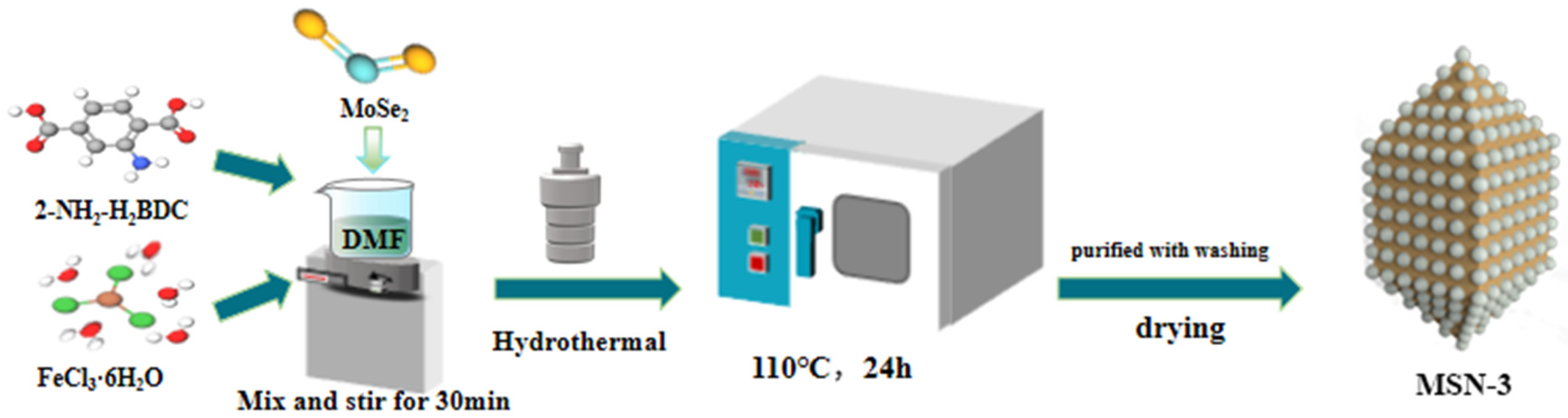
3.1. Preparation of MSN Composite Materials
3.2. Catalytic Degradation Experiment
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, P.; Chang, Q.; Jiang, G.; Xiao, K.; Wang, X. MIL-101(FeII3,Mn) with dual-reaction center as Fenton-like catalyst for highly efficient peroxide activation and phenol degradation. Sep. Purif. Technol. 2023, 306, 122582. [Google Scholar] [CrossRef]
- Hubab, M.; Gilani, I.E.; Al-Ghouti, M.A. Metal-organic frameworks: A promising solution for addressing phenol pollution and promoting environmental sustainability. Environ. Technol. Innov. 2025, 37, 104004. [Google Scholar] [CrossRef]
- Grace Pavithra, K.; Sundar Rajan, P.; Arun, J.; Brindhadevi, K.; Hoang Le, Q.; Pugazhendhi, A. A review on recent advancements in extraction, removal and recovery of phenols from phenolic wastewater: Challenges and future outlook. Environ. Res. 2023, 237, 117005. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Mishra, S.R.; Gadore, V.; Yadav, G.; Roy, S.; Bhattacharjee, B.; Bhuyan, A.; Hazarika, B.; Darabdhara, J.; Kumari, K. Phenolic compounds in water: From toxicity and source to sustainable solutions—An integrated review of removal methods, advanced technologies, cost analysis, and future prospects. J. Environ. Chem. Eng. 2024, 12, 112964. [Google Scholar] [CrossRef]
- Mumtaz, F.; Li, B.; Al Shehhi, M.R.; Feng, X.; Wang, K. Treatment of phenolic-wastewater by hybrid technologies: A review. J. Water Process Eng. 2024, 57, 104695. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Z.; Luo, Y.; Bai, Y.; Fan, J. Bioremediation of phenolic pollutants by algae-current status and challenges. Bioresour. Technol. 2022, 350, 126930. [Google Scholar] [CrossRef]
- Bibi, A.; Bibi, S.; Abu-Dieyeh, M.; Al-Ghouti, M.A. Towards sustainable physiochemical and biological techniques for the remediation of phenol from wastewater: A review on current applications and removal mechanisms. J. Clean. Prod. 2023, 417, 137810. [Google Scholar] [CrossRef]
- Yang, J.-N.; Tian, H.; Li, S.-Q.; Wang, K.-X.; Chen, J.-S. MoSe2 hybrid superlattice with expanded interlayer spacing and enriched 1T phase for aqueous zinc ion batteries. Chem. Eng. J. 2025, 516, 164105. [Google Scholar] [CrossRef]
- Kumar, G.; Bhargav, P.B.; Ahmed, N.; Selvaraj, S. Mn and rGO functionalized MoSe2: A dual-role catalyst for streamlining green hydrogen generation and industrial wastewater remediation. J. Water Process Eng. 2025, 71, 107258. [Google Scholar] [CrossRef]
- Wang, L.; Luo, D.; Yang, J.; Wang, C. Metal-organic frameworks-derived catalysts for contaminant degradation in persulfate-based advanced oxidation processes. J. Clean. Prod. 2022, 375, 134118. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Zhao, G.; Zhang, Z.; Su, P.; Li, Y.; Mu, Y.; Zhou, W. A critical review on the activation of peroxymonosulfate by MOFs for antibiotics degradation: Affecting factor, performance and mechanism. J. Environ. Chem. Eng. 2024, 12, 113634. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Meng, Q.; Jing, K.; Zhang, J.; Guan, Q. Enhanced photocatalytic performance of 0.1Bi-MIL-101-NH2 after phosphorus adsorption: Synergistic effect of adsorption and photocatalysis. Appl. Catal. B Environ. Energy 2024, 359, 124487. [Google Scholar] [CrossRef]
