Abstract
Sebum secreted by sebaceous glands mixes with sweat to form a protective film that aids in maintaining skin health. Reduced sebum production compromises such barrier functions, potentially leading to severe itchiness and inflammation. Therefore, incorporating moisturizers with ingredients promoting sebum secretion is desirable. Wild watermelon possesses moisturizing and antioxidant properties, and its extracts are utilized in skin cosmetics and supplements. This study investigates whether seed watermelon (Citrullus mucosospermus (Fursa))—a species closely related to wild watermelon—influences sebum synthesis and can serve as a skin cosmetic raw ingredient. Several bioactive compounds—including coniferyl alcohol, coniferin, and p-coumaryl alcohol—were identified in the active third fraction of the fruit extract. Subsequently, SZ95 sebocytes stimulated with linoleic acid were stained using Oil Red O to detect lipogenesis facilitated by the identified bioactive compounds. Coniferyl alcohol promoted linoleic acid-stimulated lipogenesis by approximately 2.2-fold at a concentration of 300 µM. Lipidomic analyses confirmed an increase in total lipid content following coniferyl alcohol treatment, with notable increases in cholesterol ester, cardiolipin, and simple lipid content. Overall, these findings suggest that seed watermelon contains compounds that do influence sebum synthesis. Consequently, skin cosmetics containing seed watermelon fruit extracts with linoleic acid may benefit individuals with dry skin.
1. Introduction
The sebaceous gland is a skin appendage comprising two types of tissues: sebaceous gland lobules and sebaceous gland ducts. Sebocytes differentiated in the basal cell layer synthesize and accumulate sebum in the sebaceous gland lobules. Thereafter, they are destroyed upon apoptosis, and their sebum contents are entirely released into the sebaceous gland ducts (a type of holocrine secretion) [1]. Human sebum is primarily composed of triglycerides and fatty acids (57.7%), wax esters (26%), and squalene (12%) [2]. When secreted from the glands, sebum mixes with sweat released from the apocrine glands and forms a sebum barrier on the skin surface. Notably, exposure to external factors, such as dryness, ultraviolet (UV) rays, chemical substances, and pathogenic microorganisms, poses risks to the skin surface and overall skin health. However, the sebum barrier mitigates the loss of body moisture through evaporation, effects of external physical stimuli and chemical substances, and invasion of pathogenic microorganisms [3,4].
Sebum secretion from sebocytes is regulated by the male hormone testosterone, which is converted to 5α-dihydrotestosterone (DHT) by the enzyme 5α-reductase [5]. DHT, in turn, stimulates the sebaceous glands, promoting sebocyte proliferation and thereby increasing sebum production and secretion [6]. In general, sebum secretion is regulated by various factors such as androgens [6], growth factors [7], neurotransmitters [8], and fatty acids [9,10,11]. Additionally, age influences the amount of sebum secreted from the sebaceous glands in humans. Sebum production peaks between the ages of 17 and 20 years and gradually decreases with age [12]. Decreased sebum synthesis can lead to skin conditions, such as asteatotic eczema, xeroderma, and dry skin, with one in three elderly people being affected by dry skin [13,14]. While symptom management is commonly addressed via the use of medical-grade moisturizers, reactivating diminished sebaceous gland function has also been reported as a viable therapeutic approach [15,16]. For example, the administration of dehydroepiandrosterone has shown efficacy in individuals with significantly reduced sebum production due to menopause or ageing [17,18]. Although topical corticosteroids are frequently prescribed to treat various dermatologic conditions, concerns have been raised regarding their adverse effects under excessive use [19,20]. In some cases, steroid-induced side-effects can exacerbate xeroderma, complicating treatment approaches and increasing the likelihood of recurrence [21]. In this regard, non-steroidal compounds that have been shown to sustainably and fundamentally enhance sebum production and secretion include undecylenic acid and bryonolic acid [22,23]. Previous studies have shown that the topical application of creams containing undecylenic acid promotes the enlargement of sebaceous glands inside hamster ears and improves the content of epidermal lipids on the human forehead [22]. Similarly, treatments with bryonolic acid not only promote the enlargement of sebaceous glands inside hamster ears but also increase squalene levels in their epidermal lipids [23]. Bryonolic acid is an acidic pentacyclic triterpenol compound produced by Cucurbitaceae plants, such as loofah and melon; this suggests that other Cucurbitaceae plant species may also contain compounds that can promote sebum synthesis.
Watermelon (Citrullus lanatus), a member of the Cucurbitaceae family, is cultivated worldwide and known for its sweet fruits containing red or yellow flesh. Contrastingly, wild watermelon (Citrullus lanatus sp.), believed to have originated from the Kalahari Desert in central South Africa, is characterized by its white, unsweetened flesh [24]. In the Republic of Botswana, of which the Kalahari Desert occupies a large part, the indigenous people store the fruits of wild watermelon as a valuable water resource for the dry season and also utilize them for food and washing their bodies [25]. Wild watermelon can be stored for long periods without spoiling; this suggests that its fruit contains various functional compounds [26,27]. Notably, it is already being used as a raw material in the development of skin supplements and cosmetics. Moreover, it demonstrates remarkable properties, such as disease resistance and heat tolerance, and is used as a breeding material to develop other watermelon cultivars [28,29]. One such cultivar, the seed watermelon (Citrullus mucosospermus (Fursa)), is a species that retains many wild-type characteristics. It is primarily consumed in China, where the seed ovules are eaten similarly to nuts. It shares several characteristics with the wild watermelon, such as the appearance, flesh colour, and drought resistance, suggesting that it may also contain natural compounds proving effective for the skin. However, the potential of seed watermelon as a cosmetic ingredient has not been previously investigated, and it has received little attention in the field of cosmetic science. Therefore, in this study, we investigate the possibility of developing skin cosmetics using the seed watermelon fruit as a raw ingredient and also seek to identify bioactive compounds in the fruit that can directly activate sebocytes.
