Abstract
Herein, we disclose a novel and efficient cobalt-catalyzed cross-coupling strategy for picolinamide-directed direct C(sp3)-H bond formation with N-(phenylsulfanyl)succinimides. This method enables the direct construction of C(sp3)–S bonds under mild conditions and exhibits excellent functional group tolerance along with a broad substrate scope. Notably, the catalytic system achieves oxidative C–H functionalization without relying on costly or environmentally detrimental oxidants, offering a more sustainable and practical alternative for C–S bond formation.
1. Introduction
Aromatic chalcogen-based frameworks, particularly aryl thioethers, constitute a class of highly valuable molecular scaffolds that are widely present in numerous biologically active natural products [1,2,3], pharmaceutical agents [4,5,6], and functional materials [7,8]. Owing to the critical importance of these organosulfur compounds, significant research efforts have been directed toward developing efficient synthetic routes [9,10,11]. Conventional strategies for constructing C-S bonds have largely relied on transition metal-catalyzed cross-coupling reactions between aryl halides and thiols. However, these methods often suffer from narrow substrate scope, prefunctionalization requirements, and byproduct formation, driving the development of innovative catalytic strategies that offer milder conditions, broader compatibility, and improved sustainability.
In recent years, with the participation of N,N-bidentate [12,13,14,15,16] coordination system, transition-metal-catalyzed C-H bond activation with a cyclic metallacycle intermediate has emerged as a useful pathway for novel and efficient strategy in the construction of C-X bonds (X = C, N, O, S). This approach eliminates circumventing the reliance on aryl halides required in traditional cross-coupling reactions, and significantly reduces halogenated byproducts, thereby improving atom economy and synthetic efficiency. Despite significant advances in C-H functionalization, research efforts have predominantly centered on C(sp2)-H bonds, whereas the functionalization of more inert C(sp3)-H bonds remains less explored [17,18,19,20]. Notably, palladium-catalyzed C(sp3)-H functionalization has been established as a robust strategy for constructing diverse organic molecules. For instance, in 2012, Zhang and colleagues reported a Pd-catalyzed arylation/oxidation of benzylic C-H bonds [21]. Subsequently, the same group achieved Pd-catalyzed acetoxylation of C(sp3)-H bonds to afford benzyl esters [22,23]. Related acetoxylation reactions were also documented by the research groups Huang [24]. In 2020, Xie and co-workers established the Pd-catalyzed direct benzylic C(sp3)-H chalcogenation with diaryl disulfides and diphenyl diselenide [25] (Figure 1). While significant progress has been made in picolinamide-directed C(sp3)-H functionalization, the field remains heavily reliant on expensive palladium catalysts, a reliance that substantially limits its economic viability and broader applicability. Research efforts are increasingly directed toward cobalt-catalyzed C-H functionalization, driven by the metal’s economic advantage and inherently sustainable profile compared to precious metal catalysts [26,27,28].
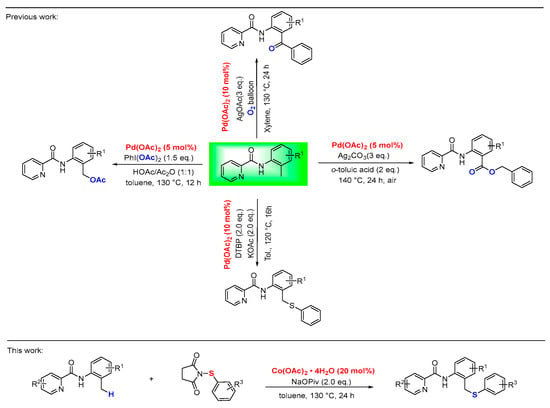
Figure 1.
Picolinamide-directed functionalization of γ-C(sp3)-H bonds.
Herein, we report the first Co(II)-catalyzed, picolinamide-directed sulfurization of benzylic C(sp3)-H bonds, employing N-(phenylsulfanyl)succinimides as a source of sulfur ethers. This method exhibits excellent functional group tolerance and delivers diverse products in moderate to excellent yields.
2. Results and Discussion
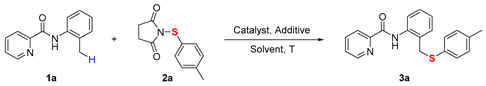
We initiated our investigation using N-(o-tolyl)picolinamide (1a) and 1-(p-tolylthio)pyrrolidine-2,5-dione (2a) as model substrates, employing Co(OAc)2 as catalyst and KOAc as additive in toluene at 130 °C for 24 h. Satisfactorily, the desired product (3a) was obtained in 57% yield (Table 1, entry 1). Encouraged by this result, we screened various metal catalysts including CoBr2, Co(acac)2, NiCl2, CuBr2, Cu(OAc)2 and Fe(OAc)2. The comparative studies consistently identified Co(OAc)2 as the most effective catalyst for this specific transformation. In contrast, no reaction occurred in the absence of catalyst (Table 1, entries 2–8). Subsequently, a systematic examination on the impact of various additives was conducted, including NaOAc, K2CO3, Na2CO3 and NaOPiv, which were evaluated as alternatives to KOAc. Among them, NaOPiv proved superior to the others, yielding the target product in 72% (Table 1, entries 9–13). We also investigated other solvents, including p-xylene, DMSO, DMF and mesitylene. However, these solvent systems proved less favorable, leading to a concomitant decrease in yield to 58%, 43%, 48% and 61%, respectively (Table 1, entries 14–17). Finally, reducing the catalyst loading or changing the air atmosphere resulted in yield rates of 60, 13, and 76, respectively (Table 1, entris 18–20).

Table 1.
Optimization of reaction conditions a,b.
