Direct Cyclization/Chlorination Strategy of Hydrazines for Synthesis of 4-Chloropyrazoles by TCCA
Abstract
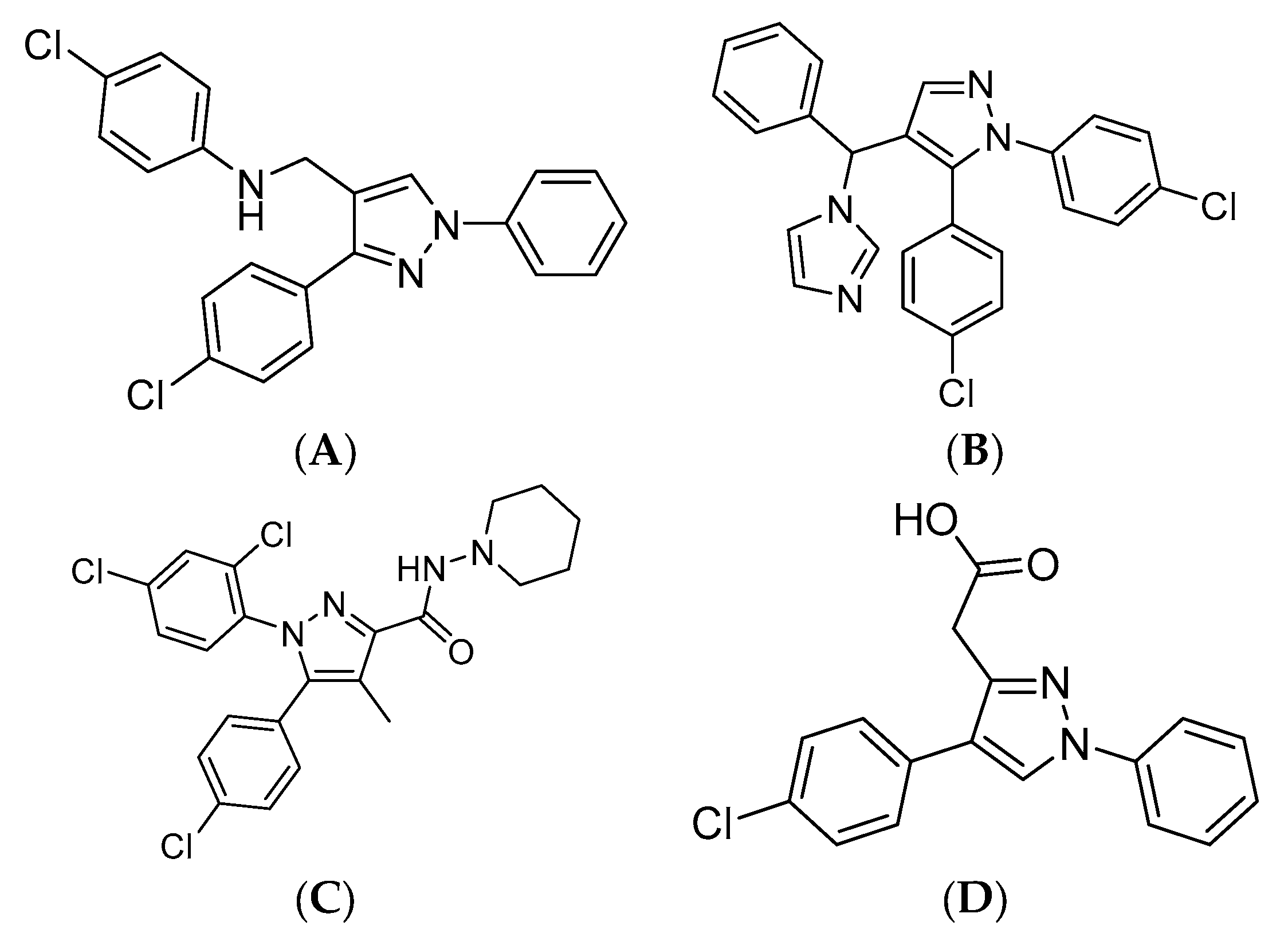
1. Introduction
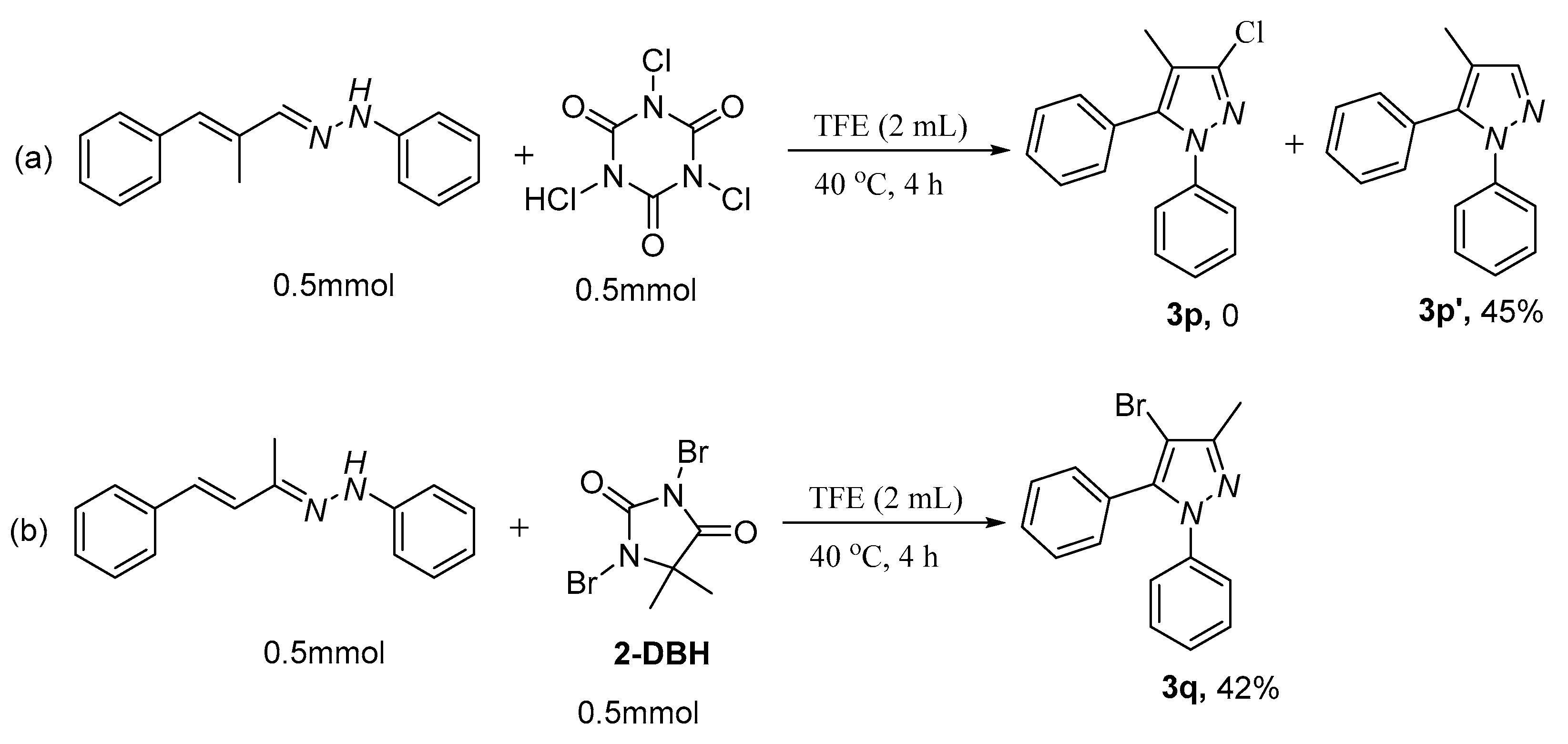
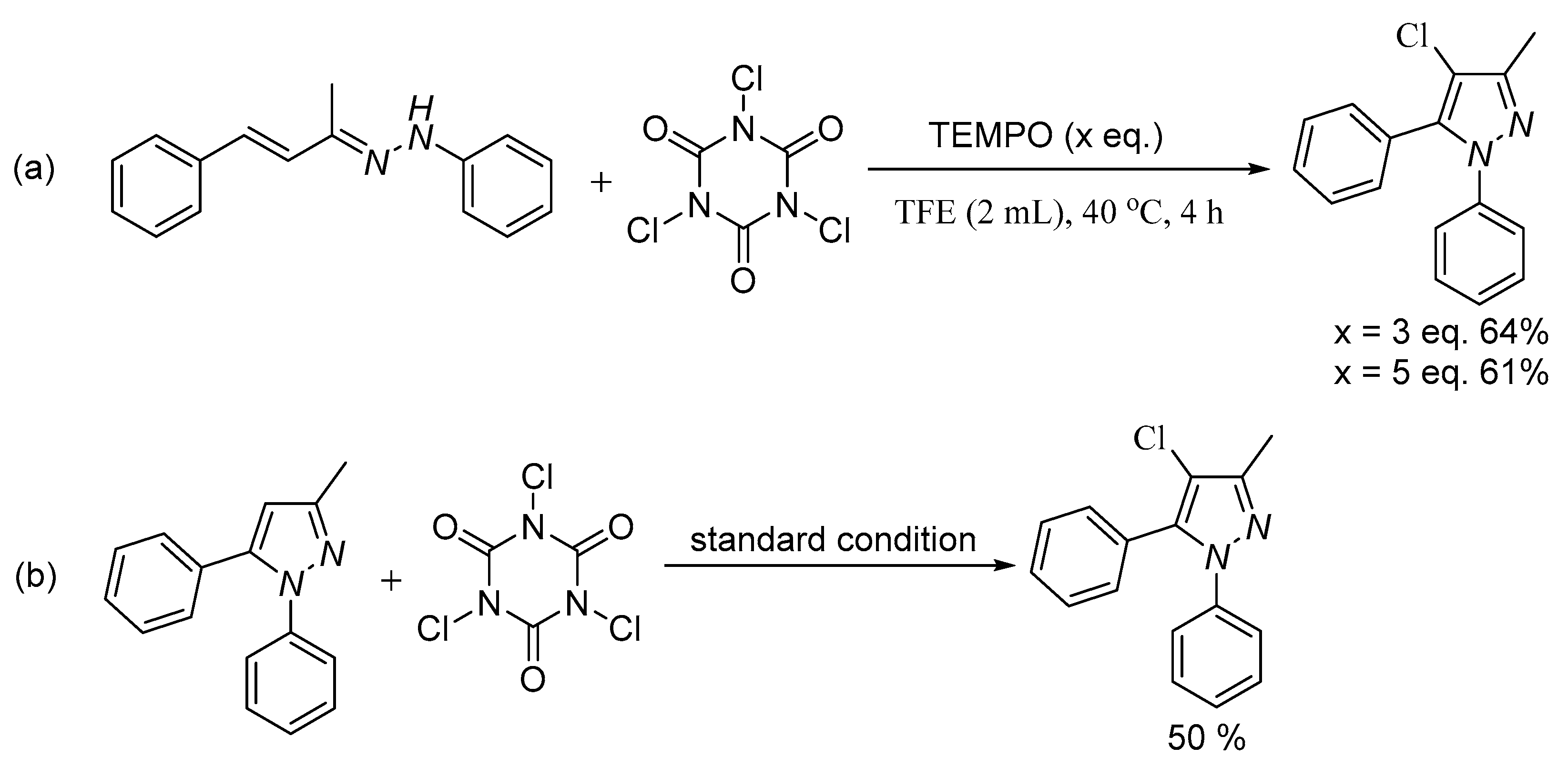
2. Results and Discussion
3. Conclusions
4. Experimental
4.1. General Information
4.2. General Procedure for the Synthesis of Pyrazole Derivatives (3)
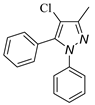
4.3. 4-Chloro-3-methyl-1,5-diphenyl-1H-pyrazole (3a)
4.4. General Procedure for the Synthesis of Arylated Pyrazole Derivatives (4)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faisal, M.; Saeed, A.; Hussain, S.; Dar, P.; Larik, F.A. Recent developments in synthetic chemistry and biological activities of pyrazole derivatives. J. Chem. Sci. 2019, 131, 1–30. [Google Scholar] [CrossRef]
- Menozzi, G.; Merello, L.; Fossa, P.; Schenone, S.; Ranise, A.; Mosti, A.; Bondavalli, F.; Loddo, R.; Murgioni, C.; Mascia, V.; et al. Synthesis, antimicrobial activity and molecular modeling studies of halogenated 4-[1H-imidazol-1-yl(phenyl)methyl]-1,5-diphenyl-1H-pyrazoles. Bioorganic Med. Chem. 2004, 12, 5465–5483. [Google Scholar] [CrossRef]
- Huang, X.-F.; Lu, X.; Zhang, Y.; Song, G.-Q.; He, Q.-L.; Li, Q.-S.; Yang, X.-H.; Wei, Y.; Zhu, H.-L. Synthesis, biological evaluation, and molecular docking studies of N-((1,3-diphenyl-1H-pyrazol-4-yl)methyl)aniline derivatives as novel anticancer agents. Bioorganic Med. Chem. 2012, 20, 4895–4900. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Abuo-Rahma, G.E.-D.A.; Hassan, A.A. Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities. Eur. J. Med. Chem. 2009, 44, 3480–3487. [Google Scholar] [CrossRef]