- Xing, J.; Huang, J.; Wang, X.; Yang, F.; Bai, Y.; Li, S.; Zhang, X. Removal of low-concentration tetracycline from water by a two-step process of adsorption enrichment and photocatalytic regeneration. J. Environ. Manag. 2023, 343, 118210. [Google Scholar] [CrossRef]
- Wang, C.; Hou, S.; Ma, M.; Ji, Z.; Wang, P.; Wang, Y.; Su, Y.; Zhou, Y.; Li, M. Recent development of MIL-101(Cr) for photocatalysis: A mini review. J. Water Process Eng. 2024, 66, 106040. [Google Scholar] [CrossRef]
- Xiang, Y.; Wei, S.; Wang, T.; Li, H.; Luo, Y.; Shao, B.; Wu, N.; Su, Y.; Jiang, L.; Huang, J. Transformation of metal-organic frameworks (MOFs) under different factors. Coord. Chem. Rev. 2025, 523, 216263. [Google Scholar] [CrossRef]
- Simonescu, C.M.; Culita, D.C.; Marinescu, G.; Atkinson, I.; Marinescu, V.; Oprea, O.; Stanica, N. Novel Magnetically Recoverable Amino-Functionalized MIL-101(Fe) Composite with Enhanced Adsorption Capacity for Pb(II) and Cd(II) Ions. Molecules 2025, 30, 2879. [Google Scholar] [CrossRef]
- Pan, Y.; Sanati, S.; Abazari, R.; Jankowska, A.; Goscianska, J.; Srivastava, V.; Lassi, U.; Gao, J. Vanadium- and manganese-based metal-organic frameworks for potential environmental and catalysis applications. Coord. Chem. Rev. 2025, 522, 216231. [Google Scholar] [CrossRef]
- Tong, J.; Xiang, Y.; Li, N.; Xu, Z.; Yang, Z.; Peng, H. Catalytic microenvironment regulation by introducing biochar into MIL-101Fe derivative for enhanced persulfate activation: Efficient antibiotic removal and RSM optimization. Sep. Purif. Technol. 2024, 331, 125719. [Google Scholar] [CrossRef]
- Cheang, T.; Huang, W.; Li, W.; Ren, S.; Wen, H.; Zhou, T.; Zhang, Y.; Lin, W. Exposed carboxyl functionalized MIL-101 derivatives for rapid and efficient extraction of heavy metals from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129517. [Google Scholar] [CrossRef]
- Feng, H.; Xu, Q.; Lv, T.; Liu, H. Bimetallic NH2-MIL-101(Fe, Co) as highly efficient photocatalyst for nitrogen fixation. Appl. Catal. B Environ. Energy 2024, 351, 123949. [Google Scholar] [CrossRef]
- Bu, F.; Huang, W.; Xian, M.; Zhang, X.; Liang, F.; Liu, X.; Sun, X.; Feng, D. Magnetic carboxyl-functionalized covalent organic frameworks for adsorption of quinolones with high capacities, fast kinetics and easy regeneration. J. Clean. Prod. 2022, 336, 130485. [Google Scholar] [CrossRef]
- Li, S.; Dong, K.; Cai, M.; Li, X.; Chen, X. A plasmonic S-scheme Au/MIL-101(Fe)/BiOBr photocatalyst for efficient synchronous decontamination of Cr(VI) and norfloxacin antibiotic. eScience 2024, 4, 100208. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Y.; Zhang, Z.; Song, D.; Cao, J.; Yu, H.; Yu, G.; Zhou, L.; Su, Y.; Lv, Y.; et al. Highly efficient removal of oxytetracycline using activated magnetic MIL-101(Fe)/γ-Fe2O3 heterojunction catalyst. J. Environ. Manag. 2022, 317, 115327. [Google Scholar] [CrossRef]