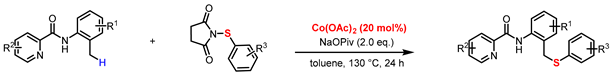
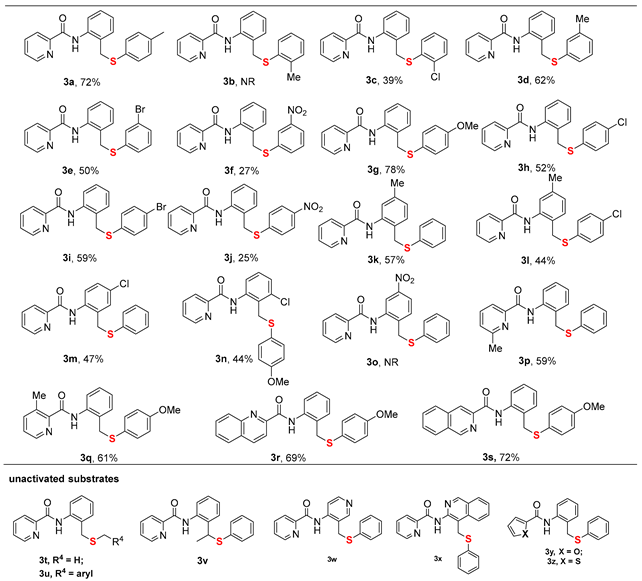
With the optimized reaction conditions established, we subsequently explored the substrate scope of sulfurization substrates (Table 2). The reaction demonstrated good functional group tolerance, accommodating a range of N-(phenylsulfanyl)succinimides bearing either electron-donating groups (EDGs) (3a, 3d, 3g) or electron-withdrawing groups (3c, 3e, 3f, 3h, 3i, 3j, 3k), furnishing the target materials in yields ranging from 25% to 78%. Substrates featuring EDGs generally exhibited higher reaction efficiency, and a more pronounced electronic effect (whether donating or withdrawing) correlated with a greater influence on the yield. Notably, steric hindrance was found to have a notable influence on the reaction efficiency. Specifically, substrates with substituents at the ortho-position (3b, 3c) proved to be recalcitrant, yielding a maximum of 39%. Similarly, the parallel trend was noted for the amide substrates bearing such substituents, reinforcing the pivotal role of electronic effects (3k–3o). Ulteriorly, investigation on directing group scopes demonstrated that while modifications such as substituted pyridines, quinoline, and isoquinoline were all viable, heterocycles like furan and thiophene were completely ineffective in promoting the transformation (3p–3u). Unfortunately, alkyl sulfides, benzyl sulfides, N-(2-ethylphenyl)picolinamide, and heteroaromatic amides like N-(3-methylpyridin-4-yl)picolinamide and N-(4-methylisoquinolin-3-yl)picolinamide are not applicable to this reaction, indicating a limited scope of the method (3v–3z). This transformation is highly sensitive to both steric and electronic parameters. The facilitatory effect of EDGs and the deleterious impact of ortho-substituents and certain heterocycles highlight this dependence. Most tellingly, the incompatibility of non-aromatic sulfides and specific amides defines a narrow substrate profile, suggesting a mechanism that demands precise electronic character and possibly chelation ability from the substrates.

Table 2.
Scope of sulfurization substrates a,b.
To gain more insights into the reaction mechanism, we incorporated 5.0 equivalents TEMPO or BHT into the reaction, and no desired products were obtained. Subsequently, the thioether radical was successfully trapped by 1,1-diphenylethylene and detected by ESI-HRMS (Figure 2).
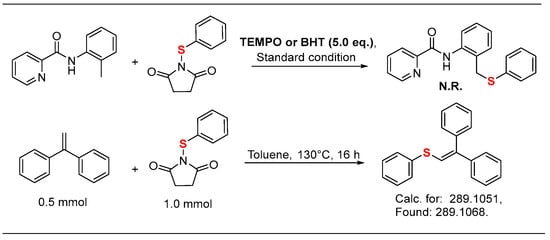
Figure 2.
Mechanistic studies.
Based on the experimental results presented and supported by previous literature [25,28,29,30,31], a plausible mechanism for this transformation via a CoII/CoIV pathway is proposed (Figure 3). The catalytic cycle is initiated by the subsequent ligand exchange of Co(OAc)2 to the nitrogen atoms of N-(o-tolyl)picolinamide (1a) and forming the complex A. Then, A oxidized by O2 to yield cobalt(III) intermediate B, which undergoes reversible C-H cobaltation to provide cobaltacycle species C. Concurrently, toluylthiether radical was generated from 1-(p-tolylthio)pyrrolidine-2,5-dione (2a) upon heating and coupling with C to afford the intermediate D. Subsequent reorganization leads to complex E, and reductive elimination from E finally releases product (3a) and regenerates the cobalt catalyst for the next catalytic cycle.
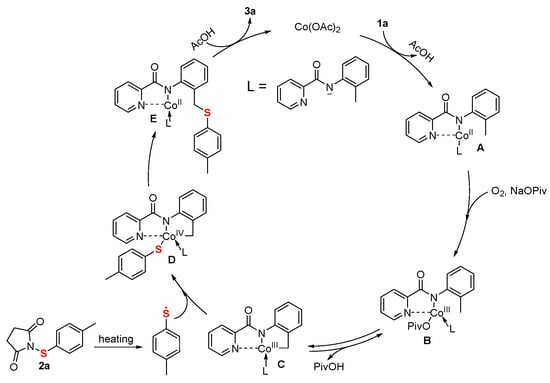
Figure 3.
Proposed mechanism pathway.
3. Conclusions
In conclusion, we have developed a novel cobalt-catalyzed cross-coupling strategy for the picolinamide-directed C(sp3)-H chalcogenation of alkylamines using N-(phenylsulfanyl)succinimides. This method facilitates the direct construction of C(sp3)-S bonds under mild conditions and exhibits a broad substrate scope with excellent functional group tolerance. Notably, the catalytic system accomplishes oxidative C-H transformation without the need for expensive or environmentally harmful oxidants, thereby offering a sustainable and efficient alternative for C-S bond formation.
4. Experimental Section
All the chemicals were obtained commercially and used without any prior purification. 1H NMR and 13C NMR spectra were recorded on Bruker Avance II 400 or 500 spectrometers. All products were isolated by short chromatography on a silica gel (200–300 mesh) column using petroleum ether (60–90 °C) and ethyl acetate, unless otherwise noted. All compounds were characterized by 1H NMR and 13C NMR, which are consistent with those reported in the literature.