- Fink, B.E.; Mortensen, D.S.; Stuffer, S.R.; Aron, Z.D.; Katzenellenbogen, J.A. Novel structural templates for estrogen-receptor ligands and prospects for combinatorial synthesis of estrogens. Chem. Biol. 1999, 6, 205–219. [Google Scholar] [CrossRef]
- Stauffer, S.R.; Coletta, C.J.; Tedesco, R.; Nishiguchi, G.; Carlson, K.; Sun, J.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A. Pyrazole Ligands: Structure-Affinity/Activity Relationships and Estrogen Receptor-α-Selective Agonists. J. Med. Chem. 2000, 43, 4934–4947. [Google Scholar] [CrossRef]
- Yang, L.; Okuda, F.; Kobayashi, K.; Nozaki, K.; Tanabe, Y.; Ishii, Y.; Haga, M. Syntheses and Phosphorescent Properties of Blue Emissive Iridium Complexes with Tridentate Pyrazolyl Ligands. Inorg. Chem. 2008, 47, 7154–7165. [Google Scholar] [CrossRef]
- Bernhammer, J.C.; Huynh, H.V. Correlation of spectroscopically determined ligand donor strength and nucleophilicity of substituted pyrazoles. Dalton Trans. 2012, 41, 8600–8608. [Google Scholar] [CrossRef]
- Mogensen, S.B.; Taylor, M.K.; Lee, J. Homocoupling Reactions of Azoles and Their Applications in Coordination Chem. Molecules 2020, 25, 5950–5979. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, Y.; Tu, Z. Pyrazole Scaffold Synthesis, Functionalization, and Applications in Alzheimer’s Disease and Parkinson’s Disease Treatment (2011–2020). Molecules 2021, 26, 1202–1239. [Google Scholar] [CrossRef] [PubMed]
- Rimi; Uttam, B.; Sharma, D.; Zhdankin, V.V.; Kumar, R. Pyrazole-tethered isoxazoles: Hypervalent iodine-mediated, metal-free synthesis and biological evaluation. Arkvoc 2024, 2024, 202412382. [Google Scholar] [CrossRef]
