Effect of Preparation Conditions of Fe@SiO2 Catalyst on Its Structure Using High-Pressure Activity Studies in a 3D-Printed SS Microreactor
Abstract
1. Introduction
2. Results and Discussion
2.1. BET Analysis
2.2. XRD Analysis
2.3. SEM Analysis
2.4. TEM Analysis
2.5. TPR Analysis
2.6. TGA-DSC Analysis
2.7. FTIR Analysis
2.8. XPS Analysis
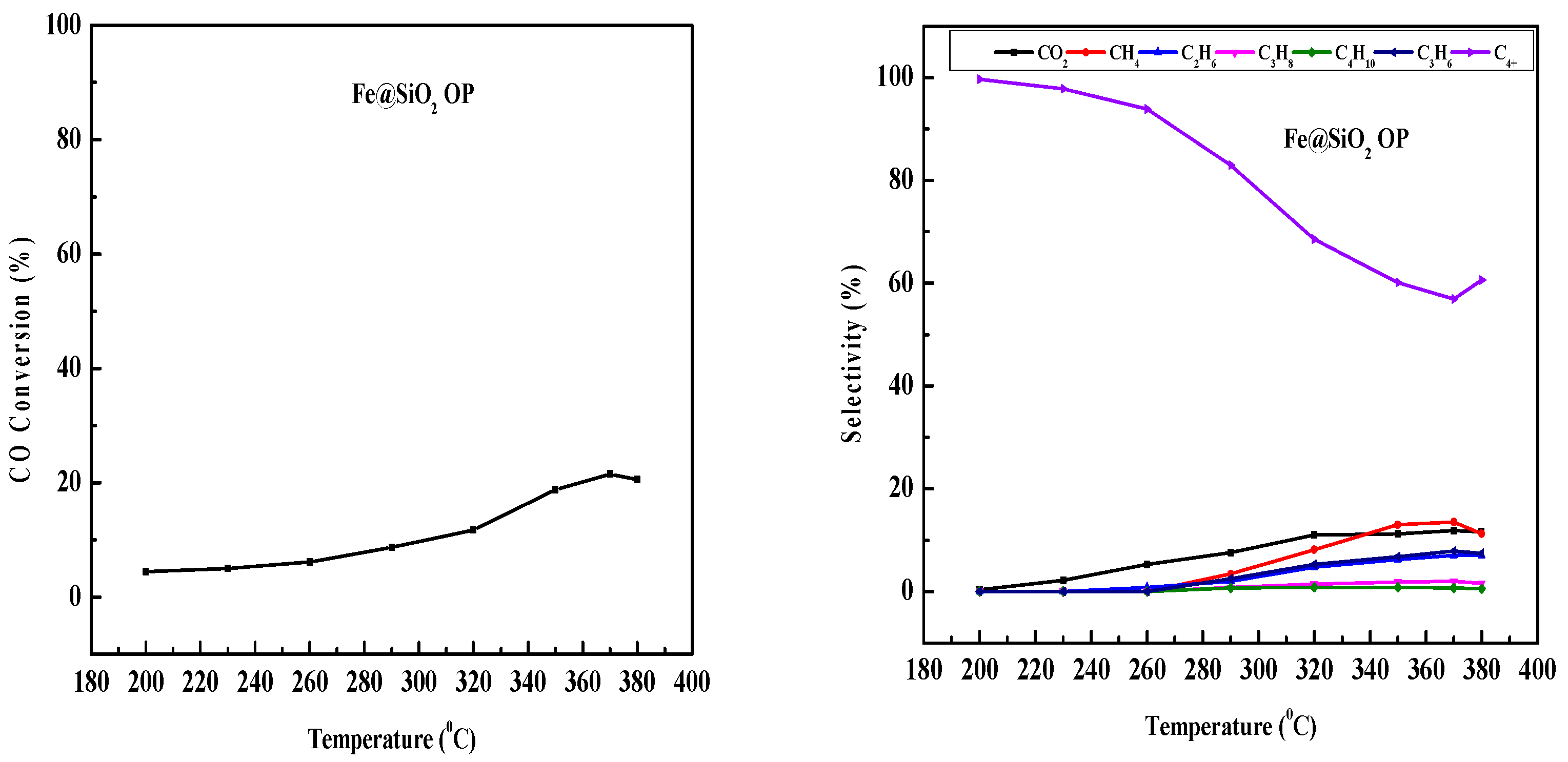
2.9. Fe-Based Core–Shell Catalysts FTS
3. Experimental
3.1. Materials
3.2. Fe@SiO2 Core–Shell Preparation Using Autoclave (AC) Procedure
3.3. Fe@SiO2 Core–Shell Preparation with One-Pot (OP) Synthesis Technique
3.3.1. Synthesis of Fe (Core) Nanoparticles
3.3.2. Preparation of SiO2 (Shell)
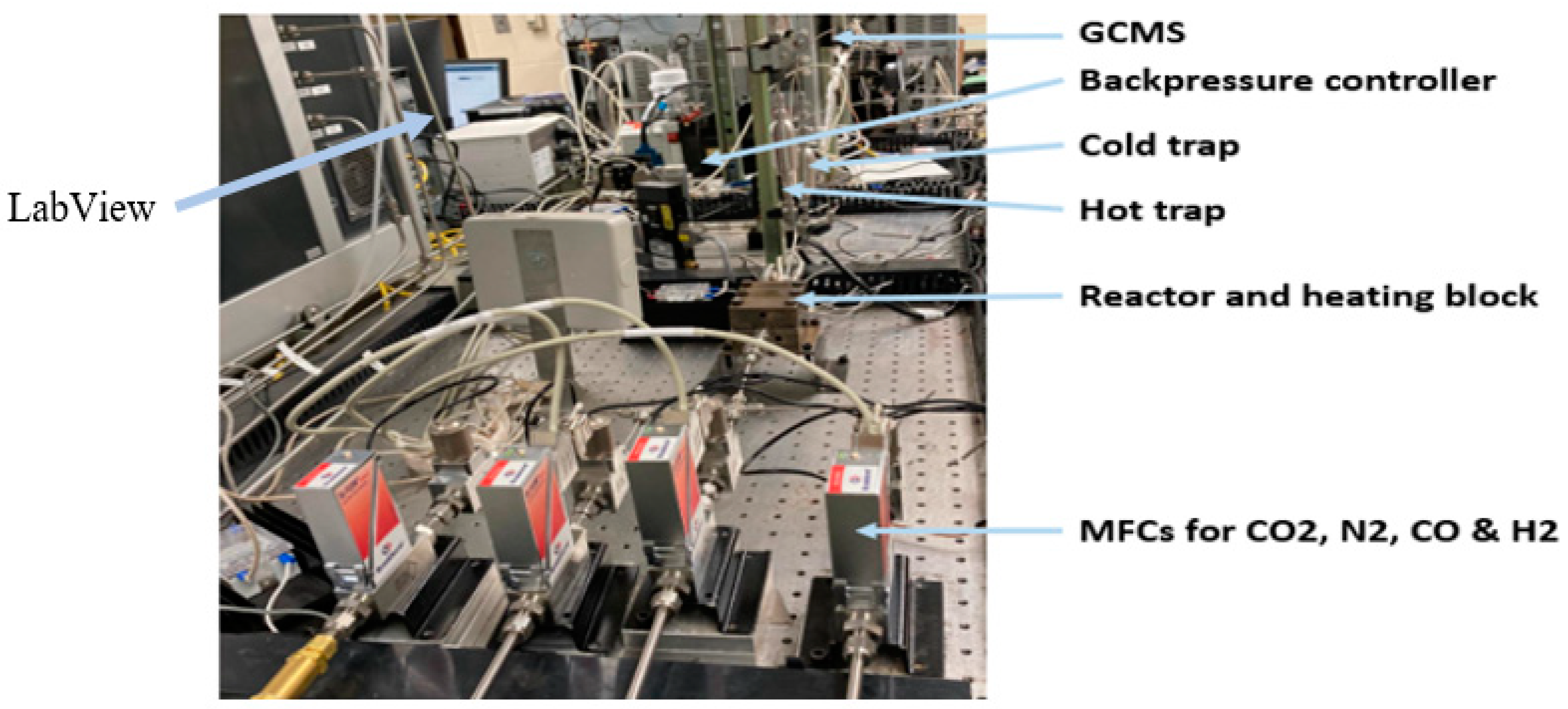
3.4. 3D-Printed Stainless Steel Microchannel Microreactor (SSMR)
3.5. Characterization Techniques
3.6. FTS Process
4. Stability Studies of All Catalysts
4.1. Spent Catalyst Characterization
4.1.1. SEM Analysis of Spent Catalysts
4.1.2. TGA-DSC Analysis of Spent Catalysts
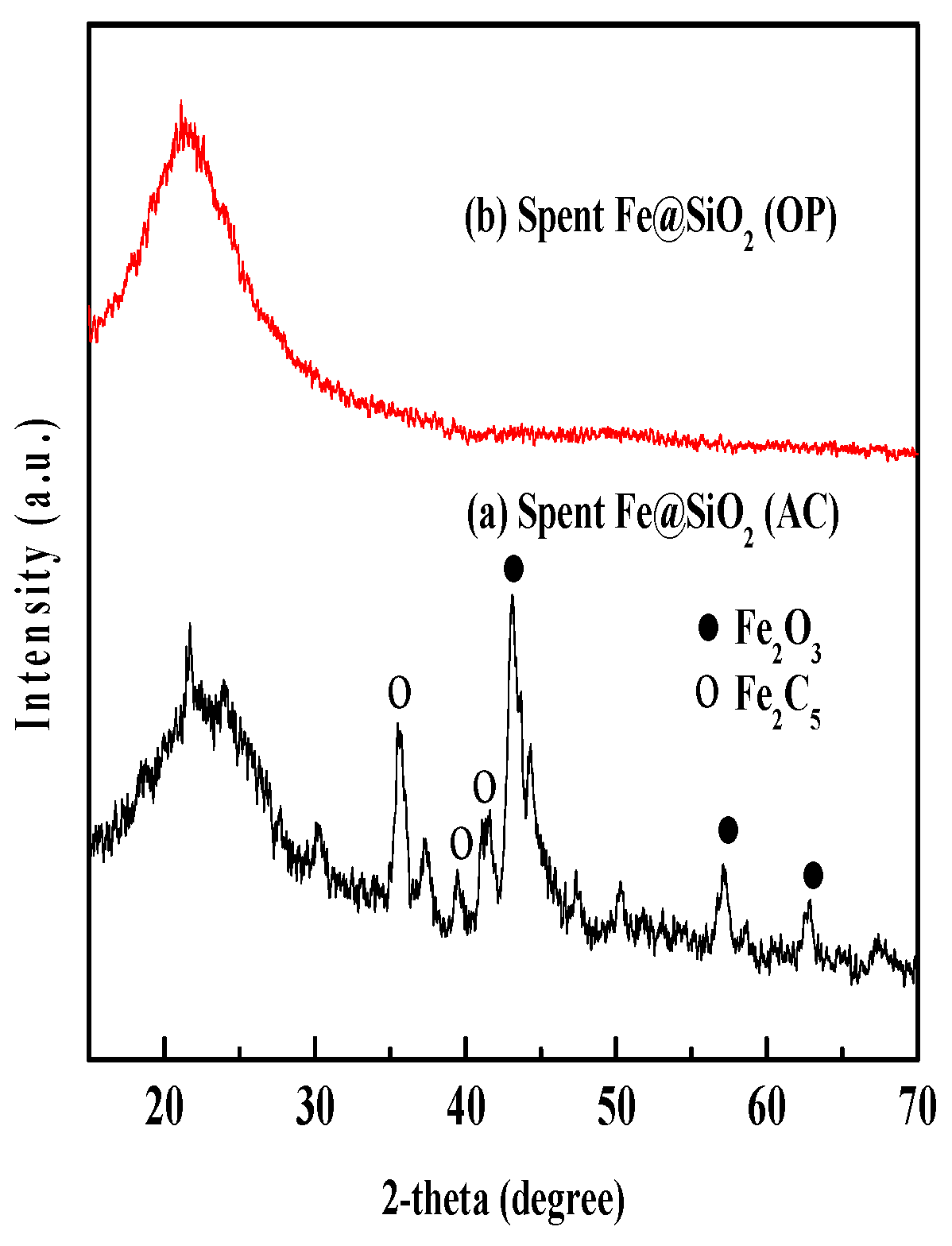
4.1.3. XRD Analysis of Spent Catalysts
4.2. Comparative Study of Activity of Different Fe-Based Catalysts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, F.; Tropsch, H. The Synthesis of Petroleum at Atmospheric Pressures from Gasification Products of Coal. Brennstoff-Chemie 1926, 7, 97–104. [Google Scholar]
- Bepari, S.; Khan, M.; Li, X.; Mohammad, N.; Kuila, D. Effect of Ce and Zn on Cu-Based Mesoporous Carbon Catalyst for Methanol Steam Reforming. Top. Catal. 2023, 66, 375–392. [Google Scholar] [CrossRef]
- Bepari, S.; Kuila, D. Steam Reforming of Methanol, Ethanol and Glycerol over Nickel-Based Catalysts—A Review. Int. J. Hydrogen Energy 2020, 45, 18090–18113. [Google Scholar] [CrossRef]
- Bej, B.; Bepari, S.; Pradhan, N.C.; Neogi, S. Production of Hydrogen by Dry Reforming of Ethanol over Alumina Supported Nano-NiO/SiO2 Catalyst. Catal. Today 2017, 291, 58–66. [Google Scholar] [CrossRef]
- Bepari, S.; Pradhan, N.C.; Dalai, A.K. Selective Production of Hydrogen by Steam Reforming of Glycerol over Ni/Fly Ash Catalyst. Catal. Today 2017, 291, 36–46. [Google Scholar] [CrossRef]
- Bepari, S.; Basu, S.; Pradhan, N.C.; Dalai, A.K. Steam Reforming of Ethanol over Cerium-Promoted Ni-Mg-Al Hydrotalcite Catalysts. Catal. Today 2017, 291, 47–57. [Google Scholar] [CrossRef]
- Bepari, S.; Sarkar, J.J.; Pradhan, N.C. Kinetics of Ethanol Steam Reforming over Ni/Olivine Catalyst. Int. J. Hydrogen Energy 2022, 47, 30843–30860. [Google Scholar] [CrossRef]
