Abstract
The Jurubatiba Sandbank National Park (PARNA Jurubatiba) is an ecological reserve characterized by harsh environmental conditions, including low rainfall, high sun exposure, and sandy soil. Among its native vegetation, Eremanthus crotonoides stands out for its richness in flavonoids, phenolic compounds, and sesquiterpene lactones. The objective of this study was to isolate and quantify sesquiterpene lactones from this species using 1H NMR and to investigate their anti-SARS-CoV-2 potential and cytotoxicity against cancer cells. UPLC-(ESI)-MS/MS analyses enabled metabolite annotation, and semi-preparative HPLC-DAD allowed the isolation of centratherin and goyazensolide, which were identified by 1D and 2D NMR. In vitro assays showed that centratherin at 10 µM concentration reduced the viability of PC-3 and HCT-116 cancer cells by 100%, while goyazensolide had no noteworthy effects. Furthermore, enzymatic inhibition assays on SARS-CoV2 targets revealed that centratherin exhibited a lower apparent IC50 of 12 µM against PLpro, while goyazensolide was more active against 3CLpro, with an IC50 of 71 µM. Notably, the dichloromethane fraction demonstrated promising activity against both enzymes, with IC50 values of 30 µM for PLpro and 11 µM for 3CLpro. This study reports, for the first time, the isolation of goyazensolide from E. crotonoides and highlights the potential of both sesquiterpene lactones as SARS-CoV-2 enzyme inhibitors. In contrast to centratherin, goyazensolide fortunately had almost no cytotoxic effects at inhibition concentration on the cells tested. This shows that anticancer and anti-SARS effects can be separated and should have different SARs, an important prerequisite for further development.
1. Introduction
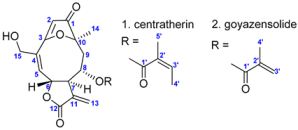
Eremanthus crotonoides (DC.) Sch.Bip. (Asteraceae) is a native Brazilian species that can be found in various regions of the northeastern and southeastern parts of the country []. Several studies have investigated the biological properties of this species. For example, the ethyl acetate fraction obtained from its leaves has shown potential as an antidiabetic agent, with flavonoids and caffeoylquinic acid derivatives identified as α-glucosidase inhibitors []. Previous research has also examined the ethanolic extract and dichloromethane fraction from the leaves, as well as on the isolated compound centratherin []. All of these samples exhibited cytotoxic activity against glioblastoma multiforme, with centratherin (10, Figure 1), a sesquiterpene lactone, showing IC50 values comparable to the commercial anticancer drug doxorubicin [].
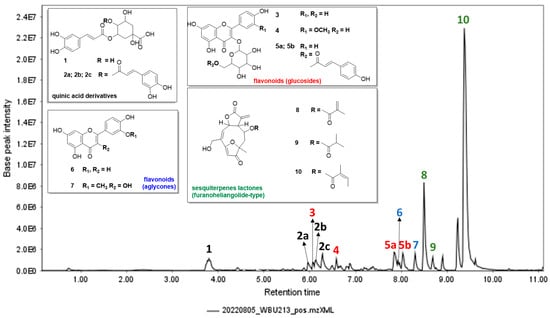
Figure 1.
UPLC-(ESI)-MS/MS total ion chromatogram of the crude extract of E. crotonoides from 0 to 11 min, highlighting the annotated compounds: chlorogenic acid (1), caffeoylquinic acid isomers (2a–2c), quercetin-3-O-glucoside (3), isorhamnetin-3-O-glucoside (4), tiliroside (5), luteolin (6), isorhamnetin (7), goyazensolide (8), budlein A isobutyrate (9), and centratherin (10). Note: Axis labels are displayed in MZmine format (e.g., 2.0E6 corresponds to 2.0 × 106).
Additionally, our research group has conducted two studies to date. The first was a bio-guided investigation that identified the dichloromethane fraction as bactericidal against methicillin-resistant Staphylococcus aureus (MRSA, ATCC 33591) at a concentration of 128 μg/mL. In this study, centratherin inhibited a clinical isolate of S. aureus at 32 µg/mL and demonstrated antibiofilm activity at 128 µg/mL []. The second study focused on the dichloromethane fraction, which showed MIC50 values of 0.95 ± 1.08 μg/mL against Mycobacterium bovis BCG and 2.17 ± 1.11 μg/mL against M. tuberculosis H37Rv []. Furthermore, this fraction inhibited NO and TNF-α production in LPS-stimulated macrophages, while remaining non-cytotoxic.
All these findings regarding the intriguing chemical and biological profile of E. crotonoides motivated this study, which aimed to investigate the main constituents of this species, specifically the sesquiterpene lactones. To this end, the chemical profile of the crude extract was explored using Ultra-Performance Liquid Chromatography coupled with Electrospray Ionization Tandem Mass Spectrometry (UPLC-(ESI)-MS/MS). Additionally, furanoheliangolide-type sesquiterpene lactones were isolated by semi-preparative High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD), identified by one-dimensional (1D) and two-dimensional (2D) Nuclear Magnetic Resonance (NMR), and quantified by quantitative proton NMR (q1H NMR) from the dichloromethane fraction (FDCM). The cytotoxicity of the E. crotonoides crude extract (EBEC), dichloromethane fraction, centratherin (10, Figure 1), and goyazensolide (8, Figure 1) against human carcinoma cells was evaluated, along with their potential enzymatic inhibition against recombinant papain-like protease (rPLpro) and 3-chymotrypsin-like protease (3CLpro).
2. Results and Discussion
Research on species from Brazil’s coastal restinga ecosystem is particularly important due to the distinctive adaptations of its flora, which often result in the production of specialized secondary metabolites with potential pharmacological applications. The analysis of E. crotonoides, a species native to this unique habitat and notable for its diverse chemical profile, enabled the identification of several compounds with promising bioactive properties. These findings encompass extraction, detailed chemical characterization, and bioactivity assays, highlighting the pharmacological relevance of these metabolites.