- Ismail, M.A.H.; Lehmann, J.; Abou El Ella, D.A.; Albohy, A.; Abouzid, K.A.M. Lonazolac analogues: Molecular modeling, synthesis, and in vivo anti-inflammatory activity. Med. Chem. Res. 2009, 18, 725–744. [Google Scholar] [CrossRef]
- Khan, M.F.; Alam, M.M.; Verma, G.; Akhtar, W.; Akhter, M.; Shaquiquzzaman, M. The therapeutic voyage of pyrazole and its analogs: A review. Eur. J. Med. Chem. 2016, 120, 170–201. [Google Scholar] [CrossRef]
- Santos, N.E.; Carreira, A.R.F.; Silva, V.L.M.; Braga, S.S. Natural and Biomimetic Antitumor Pyrazoles, A Perspective. Molecules 2020, 25, 1364–1375. [Google Scholar] [CrossRef]
- Lamberth, C. Pyrazole chemistry in crop protection. Heterocycles 2007, 71, 1467–1502. [Google Scholar] [CrossRef]
- Giornal, F.; Pazenok, S.; Rodefeld, L.; Lui, N.; Vors, J.; Leroux, F.R. Synthesis of diversely fluorinated pyrazoles as novel active agrochemical ingredients. J. Fluor. Chem. 2013, 152, 2–11. [Google Scholar] [CrossRef]
- Fioravanti, R.; Bolasco, A.; Manna, F.; Rossi, F.; Orallo, F.; Yáñez, M.; Vitali, A.; Ortuso, F.; Alcaro, S. Synthesis and molecular modelling studies of prenylated pyrazolines as MAO-B inhibitors. Bioorganic Med. Chem. Lett. 2010, 20, 6479–6482. [Google Scholar] [CrossRef]
- Dumeunier, R.; Lamberth, C.; Trah, S. Synthesis of Tetrasubstituted Pyrazoles through Different Cyclization Strategies; Isosteres of Imidazole Fungicides. Synlett 2013, 24, 1150–1154. [Google Scholar] [CrossRef]
