Abstract
The advent of nanotechnology has revolutionized the field of medicine, particularly in the development of targeted therapeutic strategies. Among these, radiolabeled nanomaterials have emerged as promising tools for both diagnostic and therapeutic applications, offering precise delivery of radiation to diseased tissues while minimizing damage to healthy ones. Notably, Lutetium-177 (177Lu) has gained significant attention due to its favorable emission properties and availability that render it suitable for imaging and therapeutic purposes. When integrated with magnetic nano-formulations, 177Lu-labeled systems combine the benefits of targeted radiation therapy (TRT) with the unique properties of magnetic nanoparticles (MNPs), such as magnetic resonance imaging (MRI) contrast enhancement and magnetically guided drug delivery to address challenges in diagnosis and treatment of diseases, such as cancer. By examining the latest advancements in their design, particularly surface functionalization and bioconjugation strategies, this study aims to highlight their efficacy in targeted therapy, imaging, and theranostic applications. Furthermore, we discuss the current challenges, such as scalability, biocompatibility, and regulatory hurdles, while proposing future directions to enhance their clinical translation. This comprehensive review underscores the transformative potential of 177Lu-labeled magnetic nano-formulations in precision medicine and their role in shaping the future of therapeutic interventions.
1. Introduction
Cancer remains a major global health threat, standing as the second most common cause of mortality worldwide with an estimated 10 million fatalities recorded in 2022 alone []. The World Health Organization projects a 77% increase in cancer cases by 2050, underscoring the pressing need for more effective treatment modalities []. While conventional approaches, including surgical resection, cytotoxic chemotherapy, and external beam radiation therapy, remain cornerstones of oncologic care, their clinical utility is often limited by significant drawbacks. These include non-specific biodistribution of chemotherapeutic agents leading to systemic toxicity, radiation damage to healthy tissues adjacent to tumors, and the emergence of multidrug resistance mechanisms in malignant cells [].
In this therapeutic landscape, TRT has emerged as a paradigm-shifting approach that combines the precision of molecular targeting with the potent cytotoxic effects of ionizing radiation []. This innovative strategy employs radiopharmaceuticals designed to selectively accumulate in tumor tissue through various mechanisms, including receptor-ligand interactions, antigen–antibody binding, or metabolic trapping []. The fundamental advantage of TRT lies in its ability to deliver lethal radiation doses specifically to cancer cells while largely sparing normal tissues, thereby achieving an improved therapeutic index compared to conventional treatments [].
177Lu has emerged as one of the most clinically valuable radionuclides for TRT and Single Photon Emission Computed Tomography (SPECT) imaging due to its ideal physico-chemical and emission properties. These properties facilitate the ‘crossfire’ effect on neighboring cancer cells while sparing the normal surrounding tissue with minimal effect []. The versatility of 177Lu, combined with its well-characterized chemistry for stable chelation with various polydentate macrocyclic ligands (e.g., tetraxetan), makes it a cornerstone of modern TRT development, with ongoing research focused on optimizing dosimetry, combination therapies, and personalized treatment approaches [,]. The success of 177Lu-labeled compounds, particularly [177Lu]Lu-DOTATATE (Lutathera®) for neuroendocrine tumors [] and [177Lu]Lu-Vivipotide tetraxetan (Pluvicto®) for the treatment of prostate tumors expressing somatostatin [], has demonstrated the clinical potential of this radionuclide in peptide receptor radionuclide therapy (PRRT) and radioligand therapy, showing significant improvements in progression-free survival with favorable safety profiles [].
Recent advances in nanotechnology have further enhanced the potential of 177Lu-based therapies by incorporating MNPs as multifunctional carriers. These nano-formulations combine the benefits of radiation therapy with magnetic targeting, enabling improved tumor accumulation and retention []. MNPs, particularly those based on iron oxides (e.g., Fe3O4), exhibit excellent biocompatibility, superparamagnetic behavior, and surface functionalization versatility, rendering them ideal for theranostic applications []. When labeled with 177Lu, these NPs can be guided to tumor sites using external magnetic fields, enhancing therapeutic precision while reducing off-target effects [].
The synthesis of 177Lu-labeled magnetic nano-formulations involves critical steps, including NP preparation, surface modification, radiolabeling optimization, and thorough physico-chemical characterization []. Key parameters such as particle size, surface charge, colloidal stability, radiolabeling efficiency, and in vitro/in vivo behavior must be meticulously evaluated to ensure therapeutic efficacy []. Additionally, the integration of targeting ligands (e.g., peptides, antibodies, or small molecules) can further enhance tumor specificity, paving the way for personalized cancer therapy [].
This review article provides, for the first time, a comprehensive analysis of 177Lu-labeled magnetic nano-formulations, consolidating current knowledge on their synthesis strategies, radiochemical and physico-chemical properties, and biological applications. Furthermore, we discuss current challenges, such as scalability, long-term stability, functional implications, and potential toxicity, while exploring future perspectives, including combination therapies, multimodal imaging, and smart stimulus-responsive designs. By critically evaluating recent advancements, this review aims to highlight the transformative potential of 177Lu-labeled magnetic nano-formulations in oncology and inspire further research toward clinical translation.
2. 177Lutetium
2.1. 177Lutetium Chemistry
Lutetium (Lu), the final element in the lanthanide series, possesses 71 electrons with an electronic configuration of [Xe]4f145d16s2. In chemical reactions, Lu typically loses its two 6s electrons and the single 5d electron, forming a trivalent (+3) cation. This +3 oxidation state is dominant across lutetium’s compounds, including oxides and halides, aligning it with conventional lanthanide behavior. Among the lanthanides, Lu(III) has the smallest atomic radius due to lanthanide contraction, contributing to its exceptional hardness and density. While most lanthanides belong to the f-block, lutetium’s filled 4f orbital (14 electrons) allows it to also be classified as the first d-block element in the sixth period. In the +3 state, Lu(III) retains empty s, p, and d orbitals while maintaining a closed 4f shell. The tightly bound f-electrons, shielded by a highly effective nuclear charge, do not participate in bonding, making Lu(III) a hard Lewis acid whose chemistry is primarily dictated by its vacant s, p, and d orbitals. With an ionic radius of just 86.1 pm, the smallest of all lanthanides, Lu(III), accommodates fewer ligands due to spatial constraints. Coordination geometry is largely determined by ligand-ligand repulsions rather than directional bonding contributions from s, p, or d orbitals. Lu(III) complexes exhibit variable coordination numbers, typically ranging from 6 to 9, demonstrating the element’s versatile bonding characteristics [].
The International Commission on Radiological Protection (ICRP) has catalogued over 1200 radionuclides, yet only a few dozen are routinely used in clinical and scientific settings []. As shown in Table 1, these radionuclides possess distinct physical and biochemical properties, including half-life, decay mode, radiation energy, and particle range, which determine their specific applications. The table also indicates their common production methods and reactions. For clinical purposes, radionuclides are broadly categorized as either imaging isotopes or therapeutic isotopes, which correspond to their primary decay modes (e.g., γ-emitters for imaging and α−, β−, or Auger electron emitters for therapy) [,].
The selection of an optimal radionuclide is a multifactorial decision, extending beyond the general category of radiation. Critical considerations involve: (i) ensuring the emission range is appropriate for the dimensions of the target structures, with longer-range emitters potentially compensating for heterogeneous uptake of the carrier molecule, (ii) aligning the radionuclide’s physical half-life with the carrier’s pharmacokinetic profile to maximize energy deposition within the target at the critical time, (iii) accounting for the abundance of photon emissions, which is relevant for both diagnostic imaging and radiation protection, and (iv) evaluating the implications of any radioactive daughter nuclides, which may contribute to the overall dose but also pose a risk of redistributing away from the intended site [].

Table 1.
Radionuclide properties. Data from [,].
Table 1.
Radionuclide properties. Data from [,].
| Radionuclides for SPECT Imaging | |||||
| Radionuclide | Haf-Life | Max Energy (keV) | Decay | Production | Common Production Reaction |
| Au-198 | 2.7 days | 960 | β−, γ | Cyclotron | 197Au(n,g)198Au |
| Au-199 | 3.1 days | 452.6 | β−, γ | Cyclotron | 198Au(n,g)199Au |
| Co-57 | 270 days | 692 | Electron capture, γ | Cyclotron | 56Fe(d,n)57Co |
| Fe-59 | 44.5 days | 1291 | β−, γ | Cyclotron | 59Co(p,n)59Fe |
| Ga-67 | 78.3 h | 300 | Auger e−, γ | Cyclotron | 68Zn(p,2n)67Ga |
| Gd-153 | 240.4 days | 103 | Electron capture, γ | Cyclotron | 152Gd(n,g)153Gd |
| In-111 | 2.81 days | 245 | γ | Cyclotron | 111Cd(p,n)111In |
| I-123 | 13.3 h | 159 | Auger e−, γ | Cyclotron | 127I(p,5n)123Xe |
| Re-186 | 91 h | 1080 | β−, γ | Cyclotron | 186W(p,n)186Re |
| Tc-99m | 6.0 h | 140 | γ | Generator | 99Mo/99mTc |
| Tl-201 | 3.0 days | 71 | γ | Cyclotron | 203Tl(p,3n)201Pb |
| Radionuclides for PET imaging | |||||
| As-72 | 25.9 h | 3320 | β+ | Cyclotron | 72Ge(p,n)72As |
| Br-76 | 16 h | 3980 | β+ | Cyclotron | 76Se(p,n)76Br |
| C-11 | 20.4 min | 961 | β+ | Cyclotron | 14N(p,a)11C |
| Cu-62 | 9.7 min | 2926 | β+ | Generator | 62Zn/62Cu |
| Cu-64 | 12.7 h | 656 | Electron capture, β+, β− | Cyclotron | 64Ni(p,n)64Cu |
| F-18 | 109.7 min | 634 | Electron capture, β+ | Cyclotron | 18F(F−):18O(p,n)18F |
| Ga-68 | 67.6 min | 1899 | Electron capture, β+ | Generator/ Cyclotron | 68Ge/68Ga |
| Ge-69 | 39.1 h | 1205 | β+ | Cyclotron | 69Ga(p,n)69Ge |
| I-124 | 4.2 days | 2100 | Electron capture, β+ | Cyclotron | 124Te(p,n)124I |
| Mn-52 | 5.6 days | 1434 | β+ | Cyclotron | 52Cr(p,n)52Mn |
| N-13 | 9.9 min | 1199 | β+ | Cyclotron | 16O(p,a)13N |
| O-15 | 2.1 min | 1732 | β+ | Cyclotron | 15N(p,n)15O |
| Rb-82 | 1.3 min | 3378 | Electron capture, β+ | Generator | 82Sr/82Rb |
| Y-86 | 14.7 h | 3150 | β+ | Cyclotron | 86Sr(p,n)86Y |
| Zr-89 | 78.4 h | 900 | Electron capture, β+ | Cyclotron | 89Y(p,n)89Zr |
| Radionuclides for therapy applications (β-emission, Linear energy transfer ~0.2 keV.μm−1) | |||||
| Radionuclide | Haf-life | Maximum energy (keV) | Decay | Production | Maximum particle range |
| Au-198 | 2.7 days | 960 | β−, γ | Cyclotron | 4.0 mm |
| Y-90 | 64.0 h | 2280 | β− | Generator | 12.0 mm |
| Lu-177 | 6.7 days | 500 | β−, γ | Cyclotron | 1.5 mm |
| I-131 | 8.0 days | 610 | β−, γ | Fission | 2.0 mm |
| Cu-67 | 62 h | 577 | β−, γ | Cyclotron | 1.8 mm |
| Re-186 | 91 h | 1080 | β−, γ | Cyclotron | 5.0 mm |
| Re-188 | 16.9 h | 2120 | β−, γ | Generator | 10.0 mm |
| Radionuclides for therapy applications (α-emission, Linear energy transfer ~80 keV.μm−1) | |||||
| At-211 | 7.2 h | 6000 | α | Cyclotron | 0.08 mm |
| Ac-225 | 10 days | 8000 | α, β− | Cyclotron | 0.1 mm |
| Bi-212 | 60.6 min | 6000 | α, β− | Cyclotron | 0.09 mm |
| Bi-213 | 46 min | 6000 | α, β− | Cyclotron | <0.1 mm |
| Ra-223 | 11.4 days | 7000 | α, β− | Cyclotron | <0.1 mm |
| Pb-212 | 10.6 h | 7800 | α, β− | Cyclotron | <0.1 mm |
| Tb-149 | 4.2 h | 400 | α | Cyclotron | <0.1 mm |
| Radionuclides for therapy applications (Auger-emission, Linear energy transfer ~4–26 keV.μm−1) | |||||
| Ga-67 | 78.3 h | 300 | Auger e−, γ | Cyclotron | 10 nm |
| I-123 | 13.3 h | 159 | Auger e−, γ | Cyclotron | 10 nm |
| I-125 | 60.5 days | 27 | Auger e−, γ | Neutron activation | 10 nm |
The two main forms of radionuclide imaging are SPECT and Positron Emission Tomography (PET), relying on single-photon and positron-emitting radionuclides []. The short half-lives of these isotopes help limit radiation dose to patients. In contrast, choosing a radionuclide for therapy requires different criteria. An ideal therapeutic agent has a half-life between 6 h and 10 days [] and emits high linear energy transfer radiation for potent cell killing []. These requirements are met by three primary types of radionuclides: β-emitters, α-emitters, and Auger electron emitters. The basic characteristics of these types of particles are presented in Table 2 [].

Table 2.
Physical and biological characteristics of α, β particles, and Auger electrons. Data from [].
177Lu undergoes radioactive decay with a half-life of 6.646 days. In 79.4% of decay events, it emits beta particles (β−) with a maximum energy (Eβmax) of 497.1 keV, transitioning directly to the stable ground state of Hafnium-177 (177Hf). Additionally, 9.0% of decays occur via β− emission with Eβmax = 385 keV, while 11.61% involve β− emission at Eβmax = 177 keV, both leading to excited states of 177Hf at 249.67 keV and 321.32 keV above the ground state, respectively. These excited states then relax to the ground state by emitting gamma photons. The emitted gamma rays of 112.95 keV and 208.37 keV occur via an intermediate state that has a half-life of 0.583 nanoseconds []. A simplified decay scheme of 177Lu is presented below in Figure 1. The energy of beta particles enables optimal soft tissue penetration up to 2 mm, effectively destroying tumor cells while minimizing radiation exposure effects on surrounding healthy tissues [,,].
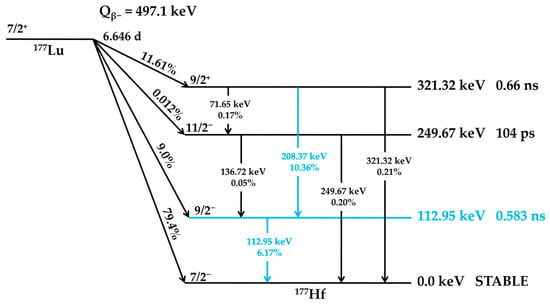
Figure 1.
Simplified decay scheme of 177Lu. Levels, spins, parities, energies, and half-lives of the excited states are based on literature-reported data [,,]. The decay pathways and energy states involved in gamma-ray cascades are highlighted in blue.
In recent years, the radionuclide 177Lu has gained significant interest across research, commercial, and clinical fields due to its potential in various diagnostic and therapeutic applications [,,,,,,,]. Although it emerged relatively late, in a short period, 177Lu has not only proven its effectiveness but also secured a prominent position in nearly all areas of in vivo radionuclide imaging and treatment, becoming a leading choice for TRT. 177Lu is ideal for radiolabeling bioactive tracer molecules, using a chemistry similar to the positron emitter 68Ga. This compatibility enables the creation of a matched diagnostic/therapeutic pair. Such a pair facilitates a theranostic strategy, where high-resolution quantitative PET imaging with 68Ga is used for patient selection, followed by targeted therapy with 177Lu for treatment and monitoring. Consequently, these properties establish 177Lu as an excellent isotope for image-guided radionuclide therapy [].
A key benefit of 177Lu-based therapies is their favorable safety profile. Unlike conventional chemotherapy, which often harms healthy tissues, 177Lu bifunctional radioligand treatments selectively target cancer cells, reducing damage to normal tissues. As a result, patients typically experience fewer side effects and an improved quality of life. This approach is particularly valuable for advanced-stage cancer patients with limited conventional treatment options []. A bifunctional radioligand (Figure 2) consists of three key components: a chelator that binds the radiometal and a pharmacophore that provides biological specificity to the final radiolabeled conjugate complex. To facilitate conjugation, chelators are functionalized with reactive groups that allow covalent attachment to biomolecules like antibodies, peptides, drugs, nucleotides, human Escherichia coli enterotoxins, or even bone pain palliation agents, particulates, steroids, porphyrins, nitroimidazoles, fullerenes, and various types of NPs []. The pharmacokinetic properties of the conjugate can be adjusted by introducing a linker between the chelator and the targeting vector. Typically composed of hydrocarbon chains (CH2)n, polyethylene glycol, or peptide sequences, these linkers affect biodistribution and pharmacokinetics by modifying charge and hydrophilicity [,,,]. Indicative examples of current 177Lu-labeled bifunctional radioligand-based radiopharmaceuticals and their treatment conditions are presented in Table 3.
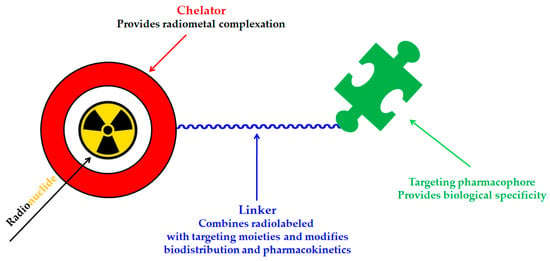
Figure 2.
Schematic representation of the key components of a bifunctional radioligand.

Table 3.
Current 177Lu-labeled bifunctional radioligand-based radiopharmaceuticals and their treatment conditions. Data from [].
177Lu bifunctional radioligand therapies are based on highly efficient and selective chemical reactions that involve binding 177Lu to a targeting molecule via a “click” reaction. In general, the concept of “click chemistry” describes a class of efficient and reliable chemical reactions ideal for creating functional materials and bioconjugates. These ligation processes typically join two molecular components under mild conditions, yielding the desired product rapidly and with high efficiency. Significant advancements over the past twenty years have broadened the scope of click reactions, with many now proceeding effectively in water without sacrificing speed or selectivity. A key advantage is the straightforward purification of the final product, as these reactions often allow for the simple removal of unreacted starting materials and byproducts, eliminating the need for complex separation techniques [].
Consequently, click chemistry has become a valuable tool across diverse fields, including biochemistry, materials science, drug development, and the synthesis of radiolabeled compounds. While other ligation methods like thiol-Michael additions [] or oxime formation exist [], they often suffer from limitations such as low specificity or instability in aqueous environments. A major breakthrough came with the introduction of the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) (Figure 3) [] as a powerful click reaction for bioconjugation. Its success is largely due to the bioorthogonality of azide and alkyne functional groups, which do not react with native biomolecules [].
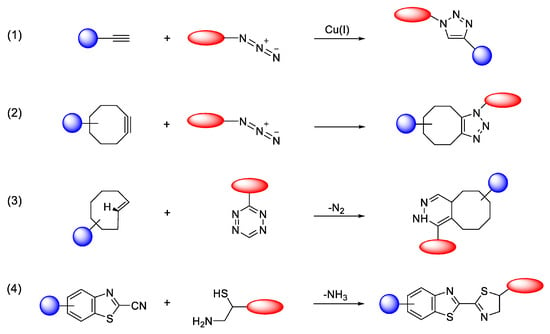
Figure 3.
Selected bioorthogonal conjugation reactions. (1) CuAAC; (2) SPAAC; (3) IEDDA; (4) condensation reaction between 2-cyanobenzothiazole (CBT) and 1,2-aminothiol (N-terminal cysteine). Adopted from Mushtaq, S.; Yun, S.-J.; Jeon, Molecules; published by MDPI, 2019 [].
However, concerns about the cytotoxicity of the required copper catalyst have prompted the development of metal-free alternatives. Reactions such as the strain-promoted azide-alkyne cycloaddition (SPAAC) [] and the inverse-electron-demand Diels–Alder (IEDDA) [] (Figure 3) have since emerged as highly useful, biocompatible click reactions and are now widely adopted in biological research [].