- Feng, S.; Liu, J.; Chen, X.; Wu, K.; Ning, F.; Yi, J.; Liu, Y. Synthesis and selenium vacancy-induced catalytic enhancement of CoSe2 for efficient electrocatalytic nitrate reduction. Sep. Purif. Technol. 2025, 371, 133373. [Google Scholar] [CrossRef]
- Singh, P.; Devi, P. Deciphering the photoelectrochemical behaviour of MoSe2-decorated BiVO4 as a dual system for concurrent degradation of methylene blue and hydrogen generation. J. Clean. Prod. 2024, 448, 141502. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, Q.; Wu, Y.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Huang, B.; Wang, P. Composite of mixed-cation perovskite MA1-xFAxPbI3 with MoSe2 for enhanced photocatalytic H2 evolution. Chem. Eng. J. 2025, 511, 162120. [Google Scholar] [CrossRef]
- Chen, X.; Li, A.; Xing, L.; Wang, J.; Sun, Y.; Wang, Y.; Chen, G.; Xing, T.; Xu, L. Piezo-photocatalytic degradation and mechanism of rhodamine B by flexible MoSe2/PVDF composite foam. J. Water Process Eng. 2024, 59, 105015. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, X.; Yao, Y.; Ling, C. Construction of MoSe2/Bi3O4Cl Z-scheme 2D/3D heterojunction for enhanced photocatalytic performance. J. Environ. Chem. Eng. 2025, 13, 117802. [Google Scholar] [CrossRef]
- Dai, T.-J.; Fan, Z.-Y.; Peng, C.-F.; Xiao, X.; Zhou, Y.; Sun, J.; Zhou, Z.-Y.; Guo, X.; Liu, X.-F.; Niu, X.-H. Electric Field Modulation and Ultrafast Photogenerated Electron-Hole Dynamics in MoSe2/WSe2 van der Waals Heterostructures. Molecules 2025, 30, 3840. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Qin, W.; Gan, Y.; Huang, Y.; Jiang, Z.; Chen, Y.; Li, X.; Yan, K. Acceleration of Fe3+/Fe2+ cycle in garland-like MIL-101(Fe)/MoS2 nanosheets to promote peroxymonosulfate activation for sulfamethoxazole degradation. Chem. Eng. J. 2023, 470, 144190. [Google Scholar] [CrossRef]
- Kang, B.; Li, J.; Wei, S.; Hu, X.; Zeng, L.; Lai, W.; Xiao, F.; Xiao, L.; Chen, Q.; Qian, Q.; et al. Antimony-mediated few-layer metallic MoSe2 with rich selenium vacancies for ultrafast sodium/potassium storage. Chem. Eng. J. 2024, 499, 156305. [Google Scholar] [CrossRef]
- Du, P.; Wen, T.; Gao, X.; Zhao, X.; Wang, M.; Huang, H. Metal cluster-dependent adsorption for rocephin antibiotic in MIL-101-NH2 metal–organic frameworks. Sep. Purif. Technol. 2025, 354, 129149. [Google Scholar] [CrossRef]
- Lv, Y.; Xue, J.; Chen, Z.; Qu, J.; Huang, K.; Wang, M.; Sun, W. Development of hydrothermal carbonaceous carbon/NH2-MIL-101(Fe) composite photocatalyst with in-situ production and activation of H2O2 capabilities for effective sterilization. Chem. Eng. J. 2024, 498, 155263. [Google Scholar] [CrossRef]
- Wan, Z.; Xu, X.; Bi, Z.; Jiajia, D.; Li, Y.; Chen, M.; Huang, Z. Gadolinium doping-induced electronic structure optimization of MIL-101-NH2: Efficient adsorption of arsenic (V) and phosphorus and electrochemical regeneration. Sep. Purif. Technol. 2025, 357, 130133. [Google Scholar] [CrossRef]