2.1. Extraction Yield and Fractionation of Leaves
From 34 g of dried leaves, 2.7 g of freeze-dried crude extract was obtained after extraction with ethanol. Of this total, 1 g was subjected to liquid–liquid partitioning, yielding fractions in n-hexane, dichloromethane, and ethyl acetate with yields of 7.4%, 15.2%, and 4.8%, respectively, relative to the dried extract weight. For this study, only the dichloromethane fraction and the crude extract were selected, as they showed greater potential for subsequent bioactivity assays.
2.2. Chemical Characterization of E. crotonoides Crude Extract
UPLC-(ESI)-MS/MS analysis of the crude extract of E. crotonoides (EBEC) enabled the characterization of 10 compounds (Figure 1 and Table S1). Overall, the extract appears to be rich in quinic acid derivatives, flavonoids, and furanoheliangolide-type sesquiterpene lactones. Based on dereplication studies using the GNPS platform, the literature, and fragmentation pathway analysis, the annotated compounds were chlorogenic acid (1), caffeoylquinic acid (2), quercetin-3-O-glucoside (3), isorhamnetin-3-O-glucoside (4), tiliroside (5), luteolin (6), isorhamnetin (7), goyazensolide (8), budlein A isobutyrate (9), and centratherin (10). Additionally, all the phenolic compounds mentioned were previously identified and isolated in the ethyl acetate fraction of E. crotonoides [].
The MS2 spectrum of compound 1 (m/z 355.1019 [M+H]+) showed a fragment ion at m/z 163.0376 [caffeic acid–H2O+H]+, indicating a neutral loss of 192 Da, characteristic of the quinic acid moiety, leading to the annotation as chlorogenic acid (Table S1; Figure S1) []. For the isomeric forms of compound 2 (2a: m/z 517.1345 [M+H]+; 2b: m/z 517.1326 [M+H]+; 2c: m/z 517.1337 [M+H]+, a fragment ion at m/z 163.0378 was also observed, corresponding to the losses of 162 (caffeic acid–H2O) and 192 Da (quinic acid moiety). Thus, compound 2 was annotated as caffeoylquinic acid (Table S1; Figures S2, S3 and S5) [].
Compounds 3 (m/z 465.1027 [M+H]+) and 4 (m/z 479.1178 [M+H]+) exhibited fragment ions in their MS2 spectra corresponding to a neutral loss of 162 Da, suggesting the presence of a hexose moiety. These were identified as the O-glycosylated flavonoids quercetin-3-O-glucoside and isorhamnetin-3-O-glucoside, respectively (Table S1 and Figures S4–S6) [,]. Furthermore, compound 5 (m/z 595.1450 [M+H]+) showed in fragment ions at m/z 309.0968 and m/z 287.0551, corresponding to coumaroyl glucoside moiety and kaempferol aglycone, respectively, and was annotated as tiliroside (Table S1 and Figure S7) [].
The spectrum of compound 6 (m/z 287.0565 [M+H]+) showed characteristic fragments ions at m/z 241.0481, 213.0520, and 153.0166, typical for luteolin. The fragment at m/z 153.0166 corresponds to the 1,3A+ Retro-Diels-Alder (RDA) fragment of this flavonoid (Table S1 and Figure S8) []. For compound 7 (m/z 317.0670 [M+H]+) the MS/MS spectrum displayed a fragment ion at m/z 302.0404, indicating a loss of 15 Da, characteristic of a methyl group, suggesting that this compound corresponds to isorhamnetin (Table S1 and Figure S9) [].
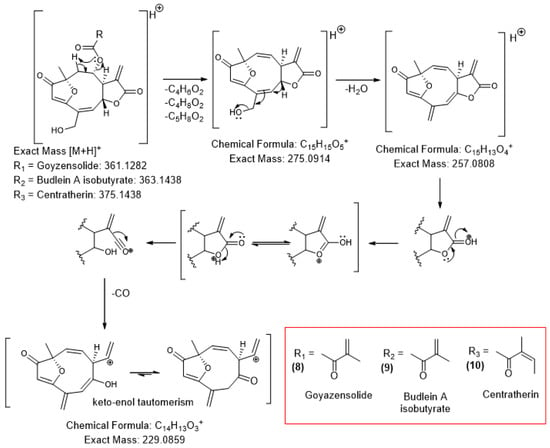
The three characterized sesquiterpene lactones exhibit similar fragmentation patterns, with their MS2 spectra presented in Figures S10–S12. The proposed fragmentation pathway, depicted in Scheme 1, suggests that all three compounds share the same main skeleton, with the daughter ion m/z 275.09 originating from the loss of the radical attached to C-8. This allows the characterization of compound 8 as goyazensolide, compound 9 as budlein A isobutyrate, and compound 10 as centratherin. Further successive fragmentations include the loss of water (m/z 257.08) and ultimately carbon monoxide (CO) (m/z 229.08) from the lactonic ring, based on fragmentations studies of five-membered lactones by electrospray ionization [].
Scheme 1.
Proposed fragmentation pathway of furanoheliagolide-type sesquiterpene lactones goyazensolide (8), budlein A isobutyrate (9), and centratherin (10). The substituents displayed in the red box correspond to the R (R1, R2, R3) groups indicated in the chemical structures.
This represents the first proposed fragmentation pathway for the sesquiterpene lactones under study, as well as the first characterization of budlein A isobutyrate in E. crotonoides, which had previously been described only in species of Helianthus [,,], also from the Asteraceae family.
In summary, concerning the characterization of the crude extract of the studied species, GNPS facilitated the dereplication of phenolic acids and flavonoids, supported by high cosine values (0.93–0.99, Table S1). For sesquiterpene lactones, however, the results were less straightforward: goyazensolide showed only a moderate score (cosine = 0.73, Table S1) while budlein A isobutyrate and centratherin showed no library matches. This study therefore illustrates that, although GNPS is a useful tool to accelerate dereplication, its application must be combined with manual inspection of MS2 data to ensure reliable identification, especially for compounds underrepresented in spectral libraries. Importantly, this represents the first dereplication study reported for Eremanthus crotonoides, providing a chemical baseline that supported the subsequent biological assays.