The principles of click chemistry are increasingly being adopted for synthesizing radioisotope-labeled compounds used in nuclear medicine for both diagnostic imaging (PET and SPECT) and therapy. This application is particularly vital for short-lived isotopes. Metal-free click reactions are exceptionally well-suited for this purpose, as they can label sensitive small molecules and biomacromolecules under mild conditions without requiring high temperatures, extreme pH, or cytotoxic metal catalysts. Beyond their use in test tube (in vitro) radiolabeling, these bioorthogonal ligation methods are also being explored for in vivo pre-targeting strategies. This approach aims to improve the specificity of imaging and cancer radiotherapy in animal models [,,].
Moreover, click chemistry allows the chelator to be radiolabeled independently before attaching it to the binding molecule, rather than labeling the entire chelator-targeting molecule complex. This method is beneficial because the radiolabeling process could potentially disrupt the structural integrity of the targeting molecule. Unlike alternative techniques, this type of 177Lu chelation provides benefits such as speed, high efficiency, and precise attachment to different targeting molecules. These click reactions are exceptionally selective, ensuring 177Lu binds only to a designated site on the targeting molecule, thereby reducing the likelihood of nonspecific interactions [].
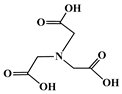
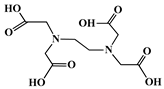
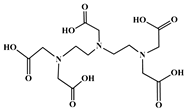
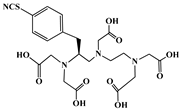
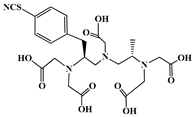
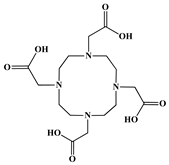
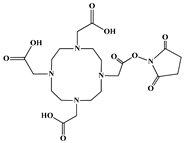
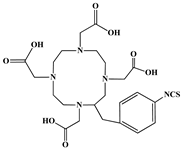
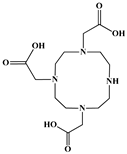
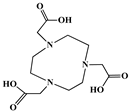
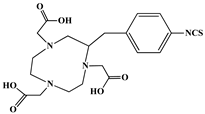
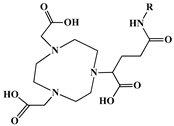
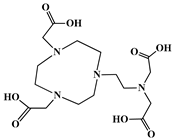
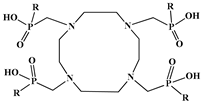
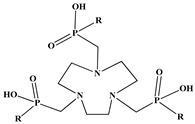
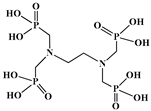
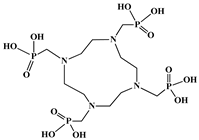
The identification of the most suitable class of chelators for the binding of 177Lu(III) ions plays a crucial role in the efficacy of the produced radioligands. As demonstrated by the ionic model, selection possibilities are considerably constrained, with optimal candidates being chelators that combine strong ionic binding capacity with the ability to form a complete coordination shell around the metal ion. Monodentate ligands are suboptimal for practical applications due to the omnipresent competition from hydroxide ions (OH−) in aqueous solutions, which form the most stable complexes with metal ions. Additionally, monodentate ligands cannot exploit the chelating effect, a key advantage offered by polydentate ligands []. Consequently, polycarboxylate-based chelators, attached to either carbon or nitrogen backbones, are commonly used in the synthesis of 177Lu radiopharmaceuticals for their superior binding properties. These ligands are generally categorized as either acyclic orcyclic polyaminopolycarboxylate-type chelators with 8 or 9 donor atoms. Among these chelators, DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) and its derivatives are the most widely used chelating agents for 177Lu, providing complexation of significantly high kinetic inertness and thermodynamic stability []. Table 4 demonstrates the structures of the most widely used 177Lu chelators employed in the development of bifunctional radioligands.

Table 4.
Structures of 177Lu chelators employed in the development of bifunctional radioligands [,].
In the design of metal-chelators for biological systems, kinetic inertness and thermodynamic stability are paramount. Although acyclic chelators are conventionally considered less inert and faster-binding than their macrocyclic counterparts, this distinction is overly simplistic. Experimental evidence, particularly from X-ray crystallography, shows that trivalent radiometals (e.g., Ga(III), Y(III), In(III), Lu(III)) are not encapsulated by the macrocyclic cavity. The macrocycle’s key role is instead to support the carboxylic acid pendant arms, optimizing their interaction with the metal ion. Since this coordination geometry is consistent across these ions, the traditional classification of chelators into rigid cyclic and flexible acyclic categories may have limited fundamental relevance [,,].
To clarify this point, the negatively charged oxygen atoms in polycarboxylate ligands create a powerful ionic attraction with the positively charged metal ion. This bond must be strong enough to displace the hydroxide (OH−) groups from the metal’s hydration sphere. This displacement is a key enthalpic driver that lowers the reaction’s free energy, making the process thermodynamically favorable. The ligand’s backbone—whether cyclic or acyclic—functions to pre-organize these carboxylate groups, positioning them near the metal ion to facilitate binding. While this pre-organization reduces entropy, the effect is counterbalanced by the release of water molecules from the metal’s hydration shell. Furthermore, the strong electrostatic attraction between the Lu(III) ion and the oxygen atoms provides a significant enthalpic gain. The net result is a substantial decrease in free energy, which is empirically demonstrated by the high stability constants of Lu(III) complexes with both open-chain and macrocyclic ligands (Table 5) [,,].

Table 5.
Log stability constants of Lu(III) complexes with well-known chelating agents. Data from [,].
The kinetic profile of the coordination reaction is directly influenced by the ligand’s structural rigidity. To achieve optimal metal binding, the ligand backbone must reconfigure, a process that is inherently more facile for flexible acyclic chelators than for conformationally constrained macrocyclic ones. This fundamental difference explains why macrocycles like DOTA require elevated temperatures for radiolabeling [,,].
This thermal requirement is often misinterpreted as necessary for the metal to enter the macrocyclic cavity, suggesting a need for size compatibility. However, empirical evidence from X-ray diffraction reveals that the Lu(III) ion in the DOTA complex is not encapsulated within the ring but is capped by it (Figure 4). The actual function of the heat is to provide the energy needed to distort the macrocyclic ring into its active binding conformation. This distortion facilitates the proper spatial arrangement of donor atoms, including the ring nitrogens, to form the stable, square antiprismatic coordination sphere observed in the final complex [,,].
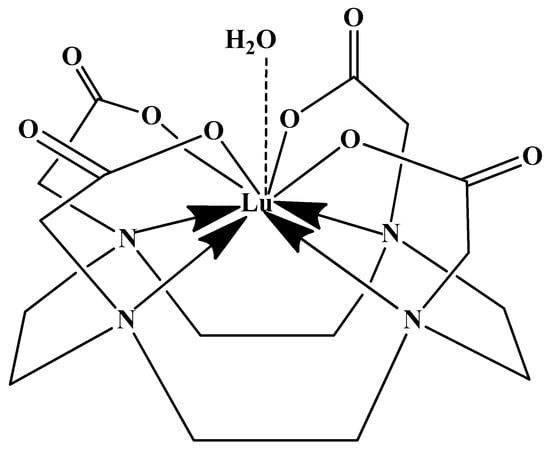
Figure 4.
Schematic of the Lu(III) coordination sphere in the DOTA complex Na[Lu(DOTA)(H2O)]·4H2O, based on X-ray crystallographic data [].
In synthesizing bifunctional radioligands, standard approaches often functionalize a carboxylate group on DOTA for bioconjugation, which reduces its denticity and can destabilize the resulting metal complex. To circumvent this, platforms such as p-SCN-Bn-DOTA enable linkage via a non-coordinating side chain, thereby preserving the complete octadentate coordination sphere and the superior stability of the parent chelator [,,].
Emerging research focuses on novel DOTA ligands where phosphinic acid groups substitute the traditional carboxylates, presenting distinct coordination behavior. Nevertheless, despite its slow complexation kinetics, DOTA continues to be the leading chelator for 177Lu radiopharmaceuticals. The pursuit of a derivative that enables rapid labeling under mild conditions remains an ongoing and unresolved challenge in the field [,,].
The macrocyclic chelator NOTA is a hexadentate ligand that coordinates metal ions through its three ring nitrogen atoms and three pendant carboxylate oxygen atoms (N3O3-type). Compared to the larger DOTA macrocycle, NOTA’s smaller ring size requires less energy to distort into its optimal binding conformation, resulting in significantly faster radiolabeling kinetics. However, this comes with a trade-off: the N3O3 system provides only six donor atoms, which is insufficient to fully saturate the coordination sphere of the Lu(III) ion. This incomplete coordination can compromise the complex’s stability, a problem that is exacerbated when one carboxylate arm is used for bioconjugation, reducing the available donor atoms to just five. To resolve this limitation, the NETA chelator was developed. NETA incorporates an additional carboxylate group on a side chain, increasing its denticity. This design combines the fast labeling kinetics of an acyclic chelator with the enhanced in vivo stability provided by a macrocyclic framework [,,]. It has to be noted that the octadentate NETA-trastuzumab conjugate instantly bound 90Y and 177Lu, forming radiolabeled complexes that remained stable in human serum and in tumor-bearing mice [].
As a first-generation acyclic chelator, DTPA binds rapidly with various radiometals at ambient temperature. Its structure, consisting of a diethylenetriamine core with five carboxylate groups, allows it to act as an octadentate (N3O5) ligand. Despite a large body of stability data for DTPA complexes with isotopes from 99mTc to lanthanides, the results are often difficult to generalize. Consequently, the efficacy of a novel DTPA-derived bifunctional radioligand must be validated on a case-by-case basis. This is illustrated by the unexpected stability difference between DTPA complexes of Y(III) and Lu(III). Despite their nearly identical coordination numbers and ionic radii, the Y(III) complex demonstrates superior stability, implicating other, less-easily measured physico-chemical parameters in the final complex stability [,,].
Despite these limitations, researchers have developed novel DTPA derivatives to overcome its shortcomings. One such compound is 1B4M-DTPA, a bifunctional version featuring a methyl group on one of its carbon backbones. It is proposed that this methyl group introduces greater structural rigidity to the ligand, thereby enhancing the stability of its metal complexes [,,]. An indicative example is the radiopharmaceutical Zevalin®, which is currently commercially available for radioimmunotherapy and was developed using 1B4M-DTPA. This analogue was selected for its efficient binding kinetics with the radioactive isotope 90Y [].
In the design of radiotherapeutic agents for bone pain palliation, multidentate polyaminophosphonic acids serve as excellent carrier ligands. The therapeutic potential of 177Lu complexes with these radioligands was first explored in bone palliation agents with 177Lu-EDTMP [], a finding later advanced into patient applications []. Their efficacy is attributed to their high affinity for skeletal tissue, selective localization to sites of heightened metabolic activity, and the formation of exceptionally stable in vivo chelates, particularly with radiolanthanides. Systematic evaluation of various cyclic and acyclic polyaminopolyphosphonate ligands complexed with 177Lu has revealed distinct advantages for cyclic structures. These cyclic phosphonates form complexes more efficiently, requiring lower ligand-to-metal ratios, and result in products with superior thermodynamic stability and kinetic inertness. Among acyclic ligands, EDTMP has shown effective complexation at a 20:1 ratio. A direct comparison between the 177Lu complexes of EDTMP and the cyclic DOTMP found that both could be synthesized in near-quantitative yields using low ligand concentrations. Biodistribution studies in Wistar rats indicated that both complexes have favorable pharmacokinetics. However, 177Lu-DOTMP cleared from the blood more rapidly and was retained less in the liver and kidneys than 177Lu-EDTMP. Conversely, 177Lu-EDTMP demonstrated consistently higher bone accumulation over time [,,].
Nevertheless, the broader significance of 177Lu complexes with bifunctional radioligands was truly established with the development of 177Lu-DOTATATE, a radiopharmaceutical that targets somatostatin receptors for the treatment of neuroendocrine tumors [,]. Its success under the brand name Lutathera® established PRRT as a major pillar in precision oncology.
2.2. 177Lutetium Production
The growing availability and supporting data for 177Lu-based radionuclide therapy justify an evaluation of both existing and emerging production methods. While 177Lu can be generated using a cyclotron via charged particle acceleration, the most feasible and economical approach is through neutron irradiation in a nuclear reactor. The cyclotron-based method yields significantly lower radioactivity levels and is more costly, rendering it impractical for large-scale production. In a reactor, 177Lu can be produced through two primary pathways: direct neutron activation of enriched Lutetium-176 (176Lu) or indirectly by irradiating Ytterbium-176 (176Yb), which subsequently undergoes β− decay to form 177Lu (Figure 5) [,,,].
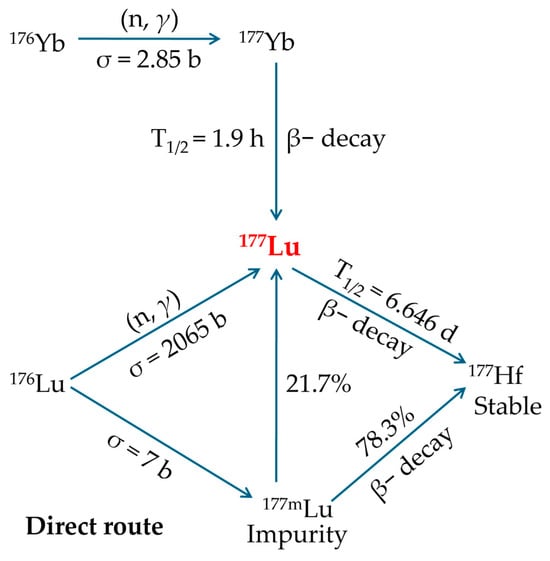
Figure 5.
Dual distinct reactor-based production pathways for 177Lu generation. Reproduced with permission from Banerjee, S.; Pillai, M.R.; Knapp, F.F., Chem. Rev.; published by ACS, 2015 [].
The direct, carrier-added production involves irradiating a stable 176Lu target (176L2O3) with neutrons according to the following nuclear reaction:
To maximize yield and minimize the formation of undesirable radionuclides, the target is enriched in 176Lu [,,,].
This method offers the following benefits [,,,]:
- This is the most straightforward production method, requiring only minor adjustments to existing reactor and processing facilities.
- It employs a 176Lu2O3 target, which maintains excellent chemical and thermal stability during reactor irradiation.
- This route represents the most inexpensive option for obtaining 177Lu with the required purity.
- The nuclear reaction has a high cross-section (~2065 barn) and a neutron capture resonance integral of ~1087 barn, resulting in enhanced production yields and elevated specific activity, meaning just 1 mg of enriched 176Lu can yield roughly 50 patient doses.
- Significant specific activities of 177Lu are attainable by irradiating highly enriched targets in research reactors with flux levels in the medium-high range.
- Enables production scaling by simply modifying target dimensions to meet fluctuating demand.
- Post-irradiation processing of the target is straightforward, requiring only the conversion to 177LuCl3 and without the need for further refinement or preparation prior to clinical administration.
However, this approach has significant limitations [,,,]:
- Only about 30% of the irradiated 176Lu becomes the desired 177Lu, and this proportion decreases further due to radioactive decay before administration. Therefore, the highest achievable specific activity requires irradiation in high-flux reactor facilities.
- To significantly increase both the production yield and specific activity of 177Lu, it is essential to use targets enriched in the 176Lu isotope, as its natural abundance is only 2.6%.
- In practice, the specific activity of 177Lu from this route is 740–1110 GBq·mg−1, only about 18–27% of the theoretical 4.07 TBq/mg. This is because the product mixture contains just 25% radioactive 177Lu atoms, with the rest being stable lutetium isotopes. Therefore, the maximum achievable specific activity, even under ideal conditions in high-flux reactors, caps at around 70% of the theoretical limit.
- Directly produced 177Lu achieves an initial specific activity of 740–1110 GBq·mg−1 (20–30 Ci·mg−1), which may be suitable for PPRT. However, the continual decrease in specific activity due to radioactive decay limits its shelf-life, making it less ideal for procedures requiring consistently high specific activity.
- The process also generates 177mLu, an unwanted beta-emitting byproduct with a 160-day half-life, complicating waste handling and disposal.
The production of 177Lu for therapy relies on the natural isotope 176Lu, which has a low abundance of 2.6% and a very long half-life. The process uses Lu2O3 as a target material for its stability and solubility. To obtain a high-specific-activity product suitable for therapy, it is essential to use targets that are both highly enriched in 176Lu and exceptionally pure. This high purity is critical because impurities can absorb neutrons in the reactor flux, diminishing the final product’s specific radioactivity [].
The concentration of the 177mLu impurity depends on both the irradiation time and the cooling period post-irradiation. Although the activity levels of 177mLu are low due to its long half-life and low formation cross-section, its presence can raise regulatory concerns in some countries and creates a long-term radioactive waste issue. Nevertheless, studies have confirmed that the resulting increase in radiation dose from 177mLu is negligible at clinical dose levels, particularly for PRRT. Under optimized production conditions, the 177mLu/177Lu ratio is typically maintained at a very low 0.01% to 0.02% at the end of bombardment [].
The practical maximum specific activity (SA) achievable via the direct production route is approximately 70% of the theoretical value, a level only attainable in high-flux nuclear reactors found in a limited number of countries. Reported SA values can reach 1850–2405 GBq·mg−1 (50–65 Ci·mg−1) in such facilities. However, the use of 176Lu-enriched targets (60–80%) in more widely available medium-flux reactors can reliably yield 177Lu with SA values of 740–1110 GBq·mg−1 (20–30 Ci·mg−1), which is adequate for all established radionuclide therapy applications [].
Conclusively, although the patient radiation dose from the 177mLu impurity is considered negligible, its management presents a significant logistical challenge for hospitals. The safe handling and long-term disposal of 177mLu-contaminated waste are difficult to manage within standard hospital storage protocols. Despite this drawback, the direct production route remains the preferred choice for many facilities due to its cost-effectiveness and reliable supply of 177Lu in sufficient quality and quantity. This established supply chain is a critical foundation for expanding the radiopharmaceutical applications of 177Lu [].
The indirect, no-carrier-added production utilizes neutron irradiation of 176Yb as the starting material. To optimize yield and minimize unwanted radionuclide formation, such as Ytterbium-169 (169Yb) and Ytterbium-175 (175Yb), the ytterbium is typically enriched to >99% in 176Yb. During irradiation, short-lived Ytterbium-177 (177Yb) (t1/2 < 2 h) forms and subsequently decays to 177Lu [,,,].
This approach offers the following advantages [,,,]:
- After radiochemical separation from 177Yb, the final product contains only pure 177Lu with exceptionally high specific activity.
- The production pathway avoids creation of the problematic 177mLu byproduct.
- SA does not depend on neutron flux.
- The radiochemical performance is sufficient.
- This production method yields 177Lu with extended viability (approximately 14 days) due to minimal SA loss over time.
However, this method presents some important challenges [,,,]:
- The nuclear reaction has a low cross-section (2.85 barn), requiring approximately 1 g of enriched 176Yb to generate activity equivalent to that produced from just 1 mg of enriched 176Lu in the carrier-added route. Therefore, to achieve sufficient yields and ensure efficient use of the enriched target material, neutron irradiation of the 176Yb2O3 target must be performed in a high-flux nuclear reactor.
- Using enriched targets with low neutron-capture cross-sections proves economically inefficient, as much of the material remains unactivated and requires expensive recovery and rigorous radiochemical processing to ensure complete 176Yb removal, further increasing complexity and cost.
- Extracting 177Lu from 177Yb is highly complex due to its extremely low concentration (~1 177Lu atom per 5000 177Yb atoms), necessitating an exceptionally efficient combination of purification methods, such as column chromatography, solvent extraction, supported liquid membrane extraction, extraction chromatography, or even electrochemical separation, cementation, and electro-amalgamation processes.
- The maximum achievable yield (saturation yield) of 177Lu requires irradiation lasting approximately 5–6 half-lives, typically necessitating several weeks of continuous neutron exposure. As a result, several factors contribute to substantially higher production costs, such as the requirement of isotopically enriched target material, larger irradiation volumes, extended irradiation periods, and complex post-irradiation radiochemical separation processes.
- This production method is significantly more expensive than alternative routes for obtaining 177Lu of the required purity.
