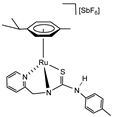
Abstract
Pyridyl-thiourea complexes of formula [(Cym)MCl(κ2Npy,S-H2NNS)][SbF6] (Cym = η6-p-MeC6H4iPr; H2NNS = N-(p-tolyl)-N′-(2-pyridylmethyl)thiourea); M = Ru (1), Os (2)) were synthesized by reacting the corresponding metal dimers [{(Cym)MCl}2(μ-Cl)2] with H2NNS in the presence of NaSbF6. Subsequent chloride abstraction with AgSbF6, followed by NH deprotonation using NaHCO3, afforded the cationic complexes [(Cym)M(κ3Npy,Namide,S-HNNS)][SbF6] (M = Ru (5a), (5c); M = Os (6a, 6c)) and [(Cym)M(κ2Namide,S-HNNS)][SbF6] (M = Ru (5b); M = Os (6b)). The proposed structures for the prepared compounds are based on NMR data. Complexes 5a, 5b, and 6a, 6b evolve to the thermodynamically more stable species 5c and 6c, respectively, in which the deprotonated ligand HNNS adopts a κ3Npy,Namide,S coordination mode. Complexes 5c and 6c activate H2, behaving as frustrated Lewis pair (FLP) species, and catalyze (5c and/or 6c) the hydrogenation of polar multiple bonds, including the C=N bonds of N-benzylideneaniline and quinoline, the C=C bond of methyl acrylate, and the C=O bond of 2,2,2-trifluoroacetophenone.
1. Introduction
There are known examples of intra- and intermolecular combinations of Lewis acids and bases that, in solution, for steric, electronic reasons, or both, do not form the corresponding Lewis adducts. These acid-base pairs are referred to as frustrated Lewis pairs (FLP). A milestone in the development of such systems was the discovery in 2006 by Stephan and coworkers that such species, in which no metal was present, were capable of activating the hydrogen molecule heterolytically and reversibly under mild conditions [1]. A few years later, it was found that the acidic and basic components of FLPs could activate many other small (CO2, CO, SO2, N2O, NO) and organic (olefins, alkynes) molecules in a cooperative and concerted manner, following new reaction pathways [2,3,4,5,6,7,8,9,10,11,12].
Much less developed, but becoming increasingly important, are FLPs in which one of the two FLP components is a transition metal fragment. Pioneering work from Wass [13] and Erker’s [14] groups on zirconium-phosphane combinations was shortly followed by notable metal examples, demonstrating the potential of transition-metal frustrated Lewis pairs (TMFLPs) in small molecule activation and catalysis. Introducing a transition metal into the system provides the FLP with greater structural diversity, enabling access to transition metal fundamental catalytic reactions [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31].
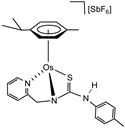
In particular, we have recently prepared the phosphane-guanidinate, pyridyl-guanidinate, and phosphane-thiourea complexes of ruthenium and osmium [32,33,34] in which the ligands adopt a fac coordination (Figure 1). This coordination forces the central nitrogen atom (N1) to take on sp3 hybridization. Under these conditions, the four-membered cycles M−N1−C−N2 and M−N1−C−S support a strong ring strain that can be relaxed upon breaking the M−N1 (thiourea complexes) or M−N2 bond (guanidinate complexes), giving rise to active FLP species. However, in this context, in the course of the study of the catalytic activity in hydrogenation reactions, we detected that in the pyridyl-guanidinate complexes, the presence of protons in the 2 and 6 positions of the p-tolyl substituent gives rise to metallation reactions that preclude FLP reactivity, due to the change in the metalacycle from four members into a five-membered M−N1−C−N2−C [32].
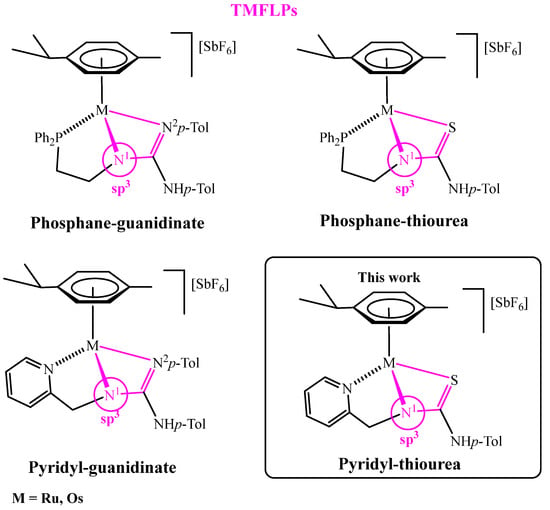
Figure 1.
TMFLPs compounds based on phosphane-guanidinate, pyridyl-guanidinate, and phosphane-thiourea ligands.
Taking these aspects into consideration, in this work we report the synthesis and characterization of pyridyl-based complexes in which the guanidine moiety (N2p-Tol) of the previously studied tridentate ligand has been replaced by a more robust thiourea fragment (Figure 1) [35,36,37,38]. A characteristic feature of these compounds—whether bearing the neutral H2NNS or the anionic HNNS pyridyl-thiourea ligand—is the existence of a dynamic equilibrium between molecular species exhibiting distinct coordination modes (Figure 2). When the deprotonated monoanionic ligand HNNS adopts a κ3Npy,Namide,S coordination mode (as in complexes 5c and 6c), the resulting M−Namide−C−S four-membered ring can undergo structural relaxation via cleavage of the metal–amide nitrogen bond. This process leads to the generation of frustrated Lewis pair (FLP) species. The FLP behaviour of complexes 5c and 6c has been investigated in the context of hydrogenation catalysis, demonstrating their ability to heterolytically activate molecular hydrogen and promote the reduction in unsaturated substrates.
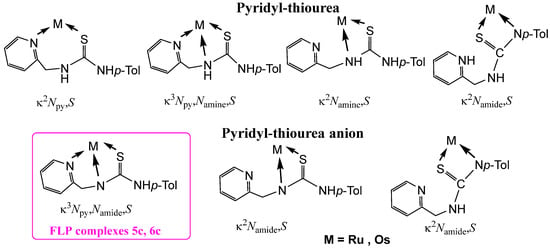
Figure 2.
Coordination modes of pyridyl-thiourea ligands in the work.
2. Results and Discussion
2.1. Synthesis of the Ligand
The pyridyl-thiourea ligand H2NNS has been prepared in high yield by reacting 2-pyridylmethanamine with p-tolyl-isothiocyanate in dry THF (Scheme 1) following literature procedures (see Materials and Methods) [32,33,34,39].
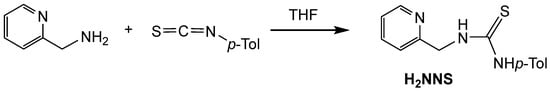
Scheme 1.
Preparation of H2NNS ligand.
2.2. Synthesis of the Chlorido Complexes [(Cym)MCl(κ2Npy,S-H2NNS)][SbF6] (M = Ru (1), Os (2))
The chlorido complexes 1 and 2 were prepared, with a yield of 96% (1) and 90% (2), by treating the dimers [{(Cym)MCl}2(μ-Cl)2] [40,41] with stoichiometric amounts of the ligand H2NNS in methanol in the presence of NaSbF6 (Scheme 2).
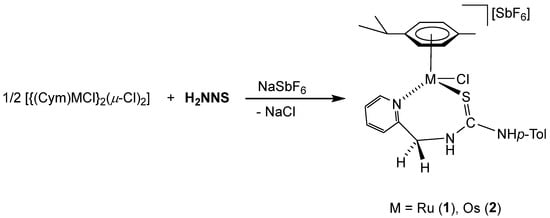
Scheme 2.
Preparation of complexes 1 and 2.
The complexes were characterised by analytical and spectroscopic means (see Materials and Methods). Two-dimensional homonuclear and heteronuclear correlations verified the assignment of the NMR signals. At this point, it should be noted that for compound 1, the presence in the 1H NMR spectra of only one species has been observed in CD2Cl2, (CD3)2CO, and CD3OD. However, for the compound 2 in CD2Cl2, signals corresponding to four species are identified, and these evolve to a unique set of signals in more polar solvents, such as (CD3)2CO and CD3OD. This behaviour is consistent with a change in the coordination mode of the H2NNS ligand. The mass spectrum of ruthenium compound 1 agrees with the structure shown in Scheme 2, where the chlorine atom is coordinated to the ruthenium centre. The similarity of the NMR spectra of compounds 1 and 2 in polar solvents allows us to propose the same structure for the two compounds (Scheme 2). Coordination of the pyridyl nitrogen to the metal in complexes is supported by a strong deshielding of the H6 proton of the pyridyl moiety, from 8.44 (free ligand) to 9.52 (complex 1) and 9.42 ppm (complex 2). Additionally, in a similar way to the related ruthenium and osmium phosphane-thiourea complexes published by us [32], the coordination through the sulphur atom in both complexes gives rise to the seven-membered metallacycles. The metal is a stereogenic centre, and accordingly, the methylene hydrogens are diastereotopic and resonate as a pair of signals in each case, 5.69 and 4.79 ppm (complex 1) and 5.65 and 4.74 ppm (complex 2) (see Materials and Methods).
2.3. Synthesis of Dicationic Ruthenium Complexes 3
A mixture of dicationic complexes 3a–3e was obtained by treating the chlorido complex 1 with AgSbF6, in acetone. The 1H NMR spectrum of the isolated solid in (CD3)2CO shows the presence of two major compounds, 3a (39%) and 3b (42%), together with three minor compounds, 3c–3e (19%) (Scheme 3, Materials and Methods and Supplementary Materials). The presence of trace amounts of water in the solvent is sufficient for 3a aqua-complex to form. By variable temperature 1H NMR in (CD3)2CO we studied the behaviour in solution. At −70 °C, the spectrum showed the presence of the five compounds 3a (47%), 3b (34%), and 3c–3e (19%). When the temperature was raised to 50 °C, the proportion of compound 3a decreased at the expense of 3b (3a (15%), 3b (66%), and 3c–3e (19%)) (Scheme 3). In addition, when 100 μL of NCMe are added to the mixture of five compounds in (CD3)2CO, the formation of a new compound with NCMe coordinated of stoichiometry [(Cym)Ru(NCMe)(κ2Npy,S-H2NNS)][SbF6]2 (3f, 95% abundance) is essentially observed (Scheme 3). This behaviour indicates the presence of different dicationic species (3a–3e) according to different forms of coordination κ2 or κ3 of the H2NNS ligand and the coordination or not of the solvent molecules, such as H2O [32,33].
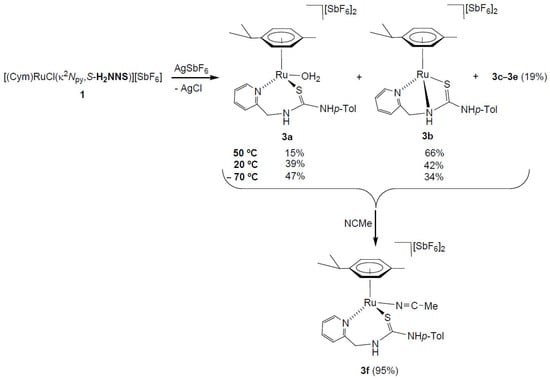
Scheme 3.
Preparation of dicationic ruthenium complexes 3.