- Merimi, I.; Touzani, R.; Aouniti, A.; Chetouani, A.; Hammouti, A. Pyrazole derivatives efficient organic inhibitors for corrosion in aggressive media: A comprehensive review. Int. J. Corros. Scale Inhib. 2020, 4, 1237–1260. [Google Scholar] [CrossRef]
- Boyd, S.T.; Fremming, B.A. Rimonabant-a selective CB1 antagonist. Ann. Pharmacother. 2005, 39, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Emtiazi, H.; Amrollahi, M.A.; Mirjalili, B.B.F. Nano-silica sulfuric acid as an efficient catalyst for the synthesis of substituted pyrazoles. Arab. J. Chem. 2015, 8, 793–797. [Google Scholar] [CrossRef]
- Li, M.; Zhao, B.X. Progress of the synthesis of condensed pyrazole derivatives (from 2010 to mid-2013). Eur. J. Med. Chem. 2014, 85, 311–340. [Google Scholar] [CrossRef]
- Santos, F.; María, S.-R.; Pablo, B.; Antonio, S.-F. From 2000 to Mid-2010: A Fruitful Decade for the Synthesis of Pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cheng, Y.; Yang, Y.; Rao, Y. A general and efficient approach to 2H-indazoles and 1H-pyrazoles through copper-catalyzed intramolecular N–N bond formation under mild conditions. Chem. Commun. 2011, 47, 10133–10135. [Google Scholar] [CrossRef]
- Yang, Y.; Kuang, C.; Jin, H.; Yang, Q.; Zhang, Z. Efficient synthesis of 1,3-diaryl-4-halo-1H-pyrazoles from 3-arylsydnones and 2-aryl-1,1-dihalo-1-alkenes. Beilstein J. Org. Chem. 2011, 7, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, L.; Chen, H.; Wu, W.; Jiang, H. Copper-Catalyzed Aerobic C(sp2)–H Functionalization for C–N Bond Formation: Synthesis of Pyrazoles and Indazoles. J. Org. Chem. 2013, 78, 3636–3646. [Google Scholar] [CrossRef] [PubMed]