- Maschio, G.; Lucchesi, A.; Stoppato, G. Production of Syngas from Biomass. Bioresour. Technol. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Rauch, R.; Hrbek, J.; Hofbauer, H. Biomass Gasification for Synthesis Gas Production and Applications of the Syngas. Wiley Interdiscip. Rev. Energy Envrion. 2014, 3, 343–362. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More Efficient Biomass Gasification via Torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- van Dyk, J.C.; Keyser, M.J.; Coertzen, M. Syngas Production from South African Coal Sources Using Sasol–Lurgi Gasifiers. Int. J. Coal Geol. 2006, 65, 243–253. [Google Scholar] [CrossRef]
- Joos, L.; Filot, I.A.W.; Cottenier, S.; Hensen, E.J.M.; Waroquier, M.; Van Speybroeck, V.; Van Santen, R.A. Reactivity of CO on Carbon-Covered Cobalt Surfaces in Fischer-Tropsch Synthesis. J. Phys. Chem. C 2014, 118, 5317–5321. [Google Scholar] [CrossRef]
- Mohammad, N.; Aravamudhan, S.; Kuila, D. Atomic Layer Deposition of Cobalt Catalyst for Fischer–Tropsch Synthesis in Silicon Microchannel Microreactor. Nanomaterials 2022, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.; Abrokwah, R.Y.; Stevens-Boyd, R.G.; Aravamudhan, S.; Kuila, D. Fischer-Tropsch Studies in a 3D-Printed Stainless Steel Microchannel Microreactor Coated with Cobalt-Based Bimetallic-MCM-41 Catalysts. Catal. Today 2020, 358, 303–315. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer–Tropsch Synthesis: Current Mechanism and Futuristic Needs. Fuel Process. Technol. 2001, 71, 157–166. [Google Scholar] [CrossRef]
- Anderson, R.B.; Friedel, R.A.; Storch, H.H. Fischer-Tropsch Reaction Mechanism Involving Stepwise Growth of Carbon Chain. J. Chem. Phys. 1951, 19, 313–319. [Google Scholar] [CrossRef]
- Abelló, S.; Montané, D. Exploring Iron-Based Multifunctional Catalysts for Fischer–Tropsch Synthesis: A Review. ChemSusChem 2011, 4, 1538–1556. [Google Scholar] [CrossRef]
- Santos, V.P.; Wezendonk, T.A.; Jaén, J.J.D.; Dugulan, A.I.; Nasalevich, M.A.; Islam, H.U.; Chojecki, A.; Sartipi, S.; Sun, X.; Hakeem, A.A.; et al. Metal Organic Framework-Mediated Synthesis of Highly Active and Stable Fischer-Tropsch Catalysts. Nat. Commun. 2015, 6, 6451. [Google Scholar] [CrossRef]
- Jung, J.S.; Kim, S.W.; Moon, D.J. Fischer–Tropsch Synthesis over Cobalt Based Catalyst Supported on Different Mesoporous Silica. Catal. Today 2012, 185, 168–174. [Google Scholar] [CrossRef]
- Panpranot, J.; Goodwin, J.G.; Sayari, A. Synthesis and Characteristics of MCM-41 Supported CoRu Catalysts. Catal. Today 2002, 77, 269–284. [Google Scholar] [CrossRef]
- Wielers, A.F.H.; Kock, A.J.H.M.; Hop, C.E.C.A.; Geus, J.W.; van Der Kraan, A.M. The Reduction Behavior of Silica-Supported and Alumina-Supported Iron Catalysts: A Mössbauer and Infrared Spectroscopic Study. J. Catal. 1989, 117, 1–18. [Google Scholar] [CrossRef]
