Unveiling the Power of Computational Tools in Chiral Liquid Chromatography
Abstract
1. Introduction
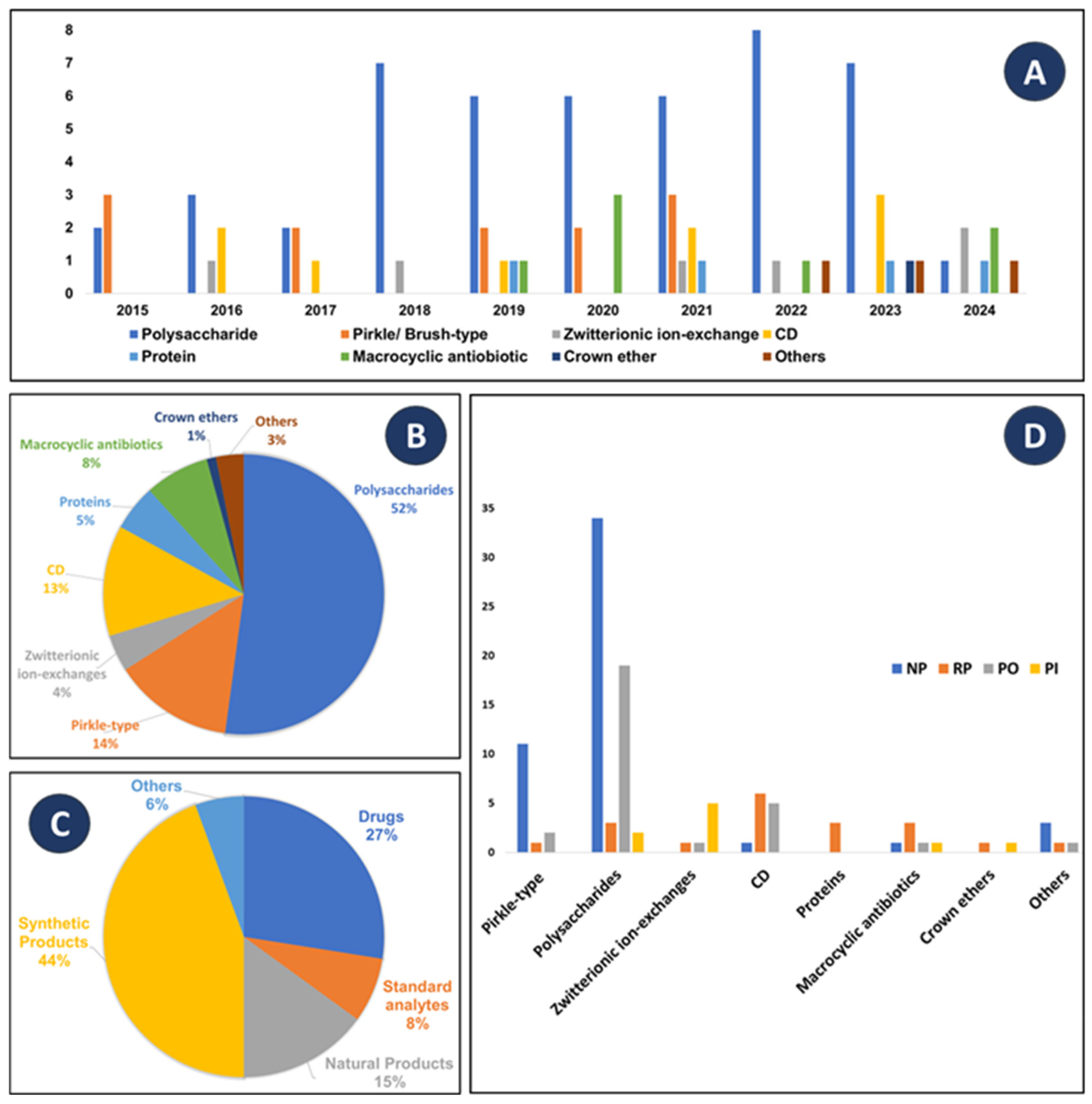
2. Molecular Modeling in Chiral Liquid Chromatography
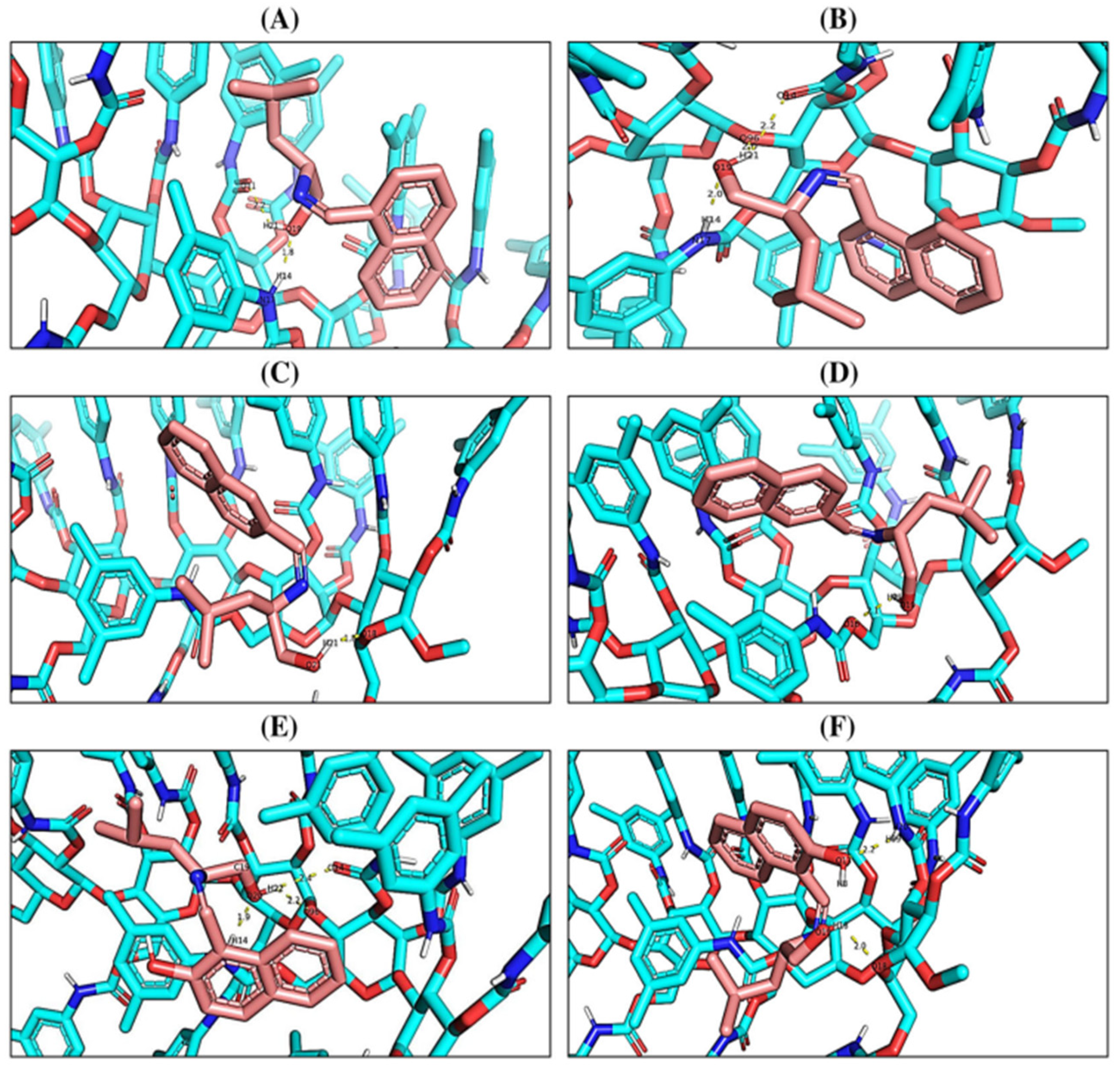
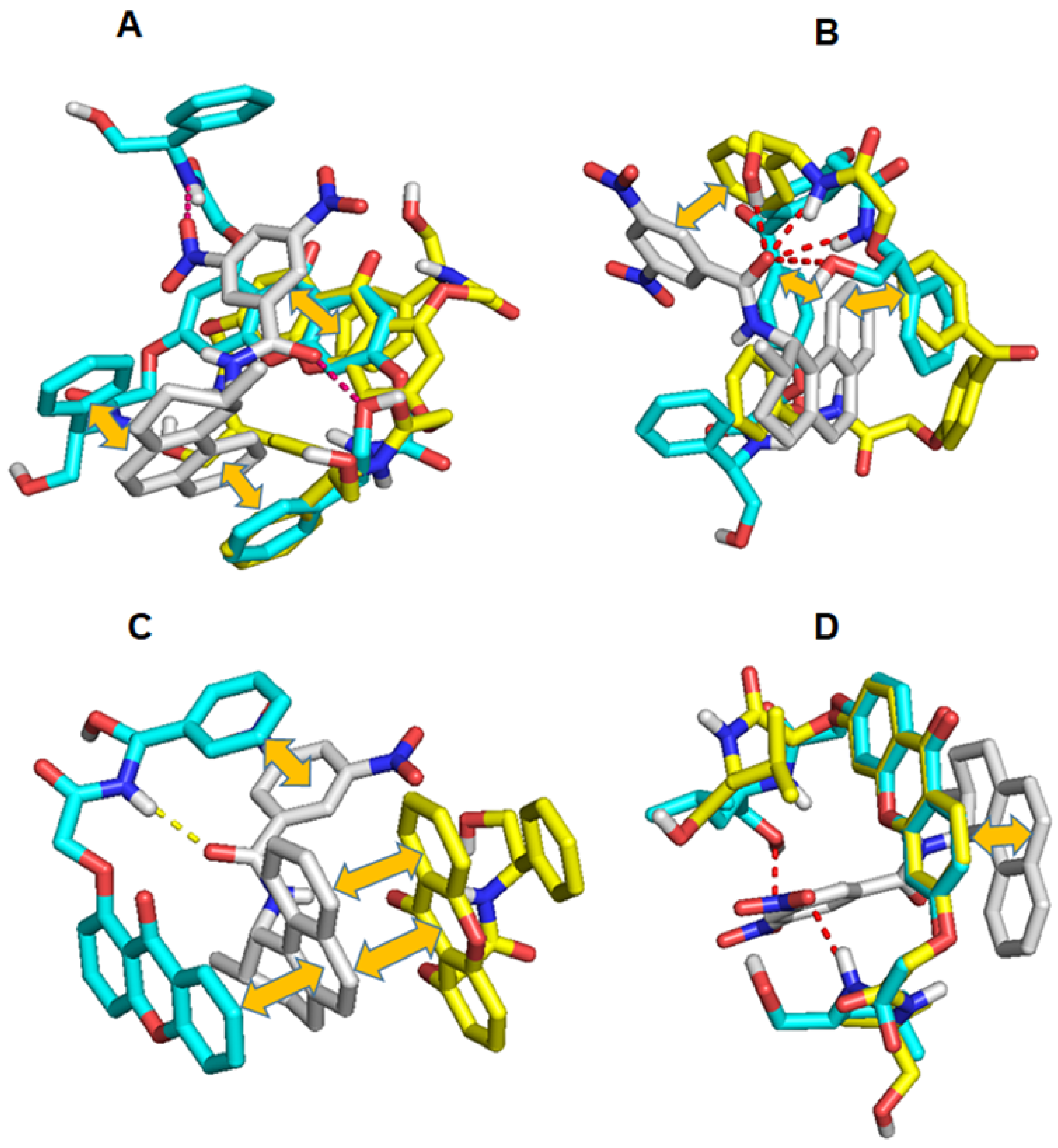
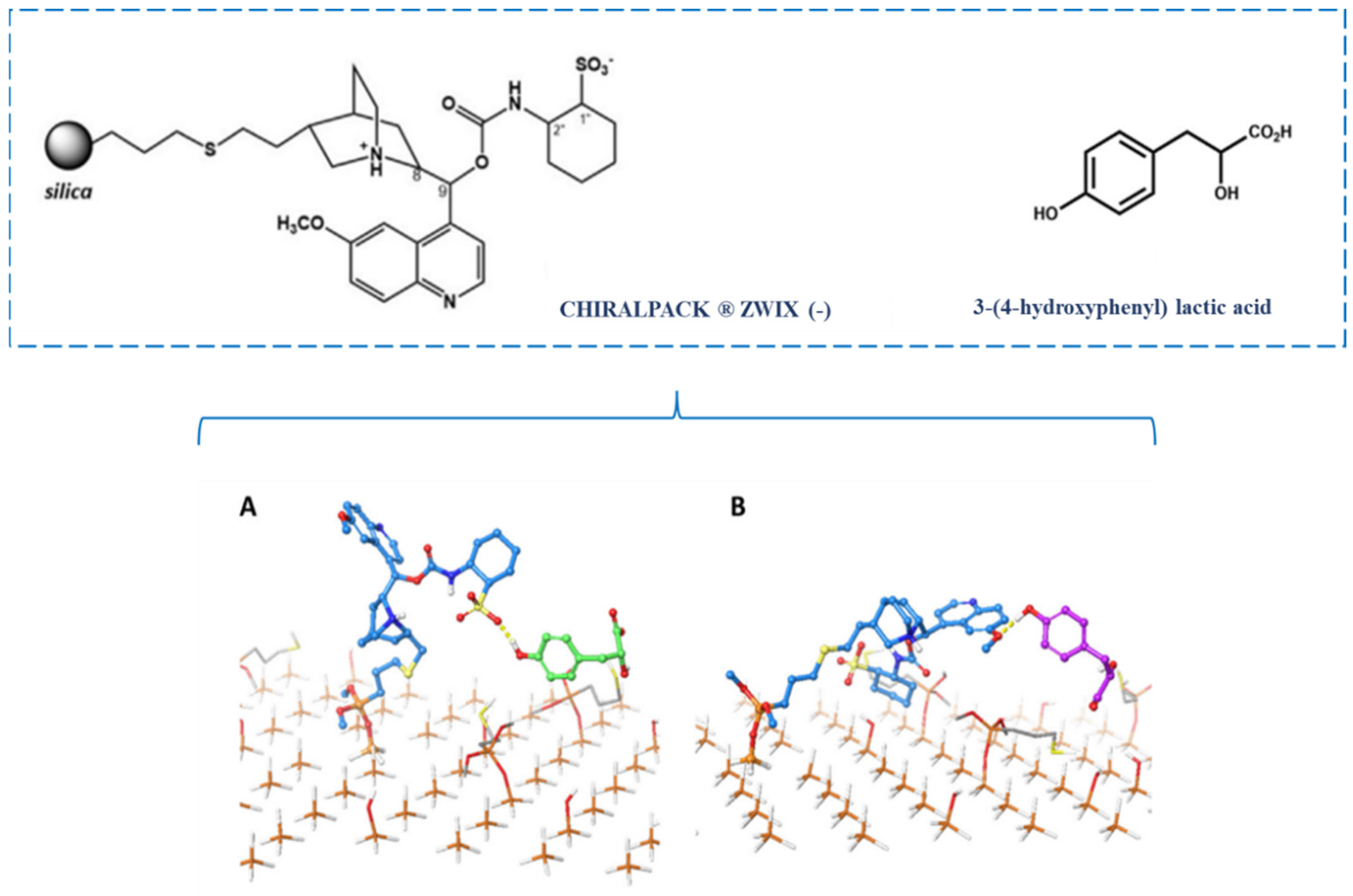
2.1. Molecular Docking
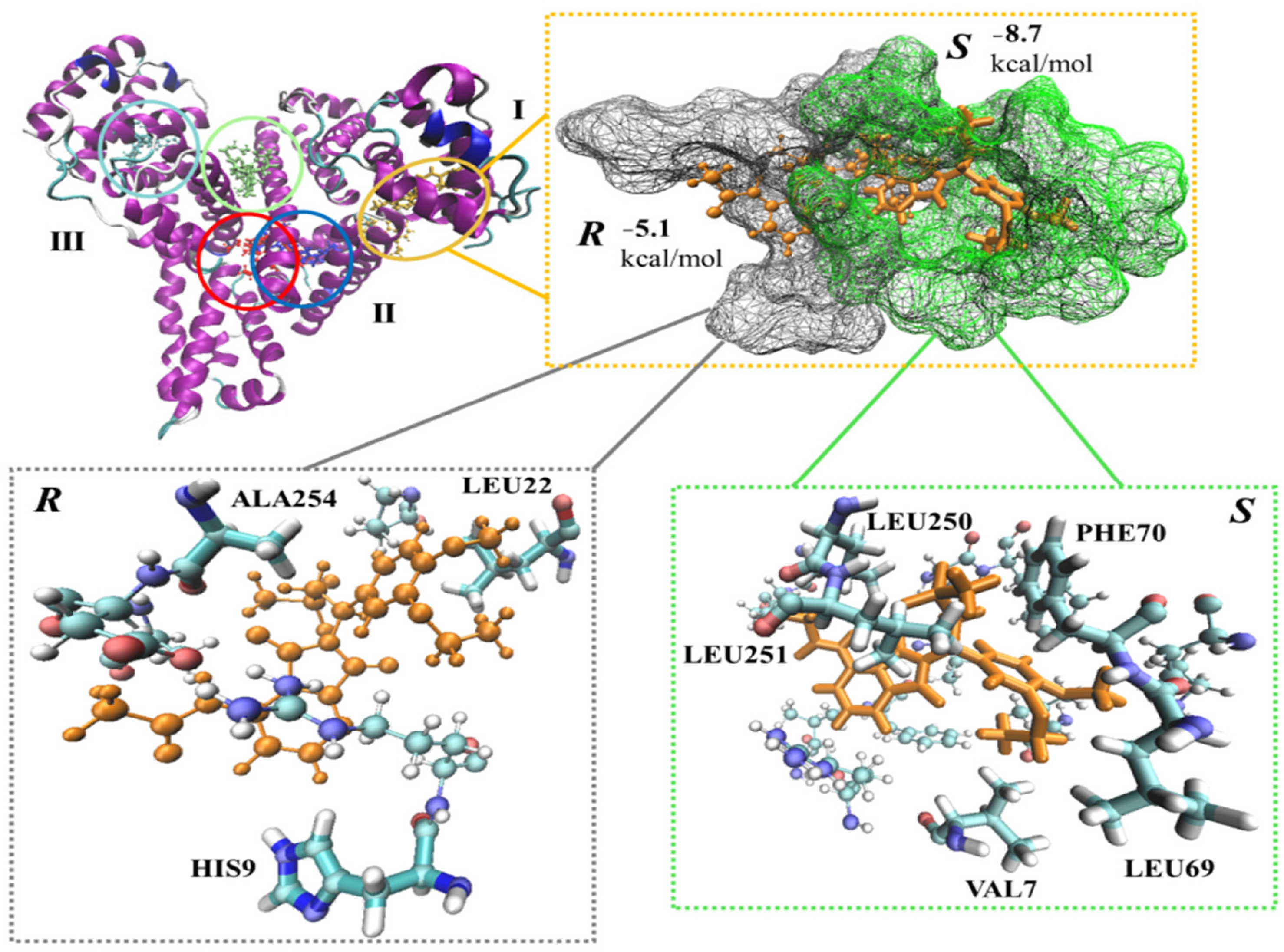
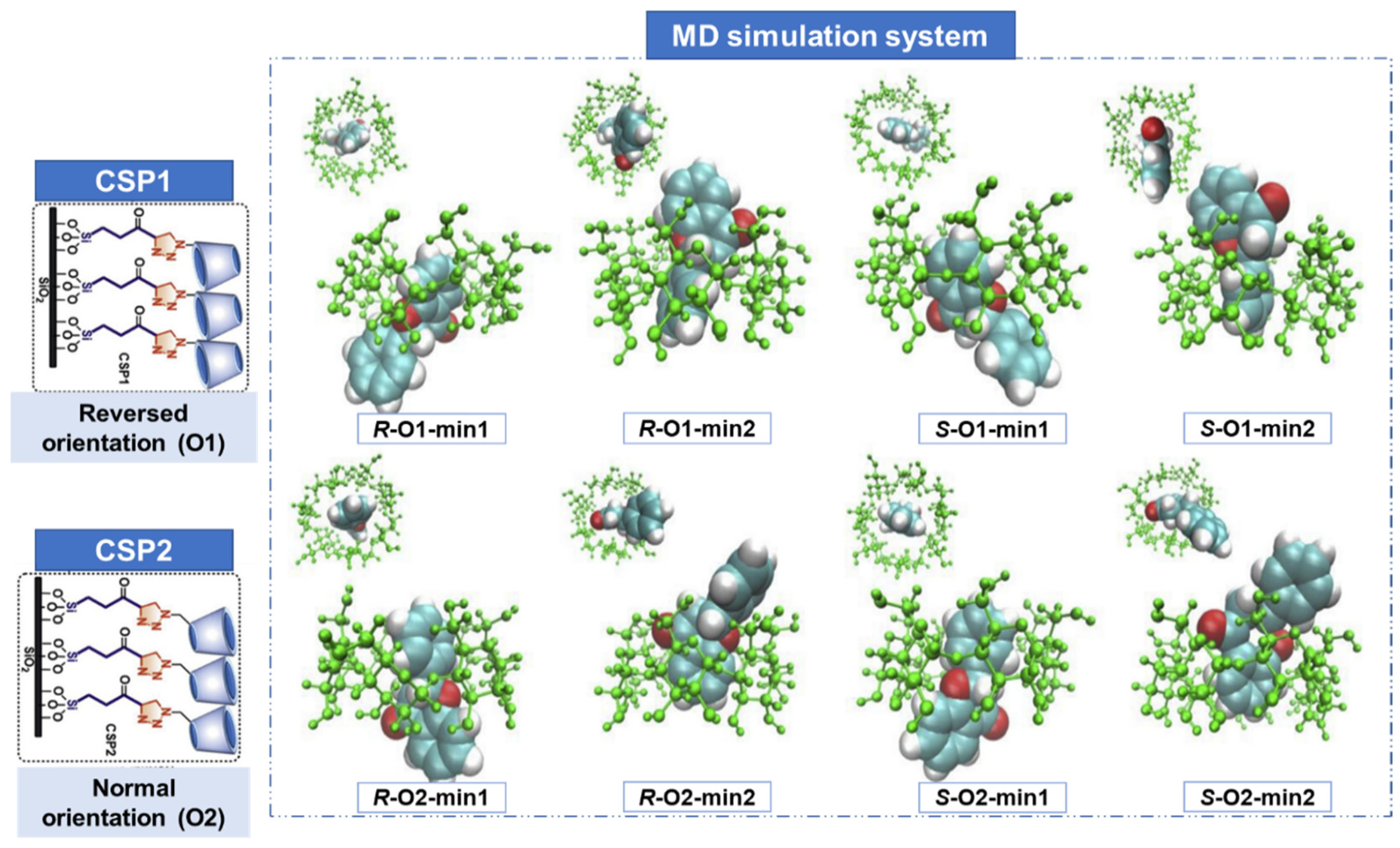
2.2. Molecular Dynamics
2.3. Other Computational Approaches
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rocco, A.; Aturki, Z.; Fanali, S. Chiral separations in food analysis. TrAC Trends Anal. Chem. 2013, 52, 206–225. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Cifuentes, A. Chiral analysis in food science. TrAC Trends Anal. Chem. 2020, 123, 115761. [Google Scholar] [CrossRef]
- Sardella, R.; Ianni, F.; Marinozzi, M.; Macchiarulo, A.; Natalini, B. Laboratory-scale preparative enantioseparations of pharmaceutically relevant compounds on commercially available chiral stationary phases for HPLC. Curr. Med. Chem. 2017, 24, 796–817. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Liu, X.; Yang, X.; Zhao, Z. Recent application of chiral liquid chromatography–tandem mass spectrometric methods for enantiomeric pharmaceutical and biomedical determinations. J. Chromatogr. Sci. 2013, 51, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Maia, A.S.; Ribeiro, C.; Tiritan, M.E. Analysis of chiral drugs in environmental matrices: Current knowledge and trends in environmental, biodegradation and forensic fields. TrAC Trends Anal. Chem. 2020, 124, 115783. [Google Scholar] [CrossRef]
- Barreiro, J.C.; Tiritan, M.E.; Cass, Q.B. Challenges and innovations in chiral drugs in an environmental and bioanalysis perspective. TrAC Trends Anal. Chem. 2021, 142, 116326. [Google Scholar] [CrossRef]
- Coelho, M.M.; Fernandes, C.; Remião, F.; Tiritan, M.E. Enantioselectivity in drug pharmacokinetics and toxicity: Pharmacological relevance and analytical methods. Molecules 2021, 26, 3113. [Google Scholar] [CrossRef]
- Almeida, A.S.; Silva, B.; Pinho, P.G.; Remião, F.; Fernandes, C. Synthetic cathinones: Recent developments, enantioselectivity studies and enantioseparation methods. Molecules 2022, 27, 2057. [Google Scholar] [CrossRef]
- Miller, L.; Potter, M. Preparative chromatographic resolution of racemates using HPLC and SFC in a pharmaceutical discovery environment. J. Chromatogr. B 2008, 875, 230–236. [Google Scholar] [CrossRef]
- Leek, H.; Andersson, S. Preparative scale resolution of enantiomers enables accelerated drug discovery and development. Molecules 2017, 22, 158. [Google Scholar] [CrossRef]
- Li, M.; Luo, S.; Di, X.; Cui, Y. Ultrasound-assisted extraction coupling to high performance liquid chromatography for enantiomerically quantitative analysis of two preservatives in cosmetics and the potentially cytotoxic study. Microchem. J. 2022, 172, 106937. [Google Scholar] [CrossRef]
- Blehaut, J.; Franco, P.; Zhang, T.; Lang, E.; Valery, E.; Marcoux, J.F. 9.17 Industrial applications of chiral chromatography. Compr. Chirality 2012, 9, 400–456. [Google Scholar]
- Snyder, L.R.; Kirkland, J.J.; Dolan, J.W. Introduction to Modern Liquid Chromatography; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Pinto, M.M.; Fernandes, C.; Tiritan, M.E. Chiral separations in preparative scale: A medicinal chemistry point of view. Molecules 2020, 25, 1931. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, J.; Zhuang, S.; Liu, W. Development of chiral stationary phases for high-performance liquid chromatographic separation. TrAC Trends Anal. Chem. 2012, 39, 180–194. [Google Scholar] [CrossRef]
- Zhang, J.H.; Xie, S.M.; Yuan, L.M. Recent progress in the development of chiral stationary phases for high-performance liquid chromatography. J. Sep. Sci. 2022, 45, 51–77. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.; Fernandes, C. Chiral stationary phases for liquid chromatography: Recent developments. Molecules 2019, 24, 865. [Google Scholar] [CrossRef] [PubMed]
- Nazario, C.E.; Silva, M.R.; Franco, M.S.; Lancas, F.M. Evolution in miniaturized column liquid chromatography instrumentation and applications: An overview. J. Chromatogr. A 2015, 1421, 18–37. [Google Scholar] [CrossRef]
- Fekete, S.; Kohler, I.; Rudaz, S.; Guillarme, D. Importance of instrumentation for fast liquid chromatography in pharmaceutical analysis. J. Pharm. Biomed. Anal. 2014, 87, 105–119. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Z.; Chen, J.; Wang, J.; Qiu, H. Recent advances in chiral liquid chromatography stationary phases for pharmaceutical analysis. J. Chromatogr. A 2023, 1708, 464367. [Google Scholar] [CrossRef]
- Fernandes, C.; Lima, R.; Pinto, M.M.; Tiritan, M.E. Chromatographic supports for enantioselective liquid chromatography: Evolution and innovative trends. J. Chromatogr. A 2022, 1684, 463555. [Google Scholar] [CrossRef]
- Fernandes, C.; Teixeira, J.; Pinto, M.M.; Tiritan, M.E. Strategies for preparation of chiral stationary phases: Progress on coating and immobilization methods. Molecules 2021, 26, 5477. [Google Scholar] [CrossRef] [PubMed]
- Sardella, R.; Camaioni, E.; Macchiarulo, A.; Gioiello, A.; Marinozzi, M.; Carotti, A. Computational studies in enantioselective liquid chromatography: Forty years of evolution in docking-and molecular dynamics-based simulations. TrAC Trends Anal. Chem. 2020, 122, 115703. [Google Scholar] [CrossRef]
- Peluso, P.; Chankvetadze, B. Recent developments in molecular modeling tools and applications related to pharmaceutical and biomedical research. J. Pharm. Biomed. Anal. 2024, 238, 115836. [Google Scholar] [CrossRef] [PubMed]
- Peluso, P.; Dessì, A.; Dallocchio, R.; Mamane, V.; Cossu, S. Recent studies of docking and molecular dynamics simulation for liquid-phase enantioseparations. Electrophoresis 2019, 40, 1881–1896. [Google Scholar] [CrossRef]
- Sardella, R.; Ianni, F.; Macchiarulo, A.; Pucciarini, L.; Carotti, A.; Natalini, B. Elucidation of the chromatographic enantiomer elution order through computational studies. Mini Rev. Med. Chem. 2018, 18, 88–97. [Google Scholar] [CrossRef]
- Dallocchio, R.; Dessì, A.; Sechi, B.; Peluso, P. Molecular dynamics simulations of amylose-and cellulose-based selectors and related enantioseparations in liquid phase chromatography. Molecules 2023, 28, 7419. [Google Scholar] [CrossRef]