- Sar, D.; Bag, R.; Yashmeen, A.; Bag, S.S.; Punniyamurthy, T. Synthesis of Functionalized Pyrazoles via Vanadium-Catalyzed C–N Dehydrogenative Cross-Coupling and Fluorescence Switch-On Sensing of BSA Protein. Org. Lett. 2015, 17, 5308–5311. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, J.; Niu, P.; Wu, J.; Yu, W.; Chang, J. I2-Mediated Oxidative C−N Bond Formation for Metal-Free One-Pot Synthesis of Di-, Tri-, and Tetrasubstituted Pyrazoles from α,β-Unsaturated Aldehydes/Ketones and Hydrazines. J. Org. Chem. 2014, 79, 10170–10178. [Google Scholar] [CrossRef]
- Kashiwa, M.; Kuwata, Y.; Sonoda, M.; Tanimori, S. Oxone-mediated facile access to substituted pyrazoles. Tetrahedron 2016, 72, 304–311. [Google Scholar] [CrossRef]
- Liang, D.; Zhu, Q. A Facile Synthesis of Pyrazoles through Metal-Free Oxidative C(sp2)-H Cycloamination of Vinyl Hydrazo. Asian J. Org. Chem. 2015, 4, 42–45. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, R.; Marrot, J.; Coeffard, V.; Xiong, Y. Hypervalent iodine-mediated synthesis of benzoxazoles and benzimidazoles via an oxidative rearrangement. Tetrahydron 2015, 71, 700–708. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; He, Y.; Hussain, M.I.; Hu, W.; Xiong, Y.; Zhu, X. Oxidative rearrangement strategy for synthesis of 2,4,5-trisubstituted oxazoles utilizing hypervalent iodine reagent. Tetrahydron 2016, 72, 5749–5753. [Google Scholar] [CrossRef]
- Hu, L.; Pan, J.; Zhang, X.; Hu, W.; Xiong, Y.; Zhu, X. Synthesis of N, O π-conjugated boron complexes and the reactivity of Suzuki cross-coupling. Tetrahydron 2017, 73, 223–229. [Google Scholar] [CrossRef]