- Zhang, C.H.; Yang, Y.; Teng, B.T.; Li, T.Z.; Zheng, H.Y.; Xiang, H.W.; Li, Y.W. Study of an Iron-Manganese Fischer–Tropsch Synthesis Catalyst Promoted with Copper. J. Catal. 2006, 237, 405–415. [Google Scholar] [CrossRef]
- Ni, Z.; Qin, H.; Kang, S.; Bai, J.; Wang, Z.; Li, Y.; Zheng, Z.; Li, X. Effect of Graphitic Carbon Modification on the Catalytic Performance of Fe@SiO2-GC Catalysts for Forming Lower Olefins via Fischer-Tropsch Synthesis. J. Colloid Interface Sci. 2018, 516, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yu, G.; Lin, J.; Xu, K.; Pei, Y.; Yan, S.; Qiao, M.; Fan, K.; Zhang, X.; Zong, B. A Highly Selective Raney Fe@HZSM-5 Fischer–Tropsch Synthesis Catalyst for Gasoline Production: One-Pot Synthesis and Unexpected Effect of Zeolites. Catal. Sci. Technol. 2012, 2, 1625–1629. [Google Scholar] [CrossRef]
- Su, L.; Jing, Y.; Zhou, Z. Li Ion Battery Materials with Core–Shell Nanostructures. Nanoscale 2011, 3, 3967–3983. [Google Scholar] [CrossRef]
- Song, F.; Yong, X.; Wu, X.; Zhang, W.; Ma, Q.; Zhao, T.; Tan, M.; Guo, Z.; Zhao, H.; Yang, G.; et al. FeMn@HZSM-5 Capsule Catalyst for Light Olefins Direct Synthesis via Fischer-Tropsch Synthesis: Studies on Depressing the CO2 Formation. Appl. Catal. B 2022, 300, 120713. [Google Scholar] [CrossRef]
- Hassan, S.; Arslan, M.; Shajahan, J.; Bepari, S.; Vidanapathirana, P.; Kuila, D. Fischer-Tropsch Synthesis of Fuels and Olefins in 3D Printed SS Microreactor Using Iron/Graphene Oxide Catalysts with Mn- and Na-Metal Promoters. Int. J. Hydrogen Energy 2024, 67, 1248–1261. [Google Scholar] [CrossRef]
- Ni, Z.; Zhang, X.; Bai, J.; Wang, Z.; Li, X.; Zhang, Y. Potassium Promoted Core–Shell-Structured FeK@SiO2-GC Catalysts Used for Fischer–Tropsch Synthesis to Olefins without Further Reduction. New J. Chem. 2019, 44, 87–94. [Google Scholar] [CrossRef]
- Qiu, T.; Wang, L.; Lv, S.; Sun, B.; Zhang, Y.; Liu, Z.; Yang, W.; Li, J. SAPO-34 Zeolite Encapsulated Fe3C Nanoparticles as Highly Selective Fischer-Tropsch Catalysts for the Production of Light Olefins. Fuel 2017, 203, 811–816. [Google Scholar] [CrossRef]
- Xiang, M.; Huang, M.; Li, H.; Wang, W.; Huang, Y.; Lu, Z.; Wang, C.; Si, R.; Cao, W. Nanoscale Zero-Valent Iron/Cobalt@mesoporous Hydrated Silica Core–Shell Particles as a Highly Active Heterogeneous Fenton Catalyst for the Degradation of Tetrabromobisphenol A. Chem. Eng. J. 2021, 417, 129208. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, J.; Basu, S. Efficient Metal Ion Adsorption and Photodegradation of Rhodamine-B by Hierarchical Porous Fe-Ni@SiO2 Monolith. Microchem. J. 2019, 145, 708–717. [Google Scholar] [CrossRef]