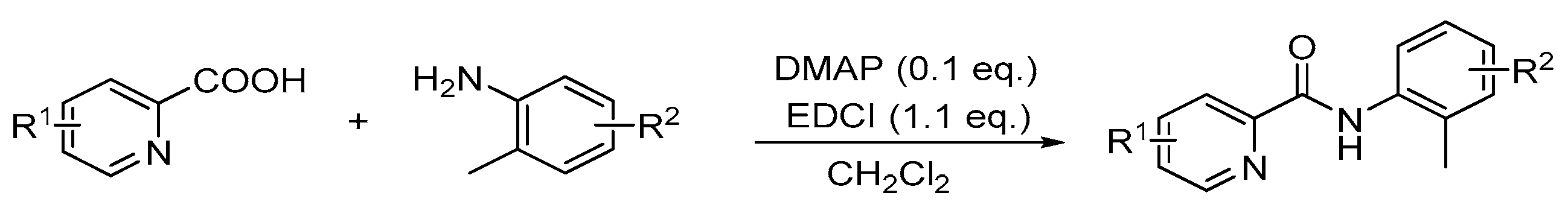
The typical procedure to make them: A 100 mL two-necked round-bottom flask was equipped with magnetic stir bar and charged with amine (20 mmol), picolinic acid (1.1 equiv), N,N-dimethyl-4-aminopyridine (DMAP, 0.1 equiv) dissolved in 30 mL anhydrous CH2Cl2 at 0 °C. Then EDCI (1.1 equiv) in CH2Cl2 (20 mL) was dropwise added to the solution under a nitrogen atmosphere. After the addition, the reaction was then warmed to room temperature, stirred for 12h and quenched with water (30 mL). Then the reaction mixture was extracted with ethyl acetate (20 × 3 mL) and the combined organic solvent was dried over Na2SO4, filtered and concentrated under reduced pressure. The resulting residue was purified by column chromatography using PE/AcOEt (15:1) as an eluent.
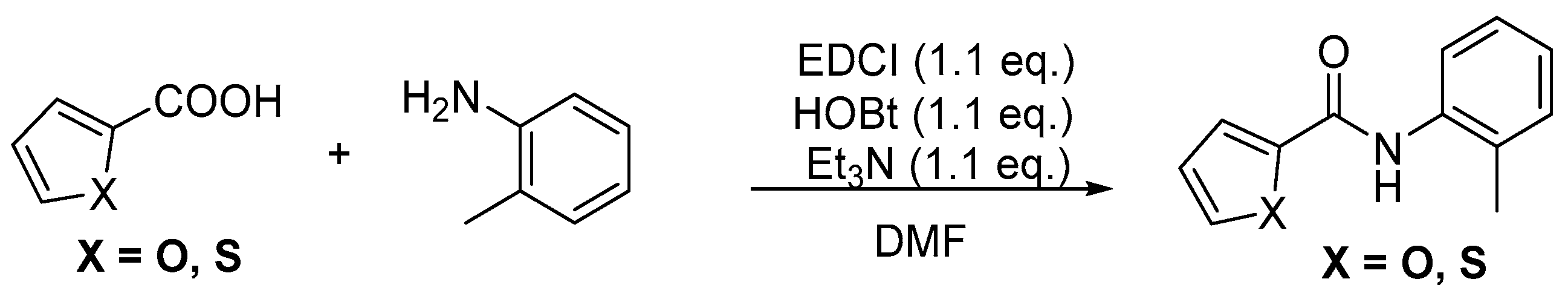
The typical procedure to make them: A 100 mL two-necked round-bottom flask was equipped with magnetic stir bar and charged with amine (5 mmol), acid (1.1 equiv), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI, 5.5 mmol, 1.1 equiv.) and 1-Hydroxybenzotriazole (EDCI, 5.5 mmol, 1.1 equiv.) dissolved in 10 mL DMF. The reaction mixture was stirred at room temperature for 12 h, then poured into H2O (50 mL). The mixture was washed with 1.0 M HCl (15 mL), saturated aqueous NaHCO3 (15 mL), and brine (15 mL). Then the reaction mixture was extracted with ethyl acetate (20 × 3 mL) and the combined organic solvent was dried over Na2SO4, filtered and concentrated under reduced pressure. The resulting residue was purified by column chromatography using PE/AcOEt (15:1) as an eluent.
- Preparation of N-aryl/alkylthiosuccinimide (2) [34]
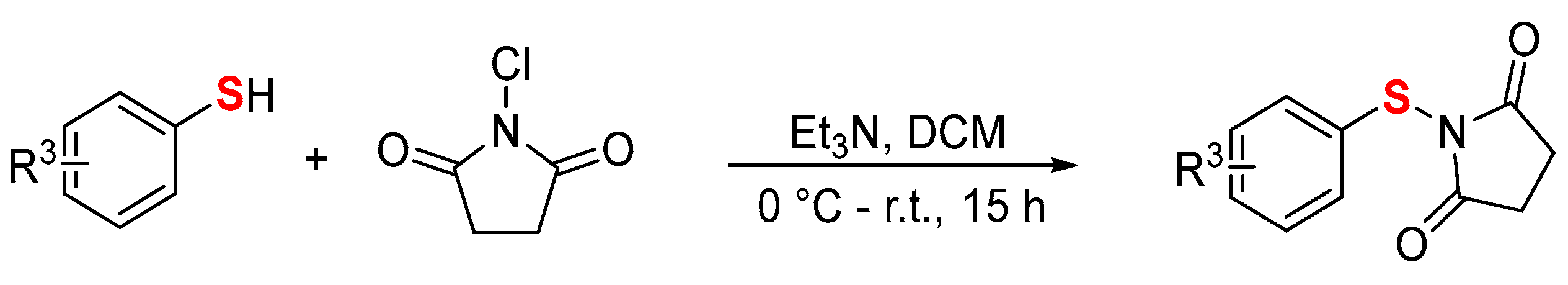
To a solution of N-chlorosuccinimide (NCS) (1.0 equiv) in CH2Cl2 (5.0 mL for 2.0 mmol) was added thiophenols (1.0 equiv) and Et3N (1.0 equiv) drop-wise under an argon atmosphere at 0 °C. The resulting mixture was stirred for 12 h at rt. After completion, the reaction mixture was quenched with saturated aqueous NH4Cl solution. The organic layer was separated; the aqueous layer was extracted with CH2Cl2 (20 × 3 mL). The combined extracts were washed with brine. The organic layer was dried over Na2SO4. Solvent was filtered and evaporated under reduced pressure. The crude residue was purified using column chromatography on silica gel using PE/AcOEt (5:1) as an eluent.