- Shen, H.; Deng, Q.; Liu, R.; Feng, Y.; Zheng, C.; Xiong, Y. Intramolecular aminocyanation of alkenes promoted by hypervalent iodine. Org. Chem. Front. 2017, 4, 1806–1811. [Google Scholar] [CrossRef]
- Zhang, T.; Bao, W. Synthesis of 1H-Indazoles and 1H-Pyrazoles Via Febr3 /O2 Mediated Intramolecular C-H Amination. J. Org. Chem. 2013, 78, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, E.; Jiang, J.; Huang, Z.; Zheng, L.; Liu, Z.-Q. Copper-mediated tandem ring-opening/cyclization reactions of cyclopropanols with aryldiazonium salts: Synthesis of N-arylpyrazoles. Chem. Commun. 2020, 56, 2202–2205. [Google Scholar] [CrossRef]
- Roy, S.; Chatterjee, R.; Kisan, P.; Dandela, R. Ultrasound-assisted synthesis of 1,5-disubstituted pyrazoles via HFIP-mediated cascade cyclization of enaminones with aryl hydrazine. Tetrahedron Lett. 2024, 149, 155277–155281. [Google Scholar] [CrossRef]

 | |||||
|---|---|---|---|---|---|
| Entry | Solvent (mL) | t/h | T/°C | TCCA (eq.) | Yield b (%) |
| 1 | TFE | 4 | 30 | 1.0 | 69 |
| 2 | MeOH | 4 | 30 | 1.0 | 8 |
| 3 | EtOH | 4 | 30 | 1.0 | 38 |
| 4 | HFIP | 4 | 30 | 1.0 | 40 |
| 5 | DMF | 4 | 30 | 1.0 | 32 |
| 6 | HOAc | 4 | 30 | 1.0 | 25 |
| 7 | 1,4-Dioxane | 4 | 30 | 1.0 | 14 |
| 8 | DCM | 4 | 30 | 1.0 | 6 |
| 9 | TFE | 4 | 0 | 1.0 | 64 |
| 10 | TFE | 4 | 40 | 1.0 | 75, (65) c |
| 11 | TFE | 4 | 60 | 1.0 | 70 |
| 12 | TFE | 2 | 40 | 1.0 | 70 |
| 13 | TFE | 6 | 40 | 1.0 | 68 |
| 14 | TFE | 4 | 40 | 0.8 | 68 |
| 15 | TFE | 4 | 40 | 1.2 | 58 |
 | ||
|---|---|---|
| Entry | Additive (5 wt%) | Yield b (%) |
| 1 | 4 Å MS | 47 |
| 2 | CH3COOH | 57 |
| 3 | Pyridine | 63 |
| 4 | K2CO3 | 49 |
| 5 | Zn(OAc)2·2H2O | 63 |