- Ziyadi, H.; Baghali, M.; Heydari, A. The Synthesis and Characterization of Fe2O3@SiO2–SO3H Nanofibers as a Novel Magnetic Core-Shell Catalyst for Formamidine and Formamide Synthesis. Heliyon 2021, 7, e07165. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Kang, S.; Bai, J.; Li, Y.; Huang, Y.; Wang, Z.; Qin, H.; Li, X. Uniformity Dispersive, Anti-Coking Core@double-Shell-Structured Co@SiO2@C: Effect of Graphitic Carbon Modified Interior Pore-Walls on C5+ Selectivity in Fischer-Tropsch Synthesis. J. Colloid Interface Sci. 2017, 505, 325–331. [Google Scholar] [CrossRef]
- González, O.; Pérez, H.; Navarro, P.; Almeida, L.C.; Pacheco, J.G.; Montes, M. Use of Different Mesostructured Materials Based on Silica as Cobalt Supports for the Fischer–Tropsch Synthesis. Catal. Today 2009, 148, 140–147. [Google Scholar] [CrossRef]
- Chen, W.; Bao, Y.; Li, X.; Huang, J.; Tang, Y.; Li, L. Mineralization of Salicylic Acid via Catalytic Ozonation with Fe-Cu@SiO2 Core-Shell Catalyst: A Two-Stage First Order Reaction. Chemosphere 2019, 235, 470–480. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Tu, J.; Ding, M.; Zhang, Y.; Li, Y.; Wang, T.; Ma, L.; Wang, C.; Li, X. Synthesis of Fe3O4-Nanocatalysts with Different Morphologies and Its Promotion on Shifting C5+ Hydrocarbons for Fischer–Tropsch Synthesis. Catal. Commun. 2015, 59, 211–215. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Wei, J.; Wen, Z.; Xu, H.; Ge, Q. Fischer–Tropsch Synthesis over Iron Catalysts with Corncob-Derived Promoters. J. Energy Chem. 2017, 26, 632–638. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Y.; Nisa, M.U.; Li, X.; Zhao, N.; Li, Z. Highly Active FexOy@SiO2 Catalyst for Fischer-Tropsch Synthesis through the Confinement Effect of Metal Organic Frameworks Material: Preparation and Structure-Activity Relationship. Mol. Catal. 2021, 513, 111813. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer–Tropsch Synthesis: Reaction Mechanisms for Iron Catalysts. Catal. Today 2009, 141, 25–33. [Google Scholar] [CrossRef]
- Luo, M.; O’Brien, R.; Davis, B.H. Effect of Palladium on Iron Fischer-Tropsch Synthesis Catalysts. Catal. Lett. 2004, 98, 17–22. [Google Scholar] [CrossRef]
- Kang, S.H.; Bae, J.W.; Prasad, P.S.S.; Jun, K.W. Fischer-Tropsch Synthesis Using Zeolite-Supported Iron Catalysts for the Production of Light Hydrocarbons. Catal. Lett. 2008, 125, 264–270. [Google Scholar] [CrossRef]
- Chun, D.H.; Park, J.C.; Hong, S.Y.; Lim, J.T.; Kim, C.S.; Lee, H.T.; Yang, J.I.; Hong, S.; Jung, H. Highly Selective Iron-Based Fischer–Tropsch Catalysts Activated by CO2-Containing Syngas. J. Catal. 2014, 317, 135–143. [Google Scholar] [CrossRef]