General procedure for synthesis of 3: A mixture of 1 (0.2 mmol), 2 (1.5 equiv.), Co(OAc)2·4H2O (20 mol%), NaOPiv (2.0 equiv), toluene (2.0 mL), stirred at 130 °C, under air, 24 h. Then the mixture was cooled to room temperature and concentrated under vacuum after filtration. The product 3 was purified by silica gel column flash chromatography using PE/AcOEt (30:1) as an eluent.
N-(2-((p-tolylthio)methyl)phenyl)picolinamide (3a): Colourless liquid, 72% yield; 1H NMR (400 MHz, CDCl3) δ 10.56 (s, 1H), 8.53 (d, J = 4.4 Hz, 1H), 8.23 (d, J = 7.8 Hz, 1H), 8.15 (d, J = 8.1 Hz, 1H), 7.83 (t, J = 7.2 Hz, 1H), 7.42–7.39 (m, 1H), 7.31 (d, J = 7.9 Hz, 2H), 7.26 (d, J = 7.6 Hz, 1H), 7.07 (d, J = 7.2 Hz, 1H), 7.01 (d, J = 8.2 Hz, 2H), 6.97 (d, J = 7.4 Hz, 1H), 4.08 (s, 2H), 2.24 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 162.50, 150.22, 148.27, 137.73, 137.68, 136.24, 132.71, 131.41, 130.62, 129.81, 128.68, 127.82, 126.55, 124.78, 123.11, 122.63, 38.21, 21.26. HRMS(ESI+): Calculated for C20H19N2OS+, [M+H]+ 335.1218. Found 335.1221.
N-(2-(((2-chlorophenyl)thio)methyl)phenyl)picolinamide (3c): Obtained as a colourless liquid, 39% yield; 1H NMR (400 MHz, CDCl3) δ 10.49 (s, 1H), 8.44 (d, J = 4.0 Hz, 1H), 8.21 (d, J = 7.7 Hz, 1H), 8.11 (d, J = 8.0 Hz, 1H), 7.82 (d, J = 7.5 Hz, 1H), 7.39–7.36 (m, 1H), 7.35–7.29 (m, 2H), 7.26 (d, J = 7.6 Hz, 1H), 7.14 (d, J = 7.5 Hz, 1H), 7.10–7.06 (m, 2H), 6.99 (s, 1H), 4.16 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 162.54, 150.04, 148.24, 137.66, 136.27, 135.89, 134.42, 132.34, 130.60, 129.83, 128.88, 128.23, 127.23, 127.08, 126.55, 124.97, 123.30, 122.55, 77.48, 77.16, 76.84, 35.55. HRMS(ESI+): Calculated for C19H16ClN2OS+, [M+H]+ 355.0672. Found 355.0679.
N-(2-((m-tolylthio)methyl)phenyl)picolinamide (3d): Colourless liquid, 62% yield; 1H NMR (400 MHz, CDCl3) δ 10.54 (s, 1H), 8.46 (d, J = 4.4 Hz, 1H), 8.21 (d, J = 7.7 Hz, 1H), 8.13 (d, J = 8.1 Hz, 1H), 7.79 (d, J = 7.7 Hz, 1H), 7.38–7.35 (m, 1H), 7.26 (t, J = 7.5 Hz, 1H), 7.19 (s, 1H), 7.12–7.06 (m, 2H), 7.01–6.91 (m, 3H), 4.10 (s, 2H), 2.20 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 162.50, 150.17, 148.24, 138.77, 137.67, 136.26, 134.98, 132.53, 130.61, 128.89, 128.83, 128.72, 128.25, 127.67, 126.53, 124.81, 123.13, 122.60, 37.50, 21.39. HRMS(ESI+): Calculated for C20H19N2OS+, [M+H]+ 335.1218. Found 335.1210.
N-(2-(((3-bromophenyl)thio)methyl)phenyl)picolinamide (3e): Colourless liquid, 50% yield; 1H NMR (400 MHz, CDCl3) δ 10.51 (s, 1H), 8.52 (d, J = 4.5 Hz, 1H), 8.23 (d, J = 7.8 Hz, 1H), 8.15 (d, J = 8.1 Hz, 1H), 7.86–7.81 (m, 1H), 7.61 (s, 1H), 7.41 (dd, J = 7.0, 5.1 Hz, 1H), 7.31–7.28 (m, 3H), 7.14 (d, J = 7.2 Hz, 1H), 7.08 (d, J = 7.9 Hz, 1H), 7.05–7.01 (m, 1H), 4.15 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 162.46, 150.03, 148.86, 148.37, 137.74, 136.03, 134.21, 130.61, 130.43, 130.33, 130.16, 129.06, 126.95, 126.65, 124.96, 123.27, 122.65, 120.39, 37.49. HRMS(ESI+): Calculated for C19H16BrN2OS+, [M+H]+ 399.0167. Found 399.0176.
N-(2-(((3-nitrophenyl)thio)methyl)phenyl)picolinamide (3f): Colourless liquid, 27% yield; 1H NMR (400 MHz, CDCl3) δ 10.42 (s, 1H), 8.47 (d, J = 4.4 Hz, 1H), 8.26–8.20 (m, 2H), 8.09 (d, J = 8.1 Hz, 1H), 8.00–7.94 (m, 1H), 7.85–7.81 (m, 1H), 7.61 (d, J = 7.7 Hz, 1H), 7.43–7.39 (m, 1H), 7.36 (t, J = 8.1 Hz, 1H), 7.30 (t, J = 7.7 Hz, 1H), 7.16 (s, 1H), 7.04 (d, J = 7.5 Hz, 1H), 4.22 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 162.46, 148.32, 137.79, 137.00, 132.47, 130.61, 129.70, 129.22, 126.74, 125.66, 125.18, 123.59, 122.65, 122.02, 121.73, 117.97, 114.82, 113.91, 36.99. HRMS(ESI+): Calculated for C19H16N3O3S+, [M+H]+ 366.0912. Found 366.0920.