Despite existing challenges, the potential of no-carrier-added 177Lu for TRT is significant, driving active research at many institutions. The success of this indirect production method hinges on a critical step: the efficient chemical separation of pure 177Lu from the much larger mass of the irradiated Yb target. This separation is particularly difficult because Yb and Lu share nearly identical coordination chemistry with the chelating agents used in radiopharmaceuticals. Therefore, evaluating the use of highly enriched 176Yb (up to ~97%) is not just promising but essential. Since very little of the target material is consumed during irradiation, developing a method to recover and recycle the unused, enriched ytterbium is a critical factor for making this indirect production route economically viable [].
2.3. Cost-Effectiveness and Distribution Efficiency of 177Lu
A significant factor in the global adoption of 177Lu for therapy is its reliable production in high quantities and sufficient specific activity from numerous medium- to high-flux reactors worldwide. By optimizing irradiation parameters and using enriched targets, reactors with a neutron flux above 1 × 1014 n·cm−2·s−1 can consistently produce 177Lu with a specific activity exceeding 740 GBq·mg−1. This favorably contrasts with 131I, a widely used radionuclide, whose production—whether by neutron activation or fission—cannot achieve an isotopic abundance greater than 20% [].
The indirect method for producing no-carrier-added 177Lu achieves a high theoretical SA but is an expensive process. This high cost stems from several factors: the price of the enriched 176Yb target material, low production yields due to a small nuclear cross-section, and the complex, costly procedures required to process the radioactive target and recover the valuable 176Yb for reuse. Because a significant portion of the expensive target is not activated, the process is often economically inefficient. Consequently, many countries are exploring the direct production route for 177Lu. The indirect method is typically reserved for situations demanding the highest SA, such as certain targeted therapies, where its benefits outweigh the substantial production costs [].
The 6.7-day half-life of 177Lu is a key advantage for its widespread use in radiopharmaceuticals. This duration is ideal for clinical applications, allowing sufficient time for the drug to target and treat diseased cells. Furthermore, it provides significant logistical benefits for global distribution. The slow decay rate means minimal radioactivity is lost during transport from the production facility to distant hospitals and clinics. This efficiency helps keep 177Lu cost-effective and has been a major factor in its rapid adoption worldwide. To meet this growing demand, multiple suppliers now produce both carrier-added and no-carrier-added forms of 177Lu for drug development [].
The current supply and demand for 177Lu is not entirely clear. While the approved drug Lutathera® accounts for a significant portion of current use—estimated at 10,000–15,000 doses annually—this likely represents a fraction of total production due to extensive clinical research. Forecasting future demand is challenging, but analysts predict a dramatic increase, potentially several times over current levels. This growth is primarily driven by the adoption of prostate-specific membrane antigen (PSMA)-targeted therapies for prostate cancer. With approximately 366,000 global annual deaths from this disease, the potential patient population is vast, requiring multiple treatment cycles per patient. This suggests a potential market for 177Lu-PSMA therapy that is at least an order of magnitude larger than that for Lutathera®, indicating a substantial future demand even before considering other emerging applications [].
The global market for 177Lu is highly concentrated, with the top three producers—Advanced Accelerator Applications (a Novartis company), Eckert & Ziegler Strahlen, and SHINE Technologies—collectively holding approximately 98% of the market share. Geographically, North America is the largest market, accounting for 44% of the total, followed by Europe (33%) and Asia-Pacific (18%). In terms of product and application, the market is dominated by the non-carrier-added type, which holds a 99% share, and its use in nuclear therapy, which constitutes about 98% of all applications. The 177Lu market size, estimations, and forecasts are provided in Table 6, in terms of output/shipments (Doses) and revenue (US$ millions), considering 2025 as the base year, with history and forecast data for the period from 2025 to 2032 [,].

Table 6.
Global 177Lu market research report 2025. Data from [,].
2.4. 177Lutetium Clinical Advancements
Radionuclide therapy using therapeutic radiopharmaceuticals offers considerable benefits compared to conventional treatments, as it enables precise targeting of cancerous or diseased tissues while minimizing damage to surrounding healthy cells. Researchers have recognized the potential of 177Lu and have begun exploring its applications, particularly in oncology []. The combined action of the targeting molecule and the particle-emitting radionuclide enhances cell destruction, improving treatment efficacy for cancer and other conditions. Despite ongoing research into radiolabeled compounds for therapeutic use, a key hurdle remains in translating findings from preclinical animal studies to human clinical trials. However, following the approval of Lutathera® and Pluvicto® radiopharmaceuticals, extensive research is underway to explore various methods of binding and delivering 177Lu to different tumor types using diverse labeling techniques and biological mechanisms []. Table 7 presents a detailed overview of the most recent ongoing, terminated, or completed clinical trials with valuable results on the investigation of 177Lu radiopharmaceuticals under varying treatment conditions.

Table 7.
Active, terminated, or completed recent clinical trials on 177Lu-based radiopharmaceuticals. Data from [].
3. Magnetic Nano-Formulations
3.1. Magnetic Nano-Formulations as Theranostic Agents
The unique properties of nanomaterials—characterized by at least one dimension below 100 nm—have positioned them as transformative tools in modern medicine. Their distinct optical, magnetic, and electronic properties, coupled with an exceptional surface-to-volume ratio and tunable surface chemistry, render them particularly valuable for medical applications []. These materials are broadly classified into organic variants (liposomes, micelles, polymers) and inorganic forms (gold NPs, carbon allotropes, metal oxides), each with distinct preparation methods and structural characteristics.
Inorganic NPs are typically produced through precipitation or reduction reactions, while organic NPs form via self-assembly of molecular building blocks—with the notable exception of covalently structured dendrimers []. This fundamental difference in structure leads to varying stability profiles, with organic NPs exhibiting dynamic behavior in biological environments compared to their inorganic counterparts.
The clinical success of liposomal and polymeric nano-formulations [,] has spurred interest in developing theranostic versions that combine therapeutic and diagnostic capabilities. However, the incorporation of imaging components (radioactive tracers, fluorescent dyes, MRI contrast agents) presents significant challenges, particularly regarding the potential dissociation of diagnostic and therapeutic components in vivo [,,,] (Figure 6). This has driven the search for intrinsically multifunctional nanomaterials that combine imaging capability, drug-loading capacity, and biological compatibility—especially important for cancer therapies requiring repeated administration [].
Certain inorganic NPs demonstrate particular interest due to their inherent optoelectronic properties. These materials can serve dual roles as therapeutic enhancers [] and diagnostic agents []. Among these types of NPs, iron oxide NPs (IONPs), including superparamagnetic IONPs (SPIONPs), magnetite (Fe3O4), and maghemite (γ-Fe2O3), are widely utilized for magnetic targeting of tumors or cells under an external magnetic field, facilitating drug delivery []. Their inherent magnetic properties, biocompatibility, and functional versatility render them valuable as MRI contrast agents for diagnostic imaging []. Additionally, IONPs show potential in multimodal therapies, combining radionuclide treatment, magnetic hyperthermia, and photothermal therapy []. A key advantage of IONPs is their versatile surface chemistry, which allows straightforward functionalization with biomolecules, including antibodies, peptides, nucleic acid structures, or other therapeutic agents to enhance targeting and efficacy, enabling precise interaction with overexpressed receptors on tumor cells and associated vasculature, representing a significant step toward personalized cancer theranostics [].
Polymers and stabilizing agents as IONP coatings serve a dual function: they prolong blood circulation by inhibiting particle clumping and protein accumulation while also binding metal ions, eliminating the requirement for specialized chelating agents []. Furthermore, particle geometry optimization has enhanced circulation time and tissue penetration []. The development of ultra-small (<5 nm) IONPs has been particularly noteworthy, combining improved tumor accumulation via the permeability and retention (EPR) effect [], with enhanced MRI contrast capabilities [].
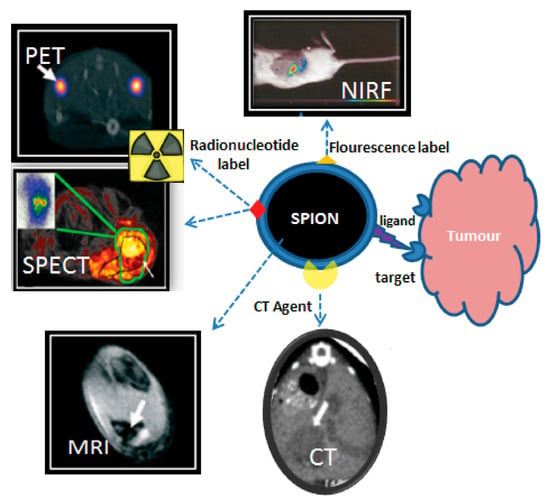
Figure 6.
Theranostic applications of SPIONPs. Adopted from Thomas, R.; Park, I.-K.; Jeong, Y.Y. Int. J. Mol. Sci.; published by MDPI, 2013 [].
3.2. Radiolabeling of Magnetic Nano-Formulations
Three principal methodologies exist for incorporating radionuclides into IONPs, with the chelator-based approach representing the most versatile and widely implemented technique. This method utilizes bifunctional ligands that serve as molecular bridges between the NP surface and metallic radionuclides, as illustrated in Figure 7a. Successful implementation of this strategy requires careful consideration of two fundamental aspects: the specificity of NP-ligand binding and the stability of radionuclide-chelate coordination [,].
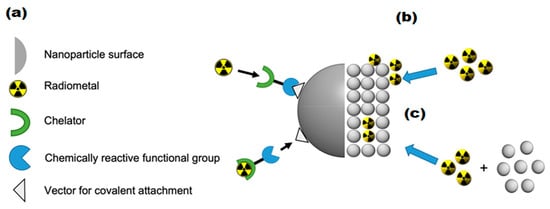
Figure 7.
Visual representation of the three primary radiolabeling techniques for IONPs using positron-emitting isotopes: (a) Chelator-mediated approach—Utilizing bifunctional ligands to bridge NPs and radionuclides, (b) Surface adsorption method—Heat-driven deposition of radiometals onto NP surfaces, (c) “Hot + cold precursor” method—Simultaneous NP formation and radiolabeling through direct incorporation of radionuclides into the NP crystal lattice. Adopted from “Radiolabeled iron oxide nanomaterials for multimodal nuclear imaging and positive contrast magnetic resonance imaging (MRI): A review,” by Pellico, J.; Ruiz-Cabello, J.; Herranz F. ACS Appl. Nano Mater. 2023, 6, 20523–20538. CC-BY-NC-ND 4.0 [].
The conjugation process must maintain the IONPs’ essential physico-chemical properties, including hydrodynamic size, surface charge, and magnetic characteristics. Conventional bioconjugation techniques typically employ amide bond formation between carboxyl and amine groups or thioether linkages through thiol-maleimide reactions []. While these methods offer the advantages of rapid conjugation under mild conditions with moderate to high yields, they often lack precise control over ligand density, which can inadvertently modify NP properties [].
Recent advances in surface functionalization have introduced click chemistry approaches that provide superior control over conjugation processes [,]. These selective reactions, such as strain-promoted azide-alkyne cycloadditions and inverse electron-demand Diels-Alder reactions, enable more precise surface modifications. However, their application is limited by the requirement for specific complementary functional groups on both the NP surface and the ligand molecules [,].
A critical consideration in radiolabeling is the maintenance of radiochemical stability, which ensures the radionuclide remains coordinated to the NP under physiological conditions. Inadequate radiochemical stability can lead to radionuclide dissociation in vivo, potentially compromising imaging accuracy by generating signals from unbound radionuclides rather than the targeted NPs [].
The field predominantly employs macrocyclic chelators, particularly DOTA and NOTA, due to their exceptional metal coordination properties. DOTA has gained widespread popularity for its ability to form stable complexes with various positron-emitting metals, though its requirement for high-temperature labeling conditions may pose challenges for biomolecule conjugation. NOTA, while operating effectively under milder conditions, exhibits a more restricted coordination sphere that limits its application to larger radionuclides such as 132/135La, 131Ba, 201Tl, 203Pb, 213Bi, 223Ra, and 225Ac [].
These chelators are commercially available with diverse functional groups, facilitating their conjugation to NP surfaces. The chelator-based approach has demonstrated remarkable versatility, with numerous successful applications in T1-weighted IONP radiolabeling studies across various nano-formulations and imaging modalities. The continued refinement of these techniques promises to enhance the precision and reliability of radiolabeled IONPs for advanced diagnostic and theranostic applications [].
In addition to chelator-based approaches, researchers have developed alternative methods for radiolabeling IONPs, including heat-induced radiolabeling (chemical adsorption) and the “hot + cold precursor” strategy. Heat-induced radiolabeling (Figure 7b) exploits the strong affinity of magnetite (Fe3O4) and maghemite (γ-Fe2O3) surfaces for certain radiometals, including 89Zr, 69Ge, and arsenic isotopes (71As, 72As, 74As). This radio-mineralization method involves heating IONPs in the presence of radiometals, leading to the deposition of radiolabeled metal oxides on the NP surface. While this technique achieves near-quantitative radiochemical stability, it typically requires high temperatures (>80 °C) [].
On the other hand, the “hot + cold precursor” method (Figure 7c) enables simultaneous NP synthesis and radiolabeling in a single step. Unlike other techniques, this strategy requires careful optimization of reaction time due to its dependence on radionuclide half-life. Traditional IONP synthesis methods often take several hours, limiting compatibility with short-lived isotopes. The key advantage of the “hot + cold precursor” method lies in the direct incorporation of radionuclides into the NP crystal lattice, ensuring exceptional in vivo stability. Moreover, this technique minimizes structural modifications to the IONPs, preserving their original biodistribution profile, a critical factor for accurate imaging interpretation. When selecting a radiolabeling strategy, researchers must consider potential alterations to NP properties, as these could affect biological behavior and imaging results. In this regard, the “hot + cold precursor” approach offers distinct advantages by maintaining the native characteristics of IONPs while achieving robust radiolabeling [].
4. 177Lu-Labeled Magnetic Nano-Formulations
4.1. Synthesis, Radio- and Physico-Chemical Characterization
The integration of 177Lu into magnetic nano-formulations presents unique physicochemical and radiochemical considerations that fundamentally influence their theranostic potential. While the nuclear properties of 177Lu are well-characterized for radiopharmaceutical applications, their manifestation in nanostructured systems introduces complex structure-property correlations that remain insufficiently explored. This section provides a comprehensive analysis of the critical parameters governing the performance of these hybrid systems, including: (i) the interplay between NP core composition and radiolabeling efficiency, (ii) the impact of surface functionalization on colloidal stability and physico-chemical characteristics, (iii) the retention of magnetic properties, and (iv) the evolution of these characteristics under physiological conditions.
In an effort to generate an effective theranostic agent to achieve optimal radiolabeling efficiency with a therapeutic and diagnostic isotope, Salvanou et al. [] developed condensed colloidal nanocrystal clusters (co-CNCs) based on two variants of magnetic IONPs: alginate-coated nanocrystallites (MA) and their PEGylated counterparts (MAPEG), the latter being modified to confer stealth properties. The newly synthesized substrates of co-CNCs magnetic IONPs, prepared via a soft biomineralization process conducted at 50 °C under ambient pressure following the alkaline precipitation method of the starting ferrous precursor, were physico-chemically characterized in terms of their hydrodynamic diameter (Dh) and surface charge (ζ-potential) via dynamic light scattering (DLS). The MA nano-formulations displayed an average Dh of 100 nm and a highly negative ζ-potential of −40 mV, consistent with the carboxylate-rich alginate coating. In contrast, the MAPEG NPs showed a slightly larger Dh of 120 nm and a near-neutral ζ-potential (−7 mV), reflecting the shielding effect of the PEG layer. The radiolabeling procedure for MA and MAPEG with 177Lu was performed through incubation of the nano-formulations with [177Lu]LuCl3 (50 µL, 10–30 MBq). Instant Thin Layer Chromatography-Silica Gel (ITLC-SG) analysis demonstrated high radiochemical yields of 95.21 ± 1.28% for [177Lu]Lu-MA and 93.65 ± 1.03% for [177Lu]Lu-MAPEG after 30 min of incubation. This efficient labeling likely results from the strong interaction between the Lu(III) cations and the negatively charged carboxylate groups (-COO−) in the alginate coating. The DLS measurements, which were performed in deionized H2O, revealed that [177Lu]Lu-MAPEG maintained its original Dh (≈119 nm) without any aggregation. Although the slightly higher pH conditions reduced immediate aggregation of [177Lu]Lu-MA (Dh ≈ 450 nm), these nano-formulations eventually precipitated after 24 h. The radiolabeled nano-formulations demonstrated excellent bench and in vitro stability when stored at room temperature, maintaining their integrity for at least 7 days post-preparation. Quantitative analysis performed via ITLC-SG, revealed retention rates of 93.97 ± 3.44% for [177Lu]Lu-MA and 95.60 ± 2.03% for [177Lu]Lu-MAPEG. In serum stability studies conducted at 37 °C (using a 1:10 v/v NP/serum ratio), both formulations showed gradual degradation over time. The [177Lu]Lu-MA sample decreased from 79.81 ± 1.28% intact at 2 h to 73.24 ± 2.59% after 7 days. Similarly, the [177Lu]Lu-MAPEG sample exhibited comparable stability, with intact percentages declining from 77.29 ± 1.69% (2 h) to 70.72 ± 1.75% (7 days post-labeling). Additional stability testing in aqueous solution (prepared at the same concentration used for biodistribution studies) showed remarkable stability, with >90% of the radiotracer remaining intact after 5 days of storage [].
To overcome limitations of conventional brachytherapy, intratumoral administration of radionuclide-loaded nano-formulations (nanobrachytherapy, NBT) has emerged as a promising strategy for targeted radiotherapy of solid tumors, offering advantages over systemic intravenous delivery. To that end, Stanković et al. [] developed 177Lu-labeled SPIONPs functionalized with meso-1,2-dimercaptosuccinic acid (DMSA) to create 177Lu-DMSA@SPIONPs as a potential nanobrachytherapy agent for localized tumor treatment. Following synthesis via the chemical co-precipitation of Fe(II)/Fe(III) cations, the structural properties of uncoated SPIONPs were analyzed using transmission electron microscopy (TEM). TEM images revealed a spherical nano-formulation morphology with an average diameter of 11.4 ± 3.2 nm and a polydispersity index (PDI) of 28.3% suggesting a moderate particle stability and a propensity for NP aggregation. X-ray diffraction (XRD) analysis of both uncoated SPIONPs and DMSA@SPIONPs demonstrated distinct crystalline patterns, representing a mixture of magnetite and maghemite phases. Crystallite size determination at 2θ ~36° yielded a mean value of 12.2 nm, showing excellent agreement with the TEM measurements. The Dh of both uncoated and DMSA-coated SPIONPs was evaluated using DLS. For uncoated SPIONPs, DLS analysis revealed a Dh of 46.1 ± 3.8 nm, which was notably larger than the core sizes obtained from TEM measurements (p < 0.05). This discrepancy likely resulted from NP aggregation in suspension, reflecting the system’s limited colloidal stability. Following DMSA coating, DLS measurements demonstrated a significant increase in Dh to 140.3 ± 6.5 nm (p < 0.05), consistent with the expected size expansion due to surface functionalization. The substantial difference between DLS and TEM-derived dimensions underscores the importance of considering both core size and hydrodynamic volume when characterizing NP systems. The ζ-potential measurements revealed that the uncoated SPIONPs exhibited a ζ-potential of +12.5 mV, which shifted to −35.1 mV after DMSA functionalization. This significant change to a negative potential reflects the introduction of carboxyl and thiol groups on the NP surface, substantially improving their suspension stability compared to bare SPIONPs. Fourier-transform infrared (FT-IR) spectroscopy provided further evidence of successful DMSA coating. The spectrum of DMSA@SPIONPs displayed characteristic absorption bands confirming the covalent attachment of DMSA molecules to the iron oxide surface. The spectral data support a proposed coating mechanism where (i) polar Fe-O-C bonds form through a condensation reaction with H2O elimination, and (ii) free thiol groups undergo oxidative coupling to form disulfide bridges in the coating layer. The DMSA-functionalized SPIONPs were effectively radiolabeled with 177Lu(III) ions using a direct labeling methodology. The radiolabeling procedure yielded 86.6 ± 2.1% incorporation efficiency, and subsequent magnetic separation produced 177Lu-DMSA@SPIONPs with radiochemical purity exceeding 99%, as verified by ITLC-SG analysis. The stability profile of the radiolabeled nano-formulations was systematically investigated under different storage conditions, including ambient temperature, human serum at 37 °C, and saline solution at 37 °C. Radiochromatographic monitoring over a 144 h period demonstrated excellent stability characteristics. The maximum observed release of free 177Lu(III) ions was limited to 2.2 ± 0.5% for room temperature storage, 3.6 ± 0.7% in human serum, and 4.2 ± 1.0% in saline solution at 37 °C [].