- Chun, D.H.; Park, J.C.; Rhim, G.B.; Lee, H.T.; Yang, J.I.; Hong, S.J.; Jung, H. Nanocrystalline Ferrihydrite-Based Catalysts for Fischer-Tropsch Synthesis: Part I. Reduct. Carburization Behavior. J. Nanosci. Nanotechnol. 2016, 16, 1660–1664. [Google Scholar] [CrossRef]
- An, X.; Wu, B.; Hou, W.; Wan, H.; Tao, Z.; Li, T.; Zhang, Z.; Xiang, H.; Li, Y.; Xu, B.; et al. The Negative Effect of Residual Sodium on Iron-Based Catalyst for Fischer–Tropsch Synthesis. J. Mol. Catal. A Chem. 2007, 263, 266–272. [Google Scholar] [CrossRef]
- Wu, B.; Bai, L.; Xiang, H.; Li, Y.W.; Zhang, Z.; Zhong, B. An Active Iron Catalyst Containing Sulfur for Fischer–Tropsch Synthesis. Fuel 2004, 83, 205–212. [Google Scholar] [CrossRef]
- Ubilla, P.; García, R.; Fierro, J.L.G.; Escalona, N. Hydrocarbons Synthesis from a Simulated Biosyngas Feed Over Fe/SIO2, Catalysts. J. Chil. Chem. Soc. 2010, 55, 35–38. [Google Scholar] [CrossRef]
- Wan, H.J.; Wu, B.S.; Zhang, C.H.; Teng, B.T.; Tao, Z.C.; Yang, Y.; Zhu, Y.L.; Xiang, H.W.; Li, Y.W. Effect of Al2O3/SiO2 Ratio on Iron-Based Catalysts for Fischer–Tropsch Synthesis. Fuel 2006, 85, 1371–1377. [Google Scholar] [CrossRef]
- Li, Y.P.; Wang, T.J.; Wu, C.Z.; Qin, X.X.; Tsubaki, N. Effect of Ru Addition to Co/SiO2/HZSM-5 Catalysts on Fischer–Tropsch Synthesis of Gasoline-Range Hydrocarbons. Catal. Commun. 2009, 10, 1868–1874. [Google Scholar] [CrossRef]
- Adeli, M.; Seyedein, S.H.; Aboutalebi, M.R.; Kobashi, M.; Kanetake, N. Implementation of DSC Analysis in Reaction Kinetics during Heating of Ti–50 at.%Al Powder Mixture. J. Therm. Anal. Calorim. 2017, 128, 867–874. [Google Scholar] [CrossRef]
- Zemenová, P.; Král, R.; Nitsch, K.; Knížek, K.; Cihlář, A.; Bystřický, A. Characterization and Crystallization Kinetics of Er-Doped Li2O–Y2O3–P2O5 Glass Studied by Non-Isothermal DSC Analysis. J. Therm. Anal. Calorim. 2016, 125, 1431–1437. [Google Scholar] [CrossRef]
- Abbasi, M.; Mirzaei, A.A.; Atashi, H. Hydrothermal Synthesis of Fe-Ni-Ce Nano-Structure Catalyst for Fischer-Tropsch Synthesis: Characterization and Catalytic Performance. J. Alloys Compd. 2019, 799, 546–555. [Google Scholar] [CrossRef]
- Pejova, B.; Isahi, A.; Najdoski, M.; Grozdanov, I. Fabrication and Characterization of Nanocrystalline Cobalt Oxide Thin Films. Mater. Res. Bull. 2001, 36, 161–170. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, H.; Hou, Y.; Ma, D. Fe 5C 2 Nanoparticles: A Facile Bromide-Induced Synthesis and as an Active Phase for Fischer-Tropsch Synthesis. J. Am. Chem. Soc. 2012, 134, 15814–15821. [Google Scholar] [CrossRef]