N-(2-(((4-methoxyphenyl)thio)methyl)phenyl)picolinamide (3g): Colourless liquid, 78% yield; 1H NMR (400 MHz, CDCl3) δ 10.54 (s, 1H), 8.61–8.54 (m, 1H), 8.24 (d, J = 7.8 Hz, 1H), 8.16 (d, J = 8.0 Hz, 1H), 7.84 (td, J = 7.7, 1.7 Hz, 1H), 7.44–7.39 (m, 1H), 7.37–7.31 (m, 2H), 7.29–7.23 (m, 1H), 7.01–6.93 (m, 2H), 6.76–6.68 (m, 2H), 4.02 (s, 2H), 3.70 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 162.43, 159.82, 150.18, 148.22, 137.70, 136.12, 135.45, 130.65, 128.57, 128.00, 126.56, 125.07, 124.70, 123.06, 122.64, 114.58, 55.42, 39.14. HRMS(ESI+): Calculated for C20H19N2O2S+, [M+H]+ 351.1167. Found 351.1158.
N-(2-(((4-chlorophenyl)thio)methyl)phenyl)picolinamide (3h): Colourless liquid, 52% yield; 1H NMR (400 MHz, CDCl3) δ 10.38 (s, 1H), 8.40 (d, J = 4.4 Hz, 1H), 8.14 (d, J = 7.8 Hz, 1H), 8.04 (d, J = 8.1 Hz, 1H), 7.77–7.73 (m, 1H), 7.32 (dd, J = 6.8, 5.0 Hz, 1H), 7.21 (t, J = 6.0 Hz, 3H), 7.09 (d, J = 5.9 Hz, 2H), 6.99 (d, J = 7.0 Hz, 1H), 6.91 (t, J = 7.3 Hz, 1H), 4.01 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 162.44, 150.06, 148.20, 137.75, 136.16, 133.63, 133.40, 130.58, 129.15, 128.90, 127.43, 126.63, 124.94, 123.31, 122.66, 37.70. HRMS(ESI+): Calculated for C19H16ClN2OS+, [M+H]+ 355.0672. Found 355.0677.
N-(2-(((4-bromophenyl)thio)methyl)phenyl)picolinamide (3i): Colourless liquid, 59% yield; 1H NMR (400 MHz, CDCl3) δ 10.46 (s, 1H), 8.47 (d, J = 4.4 Hz, 1H), 8.22 (d, J = 7.8 Hz, 1H), 8.12 (d, J = 8.1 Hz, 1H), 7.86–7.81 (m, 1H), 7.40 (dd, J = 6.8, 5.1 Hz, 1H), 7.30 (t, J = 7.8 Hz, 3H), 7.23 (d, J = 8.4 Hz, 2H), 7.08 (d, J = 7.0 Hz, 1H), 7.00 (t, J = 7.3 Hz, 1H), 4.10 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 162.44, 150.05, 148.23, 137.76, 136.17, 134.39, 133.50, 132.10, 130.58, 128.93, 127.40, 126.65, 124.97, 123.34, 122.67, 121.62, 77.48, 77.16, 76.84, 37.52. HRMS(ESI+): Calculated for C19H16BrN2OS+, [M+H]+ 399.0167. Found 399.0161.
N-(2-(((4-nitrophenyl)thio)methyl)phenyl)picolinamide (3j): Light yellow liquid, 25% yield; 1H NMR (400 MHz, CDCl3) δ 10.36 (s, 1H), 8.34 (d, J = 4.1 Hz, 1H), 8.21 (d, J = 7.7 Hz, 1H), 8.05 (d, J = 8.6 Hz, 3H), 7.83 (t, J = 7.5 Hz, 1H), 7.40 (d, J = 8.4 Hz, 3H), 7.34–7.26 (m, 2H), 7.08 (t, J = 7.4 Hz, 1H), 4.27 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 162.43, 149.76, 148.17, 141.96, 137.89, 136.19, 130.48, 129.38, 128.42, 126.80, 126.61, 125.50, 124.06, 123.84, 122.71, 117.37, 77.48, 77.16, 76.84, 35.22. HRMS(ESI+): Calculated for C19H16N3O3S+, [M+H]+ 366.0912. Found 366.0922.
N-(5-methyl-2-((phenylthio)methyl)phenyl)picolinamide (3k): Colourless liquid, 57% yield; 1H NMR (400 MHz, CDCl3) δ 10.55 (s, 1H), 8.49 (d, J = 4.3 Hz, 1H), 8.25 (d, J = 7.6 Hz, 1H), 8.01 (s, 1H), 7.86 (d, J = 7.0 Hz, 1H), 7.45–7.40 (m, 3H), 7.24–7.19 (m, 2H), 7.16 (s, 1H), 7.03 (d, J = 7.7 Hz, 1H), 6.83 (d, J = 7.5 Hz, 1H), 4.14 (s, 2H), 2.32 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 162.46, 150.20, 148.24, 138.82, 137.69, 136.02, 135.57, 131.73, 130.40, 129.00, 127.28, 126.52, 125.67, 124.49, 123.71, 122.58, 37.24, 21.56 HRMS(ESI+): Calculated for C20H19N2OS+, [M+H]+ 335.1218. Found 335.1228.
N-(2-(((4-chlorophenyl)thio)methyl)-5-methylphenyl)picolinamide (3l): Colourless liquid, 44% yield; 1H NMR (400 MHz, CDCl3) δ 10.48 (s, 1H), 8.50 (d, J = 4.3 Hz, 1H), 8.25 (d, J = 7.8 Hz, 1H), 8.00 (s, 1H), 7.87 (dd, J = 11.0, 4.3 Hz, 1H), 7.45–7.42 (m, 1H), 7.34 (d, J = 8.4 Hz, 2H), 7.22–7.19 (m, 2H), 7.00 (d, J = 7.7 Hz, 1H), 6.84 (d, J = 7.6 Hz, 1H), 4.10 (s, 2H), 2.33 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 162.39, 150.06, 148.20, 138.98, 137.75, 135.90, 133.86, 133.48, 133.23, 130.40, 129.13, 126.61, 125.76, 124.29, 123.84, 122.61, 77.48, 77.16, 76.84, 37.44, 21.55. HRMS(ESI+): Calculated for C20H18ClN2OS+, [M+H]+ 369.0828. Found 369.0825.