2. Results
2.1. Isolation of Cinnamyl Alcohol Analogues from Seed Watermelon Fruit
The crude extract of seed watermelon fruits was separated using the InertSep C18 column (GL Sciences Inc., Tokyo, Japan), to yield seven fractions (100% H2O; 9:1, 4:1, 7:3, 3:2, and 1:1 (v/v) H2O–CH3OH; and 100% CH3OH). The activity of all fractions for linoleic acid (LA)-stimulated lipogenesis was checked. All fractions showed this activity except for the 100% H2O fraction (Figure S1). Because of high yield, the third fraction (56.2 mg) was selected and used for further experiments. This material obtained in 4:1 H2O–CH3OH, was further purified via reverse-phase high-performance liquid chromatography (HPLC) employing a C-18 packed column. The activity of all peaks in the HPLC chromatogram was checked, and three compounds were confirmed to be active (Figure S2). Coniferin (1), p-coumaryl alcohol (2), and coniferyl alcohol (3) were eluted after 15, 22, and 26 min, respectively (Figure 1). The fractions corresponding to the peaks were collected and concentrated to yield colourless powders of pure 1 (3.2 mg), 2 (0.7 mg), and 3 (1.0 mg). All spectral data obtained for these compounds were identical to the commercially available standards or those reported previously, as shown in Figure 1 [30].
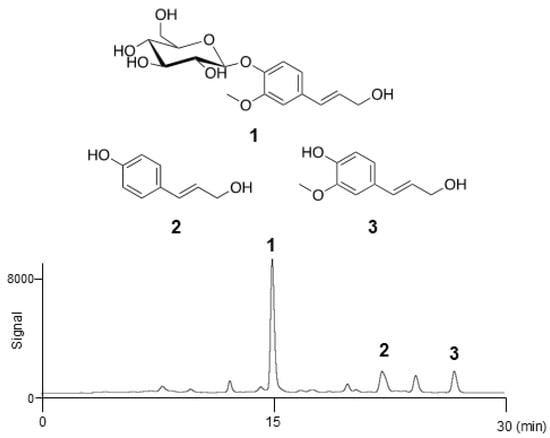
Figure 1.
Structures of coniferin (1), p-coumaryl alcohol (2), and coniferyl alcohol (3), and the HPLC chromatogram.
2.2. Assessment of Cytotoxicity of Isolated Cinnamyl Alcohol Analogues in Terms of Effect on SZ95 Sebocyte Viability
To evaluate the cytotoxicity of the isolated compounds (coniferyl alcohol, coniferin, and p-coumaryl alcohol) and the coniferyl alcohol-related compound (sinapyl alcohol), we assessed their effects on SZ95 sebocyte viability (Figure 2). Remarkably, none of the tested compounds exhibited cytotoxic effects, and more than 80% of the SZ95 cells remained viable after treatment for 24 h with 100, 200, and 300 µM of these compounds.
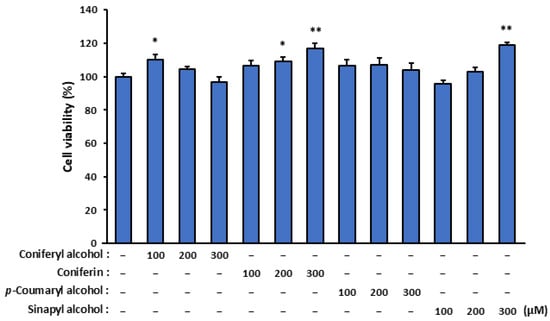
Figure 2.
The effect of isolated cinnamyl alcohol analogues on the viability of SZ95 sebocytes. The SZ95 cells were treated with specific concentrations (100, 200, and 300 µM) of the analogues—coniferyl alcohol, coniferin, p-coumaryl alcohol, and sinapyl alcohol—for 24 h. Cell viability was assessed using a WST-1 assay. The data are presented as means ± standard error (n = 12) and were analyzed for statistically significant differences using Student’s t-test, with different letters indicating the differences at * p < 0.05 and ** p < 0.01 vs. SZ95 cells with no cinnamyl alcohol analogue treatment.
2.3. Induction of Lipogenesis by Isolated Coniferyl Alcohol in LA-Stimulated SZ95 Sebocytes
LA strongly induces in vitro differentiation and lipid metabolism in sebocytes; specifically, it upregulates the genes involved in fatty acid metabolism, which leads to an increase in the intracellular levels of neutral lipids and thereby an enlargement in the associated lipid droplets. As detailed in this sub-section, we investigated whether the isolated compounds (coniferyl alcohol, coniferin, and p-coumaryl alcohol) and coniferyl alcohol-related compound (sinapyl alcohol) promoted lipogenesis in LA-stimulated SZ95 cells. Treatment with 100 µM LA alone resulted in a 1.6-fold increase in lipogenesis in the SZ95 cells (Figure 3A). Among the tested compounds, coniferyl alcohol significantly enhanced LA-induced lipogenesis in a dose-dependent manner, with 300 µM coniferyl alcohol leading to an approximately 2.2-fold increase in lipogenesis relative to the case of LA stimulation alone. These findings were further supported by microscopic analyses employing Oil Red O staining, which revealed enlarged lipid droplets in the LA-stimulated SZ95 cells treated with coniferyl alcohol (Figure 3B). Contrastingly, coniferin and p-coumaryl alcohol did not significantly influence the lipid synthesis. Sinapyl alcohol exhibited inhibitory effects on lipogenesis at concentrations of 100 and 200 µM; however, these effects were not observed at a concentration of 300 µM.