For the major compounds 3a and 3b we propose the structures drawn in Scheme 3, based on related ruthenium complexes with pyridyl-guanidine [33] [(Cym)Ru(H2O)(κ2N,N-H2NNN)][SbF6]2 and phosphane-thiourea [32] [(Cym)Ru(κ3P,N,S-H2PNS)][SbF6]2 ligands, informed previously by us. A significant difference between these two compounds is the chemical shift of the methylene carbon CH2, 44.25 ppm in 3a and 61.40 ppm in 3b. The chemical shifts of the methylene carbons of complexes prepared in this work resonate in the 61.20–63.08 ppm range when the ligand is κ3 coordinated and at a lower chemical shift (44.25–53.24 ppm) when it is κ2 coordinated. Most probably, in complex 3b, where the sp3 nitrogen is coordinated to the metal, it shares a lower electron density with the methylene carbon.
2.4. Synthesis of the Dicationic Complexes [(Cym)M(NCMe)(κ2Npy,S-H2NNS)][SbF6]2 (M = Ru (3f), Os (4a))
The chloride ligand in complexes 1 and 2 was eliminated as AgCl by treatment with AgSbF6 in NCMe. The presence of NCMe allows the isolation of the dicationic complexes [(Cym)M(NCMe)(κ2Npy,S-H2NNS)][SbF6]2 (M = Ru (3f), Os (4a)) in high yield (Scheme 4).
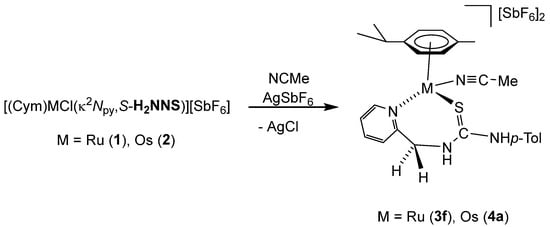
Scheme 4.
Synthesis of complexes 3f and 4a.
Complexes 3f and 4a were characterised by analytical and spectroscopic means (see Materials and Methods). In proton NMR, strong deshielding was observed for the H6 proton of the pyridine moiety, δH6 = 9.17 ppm (3f) and 9.12 ppm (4a). The NH bonded to the p-tolyl group resonates at 8.22 ppm (3f) and 8.16 ppm (4a), and the NH bonded to the methylene group resonates as a broad singlet at 7.13 ppm (3f) and as a broad triplet at 7.24 ppm (4a), due to the coupling with the CH2 protons. The observed NOEs are in agreement with the proposed structure, where the ligand is κ2 coordinated through the pyridyl N and S atoms.
2.5. Synthesis of the Monocationic Ruthenium Complexes 5
The addition of solid NaHCO3 to a solution of a mixture of complexes 3a–3e in methanol (Scheme 5) results in the formation of the monocationic complexes 5a–5c in which the coordinated ligand H2NNS is monodeprotonated.
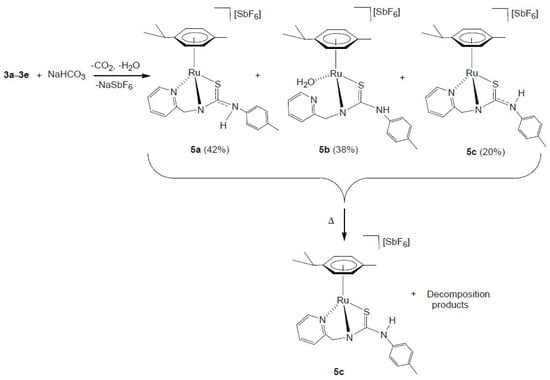
Scheme 5.
Synthesis of the monocationic ruthenium complexes 5.
The initial proportion of complexes 5a/5b/5c (42/38/20 molar ratio) changes to 27/24/49, with a 13% decomposition of the products, after maintaining the acetone solution for 5 h at 50 °C. Reacting the mixture of complexes 5a–5c with H2 at RT, in THF-d8/D2O, gives the compound 5c pure (see below).
Complexes 5a–5c were characterised by analytical and spectroscopic means (see Materials and Methods and Supplementary Materials). Coordination of ligand by the pyridyl nitrogen in complexes 5a and 5c is supported by the deshielding of the H6 proton of the pyridyl moiety to 9.28 (complex 5a) and 9.05 ppm (complex 5c) and can be assessed through NOE experiments (Figure 3). In complex 5b, the chemical shift of H6 from the pyridyl group, together with the no NOE observed between this proton and the p-cymene group, indicates that the pyridyl moiety is not coordinated to ruthenium. In addition, NOE was observed between the CH2 protons and the H5 of the pyridyl moiety (Figure 3). The absence of chemical coupling between CH2 and NH protons indicates that NH is bonded to the p-tolyl group in the three complexes 5a–5c.
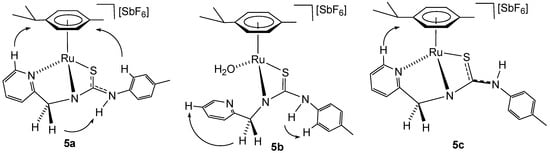
Figure 3.
Selected NOE for the complexes 5a–5c.
All this data support the proposed structures for compounds 5a and 5c, in which the pyridyl-thiourea HNNS ligand is coordinated in a κ3 fashion, whereas in compound 5b, a κ2 coordination mode involving the amide nitrogen and sulphur atoms is observed. A water molecule likely completes the coordination sphere of the metal centre in 5b. This interpretation is consistent with the chemical shifts observed in the 13C NMR spectra for the methylene carbon; compounds 5a and 5c display similar resonances at 62.21 ppm and 61.20 ppm, respectively, while compound 5b shows a signal significantly upfield at 48.38 ppm.
We propose that compounds 5a and 5c exist as two distinct rotamers around the C(S)–N(H) bond, separated by a relatively high rotational energy barrier. This is consistent with the sharp signals observed in the NMR spectra for 5a and 5c, indicative of slow exchange on the NMR timescale. Additional support for this structural assignment comes from NOE experiments. Only in compound 5a, an NOE between the aromatic proton of the p-tolyl group and the p-cymene ligand is observed (Figure 3). These findings agree with previously reported DFT calculations on structurally related ruthenium complexes [32,33]. For example, in the case of [(Cym)Ru(κ3P,N,S-HPNS)][SbF6], containing a phosphane-thiourea ligand [32], a fluxional behaviour is observed in solution. The C(S)–N(H) bond in this complex exhibits an intermediate bond length between a typical single and double bond, and two conformers are detected in the NMR spectra. Similarly, in one of the crystallographically characterised conformers of [(Cym)RuCl(κ2N,N-H2NNN)][SbF6] with a pyridyl-guanidine ligand [33]. the C–N bond also displays a partial double-bond character, further reinforcing the hypothesis of restricted rotation and conformational stability in these types of complexes.
2.6. Syntheses of the Monocationic Osmium Complexes 6
Osmium compound [(Cym)OsCl(κ2Npy,S-H2NNS)][SbF6] (2) reacts with AgSbF6 in acetone, rendering a mixture of the monocationic complexes [(Cym)Os(κ3Npy,Namide,S-HNNS)][SbF6] (6a and 6c) and the dicationic complex [(Cym)Os(H2O)(κ2Namide,S-H2NNS)][SbF6]2 (4b). Addition of NaHCO3 to the mixture yields the monocationic complexes 6a-6c. The experimental data support the structural formulations (Scheme 6).
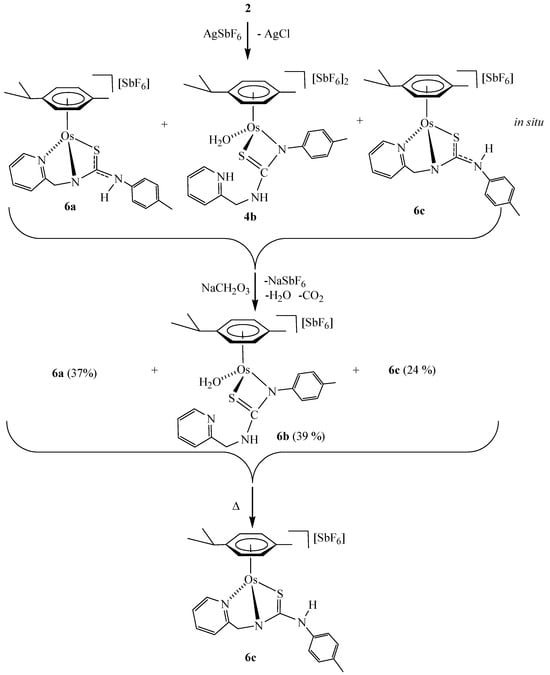
Scheme 6.
Synthesis of the monocationic osmium complexes 6.
The initial proportion of complexes 6a/6b/6c (37/39/24 molar ratio) changes to 12/16/72, after maintaining the methanol solution at 60 °C for 24 h. The lower solubility of compound 6c in methanol allowed isolating it as a yellow solid with 98% purity (see Materials and Methods).
Osmium complexes 6a–6c were characterised by analytical and spectroscopic means. Spectroscopic data indicate that compounds 6a and 6c show a similar structure to ruthenium compounds 5a and 5c, respectively. Coordination of the pyridyl nitrogen of the ligand at the metal in complexes 6a and 6c is supported by a deshielding of the H6 proton of the pyridyl moiety to 9.00 (complex 6a) and 8.87 ppm (complex 6c). In complex 6b, the chemical shift of H6 from the pyridyl group (8.39 ppm) indicates that the pyridyl moiety is not coordinated to osmium, and the NH group resonates as a broad triplet at 4.73 ppm, indicating that the NH is bonded to the methylene group. The chemical shift of the methylene carbon 13C NMR in compounds 6a, 6b, and 6c is 62.46, 46.09, and 62.66 ppm, respectively. The most representative observed NOEs are shown in Figure 4, which are in agreement with the proposed structures.
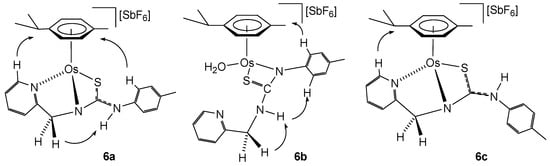
Figure 4.
Selected NOE for the complexes 6a–6c.
At this point, it should be noted that the 1H NMR spectrum of osmium compound 4b shows the same signals as 6b except for the NH and H6 of the pyridinium group in 4b. For compound 4b, a broad 1H singlet at 12.45 ppm coupled with H6 is attributed to the NH functionality of the pyridinium group. Consequently, the H6 of the pyridinium group resonates at 8.35 ppm as a pseudo triplet (J = 5.0 Hz).
Additionally, the reaction for the formation of compound 4a from 6a–6c in the presence of HSbF6 and NCMe was carried out, as well as the formation of 6a–6c from 4a in methanol in the presence of NaHCO3 (Scheme 7).
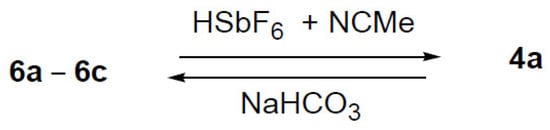
Scheme 7.
Conversion reactions between 4a and 6a–6c.