- Bukur, D.B.; Nowicki, L.; Manne, R.K.; Lang, X.S. Activation Studies with a Precipitated Iron Catalyst for Fischer-Tropsch Synthesis: II. Reaction Studies. J. Catal. 1995, 155, 366–375. [Google Scholar] [CrossRef]
- Bukur, D.B.; Okabe, K.; Rosynek, M.P.; Li, C.P.; Wang, D.J.; Rao, K.R.P.M.; Huffman, G.P. Activation Studies with a Precipitated Iron Catalyst for Fischer-Tropsch Synthesis: I. Characterization Studies. J. Catal. 1995, 155, 353–365. [Google Scholar] [CrossRef]
- Galakhov, V.R.; Shkvarin, A.S.; Semenova, A.S.; Uimin, M.A.; Mysik, A.A.; Shchegoleva, N.N.; Yermakov, A.Y.; Kurmaev, E.Z. Characterization of Carbon-Encapsulated Nickel and Iron Nanoparticles by Means of X-Ray Absorption and Photoelectron Spectroscopy. J. Phys. Chem. C 2010, 114, 22413–22416. [Google Scholar] [CrossRef]
- Nohira, H.; Tsai, W.; Besling, W.; Young, E.; Petry, J.; Conard, T.; Vandervorst, W.; De Gendt, S.; Heyns, M.; Maes, J.; et al. Characterization of ALCVD-Al2O3 and ZrO2 Layer Using X-Ray Photoelectron Spectroscopy. J. Non Cryst. Solids 2002, 303, 83–87. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Barbosa, I.A.; de Sousa Filho, P.C.; da Silva, D.L.; Zanardi, F.B.; Zanatta, L.D.; de Oliveira, A.J.A.; Serra, O.A.; Iamamoto, Y. Metalloporphyrins Immobilized in Fe3O4@SiO2 Mesoporous Submicrospheres: Reusable Biomimetic Catalysts for Hydrocarbon Oxidation. J. Colloid. Interface Sci. 2016, 469, 296–309. [Google Scholar] [CrossRef]
- Arslan, M.; Bepari, S.; Abrokwah, R.; Mohammad, N.; Shajahan, J.; Kuila, D. Effect of Al2O3 Support on Co-Based SiO2 Core–Shell Catalysts for Fischer–Tropsch Synthesis in 3D Printed SS Microchannel Microreactor. Top. Catal. 2022, 66, 477–497. [Google Scholar] [CrossRef]
- Bepari, S.; Li, X.; Abrokwah, R.; Mohammad, N.; Arslan, M.; Kuila, D. Co-Ru Catalysts with Different Composite Oxide Supports for Fischer–Tropsch Studies in 3D-Printed Stainless Steel Microreactors. Appl. Catal. A Gen. 2020, 608, 117838. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, K.; Lin, M.; Hou, B.; Zhong, L.; Zhu, Y.; Wei, W.; Sun, Y. Controlled Fabrication of Iron Oxide/Mesoporous Silica Core-Shell Nanostructures. J. Phys. Chem. C 2013, 117, 21529–21538. [Google Scholar] [CrossRef]
- Ye, F.; Laurent, S.; Fornara, A.; Astolfi, L.; Qin, J.; Roch, A.; Martini, A.; Toprak, M.S.; Muller, R.N.; Muhammed, M. Uniform Mesoporous Silica Coated Iron Oxide Nanoparticles as a Highly Efficient, Nontoxic MRI T 2 Contrast Agent with Tunable Proton Relaxivities. Contrast Media Mol. Imaging 2012, 7, 460–468. [Google Scholar] [CrossRef]
- Arslan, M. Development of Core-Shell Catalysts for Fischer-Tropsch Synthesis in 3D Printed SS Microchannel Microreactor and Tubular Reactor. In Proceedings of the 6th International Conference on Catalysis and Chemical Engineering, Las Vegas, NV, USA, 20–24 February 2023. [Google Scholar]