- Peluso, P.; Chankvetadze, B. Recognition in the domain of molecular chirality: From noncovalent interactions to separation of enantiomers. Chem. Rev. 2022, 122, 13235–13400. [Google Scholar] [CrossRef]
- Mcconnell, O.; Bach, A.; Balibar, C.; Byrne, N.; Cai, Y.; Carter, G.; Chlenov, M.; Di, L.; Fan, K.; Goljer, I.; et al. Enantiomeric separation and determination of absolute stereochemistry of asymmetric molecules in drug discovery—Building chiral technology toolboxes. Chirality 2007, 19, 658–682. [Google Scholar] [CrossRef]
- Jin, M.Y.; Zhen, Q.; Xiao, D.; Tao, G.; Xing, X.; Yu, P.; Xu, C. Engineered non-covalent π interactions as key elements for chiral recognition. Nat. Commun. 2022, 13, 3276. [Google Scholar] [CrossRef]
- Chen, W.; Fu, L.; Zhu, Z.; Liu, J.; Cheng, L.; Xu, Z.; Dong, H.; Ma, J.; Li, Y.; Fan, X. Synergistic regulation of intermolecular interactions to control chiral structures for chiral recognition. Chin. Chem. Lett. 2023, 34, 107713. [Google Scholar] [CrossRef]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef] [PubMed]
- Cavazzini, A.; Nadalini, G.; Dondi, F.; Gasparrini, F.; Ciogli, A.; Villani, C. Study of mechanisms of chiral discrimination of amino acids and their derivatives on a teicoplanin-based chiral stationary phase. J. Chromatogr. A 2004, 1031, 143–158. [Google Scholar] [CrossRef] [PubMed]
- He, S.; He, Y.; Cheng, L.; Wu, Y.; Ke, Y. Novel chiral ionic liquids stationary phases for the enantiomer separation of chiral acid by high-performance liquid chromatography. Chirality 2018, 30, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Siret, L.; Tambuté, A.; Bégos, A.; Rouden, J.; Caude, M. Steric hindrance influence on the enantiorecognition ability of tyrosine-derived chiral stationary phases. Chirality 1991, 3, 427–435. [Google Scholar] [CrossRef]
- Fernandes, C.; Phyo, Y.Z.; Silva, A.S.; Tiritan, M.E.; Kijjoa, A.; Pinto, M.M. Chiral stationary phases based on small molecules: An update of the last 17 years. Sep. Purif. Rev. 2018, 47, 89–123. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Kannappan, V.; Kanthiah, S. Impact of cyclofructan derivatives as efficient chiral selector in chiral analysis: An overview. Chirality 2022, 34, 364–373. [Google Scholar] [CrossRef]
- Maier, N.M.; Lindner, W. Chiral recognition applications of molecularly imprinted polymers: A critical review. Anal. Bioanal. Chem. 2007, 389, 377–397. [Google Scholar] [CrossRef]
- De Gauquier, P.; Vanommeslaeghe, K.; Vander Heyden, Y.; Mangelings, D. Modelling approaches for chiral chromatography on polysaccharide-based and macrocyclic antibiotic chiral selectors: A review. Anal. Chim. Acta 2022, 1198, 338861. [Google Scholar] [CrossRef]
- Junior, F.M.; Junior, J.M. Absolute configuration from chiroptical spectroscopy. In Chiral Separations and Stereochemical Elucidation: Fundamentals, Methods, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 551–591. [Google Scholar]
- Schneider, H.J. Mechanisms of molecular recognition: Investigations of organic host–guest complexes. Angew. Chem. Int. Ed. 1991, 30, 1417–1436. [Google Scholar] [CrossRef]
- Lipkowitz, K.B. Theoretical studies of type II–V chiral stationary phases. J. Chromatogr. A 1995, 694, 15–37. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Pochapsky, T.C. Considerations of chiral recognition relevant to the liquid chromatography separation of enantiomers. Chem. Rev. 1989, 89, 347–362. [Google Scholar] [CrossRef]
- Bentley, R. Diastereoisomerism, contact points, and chiral selectivity: A four-site saga. Arch. Bichem Biophys. 2003, 414, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Easson, L.H.; Stedman, E. Studies on the relationship between chemical constitution and physiological action: Molecular dissymmetry and physiological activity. Biochem. J. 1933, 27, 1257. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, S.; Abrol, R. Chiral graphs: Reduced representations of ligand scaffolds for stereoselective biomolecular recognition, drug design, and enhanced exploration of chemical structure space. Chem. Med. Chem. 2019, 14, 798–809. [Google Scholar] [CrossRef]
- Ogston, A.G. Interpretation of experiments on metabolic processes, using isotopic tracer elements. Nature 1948, 162, 963. [Google Scholar] [CrossRef]
- Topiol, S.; Sabio, M. Interactions between eight centers are required for chiral recognition. J. Am. Chem. Soc. 1989, 111, 4109–4110. [Google Scholar] [CrossRef]
- Mesecar, A.D.; Koshland Jr, D.E. A new model for protein stereospecificity. Nature 2000, 403, 614–615. [Google Scholar] [CrossRef]
- Dalgliesh, C.E. 756. The optical resolution of aromatic amino-acids on paper chromatograms. J. Chem. Soc. 1952, 3940–3942. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Pochapsky, T.C. A new, easily accessible reciprocal chiral stationary phase for the chromatographic separation of enantiomers. J. Am. Chem. Soc. 1986, 108, 352–354. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Pochapsky, T.C. Chiral molecular recognition in small bimolecular systems: A spectroscopic investigation into the nature of diastereomeric complexes. J. Am. Chem. Soc. 1987, 109, 5975–5982. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Finn, J. 6-Separation of Enantiomers by Liquid Chromatographic Methods. In Asymmetric Synthesis; Morrison, J.D., Ed.; Academic Press: Cambridge, MA, USA, 1983; pp. 87–124. [Google Scholar]
- Davankov, V.A. The nature of chiral recognition: Is it a three-point interaction? Chirality 1997, 9, 99–102. [Google Scholar] [CrossRef]
- Booth, T.D.; Wahnon, D.; Wainer, I.W. Is chiral recognition a three-point process? Chirality 1997, 9, 96–98. [Google Scholar] [CrossRef]
- Kafri, R.; Lancet, D. Probability rule for chiral recognition. Chirality 2004, 16, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Scriba, G.K. Chiral recognition mechanisms in analytical separation sciences. Chromatographia 2012, 75, 815–838. [Google Scholar] [CrossRef]
- Jozwiak, K.; Moaddel, R.; Ravichandran, S.; Plazinska, A.; Kozak, J.; Patel, S.; Yamaguchi, R.; Wainer, I.W. Exploring enantiospecific ligand–protein interactions using cellular membrane affinity chromatography: Chiral recognition as a dynamic process. J. Chromatogr. B 2008, 875, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; House, D.W.; Oroskar, P.A.; Oroskar, A.; Oroskar, A.; Jameson, C.J.; Murad, S. Molecular dynamics simulations of the chiral recognition mechanism for a polysaccharide chiral stationary phase in enantiomeric chromatographic separations. Mol. Phys. 2019, 117, 3569–3588. [Google Scholar] [CrossRef]