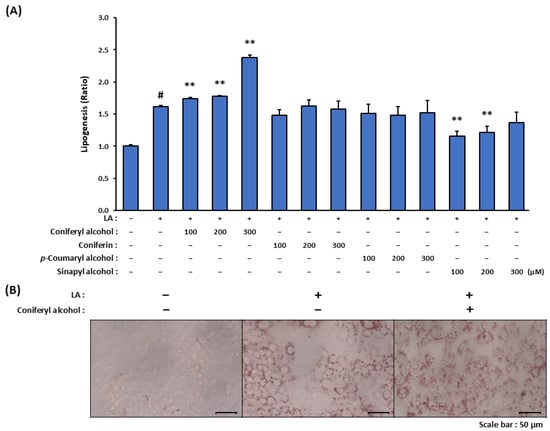
Figure 3.
The effect of coniferyl alcohol and other cinnamyl alcohol analogues isolated from seed watermelon fruit on lipogenesis in LA-stimulated SZ95 sebocytes. (A) SZ95 cells were pre-treated with cinnamyl alcohol analogues (100, 200, and 300 µM; coniferyl alcohol, coniferin, p-coumaryl alcohol, and sinapyl alcohol) for 2 h. The cells were then stimulated with and without LA (100 µM) for 24 h. Following incubation, the cells were stained with Oil Red O, and the dye dissolved in the lipid droplets was extracted and measured using a colorimetric microplate reader. The data are presented as means ± standard error (n = 12) and were analyzed for statistically significant differences using Student’s t-test, with different letters indicating the differences at # p < 0.05, vs. SZ95 cells without both LA-stimulation and cinnamyl alcohol analogue treatment, ** p < 0.01 vs. LA-stimulated SZ95 cells with no cinnamyl alcohol analogue treatment. (B) SZ95 cells were pre-treated with coniferyl alcohol (300 µM) for 2 h. The cells were then stimulated with and without LA (100 µM) for 24 h. Following incubation, the cells were stained with Oil Red O and observed using a microscope.
2.4. Influence of Isolated Coniferyl Alcohol on Lipid Profile of LA-Stimulated SZ95 Sebocytes
We performed lipidomic analyses to investigate whether coniferyl alcohol alters the lipid composition and promotes lipid synthesis in LA-stimulated SZ95 sebocytes. We observed that LA stimulation significantly increased the cellular levels of various lipid species including cholesterol ester, cardiolipin, diglyceride, triglyceride, phosphatidylethanolamine, phosphatidylglycerol, and phosphatidylserine (Figure 4). Additionally, coniferyl alcohol treatment enhanced the accumulation of various lipid species including cholesterol ester, cardiolipin, simple lipid, and sphingomyelin. When comparing the contents of lipid species with and without LA, we found that the levels of LA-containing cardiolipin, phosphatidylglycerol, and phosphatidylinositol increased after coniferyl alcohol pretreatment and LA stimulation. Contrastingly, the levels of their non-LA-containing counterparts decreased or remained unchanged.
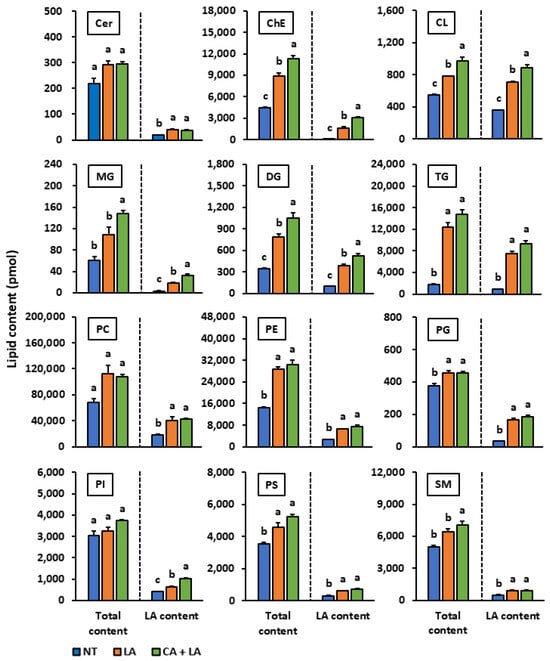
Figure 4.
The effect of coniferyl alcohol isolated from watermelon fruit on the lipid profile in LA-stimulated SZ95 sebocytes. SZ95 cells were first pre-treated with coniferyl alcohol (300 µM) for 2 h and then stimulated with and without LA (100 µM) for 24 h. Following incubation, lipids were extracted from the cells using the Bligh and Dyer method. The 12 lipid profile classes [ceramide (Cer), cholesterol ester (ChE), cardiolipin (CL), monoglyceride (MG), diglyceride (DG), triglyceride (TG), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylserine (PS), and sphingomyelin (SM)] were analyzed using liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI/MS/MS). Total content: the total content of all lipid species including those containing various fatty acid components such as palmitic acid, stearic acid, oleic acid, and LA; LA content: content of lipid species that contain LA as one of their fatty acid components. The data are presented as means ± standard error (n = 3) and were analyzed for statistically significant differences using the Tukey–Kramer method, with different alphabets indicating the differences at p < 0.05. NT: SZ95 cells without both LA-stimulation and coniferyl alcohol treatment, LA: LA-stimulated SZ95 cells with no coniferyl alcohol treatment, CA + LA: LA-stimulated SZ95 cells with 300 µM coniferyl alcohol treatment.
3. Discussion
In humans, sebum synthesis and secretion peak during adolescence and decline with age thereafter, resulting in approximately one in three elderly individuals experiencing the condition of dry skin [13,14]. Sebum lubricates the skin to protect against friction and makes it more impervious to moisture. Furthermore, the sebaceous gland transports antioxidants in and on the skin and exhibits a natural light protective activity [31]. It possesses an innate antibacterial activity and has a pro- and anti-inflammatory function [32]. It can regulate the activity of xenobiotics and is actively involved in the wound healing process [33]. In this study, we searched for natural compounds present in seed watermelon fruits that can help fundamentally improve sebum production. The active third fraction, obtained via solid-phase extraction using 20% CH3OH, contained several compounds including coniferyl alcohol, coniferin, and p-coumaryl alcohol (Figure 1). These compounds, classified as cinnamyl alcohol analogues, are precursors of lignin, a major plant cell wall component synthesized from the amino acids phenylalanine and tyrosine. Coniferyl alcohol is poorly water-soluble and possesses a free phenolic hydroxyl group with a high oxidative potential. In fresh shoots of Ginkgo biloba L. and Magnolia liliiflora, this compound undergoes the glycosylation of its phenolic hydroxyl group to give rise to coniferin, which results in increased water solubility and reduced oxidative activity [34]. This modification also facilitates the transport of coniferyl alcohol to the sites of lignin deposition [35].