The study of Ognjanović et al. [] focused on the development of radiolabeled IONPs with a well-defined nanoflower-like morphology and precisely controlled dimensions for possible diagnostic applications or combined cancer treatment involving hyperthermia and radionuclide therapy. The IONPs were synthesized following a solvothermal approach of the polyol method, which involved alkaline co-precipitation of Fe(II)/Fe(III) salts in a solvent mixture of N-methyldiethanolamine and diethylene glycol. The synthesized IONPs were surface-coated with citric acid (CA), poly(acrylic acid) (PAA) or PEG to prevent aggregation while enhancing biocompatibility and facilitating straightforward radiolabeling. The morphology and size distribution of the IONPs were analyzed using TEM. The images revealed NPs with particle shapes between spherical and rounded cubes and an average size of 13.5 (±1.2) nm, which tended to form small agglomerates. The agglomerate sizes followed a log-normal distribution, with a Dh of 24.8 (±4.4) nm and a PDI of 18%. A combination of selected-area electron diffraction (SAED) and powder XRD analyses confirmed that the synthesized NPs likely consist of a mixed phase of magnetite and maghemite. The magnetic properties of the IONPs were comprehensively evaluated using multiple techniques. Isothermal magnetization measurements were conducted at both cryogenic (5 K) and room temperature (300 K) conditions, with applied fields reaching up to 3988.5 kA·m−1. The saturation magnetization (Ms) values were determined to be approximately 77 emu·g−1 at 5 K and 70.5 emu·g−1 at 300 K. The room temperature magnetization curve exhibited characteristic superparamagnetic behavior, as evidenced by the zero-coercivity hysteresis loop passing through the origin. In contrast, at 5 K the IONPs demonstrated ferromagnetic properties with a measurable coercivity of 231.3 kA·m−1. This temperature-dependent magnetic behavior confirmed the superparamagnetic nature of the synthesized IONPs at physiological temperatures, while maintaining significant magnetic responsiveness at cryogenic conditions. The radiolabeling of the coated IONPs was performed by applying indirect radiolabeling approaches under mild conditions, taking advantage of the radionuclides’ ability to form stable complexes with functional groups present on the NP coatings. The methodology demonstrated several key advantages, including a simplified radiolabeling procedure applicable to multiple radionuclides, compatibility with various surface coatings, and the potential for combined diagnostic and therapeutic applications while maintaining NP functionality. The radiolabeling efficiency of coated IONPs with 177Lu exceeded 98% when conducted at 25 °C for 30 min, with no significant improvement observed upon extending the reaction time to 60 min. This high labeling efficiency was attributed to electrostatic interactions between the positively charged radionuclides and negatively charged functional groups on the NP surfaces: carboxylate groups in PAA@IONPs and CA@IONPs, and hydroxyl groups in PEG@IONPs. The PEG coating serves dual functions—its hydrophilic nature ensures aqueous dispersibility of the IONPs, while its steric hindrance effect prevents NP aggregation through interparticle repulsion forces. The radiolabeled coated IONPs were subjected to stability testing in both saline and human serum environments. Samples were analyzed using ITLC at predetermined intervals (1, 2, and 24 h post-preparation). Results demonstrated exceptional stability across all coating types (CA, PAA, and PEG@IONPs), with 177Lu-labeled NPs maintaining > 95% radiochemical purity in physiological media even after prolonged incubation (96 h). The effective functionalization of IONPs with PAA was verified through ζ-potential measurements, which showed a significant shift in the isoelectric point toward more acidic pH values. Specifically, the surface charge decreased from +14 mV to −26 mV at physiological pH (7.5), confirming successful PAA coating [].
In an effort to support the notion that traditional cancer treatments like chemotherapy and radiotherapy, along with techniques such as brachytherapy, combined with the distinctive characteristics of IONPs, may lead to the creation of innovative theranostic agents, Salvanou et al. [] assessed the capability of IONPs coated with alginic acid (AA) and PEG, functionalized with the chemotherapy drug doxorubicin (DOX) and the monoclonal antibody bevacizumab (BVCZ), to act as a nanoradiopharmaceutical for breast cancer treatment. The functionalization of the coated IONPs was achieved through the utilization of the carboxyl groups present in alginic acid. The coupling reaction was facilitated by the crosslinking agents N-ethyl-N′-(3-(dimethylamino)propyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS) at physiological pH (Figure 8).
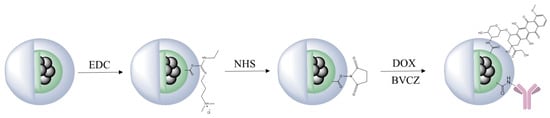
Figure 8.
Functionalization scheme of PEG-stabilized AA-coated IONPs with DOX and BVCZ. Adopted from Salvanou, E.-A.; Kolokithas-Ntoukas, A.; Prokopiou, D.; Theodosiou, M.; Efthimiadou, E.; Koźmiński, P.; Xanthopoulos, S.; Avgoustakis, K.; Bouziotis, P. Molecules; published by MDPI, 2024 [].
The confirmation and quantification of the effective conjugation of the antibody and drug to the coated IONPs was performed via high-performance liquid chromatography (HPLC) and thermogravimetric analysis (TGA). The experimental results demonstrated high DOX loading efficiency in the nano-formulation, achieving 82.67 ± 3.87% by weight. Interestingly, PEG-stabilized AA-coated IONPs functionalized solely with DOX exhibited marginally greater encapsulation efficiency (88.99%) compared to those conjugated with both BVCZ and DOX (82.70%). Correspondingly, the conjugation efficiency of BVCZ to the alginate-coated NPs demonstrated substantial binding (41.84%) and encapsulation (35.97%) capabilities. The observed high loading capacities were attributed to the covalent bonding of the antibody and the drug to the coating of the IONPs. The TGA thermograms indicated a ~64.2% mass loss in the temperature range between 150 and 800 °C, which was assigned to the coating degradation in combination with the destruction of the conjugated DOX and BVCZ molecules. The particle size distribution and surface charge characteristics of the fabricated nano-formulations were analyzed through DLS measurements. The results revealed an average Dh of 474.6 ± 13.8 nm with a corresponding ζ-potential value of +16.7 mV. The measured high Dh and ζ-potential values were also attributed to the functionalization of the coated nano-formulations with the antibody and the drug. The in vitro DOX release under different physiological conditions was determined via reverse-phase high-performance liquid chromatography (RP-HPLC). Using phosphate-buffered saline (PBS) at both physiological (pH 7.4) and acidic (pH 5.5) conditions, initial drug release remained limited during the first hour (5.75 ± 0.93% and 6.69 ± 4.00%, respectively) mainly due to the conjugation of DOX, with only modest increases observed after 12 h (12.65 ± 0.42% and 12.06 ± 0.73%). Remarkably, the nano-formulations retained >70% of their drug payload (70.99% at pH 7.4; 71.51% at pH 5.5) following 72 h incubation at 37 °C. The introduction of pronase (a proteolytic enzyme targeting amide linkages between DOX and NPs) in acidic PB (pH 5.5) significantly altered the release kinetics. Drug liberation increased to 37.68 ± 3.94% by 12 h, demonstrating enzyme-mediated cleavage of drug-carrier bonds. Cumulative release progressed to 45.93 ± 6.32% at 24 h, 62.32 ± 7.38% at 48 h, and ultimately reached 90.94 ± 3.40% after 72 h. These findings clearly demonstrated pronase’s catalytic role in accelerating DOX release from the nano-formulation. The radiolabeling of the developed nano-formulations, functionalized and non-functionalized, occurred after direct incubation with 177Lu at 75 °C for 30 min. The radiolabeling yield and purity were determined via ITLC-SG. The measurements showed a 93.65 ± 1.03% yield for the 177Lu-labeled non-functionalized nano-formulations, which maintained 95.60 ± 2.03% stability after 7 days in serum (1:10 v/v, 37 °C). Correspondingly, the antibody-functionalized nano-formulations showed a radiochemical yield of 93.41 ± 2.59% after incubation at 40 °C and exhibited excellent in vitro integrity at room temperature, with 89.12 ± 2.99% remaining intact even after 14 days post-radiolabeling. In serum stability studies, conducted at physiological temperature (37 °C) using a 1:10 v/v NP-to-serum ratio, the conjugate demonstrated good stability over time with 84.80 ± 3.75% of the radiolabeled nano-formulations remaining intact after 1 day, gradually decreasing to 72.16 ± 3.05% by day 14 [].
Gholami et al. [] employed a chelate-free, heat-induced radiolabeling (HIR) method to develop 177Lu-labeled Feraheme (FH) nano-formulations for diagnostic imaging and radiotherapeutic applications. FH, an approved drug against iron anemia, consists of an iron oxide core of ~5 nm diameter coated with a thick carboxymethyldextran (CMD) layer, thus forming a nanoconstruct of ~25 nm overall diameter. The Dh of the synthesized FH NPs with incorporated non-radioactive Lu(III) was estimated via DLS at around 26.70 ± 0.45 nm, whilst the Lu(III) incorporation percentage exceeded 75% according to inductively coupled plasma mass spectrometry (ICP-MS) measurements. Taking into consideration that therapeutic applications have significantly different specific radioactivity requirements compared to imaging, the authors optimized radiolabeling by applying modifications to the HIR method, such as heated vortex mixing, temperature increase, short radiolabeling duration, or an increase in the initial radioactivity. In the case of 177Lu, the radiolabeling process was performed at a stable temperature (140 °C) under vortexing, the reaction duration was 2 h, and the initial radioactivity was around 15 MBq. Size exclusion chromatography (SEC) and TLC measurements demonstrated that 177Lu(III) cations reacted thermally with FH and resulted in high radiolabeling yield (91.0 ± 0.7%) and radiochemical purity (95.0 ± 0.7%) [].
In an effort to showcase the broad applicability of Ligand Anchoring Group-Mediated Radiolabeling (LAGMERAL), a straightforward yet highly effective technique recently introduced for creating nuclear imaging nanoprobes, Ge et al. [] developed 177Lu-labeled diphosphonate-polyethylene glycol (DP-PEG) coated Fe3O4 NPs suitable for MRI and therapeutic SPECT modalities. The Fe3O4 NPs, averaging 3.0 nm in size, were synthesized using a flow-based method involving the thermal decomposition of ferric acetylacetonate. Subsequently, the original hydrophobic oleate ligand was replaced with a diphosphonate-terminated PEG ligand (DP-PEG, Mn ≈ 2000), which features a methoxy group at the opposite end. Due to the strong binding affinity of diphosphonate groups for ferric ions, the resulting PEG-coated nano-formulations demonstrated excellent H2O dispersibility with no significant aggregation. DLS analysis revealed a narrow hydrodynamic size distribution centered at around 15.7 nm and a ζ-potential of 6.7 mV. MRI characterization showed that the nano-formulations exhibited longitudinal (r1) and transverse (r2) relaxivities of 7.8 and 32.7 m·M−1·s−1, respectively, at 3 T. Furthermore, the relatively low r2/r1 ratio (4.2) combined with the high r2 value indicated that these nano-formulations could serve as an effective dual-mode T1/T2 contrast agent for MRI applications. Subsequent radiolabeling with 177Lu resulted in an average radiolabeling rate of 50.9% and the experiments showed that the radiolabeling efficiency depends significantly on the ratio of radioactive atoms to NPs. Furthermore, the nano-formulations maintained excellent colloidal stability throughout the labeling process and subsequent purification steps, as evidenced by consistent relaxometric properties, hydrodynamic size distributions, and ζ-potential measurements before and after treatment. The radiolabeling stability was assessed by measuring with a gamma counter the radiochemical purity of radioactive nano-formulations in fetal bovine serum (FBS) after ultrafiltration. Following 6 h of incubation, the nano-formulations demonstrated excellent stability, with radiochemical purity levels at around 99.5%. Long-term stability tests revealed that 177Lu-labeled NPs maintained consistent purity (>99%) for over 72 h [].
Rasaneh et al. [] developed a novel dual-modality radiopharmaceutical for breast cancer treatment that enables simultaneous monitoring through both SPECT and MRI. DLS analysis revealed a Dh of 41 ± 15 nm, while the core size averaged 9.0 ± 2.5 nm. The radiolabeling efficiency was determined to be 61 ± 2%. Stability studies demonstrated that 86 ± 5% of 177Lu-trastuzumab remained intact in phosphate buffer, while 80 ± 3% was stable in human serum over 7 days. Additionally, the trastuzumab-functionalized nano-formulation conjugate exhibited high stability in phosphate buffer for 8 days, with only a 4% increase in size and no detectable free trastuzumab in PBS [].
Shanehsazzadeh et al. [] assessed the suitability of SPIONPs radiolabeled with 177Lu as a promising theranostic agent for combined SPECT/MRI applications. The SPIONPs consisted of dextran-coated iron oxide cores crosslinked with surface-exposed amine (-NH2) groups. The bifunctional chelator cyclohexane-1,4-diyldinitrilo)tetraacetic acid dianhydride (ccDTPA) was coupled to the SPIONPs via a modified cyclic anhydride-mediated conjugation approach, using a 1:2 molar ratio (SPIONPs:ccDTPA) for optimal functionalization (Figure 9).
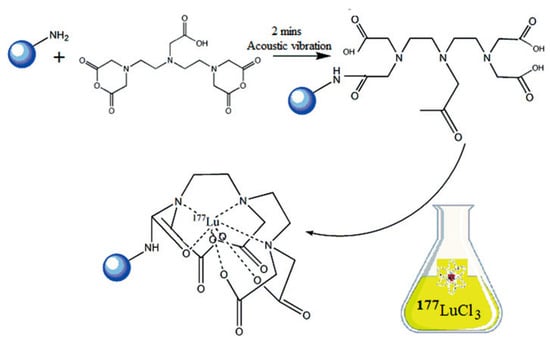
Figure 9.
Conjugation of cDTPA to aminated dextran-coated IONPs and subsequent radiolabeling with 177Lu. Adopted with permission from Shanehsazzadeh, S.; Grüttner, C.; Yousefnia, H.; Lahooti, A.; Gholami, A.; Nosrati, S.; Zolghadri, S.; Anijdan, S.H.M.; Lotfabadi, A.; Varnamkhasti, B.S.; Daha, F.J.; Jalilian. A.R., Radiochim. Acta; published by De Gruyter Brill, 2016 [].
After radiolabeling of the functionalized nano-formulations with 177Lu, a purification process was performed by employing a magnetic assorting cell separation (MACS) column to remove unbound 177Lu (Figure 10).
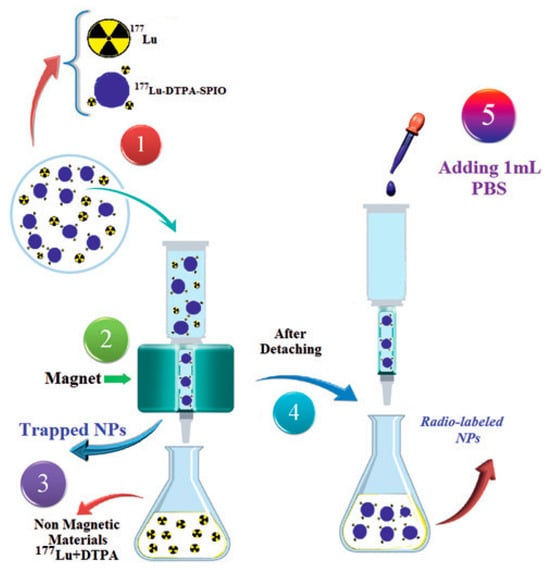
Figure 10.
Descriptive scheme of the magnetic purification process of 177Lu-labeled functionalized nano-formulations via a MACS column to isolate the radiolabeled product from free 177Lu. Step 1: The crude reaction mixture containing both 177Lu-labeled magnetic nano-formulations and free 177Lu is loaded onto the MACS column placed in a magnetic field. Step 2: The column is washed with PBS to elute non-magnetic components, primarily free 177Lu. The MNPs are retained within the column. Step 3: The flow-through containing the free radionuclide is collected and discarded. Step 4: The column is removed from the magnetic field and allowed to stand for 1–2 min. Step 5: Elution of the final product. Adopted with permission from Shanehsazzadeh, S.; Grüttner, C.; Yousefnia, H.; Lahooti, A.; Gholami, A.; Nosrati, S.; Zolghadri, S.; Anijdan, S.H.M.; Lotfabadi, A.; Varnamkhasti, B.S.; Daha, F.J.; Jalilian. A.R., Radiochim. Acta; published by De Gruyter Brill, 2016 [].
The functionalized nano-formulations exhibited a narrow size distribution with an average crystalline core diameter of ~9 nm according to TEM analysis. Photon correlation spectroscopy (PCS) analysis revealed a Dh of approximately 67 nm. Following radiolabeling, the magnetic nanoconstructs initially demonstrated 73% radionuclide purity, which increased to 98.5% after purification via MACS column separation. At 48 h post-labeling, the radiochemical purity remained high, with >93% stability in reference buffer and >78% in human plasma. Dh analysis confirmed that the nano-formulation size distribution remained unchanged after radiolabeling, indicating excellent colloidal stability [].
To explore radiolabeling potential and design novel theranostic agents for combined cancer diagnosis and radionuclide-enhanced hyperthermia therapy, Mirković et al. [] synthesized two types of MNPs: a poly-L-lysine-coated (PLL) magnetite system and an amino acid-decorated iron oxide platform. The amino acid-modified nano-formulations originated from perchloric acid-stabilized IONPs, which subsequently underwent surface engineering through adsorption of proline (Pro) and tryptophan (Trp) molecules. A systematic optimization study was conducted to determine the ideal Trp, Pro, and PLL loading on the MNPs. The loading was determined via Ultraviolet-Visible (UV-Vis) spectrometry. The measurements showed that the Pro formulation prepared with an input mass ratio of 5:1 demonstrated an adsorption efficiency of approximately 17% (w/w). In the case of Trp, the formulation prepared with a Trp-to-MNP mass ratio of 7:1 exhibited an adsorption efficiency of 3.5% (w/w). Correspondingly, the PLL formulation with a PLL-to-MNP mass ratio of 2:1 exhibited the highest PLL adsorption efficiency. A comprehensive suite of analytical techniques, such as TEM, DLS, differential centrifugal sedimentation (DCS), and thermomagnetic analysis, was employed to characterize the nano-formulations. TEM micrographs of the as-prepared samples revealed irregularly shaped particles with some degree of agglomeration attributable to sample preparation artifacts and average core diameters of 7–9 nm for non-functionalized, Pro- and Trp-functionalized formulations, respectively. The PLL-coated nano-formulations exhibited a slightly larger core diameter of approximately 11 nm. The DLS analysis showed that PLL MNPs displayed a monodisperse distribution with a peak at 112.6 nm, while uncoated, Pro-, and Trp-coated formulations showed respective peaks at 34.2 nm, 41.5 nm, and 49.7 nm. The observed size increases correlated well with DCS measurements (27.3 nm for uncoated, 53.8 nm for Pro-, 58.5 nm for Trp-coated MNPs) and confirmed successful amino acid functionalization. Notably, unmodified MNPs exhibited significant aggregation (Dh = 91.2 nm, PDI = 0.256), whereas PLL coating improved colloidal stability (PDI = 0.114), likely due to both the polymer’s molecular dimensions and its ability to form three-dimensional hydrophilic structures. Surface charge analysis via ζ-potential measurements revealed enhanced values for functionalized samples, confirming successful surface modification. All modified systems demonstrated excellent colloidal stability in 10 mM NaCl, with ζ-potential magnitudes exceeding 25 mV—the threshold for effective electrostatic stabilization. Magnetic characterization confirmed superparamagnetic behavior at 300 K for all samples, with no observable hysteresis. Saturation magnetization values were determined as 53.41 emu·g−1 (Pro MNPs), 47.11 emu·g−1 (Trp MNPs), and 57.17 emu·g−1 (PLL MNPs). The reduced magnetization relative to unmodified samples (uncoated MNPs = 60.6 emu·g−1, unstabilized MNPs = 75.6 emu·g−1) reflects the increased mass fraction of non-magnetic coating materials, as further supported by TGA. Magnetic core diameters derived from magnetization curves showed close agreement with TEM measurements, validating the consistency of the characterization methods. Under alternating magnetic fields (15.9 kA·m−1, 252 kHz), all functionalized nano-formulations generated heat within the therapeutic range (42–46 °C). PLL MNPs showed superior heating capacity with specific absorption rate (SAR) = 99.7 W·g−1 and intrinsic loss power (ILP) = 1.56 nH·m2·kg−1, significantly outperforming Pro MNPs and Trp MNPs. This enhanced performance stems from their optimized surface chemistry and magnetic properties, making PLL MNPs particularly suitable for magnetic hyperthermia applications. PLL MNPs demonstrated superior performance even under static magnetic field conditions. The radiolabeling of the produced nano-formulations with 177Lu (~50 μCi, 1.85 MBq) was performed at two different temperatures (room temperature and 80 °C) and pH 4.5–5, following a direct method. The exceptional colloidal stability of 177Lu-labeled formulations precluded radiochemical yield determination via magnetic separation. Consequently, radiochemical purity was assessed by ITLC-SG immediately post-labeling. Notably, PLL MNPs demonstrated superior radiochemical purity values (>98%) at both evaluated temperatures compared to the other functionalized formulations. Stability assessments of 177Lu-labeled PLL MNPs (80 °C radiolabeling) in physiological media demonstrated exceptional performance, maintaining >98% radiochemical purity in both saline and human serum at 37 °C over 96 h. Room temperature-labeled counterparts showed slightly reduced yet substantial stability, retaining > 80% integrity throughout the evaluation period [].