- Davankov, V.A. 50 years of chiral liquid Chromatography. How it started. J. Chromatogr. A 2022, 1668, 462910. [Google Scholar] [CrossRef]
- Tiritan, M.E.; Pinto, M.M.; Fernandes, C. Pirkle type. In Chiral Separations and Stereochemical Elucidation: Fundamentals, Methods, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Fernandes, C.; Tiritan, M.E.; Pinto, M.M. Small molecules as chromatographic tools for HPLC enantiomeric resolution: Pirkle-type chiral stationary phases evolution. Chromatographia 2013, 76, 871–897. [Google Scholar] [CrossRef]
- Scriba, G.K. Chiral recognition in separation sciences. Part II: Macrocyclic glycopeptide, donor-acceptor, ion-exchange, ligand-exchange and micellar selectors. TrAC Trends Anal. Chem. 2019, 119, 115628. [Google Scholar] [CrossRef]
- Scriba, G.K. Update on chiral recognition mechanisms in separation science. J. Sep. Sci. 2024, 47, 2400148. [Google Scholar] [CrossRef]
- Li, G.W.; Wang, X.J.; Cui, D.D.; Zhang, Y.F.; Xu, R.Y.; Shi, S.H.; Liu, L.T.; Wang, M.C.; Liu, H.M.; Lei, X.X. Azaheterocyclic diphenylmethanol chiral solvating agents for the NMR chiral discrimination of alpha-substituted carboxylic acids. RSC Adv. 2020, 10, 34605–34611. [Google Scholar] [CrossRef] [PubMed]
- Marta, T.B.; Argondizzo, A.C.; da Silva Oliboni, R.; Silva, M.S. NMR chiral recognition of lipoic acid by cinchonidine CSA: A stereocenter beyond the organic function. Chirality 2023, 35, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S. Recent advances in multinuclear NMR spectroscopy for chiral recognition of organic compounds. Molecules 2017, 22, 247. [Google Scholar] [CrossRef] [PubMed]
- Uccello-Barretta, G.; Vanni, L.; Balzano, F. Nuclear magnetic resonance approaches to the rationalization of chromatographic enantiorecognition processes. J. Chromatogr. A 2010, 1217, 928–940. [Google Scholar] [CrossRef]
- Scriba, G.K. Chiral recognition in separation science–an update. J. Chromatogr. A 2016, 1467, 56–78. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Murray, P.G.; Wilson, S.R. X-ray crystallographic evidence in support of a proposed chiral recognition mechanism. J. Org. Chem. 1996, 61, 4775–4777. [Google Scholar] [CrossRef]
- Chankvetadze, B.; Burjanadze, N.; Pintore, G.; Bergenthal, D.; Bergander, K.; Mühlenbrock, C.; Breitkreuz, J.; Blaschke, G. Separation of brompheniramine enantiomers by capillary electrophoresis and study of chiral recognition mechanisms of cyclodextrins using NMR-spectroscopy, UV spectrometry, electrospray ionization mass spectrometry and X-ray crystallography. J. Chromatogr. A 2000, 875, 471–484. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ikai, T. Chiral HPLC for efficient resolution of enantiomers. Chem. Soc. Rev. 2008, 37, 2593–2608. [Google Scholar] [CrossRef]
- Ward, T.J.; Ward, K.D. Chiral separations: A review of current topics and trends. Anal. Chem. 2012, 84, 626–635. [Google Scholar] [CrossRef]
- Haginaka, J. Protein-based chiral stationary phases for high-performance liquid chromatography enantioseparations. J. Chromatogr. A 2001, 906, 253–273. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Ward, T.J.; Armstrong, R.D.; Beesley, T.E. Separation of drug stereoisomers by the formation of β-cyclodextrin inclusion complexes. Science 1986, 232, 1132–1135. [Google Scholar] [CrossRef]
- Weinstein, S.; Leiserowitz, L.; Gil-Av, E. Chiral secondary amides. 2. Molecular packing and chiral recognition. J. Am. Chem. Soc. 1980, 102, 2768–2772. [Google Scholar] [CrossRef]
- Lipkowitz, K.B. Atomistic modeling of enantioselection in chromatography. J. Chromatogr. A 2001, 906, 417–442. [Google Scholar] [CrossRef]
- Lipkowitz, K.B. Theoretical studies of brush-type chiral stationary phases. J. Chromatogr. A 1994, 666, 493–503. [Google Scholar] [CrossRef]
- Lipkowitz, K.B.; Baker, B. Computational analysis of chiral recognition in Pirkle phases. Anal. Chem. 1990, 62, 770–774. [Google Scholar] [CrossRef]
- Scriba, G.K. Chiral recognition in separation sciences. Part I: Polysaccharide and cyclodextrin selectors. TrAC Trends Anal. Chem. 2019, 120, 115639. [Google Scholar] [CrossRef]
- Meng, M.; Wang, L.; Reuschel, S. Computational approaches in developing accelerated chiral liquid chromatography techniques for mass spectral assays. In Advanced LC-MS Applications in Bioanalysis; Future Science Ltd.: London, UK, 2015; pp. 117–131. [Google Scholar]
- Scriba, G.K. Recognition mechanisms of chiral selectors: An overview. Chiral Sep. Meth Prot. 2019, 1985, 1–33. [Google Scholar]
- Ikai, T.; Okamoto, Y. Preparation and chiral recognition of polysaccharide-based selectors. In Chiral Recognition in Separation Methods: Mechanisms and Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 33–52. [Google Scholar]
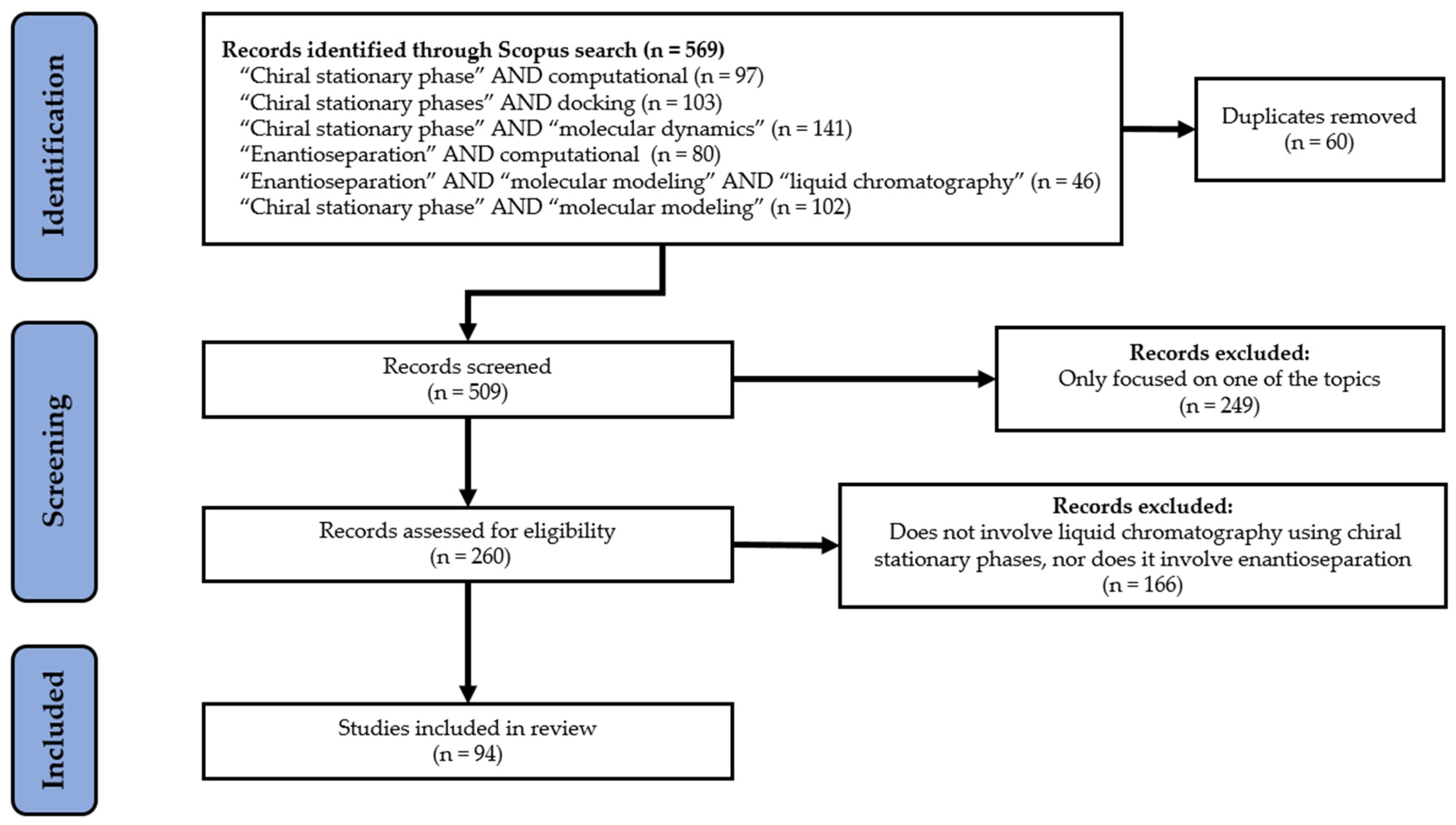
- Page, M.J.; Mckenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffman, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Haque, A.; Al-Othman, Z.A.; Al-Warthan, A.; Asnin, L. Stereoselective interactions of chiral dipeptides on amylose based chiral stationary phases. Sci. China Chem. 2015, 58, 519–525. [Google Scholar] [CrossRef]
- Ashtari, M.; Cann, N.M. The docking of chiral analytes on proline-based chiral stationary phases: A molecular dynamics study of selectivity. J. Chromatogr. A 2015, 1409, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Su, X.; Wang, Y.; Chen, Y.; Shen, W. Studies on pidotimod enantiomers with Chiralpak-IA: Crystal structure, thermodynamic parameters and molecular docking. Chirality 2015, 27, 802–808. [Google Scholar] [CrossRef] [PubMed]
- He, Z.J.; Song, H.; Zhang, Y.W.; Wang, D.C.; Yao, S. Chiral stationary phases and their relationship with enantiomer structures in enantioseparation research of analytical laboratory. J. Mex. Chem. Soc. 2015, 59, 43–49. [Google Scholar]
- Hu, G.X.; Luo, C.C.; Pan, S.F.; Jiang, Y.J.; Zou, J.W. Predicting retention and separation factors of chiral diarylmethane derivates by QSPR models. Acta Phys-Chim. Sin. 2015, 31, 73–82. [Google Scholar]