N-(4-chloro-2-((phenylthio)methyl)phenyl)picolinamide (3m): Colourless liquid, 47% yield; 1H NMR (400 MHz, CDCl3) δ 10.52 (s, 1H), 8.48 (d, J = 4.5 Hz, 1H), 8.21 (d, J = 7.8 Hz, 1H), 8.12 (d, J = 8.7 Hz, 1H), 7.83 (t, J = 7.7 Hz, 1H), 7.42–7.38 (m, 3H), 7.24–7.20 (m, 3H), 7.18 (s, 1H), 7.06 (d, J = 2.3 Hz, 1H), 4.06 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 162.48, 149.82, 148.29, 137.78, 134.80, 134.58, 132.21, 130.31, 129.71, 129.35, 129.15, 128.63, 127.80, 126.73, 124.20, 122.65, 37.24. HRMS(ESI+): Calculated for C19H16ClN2OS+, [M+H]+ 355.0672. Found 355.0679.
N-(3-chloro-2-(((4-methoxyphenyl)thio)methyl)phenyl)picolinamide (3n): Colourless liquid, 44% yield; 1H NMR (400 MHz, CDCl3) δ 10.46 (s, 1H), 8.55 (d, J = 4.4 Hz, 1H), 8.20 (d, J = 7.8 Hz, 1H), 8.05 (d, J = 8.1 Hz, 1H), 7.85–7.81 (m, 1H), 7.44–7.42 (m, 1H), 7.39 (d, J = 8.8 Hz, 2H), 7.16 (s, 1H), 7.09 (d, J = 7.9 Hz, 1H), 6.70 (d, J = 8.7 Hz, 2H), 4.21 (s, 2H), 3.69 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 162.52, 160.12, 149.86, 148.23, 137.75, 137.69, 136.06, 134.96, 128.79, 126.74, 125.93, 124.52, 122.70, 121.70, 114.93, 114.61, 55.43, 35.28. HRMS(ESI+): Calculated for C20H18ClN2OS+, [M+H]+ 385.0778. Found 385.0782.
6-methyl-N-(2-((phenylthio)methyl)phenyl)picolinamide (3p): White solid, 59% yield; 1H NMR (400 MHz, CDCl3) δ 10.68 (s, 1H), 8.20 (d, J = 8.1 Hz, 1H), 8.07 (d, J = 7.6 Hz, 1H), 7.74 (t, J = 7.7 Hz, 1H), 7.36 (d, J = 7.2 Hz, 2H), 7.32–7.24 (m, 3H), 7.12–7.19 (m, 2H), 7.12 (d, J = 7.3 Hz, 1H), 7.02 (t, J = 7.4 Hz, 1H), 4.18 (s, 2H), 2.43 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 162.68, 157.46, 149.37, 137.88, 136.37, 135.43, 130.98, 130.67, 129.01, 128.77, 127.32, 127.08, 126.30, 124.69, 123.07, 119.68, 36.74, 24.18. HRMS(ESI+): Calculated for C20H19N2OS+, [M+H]+ 335.1218. Found 335.1223.
N-(2-(((4-methoxyphenyl)thio)methyl)phenyl)-3-methylpicolinamide (3q): Colourless liquid, 61% yield; 1H NMR (400 MHz, CDCl3) δ 10.64 (s, 1H), 8.39 (d, J = 4.1 Hz, 1H), 8.08 (d, J = 8.1 Hz, 1H), 7.57 (d, J = 7.6 Hz, 1H), 7.31–7.28 (m, 3H), 7.24 (t, J = 7.3 Hz, 1H), 6.99–6.92 (m, 2H), 6.70 (d, J = 8.5 Hz, 2H), 4.00 (s, 2H), 3.70 (s, 3H), 2.74 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.08, 159.79, 147.25, 145.65, 141.30, 136.42, 136.34, 135.33, 130.64, 128.46, 128.25, 126.12, 125.29, 124.54, 123.20, 114.59, 55.45, 39.09, 20.92. HRMS(ESI+): Calculated for C21H21N2O2S+, [M+H]+ 365.1324. Found 365.1328.
N-(2-(((4-methoxyphenyl)thio)methyl)phenyl)quinoline-2-carboxamide (3r): White solid, 69% yield; 1H NMR (400 MHz, CDCl3) δ 10.82 (s, 1H), 8.32 (q, J = 8.5 Hz, 2H), 8.19 (d, J = 8.0 Hz, 1H), 8.01 (d, J = 8.5 Hz, 1H), 7.84 (d, J = 7.5 Hz, 1H), 7.69–7.67 (m, 1H), 7.60–7.53 (m, 1H), 7.34–7.26 (m, 3H), 6.97 (d, J = 4.1 Hz, 2H), 6.71–6.66 (m, 2H), 4.08 (s, 2H), 3.68 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 162.65, 159.74, 149.96, 146.52, 137.88, 136.23, 135.09, 130.79, 130.31, 130.14, 129.58, 128.63, 128.29, 128.03, 127.85, 125.07, 124.68, 123.03, 118.98, 114.64, 55.43, 38.84. HRMS(ESI+): Calculated for C24H21N2O2S+, [M+H]+ 401.1324. Found 401.1330
N-(2-(((4-methoxyphenyl)thio)methyl)phenyl)isoquinoline-3-carboxamide (3s): White solid, 72% yield; 1H NMR (400 MHz, CDCl3) δ 10.76 (s, 1H), 9.70–9.60 (m, 1H), 8.47 (d, J = 5.5 Hz, 1H), 8.16 (d, J = 8.1 Hz, 1H), 7.82–7.78 (m, 2H), 7.69–7.61 (m, 2H), 7.35–7.26 (m, 3H), 7.04–6.94 (m, 2H), 6.75–6.68 (m, 2H), 4.05 (s, 2H), 3.67 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.13, 159.78, 147.92, 140.29, 137.74, 136.32, 135.34, 130.71, 128.99, 128.53, 128.50, 127.93, 127.48, 127.06, 125.14, 124.94, 124.77, 123.37, 114.58, 77.48, 77.16, 76.84, 55.41, 39.12. HRMS(ESI+): Calculated for C24H21N2O2S+, [M+H]+ 401.1324. Found 401.1329.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30224462/s1, Figure S1: 1H NMR Spectra of Compound 3a; Figure S2: 13C NMR Spectra of Compound 3a; Figure S3: 1H NMR Spectra of Compound 3c; Figure S4:13C NMR Spectra of Compound 3c; Figure S5: 1H NMR Spectra of Compound 3d; Figure S6: 13CNMR Spectra of Compound 3d; Figure S7: 1H NMR Spectra of Compound 3e; Figure S8: 13C NMR Spectra of Compound 3e; Figure S9: 1H NMR Spectra of Compound 3f; Figure S10: 13C NMR Spectra of Compound 3f; Figure S11: 1H NMR Spectra of Compound 3g; Figure S12: 13C NMR Spectra of Compound 3g; Figure S13: 1H NMR Spectra of Compound 3h; Figure S14: 13C NMR Spectra of Compound 3h; Figure S15: 1H NMR Spectra of Compound 3i; Figure S16: 13C NMR Spectra of Compound 3i; Figure S17: 1H NMR Spectra of Compound 3j; Figure S18: 13C NMR Spectra of Compound 3j; Figure S19: 1H NMR Spectra of Compound 3k; Figure S20: 13C NMR Spectra of Compound 3k; Figure S21: 1H NMR Spectra of Compound 3l; Figure S22: 13C NMR Spectra of Compound 3l; Figure S23: 1H NMR Spectra of Compound 3m; Figure S24: 13C NMR Spectra of Compound 3m; Figure S25: 1H NMR Spectra of Compound 3n; Figure S26: 13C NMR Spectra of Compound 3n; Figure S27: 1H NMR Spectra of Compound 3p; Figure S28: 13C NMR Spectra of Compound 3p; Figure S29: 1H NMR Spectra of Compound 3q; Figure S30: 13C NMR Spectra of Compound 3q; Figure S31: 1H NMR Spectra of Compound 3r; Figure S32: 13C NMR Spectra of Compound 3r; Figure S33: 1H NMR Spectra of Compound 3s; Figure S34: 13C NMR Spectra of Compound 3s.