We herein examined whether lipogenesis in LA-stimulated SZ95 sebocytes was promoted by the coniferyl alcohol, coniferin, p-coumaryl alcohol, and coniferyl alcohol-related compound sinapyl alcohol. According to our observations, coniferyl alcohol enhanced LA-induced lipogenesis in a dose-dependent manner (Figure 3A). Contrastingly, coniferin and p-coumaryl alcohol did not significantly influence lipid synthesis. The lack of activity observed for coniferin is presumably because of the glycosylation of its phenolic hydroxyl group, which likely reduced membrane permeability and cellular uptake [36,37]. Furthermore, we hypothesized that the presence of a methoxy group adjacent to the phenolic hydroxyl group enhanced lipid synthesis in the LA-stimulated SZ95 cells.
We subsequently conducted lipidomic analyses to elucidate these effects further and assess the influence of LA stimulation and coniferyl alcohol pretreatment on sebum synthesis. The total lipid content increased under LA stimulation, and coniferyl alcohol pretreatment further amplified this increase (Figure 4), which was consistent with the observations obtained upon Oil Red O staining. LA functions as a ligand for G protein-coupled receptor 120 (GPR120), which is expressed in various tissues including the small intestine, spleen, adipose tissue, and taste buds [38,39]. When LA binds to GPR120 in 3T3L1 cells, adipogenesis is triggered via the activation of peroxisome proliferator-activated receptor γ and upregulation of key adipogenic genes through intracellular calcium signalling and the extracellular signal-regulated kinase 1/2 signal pathway [40]. LA is also taken up by fatty acid transporters, serving as both an energy source and a major structural component of lipid membranes [41,42]. Once internalized, LA is rapidly converted to fatty acyl-CoA by acyl-CoA synthetase [43,44]. While some quantity of the fatty acyl-CoA is utilized for mitochondrial energy production, the remaining is stored in the form of lipid droplets. Our study showed that the levels of non-LA-containing cardiolipin, phosphatidylglycerol, and phosphatidylinositol either decreased or remained unchanged following LA stimulation and coniferyl alcohol pre-treatment, whereas the levels of LA-containing compounds increased (Figure 4). These results suggest that LA was selectively taken up and incorporated into specific lipid species in the SZ95 sebocytes, thereby directly contributing to lipid synthesis. We intend to continue investigating the mechanisms governing LA-induced lipogenesis in SZ95 sebocytes as well as the lipogenesis-promoting effects of coniferyl alcohol under LA stimulation.
Overall, our studies demonstrated that coniferyl alcohol isolated from the seed watermelon fruit promoted lipogenesis in LA-stimulated SZ95 sebocytes. These findings suggest that seed watermelon can serve as a raw ingredient in the development of newer skin topical applications and that remedies containing seed watermelon fruit extracts in combination with LA may benefit individuals with dry skin. These identified active compounds are lignin precursors, which is significant as lignin is a component of the seed coat. Related studies have shown that pomegranate cultivars possessing hard seeds accumulated higher levels of coniferyl alcohol and sinapyl alcohol in the inner seed coat than soft-seeded varieties did [45]. The seed size and seed coat thickness in watermelons vary depending on the cultivated variant, with seed watermelons possessing a relatively thicker seed coat than that of other variants [46,47]. If watermelons exhibit a trend similar to that of pomegranates, varietal differences in lignin precursor contents can be expected. Therefore, we aim to continue screening and selecting watermelon varieties to identify those with the optimal potential to promote sebum synthesis.
4. Materials and Methods
4.1. Materials
Coniferin was purchased from MedChemExpress (Monmouth Junction, NJ, USA). Coniferyl alcohol was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Osaka, Japan). Sinapyl alcohol was purchased from Cayman Chemical (Ann Arbor, MI, USA).
4.2. Isolation
Whole seed watermelon fruits were crushed, pressed, and concentrated to obtain the seed watermelon fruit extract. The crude extract was dissolved in a small volume of H2O, applied to an InertSep C18 column (10 g), and eluted stepwise with the following solvents: 100% H2O; 9:1, 4:1, 7:3, 3:2, and 1:1 (v/v) H2O–CH3OH; and 100% CH3OH (60 mL each). The third fraction obtained in 4:1 H2O–CH3OH was further purified via reverse-phase HPLC employing a C-18 packed column (10 mm × 250 mm; PEGASIL ODS sp100, Senshu Scientific Co., Tokyo, Japan) under the following conditions: 20–40% aq. CH3OH used as solvent, linear gradient of 30 min; flow rate of 3.0 mL/min, and detection using ultraviolet (UV) light at a wavelength of 254 nm.
4.3. Cell Culture
The immortalized human sebaceous gland cell line SZ95 was obtained from Professor Christos C. Zouboulis (Staedtisches Klinikum Dessau, Dessau, Germany) [48]. The cells were cultured in EpiLifeTM medium supplemented with 60 µM calcium (Thermo Fisher Scientific, Waltham, MA, USA), 10% heat-inactivated fetal bovine serum (CORNING, Corning, NY, USA), penicillin (50 U/mL), streptomycin (50 µg/mL), and recombinant human epidermal growth factor (0.2 µg/mL, Thermo Fisher Scientific) in a humidified atmosphere containing 5% CO2 at 37 °C.