- Grecsó, N.; Kohout, M.; Carotti, A.; Sardella, R.; Natalini, B.; Fülöp, F.; Lindner, W.; Péter, A.; Ilisz, I. Mechanistic considerations of enantiorecognition on novel Cinchona alkaloid-based zwitterionic chiral stationary phases from the aspect of the separation of trans-paroxetine enantiomers as model compounds. J. Pharm. Biomed. Anal. 2016, 124, 164–173. [Google Scholar] [CrossRef]
- Hu, G.; Huang, M.; Luo, C.; Wang, Q.; Zou, J.W. Interactions between pyrazole derived enantiomers and Chiralcel OJ: Prediction of enantiomer absolute configurations and elution order by molecular dynamics simulations. J. Mol. Graph. Mod. 2016, 66, 123–132. [Google Scholar] [CrossRef]
- Peluso, P.; Mamane, V.; Aubert, E.; Dessì, A.; Dallocchio, R.; Dore, A.; Pale, P.; Cossu, S. Insights into halogen bond-driven enantioseparations. J. Chromatogr. A 2016, 1467, 228–238. [Google Scholar] [CrossRef]
- Szabó, Z.I.; Mohammadhassan, F.; Szőcs, L.; Nagy, J.; Komjáti, B.; Noszál, B.; Tóth, G. Stereoselective interactions and liquid chromatographic enantioseparation of thalidomide on cyclodextrin-bonded stationary phases. J. Incl. Phenom. Macrocyl Chem. 2016, 85, 227–236. [Google Scholar] [CrossRef]
- Szabó, Z.I.; Szőcs, L.; Horváth, P.; Komjáti, B.; Nagy, J.; Jánoska, Á.; Muntean, D.L.; Noszál, B.; Tóth, G. Liquid chromatography with mass spectrometry enantioseparation of pomalidomide on cyclodextrin-bonded chiral stationary phases and the elucidation of the chiral recognition mechanisms by NMR spectroscopy and molecular modeling. J. Sep. Sci. 2016, 39, 2941–2949. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, L.; Liu, K.; Guo, F.; Liu, W. Enantioseparation of four amide herbicide stereoisomers using high-performance liquid chromatography. J. Chromatogr. A 2016, 1471, 145–154. [Google Scholar] [CrossRef]
- Çakmak, R.; Ercan, S.; Sünkür, M.; Yılmaz, H.; Topal, G. Design, preparation and application of a Pirkle-type chiral stationary phase for enantioseparation of some racemic organic acids and molecular dynamics studies. Org. Commun. 2017, 10, 216–227. [Google Scholar] [CrossRef]
- Carraro, M.L.; Palmeira, A.; Tiritan, M.E.; Fernandes, C.; Pinto, M.M. Resolution, determination of enantiomeric purity and chiral recognition mechanism of new xanthone derivatives on (S,S)-whelk-O1 stationary phase. Chirality 2017, 29, 247–256. [Google Scholar] [CrossRef]
- Li, X.; Yao, X.; Xiao, Y.; Wang, Y. Enantioseparation of single layer native cyclodextrin chiral stationary phases: Effect of cyclodextrin orientation and a modeling study. Anal. Chim. Acta 2017, 990, 174–184. [Google Scholar] [CrossRef]
- Rossi, D.; Nasti, R.; Collina, S.; Mazzeo, G.; Ghidinelli, S.; Longhi, G.; Memo, M.; Abbate, S. The role of chirality in a set of key intermediates of pharmaceutical interest, 3-aryl-substituted-γ-butyrolactones, evidenced by chiral HPLC separation and by chiroptical spectroscopies. J. Pharm. Biomed. Anal. 2017, 144, 41–51. [Google Scholar] [CrossRef]
- Zhao, B.; Oroskar, P.A.; Wang, X.; House, D.; Oroskar, A.; Oroskar, A.; Jameson, C.; Murad, S. The composition of the mobile phase affects the dynamic chiral recognition of drug molecules by the chiral stationary phase. Langmuir 2017, 33, 11246–11256. [Google Scholar] [CrossRef] [PubMed]
- Dallocchio, R.; Dessì, A.; Solinas, M.; Arras, A.; Cossu, S.; Aubert, E.; Mamane, V.; Peluso, P. Halogen bond in high-performance liquid chromatography enantioseparations: Description, features and modelling. J. Chromatogr. A 2018, 1563, 71–81. [Google Scholar] [CrossRef]
- Li, M.; Zhang, B.; Yu, J.; Wang, J.; Guo, X. Enantiomeric separation and simulation study of eight anticholinergic drugs on an immobilized polysaccharide-based chiral stationary phase by HPLC. New J. Chem. 2018, 42, 11724–11731. [Google Scholar] [CrossRef]
- Peluso, P.; Gatti, C.; Dessì, A.; Dallocchio, R.; Weiss, R.; Aubert, E.; Pale, P.; Cossu, S.; Mamane, V. Enantioseparation of fluorinated 3-arylthio-4,4’-bipyridines: Insights into chalcogen and π-hole bonds in high-performance liquid chromatography. J. Chromatogr. A 2018, 1567, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Peluso, P.; Mamane, V.; Dallocchio, R.; Dessì, A.; Villano, R.; Sanna, D.; Aubert, E.; Pale, P.; Cossu, S. Polysaccharide-based chiral stationary phases as halogen bond acceptors: A novel strategy for detection of stereoselective σ-hole bonds in solution. J. Sep. Sci. 2018, 41, 1247–1256. [Google Scholar] [CrossRef]
- Pisani, L.; Rullo, M.; Catto, M.; de Candia, M.; Carrieri, A.; Cellamare, S.; Altomare, C.D. Structure–property relationship study of the HPLC enantioselective retention of neuroprotective 7-[(1-alkylpiperidin-3-yl)methoxy]coumarin derivatives on an amylose-based chiral stationary phase. J. Sep. Sci. 2018, 41, 1376–1384. [Google Scholar] [CrossRef]
- Sardella, R.; Macchiarulo, A.; Urbinati, F.; Ianni, F.; Carotti, A.; Kohout, M.; Lindner, W.; Péter, A.; Ilisz, I. Exploring the enantiorecognition mechanism of Cinchona alkaloid-based zwitterionic chiral stationary phases and the basic trans-paroxetine enantiomers. J. Sep. Sci. 2018, 41, 1199–1207. [Google Scholar] [CrossRef]
- Xiong, F.; Yang, B.B.; Zhang, J.; Li, L. Enantioseparation, stereochemical assignment and chiral recognition mechanism of sulfoxide-containing drugs. Molecules 2018, 23, 2680. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhao, F.; Yu, J.; Wang, Z.; Song, Y.; Li, Q. Chiral separation and a molecular modeling study of eight azole antifungals on the cellulose tris(3,5-dichlorophenylcarbamate) chiral stationary phase. New J. Chem. 2018, 42, 13421–13429. [Google Scholar] [CrossRef]
- Barfeii, H.; Garkani-Nejad, Z.; Saheb, V. Investigation of the mechanism of enantioseparation of some drug compounds by considering the mobile phase in HPLC by molecular dynamics simulation. J. Mol. Model. 2019, 25, 297. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, J.P.; Phyo, Y.Z.; Palmeira, A.; Tiritan, M.E.; Afonso, C.; Kijjoa, A.; Pinto, M.M.; Fernandes, C. Enantioseparation, recognition mechanisms and binding of xanthones on human serum albumin by liquid chromatography. Bioanalysis 2019, 11, 1255–1274. [Google Scholar] [CrossRef]
- He, Z.; Wu, F.; Xia, W.; Li, L.; Hu, K.; Kaziem, A.E.; Wang, M. Separation and detection of cyproconazole enantiomers and its stereospecific recognition with chiral stationary phase by high-performance liquid chromatography. Analyst 2019, 144, 5193–5200. [Google Scholar] [CrossRef]
- Knežević, A.; Novak, J.; Vinković, V. New brush-type chiral stationary phases for enantioseparation of pharmaceutical drugs. Molecules 2019, 24, 823. [Google Scholar] [CrossRef]