Concerning the observed equilibria and structural diversity in complexes 5 and 6, the greater lability of the pyridyl–metal bond in the pyridyl-thiourea complexes, compared to the related pyridyl-guanidinate analogues previously reported [32,33,34], may be attributed to the stronger M–S bond in the thiourea moiety, which can reinforce coordination to the metal centre and disfavour simultaneous tight binding of all three donor atoms (Figure 5). This difference in bonding behaviour appears to facilitate reversible coordination-decoordination processes of the pyridyl group, which are not observed in the more rigid pyridyl-guanidinate systems. This hemilability plays a critical role in the formation of frustrated Lewis pair species. At this point, it is necessary to point out that some FLP systems can show their activity even if the classical Lewis acid–base adduct is stable, provided that the dissociated form is thermally accessible. Such a type of FLP is denominated as a masked FLP [26].
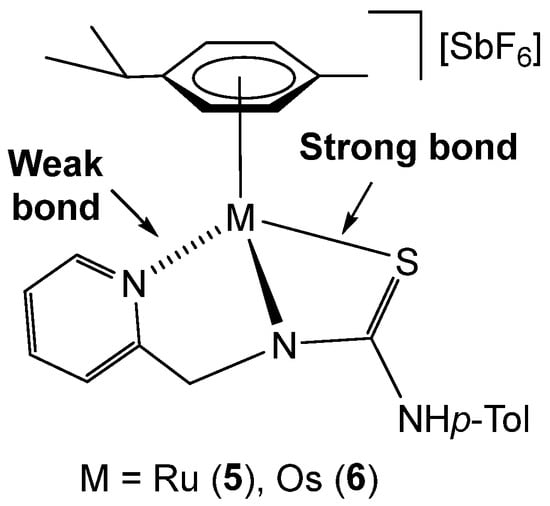
Figure 5.
Relative bond strength in complexes 5 and 6.
2.7. Catalytic Hydrogenation Assays
The chemical behaviour of complexes 5 and 6, previously discussed, allows us to have complexes 5c and 6c that show structural characteristics of FLP. This is a consequence of the strained four-membered M−N−C−S metallacycle in the fac κ3N,N,S coordination mode of the ligand. This metallacycle can be opened, giving rise to FLP species, in which the metal and nitrogen atoms play the roles of Lewis acid and Lewis base components, respectively, activating the hydrogen molecule. Complexes 5 and 6 have been tested as catalysts in the hydrogenation of benchmark substrates such as the C=C double bond in the methyl acrylate [42,43], the C=O bond in the 2,2,2-trifluoroacetophenone [44,45,46,47,48] and the C=N bond in the N-benzylideneaniline and quinoline [44,46,49]. It should be noted that similar results are obtained using mixtures of complexes 5 and 6, or pure complexes 5c and 6c. The hydrogenation reactions were carried out at 60 °C, in THF-d8 with a catalyst/substrate molar ratio of 1/20 under 5 bar of H2. For ruthenium complexes 5, from 2.5 to 9 days were needed to achieve complete conversion in the hydrogenation of methyl acrylate, N-benzylideneaniline, and quinoline (Table 1, entries 1–3). For the substrate 2,2,2-trifluoroacetophenone, no quantitative conversions are achieved, and a decrease in the rate of the reaction is observed while free p-cymene is progressively formed due to catalyst decomposition (Table 1, entry 4). The osmium complex 6c showed lower activity than complex 5c (Table 1, entry 6), and in a similar way to the ruthenium catalyst, no quantitative conversions are obtained with substrates with carbonyl groups (Table 1, entries 5 and 7). Only complexes 5 or 6 were detected under catalytic conditions, indicating that catalyst hydrogenation is the slow step in the catalytic cycle, being the resting state 5c or 6c.

Table 1.
Hydrogenation of unsaturated substrates 1.
When we explored the reactivity of complexes 5 and 6c with molecular hydrogen (5 bar, 60 °C) in THF-d8, the corresponding hydride complex [(Cym)MH(κ2Npy,S-H2NNS)][SbF6] was not observed. Then, we tested the isotopic reaction exchange from H2 to HD in the presence of D2O as a deuterium source. Effectively, at RT, this exchange reaction is observed when compounds 5 and 6c are exposed to 5 bar of H2 in a mixture of THF-d8/D2O (0.35 mL/0.10 mL) for 30 min and 5 days, respectively. The appearance of HD implies that the activation of H2, leading to the formation of hydride complexes [(Cym)MH(κ2Npy,S-H2NNS)][SbF6], is a reversible reaction, and the equilibrium is strongly shifted towards the starting complexes (Scheme 8). Furthermore, as we have previously mentioned, the most stable compound 5c is obtained purely by reacting mixture 5a–5c in THF-d8/D2O, 0.35 mL/0.1 mL, with H2 at RT for 5 days. Most probably, complex 5 reacts with H2 to give the hydride species, and this favours the formation of 5c complex at the expense of 5a and 5b (Scheme 8).
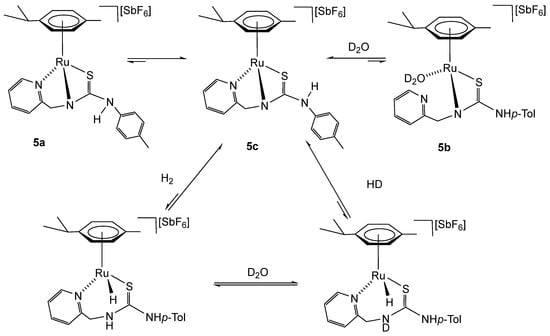
Scheme 8.
Reaction with H2 of complexes 5a–5c in THF-d8/D2O.
The lability of the pyridyl–metal bond and the resulting formation of species such as 5b, featuring a κ2 coordinated ligand, may account for the progressive decomposition of the catalyst over time and thus, for the relatively slow hydrogenation rates observed under catalytic conditions. Accordingly, species with carbonyl groups are well suited to compete with the pyridine group in the coordination to the metal and modify the catalyst (Table 1, entries 4, 5, and 7). The characterisation of compounds 5b and 6b provides further insight into the delicate equilibrium between different coordination modes and highlights that FLP behaviour in these systems arises from a finely tuned balance of bond strengths between the metal centre and the three donor atoms of the ligand in a facial κ3 arrangement. Slight deviations in this balance, particularly a weakening of the metal–pyridyl interaction, can shift the complex toward less stable κ2 species, which compromises both catalytic activity and long-term catalyst stability. These findings underscore the importance of ligand design in TMFLP systems, where controlled hemilability can promote cooperative reactivity, but excessive flexibility may lead to ligand decoordination and catalyst degradation.
3. Conclusions
A key feature of ruthenium and osmium complexes bearing pyridyl-thiourea ligands is the remarkable versatility in coordination modes exhibited by the H2NNS and HNNS ligands. These ligands can bind to the metal centre in several fashions, including κ2Npy,S (complexes 1, 2, 3a, 3f, 4a), κ3Npy,Namine,S (complex 3b), κ3Npy,Namide,S (complexes 5a, 5c, 6a, 6c) and κ2Namide,S (complexes 4b, 5b, 6b). Among these, complexes [(Cym)M(κ3Npy,Namide,S-HNNS)][SbF6] (M = Ru (5c), Os (6c)), in which the ligand adopts a facial κ3 coordination mode, have demonstrated the ability to heterolytically activate molecular hydrogen, behaving as transition-metal frustrated Lewis pairs. These species catalyse the hydrogenation of polar unsaturated bonds, including C=N bonds in N-benzylideneaniline and quinoline, C=C bonds in methyl acrylate, and C=O bonds in 2,2,2-trifluoroacetophenone. Notably, both the catalytic performance and stability of these complexes are strongly influenced by the hemilability of the pyridyl–metal bond, which plays a pivotal role in generating the active FLP form. This dynamic coordination behaviour appears to be crucial for efficient hydrogen activation and effective substrate conversion.
4. Materials and Methods
All preparations have been carried out under argon. All solvents were treated in a PS-400–6 Innovative Technologies Solvent Purification System (SPS, Innovative Technologies, Wilmington, DE, USA) and degassed before use. Infrared spectra were recorded on a Perkin-Elmer Spectrum-100 (ATR mode) FT-IR spectrometer. Carbon, hydrogen, nitrogen, and sulphur analyses were performed using a Perkin-Elmer 240 B microanalyser (Perkin-Elmer, Shelton, CT, USA). 1H and 13C NMR spectra were recorded on a Bruker AV-300 spectrometer (300.13 MHz), Bruker AV-400 (400.16 MHz) or Bruker AV-500 (500.13 MHz) (Bruker, Billerica, MA, USA). In both 1H NMR and 13C NMR measurements, the chemical shifts are expressed in ppm downfield from SiMe4. J values are given in Hz (the coupling constants are values measured experimentally, accordingly, the difference observed between the values of the constants of the hydrogens in the pyridyl group). NOESY, 13C, and 1H correlation spectra were obtained using standard procedures. Mass spectra were obtained with a Micro Tof-Q Bruker Daltonics spectrometer (Bruker, Billerica, MA, USA).
4.1. Preparation of H2NNS
At RT, a mixture of 2-pyridylmethanamine (1000.0 mg, 9.26 mmol) and p-tolyl-isothiocyanate (1151.4 μL, 9.26 mmol) in dry THF (10 mL) was stirred for 15 h. During this time, a white solid precipitated, and the THF was removed by vacuum filtration. The resulting white solid was washed with n-hexane (3 × 5 mL) and vacuum-dried.
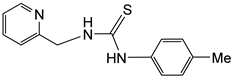
H2NNS. Yield: 2268 mg, 95%. Anal. Calcd for C14H15N3S: C, 65.34; H, 5.87; N, 16.33; S, 12.46. Found: C, 65.20; H, 5.79; N, 16.20; S, 12.34. HRMS (μ-TOF), C14H16N3S, [M + H]+, calcd: 258.1059, found: 258.1068. IR (cm−1): ν(NH) 3323 (m). 1H NMR (500.10 MHz, CD2Cl2, RT): δ = 8.44 (bs, 1H, H6 Py), 8.03 (bs, 1H, NHp-Tol), 7.69 (t, J = 7.8 Hz, 1H, H4 Py), 7.65 (bs, 1H, NHCH2), 7.31 (d, J = 7.8 Hz, 1H, H3 Py), 7.26 (d, J = 8.3 Hz, 2H, CH p-Tol), 7.22 (d, 2H, CH p-Tol), 7.21 (t, J = 7.5 Hz, 1H, H5 Py), 4.89 (bs, 2H, CH2) and 2.38 (s, 3H, Me). 13C{1H} NMR (125.77 MHz, CD2Cl2, RT): δ = 181.09 (C=S), 156.60 (C2 Py), 149.54 (C6 Py), 137.54 (C4 Py), 136.55, 133.89, 131.22, 125.50 (CH p-Tol), 123.19 (C5 Py), 122.71 (C3 Py), 50.60 (CH2) and 21.54 (Me).
4.2. Preparation of the Complexes [(Cym)MCl(κ2Npy,S-H2NNS)][SbF6] (M = Ru (1), Os (2)
To a suspension of the corresponding dimer [{(Cym)MCl}2(μ-Cl)2] (0.5 mmol) in methanol (10 mL), 257.3 mg (1.0 mmol) of the H2NNS ligand and 258.7 mg (1.0 mmol) of NaSbF6 were added. The resulting solution was stirred for 5 h and vacuum-evaporated to dryness. The residue was extracted with dichloromethane, and the solution was concentrated under reduced pressure to ca. 2 mL. The slow addition of n-hexane led to the precipitation of orange (1) or yellow-brown (2) solids, which were washed with n-hexane (3 × 10 mL) and vacuum-dried.