4.4. Cell Viability Assay
The SZ95 cells were seeded into 24-well plates (2 × 105 cells/well) and cultured for 48 h. After 48 h of incubation, the cells were treated with coniferyl alcohol, coniferin, p-coumaryl alcohol, and sinapyl alcohol for 24 h at final concentrations of 100, 200, and 300 µM. Subsequently, the culture medium was replaced with fresh medium containing 10% WST-1 (Premix WST-1 Cell Proliferation Assay System, Takara Bio Inc., Ohtsu, Shiga, Japan). After incubation at 37 °C for 1 h, the absorbance was measured at wavelengths of 450 and 690 nm using a colorimetric microplate reader (Varioskan LUX, Thermo Fisher Scientific). The percentage of viable cells was calculated using Equation (1) as follows.
4.5. Oil Red O Staining
The SZ95 cells were seeded into 24-well plates (2 × 105 cells/well) and cultured for 48 h. After 48 h of incubation, the cells were pre-treated with coniferyl alcohol, coniferin, p-coumaryl alcohol, and sinapyl alcohol for 2 h at final concentrations of 100, 200, and 300 µM. Subsequently, the cells were stimulated with and without LA at a final concentration of 100 µM (Sigma-Aldrich, St. Louis, MO, USA) for 24 h. After 24 h of incubation, the culture medium was replaced with fresh medium containing 10% WST-1 (Premix WST-1 Cell Proliferation Assay System, Takara Bio Inc.). After incubation at 37 °C for 1 h, the absorbance was measured at wavelengths of 450 and 690 nm using a colorimetric microplate reader (Varioskan LUX, Thermo Fisher Scientific). The cells were then washed twice using phosphate-buffered saline (PBS)(-) and fixed in 10% formalin at 4 °C overnight. The fixed cells were also washed twice using PBS(-) and stained for 1 h using a staining solution composed of Oil Red O dye (Cosmo Bio Co., Ltd., Tokyo, Japan) and ultrapure H2O in a v/v ratio of 3:2. After the staining process, the cells were washed using distilled H2O and dried at 25 °C for 1 h. The cells stained with Oil Red O were observed under a microscope (BZ-X800, Keyence Co., Ltd., Osaka, Japan) with a 40× objective lens. The Oil Red O content dissolved in the lipid droplets was extracted using 2-propanol, after which the absorbance was measured at a wavelength of 540 nm using a colorimetric microplate reader (Varioskan LUX, Thermo Fisher Scientific). The lipogenesis (ratio) was calculated using Equation (2) as follows.
4.6. Non-Targeted Lipidomics Analysis (LC-ESI/MS/MS Analysis)
4.6.1. Lipid Preparation
Methanol, isopropanol, and chloroform of ultra-performance LC/MS quality were obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Ultrapure water was obtained from a Milli-Q water system (Millipore, Milford, MA, USA). Lipidome analysis was conducted using the Lipidome lab Non-targeted Lipidome Scan package (Lipidome lab, Akita, Japan) via LC orbitrap MS, as per methods described previously [49,50]. As part of the extraction of lipid metabolites from the SZ95 sebocytes, the cells were seeded in 6-well plates at a density of 2 × 105 cells/mL and treated with and without coniferyl alcohol at a final concentration of 300 µM following LA stimulation for 24 h. The cells were then washed with PBS(-), harvested, dissolved with methanol, and homogenized. The total lipid content in this solution was extracted using the Bligh and Dyer liquid–liquid extraction methods. An aliquot of the lower/organic phase was evaporated to dryness under nitrogen gas, and the residue was re-dissolved in methanol for LC-MS/MS measurements.
4.6.2. Instrumental Analysis and Data Processing
All internal standard reagents were purchased from Avanti Polar Lipids (Alabaster, AL, USA). LC-ESI/MS/MS was performed using a Q-Exactive Plus mass spectrometer with an UltiMate 3000 LC system (Thermo Fisher Scientific). Samples were separated on an L-column3 C18 metal-free column (2.0 µm, 2.0 mm × 100 mm i.d.) at 40 °C using a gradient solvent system. Mobile phase A comprised a mixture of isopropanol, methanol, and water (5/1/4 v/v/v) supplemented with 5 mM ammonium formate and 0.05% ammonium hydroxide (28% in water). Mobile phase B comprised isopropanol supplemented with 5 mM ammonium formate and 0.05% ammonium hydroxide (28% in water). The gradient flow consisted of the following ratios: 60% A/40% B (0 min), 40% A/60% B (0–1 min), 20% A/80% B (1–9 min), 5% A/95% B (9–11 min), 5% A/95% B (11–22 min), 95% A/5% B (22–22.1 min), 95% A/5% B (22.1–25 min), 60% A/40% B (25–25.1 min), and 60% A/40% B (25.1–30 min). The injection volume was 10 µL, and the flow rate was 0.1 mL/min. Conditions for the heated electrospray ionization source were as follows: ionization mode, positive or negative; sheath gas, 60 arbitrary units; auxiliary gas, 10 arbitrary units; sweep gas, 0 arbitrary units; spray voltage, 3.2 kV in positive mode and −3.0 kV in negative mode; heater temperature, 325 °C; ion transfer capillary temperature, 300 °C in positive mode and −320 °C in negative mode; and S-lens RF level, 50. The Orbitrap mass analyzer was operated at a resolving power of 70,000 in full-scan mode (scan range: 200–1800 m/z in both positive and negative modes; automatic gain control (AGC) target of 1e6 in positive mode and 3e6 in negative mode) and a resolving power of 17,500 in positive mode and 35,000 in negative mode in the Top 20 data-dependent MS2 mode (stepped normalized collision energy: 20, 30, and 40; isolation window: 4.0 m/z; AGC target: 1e5) with a dynamic exclusion setting of 10.0 s.