- Li, J.; Liu, R.; Wang, L.; Liu, X.; Gao, H. Enantioseparation of chiral pharmaceuticals by vancomycin-bonded stationary phase and analysis of chiral recognition mechanism. Chirality 2019, 31, 236–247. [Google Scholar] [CrossRef]
- Luo, X.; Fang, C.; Mi, J.; Xu, J.; Lin, H. Enantiomeric resolution, thermodynamic parameters, and modeling of clausenamidone and neoclausenamidone on polysaccharide-based chiral stationary phases. Chirality 2019, 31, 423–433. [Google Scholar] [CrossRef]
- Phyo, Y.Z.; Teixeira, J.; Tiritan, M.E.; Cravo, S.; Palmeira, A.; Gales, L.; Silva, A.M.; Pinto, M.M.; Kijjoa, A.; Fernandes, C. New chiral stationary phases for liquid chromatography based on small molecules: Development, enantioresolution evaluation and chiral recognition mechanisms. Chirality 2020, 32, 81–97. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, J.; Chen, L.; Dong, S.; Li, H.; Qiu, H.; Zhao, L. Graphene quantum dots functionalized β-cyclodextrin and cellulose chiral stationary phases with enhanced enantioseparation performance. J. Chromatogr. A 2019, 1600, 209–218. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Wang, X.; Yu, J.; Song, Y.; Guo, X. Enantioseparation and molecular modeling study of five β-adrenergic blockers on C hiralpak IC column. Chirality 2019, 31, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Wang, F.; Han, J.; Liu, B.; Shen, J.; Zhang, L.; Okamoto, Y. Influence of the substituents on phenyl groups on enantioseparation property of amylose phenylcarbamates. Carbohydr. Polym. 2020, 241, 116372. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Xue, M.; Lun, J.; Li, S.; Yu, J.; Guo, X. Enantioseparation and molecular modeling study of eight psychoactive drugs on a coated polysaccharide-based chiral stationary phase. Electrophoresis 2020, 41, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Ianni, F.; Cerra, B.; Shandiz, S.T.; Di Michele, A.; Saluti, G.; Galarini, R.; Gioiello, A.; Sardella, R.; Carotti, A. Integrating experimental and computational techniques to study chromatographic enantioresolutions of chiral tetrahydroindazole derivatives. J. Chromatogr. A 2020, 1625, 461310. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Z.; Di, X.; Song, Y. Enantiomeric separation of six beta-adrenergic blockers on Chiralpak IB column and identification of chiral recognition mechanisms by molecular docking technique. Biomed. Chromatogr. 2020, 34, 4803. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, L.; Lun, J.; Zhao, M.; Guo, X. Enantiomeric separation and molecular docking study of seven imidazole antifungal drugs on a cellulose tris-(3,5-dimethylphenylcarbamate) chiral stationary phase. New J. Chem. 2020, 44, 18337–18346. [Google Scholar] [CrossRef]
- Papp, L.A.; Foroughbakhshfasaei, M.; Fiser, B.; Horváth, P.; Kiss, E.; Sekkoum, K.; Gyéresi, Á.; Hancu, G.; Noszál, B.; Szabó, Z.I.; et al. Reversed-phase HPLC enantioseparation of pantoprazole using a teicoplanin aglycone stationary phase—Determination of the enantiomer elution order using HPLC-CD analyses. Chirality 2020, 32, 158–167. [Google Scholar] [CrossRef]
- Sardella, R.; Ianni, F.; Cossignani, L.; Aldini, G.; Carotti, A. Binding modes identification through molecular dynamic simulations: A case study with carnosine enantiomers and the Teicoplanin A2-2-based chiral stationary phase. J. Sep. Sci. 2020, 43, 1728–1736. [Google Scholar] [CrossRef]
- Shahnani, M.; Sefidbakht, Y.; Maghari, S.; Mehdi, A.; Rezadoost, H.; Ghassempour, A. Enantioseparation of mandelic acid on vancomycin column: Experimental and docking study. Chirality 2020, 32, 1289–1298. [Google Scholar] [CrossRef]
- Shi, G.; Dai, X.; Zhou, Y.; Zhang, J.; Shen, J.; Wan, X. Synthesis and enantioseparation of proline-derived helical polyacetylenes as chiral stationary phases for HPLC. Polym. Chem. 2020, 11, 3179–3187. [Google Scholar] [CrossRef]
- Wang, X.; Jameson, C.J.; Murad, S. Modeling enantiomeric separations as an interfacial process using amylose tris(3,5-dimethylphenyl carbamate)(ADMPC) polymers coated on amorphous silica. Langmuir 2020, 36, 1113–1124. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, J.; Fang, H.; Hou, X.; Hou, Z.; Sang, L.; Yang, X. Enantioseparation of lysine derivatives on amylose tris(3,5-dimethylphenylcarbamate) as chiral stationary phase with high separation factor. J. Chromatogr. A 2020, 1632, 461598. [Google Scholar] [CrossRef]
- Dallocchio, R.; Sechi, B.; Dessì, A.; Chankvetadze, B.; Cossu, S.; Mamane, V.; Weiss, R.; Pale, P.; Peluso, P. Enantioseparations of polyhalogenated 4, 4’-bipyridines on polysaccharide-based chiral stationary phases and molecular dynamics simulations of selector–selectand interactions. Electrophoresis 2021, 42, 1853–1863. [Google Scholar] [CrossRef]
- Franzini, R.; Pierini, M.; Mazzanti, A.; Iazzetti, A.; Ciogli, A.; Villani, C. Molecular recognition of the HPLC Whelk-O1 selector towards the conformational enantiomers of nevirapine and oxcarbazepine. Int. J. Mol. Sci. 2020, 22, 144. [Google Scholar] [CrossRef] [PubMed]
- Haginaka, J.; Yamashita, T.; Tsujino, H.; Arisawa, M. Revisiting Chiral Recognition Mechanism on Chicken Alpha 1-Acid Glycoprotein: Location of Chiral Binding Sites and Insight into Chiral Binding Mechanism. Separations 2021, 8, 73. [Google Scholar] [CrossRef]
- Hoyas, S.; Roscioni, O.M.; Tonneaux, C.; Gerbaux, P.; Cornil, J.; Muccioli, L. Peptoids as a chiral stationary phase for liquid chromatography: Insights from molecular dynamics simulations. Biomacromolecules 2021, 22, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, X.; Di, X.; Jiang, Z. Enantioseparation on a new synthetic β-cyclodextrin chemically bonded chiral stationary phase and molecular docking study. Anal. Bioanal. Chem. 2021, 413, 3933–3944. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Z.; Guo, X.; Di, X.; Yu, J. Enantioseparation and modelling study of six proton pump inhibitors on a novel 3,5-dichloro-phenylcarbamated β-cyclodextrin chemically bonded chiral stationary phase by high performance liquid chromatography. Microchem. J. 2021, 166, 106211. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Yu, J.; Guo, X. Chiral separation and molecular simulation study of six antihistamine agents on a coated cellulose tris-(3,5-dimethylphenycarbamate) column (Chiralcel OD-RH) and its recognition mechanisms. Electrophoresis 2021, 42, 1461–1472. [Google Scholar] [CrossRef]