2.3. Isolation and Identification of Sesquiterpene Lactones
Following the chemical characterization of the species, efforts were focused on the analysis of the dichloromethane extract of E. crotonoides due to the promising nature of sesquiterpene lactones as bioactives. Figure S13 shows the overlapped 1H NMR spectra of the crude extract and the dichloromethane fraction, highlighting the main peaks in each sample.
The dichloromethane fraction of E. crotonoides (FDEC) was subjected to isolation by semi-preparative HPLC-DAD monitored at λ = 269 nm, yielding six collected peaks as shown in Figure S14. All these peaks were analyzed by electrospray ionization mass spectrometry (direct injection) in both positive and negative ionization modes. Peaks 1 (rt = 4.02 min) and 2 (rt = 4.48 min) ionized more effectively in negative mode, showing [M−H]− ions at m/z 285.3 and m/z 315.2, respectively, and exhibited very similar ultraviolet profiles. The m/z 285.3 ion is suggestive of kaempferol, which had already been identified during dereplication studies. Peaks 3 to 6 showed better ionization in positive mode, with m/z values of 383.1 (peak 3, rt = 4.98 min, [M+Na]+), 385.2 (peak 4, rt = 5.15 min, [M+Na]+), 397.7 (peak 5, rt 6.12 min, [M+Na]+), and 397.5 (peak 6, rt = 6.38 min, [M+Na]+), and also displayed similar ultraviolet profiles.
All peaks were inspected by 1H NMR, and peaks 3 and 6 showed the best spectral resolution; these were therefore selected for comprehensive 1D and 2D NMR analysis (Figures S15–S27). Subsequent isolation afforded goyazensolide (11 mg) and centratherin (38 mg), whose structures were assigned from 1H and 13C NMR data (Table 1). GNPS library matching had previously suggested the presence of goyazensolide in E. crotonoides; the present work documents its first isolation and full NMR characterization from this species. Centratherin had been reported in this species by our group []. The 1D and 2D NMR datasets are consistent with literature values for these sesquiterpene lactones isolated from other Asteraceae species, including Soares et al. (2012) [] and Santos Junior et al. (2015) [], the latter establishing the absolute configuration of centratherin by ECD and supporting the assignments presented here.

Table 1.
NMR chemical shifts (1H and 13C) of sesquiterpene lactones isolated from dichloromethane fraction of E. crotonoides.
As previously shown in Figure S14, the HPLC-DAD analysis monitored at λ = 269 nm identified centratherin (10) and goyazensolide (8) as main constituents of FDEC. Both compounds were quantified by 1H NMR (10 mg/mL). This non-destructive technique offers several advantages, such as shorter analysis time compared to conventional chromatographic methods, albeit with lower sensitivity. Additionally, unlike chromatographic or MS-based methods, the detection of compounds by 1H NMR does not dependent on their polarity, ionization efficiency, or light absorption.
Figure 2 presents the 1H NMR spectra of centratherin, goyazensolide and FDEC. The non-overlapping signals of H-4′ of goyazensolide and H-5′ of centratherin allow quantification throughout signal integration. In this case, the integrated signals show a ratio of 0.16:1 (goyazensolide–centratherin), indicating that centratherin is present at approximately 6.25 times the concentration of goyazensolide.
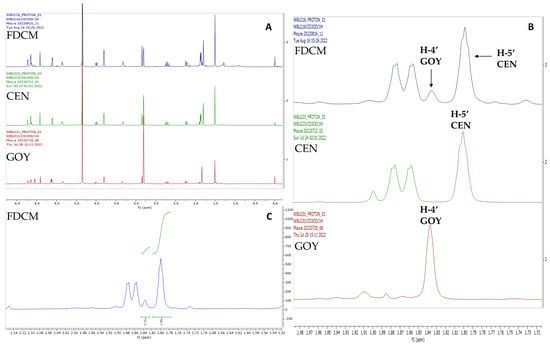
Figure 2.
Quantitative 1H NMR. Overlap of spectra (δH 0.0 to 6.75 ppm) of the dichloromethane fraction (FDCM), goyazensolide (GOY), and centratherin (CEN) (A). Expansion (δH 1.72 to 1.98 ppm) of compounds and fraction spectra to highlight H-4′ of goyazensolide and H-5′ of centratherin in the dichloromethane fraction, isolated goyazensolide and centratherin (B) and expansion (δH 1.52 to 2.14 ppm) of 1H NMR spectrum of dichloromethane fraction (C).
2.4. Cytotoxicity Against Human Carcinoma Cells
The cell viability of carcinoma cells tested in vitro with EBEC, FDEC, centratherin and goyazensolide was evaluated using MTT and crystal violet (CV) assays, and the results are presented in Table 2. Both EBEC and FDEC, at a concentration of 50 µg/mL, showed promising activity by reducing cancer viability by 100%. Regarding the cytotoxicity of the isolated compounds, goyazensolide was unable to decrease cell viability after 48 h at concentrations ≤ 10 µM; however, centratherin was able to reduce cell viability of both PC-3 and HCT-116 cell lines by 100%. Therefore, it can be inferred that the cytotoxic activity of EBEC and FDEC against the evaluated strains is primarily correlated with centratherin.

Table 2.
Cell viability (%) of prostate cancer PC-3 and colon cancer HCT-116 cells treated for 48 h with EBEC, FDEC, centratherin and goyazensolide, determined by MTT and crystal violet assays. Data represented by the average of the biological triplicates (n = 3) and the standard deviation (±).
The α-methylene-γ-lactone ring of sesquiterpene lactones (SQLs) is well recognized for its pivotal role in imparting cytotoxic effects against (cancer) cells. For instance, centratherin and goyazensolide have previously demonstrated promising activity against glioma (U251 and U87-MG) [], lung carcinoma (NCI-H187) [], and colon cancer (HT-29) [] cell lines, with results comparable to commercial standards. However, to the best of our knowledge, this is the first study to evaluate the anticancer potential of E. crotonoides and its sesquiterpene lactones against PC-3 and HCT-116 cells. This novel insight enriches the scientific literature and further highlights E. crotonoides as a potential source of anticancer agents, and with some cell type specificity of the different derivatives.