Post-processing of the raw data files was performed using the lipid molecular identification software, Lipid Search 5.1 (Mitsui Knowledge Industries Co., Ltd., Tokyo, Japan). This software identifies individual intact lipid molecules based on their molecular weight, fragmentation patterns, and headgroup and fatty acid compositions. In this method, biological matrix effects cannot be normalized across all detected peaks, because it is not possible to prepare appropriate internal standards for all the detected peaks. The relative values were calculated using the ratios of the chromatographic peak areas of each analyte to that of the total analyte peak. However, for some lipid classes, values were calculated by normalizing them using internal standards. The quantification and annotation methods used in this study correspond to the “absolute quantification Level 2 or 4” and “Fatty Acyl/Alkyl Level or Hydroxyl Group Level” defined by the Lipidomics Standard initiative, respectively [51].
4.7. Statistical Analysis
All data were analyzed using the Mac statistical analysis software package for Macintosh (version 2.0; Esumi Co., Tokyo, Japan). All data and results are expressed as means ± standard error. Statistical significance was assessed using the Tukey–Kramer test or Student’s t-test, with * p values < 0.05 and ** p values < 0.01 considered as statistically significant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30163360/s1, Figure S1: The effect of each fraction separated from seed watermelon fruit on lipogenesis in LA-stimulated SZ95 sebocytes, Figure S2: The effect of each fraction separated from the third Fr. on lipogenesis in LA-stimulated SZ95 sebocytes.
Author Contributions
Conceptualization, S.F. and T.I.; methodology, T.F. and T.I.; software, T.F. and T.I.; validation, S.I., T.F. and T.I.; formal analysis, S.F.; investigation, S.I., T.F. and T.I.; resources, S.F., C.C.Z., T.F., T.H. and T.I.; data curation, S.F.; writing—original draft preparation, S.F., T.F. and T.I.; writing—review and editing, S.F., T.F. and T.I.; visualization, S.F. and T.F.; supervision, T.H. and T.I.; project administration, S.F. and T.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the article and Supplementary Materials.
Conflicts of Interest
Authors Shingo Fujita and Toshiharu Hashizume were employed by the Hagihara Farm Co., Ltd. Christos C. Zouboulis has received thematically relevant honoraria as a consultant for AccureAcne, Almirall, Estée Lauder, L’Oréal, NAOS-BIODERMA, and PPM, and lecture honoraria from NAOS-BIODERMA and L’Oréal. His departments have received grants from his participation as a research investigator for Brandenburg Medical School Theodor Fontane, EADV, European Union, German Federal Ministry of Education and Research, and Relaxera. He is President of the EHSF e.V., President of the Deutsches Register Morbus Adamantiades-Behçet e.V., Board member of the International Society for Behçet’s Disease, coordinator of the ALLOCATE Skin group of the ERN Skin, and chair of the ARHS Task Force group of the EADV. He is Editor of the EADV News, and co-copyright holder of IHS4 on behalf of the EHSF e.V. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| UV | Ultraviolet |
| DHT | 5α-dihydrotestosterone |
| LA | Linoleic acid |
| HPLC | High-performance liquid chromatography |
| CA | Coniferyl alcohol |
| Cer | Ceramide |
| ChE | Cholesterol ester |
| CL | Cardiolipin |
| MG | Monoglyceride |
| DG | Diglyceride |
| TG | Triglyceride |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| PI | Phosphatidylinositol |
| PS | Phosphatidylserine |
| SM | Sphingomyelin |
| LC-ESI/MS/MS | Liquid chromatography-electrospray ionization tandem mass spectrometry |
| GPR120 | G protein-coupled receptor 120 |
| PBS | Phosphate-buffered saline |
| AGC | Automatic gain control |
References
- Wróbel, A.; Seltmann, H.; Fimmel, S.; Müller-Decker, K.; Tsukada, M.; Bogdanoff, B.; Mandt, N.; Blume-Peytavi, U.; Orfanos, C.E.; Zouboulis, C.C. Differentiation and apoptosis in human immortalized sebocytes. J. Investig. Dermatol. 2003, 120, 175–181. [Google Scholar] [CrossRef]
- Picardo, M.; Ottaviani, M.; Camera, E.; Mastrofrancesco, A. Sebaceous gland lipids. Derm. Endocrinol. 2009, 1, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, C.; Schneider, M.R. Genetically modified laboratory mice with sebaceous glands abnormalities. Cell. Mol. Life Sci. 2016, 73, 4623–4642. [Google Scholar] [CrossRef]
- Nikkari, T. Comparative chemistry of sebum. J. Investig. Dermatol. 1974, 62, 257–267. [Google Scholar] [CrossRef]
- Fritsch, M.; Orfanos, C.E.; Zouboulis, C.C. Sebocytes are the key regulators of androgen homeostasis in human skin. J. Investig. Dermatol. 2001, 116, 793–800. [Google Scholar] [CrossRef]
- Akamatsu, H.; Zouboulis, C.C.; Orfanos, C.E. Control of human sebocyte proliferation in vitro by testosterone and 5-alpfa-dihydrotestosterone is dependent on the localization of the sebaceous glands. J. Investig. Dermatol. 1992, 99, 509–511. [Google Scholar] [CrossRef]