Author Contributions
Conceptualization, writing—original draft preparation, J.Q.; Writing—review and editing, S.Z. and J.L.; Supervision, G.W.; project administration, K.W.; funding acquisition, K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2022QB125).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Authors Jinjing Qin and Jinwen Luo were employed by the company Zhejiang Anglikang Pharmaceutical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhao, C.; Rakesh, K.P.; Ravidar, L.; Fang, W.Y.; Qin, H.L. Pharmaceutical and medicinal significance of sulfur (SVI)-Containing motifs for drug discovery: A critical review. Eur. J. Med. Chem. 2019, 162, 679–734. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.C.; Mano, R.A.; Oliveira, D.H.; Maia, D.S.V.; Silva, W.P.; Savegnago, L.; Lenardão, E.J.; Jacob, R.G. Synthesis, antimicrobial, and antioxidant activities of chalcogen-containing nitrone derivatives from (R)-citronellal. Medicines 2017, 4, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Amlashi, D.M.; Mobini, S.; Shahedi, M.; Habibi, Z.; Bavandi, H.; Yousef, M. Biocatalytic synthesis of oxa(thia)diazole aryl thioethers. Sci. Rep. 2024, 14, 19468. [Google Scholar]
- Wang, F.; Langley, R.; Gulten, G.L.; Dover, G.; Besra, G.S.; Jacobs, W.R.; Sacchettini, J.C. Mechanism of thioamide drug action against tuberculosis and leprosy. J. Exp. Med. 2007, 204, 73–78. [Google Scholar] [CrossRef]
- Thomas, G.L.; Spandl, R.J.; Glansdorp, F.G.; Welch, M.; Bender, A.; Cockfield, J.; Lindsay, J.A.; Bryant, C.; Brown, D.F.J.; Loiseleur, O.; et al. Anti-MRSA agent discovery using diversity-oriented synthesis. Angew. Chem. Int. Ed. 2008, 47, 2808–2812. [Google Scholar] [CrossRef]
- Woo, C.M.; Gholap, S.L.; Herzon, S.B. Insights into lomaiviticin biosynthesis. isolation and structure elucidation of (-)-homoseongomycin. J. Nat. Prod. 2013, 76, 1238–1241. [Google Scholar] [CrossRef]
- Ma, N.N.; Hu, X.B.; Wu, Y.S.; Zheng, Y.W.; Ma, M.T.; Chu, X.Q.; Xu, H.; Luo, H.Q.; Shen, Z.L. Nickel-catalyzed direct cross-coupling of aryl thioether with aryl bromide. Org. Lett. 2023, 25, 1771–1775. [Google Scholar] [CrossRef]
- Nair, D.P.; Podgorski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The thiol-michael addition click reaction: A powerful and widely used tool in materials chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Luz, E.Q.; Seckler, D.; Araújo, J.S.; Angst, L.; Lima, D.B.; Rios, E.A.M.; Ribeiro, R.R.; Rampona, D.S. Fe(III)-catalyzed direct C3 chalcogenylation of indole: The effect of iodide ions. Tetrahedron 2019, 75, 1258–1266. [Google Scholar] [CrossRef]
- Nalbandian, C.J.; Miller, E.M.; Toenjes, S.T.; Gustafson, J.L. A conjugate lewis base-brønsted acid catalyst for the sulfenylation of nitrogen containing heterocycles under mild conditions. Chem. Commun. 2017, 53, 1494–1497. [Google Scholar] [CrossRef]
- Schlosser, K.M.; Krasutsky, A.P.; Hamilton, H.W.; Reed, J.E.; Sexton, K. A highly efficient procedure for 3-sulfenylation of ondole-2-carboxylates. Org. Lett. 2004, 6, 819–821. [Google Scholar] [CrossRef]
- Tang, Q.; Song, D.G.; Zhang, K.L.; Mao, W.H.; Zhao, X.H.; Du, D.; Ling, F.; Zhong, W.H. Development of an imidazole-based N,N-bidentate ligand for the manganese catalyzed direct coupling of nitriles with alcohols. RSC Adv. 2024, 14, 12978–12982. [Google Scholar] [CrossRef]
- Li, M.Y.; Eisen, M.S.; Cai, Z.G. Robust cobalt catalysts with N,N-bidentate aldimine imidazolidine-2-imine/guanidine ancillary ligand for isoprene polymerization. J. Catal. 2024, 435, 115581. [Google Scholar] [CrossRef]
- Wu, W.R.; Zhao, X.F.; Chen, G.; Liu, L.J.; Li, Y.L.; Chen, T.; James, T.D.; Liu, Y.X. Overlooked potential of N,N-bidentate directing-groups in Ni-catalyzed C-H functionalization of benzamides. Chem. Commun. 2023, 59, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Pati, B.V.; Sagara, P.S.; Ghosh, A.; Adhikari, G.K.D.; Ravikumar, P.C. Ruthenium-catalyzed regioselective C(sp2)-H activation/annulation of N-(7-Azaindole)amides with 1,3-diynes using N-amino-7-azaindole as the N,N-bidentate directing group. J. Org. Chem. 2021, 86, 9428–9443. [Google Scholar] [CrossRef] [PubMed]