- Mohammad, N. Catalyst and Microreactor Development for Atmospheric and High-Pressure Fischer-Tropsch Synthesis. Ph.D. Thesis, North Carolina Agricultural and Technical State University, Greensboro, NC, USA, 2020. [Google Scholar]
- Esmaeilpour, M.; Javidi, J.; Nowroozi Dodeji, F.; Mokhtari Abarghoui, M. Facile Synthesis of 1- and 5-Substituted 1H-Tetrazoles Catalyzed by Recyclable Ligand Complex of Copper(II) Supported on Superparamagnetic Fe3O4@SiO2 Nanoparticles. J. Mol. Catal. A Chem. 2014, 393, 18–29. [Google Scholar] [CrossRef]
- de Mendonça, E.S.D.T.; de Faria, A.C.B.; Dias, S.C.L.; Aragón, F.F.H.; Mantilla, J.C.; Coaquira, J.A.H.; Dias, J.A. Effects of Silica Coating on the Magnetic Properties of Magnetite Nanoparticles. Surf. Interfaces 2019, 14, 34–43. [Google Scholar] [CrossRef]
- He, T.; Chen, D.; Jiao, X. Controlled Synthesis of Co3O4 Nanoparticles through Oriented Aggregation. Chem. Mater. 2004, 16, 737–743. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, D.; Jiao, X. Fabrication and Characterization of Mesoporous Co3O4 Core/Mesoporous Silica Shell Nanocomposites. J. Phys. Chem. B 2006, 110, 15212–15217. [Google Scholar] [CrossRef]
- Bae, J.S.; Hong, S.Y.; Park, J.C.; Rhim, G.B.; Youn, M.H.; Jeong, H.; Kang, S.W.; Yang, J.I.; Jung, H.; Chun, D.H. Eco-Friendly Prepared Iron-Ore-Based Catalysts for Fischer-Tropsch Synthesis. Appl. Catal. B 2019, 244, 576–582. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Wang, X.; Lamey, D.; Li, M.; Keane, M.A.; Kiwi-Minsker, L. An Examination of Catalyst Deactivation in P-Chloronitrobenzene Hydrogenation over Supported Gold. Chem. Eng. J. 2014, 255, 695–704. [Google Scholar] [CrossRef]
- Tengku-Rozaina, T.M.; Birch, E.J. Thermal Oxidative Stability Analysis of Hoki and Tuna Oils by Differential Scanning Calorimetry and Thermogravimetry. Eur. J. Lipid Sci. Technol. 2016, 118, 1053–1061. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Abbas, M.; Zhang, J.; Huang, Z.; Kawi, S.; Chen, J. Eco-Friendly Solid-State Synthesis of Na-Promoted Mn-Fe/ZrO2 Catalyst for Fischer-Tropsch Synthesis. Fuel 2024, 363, 131013. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, Q.; Lyu, S.; Li, X.; Song, S.; Ding, T.; Tian, Y.; Li, X. Strategic Assembly of Active Phases on Co-Fe Bimetallic Catalysts for Efficient Fischer-Tropsch Synthesis. Chem. Eng. J. 2024, 494, 152936. [Google Scholar] [CrossRef]