- Liu, Z.; Su, R.; Sun, X.; Zhou, W.; Gao, B.; Yue, Q.; Li, Q. The obvious advantage of amino-functionalized metal-organic frameworks: As a persulfate activator for bisphenol F degradation. Sci. Total Environ. 2020, 741, 140464. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Goh, K.; Ng, D.Y.F.; Jiang, X.; Chuah, C.Y.; Chew, J.W.; Wang, R. Alternating spin-and-spray electrospun scaffold membranes with fractionated MIL-101(Cr) adsorbent for high-performance single-pass dye adsorption process. Chem. Eng. J. 2022, 450, 137963. [Google Scholar] [CrossRef]
- Liu, R.; Chi, L.; Wang, X.; Wang, Y.; Sui, Y.; Xie, T.; Arandiyan, H. Effective and selective adsorption of phosphate from aqueous solution via trivalent-metals-based amino-MIL-101 MOFs. Chem. Eng. J. 2019, 357, 159–168. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Huang, X. Hydroxylamine hydrochloride-driven activation of NiFe2O4 for the degradation of phenol via peroxymonosulfate. Environ. Res. 2024, 263, 120057. [Google Scholar] [CrossRef]
- Wang, S.; Dai, Y.; Wang, H.; Dong, X. Activation of peroxymonosulfate by Fe based metal organic framework for selective degradation of phenol in water. J. Water Process Eng. 2024, 68, 106454. [Google Scholar] [CrossRef]
- Mo, L.; Chen, G.; Xu, B. Degradation of phenol by peroxymonosulfate catalyzed by cerium-doped amino-functionalized metal-organic frameworks (NH2-MIL-101 (Fe, Ce)). J. Environ. Chem. Eng. 2024, 12, 113256. [Google Scholar] [CrossRef]
- Jiang, X.; Xing, Y.; Jin, X.; Kou, B.; Yang, R.; Ni, G. Activation of peroxymonosulfate by MnOOH/g-C3N5: Study on highly selective removal of phenolic pollutants and its non-radical pathway. J. Environ. Chem. Eng. 2024, 12, 114636. [Google Scholar] [CrossRef]
- Yue, L.; Hao, L.; Zhang, J.; Piao, X.; Chen, C. Oxygen-enriched vacancy Co2MnO4 spinel catalyst activated peroxymonosulfate for degradation of phenol: Non-radical dominated reaction pathway. J. Water Process Eng. 2023, 53, 103807. [Google Scholar] [CrossRef]
- Xing, Z.; Fan, M.; Liu, J.; Wang, Y.; Zhang, X.; Li, R.; Wang, Y.; Fan, C. A novel Fenton-like catalyst and peroxymonosulfate activator of Mn3O4/λ-MnO2 for phenol degradation: Synergistic effect and mechanism. Inorg. Chem. Commun. 2023, 150, 110396. [Google Scholar] [CrossRef]
- Huang, Z.; Lai, Z.; Zhu, D.; Wang, H.; Zhao, C.; Ruan, G.; Du, F. Electrospun graphene oxide/MIL-101(Fe)/poly(acrylonitrile-co-maleic acid) nanofiber: A high-efficient and reusable integrated photocatalytic adsorbents for removal of dye pollutant from water samples. J. Colloid Interface Sci. 2021, 597, 196–205. [Google Scholar] [CrossRef]
- Zhao, K.; Li, X.; Cheng, G.; Liu, L.; Chen, R.; Jiao, Y.; Liu, Y.; Zhu, G. Rapid and selective removal of bisphenol S from environmental samples by surface-imprinted polymer synthesized based on metal-organic framework MIL-101(Cr). J. Environ. Chem. Eng. 2024, 12, 113569. [Google Scholar] [CrossRef]