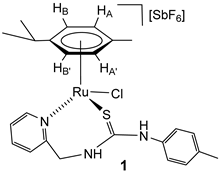
Complex 1. Yield: 733 mg, 96%. Anal. Calcd for C24H29N3ClF6RuSSb: C, 37.74; H, 3.83; N, 5.50; S, 4.20. Found: C, 38.11; H, 3.78; N, 5.69; S, 4.27. HRMS (μ-TOF), C24H29N3ClRuS, [M − SbF6]+, calcd: 528.0809, found: 528.0790. IR (cm−1): ν(NH) 3340–3195 (br), ν(SbF6) 651 (s). 1H NMR (500.10 MHz, (CD3)2CO, RT): δ = 9.81 (s, 1H, NHp-Tol), 9.52 (d, J = 5.8 Hz, 1H, H6 Py), 8.36 (bt,1H, NHCH2), 8.06, (td, J = 7.6, J = 1.6 Hz, 1H, H4 Py), 7.62 (dd, J = 7.4 Hz, J = 1.4, 1H, H3 Py), 7.61 (td, J = 7.5, J = 1.5 Hz 1H, H5 Py), 7.25 (d, J = 8.2 Hz, 2H, CHCMe p-Tol), 7.16 (dd, J = 1.6 Hz, 2H, CHCN p-Tol), 5.96 (d, 2H, HB, HB’), 5.80 (d, J = 6.1 Hz, 1H, HA), 5.68 (d, J = 6.1 Hz, 1H, HA’), 5.69 (overlapped, CHH), 4.79 (dd, J = 14.4 Hz, J = 4.9 Hz, 1H, CHH), 3.01 (sp, 1H, CH iPr), 2.35 (s, 3H, Me p-Tol), 1.92 (s, 3H, Me Cym), 1.36 and 1.35 (2 × d, J = 6.9 Hz, 6H, Me iPr).13C NMR (125.77 MHz, (CD3)2CO, RT): δ = 178.49 (C=S), 160.50 (C2 Py), 160.37 (C6 Py), 141.34 (C4 Py), 131.98 (CHCMe p-Tol), 127.97 (C3 Py), 126.91 (CHCN p-Tol), 126.42 (C5 Py), 105.71 (CiPr), 102.99 (CMe Cym), 90.00 (CHB), 787.25 (CHA’, CHB’), 85.53 (CHA’), 53.24 (CH2), 32.05 (CH iPr), 23.14, 23.10 (Me iPr), 21.69 (Me p-Tol) and 18.57 (Me Cym).
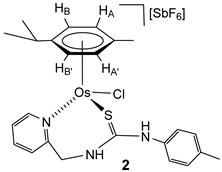
Complex 2. Yield: 768 mg, 90%. Anal. Calcd for C24H29N3ClF6OsSSb: C, 33.8; H, 3.4; N, 4.95; S, 3.8. Found: C, 33.7; H, 3.7; N, 5.0; S, 4.0. HRMS (μ-TOF), C24H28N3OsS, [M − H − Cl − SbF6]+, calcd: 582.1612, found: 582.1622. IR (cm−1): ν(NH) 3400–3000 (br), ν(SbF6) 653 (s). 1H NMR (500.10 MHz, (CD3)2CO, RT (NH resonances at −80 °C)): δ = 9.70 (s, 1H, NHp-Tol), 9.42 (d, J = 6.0 Hz, 1H, H6 Py), 8.30 (bt,1H, NHCH2), 8.00, (t, J = 7.5 Hz, 1H, H4 Py), 7.63 (d, J = 7.5 Hz, 1H, H3 Py), 7.56 (t, J = 7.1 Hz 1H, H5 Py), 7.25, 7.20 (2 × d, J = 8.4 Hz, 4H, CH p-Tol), 6.21 (d, J = 5.8 Hz, 2H, HB, HB’), 6.12 (d,1H, HA’), 5.87 (d,1H, HA), 5.65 (d, J = 14.5 Hz, 1H, CHHpro-S), 4.74 (d, 1H, CHHpro-R), 2.89 (spt, 1H, CH iPr), 2.35 (s, 3H, Me p-Tol), 1.92 (s, 3H, Me Cym), 1.36 and 1.32 (2 × d, J = 6.8 Hz, 6H, Me iPr).13C NMR (125.77 MHz, (CD3)2CO, RT): δ = 177.92 (C=S), 161.06 (C6 Py), 159.43 (C2 Py), 141.45 (C4 Py), 139.00, 134.76, 131.84 (CH p-Tol), 127.60 (C3 Py), 126.81 (CH p-Tol), 126.73 (C5 Py), 96.67 (CMe Cym), 94.85 (CiPr), 82.51 (CHB), 78.98 (CHA), (CHB’), 77.04 (CHA’), 53.20 (CH2), 32.06 (CH iPr), 23.39, 23.28 (Me iPr), 21.68 (Me p-Tol) and 18.37 (Me Cym).
4.3. Preparation of the Complexes 3
To a solution of the complex [(Cym)RuCl(κ2Npy,S-H2NNS)][SbF6] (1) (550.0 mg, 0.720 mmol) in 10 mL of acetone was added 259.3 mg (0.756 mmol) of AgSbF6. The resulting suspension was stirred for 2 h. The AgCl formed was separated via cannula, and the filtrate was concentrated under pressure to ca. 2 mL. The slow addition of n-hexane led to the precipitation of an orange solid, which was washed with n-hexane (3 × 10 mL) and vacuum-dried. A mixture of [(Cym)Ru(H2O)(κ2Npy,S-H2NNS)][SbF6]2 (3a, 39%), [(Cym)Ru(κ3Npy,Namine,S-H2NNS)][SbF6]2 (3b, 42%), 3c (7%), 3d (6%) and 3e (6%), was obtained. Addition of 100 μL acetonitrile to 15 mg of the solid obtained dissolved in (CD3)2CO results in the formation of the 3f complex.
Preparation of [(Cym)Ru(NCMe)(κ2Npy,S-H2NNS)][SbF6]2 (3f). To a solution of the complex [(Cym)RuCl(κ2Npy,S-H2NNS)][SbF6] (1) (75.0 mg, 0.098 mmol) in 10 mL of NCMe was added 33.7 mg (0.098 mmol) of AgSbF6. The resulting suspension was stirred for 2 h. The AgCl formed was separated with a cannula, and the filtrate was concentrated under reduced pressure to ca. 2 mL. The slow addition of n-hexane led to the precipitation of a yellow solid, which was washed with n-hexane (3 × 5 mL) and vacuum-dried.
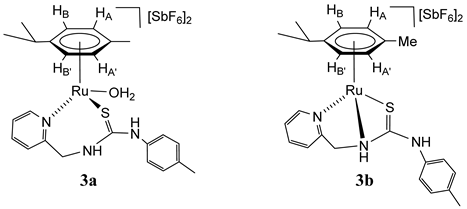
Complex 3. Yield: 640 mg, 92% (assuming the solid as 3b). HRMS (μ-TOF), C24H28N3RuS, [M − H − 2 SbF6]+ and [M − H2O − H − 2 SbF6]+, calcd: 492.1042, found: 492.1059. IR (cm−1): ν(NH) 3630–3090 (br), ν(SbF6) 652 (s). 3a. 1H NMR (500.10 MHz, (CD3)2CO, RT): δ = 9.12 (d, J = 5.7 Hz, 1H, H6 Py), 8.91 (t, J = 7.9 Hz, 1H, H4 Py), 8.31 (t, J = 6.8 Hz, 1H, H5 Py), 8.26 (overlapped, 1H, H3 Py), 7.47 (d, J = 8.5 Hz, 2H, CHCN p-Tol), 7.39 (d, 2H, CHCMe p-Tol), 6.41 (d, J = 6.0 Hz, 1H, HB), 6.02 (d, J = 6.0 Hz, 2H, HA, HB’), 5.82 (d, 1H, HA’), 5.38 (d, J = 16.5 Hz, 1H, CHHpro-S), 4.81 (d, 1H, CHHpro-R), 2.94 (sp, 1H, CH iPr), 2.39 (s, 3H, Me p-Tol), 2.28 (s, 3H, Me Cym), 1.35 and 1.34 (2 × d, J = 6.9 Hz, 6H, Me iPr). 13C NMR (125.77 MHz, THF-d8, RT): δ = 169.03 (C=S), 150.28 (C4 Py), 144.01 (C6 Py), 142.65 (C3 Py), 143.72 (CN p-Tol), 139.64 (CMe p-Tol), 132.08 (CHCMe p-Tol), 129.00 (C5 Py), 124.38 (CHCN p-Tol), 109.43 (CiPr), 105.86 (CMe Cym), 86.23, 85.24 (CHA, CHB’), 86.08 (CHA’), 86.06 (CHB), 44.25 (CH2), 33.03 (CH iPr), 24.11, 23.28 (Me iPr), 21.87 (Me p-Tol) and 19.45 (Me Cym). 3b. 1H NMR (500.10 MHz, (CD3)2CO, RT): δ = 9.51 (d, J = 5.7 Hz, 1H, H6 Py), 8.23 (td, J = 7.7 Hz, J = 1.4 Hz, 1H, H4 Py), 7.87 (d, J = 7.7 Hz 1H, H3 Py), 7.78 (t, J = 6.2 Hz, 1H, H5 Py), 7.28 (d, J = 8.3 Hz, 2H, CHCMe p-Tol), 7.11 (d, 2H, CHCN p-Tol), 6.45 (d, J = 6.2 Hz, 1H, HB), 6.38 (d, J = 6.2 Hz, 1H, HA’), 6.28 (d, 1H, HB’), 6.17 (d,1H, HA), 5.25 (bs, 2H, CH2), 2.94 (sp, 1H, CH iPr), 2.37 (s, 3H, Me Cym), 2.35 (s, 3H, Me p-Tol), 1.31 and 1.25 (2 × d, J = 6.9 Hz, 6H, Me iPr). 13C NMR (125.77 MHz, THF-d8, RT): δ = 193.66 (C=S), 156.82 (C6 Py), 142.09 (C4 Py), 140.09 (CMe p-Tol), 138.86 (CN p-Tol), 132.08 (CHCMe p-Tol), 132.21 (CHCMe p-Tol), 128.11 (C5 Py), 127.08 (CHCN p-Tol), 125.94 (C3 Py), 110.48 (CiPr), 103.47 (CMe Cym), 86.79 (CHA), 86.70 (CHB’), 85.96 (CHA’), 85.24 (CHB), 61.40 (CH2), 32.96 (CH iPr), 23.53, 23.06 (Me iPr), 21.77 (Me p-Tol) and 19.64 (Me Cym). 3c. 1H NMR (500.10 MHz, (CD3)2CO, RT): δ = 9.37 (d, J = 5.5 Hz, 1H, H6 Py), 8.05 (td, J = 7.7 Hz, J = 1.4 Hz, 1H, H4 Py), 7.66 (d, J = 7.7 Hz, 1H, H3 Py), 7.57 (t, J = 6.5 Hz, 1H, H5 Py), 7.36 (J = 8.0 Hz, 2H, CHCN p-Tol), 7.29 (d, 2H, CHCMe p-Tol), 6.22 (d, J = 6.1 Hz, 1H, HB), 5.95 (d, J = 6.4 Hz, HA’), 5.87 (d, 2H, HB’), 5.66 (d, 1H, HA), 5.46, 5.27 (d, J = 14.3 Hz, 2H, CH2), 3.26 (sp, 1H, CH iPr), 2.65 (s, 3H, Me Cym), 2.42 (s, 3H, Me p-Tol), 1.59 and 1.51 (2 × d, J = 6.9 Hz, 6H, Me iPr). 13C NMR (125.77 MHz, THF-d8, RT): δ = 163.90 (C=S), 157.39 (C6 Py), 141.69 (C4 Py), 127.42 (C5 Py), 123.52 (C3 Py), 138.86 (CN p-Tol), 137.8 (CMe p-Tol), 130.45 (CHCMe p-Tol), 128.68 (CHCN p-Tol), 109.40 (CiPr), 106.49 (CMe Cym), 92.01 (CHB), 91.71 (CHA’), 90.85 (CHB’), 89.20 (CHA), 63.08 (CH2), 32.90 (CH iPr), 23.84, 23.78 (Me iPr) and 19.31 (Me Cym). 3d. 1H NMR (500.10 MHz, (CD3)2CO, RT): δ = 3.06 (m, 1H, CH iPr), 1.40 (bd, J = 6.3 Hz, 6H, Me iPr). 3e. 1H NMR (500.10 MHz, (CD3)2CO, RT): δ = 2.75 (sp, 1H, CH iPr), 1.22 and 0.89 (2 × d, J = 6.9 Hz, 6H, Me iPr).