- Xi, M.; Cheng, Y.; Meng, F.; Yao, X.; Nawaz, M.A.; Saif, M.; Li, Z.; Reina, T.R. Densely Embedded Homogeneous FeOx Nanoparticles in N-Doped Carbon Frameworks: A 3D Scaffold Catalyst for High-Temperature Fischer-Tropsch Synthesis. Chem. Eng. J. 2025, 503, 158333. [Google Scholar] [CrossRef]
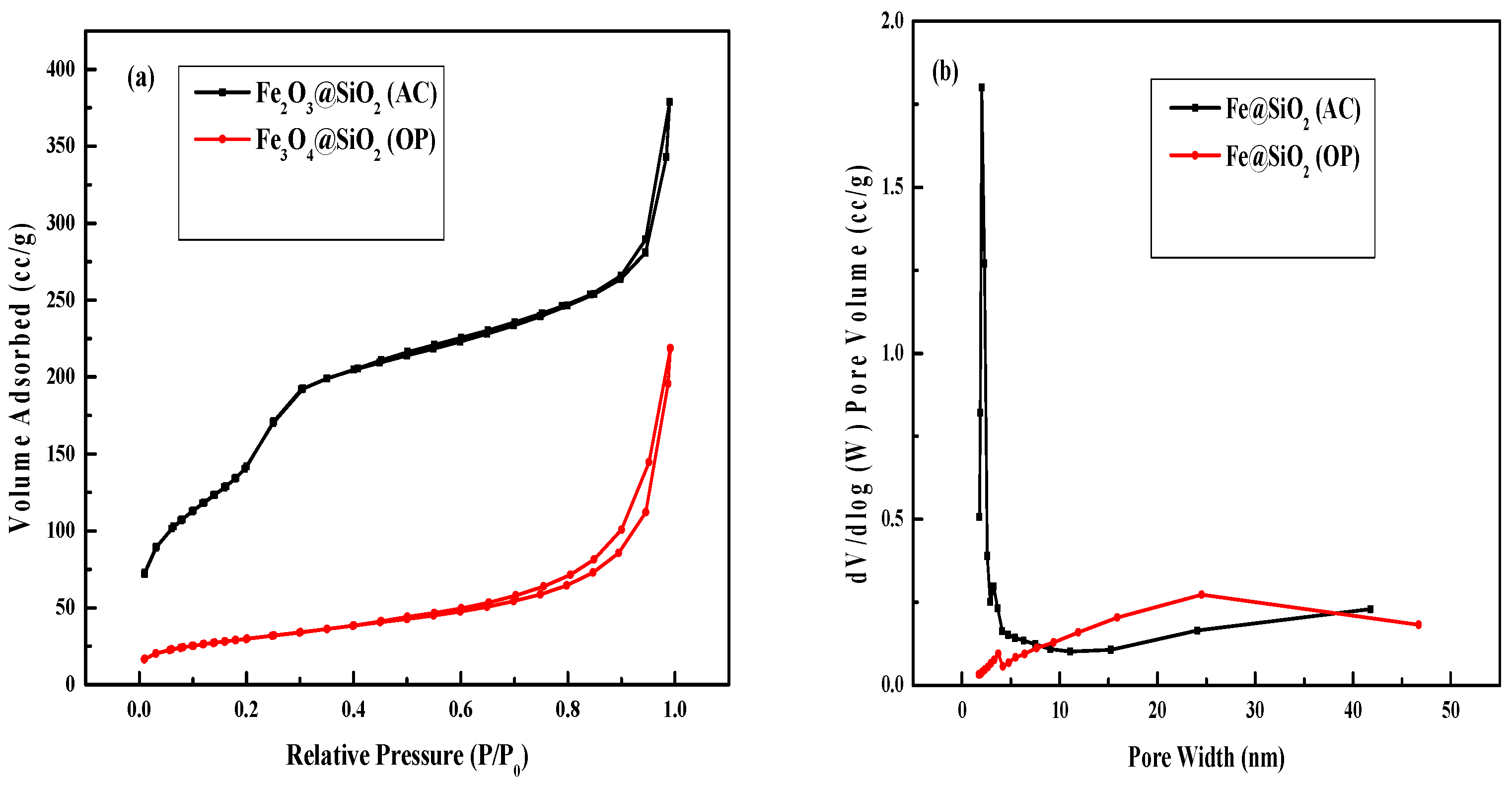





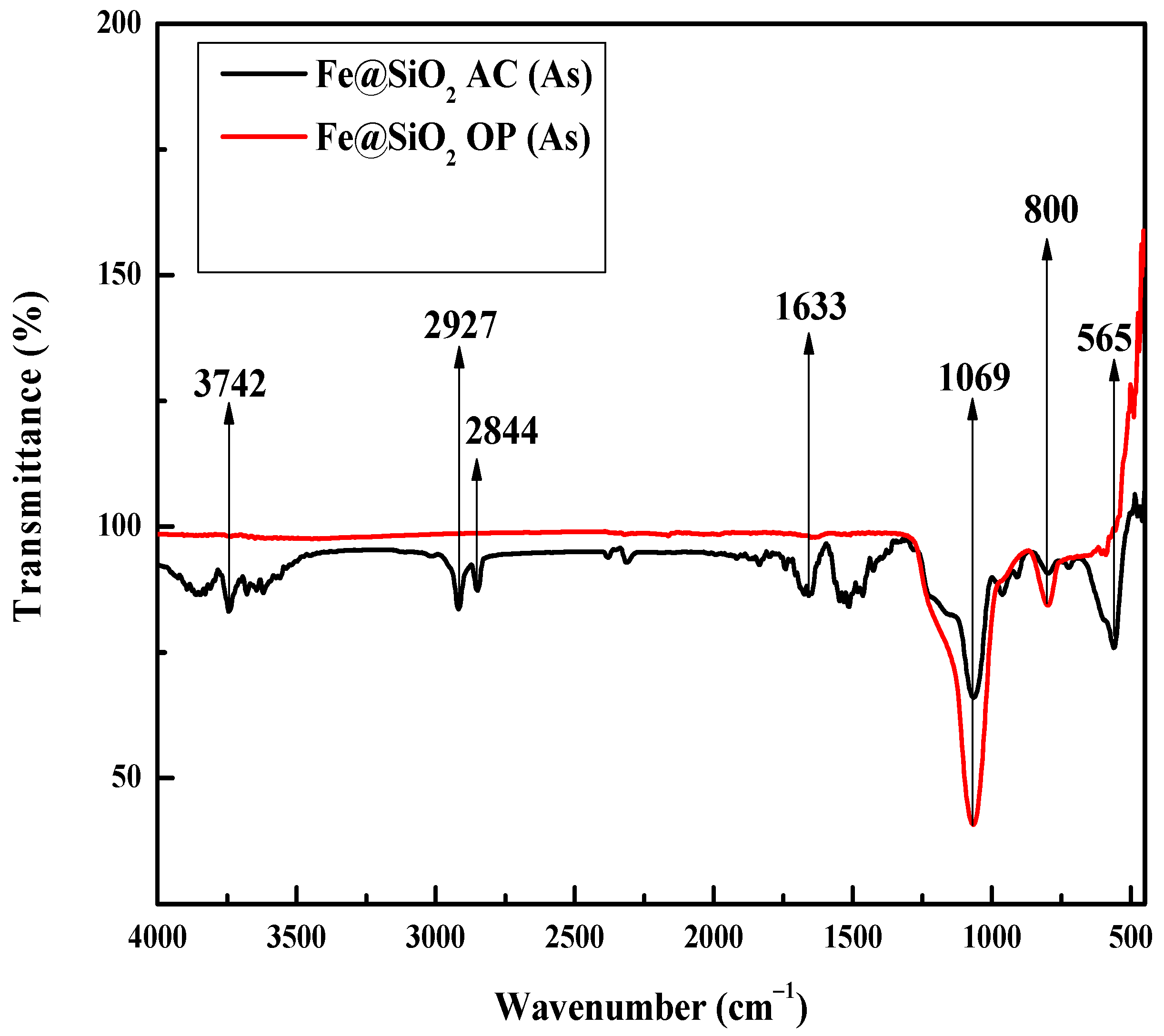







| Catalyst | Surface Area (m 2/g) | Pore Volume (cc/g) | Pore Diameter (nm) |
|---|---|---|---|
| Fe@SiO2 (OP) | 106 | 0.33 | 12.6 |
| Fe@SiO2 (AC) | 617 | 0.58 | 3.8 |
| Catalyst | Avg. Fe2O3 Crystal Size (nm) | Avg. Fe3O4 Crystal Size (nm) |
|---|---|---|
| Fe@SiO2 (OP) | - | 20.34 |
| Fe@SiO2 (AC) | 20.45 | - |
| Catalyst | Metal Loading (wt. %) Fe | Silica (Si) Loading (wt. %) | Oxygen (O) Loading (wt. %) |
|---|---|---|---|
| Fe@SiO2 (OP) | 28.92 | 19.1 | 51.98 |
| Fe@SiO2 (AC) | 26.87 | 27.79 | 45.34 |
| Catalyst | H2 Consumption (mmol/g) a | Reduction Degree (%) b |
|---|---|---|
| Fe@SiO2 (AC) | 0.27 | 55.64 |
| Fe@SiO2 (OP) | 0.13 | 14.11 |
| Catalyst | Synthesis Method | CO Conversion (%) | Product Selectivity | Reference |
|---|---|---|---|---|
| FeK@SiO2-GC (Graphite carbon) | Graphitic carbon coating | 80–90 | High olefin (approx. 20%) | [28] |
| SAPO-34@Fe3C | Zeolite encapsulation | 70 | Light olefins (>18%) | [29] |
| Na-Mn-Fe/ZrO2 | Solid state | 42 | High C5+ (60%) | [74] |
| CoFe/NC | Hydrothermal | 40.9 | High C5+ (66.1%) | [75] |
| Fe@NC/CS | Sol impregnation | 56.2 | High olefin (approx. 19.6%) | [76] |
| Fe@SiO2 (AC) | Autoclave | 85 | High olefin (16% propene), moderate C4+ | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arslan, M.; Bepari, S.; Shajahan, J.; Hassan, S.; Kuila, D. Effect of Preparation Conditions of Fe@SiO2 Catalyst on Its Structure Using High-Pressure Activity Studies in a 3D-Printed SS Microreactor. Molecules 2025, 30, 280. https://doi.org/10.3390/molecules30020280
Arslan M, Bepari S, Shajahan J, Hassan S, Kuila D. Effect of Preparation Conditions of Fe@SiO2 Catalyst on Its Structure Using High-Pressure Activity Studies in a 3D-Printed SS Microreactor. Molecules. 2025; 30(2):280. https://doi.org/10.3390/molecules30020280
Chicago/Turabian StyleArslan, Meric, Sujoy Bepari, Juvairia Shajahan, Saif Hassan, and Debasish Kuila. 2025. "Effect of Preparation Conditions of Fe@SiO2 Catalyst on Its Structure Using High-Pressure Activity Studies in a 3D-Printed SS Microreactor" Molecules 30, no. 2: 280. https://doi.org/10.3390/molecules30020280
APA StyleArslan, M., Bepari, S., Shajahan, J., Hassan, S., & Kuila, D. (2025). Effect of Preparation Conditions of Fe@SiO2 Catalyst on Its Structure Using High-Pressure Activity Studies in a 3D-Printed SS Microreactor. Molecules, 30(2), 280. https://doi.org/10.3390/molecules30020280