- Bag, R.; Sharma, N.K. Pd-catalyzed picolinamide-directed late-stage chalcogenation of tryptophan-containing peptides. J. Org. Chem. 2023, 88, 15666–15686. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zeng, L.L.; Fei, Q.Y.; Ge, Y.X.; Huang, R.H.; Chen, F.E. Recent advances in remote C(sp3)–H functionalization via chelating group-assisted metal-catalyzed chain-walking reaction. Angew. Chem. Int. Ed. 2019, 58, 5633–5638. [Google Scholar] [CrossRef]
- Bhavyesh, D.; Soliya, S.; Konakanchi, R.; Begari, E.; Ashalu, K.C.; Naveen, T. The recent advances in iron-catalyzed C(sp3)-H functionalization. Chem. Asian J. 2024, 19, e202301056. [Google Scholar] [CrossRef]
- Sheng, T.; Zhuang, Z.; Zhao, Z.H.; Hoque, M.E.; Yu, J.-Q. Copper-catalyzed γ-C(sp3)-H lactamization and iminolactonization. Angew. Chem. 2025, 64, e202416634. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.-Y.; He, G.; Nack, W.A.; Chen, G. Palladium-catalyzed picolinamide-directed acetoxylation of unactivated γ-C(sp3)-H bonds of alkylamines. Chin. Chem. Lett. 2024, 35, 109647. [Google Scholar] [CrossRef]
- Xie, Y.J.; Yang, Y.Z.; Huang, L.H.; Zhang, X.B.; Zhang, Y.H. Pd-catalyzed arylation/oxidation of benzylic C-H bond. Org. Lett. 2012, 14, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Yu, M.; Zhang, J.T.; Liu, Z.X.; Zhang, Y.H. Synthesis of benzyl esters via functionalization of multiple C–H bonds by palladium catalysis. Org. Lett. 2015, 17, 5300–5303. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Yao, J.Z.; Wu, Z.H.; Liu, Z.X.; Zhang, Y.H. Palladium-catalyzed oxidative acetoxylation of benzylic C-H bond using bidentate auxiliary. J. Org. Chem. 2013, 78, 10821–10831. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Yin, W.Y.; Zhang, Y.; Zhang, Y.N.; Huang, Y. Palladium catalyzed acetoxylation of benzylic C–H bonds using a bidentate picolinamide directing group. Org. Biomol. Chem. 2014, 12, 1405–1411. [Google Scholar] [CrossRef]
- Wang, K.; Hou, J.H.; Zhang, C.; Cheng, K.; Bai, R.R.; Xie, Y.Y. Palladium-catalyzed picolinamide-directed benzylic C(sp3)-H chalcogenation with diaryl disulfides and diphenyl diselenide. Adv. Synth. Catal. 2020, 362, 2947–2952. [Google Scholar] [CrossRef]
- Yang, T.X.; Zhang, Y.B.; Dou, Y.C.; Yang, D.D.; Niu, J.L. Earth-abundant cobalt-catalyzed enantioselective C-H functionalizations. Sci. China Chem. 2025, 65, 1–21. [Google Scholar] [CrossRef]
- Meher, N.K.; Kumar, P.; Geetharani, V.K. Cobalt-catalyzed regioselective 1,2-hydroboration of N-heteroarenes. Org. Lett. 2023, 25, 87–92. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Y.; Homölle, S.L.; Oliveira, J.C.A.; Ackermann, L. Enantioselective cobaltaphotoredox-catalyzed C-H activation. J. Am. Chem. Soc. 2024, 146, 24105–24113. [Google Scholar] [CrossRef]
- Saha, A.; Ramesh, E.; Sahoo, A.K. Brønsted acid promoted sulfenylacyloxylation of alkynes: Access to 4-sulfenylisocoumarins. Adv. Synth. Catal. 2022, 364, 3496–3500. [Google Scholar] [CrossRef]
- Li, M.L.; Wang, J.J. Cobalt-catalyzed direct C-H thiolation of aromatic amides with disulfides: Application to the synthesis of quetiapine. Org. Lett. 2018, 20, 6490–6493. [Google Scholar] [CrossRef]
- Münchow, T.; Pandit, N.K.; Dana, S.; Boos, P.; Peters, S.E.; Boucat, J.; Liu, Y.R.; Scheremetjew, A.; Ackermann, L. Enantioselective C-H annulations enabled by either nickel- or cobalt-electrocatalysed C-H activation for catalyst-controlled chemodivergence. Nat. Catal. 2025, 8, 257–269. [Google Scholar] [CrossRef]
- Lan, J.Y.; Xie, H.S.; Lu, X.X.; Deng, Y.F.; Jiang, H.F.; Zeng, W. Co(II)-catalyzed regioselective cross-dehydrogenative coupling of aryl C–H bonds with carboxylic acids. Org. Lett. 2017, 19, 4279–4282. [Google Scholar] [CrossRef]
- Wu, H.X.; Guo, W.J.; Daniel, S.; Li, Y.; Liu, C.; Zeng, Z. Fluoride-catalyzed esterification of amides. Chem. Eur. J. 2018, 24, 3444–3447. [Google Scholar] [CrossRef]
- Eitzinger, A.; Otevrel, J.; Haider, V.; Macchia, A.; Massa, A.; Faust, K.; Spingler, B.; Berkessel, A.; Waser, M. Enantioselective bifunctional ammonium salt-catalyzed syntheses of 3-CF3S-, 3-RS-, and 3-F-substituted isoindolinones. Adv. Synth. Catal. 2014, 363, 1955–1962. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).