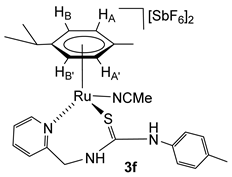
Complex 3f. Yield: 74 mg, 75%. Anal. Calcd for C26H32N4F12RuSSb2: C, 31.07; H, 3.21; N, 5.57; S, 3.19. Found: C, 31.47; H, 3.36; N, 5.84; S, 3.17. HRMS (μ-TOF), C24H29N3RuS, [M − 2 SbF6 − NCMe]2+, calcd: 246.5557, found: 246.5551. IR (cm−1): ν(NH) 3334 (br), ν(SbF6) 652 (s). 1H NMR (400.16 MHz, CD2Cl2, RT): δ = 9.17 (d, J = 6.0 Hz, 1H, H6 Py), 8.22 (s, 1H, NHp-Tol), 8.04 (td, J = 7.7 Hz, J = 1.4 Hz, 1H, H4 Py), 7.71 (d, J = 6.4 Hz, 1H, H3 Py), 7.68 (td, J = 7.7 Hz, J = 1.6 Hz, 1H, H5 Py), 7.27 (d, J = 8.2 Hz, 2H, CHCMe p-Tol), 7.08 (d, 2H, CHCN p-Tol), 7.13 (bs, 1H, NHCH2), 6.11 (d, J = 6.2 Hz, 1H, HB), 6.00 (d, J = 6.1 Hz, 1H, HB’) 5.85 (d,1H, HA), 5.64 (d, 1H, HA’), 5.18 (dd, J = 15.1 Hz, J = 7.9 Hz, 1H, CHHpro-S), 4.62 (dd, J = 5.6 Hz, 1H, CHHpro-R), 2.82 (m, 1H, CH iPr), 2.56 (s, 3H, NCMe), 2.36 (s, 3H, Me p-Tol), 1.80 (s, 3H, Me Cym), 1.32 and 1.28 (2 × d, J = 7.1 Hz, 6H, Me iPr). 13C NMR (125.77 MHz, CD2Cl2, RT): δ = 176.88 (C=S), 158.47 (C2 Py), 158.27 (C6 Py), 142.25 (C4 Py), 131.82 (CHCMe p-Tol), 128.80 (C3 Py), 128.58 (NCMe), 127.76 (C5 Py), 126.07 (CHCN p-Tol), 108.46 (CiPr), 107.47 (CMe Cym), 90.75 (CHB’), 89.20 (CHA), 88.69 (CHB), 85.03 (CHA’), 52.68 (CH2), 31.46 (CH iPr), 23.17, 22.21 (Me iPr), 21.63 (Me p-Tol), 18.45 (Me Cym) and 4.75 (NCMe).
4.4. Preparation of the Complex [(Cym)Os(NCMe)(κ2Npy,S-H2NNS)][SbF6]2 (4a)
To a solution of the complex [(Cym)OsCl(κ2Npy,S-H2NNS)][SbF6] (2) (300.0 mg, 0.352 mmol) in 10 mL of NCMe was added 120.8 mg (0.352 mmol) of AgSbF6. The resulting suspension was stirred for 2 h. The AgCl formed was separated with a cannula, and the filtrate was concentrated under reduced pressure to ca. 2 mL. The slow addition of n-hexane led to the precipitation of a yellow solid, which was washed with n-hexane (3 × 5 mL) and vacuum-dried.
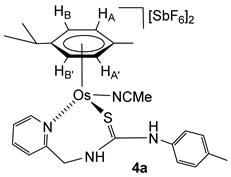
Complex 4a. Yield: 289 mg, 75%. Anal. Calcd for C26H32N4F12OsSSb2: C, 28.5; H, 2.9; N, 5.1; S, 2.9. Found: C, 28.4; H, 3.0; N, 5.0; S, 3.2. HRMS (μ-TOF), C24H28N3OsS, [M − 2 SbF6 − NCMe − H]+, calcd: 582.1612, found: 582.1635. IR (cm−1): ν(NH) 3335 (br), ν(SbF6) 651 (s). 1H NMR (500.10 MHz, CD2Cl2, RT): δ = 9.12 (d, J = 6.1 Hz, 1H, H6 Py), 8.16 (s, 1H, NHp-Tol), 8.00 (t, J = 7.7 Hz, 1H, H4 Py), 7.71 (d, J = 7.7 Hz, 1H, H3 Py), 7.63 (t, J = 6.9 Hz, 1H, H5 Py), 7.24 (bt, J = 9.3 Hz, 1H, NHCH2), 7.29 (d, J = 8.2 Hz, 2H, CHCMe p-Tol), 7.10 (d, 2H, CHCN p-Tol), 6.37 (d, J = 5.8 Hz, 1H, HB), 6.14 (d, J = 5.8 Hz, 1H, HB’) 5.93 (d,1H, HA), 5.88 (d, 1H, HA’), 5.27 (dd, J = 14.8 Hz, J = 8.0 Hz, 1H, CHHpro-S), 4.58 (dd, J = 5.7 Hz, 1H, CHHpro-R), 2.81 (s, 3H, NCMe), 2.77 (m, 1H, CH iPr), 2.37 (s, 3H, Me p-Tol), 1.83 (s, 3H, Me Cym), 1.31 and 1.28 (2 × d, J = 7.0 Hz, 6H, Me iPr). 13C NMR (125.77 MHz, CD2Cl2, RT): δ = 175.72 (C=S), 159.23 (C6 Py), 157.63 (C2 Py), 142.61 (C4 Py), 141.80 (CN p-Tol), 140.74 (CMe p-Tol), 131.88 (CHCMe p-Tol), 128.45 (C3 Py), 128.24 (C5 Py), 126.15 (CHCN p-Tol), 124.15 (NCMe), 99.64 (CiPr), 99.01 (CMe Cym), 83.78 (CHB’), 81.86 (CHB), 81.38 (CHA), 77.15 (CHA’), 52.79 (CH2), 23.29 (CH iPr), 22.60, 21.65 (Me iPr), 20.87 (Me p-Tol), 18.10 (Me Cym) and 4.71 (NCMe).
4.5. Preparation of the Complexes 5
To a solution of the complex 3 (400 mg, 0.415 mmol, assuming the solid as 3b) in methanol (20 mL), 34.9 mg (0.415 mmol) of solid NaHCO3 was added. The resulting suspension was stirred for 10 h, filtered to remove a small fraction of a dark solid in suspension, and the resulting solution was evaporated to dryness. The residue was extracted with dichloromethane, and the resulting solution was concentrated under reduced pressure to ca. 2 mL. The slow addition of n-pentane led to the precipitation of a yellow-brown solid, which was washed with n-pentane (3 × 5 mL) and vacuum-dried. A mixture of [(Cym)Ru(κ3Npy,Namide,S-H2NNS)][SbF6] (5a, 42%), [(Cym)Ru(H2O)(κ2Namide,S-H2NNS)][SbF6] (5b, 38%) and [(Cym)Ru(κ3Npy,Namide,S-H2NNS)][SbF6] (5c, 20%) was obtained. Addition of 0.1 mL of D2O and H2 (5 bar) to 15 mg of the solid obtained dissolved in THF-d8 (0.35 mL) results in the formation of 5c complex in 5 days at RT.
In an NMR tube 15 mg of a mixture of [(Cym)Ru(κ3Npy,Namide,S-HNNS)][SbF6] (5a, 42%), [(Cym)Ru(H2O)(κ2Namide,S-HNNS)][SbF6] (5b, 38%) and [(Cym)Ru(κ3Npy,Namide,S-HNNS)][SbF6] (5c, 20%) was heated in methanol at 50 °C for 5 h. The 1H NMR spectrum showed the presence of a 27/24/49 molar ratio of compounds 5a/5b/5c together with free p-cymene (13%).