2.5. Enzymatic Inhibition Against PLpro and 3CLpro
PLpro is one of the SARS-CoV-2 proteases responsible for processing the viral polyprotein (pp1ab) into single, functionally active non-structural proteins. This protease is a domain located within the nsp3 protein and exhibits papain-like activity, performing specific cleavages between nsp1 and nsp2, nsp2 and nsp3, and between nsp3 and nsp4 in pp1ab. Inhibition of PLpro activity results in deficient polyprotein processing, consequently blocking virus replication. On the other hand, the 3-Chymotrypsin-like protein (3CLpro) plays a fundamental role in peptide chain processing by cleaving the C-terminal domain at 11 sites, producing enzymes and structural proteins essential for viral function []. Given the significance of these proteases for the SARS-CoV-2 life cycle, they represent targets for the development of antiviral compounds [].
The inhibitory effects of EBEC and FDCM against both proteases are shown in Table S3, Figure 3a,b,e,f. The samples exhibited strong inhibitory potential, with a clear dose-dependent response. At the highest concentrations tested (1 µg/mL), the inhibition percentage reached nearly 100% against PLpro and 88–95% against 3CLpro. Interestingly, the dichloromethane fraction demonstrated particularly promising activity against both enzymes, with IC50 values of 30 and 10.7 µg/mL, respectively. This behavior can be attributed to the chemical profile of this fraction, which is primarily composed of sesquiterpene lactones (SQLs). This class of compounds is notable for its diverse biological properties, including antiviral activity [,,].
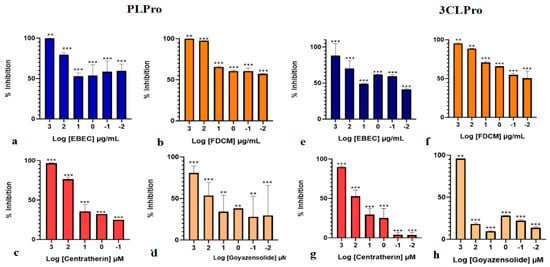
Figure 3.
Effect of (a) the crude extract (EBEC) and (b) dichloromethane fraction (FDCM) from the leaves of Eremanthus crotonoides, (c) centratherin and (d) goyazensolide on the PLpro enzyme; and (e) the crude extract (EBEC), (f) dichloromethane fraction (FDCM), (g) centratherin, and (h) goyazensolide on the 3CLpro enzyme. Enzymatic inhibition percentages were determined using the FRET assay. The bars are arranged in descending according to the logarithms of tested concentrations (1000, 100, 10, 1, 0.1, 0.01, 0.001, and 0.0001 µg/mL for the extract and fraction; 1000, 100, 10, 1, 0.1, and 0.01 µM for centratherin and goyazensolide). Values are presented as mean ± standard deviation (n = 2). Statistical significance is indicated as ** p < 0.05 and *** p < 0.0001. The control consisted of substrate, buffer, and the corresponding enzyme only.
The biological activities of SQLs are often attributed to the presence of the α-methylene-γ-butyrolactone moiety, which reacts with nucleophiles through a Michael addition and acts as an alkylating agent. Therefore, these compounds are believed to act via covalent bonding to cysteine residues in target proteins, as well as through protein inhibition and enzymatic conjugation with glutathione []. The latter process, though, can also lead to inactivation. To evaluate the potential of E. crotonoides STLs, centratherin and goyazensolide were isolated and tested for their PLpro and 3CLpro inhibitory activity.
As shown in Figure 3c,d,g,h and Table S3, both compounds have inhibitory potential against the proteases, with a clear dose-dependent response. Interestingly, the two SQLs displayed distinct behaviors against the analyzed proteases. Centratherin demonstrated superior activity against PLpro, achieving 97% inhibition at the highest concentration tested (IC50 11.9 µM), whereas goyazensolide was more effective against 3CLpro, showing 96% inhibition at the highest concentration (IC50 71 µM). These values are consistent with those reported for other natural products with significant inhibitory potential against PLpro [,]. To the best of our knowledge, there are no previous reports on the antiviral activity of centratherin, while goyazensolide has been described as an important covalent inhibitor of importin-5, a key transporter involved in viral replication [].
It is important to emphasize that the results of this study demonstrate that both compounds possess the ability to inhibit the two proteases under investigation, indicating promising antiviral potential by targeting two distinct pathways: the specific cleavages in the polyprotein (pplab) and the cleavage of the C-terminal domain. This dual inhibitory action may help explain the strong results obtained for the dichloromethane fraction of E. crotonoides leaves, which is rich in sesquiterpene lactones such as centratherin and goyazensolide. Future molecular docking analyses could provide complementary insights into the binding interactions of centratherin and goyazensolide with PLpro and 3CLpro, helping to rationalize the inhibitory profiles observed in this study.
3. Materials and Methods
3.1. Collection, Preparation and Extraction of E. crotonoides
The species was identified in situ by the botanist and plant taxonomy specialist Tatiana Ungaretti Paleo Konno, and a voucher specimen (RFA-38749) was deposited in the herbarium of the Instituto de Biodiversidade e Sustentabilidade (NUPEM) at the Federal University of Rio de Janeiro, Macaé, Brazil. The collection was authorized under SISBIO/ICMBio No 62.455-11, and the research was approved by SISGEN/MMA No: AAA989F. Aerial parts of the E. crotonoides were harvested in the National Park of Jurubatiba Sandbank (PARNA Jurubatiba, coordinates: 22.19970° S 41.47112° W, Quissamã, Rio de Janeiro, Brazil) in May 2022. Healthy leaves were selected and dried in a circulation air oven at 40 °C for three days. After drying, the leaves were powdered using a domestic processor and extracted with ethanol (99.5%). The extraction was performed over five days, with the solvent (300 mL) renewed every 24 h. Following extraction, the solution was concentrated using a rotary evaporator, and the residual extract was freeze-dried for five days.