- Peluso, P.; Chankvetadze, B. The molecular bases of chiral recognition in 2-(benzylsulfinyl) benzamide enantioseparation. Anal. Chim. Acta 2021, 1141, 194–205. [Google Scholar] [CrossRef]
- Ratih, R.; Wätzig, H.; Azminah, A.; Asmari, M.; Peters, B.; El Deeb, S. Immobilization of chondroitin sulfate a onto monolithic epoxy silica column as a new chiral stationary phase for high-performance liquid chromatographic enantioseparation. Pharm 2021, 14, 98. [Google Scholar] [CrossRef]
- Shi, G.; Dai, X.; Xu, Q.; Shen, J.; Wan, X. Enantioseparation by high-performance liquid chromatography on proline-derived helical polyacetylenes. Polym. Chem. 2021, 12, 242–253. [Google Scholar] [CrossRef]
- Varfaj, I.; Di Michele, A.; Ianni, F.; Saletti, M.; Anzini, M.; Barola, C.; Chankvetadze, B.; Sardella, R.; Carotti, A. Enantioseparation of novel anti-inflammatory chiral sulfoxides with two cellulose dichlorophenylcarbamate-based chiral stationary phases and polar-organic mobile phase (s). J. Chromatogr. Open 2021, 1, 100022. [Google Scholar] [CrossRef]
- Varfaj, I.; Protti, M.; Di Michele, A.; Macchioni, A.; Lindner, W.; Carotti, A.; Sardella, R.; Mercolini, L. Efficient enantioresolution of aromatic α-hydroxy acids with Cinchona alkaloid-based zwitterionic stationary phases and volatile polar-ionic eluents. Anal. Chim. Acta 2021, 1180, 338928. [Google Scholar] [CrossRef]
- Wang, X.; Jameson, C.J.; Murad, S. Molecular dynamics simulations of chiral recognition of drugs by amylose polymers coated on amorphous silica. Mol. Phys. 2021, 119, 1922772. [Google Scholar] [CrossRef]
- Bolognino, I.; Carrieri, A.; Purgatorio, R.; Catto, M.; Caliandro, R.; Carrozzini, B.; Belviso, B.D.; Majellaro, M.; Sotelo, E.; Cellamare, S.; et al. Enantiomeric Separation and Molecular Modelling of Bioactive 4-Aryl-3,4-dihydropyrimidin-2(1H)-one Ester Derivatives on Teicoplanin-Based Chiral Stationary Phase. Separations 2021, 9, 7. [Google Scholar] [CrossRef]
- Cao, S.; Ma, Q.; Liu, Y.; Zhang, J.; Wang, Z. Cellulose tris-(3,5-dimethyl phenyl carbamate) as a chiral stationary phase for enantiomeric determination of ofloxacin enantiomers and molecular docking study on the chiral separation mechanism. New J. Chem. 2022, 46, 9704–9709. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, Y.; Ma, Q.; Zhang, J.; Wang, Z. Experimental and computational studies of enantioseparation of three profen enantiomers with a focus on quantification of the enantiomeric impurities present in the corresponding enantiopure S-profen drugs. J. Chromatogr. A 2022, 1673, 463095. [Google Scholar] [CrossRef]
- Dallocchio, R.; Dessì, A.; Sechi, B.; Chankvetadze, B.; Cossu, S.; Mamane, V.; Aubert, E.; Rozzo, C.; Palmieri, G.; Spissu, Y.; et al. Exploring interaction modes between polysaccharide-based selectors and biologically active 4, 4′-bipyridines by experimental and computational analysis. J. Chromatogr. Open 2022, 2, 100030. [Google Scholar] [CrossRef]
- Deng, H.; Wu, X.; Zhang, L.; Shen, J.; Qiao, Y.; Wang, X.; Bai, C.; Zheng, T.; Okamoto, Y. Synthesis and application of chitosan thiourea derivatives as chiral stationary phases in HPLC. Carbohydr. Polym. 2022, 296, 119888. [Google Scholar] [CrossRef]
- Dessì, A.; Sechi, B.; Dallocchio, R.; Chankvetadze, B.; Pérez-Baeza, M.; Cossu, S.; Mamane, V.; Pale, P.; Peluso, P. Comparative enantioseparation of planar chiral ferrocenes on polysaccharide-based chiral stationary phases. Chirality 2022, 34, 609–619. [Google Scholar] [CrossRef]
- Gambacorta, N.; Özdemir, Z.; Doğan, İ.S.; Ciriaco, F.; Zenni, Y.N.; Karakurt, A.; Saraç, S.; Nicolotti, O. Integrated experimental and theoretical approaches to investigate the molecular mechanisms of the enantioseparation of chiral anticonvulsant and antifungal compounds. J. Mol. Struct. 2022, 1270, 133905. [Google Scholar] [CrossRef]
- Mousavimanesh, Z.; Shahnani, M.; Faraji-Shovey, A.; Bararjanian, M.; Sadr, A.S.; Ghassempour, A.; Salehi, P. A new chiral stationary phase based on noscapine: Synthesis, enantioseparation, and docking study. Chirality 2022, 34, 1371–1382. [Google Scholar] [CrossRef]
- Samir, L.; Hanafi, R.; El Deeb, S.; Spahn-Langguth, H. UHPLC Enantiomer Resolution for the α/β-Adrenoceptor Antagonist R/S-Carvedilol and Its Major Active Metabolites on Chiralpak IB N-5. Molecules 2022, 27, 4998. [Google Scholar] [CrossRef]
- Shi, G.; Li, Y.; Dai, X.; Shen, J.; Wan, X. Effect of pendant stereostructure on backbone conformation and enantioseparation ability of helical polyacetylene-based chiral stationary phases. Chirality 2022, 34, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, F.Z.; Belboukhari, N.; Sekkoum, K.; Aboul-Enein, H.Y. Novel Gatifloxacin3-carboxamide derivatives as anti-tumor agents: Synthesis, enantioseparation, and molecular docking. Curr. Anal. Chem. 2022, 18, 1108–1116. [Google Scholar] [CrossRef]
- Adhikari, S.; Bhujbal, S.; Paik, M.J.; Lee, W. Enantioseparation and molecular modeling study of chiral amines as three naphthaldimine derivatives using amylose or cellulose trisphenylcarbamate chiral stationary phases. Chirality 2023, 35, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Yu, Y.; Zhao, P.; Yang, Y.; Zhang, Y.; Tan, C.; Zhu, Y.; Fang, L.; Li, L. Enantioselective separation and simulation studies of five flavanone glycosides on a cellulose tris-(3,5-dichlorophenylcarbamate) chiral stationary phase. J. Mol. Liq. 2023, 374, 121260. [Google Scholar] [CrossRef]
- Dallocchio, R.; Dessì, A.; Sechi, B.; Chankvetadze, B.; Jibuti, G.; Cossu, S.; Mamane, V.; Peluso, P. Enantioseparation of planar chiral ferrocenes on cellulose-based chiral stationary phases: Benzoate versus carbamate pendant groups. Electrophoresis 2023, 44, 203–216. [Google Scholar] [CrossRef]
- Dobó, M.; Ádám, M.; Fiser, B.; Papp, L.A.; Dombi, G.; Sekkoum, K.; Szabó, Z.I.; Tóth, G. Enantioseparation and molecular docking study of selected chiral pharmaceuticals on a commercialized phenylcarbamate-β-cyclodextrin column using polar organic mode. Sci. Rep. 2023, 13, 14778. [Google Scholar] [CrossRef]