- Mirdamadi, Y.; Thielitz, A.; Wiede, A.; Goihl, A.; Papakonstantinou, E.; Hartig, R.; Zouboulis, C.C.; Reinhold, D.; Simeoni, L.; Bommhardt, U.; et al. Insulin and insulin-like growth factor-1 can modulate the phosphoinositide-3-kinase/Akt/FoxO1 pathway in SZ95 sebocytes in vitro. Mol. Cell. Endocrinol. 2015, 415, 32–44. [Google Scholar] [CrossRef]
- Li, Z.J.; Park, S.B.; Sohn, K.C.; Lee, Y.; Seo, Y.J.; Kim, C.D.; Kim, Y.S.; Lee, J.H.; Im, M. Regulation of lipid production by acetylcholine signalling in human sebaceous glands. J. Dermatol. Sci. 2013, 72, 116–122. [Google Scholar] [CrossRef]
- Seo, S.H.; Jung, J.Y.; Park, K.; Hossini, A.M.; Zouboulis, C.C.; Lee, S.E. Autophagy regulates lipid production and contributes to the sebosuppressive effect of retinoic acid in human SZ95 sebocytes. J. Dermatol. Sci. 2020, 98, 128–136. [Google Scholar] [CrossRef]
- Deplewski, D.; Qin, K.; Ciletti, N.; Rosenfield, R.L. Unique mode of lipogenic activation in rat preputial sebocytes. J. Nutr. Metab. 2011, 2011, 163631. [Google Scholar] [CrossRef]
- Dozsa, A.; Dezso, B.; Toth, B.I.; Bacsi, A.; Poliska, S.; Camera, E.; Picardo, M.; Zouboulis, C.C.; Bíró, T.; Schmitz, G.; et al. PPARγ-mediated and arachidonic acid–dependent signaling is involved in differentiation and lipid production of human sebocytes. J. Investig. Dermatol. 2014, 134, 910–920. [Google Scholar] [CrossRef]
- Pochi, P.E.; Strauss, J.S.; Downing, D.T. Age-related changes in sebazeous gland activity. J. Investig. Dermatol. 1979, 73, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, Y.; Liu, Y.; Zhu, D.; Xie, Y.; Zhao, J.; Weng, Y.; Tang, Y.; Feng, H.; Li, Y.; et al. Prevalence and associated factors of dry skin among older inpatients in hospitals and nursing homes: A multicenter cross-sectional study. Int. J. Nurs. Stud. 2022, 135, 104358. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Kirsten, N.; Körber, A.; Wilsmann-Theis, D.; Itschert, G.; Staubach-Renz, P.; Maul, J.-T.; Zander, N. Prevalence, predictors and comorbidity of dry skin in the general population. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Saeki, H.; Tsunemi, Y.; Arai, S.; Ichiyama, S.; Katoh, N.; Kikuchi, K.; Akiharu, K.; Terui, T.; Nakahara, T.; Futamaru, M.; et al. Sebum deficiency treatment guide 2021. Jpn. J. Dermatol. 2021, 131, 2255–2270. [Google Scholar] [CrossRef]
- Kobayashi, M. The effect of topically applied triterpineol ester with ferulic acid (FAE) on sebaceous glands. Ski. Res. 1979, 21, 18–34. [Google Scholar]
- Nouveau, S.; Bastien, P.; Baldo, F.; Lacharriere, O. Effects of topical DHEA on aging skin: A pilot study. Maturitas 2008, 59, 174–181. [Google Scholar] [CrossRef]
- Baulieu, E.-E.; Thomas, G.; Legrain, S.; Lahlou, N.; Roger, M.; Debuire, B.; Faucounau, V.; Girard, L.; Hervy, M.P.; Latour, F.; et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: Contribution of the DHEAge study to a sociobiomedical issue. Proc. Natl. Acad. Sci. USA 2000, 97, 4279–4284. [Google Scholar] [CrossRef]
- Coondoo, A.; Phiske, M.; Verma, S.; Lahiri, K. Side-effects of topical steroids a long overdue revisit. Indian Dermatol. Online J. 2014, 5, 416–425. [Google Scholar] [CrossRef]
- Dhar, S.; Seth, J.; Parikh, D. Systemic side-effects of topical corticosteroids. Indian J. Dermatol. 2014, 59, 460–464. [Google Scholar] [CrossRef]
- Abraham, A.; Roga, G. Topical steroid-damaged skin. Indian J. Dermatol. 2014, 59, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Matsumoto, M. Sebum Secretagogue. Japan Patent JPH082772B2, 26 September 1990. [Google Scholar]
- Hanamura, A.; Sato, A. Sebum Secretion Promoter. Japan Patent JPH07109214A, 2 June 1993. [Google Scholar]
- Malambane, G.; Madumane, K.; Sewelo, L.T.; Batlang, U. Drought stress tolerance mechanisms and their potential common indicators to salinity, insights from the wild watermelon (Citrullus lanatus): A review. Front. Plant Sci. 2022, 13, 1074395. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, T. Breeding and utilization of watermelon. Jpn. Soc. Food Sci. Technol. 2019, 66, 314–318. [Google Scholar] [CrossRef]
- Masoko, P.; Matotoka, M.M.; Mphosi, M.S. Phytochemical analysis and antibacterial activity of Citrullus lanatus var citroides (Citron watermelon) fruit and the effect of temperature on the biological activity of the rind. S. Afr. J. Bot. 2022, 150, 1111–1121. [Google Scholar]
- Gade, S.R.; Meghwal, M.; Prabhakar, P.K.; Giuffrè, A.M. A comparative study on the nutritional, antioxidant, thermal, morphological and diffraction properties of selected cucurbit seeds. Agronomy 2022, 12, 2242. [Google Scholar] [CrossRef]
- Ren, R.; Xu, J.; Zhang, M.; Liu, G.; Yao, X.; Zhu, L.; Hou, Q. Identification and molecular mapping of a gummy stem blight resistance gene in wild watermelon (Citrullus amarus) germplasm PI 189225. Plant Dis. 2020, 104, 16–24. [Google Scholar] [CrossRef]
- Kawasaki, S.; Miyake, C.; Kohchi, T.; Fujii, S.; Uchida, M.; Yokota, A. Responses of wild watermelon to drought stress: Accumulation of an ArgE homologue and citrulline in leaves during water deficits. Plant Cell Physiol. 2000, 41, 864–873. [Google Scholar] [CrossRef]