3.2. Fractionation of Crude Extracts Using the Liquid–Liquid Partitioning
The crude extract from the leaves of E. crotonoides (EBEC) was fractionated using solvents of increasing polarity: n-hexane, dichloromethane, and ethyl acetate, yielding three fractions. Among these, only the dichloromethane fraction of E. crotonoides (FDEC) was used in this present work due to its promising chemical profile.
3.3. Metabolic Profiling of the Crude Extract by High-Resolution UPLC-MSn
The crude extract (5 mg/mL) was diluted in methanol–water (4:1), centrifuged (2000 rpm), and the supernatant was analyzed by high-resolution UHPLC-MSn. Chromatographic separation was performed using an ACQUITY UPLC I-Class UHPLC System (Waters GmbH, Eschborn, Germany) under the following gradient conditions: 5%B-2 min; 5%B to 95%B-2 to 18 min; 95%B to 5%B-18 to 23 min, with a flow rate of 0.4 mL/min. The column used was an EC 150/2 Nucleoshell (C18-phase, I.D. 2 mm, 150 mm, 2.7 µm, Macherey Nagel, Düren, Germany) maintained at 40 °C. Eluents A and B were 0.3 mmol/L aqueous ammonium formate and acetonitrile, respectively. The separation system was coupled to a high-resolution TripleTOF 6600-1 mass spectrometer (AB Sciex™, Framingham, MA, USA) equipped with a DuoSpray ESI/APCI ion source, operating in positive and negative modes.
Precursor ions were selected in Data Dependent Acquisition (DDA) mode. MS experiments were conducted in TOF-scan mode with an accumulation time of 100 ms covering a m/z range of 65–1250. Tandem mass spectrometric (MS2) experiments were performed in SWATH mode, with the overall range (m/z 65–1250) divided into 48 windows of 26 m/z each, with a 1 m/z overlap. Each m/z window was acquired with a 20 ms accumulation time at a collision energy (CE) of −35 V, using a collision energy spread (CES) of 15 V and a declustering potential (DP) of −35 V. Nitrogen was used as the collision-activated dissociation (CAD) gas.
Raw data (*.wiff format) were converted to *.mzML using MSConvert (ProteoWizard; https://proteowizard.sourceforge.io/, accessed on 5 September 2022) and processed in MZmine version 2.53 (Table S2). Dereplication was performed using the GNPS platform (Global Natural Products Social Molecular Networking; https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=4f12c4f9a8f640dcb32c1633ded31735, accessed on 10 September 2022) in combination with literature data and manual interpretation of MS2 fragmentation patterns.
3.4. Isolation of Compounds
The isolation step was performed using a semi-preparative approach on an Agilent 1260 HPLC-DAD system, with the column maintained at 40 °C and monitored at wavelengths λ = 210 and 269 nm. Separations were conducted on a reverse phase C8 column (VarioPrep VP125/10 nucleodur 100_5 C8 ec, 125 × 10 MM). The injection volume was 40 µL (50 mg/mL, in methanol) with a flow rate of 7 mL/min. The mobile phase consisted of water (A) and acetonitrile (B), with the following gradient: 30%B–70%B–from 0 to 10 min; 70%B–100%B–from 10 to 12 min; 100%B–30%B–from 12 to 15 min.
3.5. NMR Analysis
One-dimensional (1D) and two-dimensional (2D) 1H and 13C spectra were acquired using an Agilent® DD2 400 MHz (9.4 Tesla) spectrometer (Santa Clara, CA, USA) operating at 400 MHz (1H) and 100 MHz (13C), or an Agilent VNMRS 600 (Varian, Palo Alto, CA, USA) operating at 599.83 MHz (1H) and 149.84 MHz (13C). The VNMRS 600 was used exclusively for quantitative analysis.
The 1D (1H, 13C) and 2D (1H-13C) gHSQCAD; (1H-13C) gHMBCAD; (1H-13C) gH2BCAD; (1H-13C) DQFCOSY; (1H-1H) zTOCSY and (1H-1H) ROESYAD NMR spectra were acquired using standard CHEMPACK 8.1 pulse sequences implemented in the VNMRJ 4.2A spectrometer software. All spectra were recorded in CD3OD at +25 °C, and quantitative CD3OD was used for quantitative measurements. 1H and 13C chemical shifts were referenced to the internal standard hexamethyl disiloxane (δH 0.062 ppm; δC 1.96 ppm). The following parameters were applied: TOCSY mixing time = 80 ms; ROESY mixing time = 300 ms; HSQC optimized for 1JCH = 146 Hz; HMBC optimized for nJCH = 8 Hz.
3.6. Biological Activities
3.6.1. Cytotoxic Activity Against Cancer Cells
The cytotoxicity of crude extract and isolated compounds were evaluated against the human tumor cell lines PC-3 (prostatic carcinoma cell) and HCT-116 (colorectal carcinoma cell). Cell maintenance was performed as described in the literature []. The extract and isolated compounds were tested at the concentrations of 0.05 and 50 μg/mL and 0.01 and 10 μM, respectively. Saponin digitonin (100 µM), a very potent permeabilizer of cell membranes, was used as positive control to induce 0% cell viability after 48 h of treatment.
Cell viability was determined by MTT and crystal violet (CV) assays after 48 h of incubation []. Absorbance was measured using an automated microplate reader at 540 nm with a reference wavelength of 670 nm. Results are expressed as percentages relative to untreated control cultures.
3.6.2. Screening of Plant Extract, Fraction and Isolated Compounds for Inhibition of SARS-CoV-2 PLpro and 3CLpro Enzymatic Assay
Recombinant SARS-CoV-2 3CLpro and PLpro, expressed in E. coli BL21(DE3) pLysS and BL21(DE3) cells (purchased from ThermoFisher, Waltham, MA, USA, USA cat C600003), respectively, were used in a fluorescence resonance energy transfer (FRET) assay. The peptides DABCYL-AVLQ↓SGFRLL-EDANS and DABCYL-ALKG↓GKIV-EDANS were employed as substrates for 3CLpro and PLpro, respectively. The 3CLpro concentration was fixed at 1.5 µM, and the substrate concentration at 50 µM. The crude extract (EBEC) and the dichloromethane fraction (FDCM) from E. crotonoides leaves were tested at concentrations ranging from 0.0001 to 1000 µg/mL. The same protocol was applied to the isolated compounds, tested over a range of 0.01 to 1000 µM.