Li et al. [] engineered a novel NP system addressing key challenges in bladder cancer therapy through simultaneous mucosal penetration, reduced systemic exposure, and localized radiotherapy, offering potential for organ-preserving treatment strategies. More specifically, three types of hyaluronicacid(HA)-coated (HA molecular weights, 3K, 10K, and 90K Da) IONPs were synthesized via the coprecipitation method. The produced formulations were further colabeled with dibenzocyclooctyne (DBCO) and 177Lu. TEM images of the produced formulations revealed average diameters of 4.9, 6.5, and 7.0 nm, respectively. The transverse relaxivity (r2) of the nanoconstructs that was determined through MRI measurements confirmed the strong potential of the nanoprobes for T2-weighted MRI applications. The hydrodynamic sizes of the produced formulations were 21.2, 29.5, and 50.8 nm, respectively, indicating the effect of the HA’s molecular weight on the size of the formulated nanoprobes. Further increase in the Dh of the formulations was also observed after modification with DBCO. The organic content of the nanoprobes was determined via TGA analyses and the respective values for the three types of formulations were 67.1, 64.7, and 62.8%, respectively. Radiochemical purity was consistently >80% for all 177Lu-labeled nanomaterials, whilst stability monitoring in biological media (PBS and 10% FBS) via gamma counter demonstrated remarkable retention of radioactivity, with <10% loss observed throughout the evaluation period (72 h) [].
4.2. Biological Applications
Recent advances in nuclear medicine and nanotechnology have converged in the development of radiolabeled nanoplatforms for targeted cancer imaging and therapy. For theranostic applications, radiolabeled inorganic NPs have emerged as promising agents for techniques like SPECT and MRI, providing critical data on pharmacokinetics and targeting efficiency. However, conventional cancer diagnostics face inherent limitations, including inadequate spatial resolution, insufficient soft-tissue contrast, and a lack of controlled drug delivery to specific sites. To address these challenges, SPIONPs functionalized with the theranostic radionuclide 177Lu have been developed. This combination enables multimodal, high-resolution imaging by enhancing MRI contrast while simultaneously permitting SPECT tracking, thereby facilitating integrated treatment planning and therapy [].
An interesting in vivo approach in developing effective nanoprobes involved labeling of HER2-positive breast cancer inhibiting antibodies with 177Lu, followed by their attachment to IONPs to form contrast agents in MRI. Radiotherapy combined with imaging methods presents several limitations regarding the accuracy in organ volume measurements and radiopharmaceutical dose. In this context, the 177Lu-trastuzumab-IONPs developed by Rasaneh et al. [] were evaluated as dual-activity agents for targeted radioimmunotherapy and effective monitoring of their biodistribution in mice with breast and liver tumors, utilizing different imaging methods. Despite the lower sensitivity of MRI relative to SPECT, 177Lu-trastuzumab-IONPs enabled enhanced MRI-based monitoring during radiotherapy. This facilitated superior dose estimation and significantly increased liver accumulation (~7% higher than the control NPs), while minimizing uptake in non-target organs (Figure 11). The combination of 177Lu’s therapeutic properties, trastuzumab’s targeted binding to overexpressed HER2 protein on cancer cells, and the imaging capability of IONPs opens new avenues for effective dosimetry tracking and tumor volume reduction.
Figure 11.
MRI images before and 1 day after injection of the 177Lu-trastuzuman-IONPs (top) and SPECT images at 1 and 7 days post injection of the 177Lu-trastuzuman-IONPs (bottom). The arrows indicate the tumors. Adopted from “Activity estimation in radioimmunotherapy using magnetic nanoparticles,” by Rasaneh, S.; Rajabi, H.; Daha, F.J. Chin. J. Cancer Res. 2015, 27, 203–208 [].
SPIONPs with polymer surface coating represent an indicative example of enhanced contrast agents for MRI applications. It is well established that surface modification of MNPs offers enhanced biocompatibility, efficient delivery, and accumulation at specific target sites. To that end, the developed by Shanehsazzadeh et al. [] promising DTPA-based 177Lu nanoplatform could be suggested as an effective theranostic agent for reticuloendothelial system (RES) studies employing SPECT and MRI. The size range and coating of the produced NPs were responsible for the accumulation in the liver and spleen and the rapid clearance from the blood. Compared with analogous SPIONPs targeting RES, accumulation of the 177Lu-labeled nano-formulations was increased from 56.64 ± 1.91 to 61.5 ± 2.9% in the liver and 12.5 ± 2.9 to 16.9 ± 1.4% in the spleen, respectively, probably due to their relatively small size, showing an increased absorption of 177Lu doses by RES compared to normal diagnostic nuclear medicine studies.
In another work, Hue et al. [] developed 177Lu-labeled thermally cross-linked spherical SPIONPs (177Lu-TCL-SPIONPs) with enhanced stability (98%) and prolonged efficiency period (21 days) that were employed as diagnostic agents for in vivo cancer imaging. Upon labeling with 177Lu, TCL-SPIONPs were injected into ICR mice and distributed from systemic circulation into intracellular tissues. The 177Lu-labeled nano-formulations were mainly accumulated in the liver and spleen (36.121 ± 8.239% ID·g−1 at one day post-injection), in contrast to free 177Lu radioactivity that was accumulated in blood and various organs, mostly in the kidneys. 177Lu-TCL-SPIONPs radioactivity rapidly declined in blood, brain, and epididymis 28 days after injection. Interestingly, in relevant studies, a second peak of radioactivity was displayed by the spleen over a period of 60 days, indicating that the iron is slowly released and “restored” in the organ, hence handled as natural iron by the human body []. These findings were quite similar to the ones obtained using 177Lu-TCL-SPIONPs, confirming thus their safety as potential chemotherapeutic carriers.
177Lu-labeled SPIONPs are considered indicative candidate nanoagents for nanobrachytherapy applications through direct injection in the tumor region. Compared to typical radiotherapy, the percentage delivered to targeted cancer cells is significantly higher, avoiding the rapid clearance by liver and spleen and the subsequent detrimental effect on these organs due to radioactivity accumulation. Stanković et al. [] investigated the in vivo pharmacokinetic behavior of 177Lu-DMSA-SPIONPs after their intratumoral injection in colorectal CT-26 and breast 4T1 tumor-bearing mice. Evaluation of tumor growth following intratumoral administration revealed a significant, dose-independent suppression compared to controls. Examination of tumor tissue indicated moderate necrotic changes, most pronounced at the injection site. A key finding was the predominant localization and retention of the nanomaterial at the injection site in both tumor types without causing adverse side effects (Figure 12). Ex vivo biodistribution studies in CT-26 and 4T1 mouse tumor xenografts after variable radiation doses and number of treatments indicated high intra-tumoral radioactivity retention (90–95% ID for 1 day), and minimal leakage in both xenograft models for 14 days, with minimal radiation exposure to healthy organs and no observed general toxicity. Significant efficacy was achieved even at a low dose of 1.85 MBq, a finding with important implications for reducing radiation exposure to surrounding healthy tissues (Figure 13).
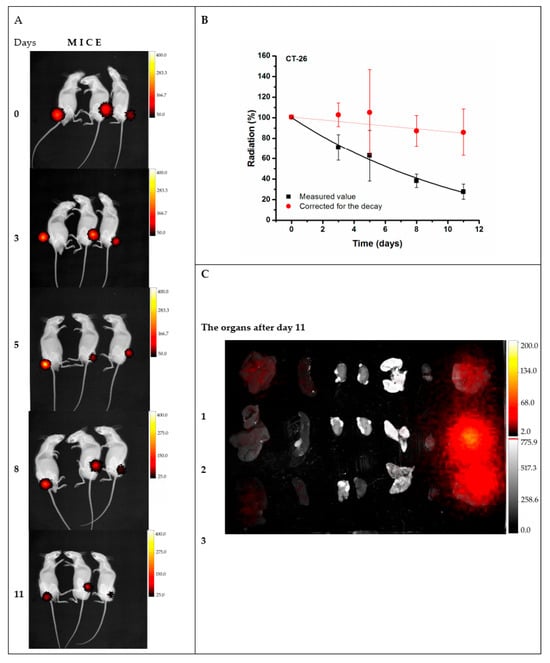
Figure 12.
Biodistribution and pharmacokinetics of 177Lu-DMSA@SPIONPs following intratumoral injection in CT-26 tumor-bearing mice. (A) In vivo whole-body imaging (radioactivity-to-light) from day 0 to day 11 post-injection. (B) Pharmacokinetic profile of the nanomaterial within the tumor, derived from the integrated signal intensities in panel A. (C) Ex vivo quantification of radioactive signal in the tumor and major organs at day 11. Adopted from Stanković, D.; Radović, M.; Stanković, A.; Mirković, M.; Vukadinović, A.; Mijović, M.; Milanović, Z.; Ognjanović, M.; Janković, D.; Antić, B.; Vranješ-Đurić, S.; Savić, M.; Prijović, Ž. Synthesis, characterization, and therapeutic efficacy of 177Lu-DMSA@SPIONs in nanobrachytherapy of solid tumors. Pharmaceutics; published by MDPI, 2023 [].
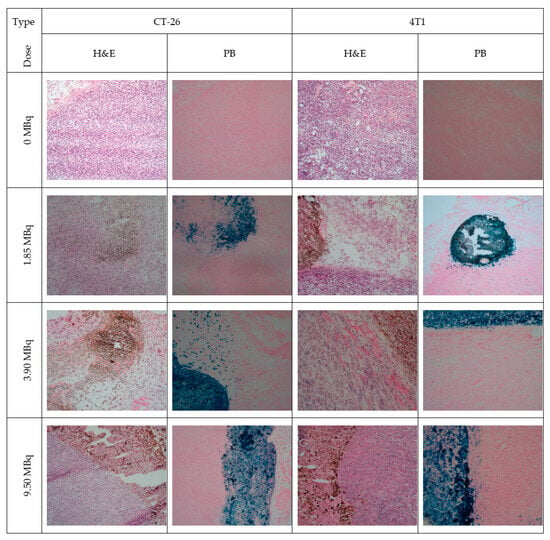
Figure 13.
Photomicrographs of CT-26 and 4T1 tumor tissues following a single intratumoral injection of 177Lu-DMSA@SPIONs at doses of 1.85, 3.70, and 9.25 MBq. Tissue sections were stained with hematoxylin and eosin and Prussian blue. Scale bar represents 100 µm (200× magnification). Adopted from Stanković, D.; Radović, M.; Stanković, A.; Mirković, M.; Vukadinović, A.; Mijović, M.; Milanović, Z.; Ognjanović, M.; Janković, D.; Antić, B.; Vranješ-Đurić, S.; Savić, M.; Prijović, Ž. Synthesis, characterization, and therapeutic efficacy of 177Lu-DMSA@SPIONs in nanobrachytherapy of solid tumors. Pharmaceutics; published by MDPI, 2023 [].
Magnetic nanomaterials open new avenues in the development of novel theranostics as contrast agents but also as carriers of drugs or radionuclides for radiotherapy and magnetic hyperthermia against cancer. Aiming to overcome limitations of radiotherapy and optimize NPs properties, such as prolonged blood circulation, aggregation, etc., several types of polymers have been utilized for surface modification.
A representative example can be found in the work of Salvanou et al. []. In their study, they performed a preliminary biological evaluation of two species of co-CNCs formulations based on: (i) surface-modified MIONPs with AA (MA), and (ii) stabilized MA NPs with PEG. In vitro evaluation of blood compatibility via hemolysis assay verified the safety profiles of both MA and MAPEG. Cytotoxicity assessment indicated that the unconjugated compounds were not markedly toxic to 4T1 cancer cells; however, the 177Lu-labeled MAPEG conjugate demonstrated a dose-dependent decrease in cell viability. Furthermore, the in vivo behavior of [177Lu]Lu-MAPEG revealed a high accumulation in the liver.
These preliminary findings prompted the same research team to optimize MAPEG by functionalizing it with targeting ligands, aiming to develop a promising theranostic agent for locoregional delivery that enables substantial tumor accumulation. To that end, Salvanou et al. [] examined a series of surface-modified species of MIONPs, such as: (i) MIONPs functionalized with the commonly used polysaccharide AA as chelator and the biocompatible polymer PEG (MAPEG), (ii) MAPEG with surface conjugated DOX and monoclonal antibody BVCZ (MAPAD), (iii) MAPEG with surface conjugated DOX (MAPEGDOX), as potential nanobracytherapy agents. The obtained nano-formulations were examined for their cytotoxicity against 4T1, MDA-MB-231, M165, MCF7, and SKBR3 cancer cells with varying levels of Vascular Endothelial Growth Factor (VEGF) expression. Evaluation via MTT assay indicated that the MAPEG NPs exhibited negligible toxicity up to 72 h post-treatment. The cytotoxic effects of both 177Lu-radiolabeled formulations, MAPEG and MAPAD, were found to be influenced by both the administered radioactivity and the duration of exposure, resulting in a time- and dose-dependent reduction in viability. Investigations into cellular uptake, using fluorescence microscopy and Prussian blue staining (Figure 14), revealed a marked difference between formulations. MAPAD NPs, functionalized with BVCZ, were internalized rapidly by 4T1 cells, with signs of nuclear localization observed after just one hour. Conversely, MAPEGDOX NPs showed predominantly cytoplasmic retention. Ex vivo biodistribution analysis up to 7 days post-injection compared three administration routes, with intratumoral delivery showing the most targeted and prolonged retention within the tumor. Ultimately, a single intratumoral administration of the functionalized 177Lu-labeled MAPAD formulations proved to have superior therapeutic efficacy in the 4T1 mouse model.
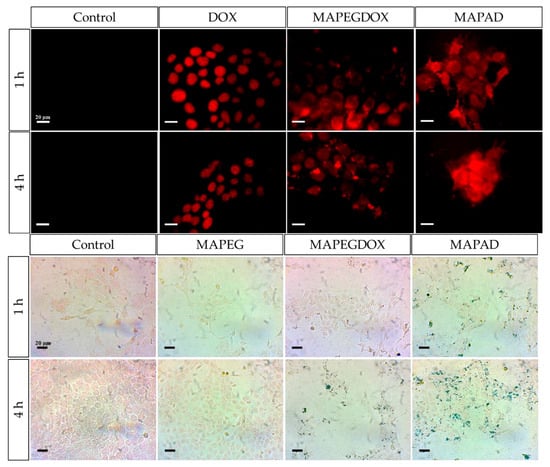
Figure 14.
Cellular uptake in 4T1 cells after 1 h and 4 h of incubation with medium, free DOX, MAPEGDOX, or MAPAD, as shown by fluorescence microscopy (scale bar: 20 µm) (top) and Prussian blue staining of 4T1 cells revealed iron uptake after 1 and 4 h of treatment with medium, MAPEG, MAPEGDOX, and MAPAD (scale bar = 20 µm) (bottom). Adopted from Salvanou, E.-A.; Kolokithas-Ntoukas, A.; Prokopiou, D.; Theodosiou, M.; Efthimiadou, E.; Koźmiński, P.; Xanthopoulos, S.; Avgoustakis, K.; Bouziotis, P. Molecules; published by MDPI, 2024 [].
Using a different polysaccharide, such as HA, Li et al. [] employed 177Lu-Fe3O4@HA/DBCO nanoprobes with biorthogonality and mucoadhesivity for the effective diagnosis and treatment of bladder cancer. This therapeutic strategy combined the excellent biocompatibility and selectivity of HA and the capacity of DBCO to participate in in vivo biorthogonal reactions that overexpress artificial receptors on cancer cells, with the theranostic properties of 177Lu. In vivo studies carried out in mice with nonmuscle-invasive (NMIBC) and muscle-invasive bladder cancer (MIBC) suggested that 177Lu-Fe3O4@HA/DBCO nano-formulations could penetrate mucosa, reaching the region of the tumor, while Fe3O4@HA NPs could only adhere to mucosa. Likewise, Fe3O4@HA demonstrated lower inhibition of NMIBC and MIBC compared to the radiolabeled 177Lu-Fe3O4@HA/DBCO formulations, which caused significant tumor shrinkage, as illustrated in Figure 15. Additional biodistribution studies revealed the increased accumulation of 177Lu in bladders compared to other organs (Figure 16), indicating that this radiolabeled biorthogonal nanoagent improves the accurate detection of the tumor while increasing selectivity, specificity, and therapeutic efficacy.
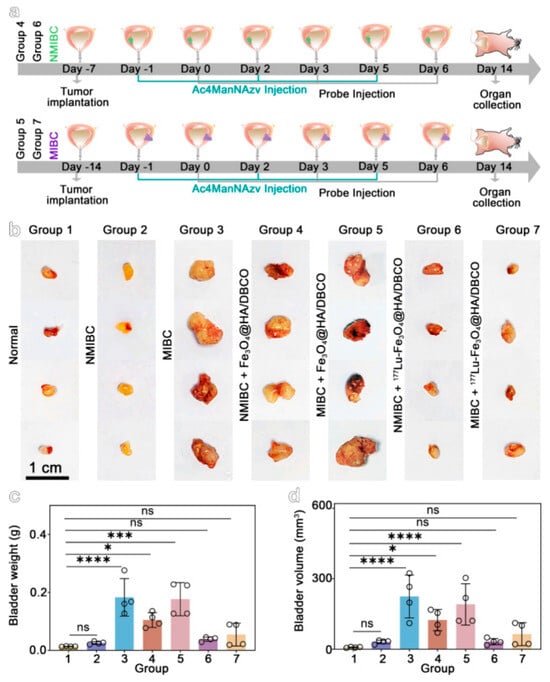
Figure 15.
Evaluation of treatment efficacy in orthotopic bladder cancer models. (a) Schematic of the treatment schedules for NMIBC and MIBC models. (b) Macroscopic appearance of bladders isolated from the different experimental groups. (c,d) Bar graphs comparing bladder weight and volume. Data are presented as mean ± SEM. p-Values were calculated using one-way ANOVA with post hoc testing (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001; ns, not significant). Adopted with permission from Li, Y.; Shan, S.; Zhang, R.; Sun, C.; Hu, X.; Fan, J.; Wang, Y.; Duan, R.; Gao, M. Imaging and downstaging bladder cancer with the 177Lu-labeled bioorthogonal nanoprobe. ACS Nano; published by American Chemical Society, 2024 [].
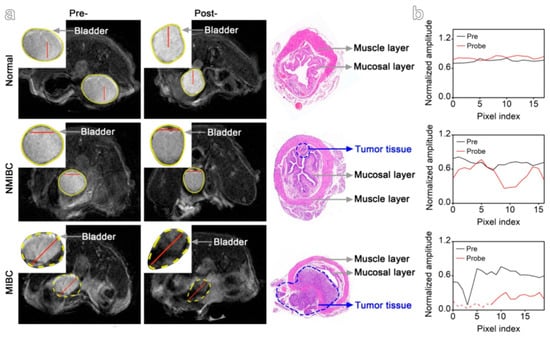
Figure 16.