| 6 | Cu(OAc)2·2H2O | 64 |
| 7 | Fe(OAc)2 | 72 |
 | |||
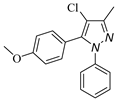
 | 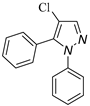 |  | 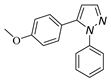 |
| 3a, 75% b | 3b, 0 | 3b′, 25% | 3c′, 45% |
 |  | 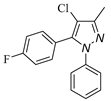 |  |
| 3d, 59% | 3e, 40% | 3f, 40% | 3g, 43% |
 |  |  | 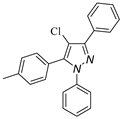 |
| 3h, 45% | 3i, 92% | 3j, 72% | 3k, 15% |
 |  |  |  |
| 3l, 18% | 3m, 18% | 3n, 25% | 3o, 15% |
 | ||
 |  |  |
| 4a, 45% b | 4b, 48% | 4c, 40% |
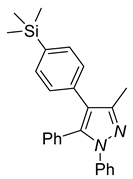 |  | 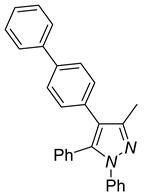 |
| 4d, 86% | 4e, 37% | 4f, 58% |
 |  |  |
| 4g, 0% | 4h, 0% | 4i, 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.; Ma, C.; Hu, L.; Xiong, Y. Direct Cyclization/Chlorination Strategy of Hydrazines for Synthesis of 4-Chloropyrazoles by TCCA. Molecules 2025, 30, 3841. https://doi.org/10.3390/molecules30193841
Deng Q, Ma C, Hu L, Xiong Y. Direct Cyclization/Chlorination Strategy of Hydrazines for Synthesis of 4-Chloropyrazoles by TCCA. Molecules. 2025; 30(19):3841. https://doi.org/10.3390/molecules30193841
Chicago/Turabian StyleDeng, Qingfu, Chenglong Ma, Liangzhen Hu, and Yan Xiong. 2025. "Direct Cyclization/Chlorination Strategy of Hydrazines for Synthesis of 4-Chloropyrazoles by TCCA" Molecules 30, no. 19: 3841. https://doi.org/10.3390/molecules30193841
APA StyleDeng, Q., Ma, C., Hu, L., & Xiong, Y. (2025). Direct Cyclization/Chlorination Strategy of Hydrazines for Synthesis of 4-Chloropyrazoles by TCCA. Molecules, 30(19), 3841. https://doi.org/10.3390/molecules30193841