The reaction mixture was incubated in 5 mM NaCl, 20 mM Tris. HCl pH 8.0, 5 mM DTT for 15 min at 37 °C prior to addition of the substrate. For PLpro, the enzyme concentration was set in 1 µM, using a reaction buffer containing 150 mM NaCl, 20 mM Tris.HCl pH 8.0, and 5 mM DTT. EDANS emission fluorescence was monitored every 30 s for 45 min, at 37 °C (λexc = 330 nm, λem = 490 nm).
Fluorescence data (RFU) were converted to specific substrate cleavage activity using a fluorescent conversion factor (FEC) previously determined for the EDANS-DABCYL fluorophore pair. Maximum enzyme activity was defined by reactions containing only vehicle (DMSO) and was used to calculate percentage inhibition by the tested samples. The concentration required to inhibit 50% of enzyme activity (IC50) was determined, and statistical analysis were performed by one-way ANOVA followed by Tukey’s test, all using GraphPad Prism 8.0.
4. Conclusions
In this work, several compounds from E. crotonoides collected in the PARNA Jurubatiba restinga were annotated. Among them, furanoheliangolide-type sesquiterpene lactones such as budlein A were characterized, and centratherin and goyazensolide were isolated and identified, with the latter being isolated for the first time in this species in quantities sufficient for unequivocal identification by NMR. Regarding the antitumoral activity of the isolated metabolites, centratherin demonstrated promising cytotoxic potential, completely inhibiting the viability of cancer cells at 10 µM, while goyazensolide had no such effect. Furthermore, enzymatic inhibition assays against SARS-CoV-2 proteases revealed significant activity of both centratherin and goyazensolide against PLpro and 3CLpro, along with promising results for the dichloromethane extract, which was shown by quantitative 1H NMR to be rich in centratherin. These findings highlight E. crotonoides extracts as a promising source of bioactive sesquiterpene lactones with notable anti-SARS-CoV-2 potential. Notably, in goyazensolide, cytotoxic and anti-SARS-CoV-2 activities appear to be structurally unrelated. This work encourages further in-depth exploration and targeted isolation of similar compounds or of semisynthetic derivatives, derived from this species and other members of the Eremanthus genus, underscoring the unique pharmacological potential within these plants. Moreover, this study is the first to reveal the anti-SARS-CoV-2 activity of E. crotonoides and its sesquiterpene lactones, opening new avenues for phytochemical and biological research in antiviral drug discovery.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30204053/s1. Table S1: Compounds characterized by UPLC-(ESI)-MS/MS in the crude extract of Eremanthus crotonoides compared to the literature, GNPS (cosine similarity), and considering fragmentation patterns. Table S2: MZmine 2.53 software parameters. Table S3: EBEC, FDCM, centratherin, and goyazensolide from the leaves of the Eremanthus crotonoides effects on the PLpro enzyme. Figure S1: Chlorogenic acid MS2 spectrum m/z 355.1019 [M+H]+. Figure S2: Caffeoylquinic acid (isomer I) MS2 spectrum m/z 517.1345 [M+H]+. Figure S3: Caffeoylquinic acid (isomer II) MS2 spectrum m/z 517.1326 [M+H]+. Figure S4: Quercetin-3-O-glucoside MS2 spectrum m/z 465.1027 [M+H]+. Figure S5: Caffeoylquinic acid (isomer III) MS2 spectrum m/z 517.1337 [M+H]+. Figure S6: Isorhamnetin-3-O-glucoside MS2 spectrum m/z 479.1178 [M+H]+. Figure S7: Tiliroside MS2 spectrum m/z 595.1450 [M+H]+. Figure S8: Luteolin MS2 spectrum m/z 287.0565 [M+H]+. Figure S9: Isorhamnetin MS2 spectrum m/z 317.0670 [M+H]+. Figure S10: Goyazensolide MS2 spectrum m/z 361.1307 [M+H]+. Figure S11: Budlein A isobutyrate MS2 spectrum m/z 363.1448 [M+H]+. Figure S12: Centratherin MS2 spectrum m/z 375.1440 [M+H]+. Figure S13: Overlapped 1H NMR spectra (δH −0.5 to 8.0 ppm) of the crude extract and the dichloromethane fraction of E. crotonoides. Figure S14: HPLC-DAD of dichloromethane fraction monitored at λ = 269 and the UV spectra of their six peaks collected. Figure S15: 1H NMR spectra (δH −0.25 to 6.5 ppm) of goyazensolide. Figure S16: 13C NMR spectra (δC 0.0 to 210.0 ppm) of goyazensolide. Figure S17: HSQC (1H-13C) NMR spectra (δH −0.25 to 6.5 ppm; δC 10 to 145 ppm) of centratherin. Figure S18: HMBC (1H-13C) NMR spectra (δH 1.2 to 6.6 ppm; δC 0.0 to 210 ppm) of centratherin. Figure S19: (1H-1H) NOESY NMR spectra (δH 0.0 to 6.5 ppm; δH 0.0 to 7.0 ppm) of centratherin. Figure S20: (1H-1H) COSY NMR spectra (δH 0.0 to 7.0 ppm; δH 0.0 to 6.5 ppm) of centratherin. Figure S21: (1H-1H) TOCSY NMR spectra (δH 0.0 to 6.5 ppm; δH 0.0 to 6.5 ppm) of centratherin. Figure S22: 1H NMR spectra (δH −0.5 to 7.0 ppm) of goyazensolide. Figure S23: 13C NMR spectra (δC 0.0 to 240.0 ppm) of goyazensolide. Figure S24: HSQC (1H-13C) NMR spectra (δH −0.25 to 6.5 ppm; δC 0 to 140 ppm) of goyazensolide. Figure S25: (1H-1H) COSY NMR spectra (δH 0.0 to 6.5 ppm; δH 1.0 to 7.5 ppm) of goyazensolide. Figure S26: (1H-13C) HMBC NMR spectra (δH −1.0 to 9.0 ppm; δC 0.0 to 210 ppm) of goyazensolide. Figure S27: (1H-1H) NOESY NMR spectra (δH −1.0 to 7.5 ppm; δH −0.5 to 7.0 ppm) of goyazensolide.