- Nair, K.M.; Thomas, N.; Pallilavalappil, S.; Mathew, S.; Deignan, K.; Hinder, S.J.; Brennan, B.; McArdle, F.; Pillai, S.C. Unravelling the impact of lower vacuum activation temperature on Fe2+/Fe3+ mixed-valence unsaturated iron centres in MIL-101(Fe) and its impact on Fenton degradation of acetaminophen. J. Environ. Chem. Eng. 2024, 12, 113615. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Li, S.; Xue, C.; Liu, D.; Huang, W. Unraveling the crucial role of Mo2N from Fe/Mo bimetal MOF-derived catalyst in initiating Fe3+/Fe2+ redox cycle to activate peroxymonosulfate for dibutyl phthalate degradation. Chem. Eng. J. 2023, 476, 146693. [Google Scholar] [CrossRef]
- Wang, H.; Huang, R.; Mao, W.; Xu, H.; Ling, C.; Zhao, J.; Yi, F.; Zhou, Y.; Zhou, J. Improving water stability and photocatalytic activity of MIL-101(Fe) via in-situ modification strategy. J. Environ. Chem. Eng. 2024, 12, 111903. [Google Scholar] [CrossRef]
- Jia, Y.; Li, H.; Duan, L.; Gao, Q.; Zhang, H.; Li, S.; Li, M. Activation of persulfate by β-PDI/MIL-101(Fe) photocatalyst under visible light toward efficient degradation of sulfamethoxazole. Chem. Eng. J. 2024, 481, 148588. [Google Scholar] [CrossRef]
- Li, T.; Pan, J.; Wang, X.; Fan, Z.; Shi, T.; Wang, L.; Gao, B. Insights into the fundamental role of Mo doping in facilitating the activation of peroxydisulfate by iron-based catalysts: Accelerating the generation of sulfate radicals. Chem. Eng. J. 2023, 477, 147000. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, B.; Wang, Z.; Li, J.; Du, Y.; He, C.; Liu, Y.; Yao, G.; Lai, B. Efficient wastewater disinfection by raised 1O2 yield through enhanced electron transfer and intersystem crossing via photocatalysis of peroxymonosulfate with CuS quantum dots modified MIL-101(Fe). Water Res. 2023, 229, 119489. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, Z.; Huang, J.; Yan, F.; Du, Y.; He, C.; Liu, Y.; Yao, G.; Lai, B. A singlet oxygen dominated process through photocatalysis of CuS-modified MIL-101(Fe) assisted by peroxymonosulfate for efficient water disinfection. Chem. Eng. J. 2022, 439, 135788. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Zhao, H.; Lu, Z.; Chen, Z. On the Role of MoSe2 in Promoting Persulfate Activation by Fe-Based Catalysts: Dual Redox Cycles and Performance and Mechanism of Efficient Phenol Degradation in Water. Molecules 2025, 30, 4466. https://doi.org/10.3390/molecules30224466
Ren Y, Zhao H, Lu Z, Chen Z. On the Role of MoSe2 in Promoting Persulfate Activation by Fe-Based Catalysts: Dual Redox Cycles and Performance and Mechanism of Efficient Phenol Degradation in Water. Molecules. 2025; 30(22):4466. https://doi.org/10.3390/molecules30224466
Chicago/Turabian StyleRen, Yirong, Hao Zhao, Zerui Lu, and Zuoyan Chen. 2025. "On the Role of MoSe2 in Promoting Persulfate Activation by Fe-Based Catalysts: Dual Redox Cycles and Performance and Mechanism of Efficient Phenol Degradation in Water" Molecules 30, no. 22: 4466. https://doi.org/10.3390/molecules30224466
APA StyleRen, Y., Zhao, H., Lu, Z., & Chen, Z. (2025). On the Role of MoSe2 in Promoting Persulfate Activation by Fe-Based Catalysts: Dual Redox Cycles and Performance and Mechanism of Efficient Phenol Degradation in Water. Molecules, 30(22), 4466. https://doi.org/10.3390/molecules30224466