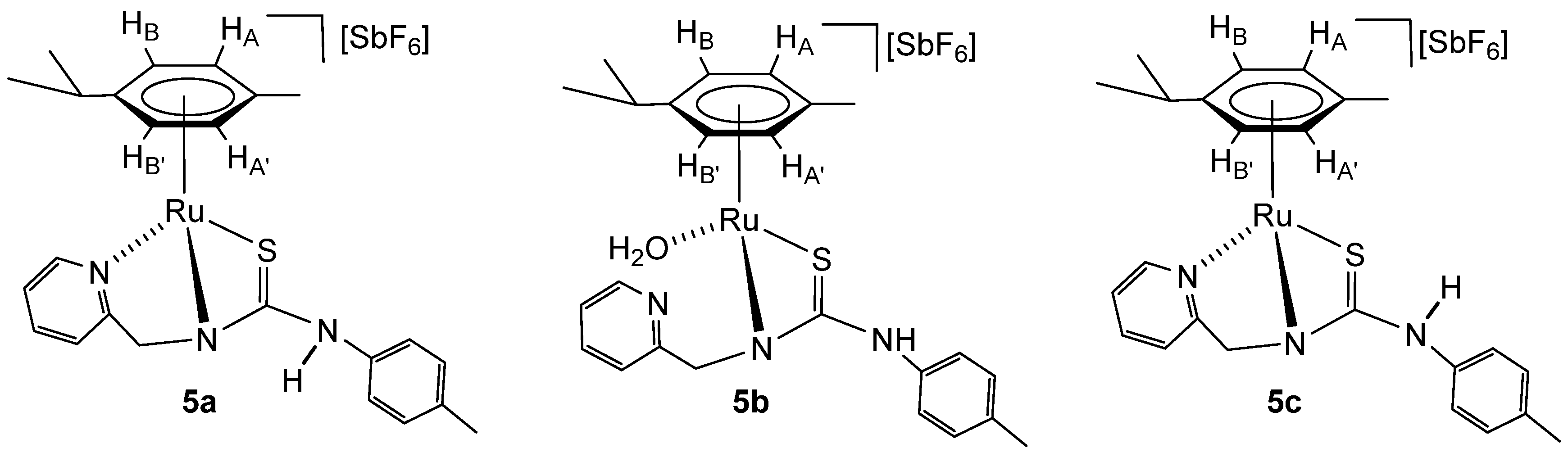
Complex 5. Yield: 242 mg, 80% (assuming the solid as 5c). Anal. Calcd for C24H28N3F6RuSSb: C, 39.63; H, 3.88; N, 5.78; S, 4.41. Found: C, 40.04; H, 3.64; N, 5.94; S, 4.37. HRMS (μ-TOF), C24H28N3RuS, [M − SbF6]+, calcd: 492.1042, found: 492.1066. IR (cm−1): ν(NH) 3368 (br), ν(SbF6) 653 (s). 5a. 1H NMR (500.10 MHz, THF-d8, RT): δ = 9.28 (bs, 1H, H6 Py), 8.83 (s, 1H, NH), 7.92 (m,1H, H4 Py), 7.60 (t, J = 6.5 Hz, 1H, H5 Py), 7.54 (m, 1H, H3 Py), 7.46 (dd, J = 8.5 Hz, J = 1.9 Hz, 2H, CHCN p-Tol), 7.33 (d, 2H, CHCMe p-Tol), 6.11(d, J = 6.2 Hz, 1H, HB), 5.87 (d, J = 5.9 Hz, 1H, HA’), 5.65 (d, 1H, HB’), 5.28 (d,1H, HA), 5.14 (d, J = 19.5 Hz, 1H, CHHpro-S), 4.76 (d, 1H, CHHpro-R), 3.07 (sp, 1H, CH iPr), 2.59 (s, 3H, Me Cym), 2.47 (s, 3H, Me p-Tol), 1.52 and 1.39 (2 × d, J = 7.1 Hz, 6H, Me iPr). 13C NMR (125.77 MHz, THF-d8, RT): δ = 163.33 (C=S), 156.52 (C6 Py), 140.57 (C4 Py), 138.37 (CN p-Tol), 136.95 (CMe p-Tol), 129.56 (CHCMe p-Tol), 128.31, 128.26 (CHCN p-Tol), 126.21 (C5 Py), 122.73 (C3 Py), 108.32 (CiPr), 105.80 (CMe Cym), 92.09 (CHB), 90.10 (CHB’), 89.51 (CHA), 89.43 (CHA’), 62.21 (CH2), 32.34 (CH iPr), 23.07, 22.87 (Me iPr), 21.15 (Me p-Tol) and 18.37 (Me Cym). 5b. 1H NMR (500.10 MHz, THF-d8, RT): δ = 8.59 (bs, 1H, H6 Py), 7.71 (t, J = 7.3 Hz, 1H, H4 Py), 7.29 (t, J = 6.0 Hz, 1H, H5 Py), 7.08 (overlapped, 1H, H3 Py), 7.09 (d, J = 8.0 Hz, 2H, CHCMe p-Tol), 7.02 (d, 2H, CHCN p-Tol), 5.42 (d, J = 6.1 Hz, 1H, HB), 5.41 (d, J = 6.0 Hz, 1H, HA’), 5.33 (d,1H, HA), 5.22 (d, 1H, HB’), 3.64 (bs, 2H, CH2), 2.79 (sp, 1H, CH iPr), 2.33 (s, 3H, Me Cym), 2.31 (s, 3H, Me p-Tol), 1.22 and 1.03 (2 × d, J = 6.9 Hz, 6H, Me iPr). 13C NMR (125.77 MHz, THF-d8, RT): δ = 177.55 (C=S), 149.81 (C6 Py), 144.68 (CN p-Tol), 137.21 (CMe p-Tol), 137.83 (C4 Py), 130.65 (CHCMe p-Tol), 124.48 (CHCN p-Tol), 123.73 (C5 Py), 122.85 (C3 Py), 108.02 (CiPr), 101.87 (CMe Cym), 84.28 (CHB’), 84.00 (CHA’), 83.81 (CHA), 83.55 (CHB), 48.38 (CH2), 31.76 (CH iPr), 23.20, 21.84 (Me iPr), 21.01 (Me p-Tol) and 18.50 (Me Cym). 5c. 1H NMR (500.10 MHz, THF-d8, RT): δ = 9.05 (d, J = 5.7 Hz, 1H, H6 Py), 7.94 (td, J = 7.8 Hz, J = 1.3 Hz, 1H, H4 Py), 7.57 (d, J = 7.8 Hz, 1H, H3 Py), 7.49 (t, J = 6.6 Hz, 1H, H5 Py), 7.00 (d, J = 8.2 Hz, 2H, CHCMe p-Tol), 6.95 (d, 2H, CHCN p-Tol), 5.97 (d, J = 5.9 Hz, 1H, HB), 5.86 (d, J = 5.9 Hz, 1H, HA’), 5.74 (d, 1H, HB’), 5.59 (d,1H, HA), 4.91 (d, J = 16.5 Hz, 1H, CHHpro-R), 4.64 (d, 1H, CHHpro-S), 2.68 (sp, 1H, CH iPr), 2.20 (s, 3H, Me p-Tol), 2.07 (s, 3H, Me Cym), 1.20 and 1.18 (2 × d, J = 6.9 Hz, 6H, Me iPr). 13C NMR (125.77 MHz, THF-d8, RT): δ = 155.54 (C6 Py), 139.56 (C4 Py), 135.22 (CMe p-Tol), 129.55 (CHCMe p-Tol), 125.48 (C5 Py), 122.46 (CHCN p-Tol), 122.37 (C3 Py), 105.90 (CiPr), 99.87 (CMe Cym), 84.45 (CHB), 84.19 (CHA), 83.90 (CHA’), 83.00 (CHB’), 61.20 (CH2), 31.12 (CH iPr), 22.63, 22.17 (Me iPr), 20.51 (Me p-Tol) and 18.41 (Me Cym).
4.6. Preparation of the Complexes 6
To a solution of the complex [(Cym)OsCl(κ2NPy,S-H2NNS)][SbF6] (2) (255.9 mg, 0.30 mmol) in 10 mL of acetone was added to 103.1 mg (0.30 mmol) of AgSbF6. The resulting suspension was stirred for 2 h. The AgCl formed was separated with a cannula, and the filtrate was concentrated under pressure to ca. 2 mL. The slow addition of n-hexane led to the precipitation of a yellow-brown solid, a mixture of complexes 6a, 6c, and 4b, which was vacuum-dried and characterised in situ by 1H NMR. Then, 25.2 mg (0.30 mmol) of NaHCO3 and 20 mL of methanol were added to the solid obtained. The resulting suspension was stirred for 10 h, and then was vacuum-evaporated to dryness, and the residue was extracted with dichloromethane. The solution was concentrated under pressure to ca. 2 mL. The slow addition of n-hexane led to the precipitation of a yellow-brown solid, which was washed with n-hexane (3 × 5 mL) and vacuum-dried. A mixture of [(Cym)Os(κ3Npy,Namide,S-HNNS)][SbF6] (6a, 37%), [(Cym)Os(H2O)(κ2Namide,S-HNNS)][SbF6]2 (6b, 39%) and [(Cym)Ru(κ3Npy,Namide,S-HNNS)][SbF6] (6c, 24%) was obtained.
In an NMR tube, 15 mg of a mixture of [(Cym)Os(κ3Npy,Namide,S-HNNS)][SbF6] (6a, 37%), [(Cym)Os(H2O)(κ2Namide,S-HNNS)][SbF6] (6b, 39%) and [(Cym)Os(κ3Npy,Namide,S-HNNS)][SbF6] (6c, 24%) was heated in methanol at 60 °C for 24 h. The 1H NMR spectrum showed the presence of a 12/16/72 molar ratio of compounds 6a/6b/6c.
The lower solubility of compound 6c in methanol allowed 6c to be separated as a yellow solid with 98% purity, and from the mother liquors, a 49/49/2 mixture of 6a/6b/6c could be isolated.
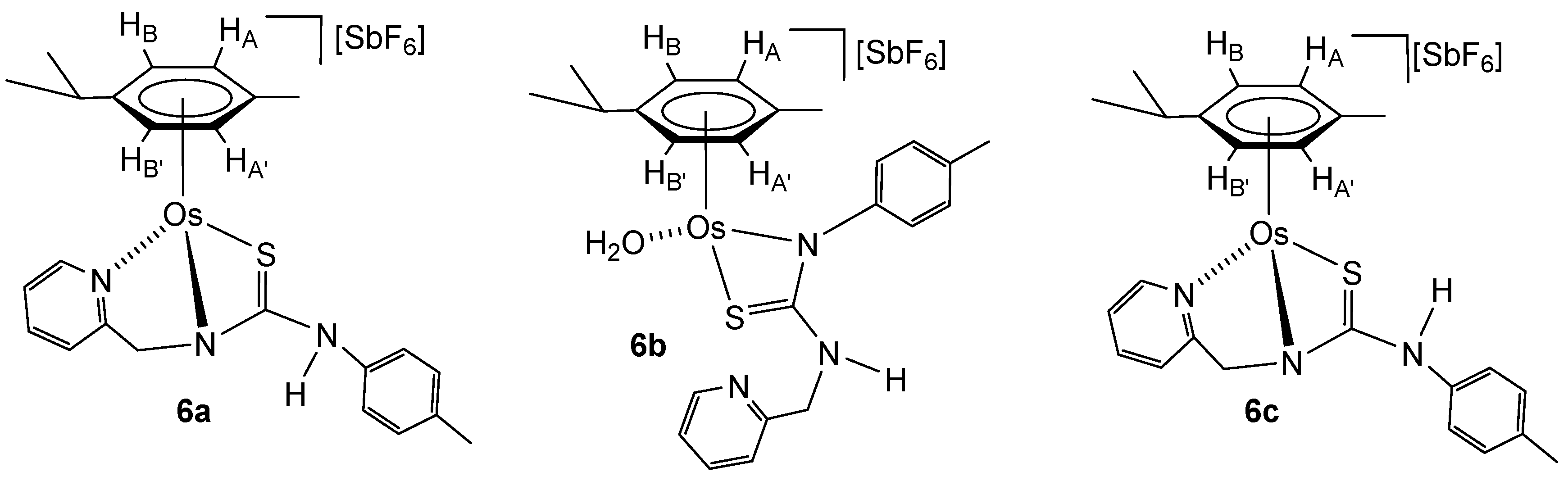
Complex 6. Yield: 194 mg, 79% (assuming the solid as 6c). Anal. Calcd for C24H28N3F6OsSSb: C, 35.3; H, 3.5; N, 5.15; S, 3.9. Found: C, 35.5; H, 3.7; N, 5.2; S, 3.9. HRMS (μ-TOF), C24H28N3OsS, [M − SbF6]+, calcd: 582.1612, found: 582.1641. IR (cm−1): ν(NH) 3600–3000 (br), ν(SbF6) 653 (s). 6a. 1H NMR (500.10 MHz, CD2Cl2, RT): δ = 9.00 (d, J = 5.7 Hz, 1H, H6 Py), 7.89 (s, 1H, NH), 7.74 (t, J = 7.5 Hz, 1H, H4 Py), 7.64 (d, J = 7.1 Hz, 1H, H3 Py), 7.47 (t, J = 6.6 Hz, 1H, H5 Py), 7.40, 7.28 (2 × d, J = 8.2 Hz, 4H, CH p-Tol), 6.01 (d, J = 5.8 Hz, 1H, HB), 5.26 (d,1H, HA), 5.77 (d, J = 5.7 Hz, 1H, HA’), 5.52 (d, 1H, HB’), 5.36 (d, J = 18.9 Hz, 1H, CHHpro-R), 4.90 (d, 1H, CHHpro-S), 2.87 (sp, 1H, CH iPr), 2.56 (s, 3H, Me Cym), 2.42 (s, 3H, Me p-Tol), 1.47, 1.37 (2 × d, J = 6.7 Hz, 6H, Me iPr). 13C NMR (125.77 MHz, CD2Cl2, RT): δ = 176.50 (C=S), 162.08 (C2 Py), 155.22(C6 Py), 141.25 (C4 Py), 138.26, 136.77, 129.85, 128.14 (CH p-Tol), 127.84 (C5 Py), 126.29 (C3 Py), 99.01 (CMe Cym), 98.37 (CiPr), 84.43 (CHB), 82.01 (CHB’), 80.96 (CHA’), 79.91 (CHA), 62.46 (CH2), 32.41 (CH iPr), 23.88, 23.09 (Me iPr), 21.58 (Me p-Tol) and 19.02 (Me Cym). 6b. 1H NMR (500.10 MHz, CD2Cl2, RT): δ = 8.39 (d, J = 5.6 Hz, 1H, H6 Py), 8.07 (t, J = 7.8 Hz, 1H, H4 Py), 7.63 (t, J = 7.4 Hz, 1H, H5 Py), 7.13 (d, J = 4.4 Hz, 1H, H3 Py), 6.89, 6.54 (2 × d, J = 7.4 Hz, 4H, CH p-Tol), 5.47 (d, J = 5.6 Hz, 1H, HA), 5.45 (d, J = 5.7 Hz, 1H, HA’), 5.23 (d,1H, HB’), 5.19 (d,1H, HB), 4.73 (bt, 1H, NH), 3.50, 3.38 (2 × dd, J = 17.1 Hz, J = 7.0 Hz, 2H, CH2), 2.50 (s, 3H, Me Cym), 2.41 (m, 1H, CH iPr), 2.22 (s, 3H, Me p-Tol), 1.10 and 0.68 (2 × d, J = 7.0 Hz, 6H, Me iPr). 