Author Contributions
Conceptualization: P.H.B.d.M., S.C.P., M.F.M., D.A., I.C.R.L.; methodology: P.H.B.d.M., N.G.d.R.X., B.B.S., L.E.C.C., S.d.S.C., I.C.R.L.; software: P.H.B.d.M., N.G.d.R.X., C.M.L., L.E.C.C., S.d.S.C.; validation: P.H.B.d.M., N.G.d.R.X., D.A., L.A.W., I.C.R.L.; formal analysis: P.H.B.d.M., N.G.d.R.X., C.M.L., L.E.C.C., S.d.S.C., S.C.P., M.F.M., D.A., L.A.W., I.C.R.L.; investigation: P.H.B.d.M., N.G.d.R.X., B.B.S., L.E.C.C., S.d.S.C., D.A., I.C.R.L.; resources: S.C.P., M.F.M., D.A., L.A.W., I.C.R.L.; data curation, P.H.B.d.M., N.G.d.R.X., L.E.C.C., S.d.S.C., D.A., I.C.R.L.; writing—original draft preparation: P.H.B.d.M., N.G.d.R.X., C.M.L., L.E.C.C., S.d.S.C.; writing—review and editing: P.H.B.d.M., D.A., L.A.W., I.C.R.L.; visualization: P.H.B.d.M., N.G.d.R.X., C.M.L., L.E.C.C., S.d.S.C.; supervision: S.C.P., M.F.M., D.A., L.A.W., I.C.R.L.; project administration: D.A., L.A.W., I.C.R.L.; funding acquisition: S.C.P., M.F.M., D.A., L.A.W., I.C.R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro), grant numbers E-26/204.263/2021 and E-26.201.168/2022. The APC was funded by Leibniz Institute of Plant Biochemistry and FAPERJ.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank Tatiana Konno (in memorian) for the collection and identification of the species. We also express our gratitude to Robert Rennert and his cell lab team, especially Martina Brode for performing the cytotoxic assays on tumor cell lines.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Loeuille, B.F.P. Eremanthus in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Disponível em. Available online: https://floradobrasil.jbrj.gov.br/FB5314 (accessed on 23 September 2022).
- Silva, E.L.; Lobo, J.F.R.; Vinther, J.M.; Borges, R.M.; Staerk, D. High-resolution α-glucosidase inhibition profiling combined with HPLC-HRMS-SPE-NMR for identification of antidiabetic compounds in Eremanthus crotonoides (Asteraceae). Molecules 2016, 21, 782. [Google Scholar] [CrossRef]
- Lobo, J.F.; Castro, E.S.; Gouvea, D.R.; Fernandes, C.P.; Almeida, F.B.D.; de Amorim, L.M.; Burth, P.; Rocha, L.; Santos, M.G.; Pinto, A.C.; et al. Antiproliferative activity of Eremanthus crotonoides extracts and centratherin demonstrated in brain tumor cell lines. Rev. Bras. De Farm. 2012, 22, 1295–1300. [Google Scholar] [CrossRef]
- Valente, J.G.; da Silva, I.C.; Muzitano, M.F.; Guimarães, D.O.; Leal, I.C. Antibacterial, Antibiofilm, and Synergistic Effects of Eremanthus crotonoides Against Multidrug-Resistant Staphylococcus strains. Rev. Bras. Farm. 2021, 31, 486–491. [Google Scholar] [CrossRef]
- Araujo, M.H.D.; Simão, T.L.B.V.; Konno, T.U.P.; Guimarães, D.O.; Leal, I.C.R.; Lasunskaia, E.; Muzitano, M.F. Anti-mycobacterial and Anti-inflammatory Activity of Restinga Plants: A Dual Approach in Searching for New Drugs to Treat Severe Tuberculosis. Rodriguésia 2021, 72, e01152019. [Google Scholar] [CrossRef]
- Ramirez, D.A. Small molecules putative structure elucidation in endemic Colombian fruits: CFM-ID approach. Int. J. Food Prop. 2022, 25, 2604–2616. [Google Scholar] [CrossRef]
- Ren, Q.; Long, S. Chemical identification and quantification of Hu-Gu capsule by UHPLC-Q-TOF-MS and HPLC-DAD. Rev. Bras. De Farm. 2017, 27, 557–563. [Google Scholar] [CrossRef]
- Lee, H.J.; Pan, C.H.; Kim, E.S.; Kim, C.Y. Online High Performance Liquid Chromatography (HPLC)-ABTS Based Assay and HPLC-Electrospray Ionization Mass Spectrometry Analysis of Antioxidant Phenolic Compounds in Salsola komarovii. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 317–321. [Google Scholar] [CrossRef]
- Jang, G.H.; Kim, H.W.; Lee, M.K.; Jeong, S.Y.; Bak, A.R.; Lee, D.J.; Kim, J.B. Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC-DAD-QTOF/MS. Saudi J. Biol. Sci. 2018, 25, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Felipe, D.F.; Brambilla, L.Z.S.; Porto, C.; Pilau, E.J.; Cortez, D.A.G. Phytochemical analysis of Pfaffia glomerata inflorescences by LC-ESI-MS/MS. Molecules 2014, 19, 15720–15734. [Google Scholar] [CrossRef]
- Abrankó, L.; Szilvássy, B. Mass spectrometric profiling of flavonoid glycoconjugates possessing isomeric aglycones. J. Mass Spectrom. 2015, 50, 71–80. [Google Scholar] [CrossRef]
- Rauha, J.P.; Vuorela, H.; Kostiainen, R. Effect of eluent on the ionization efficiency of flavonoids by ion spray, atmospheric pressure chemical ionization, and atmospheric pressure photoionization mass spectrometry. J. Mass Spectrom. 2001, 36, 1269–1280. [Google Scholar] [CrossRef]