MRI and histological analysis of bladder tumors. (a) T2-weighted MR images acquired pre- and post-intravesical administration of the targeted contrast agent (N-Azidoacetylmannosamine-tetraacylated + Fe3O4@HA/DBCO). Corresponding Hematoxylin and Eosin-stained bladder sections confirm tumor invasion status, classifying tumors as NMIBC or MIBC. (b) Post-contrast MR image with the bladder highlighted (yellow circle). The red line indicates the location for line-scan analysis of MRI signal intensity (left panel). Adopted with permission from Li, Y.; Shan, S.; Zhang, R.; Sun, C.; Hu, X.; Fan, J.; Wang, Y.; Duan, R.; Gao, M. Imaging and downstaging bladder cancer with the 177Lu-labeled bioorthogonal nanoprobe. ACS Nano; published by American Chemical Society, 2024 [].
In another study, the 177Lu-labeled surface-modified IONPs by Ognjanović et al. [] were evaluated for their potential use in magnetic hyperthermia/radionuclide cancer therapy, and the results indicated that the 177Lu-PAA@IONPs exhibited notably low cytotoxicity at physiological levels. In vitro application of the NPs on CT-26 tumor cells, using magnetic hyperthermia, showed great performance (maximal effect at 116 kHz over a period of 30 min) with remarkably high ILP values (7.3 nHm2·kg−1), inducing a significant cytotoxic effect against cancer cells.
The observed high in vitro stability of the developed by Mirković et al. [] 177Lu–PLL-MNPs urged the research team to further evaluate their in vivo behavior. The biodistribution of the 177Lu-labeled nano-formulations was evaluated in healthy Wistar rats at four time points (0.5, 3, 24, and 96 h) post-injection. The NPs accumulated primarily in the liver (69.45% ID at 0.5 h, increasing to 84.30% ID at 96 h) and the spleen (15.90% ID at 0.5 h, decreasing to 9.62% ID at 96 h), with minimal uptake in other organs (Figure 17). This pattern is typical for NPs of this size, which are rapidly cleared from the bloodstream by macrophages in the liver and spleen. The PLL coating enhanced the in vivo stability of the MNPs by preventing aggregation, as evidenced by low lung uptake. Furthermore, negligible radioactivity in the femur indicated that the 177Lu radiolabel remained stably bound to the PLL-MNPs, with no detectable free radionuclide release.
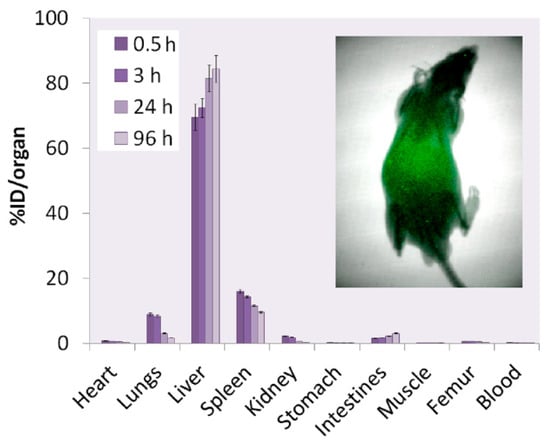
Figure 17.
Biodistribution of 177Lu–PLL-MNPs in healthy Wistar rats at 0.5, 3, 24, and 96 h post-intravenous administration. Data represent the percentage of injected dose per organ (%ID/organ), expressed as the mean ± standard deviation (n = 3–5). The inset shows a radioimage of a rat 96 h after injection. Adopted with permission from Mirković, M.; Milanović, Z.; Perić, M.; Vranješ-Đurić, S.; Ognjanović, M.; Antić, B.; Kuraica, M.; Krstić, I.; Kubovcikova, M.; Antal, I.; Sobotova, R.; Zavisova, V.; Jurikova, A.; Fabian, M.; Konerack M. Design and preparation of proline, tryptophan and poly-L-lysine functionalized magnetic nanoparticles and their radiolabeling with 131I and 177Lu for potential theranostic use. Int. J. Pharm.; published by Elsevier, 2022 [].
4.3. Analysis of Multimodal Functionality
The development of nanoscale theranostic agents that integrate multiple diagnostic and therapeutic functions into a single platform is a pivotal advancement in personalized medicine, particularly for oncology. In this context, the strategic importance of 177Lu-labeled MNFs lies in their inherent ability to exhibit synergistic multimodal activity for MRI, SPECT, and combined radiotherapy/hyperthermia. This multifunctionality allows for simultaneous and non-invasive anatomical localization via MRI, sensitive radionuclide-based tracking and dosimetry via SPECT, and the delivery of a powerful, localized therapeutic payload through the radioisotope’s beta-particle emissions complemented by magnetic hyperthermia. Such an all-in-one system enables real-time monitoring of biodistribution, precise tumor targeting, and the application of combinatorial treatments, thereby maximizing therapeutic efficacy while minimizing off-target effects and providing a comprehensive approach to cancer management.
Based on the first study of Salvanou et al. [], it has been proven possible to create effective multimodal theranostic constructs by developing alginate and PEG-coated IONPs successfully radiolabeled with both 68Ga for PET imaging and 177Lu for therapy, thereby combining MRI capability from their superparamagnetic core with PET diagnostics and radionuclide therapy in a single agent. The incorporation of 177Lu provided several key features: it enabled efficient and stable direct radiolabeling via the alginate coating without a chelator, demonstrated significant dose-dependent cytotoxicity against cancer cells in vitro, and showed a favorable biodistribution profile in vivo with high and prolonged accumulation in RES organs like the liver and spleen, supporting its potential for therapeutic applications, particularly via locoregional administration.
Their second study [] demonstrated the successful creation of a robust multimodal theranostic agent for MRI, SPECT, and therapy, based on an IONP core functionalized with DOX and BVCZ and labeled with 177Lu. From 177Lu, five critical features were leveraged: its beta emissions provided the primary therapeutic effect for tumor shrinkage, its gamma emissions enabled SPECT imaging and biodistribution tracking, its 6.7-day half-life allowed for prolonged tumor irradiation and logistical convenience, its stable direct radiolabeling to the NP’s alginate coating ensured a simple and efficient construction, and its application in a nanobrachytherapy approach via intratumoral injection resulted in exceptional tumor retention and minimized off-target effects, showcasing a potent and targeted combinational therapy.
Li et al. [] demonstrated the successful creation of an effective multimodal theranostic agent for MRI, SPECT, and therapy, the 177Lu-Fe3O4@HA/DBCO nanoprobe. This system leverages the iron oxide core for T2-weighted MRI and the radioisotope 177Lu for both therapeutic and SPECT imaging capabilities. From 177Lu, five key features were harnessed: (i) its beta emissions enable potent targeted radionuclide therapy to shrink and downstage tumors, (ii) its gamma rays permit SPECT imaging and biodistribution tracking, (iii) its favorable half-life of 6.7 days allows for sustained diagnostic and therapeutic effects, (iv) its integration with a bioorthogonal targeting system ensures precise tumor accumulation, and (v) the treatment was shown to inhibit metastasis, highlighting its comprehensive therapeutic potential.
Stanković et al. [] developed an effective multimodal theranostic agent for MRI, SPECT, and therapy using SPIONPs coated with DMSA and radiolabeled with 177Lu. The SPIONP core provides the inherent superparamagnetic properties necessary for MRI, while the177Lu radionuclide contributes several critical features: (i) its beta emissions deliver the primary therapeutic effect via a localized “cross-fire” within a ~2 mm range in tissue, (ii) its gamma emissions enable SPECT imaging and biodistribution tracking, (iii) its 6.7-day physical half-life allows for prolonged tumor irradiation and logistical convenience in handling, (iv) it achieves stable, direct chelator-free radiolabeling via the DMSA coating with high yield and in vitro stability, and (v) when administered via intratumoral injection (nanobrachytherapy), it results in exceptional tumor retention, minimal systemic leakage, and high therapeutic efficacy without signs of general toxicity, demonstrating a potent and targeted combinatorial approach.
Excellent multimodal theranostic systems for MRI, SPECT, and combined therapy using polyol-synthesized, flower-like IONPs coated with PAA were developed by Ognjanović et al. []. The SPIONP core provides the strong T2 contrast capability for MRI, while the successful radiolabeling with99mTc enables SPECT imaging. For therapy, the system leverages multiple features from 177Lu: (i) its beta emissions enable radionuclide therapy, (ii) it achieves very high radiolabeling yields (>98%) via a simple, indirect method with the PAA coating, (iii) it exhibits excellent in vitro stability with over 95% retention after 96 h in physiological media, and (iv) it is used in conjunction with the NP’s outstanding magnetic hyperthermia capability (intrinsic loss power of 7.3 nH m2·kg−1), creating a potent dual hyperthermia/radionuclide therapeutic platform.
Gholami et al. [] demonstrated the successful development of effective multimodal constructs for MRI, SPECT, and therapy using the chelate-free HIR method with FH NPs. This platform successfully incorporates a variety of diagnostic and therapeutic isotopes, including 177Lu, into the iron oxide core without the need for chelators, preserving the NP’s magnetic properties for MRI while enabling radiolabeling for nuclear imaging and targeted radionuclide therapy. For177Lu, the study demonstrated high radiochemical yields (RCY ≈ 91%) and purity (RCP ≈ 95%), confirming efficient and stable binding under HIR conditions. Additionally, the 177Lu-FH NPs exhibited minimal changes in size and relaxivity, maintained in vivo stability, and showed potential for combination with other modalities, supporting their use as versatile theranostic agents.
Based on the research presented by Ge et al. [], effective multimodal nanoprobes for MRI, SPECT, and therapy using the universal LAGMERAL method were developed. This approach successfully integrated various functional inorganic NPs, such as Fe3O4 for MRI and Cu2-xS for photoacoustic imaging, with different radionuclides. For the therapeutic isotope 177Lu, the system demonstrated several key features: (i) it achieved a radiolabeling yield of 67.4% on Cu2-xS NPs, (ii) provided excellent radiolabeling stability with 99.5% purity retained in serum after 72 h, (iii) enabled high-contrast SPECT imaging for sentinel lymph node detection, and (iv) combined the radiotherapeutic effect of 177Lu with the intrinsic photothermal properties of the NP carrier for potential synergistic therapy.
The biodistribution study by Hue et al. [] demonstrated that the 177Lu-TCL-SPIONPs show a clear potential for use in multimodal systems combining SPECT and therapy. The primary feature of 177Lu-TCL-SPIONPs is their distinct and stable biodistribution profile, characterized by high and sustained accumulation in the liver (36.12% ID·g−1 at day 1, 15.15% ID·g−1 at day 28) and spleen (15.62% ID·g−1 at 0.5 h, 9.09% ID·g−1 at day 28), which are organs of the RES. This pattern, coupled with rapid clearance from the blood and most other tissues, confirms the NP’s role as a carrier that can be tracked via the gamma emissions of 177Lu for SPECT imaging. Furthermore, the inherent superparamagnetic property of the iron oxide core provides the functionality for MRI, while the beta-particle emission from 177Lu delivers the therapeutic effect. Therefore, the system effectively integrates three key features from 177Lu: (i) its role as a SPECT radiotracer for imaging biodistribution, (ii) its function as a beta-emitter for radiotherapy, and (iii) its stable attachment to an MRI-active NP, creating a promising theranostic platform.
Rasaneh et al. [] showed the successful development of a highly effective multimodal construct for MRI, SPECT, and therapy, the 177Lu-trastuzumab-IONPs platform. This system leverages three key features from177Lu: (i) its gamma emissions enable precise tracking of the NP’s biodistribution via SPECT imaging, (ii) its beta-particle emission provides the therapeutic mechanism for radioimmunotherapy, and (iii) its stable conjugation to the antibody-NP complex ensures targeted delivery. Crucially, the IONP core simultaneously serves as a potent T2 contrast agent for high-resolution MRI. The study demonstrated that this combined approach allowed for more accurate activity estimation and dosimetry in tumors compared to SPECT alone, confirming the construct’s role as a superior theranostic platform.
As demonstrated by Shanehsazzadeh et al. [], the 177Lu-DTPA-SPIONPs system successfully harnesses three key features from177Lu: (i) its gamma emissions enable precise biodistribution tracking via SPECT imaging, (ii) its beta-particle emissions provide the therapeutic mechanism for targeted radiotherapy, and (iii) its stable conjugation via a DTPA chelator ensures the radionuclide remains attached to the NP carrier. Crucially, the SPIO core serves as a potent T2 contrast agent for high-resolution MRI. The construct exhibited a favorable biodistribution profile, with high and sustained accumulation in the liver and spleen and rapid clearance from the blood, confirming its potential as a promising theranostic platform for RES-targeted applications.
Mirković et al. [] created effective multimodal nanoconstructs suitable for MRI, SPECT, and combination therapy. The PLL-MNPs exhibited superparamagnetic behavior essential for MRI, were successfully radiolabeled with the theranostic radionuclide 177Lu, and demonstrated a high SAR for magnetic hyperthermia therapy. The 177Lu labeling provided multiple key features: (i) it enabled SPECT imaging capabilities due to its gamma emissions, (ii) delivered beta-particle therapy for localized tumor treatment, (iii) allowed for diagnostic dosimetry, and (iv) exhibited excellent in vitro and in vivo stability, which is critical for ensuring the radionuclide remains attached to the nanocarrier during diagnostic and therapeutic procedures.
5. Current Challenges
177Lu-labeled magnetic nano-formulations (177Lu-MNFs) represent a compelling theoretical platform for theranostic oncology, combining the imaging and therapeutic potential of a radionuclide with the targeting and hyperthermia capabilities of MNPs. However, their clinical translation remains a distant goal, hindered by fundamental challenges that must be systematically addressed in future preclinical studies. The critical hurdles of inherent magnetic core limitations, dosimetry, scalability, biocompatibility, and regulatory approval currently define the essential research agenda for this field.
A fundamental set of constraints arises from the inherent properties of the magnetic cores. The efficacy of magnetic targeting and hyperthermia is highly dependent on core characteristics such as composition, size, crystallinity, and magnetic saturation. A significant limitation is the potential for oxidative degradation of certain cores (e.g., magnetite) in the physiological environment, which can compromise magnetic functionality and release potentially toxic ions. Furthermore, achieving sufficient magnetic responsiveness for effective in vivo targeting against the dynamic forces of blood flow remains a major physical challenge. Future research must focus on developing more stable, high-performance magnetic materials (e.g., doped ferrites) and rigorously evaluating their structural integrity and magnetic performance post-synthesis and under physiological conditions.
A significant and complex challenge is the accurate prediction of radiation dosimetry. The unique biodistribution of 177Lu-MNFs, characterized by high and persistent accumulation in organs of the mononuclear phagocyte system (e.g., liver, spleen), could lead to unintended radiation doses to these healthy tissues. Preclinical studies must prioritize developing sophisticated pharmacokinetic models that can reliably extrapolate organ absorption and radiation doses from animal models to humans, a critical step for ensuring patient safety in future trials.
A primary obstacle is the absence of robust, scalable manufacturing protocols. The synthesis of 177Lu-MNFs with precise control over the critical magnetic core properties, in addition to size and surface chemistry, is a laboratory-scale achievement. Reproducibly scaling this synthesis under Good Manufacturing Practice (GMP) conditions for clinical-grade material is a significant unsolved problem. Therefore, a paramount future direction is to develop continuous and controlled synthesis methods that guarantee batch-to-batch consistency, a prerequisite for generating reliable preclinical data and eventual regulatory approval.
Furthermore, the biocompatibility and in vivo pharmacokinetics of these complex agents are largely unknown. Key questions regarding their stability in the bloodstream, potential for off-target accumulation, and long-term clearance pathways remain unanswered. The chemical stability of the magnetic core is directly linked to these safety concerns. Before any clinical application can be considered, extensive preclinical investigations are mandatory. These must focus on engineering coatings that minimize immune recognition and on conducting comprehensive toxicological studies to establish a foundational safety profile, which is intrinsically linked to the dosimetry concerns.
Finally, the regulatory pathway for 177Lu-MNFs is exceptionally complex due to their hybrid nature as combination products. The lack of standardized characterization methods for evaluating magnetic properties, radiochemical purity, stability, and sterility specific to these formulations presents a major barrier. A critical future direction involves proactively collaborating with regulatory agencies to define the necessary criteria, including robust dosimetry data and magnetic performance metrics, and develop the analytical protocols required to evaluate these novel agents, thereby creating a clear roadmap for development.
6. Future Perspectives
The development of 177Lu-labeled MNFs represents a compelling frontier in targeted radionuclide therapy, merging the cytotoxic power of beta radiation with the unique capabilities of nanotechnology. These “theranostic” agents are engineered to be guided by external magnetic fields to specific tumor sites, while their intrinsic magnetic properties allow for non-invasive tracking via MRI. The central hypothesis is that this dual-targeting approach—passive accumulation through enhanced EPR and magnetically controlled active guidance—could dramatically improve tumor dose delivery while minimizing irradiation of healthy tissues. This promises a significant leap beyond current 177Lu-labeled molecules, potentially unlocking treatments for cancers that are currently inaccessible.
Looking beyond the translational challenges, future perspectives for 177Lu-MNFs are expansive and hinge on advanced nanoscale engineering. Next-generation platforms could be designed for combination therapies, such as the co-delivery of chemotherapeutic agents or immunomodulators to synergize with radiotherapy and magnetic hyperthermia, potentially overcoming treatment resistance. Furthermore, engineering MNFs for multimodal imaging, by incorporating contrast agents for MRI or fluorescent tags, could provide complementary diagnostic information, enhancing tumor localization and treatment planning.
Perhaps the most promising direction lies in developing smart, stimulus-responsive designs. These “intelligent” MNFs could be engineered to release their therapeutic payload or enhance radiation dose uptake specifically in response to the unique tumor microenvironment (e.g., low pH, specific enzymes) or an externally applied stimulus (e.g., alternating magnetic field), thereby maximizing efficacy while minimizing systemic toxicity revolutionizing thus the treatment of hard-to-reach or radioresistant solid tumors, such as certain brain, pancreatic, or advanced prostate cancers, where precise localization is critical.
A promising approach involves functionalizing 177Lu-labeled NPs with targeting ligands—such as peptides, antibodies, human serum albumin, or biomimetic coatings like exosomes. This functionalization enhances both the biocompatibility and tumor-specific targeting of the platform. For instance, using a protein scaffold for 177Lu-MNFs can increase tumor accumulation while minimizing off-target cytotoxic effects, thereby concentrating the therapeutic radiation within the tumor tissue.
However, the path from concept to clinic is fraught with formidable scientific and regulatory hurdles. Key challenges include achieving scalable and reproducible synthesis of nano-formulations with uniform size, stable radiolabeling, and consistent magnetic properties. The biological behavior, including long-term biodistribution, potential immune response, and eventual clearance of these inorganic particles from the body, must be thoroughly characterized to ensure safety. Furthermore, the practical efficacy of magnetic targeting in deep-seated human tumors remains to be conclusively demonstrated, and navigating the regulatory pathway for a novel combination product (drug + device) will be complex and costly.
Despite the challenges, the question is not whether to abandon this research, but how to strategically advance it. Researchers should not “forget about it”; instead, they should focus their efforts with precision. Priority should be given to interdisciplinary collaboration between radiochemists, materials scientists, and clinicians to tackle the core issues of manufacturing and safety. Research must move beyond proof-of-concept studies to robust, pre-clinical models that realistically test targeting efficacy and therapeutic superiority over standard care. The goal should be to generate compelling data that de-risks the technology for larger-scale investment and clinical translation.
In conclusion, the pursuit of 177Lu-labeled MNFs is not just a niche interest but a high-potential, high-reward endeavor worthy of focused research. The potential to create a superior, multifunctional cancer therapeutic aligns perfectly with the goals of personalized medicine. While the obstacles are real, they are not insurmountable. Researchers should vigorously pursue this path, as success here could yield a powerful new weapon in the oncological arsenal, fundamentally changing treatment paradigms for some of the most challenging cancers.