13C NMR (125.77 MHz, CD2Cl2, RT): δ = 171.16 (C=S), 151.49 (C2 Py), 148.28 (C6 Py), 143.38 (CH p-Tol), 141.90 (C4 Py), 139.11, 130.76 (CH p-Tol), 125.06 (C3 Py), 123.72 (CH p-Tol), 122.51 (C5 Py), 97.78 (CMe Cym), 93.57 (CiPr), 77.45 (CHB’), 76.32 (CHB), 75.37 (CHA), 73.55 (CHA’), 46.09 (CH2), 31.49 (CH iPr), 23.81, 21.25 (Me iPr), 21.41 (Me p-Tol) and 19.79 (Me Cym). 6c. 1H NMR (500.10 MHz, CD2Cl2, RT): δ = 8.87 (d, J = 5.5 Hz, 1H, H6 Py), 7.81 (t, J = 7.7 Hz, 1H, H4 Py), 7.51 (d, J = 7.8 Hz, 1H, H3 Py), 7.33 (t, J = 6.6 Hz, 1H, H5 Py), 7.11, 7.05 (2 × d, J = 8.5 Hz, 4H, CH p-Tol), 5.95 (d, J = 5.4 Hz, 1H, HB), 5.75 (bs,1H, NH), 5.57 (d,1H, HA), 5.83, 5.81 (2 × d, J = 5.8 Hz, 2H, HA’, HB’), 5.10 (d, J = 16.8 Hz, 1H, CHHpro-R), 4.54 (d, 1H, CHHpro-S), 2.63 (sp, 1H, CH iPr), 2.29 (s, 3H, Me p-Tol), 2.24 (s, 3H, Me Cym), 1.23 and 1.21 (2 × d, J = 7.0 Hz, 6H, Me iPr). 13C NMR (125.77 MHz, CD2Cl2, RT): δ = 164.02 (C2 Py), 155.08 (C6 Py), 139.87 (C4 Py), 137.29, 137.01, 130.64 (CH p-Tol), 125.99 (C5 Py), 122.94 (CH p-Tol), 122.30 (C3 Py), 97.09 (CMe Cym), 91.34 (CiPr), 76.28 (CHB), 75.73, 74.57 (CHA’, CHB’), 74.69 (CHA), 62.66 (CH2), 32.85 (CH iPr), 23.79, 23.21 (Me iPr), 21.47 (Me p-Tol) and 19.79 (Me Cym).
Complex 4b. 1H NMR (500.10 MHz, CD3OD, RT): δ = 12.45 (s, 1H, NH pyridinium), 8.35 (bt, J = 5.0, 1H, H6 Py). Complex 4b was characterised in situ by 1H NMR from a mixture of 6a, 6c, and 4b. All other resonances of 4b not specified are like the resonances of 6b.
4.7. Preparation of the Complexes 6a–6c from 4a, and 4a from 6a–6c
Preparation of the complexes 6a–6c from 4a. To a solution of the complex 4a (49.2 mg, 0.045 mmol) in methanol (5 mL), 3.8 mg (0.0455 mmol) of solid NaHCO3 was added. The resulting suspension was stirred for 6 h and evaporated to dryness. The residue was extracted with dichloromethane, and the resulting solution was concentrated under reduced pressure to ca. 1 mL. The slow addition of n-pentane led to the precipitation of a yellow-brown solid (26 mg, Yield 70%), which was washed with n-pentane (3 × 3 mL) and vacuum-dried. According to NMR measurements, the solid consists of a 35/36/29, 6a/6b/6c mixture.
Preparation of the complexes 4afrom 6a–6c. To a solution of a mixture of complexes 6a–6c (50 mg, 0.047 mmol) in acetonitrile (5 mL) was added HSbF6 (3.83 μL, ρ = 2.88 g·mL−1, 0.047 mmol). The resulting solution was stirred for 5 h and concentrated under reduced pressure to ca. 1 mL. The slow addition of n-pentane led to the precipitation of a yellow solid (39 mg, yield 75%), which was washed with pentane (3 × 3 mL) and vacuum-dried.
4.8. General Procedure for the Catalytic Hydrogenation Reactions
A high-pressure NMR tube containing the catalyst (0.015 mmol) and the substrate to be hydrogenated (0.30 mmol) in THF-d8 (0.45 mL) was pressurised with hydrogen gas (5 bar). The tube was heated at the appropriate temperature, and the solution was monitored by NMR. Conversions were determined by 1H NMR.
4.9. Reaction of the Complexes 5 and 6c with H2 in the Presence of D2O
A high-pressure NMR tube containing a solution of the corresponding complexes 5 or 6c (0.015 mmol) in THF-d8/D2O (0.35 mL/0.1 mL) was pressurised with H2 (5 bar), and the resulting solution was monitored by NMR spectroscopy. After 30 min (complexes 5) or 5 days (complex 6c) at RT, HD is formed in the reaction medium.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30163398/s1, Figure S1: 1H NMR of H2NNS (500.10 MHz, CD2Cl2, RT) to Figure S22: 13C{1H} NMR of [(Cym)Os(κ3Npy,Namide,S-HNNS)][SbF6] (6c) (125.77 MHz, CD2Cl2, RT).
Author Contributions
A.G. and R.D.L.: synthesis, spectroscopic characterisation, preliminary analysis, catalytic work, and preparation of the experimental data. F.V. and R.R.: characterisation and supervision of the work. F.V., R.R. and P.L.: design, supervision of the work, visualisation, proofreading, conceptualisation, and discussion of the work. F.V., R.R. and P.L.: writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MCIN/AEI of Spain, PID2021-122406NB-100, and Gobierno de Aragón, Grupo de Referencia: Catalisis Homogénea Enantioselectiva, E05-23R. A.G. is grateful for the contract to the Programa Investigo, funded by the European Union-NextGenerationUE.
Institutional Review Board Statement
No applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We want to acknowledge the use of Servicio General de Apoyo a la Investigación-SAI, University of Zaragoza.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Welch, G.C.; Juan, R.R.S.; Masuda, J.D.; Stephan, D.W. Reversible, Metal-Free Hydrogen Activation. Science 2006, 314, 1124–1126. [Google Scholar] [CrossRef]
- Stephan, D.W. The Broadening Reach of Frustrated Lewis Pair Chemistry. Science 2016, 354, 1248–1256. [Google Scholar] [CrossRef]
- Stephan, D.W.; Erker, G. Frustrated Lewis Pair Chemistry: Development and Perspectives. Angew. Chem. Int. Ed. 2015, 54, 6400–6441. [Google Scholar] [CrossRef]
- Stephan, D.W. Frustrated Lewis Pair Catalysis: An Introduction. In Frustrated Lewis Pairs, 2nd ed.; Slootweg, J.C., Jupp, A.R., Eds.; Springer: Cham, Switzerland, 2021; Volume 3, pp. 1–28. [Google Scholar] [CrossRef]
- Jupp, A.R.; Stephan, D.W. New Directions for Frustrated Lewis Pair Chemistry. Trends Chem. 2019, 1, 35–48. [Google Scholar] [CrossRef]
- Paradies, J. From Structure to Novel Reactivity in Frustrated Lewis Pairs. Coord. Chem. Rev. 2019, 380, 170–183. [Google Scholar] [CrossRef]
- Scott, D.J.; Fuchter, M.J.; Ashley, A.E. Designing Effective ‘Frustrated Lewis Pair’ Hydrogenation Catalysts. Chem. Soc. Rev. 2017, 46, 5689–5700. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.W. Frustrated Lewis Pairs. J. Am. Chem. Soc. 2015, 137, 10018–10032. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.W. Frustrated Lewis Pairs: From Concept to Catalysis. Acc. Chem. Res. 2015, 48, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Frustrated Lewis Pairs Beyond the Main Group: Transition Metal-Containing Systems. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2013; pp. 261–280. [CrossRef]
- Frustrated Lewis Pairs I: Uncovering and Understanding; Springer: New York, NY, USA, 2013.