- Dombi, G.; Horváth, P.; Fiser, B.; Mirzahosseine, A.; Dobó, M.; Szabó, Z.I.; Tóth, G. Enantioselective Human Serum Albumin Binding of Apremilast: Liquid Chromatographic, Fluorescence and Molecular Docking Study. Int. J. Mol. Sci. 2023, 24, 2168. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, X.; Xiao, Y.; Ma, X.; Wang, Y. Investigation of the chiral recognition role of cyclodextrin hydroxyl moieties via high performance liquid chromatography. Analyst 2023, 148, 4987–4994. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Lu, X.; Sun, S.; Xiao, Y.; Wang, Y.; Jin, X.; Ma, X. Surface-up click access to allylimidazolium bridged cyclodextrin dimer phase for efficient enantioseparation. J. Sep. Sci. 2023, 46, 2300075. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Choi, Y.J.; Kim, K.H.; Song, G.Y.; Kim, H.M.; Kang, J.S. Chiral separation and molecular modeling study of decursinol and its derivatives using polysaccharide-based chiral stationary phases. J. Chromatogr. A 2023, 1705, 464165. [Google Scholar] [CrossRef]
- Núñez-Rico, J.L.; Cabezas-Giménez, J.; Lillo, V.; Balestra, S.R.; Galán-Mascarós, J.R.; Calero, S.; Vidal-Ferran, A. TAMOF-1 as a versatile and predictable chiral stationary phase for the resolution of racemic mixtures. ACS Appl. Mater. Interfaces 2023, 15, 39594–39605. [Google Scholar] [CrossRef]
- Protti, M.; Varfaj, I.; Carotti, A.; Tedesco, D.; Bartolini, M.; Favilli, A.; Gerli, S.; Mercolini, L.; Sardella, R. Microsampling and enantioselective liquid chromatography coupled to mass spectrometry for chiral bioanalysis of novel psychoactive substances. Talanta 2023, 257, 124332. [Google Scholar] [CrossRef]
- Saleh, O.A.; Badawey, A.M.; Aboul-Enein, H.Y.; Fouad, M.A. Enantioseparation, quantification, molecular docking and molecular dynamics study of five β-adrenergic blockers on Lux-Cellulose-2 column. BMC Chem. 2023, 17, 22. [Google Scholar] [CrossRef]
- Sechi, B.; Dessì, A.; Dallocchio, R.; Tsetskhladze, N.; Chankvetadze, B.; Pérez-Baeza, M.; Cossu, S.; Jibuti, G.; Mamane, V.; Peluso, P. Unravelling dispersion forces in liquid-phase enantioseparation. Part I: Impact of ferrocenyl versus phenyl groups. Anal. Chim. Acta 2023, 1278, 341725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, W.; Ning, B. Enantiomeric Resolution of Pidotimod and Its Isomers in Pidotimod Oral Solutions Using Chiral RP-HPLC with Quadrupole Dalton Analyzer Detection. Chromatographia 2023, 86, 55–62. [Google Scholar] [CrossRef]
- Varfaj, I.; Labikova, M.; Sardella, R.; Hettegger, H.; Lindner, W.; Kohout, M.; Carotti, A. A journey in unraveling the enantiorecognition mechanism of 3,5-dinitrobenzoyl-amino acids with two Cinchona alkaloid-based chiral stationary phases: The power of molecular dynamic simulations. Anal. Chim. Acta 2024, 1314, 342791. [Google Scholar] [CrossRef] [PubMed]
- Reshetova, E.N.; Barashkova, A.S.; Garifullin, B.F. Retention mechanisms of dipeptides on superficially porous particle vancomycin-and teicoplanin-based chiral stationary phases. J. Chromatogr. A 2024, 1730, 465135. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Li, Y.; Song, J.; Wang, Y. Click preparation of triazole-bridged teicoplanin-bound chiral stationary phases for efficient separating amino acid enantiomers. Talanta 2024, 274, 125984. [Google Scholar] [CrossRef] [PubMed]
- Saleh, O.A.; Badawey, A.M.; Enein, H.Y.; Mahmoud, S.T. An innovative combination of molecular modeling and green analysis approaches for the enantioseparation and quantitation of certain CNS acting drugs using HPLC. Microchem. J. 2024, 200, 110498. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Chen, J.; Yu, Y.; Chen, S.; Wang, J.; Qiu, H. Preparation and evaluation of two chiral stationary phases based on imidazolyl-functionalized bromoethoxy pillar [5] arene-bonded silica. J. Chromatogr. A 2024, 1720, 464799. [Google Scholar] [CrossRef]
- Bonafè, S.; Pagano, C.; Bianconi, E.; Mercolini, L.; Macchiarulo, A.; Perioli, L.; Sardella, R.; Carotti, A. Atypical enantioseparation of a non-ionic form of allantoin with Cinchona alkaloid-based zwitterionic chiral stationary phases. J. Chromatogr. Open 2024, 6, 100146. [Google Scholar] [CrossRef]
- Coelho, M.M.; Lima, R.; Almeida, A.S.; Fernandes, P.A.; Remião, F.; Fernandes, C.; Tiritan, M.E. Binding studies of promethazine and its metabolites with human serum albumin by high-performance affinity chromatography and molecular docking in the presence of codeine. Anal. Bioanal. Chem. 2024, 416, 4605–4618. [Google Scholar] [CrossRef]
- Sri, C.D.; Faizan, S.; Chandra, M.R.; Kumar, B.P.; Gurupadayya, B.M. Enantioselective Separation and Pharmacokinetics of a Chiral 1,4-Dihydropyrimidine Derivative in Rats: A Combined Chromatography and Docking Approach. Chirality 2024, 36, 23723. [Google Scholar] [CrossRef]
- Bai, H.; Chen, L. Stereoisomeric separation and chiral recognition mechanism study of star cyclodextrin polymer as the chiral stationary phase. Anal. Chim. Acta 2024, 1329, 343249. [Google Scholar] [CrossRef]
- Dombi, G.; Tyukodi, L.; Dobó, M.; Molnár, G.; Rozmer, Z.; Szabó, Z.I.; Fiser, B.; Tóth, G. Enantioselective Binding of Proton Pump Inhibitors to Alpha1-Acid Glycoprotein and Human Serum Albumin—A Chromatographic, Spectroscopic, and In Silico Study. Int. J. Mol. Sci. 2024, 25, 10575. [Google Scholar] [CrossRef]
- Guarducci, M.A.; Manetto, S.; Pierini, M.; Mazzoccanti, G.; Villani, C. Design, Synthesis, and Applications of Bis-Amido HPLC Pirkle-Type Chiral Stationary Phases. Chirality 2024, 36, 23715. [Google Scholar] [CrossRef]
- Muhammed, M.T.; Aki-Yalcin, E. Molecular docking: Principles, advances, and its applications in drug discovery. Lett. Drug Des. Discov. 2024, 21, 480–495. [Google Scholar] [CrossRef]
- Jameson, C.J.; Wang, X.; Murad, S. Molecular dynamics simulations of enantiomeric separations as an interfacial process in HPLC. AIChE J. 2021, 67, 17143. [Google Scholar] [CrossRef]
- Dascalu, A.E.; Speybrouck, D.; Billamboz, M.; Corens, D.; Ghinet, A.; Lipka, E. Analytical and preparative enantioseparations in supercritical fluid chromatography using different brands of immobilized cellulose tris (3,5-dichlorophenylcarbamate) columns: Some differences. J. Chromatogr. A 2020, 1622, 461125. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Saleem, K.; Hussain, I.; Gaitonde, V.D.; Aboul-Enein, H.Y. Polysaccharides chiral stationary phases in liquid chromatography. Sep. Purif. Rev. 2009, 38, 97–147. [Google Scholar] [CrossRef]
- Chen, X.; Yamamoto, C.; Okamoto, Y. Polysaccharide derivatives as useful chiral stationary phases in high-performance liquid chromatography. Pure Appl. Chem. 2007, 79, 1561–1573. [Google Scholar] [CrossRef]