Author Contributions
E.H. planned and wrote Section 1, Section 2, Section 3 and Section 4 and revised Section 5 and Section 6. D.V. planned and wrote Section 5 and Section 6. All co-authors contributed to the final version with suggestions and critical comments. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
E. Halevas acknowledges the Foundation for Education and European Culture (IPEP), founded by Nicos & Lydia Tricha.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 177Lu | Lutetium-177 |
| TRT | Targeted radiation therapy |
| MNPs | Magnetic nanoparticles |
| NPs | Nanoparticles |
| MRI | Magnetic resonance imaging |
| SPECT | Single Photon Emission Computed Tomography |
| PET | Positron Emission Tomography |
| ICRP | International Commission on Radiological Protection |
| Lutathera® | [177Lu]Lu-DOTATATE |
| Pluvicto® | [177Lu]Lu-Vivipotide tetraxetan |
| PRRT | Peptide receptor radionuclide therapy |
| CuAAC | Copper(I)-catalyzed azide-alkyne cycloaddition |
| SPAAC | Strain-promoted azide-alkyne cycloaddition |
| IEDDA | Inverse-electron-demand Diels–Alder |
| CBT | 2-cyanobenzothiazole |
| NTA | Nitrilotriacetic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| DTPA | Diethylenetriaminepentaacetic acid |
| DOTA | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DO3A | 1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic acid |
| NOTA | 1,4,7-Triazacyclononane-1,4,7-triacetic acid |
| NOTAGA | (NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid) + GA (glutaric acid)) |
| NETA | {4-[2-(bis-carboxymethylamino)-ethyl]-7-carboxymethyl-[1,4,7]-triazonan-1-yl}-acetic acid |
| DOTRP | 1,4,7,10-tetraazacyclodecane-1,4,7,10-tetra(R)ester phosphinic acid |
| NORP | 1,4,7-teriazacyclononane-1,4,7-tri(R)esterphosphinic acid |
| 177Hf | Hafnium-177 |
| 176Yb | Ytterbium-176 |
| 176Lu | Lutetium-176 |
| 169Yb | Ytterbium-169 |
| 175Yb | Ytterbium-175 |
| 177Yb | Ytterbium-177 |
| SA | Specific activity |
| GEP-NETs | Gastroenteropancreatic neuroendocrine tumors |
| PPGLs | Pheochromocytomas and paragangliomas |
| LAR | Long-acting repeatable |
| SSTR2 | Somatostatin Receptor 2 |
| mCRPC | Metastatic castration-resistant prostate cancer |
| PI(s) | Principal investigator(s) |
| PSMA | Prostate-specific membrane antigen |
| PET/CT | Positron emission tomography/Computed tomography |
| NETs | Neuroendocrine tumors |
| EPR | Enhanced permeability and retention |
| MIONPs | Magnetic iron oxide NPs |
| IONPs | Iron oxide NPs |
| SPIONPs | Superparamagnetic iron oxide NPs |
| SPIO | Superparamagnetic iron oxide |
| Fe3O4 | Magnetite |
| γ-Fe2O3 | Maghemite |
| PEG | Polyethylene glycol |
| co-CNCs | Condensed colloidal nanocrystal clusters |
| MA | Alginate-coated nanocrystallites |
| MAPEG | PEGylated MA |
| Dh | Hydrodynamic diameter |
| DLS | Dynamic light scattering |
| ITLC-SG | Instant Thin Layer Chromatography-Silica Gel |
| NBT | Nanobrachytherapy |
| DMSA | Meso-1,2-dimercaptosuccinic acid |
| TEM | Transmission electron microscopy |
| PDI | Polydispersity index |
| XRD | X-ray diffraction |
| FT-IR | Fourier-transform infrared |
| CA | Citric acid |
| PAA | Poly(acrylic acid) |
| SAED | Selected-area electron diffraction |
| MS | Saturation magnetization |
| EDC | N-ethyl-N′-(3-(dimethylamino)propyl)carbodiimide |
| NHS | N-hydroxysuccinimide |
| AA | Alginic acid |
| DOX | Doxorubicin |
| BVCZ | Bevacizumab |
| HPLC | High-performance liquid chromatography |
| TGA | Thermogravimetric analysis |
| RP-HPLC | Reverse-phase high-performance liquid chromatography |
| PBS | Phosphate-buffered saline |
| HIR | Heat-induced radiolabeling |
| FH | Feraheme |
| CMD | Carboxymethyldextran |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| SEC | Size exclusion chromatography |
| LAGMERAL | Ligand Anchoring Group-Mediated Radiolabeling |
| DP-PEG | Diphosphonate-polyethylene glycol |
| FBS | Fetal bovine serum |
| ccDTPA | Cyclohexane-1,4-diyldinitrilo)tetraacetic acid dianhydride |
| MACS | Magnetic-assorting cell separation |
| PCS | Photon correlation spectroscopy |
| PLL | Poly-L-lysine |
| SAR | Specific absorption rate |
| Pro | Proline |
| Trp | Tryptophan |
| UV-Vis | Ultraviolet-Visible |
| DCS | Differential centrifugal sedimentation |
| HA | Hyaluronic acid |
| DBCO | Dibenzocyclooctyne |
| 177Lu-TCL-SPIONPs | 177Lu-labeled thermally cross-linked SPIONPs |
| RES | Reticuloendothelial system |
| VEGF | Vascular Endothelial Growth Factor |
| NMIBC | Nonmuscle-invasivebladder cancer |
| MIBC | Muscle-invasive bladder cancer |
| 177Lu-MNFs | 177Lu-labeled magnetic nano-formulations |
| GMP | Good Manufacturing Practice |
| RCY | Radiochemical yield |
| RCP | Radiochemical purity |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 5 August 2025).
- World Health Organization. Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 5 August 2025).
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and limitations in traditional anti-cancer therapies: A comprehensive review of surgery, chemotherapy, radiation therapy, and hormonal therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef]
- Gill, M.R.; Falzone, N.; Du, Y.; Vallis, K.A. Targeted radionuclide therapy in combined-modality regimens. Lancet Oncol. 2017, 18, e414–e423. [Google Scholar] [CrossRef]
- Chakraborty, K.; Mondal, J.; An, J.M.; Park, J.; Lee, Y.K. Advances in radionuclides and radiolabelled peptides for cancer therapeutics. Pharmaceutics 2023, 15, 971. [Google Scholar] [CrossRef] [PubMed]
- Ferro-Flores, G.; Azorín-Vega, E.; Ocampo-García, B.; Luna-Gutiérrez, M.; Cruz-Nova, P.; Meléndez-Alafort, L. Effects of targeted radionuclide therapy on cancer cells beyond the ablative radiation dose. Int. J. Mol. Sci. 2025, 26, 6968. [Google Scholar] [CrossRef]
- Niu, T.; Fan, M.; Lin, B.; Gao, F.; Tan, B.; Du, X. Current clinical application of lutetium-177 in solid tumors (Review). Exp. Ther. Med. 2024, 27, 225. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, A.; Qi, X.; Han, Z.; Song, L.; Zhou, J.; Wang, G.; Zhu, R.; Li, J. Radionuclide-Labeled Biomaterials: A Novel Strategy for Tumor-Targeted Therapy. Biomimetics 2025, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, P.; Kubeil, M.; Zarschler, K.; Ullrich, M.; Brandt, F.; Anger, K.; Wadepohl, H.; Kopka, K.; Bachmann, M.; Pietzsch, J.; et al. Toward Personalized Medicine: One Chelator for Imaging and Therapy with Lutetium-177 and Actinium-225. J. Am. Chem. Soc. 2022, 144, 21555–21567. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Al-Toubah, T.; El-Haddad, G.; Strosberg, J. 177Lu-DOTATATE for the treatment of gastroenteropancreatic neuroendocrine tumors. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1023–1031. [Google Scholar] [CrossRef]
- Hennrich, U.; Eder, M. [177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-approved radiotherapeutical for treatment of prostate cancer. Pharmaceuticals 2022, 15, 1292. [Google Scholar] [CrossRef]
- Karimi, A.; Bogdani, C.; O’Dwyer, E.; Siolas, D. Emerging innovations in theranostics for pancreatic neuroendocrine tumors. NPJ Precis. Oncol. 2025, 9, 146. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Fahim, Y.A.; Hasani, I.W.; Mahmoud, R.W. Promising biomedical applications using superparamagnetic nanoparticles. Eur. J. Med. Res. 2025, 30, 441. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, Z.; Mao, H.; Yang, L. Magnetic nanoparticles for precision oncology: Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine 2016, 12, 73–87. [Google Scholar] [CrossRef]
- Bentivoglio, V.; Nayak, P.; Varani, M.; Lauri, C.; Signore, A. Methods for radiolabeling nanoparticles (Part 3): Therapeutic use. Biomolecules 2023, 13, 1241. [Google Scholar] [CrossRef]
- Chakravarty, R.; Goel, S.; Dash, A.; Cai, W. Radiolabeled inorganic nanoparticles for positron emission tomography imaging of cancer: An overview. Q. J. Nucl. Med. Mol. Imaging 2017, 61, 181–204. [Google Scholar] [CrossRef]
- Imtiaz, S.; Ferdous, U.T.; Nizela, A.; Hasan, A.; Shakoor, A.; Zia, A.W.; Uddin, S. Mechanistic study of cancer drug delivery: Current techniques, limitations, and future prospects. Eur. J. Med. Chem. 2025, 290, 117535. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, M.R.; Knapp, F.F. Lutetium-177 therapeutic radiopharmaceuticals: Linking chemistry, radiochemistry, and practical applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef]
- Eckerman, K.; Endo, A. ICRP Publication 107. Nuclear decay data for dosimetric calculations. Ann. ICRP 2008, 38, 7–96. [Google Scholar] [PubMed]
- Lyra, M.E.; Andreou, M.; Georgantzoglou, A.; Kordolaimi, S.; Lagopati, N.; Ploussi, A.; Salvara, A.L.; Vamvakas, I. Radionuclides used in nuclear medicine therapy–From production to dosimetry. Curr. Med. Imaging 2013, 9, 51–75. [Google Scholar] [CrossRef]
- Yeong, C.H.; Cheng, M.H.; Ng, K.H. Therapeutic radionuclides in nuclear medicine: Current and future prospects. J. Zhejiang Univ. Sci. B 2014, 15, 845–863. [Google Scholar] [CrossRef]
- Stokke, C.; Kvassheim, M.; Blakkisrud, J. Radionuclides for targeted therapy: Physical properties. Molecules 2022, 27, 5429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X.; Gao, X.; Chen, X.; Li, L.; Li, G.; Liu, C.; Miao, Y.; Wang, R.; Hu, K. Radiopharmaceuticals and their applications in medicine. Sig. Transduct. Target. Ther. 2025, 10, 1. [Google Scholar] [CrossRef]
- Pellico, J.; Ruiz-Cabello, J.; Herranz, F. Radiolabeled iron oxide nanomaterials for multimodal nuclear imaging and positive contrast magnetic resonance imaging (MRI): A review. ACS Appl. Nano Mater. 2023, 6, 20523–20538. [Google Scholar] [CrossRef]
- Hamoudeh, M.; Kamleh, M.A.; Diab, R.; Fessi, H. Radionuclides delivery systems for nuclear imaging and radiotherapy of cancer. Adv. Drug Deliv. Rev. 2008, 60, 1329–1346. [Google Scholar] [CrossRef] [PubMed]
- Qaim, S.M. Therapeutic radionuclides and nuclear data. Radiochim. Acta 2001, 89, 297–304. [Google Scholar] [CrossRef]
- Ersahin, D.; Doddamane, I.; Cheng, D. Targeted radionuclide therapy. Cancers 2011, 3, 3838–3855. [Google Scholar] [CrossRef]
- Salih, S.; Alkatheeri, A.; Alomaim, W.; Elliyanti, A. Radiopharmaceutical treatments for cancer therapy, Radionuclides characteristics, applications, and challenges. Molecules 2022, 27, 5231. [Google Scholar] [CrossRef]
- Browne, E. Nuclear Data Sheets for A=177. Nucl. Data Sheets 1993, 68, 747. [Google Scholar] [CrossRef]
- Welsh, J.S. Beta decay in science and medicine. Am. J. Clin. Oncol. Cancer Clin. Trials 2007, 30, 437–439. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Violet, J.; Sandhu, S.; Hofman, M.S. Prostate-specific membrane antigen theranostics: Therapy with Lutetium-177. Curr. Opin. Urol. 2018, 28, 197–204. [Google Scholar] [CrossRef]
- Read, E.D.; Eu, P.; Little, P.J.; Piva, T.J. The status of radioimmunotherapy in CD20+ Non-Hodgkin’s lymphoma. Target. Oncol. 2015, 10, 15–26. [Google Scholar] [CrossRef]
- Schötzig, U.; Schrader, H.; Schönfeld, E.; Günther, E.; Klein, R. Standardisation and decay data of 177Lu and 188Re. Appl. Radiat. Isot. 2001, 55, 89–96. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Cheng, L.; Wu, J.; Bao, T.; Yao, R.; Liu, Y. A 3-dimensional stationary cascade gamma-ray coincidence imager. Phys. Med. Biol. 2021, 66, 225001. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.S.; Hennkens, H.M.; Sisay, N.; Huclier-Markai, S.; Jurisson, S.S. Radiometals for combined imaging and therapy. Chem. Rev. 2013, 113, 858–883. [Google Scholar] [CrossRef]
- Dash, A.; Knapp, F.F.; Pillai, M.R.A. Targeted radionuclide therapy—An overview. Curr. Radiopharm. 2013, 6, 152–180. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Orvig, C. Tumour targeting with radiometals for diagnosis and therapy. Chem. Commun. 2013, 49, 4720–4739. [Google Scholar] [CrossRef]
- Volkert, W.A.; Goeckeler, W.F.; Ehrhardt, G.J.; Ketring, A.R. Therapeutic radionuclides: Production and decay property considerations. J. Nucl. Med. 1991, 32, 174–185. [Google Scholar]
- Ercan, M.T.; Caglar, M. Therapeutic radiopharmaceuticals. Curr. Pharm. Des. 2000, 6, 1085–1121. [Google Scholar]
- Guo, H.; Miao, Y. Melanoma targeting property of a Lu-177-labeled lactam bridge-cyclized alpha-MSH peptide. Bioorg. Med. Chem. Lett. 2013, 23, 2319–2323. [Google Scholar] [CrossRef] [PubMed]
- Yousefnia, H.; Jalilian, A.R.; Zolghadri, S.; Bahrami-Samani, A.; Shirvani-Arani, S.; Ghannadi-Maragheh, M. Preparation and quality control of 177Lu-[tris(1,10-phenanthroline) lutetium(III)] complex for therapy. Nucl. Med. Rev. Cent. East. Eur. 2010, 13, 49–54. [Google Scholar] [PubMed]
- Bakker, W.H.; Breeman, W.A.; Kwekkeboom, D.J.; De Jong, L.C.; Krenning, E.P. Practical aspects of peptide receptor radionuclide therapy with [177Lu][DOTA0, Tyr3]octreotate. Q. J. Nucl. Med. Mol. Imaging. 2006, 50, 265–271. [Google Scholar]
- Dash, A.; Pillai, M.R.; Knapp, F.F., Jr. Production of 177Lu for targeted radionuclide therapy: Available options. Nucl. Med. Mol. Imaging 2015, 49, 85–107. [Google Scholar] [CrossRef]
- Dadgar, H.; Jafari, E.; Ahmadzadehfar, H.; Rekabpour, S.J.; Ravanbod, M.R.; Kalantarhormozi, M.; Nabipour, I.; Assadi, M. Feasibility and therapeutic potential of the 68Ga/177Lu-DOTATATE theranostic pair in patients with metastatic medullary thyroid carcinoma. Ann. Endocrinol. 2023, 84, 45–51. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Parus, J.; Pawlak, D.; Mikolajczak, R.; Duatti, A. Chemistry and bifunctional chelating agents for binding 177Lu. Curr. Radiopharm. 2015, 8, 86–94. [Google Scholar] [CrossRef]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef]
- Wu, J.R.; Canjels, A.; Miyauchi, R.; Kwon, E.J. Impact of conjugation chemistry on the pharmacokinetics of peptide-polymer conjugates in a model of traumatic brain injury. Bioconjug. Chem. 2025, 36, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Lewis, J.S. A practical guide to the construction of radiometallated bioconjugates for positron emission tomography. Dalton Trans. 2011, 40, 6168–6195. [Google Scholar] [CrossRef] [PubMed]
- George, S.C.; Samuel, E.J.J. Developments in 177Lu-based radiopharmaceutical therapy and dosimetry. Front. Chem. 2023, 11, 1218670. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Yun, S.-J.; Jeon, J. Recent advances in bioorthogonal click chemistry for efficient synthesis of radiotracers and radiopharmaceuticals. Molecules 2019, 24, 3567. [Google Scholar] [CrossRef]
- Nair, D.P.; Podgorski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The thiol-Michael addition click reaction: A powerful and widely used tool in materials chemistry. Chem. Mater. 2013, 26, 724–744. [Google Scholar] [CrossRef]
- Crisalli, P.; Kool, E.T. Water-soluble organocatalysts for hydrazone and oxime formation. J. Org. Chem. 2013, 78, 1184–1189. [Google Scholar] [CrossRef]
- Wang, Q.; Chan, T.R.; Hilgraf, R.; Fokin, V.V.; Sharpless, K.B.; Finn, M.G. Bioconjugation by copper (I)-catalyzed azide-alkyne [3+2] cycloaddition. J. Am. Chem. Soc. 2003, 125, 3192–3193. [Google Scholar] [CrossRef] [PubMed]
- Jewett, J.C.; Bertozzi, C.R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Šečkutė, J.; Devaraj, N.K. Expanding room for tetrazine ligations in the in vivo chemistry toolbox. Curr. Opin. Chem. Biol. 2013, 17, 761–767. [Google Scholar] [CrossRef]
- Walsh, J.C.; Kolb, H.C. Applications of click chemistry in radiopharmaceutical development. CHIMIA Int. J. Chem. 2010, 64, 29–33. [Google Scholar]
- Li, Z.; Conti, P.S. Radiopharmaceutical chemistry for positron emission tomography. Adv. Drug Deliv. Rev. 2010, 62, 1031–1051. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Liu, S. Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Adv. Drug Deliv. Rev. 2008, 60, 1347–1370. [Google Scholar] [CrossRef]
- Kang, C.S.; Sun, X.; Jia, F.; Song, H.A.; Chen, Y.; Lewis, M.; Chong, H.S. Synthesis and preclinical evaluation of bifunctional ligands for improved chelation chemistry of 90Y and 177Lu for targeted radioimmunotherapy. Bioconjug. Chem. 2012, 23, 1775–1782. [Google Scholar] [CrossRef]
- Milenic, D.E.; Brady, E.D.; Brechbiel, M.W. Antibody-targeted radiation cancer therapy. Nat. Rev. Drug Discov. 2004, 3, 488–499. [Google Scholar] [CrossRef]
- Ando, A.; Ando, I.; Tonami, N.; Kinuya, S.; Kazuma, K.; Kataiwa, A.; Nakagawa, M.; Fujita, N. 177Lu-EDTMP: A potential therapeutic bone agent. Nucl. Med. Commun. 1998, 19, 587–591. [Google Scholar] [CrossRef]
- Solá, G.A.R.; Argüelles, M.G.; Bottazzini, D.L.; Furnari, J.C.; Parada, I.G.; Rojo, A.; Ruiz, H.V. Lutetium-177-EDTMP for bone pain palliation. Preparation, biodistribution and pre-clinical studies. Radiochim. Acta 2000, 88, 157–162. [Google Scholar] [CrossRef]
- Chakraborty, S.; Das, T.; Unni, P.R.; Sarma, H.D.; Samuel, G.; Banerjee, S.; Venkatesh, M.; Ramamoorthy, N.; Pillai, M.R. 177Lu labelled polyaminophosphonates as potential agents for bone pain palliation. Nucl. Med. Commun. 2002, 23, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Chakraborty, S.; Unni, P.R.; Banerjee, S.; Samuel, G.; Sarma, H.D.; Venkatesh, M.; Pillai, M.R.A. 177Lu-labeled cyclic polyaminophosphonates as potential agents for bone pain palliation. Appl. Radiat. Isot. 2002, 57, 177–184. [Google Scholar] [CrossRef]
- Chakraborty, S.; Das, T.; Sarma, H.D.; Venkatesh, M.; Banerjee, S. Comparative studies of 177Lu-EDTMP and 177Lu-DOTMP as potential agents for palliative radiotherapy of bone metastasis. Appl. Radiat. Isot. 2008, 66, 1196–1205. [Google Scholar] [CrossRef]
- Bodei, L.; Mueller-Brand, J.; Baum, R.P.; Pavel, M.E.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisio, T.M.; Howe, J.R.; Cremonesi, M.; Kwekkeboom, D.J.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Bakker, W.H.; Kooij, P.P.; Konijnenberg, M.W.; Srinivasan, A.; Erion, J.L.; Schmidt, M.A.; Bugaj, J.L.; de Jong, M.; Krenning, E.P. [177Lu-DOTAOTyr3]octreotate: Comparison with [111In-DTPAo]octreotide in patients. Eur. J. Nucl. Med. 2001, 28, 1319–1325. [Google Scholar] [CrossRef]
- Vogel, W.V.; van der Marck, S.C.; Versleijen, M.W.J. Challenges and future options for the production of lutetium-177. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Carollo, A.; Papi, S.; Chinol, M. Lutetium-177 labeled peptides: The European Institute of Oncology Experience. Curr. Radiopharm. 2016, 9, 19–32. [Google Scholar] [CrossRef]
- Global Lutetium-177 (Lu-177) Market Research Report 2025. Available online: https://reports.valuates.com/market-reports/QYRE-Auto-4H13190/global-lutetium-177-lu-177 (accessed on 26 October 2025).