- Stephan, D.W.; Erker, G. Frustrated Lewis Pairs: Metal-free Hydrogen Activation and More. Angew. Chem. Int. Ed. 2010, 49, 46–76. [Google Scholar] [CrossRef]
- Flynn, S.R.; Wass, D.F. Transition Metal Frustrated Lewis Pairs. ACS Catal. 2013, 3, 2574–2581. [Google Scholar] [CrossRef]
- Xu, X.; Kehr, G.; Daniliuc, C.G.; Erker, G. 1,1-Carbozirconation: Unusual Reaction of an Alkyne with a Methyl Zirconocene Cation and Subsequent Frustrated Lewis Pair Like Reactivity. Angew. Chem. 2013, 125, 13874–13877. [Google Scholar] [CrossRef]
- Hidalgo, N.; Moreno, J.J.; Pérez-Jiménez, M.; Maya, C.; López-Serrano, J.; Campos, J. Evidence for Genuine Bimetallic Frustrated Lewis Pair Activation of Dihydrogen with Gold(I)/Platinum(0) Systems. Chem. A Eur. J. 2020, 26, 5982–5993. [Google Scholar] [CrossRef] [PubMed]
- Zwettler, N.; Mösch-Zanetti, N.C. Interaction of Metal Oxido Compounds with B(C6F5)3. Chem. A Eur. J. 2019, 25, 6064–6076. [Google Scholar] [CrossRef]
- Zhang, S.; Appel, A.M.; Bullock, R.M. Reversible Heterolytic Cleavage of the H–H Bond by Molybdenum Complexes: Controlling the Dynamics of Exchange Between Proton and Hydride. J. Am. Chem. Soc. 2017, 139, 7376–7387. [Google Scholar] [CrossRef]
- Carmona, M.; Ferrer, J.; Rodríguez, R.; Passarelli, V.; Lahoz, F.J.; García-Orduña, P.; Cañadillas-Delgado, L.; Carmona, D. Reversible Activation of Water by an Air- and Moisture-Stable Frustrated Rhodium Nitrogen Lewis Pair. Chem. A Eur. J. 2019, 25, 13665–13670. [Google Scholar] [CrossRef]
- Ferrer-Bru, C.; Ferrer, J.; Passarelli, V.; Lahoz, F.J.; García-Orduña, P.; Carmona, D. Molecular Dihydrogen Activation by (C5Me5)M/N (M=Rh, Ir) Transition Metal Frustrated Lewis Pairs: Reversible Proton Migration to, and Proton Abstraction from, the C5Me5 Ligand. Chem. A Eur. J. 2024, 30, e202304140. [Google Scholar] [CrossRef]
- Hamilton, H.B.; King, A.M.; Sparkes, H.A.; Pridmore, N.E.; Wass, D.F. Zirconium–Nitrogen Intermolecular Frustrated Lewis Pairs. Inorg. Chem. 2019, 58, 6399–6409. [Google Scholar] [CrossRef]
- Chapman, A.M.; Haddow, M.F.; Wass, D.F. Frustrated Lewis Pairs beyond the Main Group: Cationic Zirconocene–Phosphinoaryloxide Complexes and Their Application in Catalytic Dehydrogenation of Amine Boranes. J. Am. Chem. Soc. 2011, 133, 8826–8829. [Google Scholar] [CrossRef]
- Jian, Z.; Daniliuc, C.G.; Kehr, G.; Erker, G. Frustrated Lewis Pair vs Metal–Carbon σ-Bond Insertion Chemistry at an o-Phenylene-Bridged Cp2Zr+/PPh2 System. Organometallics 2017, 36, 424–434. [Google Scholar] [CrossRef]
- Normand, A.T.; Daniliuc, C.G.; Wibbeling, B.; Kehr, G.; Le Gendre, P.; Erker, G. Phosphido- and Amidozirconocene Cation-Based Frustrated Lewis Pair Chemistry. J. Am. Chem. Soc. 2015, 137, 10796–10808. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kehr, G.; Daniliuc, C.G.; Erker, G. Reactions of a Cationic Geminal Zr+/P Pair with Small Molecules. J. Am. Chem. Soc. 2013, 135, 6465–6476. [Google Scholar] [CrossRef]
- Barnett, B.R.; Neville, M.L.; Moore, C.E.; Rheingold, A.L.; Figueroa, J.S. Oxidative-Insertion Reactivity Across a Geometrically Constrained Metal→Borane Interaction. Angew. Chem. Int. Ed. 2017, 56, 7195–7199. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Wee, G.N.J.H.; Stephan, D.W. Accessing Frustrated Lewis Pair Chemistry from a Spectroscopically Stable and Classical Lewis Acid-Base Adduct. Angew. Chem. Int. Ed. 2018, 57, 5881–5884. [Google Scholar] [CrossRef]
- Roters, S.; Appelt, C.; Westenberg, H.; Hepp, A.; Slootweg, J.C.; Lammertsma, K.; Uhl, W. Dimeric Aluminum–Phosphorus Compounds as Masked Frustrated Lewis Pairs for Small Molecule Activation. Dalton Trans. 2012, 41, 9033. [Google Scholar] [CrossRef] [PubMed]
- Boudjelel, M.; Sosa Carrizo, E.D.; Mallet−Ladeira, S.; Massou, S.; Miqueu, K.; Bouhadir, G.; Bourissou, D. Catalytic Dehydrogenation of (Di)Amine-Boranes with a Geometrically Constrained Phosphine-Borane Lewis Pair. ACS Catal. 2018, 8, 4459–4464. [Google Scholar] [CrossRef]
- Sgro, M.J.; Stephan, D.W. Frustrated Lewis Pair Inspired Carbon Dioxide Reduction by a Ruthenium Tris(Aminophosphine) Complex. Angew. Chem. Int. Ed. 2012, 51, 11343–11345. [Google Scholar] [CrossRef] [PubMed]
- Carmona, M.; Pérez, R.; Ferrer, J.; Rodríguez, R.; Passarelli, V.; Lahoz, F.J.; García-Orduña, P.; Carmona, D. Activation of H–H, HO–H, C(sp2)–H, C(sp3)–H, and RO–H Bonds by Transition-Metal Frustrated Lewis Pairs Based on M/N (M = Rh, Ir) Couples. Inorg. Chem. 2022, 61, 13149–13164. [Google Scholar] [CrossRef]
- Ferrer, C.; Ferrer, J.; Passarelli, V.; Lahoz, F.J.; García-Orduña, P.; Carmona, D. Well-Stabilized but Strained Frustrated Lewis Pairs Based on Rh/N and Ir/N Couples. Organometallics 2022, 41, 1445–1453. [Google Scholar] [CrossRef]
- Beard, S.; Grasa, A.; Viguri, F.; Rodríguez, R.; López, J.A.; Lahoz, F.J.; García-Orduña, P.; Lamata, P.; Carmona, D. Molecular Hydrogen and Water Activation by Transition Metal Frustrated Lewis Pairs Containing Ruthenium or Osmium Components: Catalytic Hydrogenation Assays. Dalton Trans. 2023, 52, 13216–13228. [Google Scholar] [CrossRef]
- Wilkinson, E.; Viguri, F.; Rodríguez, R.; López, J.A.; García-Orduña, P.; Lahoz, F.J.; Lamata, P.; Carmona, D. Strained Ruthenium Complexes Bearing Tridentate Guanidine-Derived Ligands. Helv. Chim. Acta 2021, 104, 1–17. [Google Scholar] [CrossRef]
- Parker, A.; Lamata, P.; Viguri, F.; Rodríguez, R.; López, J.A.; Lahoz, F.J.; García-Orduña, P.; Carmona, D. Half-Sandwich Complexes of Osmium Containing Guanidine-Derived Ligands. Dalton Trans. 2020, 49, 13601–13617. [Google Scholar] [CrossRef] [PubMed]
- Soriano, M.L.; Lenthall, J.T.; Anderson, K.M.; Smith, S.J.; Steed, J.W. Enhanced Anion Binding from Unusual Coordination Modes of Bis(Thiourea) Ligands in Platinum Group Metal Complexes. Chem. A Eur. J. 2010, 16, 10818–10831. [Google Scholar] [CrossRef] [PubMed]
- Sheeba, M.M.; Muthu Tamizh, M.; Farrugia, L.J.; Endo, A.; Karvembu, R. Chiral (η6-p-Cymene)Ruthenium(II) Complexes Containing Monodentate Acylthiourea Ligands for Efficient Asymmetric Transfer Hydrogenation of Ketones. Organometallics 2014, 33, 540–550. [Google Scholar] [CrossRef]
- Gatti, A.; Habtemariam, A.; Romero-Canelón, I.; Song, J.I.; Heer, B.; Clarkson, G.J.; Rogolino, D.; Sadler, P.J.; Carcelli, M. Half-Sandwich Arene Ruthenium(II) and Osmium(II) Thiosemicarbazone Complexes: Solution Behavior and Antiproliferative Activity. Organometallics 2018, 37, 891–899. [Google Scholar] [CrossRef]
- Adhikari, S.; Hussain, O.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Synthesis, Structural and Chemosensitivity Studies of Arene d6 Metal Complexes Having N-phenyl-N’-(Pyridyl/Pyrimidyl)Thiourea Derivatives. Appl. Organom. Chemis. 2018, 32, e4362. [Google Scholar] [CrossRef]
- Avila, A.; Chinchilla, R.; Gómez-Bengoa, E.; Nájera, C. Enantioselective Synthesis of Succinimides by Michael Addition of Aldehydes to Maleimides Organocatalyzed by Chiral Primary Amine-Guanidines. Eur. J. Org. Chem. 2013, 2013, 5085–5092. [Google Scholar] [CrossRef]
- Bennet, M.A.; Huang, T.N.; Matheson, T.W.; Smith, A.K. (η6-Hexamethylbenzene)Ruthenium Complexes. In Inorganic Syntheses; Fackler, J.P., Ed.; Wiley: New York, NY, USA, 1982; pp. 74–78. [Google Scholar] [CrossRef]
- Cabeza, J.A.; Maitlis, P.M. Mononuclear η6-p-Cymeneosmium(II) Complexes and Their Reactions with Al2Me6and Other Methylating Reagents. J. Chem. Soc., Dalton Trans. 1985, 3, 573–578. [Google Scholar] [CrossRef]
- Sun, T.; Wu, Z.; Wang, G.; Li, Z.; Li, C.; Wang, E. Efficient Promotional Effects of Mo on the Catalytic Hydrogenation of Methyl Acrylate over Ni-Based Catalysts under Mild Conditions. Ind. Eng. Chem. Res. 2022, 61, 152–163. [Google Scholar] [CrossRef]
- Sun, T.; Wang, G.; Guo, X.; Li, Z.; Wang, E.; Li, C. A Highly Active NiMoAl Catalyst Prepared by a Solvothermal Method for the Hydrogenation of Methyl Acrylate. Catalysts 2022, 12, 1118. [Google Scholar] [CrossRef]
- Seo, C.S.G.; Morris, R.H. Catalytic Homogeneous Asymmetric Hydrogenation: Successes and Opportunities. Organometallics 2019, 38, 47–65. [Google Scholar] [CrossRef]
- Noyori, R.; Ohkuma, T. Asymmetric Catalysis by Architectural and Functional Molecular Engineering: Practical Chemo- and Stereoselective Hydrogenation of Ketones. Angew. Chem. Int. Ed. 2001, 40, 40–73. [Google Scholar] [CrossRef]
- Clapham, S.E.; Hadzovic, A.; Morris, R.H. Mechanisms of the H2-Hydrogenation and Transfer Hydrogenation of Polar Bonds Catalyzed by Ruthenium Hydride Complexes. Coord. Chem. Rev. 2004, 248, 2201–2237. [Google Scholar] [CrossRef]
- Rautenstrauch, V.; Hoang-Cong, X.; Churlaud, R.; Abdur-Rashid, K.; Morris, R.H. Hydrogenation versus Transfer Hydrogenation of Ketones: Two Established Ruthenium Systems Catalyze Both. Chem. A Eur. J. 2003, 9, 4954–4967. [Google Scholar] [CrossRef] [PubMed]
- Meemken, F.; Baiker, A. Recent Progress in Heterogeneous Asymmetric Hydrogenation of C═O and C═C Bonds on Supported Noble Metal Catalysts. Chem. Rev. 2017, 117, 11522–11569. [Google Scholar] [CrossRef] [PubMed]
- Abdur-Rashid, K.; Lough, A.J.; Morris, R.H. RuHCl(Diphosphine)(Diamine): Catalyst Precursors for the Stereoselective Hydrogenation of Ketones and Imines. Organometallics 2001, 20, 1047–1049. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).