- Crotti, A.E.; Fonseca, T.; Hong, H.; Staunton, J.; Galembeck, S.E.; Lopes, N.P.; Gates, P.J. The fragmentation mechanism of five-membered lactones by electrospray ionisation tandem mass spectrometry. Int. J. Mass Spectrom. 2004, 232, 271–276. [Google Scholar] [CrossRef]
- Spring, O.; Schilling, E.E. The origin of Helianthus × multiflorus and H. × laetiflorus (Asteraceae). Biochem. Syst. Ecol. 1990, 18, 19–23. [Google Scholar] [CrossRef]
- Spring, O. Sesquiterpene lactones from Helianthus tuberosus. Phytochemistry 1991, 30, 519–522. [Google Scholar] [CrossRef]
- Spring, O.; Edward, E.S. The sesquiterpene lactone chemistry of Helianthus sect. Atrorubentes (Asteraceae: Heliantheae). Biochem. Syst. Ecol. 1991, 19, 59–79. [Google Scholar] [CrossRef]
- Soares, A.C.F.; Silva, A.N.; Matos, P.M.; Silva, E.H.D.; Heleno, V.C.G.; Lopes, N.P.; Sass, D.C. Complete 1H and 13C NMR Structural Assignments for a Group of Four Goyazensolide-Type Furanoheliangolides. Química Nova 2012, 35, 2205–2209. [Google Scholar] [CrossRef]
- Santos Junior, F.M.; Covington, C.L.; de Albuquerque, A.C.F.; Lobo, J.F.; Borges, R.M.; de Amorim, M.B.; Polavarapu, P.L. Absolute Configuration of (−)-Centratherin, a Sesquiterpenoid Lactone, Defined by Means of Chiroptical Spectroscopy. J. Nat. Prod. 2015, 78, 2617–2623. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Patouret, R.; Barluenga, S.; Plank, M.; Loewith, R.; Winssinger, N. Identification of a Covalent Importin-5 Inhibitor, Goyazensolide, from a Collective Synthesis of Furanoheliangolides. ACS Cent. Sci. 2021, 7, 954–962. [Google Scholar] [CrossRef]
- Kolli, E.H.; León, F.; Benayache, F.; Estévez, S.; Quintana, J.; Estévez, F.; Brouard, I.; Bermejo, J.; Benayache, S. Cytotoxic sesquiterpene lactones and other constituents of Centaurea omphalotricha. J. Braz. Chem. Soc. 2012, 23, 977–983. [Google Scholar] [CrossRef]
- Ángeles, M.A.G.; Rafael-Pita, C.; Fernández, N.; Baixinho, J.D.; Anastácio, J.; Cankar, K.; Bosch, D.; Santons, C.N. Targeting proteases involved in the viral replication of SARS-CoV-2 by sesquiterpene lactones from chicory (Cichorium intybus L.). Food Funct. 2022, 13, 8977–8988. [Google Scholar]
- Moustaqil, M.; Ollivier, E.; Chiu, H.P.; Van, T.S.; Rudolffi-Soto, P.; Stevens, C.; Bhumkar, A.; Hunter, D.J.B.; Freiberg, A.N.; Jacques, D.; et al. SARS-CoV-2 proteases PLpro and 3CLpro cleave IRF3 and critical modulators of inflammatory pathways (NLRP12 and TAB1): Implications for disease presentation across species. Emerg. Microbes Infect. 2021, 10, 178–195. [Google Scholar] [CrossRef]
- Migheli, R.; Virdis, P.; Galleri, G.; Arru, C.; Lostia, G.; Coradduzza, D.; Muroni, M.R.; Pintore, G.; Podda, L.; Fozza, C.; et al. Antineoplastic Properties by Proapoptotic Mechanisms Induction of Inula viscosa and Its Sesquiterpene Lactones Tomentosin and Inuviscolide. Biomedicines 2022, 10, 2739. [Google Scholar] [CrossRef]
- Paço, A.; Brás, T.; Santos, J.O.; Sampaio, P.; Gomes, A.C.; Duarte, M.F. Anti-Inflammatory and Immunoregulatory Action of Sesquiterpene Lactones. Molecules 2022, 27, 1142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, Y.; Yang, L.; He, J.; Li, Y.; Xia, C. Brevilin A, a Sesquiterpene Lactone, Inhibits the Replication of Influenza A Virus In Vitro and In Vivo. Viruses 2019, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cui, Q.; Cooper, L.; Zhang, P.; Lee, H.; Chen, Z.; Wang, Y.; Liu, X.; Rong, L.; Du, R. Ginkgolic acid and anacardic acid are specific covalent inhibitors of SARS-CoV-2 cysteine proteases. Cell Biosci. 2021, 11, 45. [Google Scholar] [CrossRef]
- Wasilewicz, A.; Kirchweger, B.; Bojkova, D.; Abi Saad, M.J.; Langeder, J.; Butikofer, M.; Adelsberger, S.; Grienke, U.; Jr-Cinatl, J.; Petermann, O.; et al. Identification of Natural Products Inhibiting SARS-CoV-2 by Targeting Viral Proteases: A Combined in Silico and in Vitro Approach. J. Nat. Prod. 2023, 86, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.T.; Ricardo, M.G.; Rennert, R.; Frolov, A.; Porzel, A.; Brandt, W.; Stark, P.; Westermann, B.; Arnold, N. Rare glutamic acid methyl ester peptaibols from Sepedonium ampullosporum damon KSH 534 exhibit promising antifungal and anticancer activity. Int. J. Mol. Sci. 2021, 22, 12718. [Google Scholar] [CrossRef]
- Ware, I.; Franke, K.; Hussain, H.; Morgan, I.; Rennert, R.; Wessjohann, L.A. Bioactive phenolic compounds from Peperomia obtusifolia. Molecules 2022, 27, 4363. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).