- Lutetium-177 Market Size, Share & Industry Analysis, By Drug (LUTATHERA {lutetium Lu 177 dotatate}, PLUVICTO {lutetium Lu 177 vipivotide tetraxetan}, and Others) By Age Group (Adults and Pediatrics), By Application (Neuroendocrine Tumor, Prostate Cancer, and Others) By End-User (Hospitals, Specialty Clinics, and Others), and Regional Forecast, 2025–2032. Available online: https://www.fortunebusinessinsights.com/lutetium-177-market-112740 (accessed on 26 October 2025).
- Trials, C. Clinicaltrials.gov Search Results for 177Lu. 2025. Available online: https://www.clinicaltrials.gov/search?term=177Lu&viewType=Table&aggFilters=results:with,status:com%20act%20ter&limit=50&page=1 (accessed on 26 October 2025).
- Aggarwal, R.; Starzinski, S.; de Kouchkovsky, I.; Koshkin, V.; Bose, R.; Chou, J.; Desai, A.; Kwon, D.; Kaushal, S.; Trihy, L.; et al. Single-dose 177Lu-PSMA-617 followed by maintenance pembrolizumab in patients with metastatic castration-resistant prostate cancer: An open-label, dose-expansion, phase 1 trial. Lancet Oncol. 2023, 24, 1266–1276. [Google Scholar] [CrossRef]
- Vlachostergios, P.J.; Niaz, M.J.; Skafida, M.; Mosallaie, S.A.; Thomas, C.; Christos, P.J.; Osborne, J.R.; Molina, A.M.; Nanus, D.M.; Bander, N.H.; et al. Imaging expression of prostate-specific membrane antigen and response to PSMA-targeted β-emitting radionuclide therapies in metastatic castration-resistant prostate cancer. Prostate 2021, 81, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Iravani, A.; Violet, J.; Azad, A.; Hofman, M.S. Lutetium-177 prostate-specific membrane antigen (PSMA) theranostics: Practical nuances and intricacies. PCAN 2020, 23, 38–52. [Google Scholar] [CrossRef]
- Chi, K.N.; Yip, S.M.; Bauman, G.; Probst, S.; Emmenegger, U.; Kollmannsberger, C.K.; Martineau, P.; Niazi, T.; Pouliot, F.; Rendon, R.; et al. 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: A review of the evidence and implications for Canadian clinical practice. Curr. Oncol. 2024, 31, 1400–1415. [Google Scholar] [CrossRef]
- Fizazi, K.; Herrmann, K.; Krause, B.J.; Rahbar, K.; Chi, K.N.; Morris, M.J.; Sartor, O.; Tagawa, S.T.; Kendi, A.T.; Vogelzang, N.; et al. Health-related quality of life and pain outcomes with [177Lu]Lu-PSMA-617 plus standard of care versus standard of care in patients with metastatic castration-resistant prostate cancer (VISION): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 597–610. [Google Scholar] [CrossRef]
- Herrmann, K.; Rahbar, K.; Eiber, M.; Sparks, R.; Baca, N.; Krause, B.J.; Lassmann, M.; Jentzen, W.; Tang, J.; Chicco, D.; et al. Renal and multiorgan safety of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer in the VISION dosimetry substudy. J. Nucl. Med. 2024, 65, 71–78. [Google Scholar] [CrossRef]
- Kuo, P.H.; Yoo, D.C.; Avery, R.; Seltzer, M.; Calais, J.; Nagarajah, J.; Weber, W.A.; Fendler, W.P.; Hofman, M.S.; Krause, B.J.; et al. A VISION substudy of reader agreement on 68Ga-PSMA-11 PET/CT scan interpretation to determine patient eligibility for 177Lu-PSMA-617 radioligand therapy. J. Nucl. Med. 2023, 64, 1259–1265. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Sartor, O.; de Bono, J.; Chi, K.; Fizazi, K.; Krause, B.J.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Saad, F.; et al. Association of declining prostate-specific antigen levels with clinical outcomes in patients with metastatic castration-resistant prostate cancer receiving [177Lu]Lu-PSMA-617 in the phase 3 VISION trial. Eur. Urol. 2024, 86, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Armstrong, A.J.; Krause, B.J.; Herrmann, K.; Rahbar, K.; de Bono, J.S.; Adra, N.; Garje, R.; Michalski, J.M.; Kempel, M.M.; et al. Safety analyses of the phase 3 VISION trial of [177Lu]Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur. Urol. 2024, 85, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Gafita, A.; de Bono, J.S.; Sartor, O.; Chi, K.N.; Krause, B.J.; Rahbar, K.; Tagawa, S.T.; Czernin, J.; El-Haddad, G.; et al. Multivariable models of outcomes with [177Lu]Lu-PSMA-617: Analysis of the phase 3 VISION trial. EClinicalMedicine 2024, 77, 102862. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.H.; Morris, M.J.; Hesterman, J.; Kendi, A.T.; Rahbar, K.; Wei, X.X.; Fang, B.; Adra, N.; Garje, R.; Michalski, J.M.; et al. Quantitative 68Ga-PSMA-11 PET and clinical outcomes in metastatic castration-resistant prostate cancer following 177Lu-PSMA-617 (VISION trial). Radiology 2024, 312, e233460. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; de Bono, J.; Nagarajah, J.; Sartor, O.; Wei, X.X.; Nordquist, L.T.; Koshkin, V.S.; Chi, K.N.; Krause, B.J.; Herrmann, K.; et al. Correlation analyses of radiographic progression-free survival with clinical and health-related quality of life outcomes in metastatic castration-resistant prostate cancer: Analysis of the phase 3 VISION trial. Cancer 2024, 130, 3426–3435. [Google Scholar] [CrossRef]
- Kim, C.; Liu, S.V.; Subramaniam, D.S.; Torres, T.; Loda, M.; Esposito, G.; Giaccone, G. Phase I study of the 177Lu-DOTA0-Tyr3-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung. J. Immunother. Cancer 2020, 8, e000980. [Google Scholar] [CrossRef] [PubMed]
- Dahle, J.; Repetto-Llamazares, A.H.; Mollatt, C.S.; Melhus, K.B.; Bruland, O.S.; Kolstad, A.; Larsen, R.H. Evaluating antigen targeting and anti-tumor activity of a new anti-CD37 radioimmunoconjugate against non-Hodgkin’s lymphoma. Anticancer Res. 2013, 33, 85–95. [Google Scholar]
- Repetto-Llamazares, A.H.; Larsen, R.H.; Mollatt, C.; Lassmann, M.; Dahle, J. Biodistribution and dosimetry of (177)Lu-tetulomab, a new radioimmunoconjugate for treatment of non-Hodgkin lymphoma. Curr. Radiopharm. 2013, 6, 20–27. [Google Scholar] [CrossRef]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E.; et al. International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007, 25, 579–586. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Strosberg, J.; Kunz, P.L.; Hendifar, A.; Yao, J.; Bushnell, D.; Kulke, M.H.; Baum, R.P.; Caplin, M.; Ruszniewski, P.; Delpassand, E.; et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with 177Lu-Dotatate: An analysis of the NETTER-1 study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2372–2382. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-Dotatate in the Phase III NETTER-1 trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.J.; Vallabhajosula, S.; Goldsmith, S.; Matulich, D.; Kaplan, J.; Berger, F.; Scher, H.I.; Bander, N.H.; et al. Phase II trial of 177Lutetium radiolabeled anti-prostate-specific membrane antigen (PSMA) monoclonal antibody J591 in patients with metastatic castrate-resistant prostate cancer. J. Clin. Oncol. 2008, 26 (Suppl. 15), 5140. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P.; et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 5182–5191. [Google Scholar] [CrossRef]
- Stillebroer, A.B.; Boerman, O.C.; Desar, I.M.; Boers-Sonderen, M.J.; van Herpen, C.M.; Langenhuijsen, J.F.; Smith-Jones, P.M.; Oosterwijk, E.; Oyen, W.J.; Mulders, P.F. Phase 1 radioimmunotherapy study with lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur. Urol. 2013, 64, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Stillebroer, A.B.; Zegers, C.M.; Boerman, O.C.; Oosterwijk, E.; Mulders, P.F.; O’Donoghue, J.A.; Visser, E.P.; Oyen, W.J. Dosimetric analysis of 177Lu-cG250 radioimmunotherapy in renal cell carcinoma patients: Correlation with myelotoxicity and pretherapeutic absorbed dose predictions based on 111In-cG250 imaging. J. Nucl. Med. 2012, 53, 82–89. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse applications of nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef]
- Szczyglewska, P.; Feliczak-Guzik, A.; Nowak, I. Nanotechnology–General aspects: A chemical reduction approach to the synthesis of nanoparticles. Molecules 2023, 28, 4932. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Perez-Herrero, E.; Fernandez-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Zhang, L.W.; Gao, S.; Zhang, F.; Yang, K.; Ma, Q.J.; Zhu, L. Activatable hyaluronic acid nanoparticle as a theranostic agent for optical/photoacoustic image-guided photothermal therapy. ACS Nano 2014, 8, 12250–12258. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, Z.; Deng, J.; Lemons, P.K.; Arhontoulis, D.C.; Bowne, W.B.; Cheng, H. Hyaluronidase embedded in nanocarrier PEG shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett. 2016, 16, 3268–3277. [Google Scholar] [CrossRef]
- Fu, G.; Zhu, L.; Yang, K.; Zhuang, R.; Xie, J.; Zhang, F. Diffusion weighted magnetic resonance imaging for therapy response monitoring and early treatment prediction of photothermal therapy. ACS Appl. Mater. Interfaces 2016, 8, 5137–5147. [Google Scholar] [CrossRef]
- Makino, A.; Kimura, S. Solid tumor-targeting theranostic polymer nanoparticle in nuclear medicinal fields. Sci. World J. 2014, 2014, 424513. [Google Scholar] [CrossRef]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in cancer theragnostic and drug delivery: A comprehensive review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Inorganic nanoparticles for biomedicine: Where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Raheem, M.A.; Rahim, M.A.; Gul, I.; Zhong, X.; Xiao, C.; Zhang, H.; Wei, J.; He, Q.; Hassan, M.; Zhang, C.Y.; et al. Advances in nanoparticles-based approaches in cancer theranostics. OpenNano 2023, 12, 100152. [Google Scholar] [CrossRef]
- Cho, E.C.; Glaus, C.; Chen, J.; Welch, M.J.; Xia, Y. Inorganic nanoparticle-based contrast agents for molecular imaging. Trends Mol. Med. 2010, 16, 561–573. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, T.; Zhang, S. Radionuclide-labelled nanoparticles for cancer combination therapy: A review. J. Nanobiotechnol. 2024, 22, 728. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Park, J.; Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, V.L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Nie, D.; Liu, Y.; Yu, M.; Gan, Y. Advances in particle shape engineering for improved drug delivery. Drug Discov. Today 2019, 24, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, J.; Chen, H.; Wu, H.; Xu, Y.; Li, Y.; Yi, H.; Wang, Y.A.; Yang, L.; Mao, H. Exerting enhanced permeability and retention effect driven delivery by ultrafine iron oxide nanoparticles with T1–T2 switchable magnetic resonance imaging contrast. ACS Nano 2017, 11, 4582–4592. [Google Scholar] [CrossRef]
- Laurent, S.; Boutry, S.; Mahieu, I.; Vander, E.L.; Muller, R.N. Iron oxide based MR contrast agents: From chemistry to cell labeling. Curr. Med. Chem. 2009, 16, 4712–4727. [Google Scholar] [CrossRef]
- Thomas, R.; Park, I.-K.; Jeong, Y.Y. Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930. [Google Scholar] [CrossRef]
- Núñez-Salinas, A.; Parra-Garretón, C.; Acuña, D.; Peñaloza, S.; Günther, G.; Bollo, S.; Arriagada, F.; Morales, J. Nanoradiopharmaceuticals: Design principles, radiolabeling strategies, and biomedicine applications. Pharmaceutics 2025, 17, 912. [Google Scholar] [CrossRef]
- Salehirozveh, M.; Dehghani, P.; Mijakovic, I. Synthesis, Functionalization, and Biomedical Applications of Iron Oxide Nanoparticles (IONPs). J. Funct. Biomater. 2024, 15, 340. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef]
- Elias, D.R.; Poloukhtine, A.; Popik, V.; Tsourkas, A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine 2013, 9, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; Pellico, J.; Fernandez-Barahona, I.; Martin-Salamanca, S.; Ruiz-Cabello, J.; Hidalgo, A.; Herranz, F. Thrombo-tag, an in vivo formed nanotracer for the detection of thrombi in mice by fast pre-targeted molecular imaging. Nanoscale 2020, 12, 22978–22987. [Google Scholar] [CrossRef]
- Pellico, J.; Fernández-Barahona, I.; Benito, M.; Gaitán-Simón, A.; Gutiérrez, L.; Ruiz-Cabello, J.; Herranz, F. Unambiguous detection of atherosclerosis using bioorthogonal nanomaterials. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 26–35. [Google Scholar] [CrossRef]
- Venrooij, K.R.; de Bondt, L.; Bonger, K.M. Mutually orthogonal bioorthogonal reactions: Selective chemistries for labeling multiple biomolecules simultaneously. Top. Curr. Chem. 2024, 382, 24. [Google Scholar] [CrossRef]
- Juhl, K.; Jørgensen, K.A. The First Organocatalytic enantioselective inverse-electron-demand hetero-Diels–Alder reaction. Angew. Chem. Int. Ed. 2003, 42, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Wilbur, D.S. Chemical and radiochemical considerations in radiolabeling with α-emitting radionuclides. Curr. Radiopharm. 2011, 4, 214–247. [Google Scholar] [CrossRef]
- Hu, A.; Wilson, J.J. Advancing chelation strategies for large metal ions for nuclear medicine applications. Acc. Chem. Res. 2022, 55, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, A.J.; Fallis, I.A.; Pope, S.J.A. Chelating agents for radiolanthanides: Applications to imaging and therapy. Coord. Chem. Rev. 2017, 340, 198–219. [Google Scholar] [CrossRef]
- Patrick, P.S.; Bogart, L.K.; Macdonald, T.J.; Southern, P.; Powell, M.J.; Zaw-Thin, M.; Voelcker, N.H.; Parkin, I.P.; Pankhurst, Q.A.; Lythgoe, M.F.; et al. Surface radio-mineralisation mediates chelate-free radiolabelling of iron oxide nanoparticles. Chem. Sci. 2019, 10, 2592–2597. [Google Scholar] [CrossRef]
- Pellico, J.; Gawne, P.J.; de Rosales, R.T.M. Radiolabelling of nanomaterials for medical imaging and therapy. Chem. Soc. Rev. 2021, 50, 3355–3423. [Google Scholar] [CrossRef]
- Salvanou, E.-A.; Kolokithas-Ntoukas, A.; Liolios, C.; Xanthopoulos, S.; Paravatou-Petsotas, M.; Tsoukalas, C.; Avgoustakis, K.; Bouziotis, P. Preliminary evaluation of iron oxide nanoparticles radiolabeled with 68Ga and 177Lu as potential theranostic agents. Nanomaterials 2022, 12, 2490. [Google Scholar] [CrossRef]
- Stanković, D.; Radović, M.; Stanković, A.; Mirković, M.; Vukadinović, A.; Mijović, M.; Milanović, Z.; Ognjanović, M.; Janković, D.; Antić, B.; et al. Synthesis, characterization, and therapeutic efficacy of 177Lu-DMSA@SPIONs in nanobrachytherapy of solid tumors. Pharmaceutics 2023, 15, 1943. [Google Scholar] [CrossRef]
- Ognjanović, M.; Radović, M.; Mirković, M.; Prijović, Z.; del Puerto Morales, M.; Čeh, M.; Vranješ-Đurić, S.; Antić, B. 99mTc-, 90Y-, and 177Lu-labeled iron oxide nanoflowers designed for potential use in dual magnetic hyperthermia/radionuclide cancer therapy and diagnosis. ACS Appl. Mater. Interfaces 2019, 11, 41109–41117. [Google Scholar] [CrossRef]
- Salvanou, E.-A.; Kolokithas-Ntoukas, A.; Prokopiou, D.; Theodosiou, M.; Efthimiadou, E.; Koźmiński, P.; Xanthopoulos, S.; Avgoustakis, K.; Bouziotis, P. 177Lu-labeled iron oxide nanoparticles functionalized with doxorubicin and nevacizumab as nanobrachytherapy agents against breast cancer. Molecules 2024, 29, 1030. [Google Scholar] [CrossRef]
- Gholami, Y.H.; Josephson, L.; Akam, E.A.; Caravan, P.; Wilks, M.Q.; Pan, X.Z.; Maschmeyer, R.; Kolnick, A.; El Fakhri, G.; Normandin, M.D.; et al. A chelate-free nano-platform for incorporation of diagnostic and therapeutic isotopes. Int. J. Nanomed. 2020, 15, 31–47. [Google Scholar] [CrossRef]
- Ge, J.; Chen, L.; Huang, B.; Gao, Y.; Zhou, D.; Zhou, Y.; Chen, C.; Wen, L.; Li, Q.; Zeng, J.; et al. Anchoring group-mediated radiolabeling of inorganic nanoparticles-A universal method for constructing nuclear medicine imaging nanoprobes. ACS Appl. Mater. Interfaces 2022, 14, 8838–8846. [Google Scholar] [CrossRef] [PubMed]
- Rasaneh, S.; Rajabi, H.; Daha, F.J. Activity estimation in radioimmunotherapy using magnetic nanoparticles. Chin. J. Cancer Res. 2015, 27, 203–208. [Google Scholar] [PubMed]
- Shanehsazzadeh, S.; Grüttner, C.; Yousefnia, H.; Lahooti, A.; Gholami, A.; Nosrati, S.; Zolghadri, S.; Anijdan, S.H.M.; Lotfabadi, A.; Varnamkhasti, B.S.; et al. Development of 177Lu-DTPA-SPIO conjugates for potential use as a dual contrast SPECT/MRI imaging agent. Radiochim. Acta 2016, 104, 337–344. [Google Scholar] [CrossRef]
- Mirković, M.; Milanović, Z.; Perić, M.; Vranješ-Đurić, S.; Ognjanović, M.; Antić, B.; Kuraica, M.; Krstić, I.; Kubovcikova, M.; Antal, I.; et al. Design and preparation of proline, tryptophan and poly-L-lysine functionalized magnetic nanoparticles and their radiolabeling with 131I and 177Lu for potential theranostic use. Int. J. Pharm. 2022, 628, 122288. [Google Scholar] [CrossRef]
- Li, Y.; Shan, S.; Zhang, R.; Sun, C.; Hu, X.; Fan, J.; Wang, Y.; Duan, R.; Gao, M. Imaging and downstaging bladder cancer with the 177Lu-labeled bioorthogonal nanoprobe. ACS Nano 2024, 18, 17209–17217. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Mackeyev, Y.; Krishnan, S. Radiolabeled nanomaterial for cancer diagnostics and therapeutics: Principles and concepts. Cancer Nanotechnol. 2023, 14, 15. [Google Scholar] [CrossRef]
- Hue, J.J.; Lee, H.J.; Nam, S.Y.; Kim, J.S.; Lee, B.J.; Yun, Y.W. Distribution and accumulation of 177Lu-labeled thermally cross-linked superparamagnetic iron oxide nanoparticles in the tissues of ICR mice. Korean J. Vet. Res. 2015, 55, 57–60. [Google Scholar] [CrossRef]
- Weissleder, R.; Bogdanov, A.; Neuwelt, E.A.; Papisov, M. Long-circulating iron oxides for MR imaging. Adv. Drug Deliv. Rev. 1995, 16, 321–334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).