- Whitaker, B.D.; Schmidt, W.F.; Kirk, M.C.; Barnes, S. Novel fatty acid esters of p-coumaryl alcohol in epicuticular wax of apple fruit. J. Agric. Food Chem. 2001, 49, 3787–3792. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Picardo, M.; Ju, Q.; Kurokawa, I.; Törőcsik, D.; Bíró, T.; Schneider, M.R. Beyond acne: Current aspects of sebaceous gland biology and function. Rev. Endocr. Metab. Disord. 2016, 17, 319–334. [Google Scholar] [CrossRef]
- Zouboulis, C.C. Acne and sebaceous gland function. Clin. Dermatol. 2004, 22, 360–366. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Yoshida, G.J.; Wu, Y.; Xia, L.; Schneider, M.R. Sebaceous gland: Milestones of 30-year modelling research dedicated to the “brain of the skin”. Exp. Dermatol. 2020, 29, 1069–1079. [Google Scholar] [CrossRef]
- Terashima, N.; Ko, C.; Matsushita, Y.; Westermark, U. Monolignol glucosides as intermediate compounds in lignin biosynthesis. Revisiting the cell wall lignification and new 13C-tracer experiments with Ginkgo biloba and Magnolialiliiflora. Holzforschung 2016, 70, 801–810. [Google Scholar] [CrossRef]
- Terashima, N.; Matsushita, Y.; Yagami, S.; Nishimura, H.; Yoshida, M.; Fukushima, K. Role of monolignol glucosides in supramolecular assembly of cell wall components in ginkgo xylem formation. Holzforschung 2023, 77, 485–499. [Google Scholar] [CrossRef]
- Dai, J.Y.; Yang, J.I.; Li, C. Transport and metabolism of flavonoids from Chinese herbal remedy Xiaochaihu-tang across human intestinal Caco-2 cell monolayers. Acta Pharmacol. Sin. 2008, 29, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Membrane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules 2015, 20, 18923–18966. [Google Scholar] [CrossRef]
- Mao, C.; Xiao, P.; Tao, X.-N.; Qin, J.; He, Q.-T.; Zhang, C.; Guo, S.-C.; Du, Y.-Q.; Chen, L.-N.; Shen, D.-D.; et al. Unsaturated bond recognition leads to biased signal in a fatty acid receptor. Science 2023, 380, eadd6220. [Google Scholar] [CrossRef]
- Song, T.; Yang, Y.; Zhou, Y.; Wei, H.; Peng, J. GPR120: A critical role in adipogenesis, inflammation, and energy metabolism in adipose tissue. Cell. Mol. Life Sci. 2017, 74, 2723–2733. [Google Scholar] [CrossRef]
- Song, T.; Zhou, Y.; Peng, J.; Tao, Y.X.; Yang, Y.; Xu, T.; Peng, J.; Ren, J.; Xiang, Q.; Wei, H. GPR120 promotes adipogenesis through intracellular calcium and extracellular signal-regulated kinase 1/2 signal pathway. Mol. Cell. Endocrinol. 2016, 434, 1–13. [Google Scholar] [CrossRef]
- Pohl, J.; Ring, A.; Hermann, T.; Stremmel, W. Role of FATP in parenchymal cell fatty acid uptake. BBA-Mol. Cell Biol. L. 2004, 1686, 81–87. [Google Scholar] [CrossRef]
- Abumrad, N.; Coburn, C.; Ibrahimi, A. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36, FATP and FABPm. BBA-Mol. Cell Biol. L. 1999, 1441, 4–13. [Google Scholar] [CrossRef]
- Coe, N.R.; Smith, A.J.; Frohnert, B.I.; Watkins, P.A.; Bernlohr, D.A. The fatty acid transport protein (FATP1) is a very long chain acyl-CoA synthetase. J. Biol. Chem. 1999, 274, 36300–36304. [Google Scholar] [CrossRef]
- Milger, K.; Herrmann, T.; Becker, C.; Gotthardt, D.; Zickwolf, J.; Ehehalt, R.; Watkins, P.A.; Stremmel, W.; Füllekrug, J. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J. Cell Sci. 2006, 119, 4678–4688. [Google Scholar] [CrossRef]
- Qin, G.; Liu, C.; Li, J.; Qi, Y.; Gao, Z.; Zhang, X.; Yi, X.; Pan, H.; Ming, R.; Xu, Y. Diversity of metabolite accumulation patterns in inner and outer seed coats of pomegranate: Exploring their relationship with genetic mechanisms of seed coat development. Hortic. Res. 2020, 7, 10. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, M.; Liang, X.; Xu, M.; Liu, X.; Zhang, Y.; Liu, X.; Liu, J.; Gao, Y.; Qu, S.; et al. Quantitative trait loci for seed size variation in Cucurbits—A review. Front. Plant Sci. 2020, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Zhao, S.; Yang, D.; Lu, X.; Anees, M.; He, N.; Zhu, H.; Zhao, Y.; Liu, W. Genome-wide association analysis provides molecular insights into natural variation in watermelon seed size. Hortic. Res. 2022, 9, uhab074. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Seltmann, H.; Neitzel, H.; Orfanos, C.E. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95). J. Investig. Dermatol. 1999, 113, 1011–1020. [Google Scholar] [CrossRef]
- Nishiumi, S.; Izumi, Y.; Hirayama, A.; Takahashi, M.; Nakao, M.; Hata, K.; Saigusa, D.; Hishinuma, E.; Matsukawa, N.; Tokuoka, S.M.; et al. Comparative evaluation of plasma metabolomic data from multiple laboratories. Metabolites 2022, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Takumi, H.; Kato, K.; Nakanishi, H.; Tamura, M.; Ohto-N, T.; Nagao, S.; Hirose, J. Comprehensive analysis of lipid composition in human foremilk and hindmilk. J. Oleo Sci. 2022, 71, 947–957. [Google Scholar] [CrossRef]
- Liebisch, G.; Ahrends, R.; Arita, M.; Arita, M.; Bowden, J.A.; Ejsing, C.; Griffiths, W.; Holcapek, M.; Köfeler, H.; Mitchell, T.; et al. Lipidomics needs more standardization. Nat. Metab. 2019, 1, 745–747. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).