- Ikai, T.; Ando, M.; Ito, M.; Ishidate, R.; Suzuki, N.; Maeda, K.; Yashima, E. Emergence of highly enantioselective catalytic activity in a helical polymer mediated by deracemization of racemic pendants. J. Am. Chem. Soc. 2021, 143, 12725–12735. [Google Scholar] [CrossRef]
- Cavazzini, A.; Pasti, L.; Massi, A.; Marchetti, N.; Dondi, F. Recent applications in chiral high performance liquid chromatography: A review. Anal. Chim. Acta 2011, 706, 205–222. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.; Wu, Y.; Sun, X.; Ke, Y. Enantioseparation of cloprostenol on the polysaccharide chiral stationary phase: Influence of the mobile phase on enantioselective adsorption. J. Chromatogr. A 2021, 1653, 462413. [Google Scholar] [CrossRef]
- Torres, P.H.; Sodero, A.C.; Jofily, P.; Silva-Jr, F.P. Key topics in molecular docking for drug design. Int. J. Mol. Sci. 2019, 20, 4574. [Google Scholar] [CrossRef]
- Tripathi, A.; Misra, K. Molecular docking: A structure-based drug designing approach. JSM Chem. 2017, 5, 1042–1047. [Google Scholar]
- Chaudhary, K.K.; Mishra, N. A review on molecular docking: Novel tool for drug discovery. JSM Chem. 2016, 4, 1029. [Google Scholar]
- Tao, X.; Huang, Y.; Wang, C.; Chen, F.; Yang, L.; Ling, L.; Che, Z.; Chen, X. Recent developments in molecular docking technology applied in food science: A review. Int. J. Food Sci. Technol. 2020, 55, 33–45. [Google Scholar] [CrossRef]
- Fan, J.; Fu, A.; Zhang, L. Progress in molecular docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghante, M.H.; Ajgunde, B.R. Software based approaches for drug designing and development: A systematic review on commonly used software and its applications. Bull. Fac. Pharm. 2017, 55, 203–210. [Google Scholar] [CrossRef]
- Wei, B.Q.; Baase, W.A.; Weaver, L.H.; Matthews, B.W.; Shoichet, B.K. A model binding site for testing scoring functions in molecular docking. J. Mol. Biol. 2002, 322, 339–355. [Google Scholar] [CrossRef]
- Li, J.; Fu, A.; Zhang, L. An overview of scoring functions used for protein–ligand interactions in molecular docking. Interdiscip. Sci. 2019, 11, 320–328. [Google Scholar] [CrossRef]
- Huang, S.Y.; Grinter, S.Z.; Zou, X. Scoring functions and their evaluation methods for protein–ligand docking: Recent advances and future directions. Phys. Chem. Chem. Phys. 2010, 12, 12899–12908. [Google Scholar] [CrossRef]
- Quiroga, R.; Villarreal, M.A. Vinardo: A scoring function based on autodock vina improves scoring, docking, and virtual screening. PLoS ONE 2016, 11, 0155183. [Google Scholar] [CrossRef]
- Phyo, Y.Z.; Teixeira, J.; Goncalves, R.; Palmeira, A.; Tiritan, M.E.; Bousbaa, H.; Pinto, M.M.; Fernandes, C.; Kijjoa, A. Chiral derivatives of xanthones and benzophenones: Synthesis, enantioseparation, molecular docking, and tumor cell growth inhibition studies. Chirality 2021, 33, 153–166. [Google Scholar] [CrossRef]
- Hollingsworth, S.A.; Dror, R.O. Molecular dynamics simulation for all. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef]
- Zhao, C.; Cann, N.M. The docking of chiral epoxides on the Whelk-O1 stationary phase: A molecular dynamics study. J. Chromatogr. A 2007, 1149, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Cann, N.M. Solvation of the Whelk-O1 chiral stationary phase: A molecular dynamics study. J. Chromatogr. A 2006, 1131, 110–129. [Google Scholar] [CrossRef] [PubMed]
- Nita, S.; Cann, N.M. Solvation of phenylglycine-and leucine-derived chiral stationary phases: Molecular dynamics simulation study. J. Phys. Chem. B 2008, 112, 13022–13037. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, D.; Wang, P.; Zhou, Z. Computational study of enantioseparation by amylose tris (3,5-dimethylphenylcarbamate)-based chiral stationary phase. J. Sep. Sci. 2010, 33, 3245–3255. [Google Scholar] [CrossRef]
- Bueno-Perez, R.; Balestra, S.R.; Camblor, M.A.; Min, J.G.; Hong, S.B.; Merkling, P.J.; Calero, S. Influence of Flexibility on the Separation of Chiral Isomers in STW-Type Zeolite. Chem. Eur. J. 2018, 24, 4121–4132. [Google Scholar] [CrossRef]
- Asmari, M.; Wang, X.; Casado, N.; Piponski, M.; Kovalenko, S.; Logoyda, L.; Hanafi, R.S.; El Deeb, S. Chiral monolithic silica-based HPLC columns for enantiomeric separation and determination: Functionalization of chiral selector and recognition of selector-selectand interaction. Molecules 2021, 26, 5241. [Google Scholar] [CrossRef]
- Shi, J.H.; Lin, Z.Y.; Kou, S.B.; Wang, B.L.; Jiang, S.L. Enantioseparation of mandelic acid and substituted derivatives by high-performance liquid chromatography with hydroxypropyl-β-cyclodextrin as chiral mobile additive and evaluation of inclusion complexes by molecular dynamics. Chirality 2021, 33, 675–684. [Google Scholar] [CrossRef]
- Joshi, S.Y.; Deshmukh, S.A. A review of advancements in coarse-grained molecular dynamics simulations. Mol. Simul. 2021, 47, 786–803. [Google Scholar] [CrossRef]
- Fedotov, A.; Vakhrushev, A.; Severyukhina, O.; Sidorenko, A.; Savva, Y.; Klenov, N.; Soloviev, I. Theoretical basis of quantum-mechanical modeling of functional nanostructures. Symmetry 2021, 13, 883. [Google Scholar] [CrossRef]
- Lahoz-Beltra, R. Solving the Schrödinger Equation with Genetic Algorithms: A Practical Approach. Computers 2022, 11, 169. [Google Scholar] [CrossRef]
- Chen, E.K. Realism about the wave function. Philos. Compass 2019, 14, 12611. [Google Scholar] [CrossRef]
- Car, R. Introduction to Density-Functional Theory and ab-Initio Molecular Dynamics. Quant. Struct.-Act. Relatsh. 2002, 21, 97–104. [Google Scholar] [CrossRef]
- Kumar, A.; Toal, S.E.; DiGuiseppi, D.; Schweitzer-Stenner, R.; Wong, B.M. Water-mediated electronic structure of oligopeptides probed by their UV circular dichroism, absorption spectra, and time-dependent DFT calculations. J. Phys. Chem. B 2020, 124, 2579–2590. [Google Scholar] [CrossRef]
- Kumar, A.; Schweitzer-Stenner, R.; Wong, B.M. A new interpretation of the structure and solvent dependence of the far UV circular dichroism spectrum of short oligopeptides. Chem. Comm. 2019, 55, 5701–5704. [Google Scholar] [CrossRef]
- Miyahara, T.; Nakatsuji, H. Accuracy of TD-DFT in the ultraviolet and circular dichroism spectra of deoxyguanosine and uridine. J. Phys. Chem. A 2018, 122, 100–118. [Google Scholar] [CrossRef]



| Analytes | CSP | Elution Mode/Mobile Phase Solvents | Computa-tional Approach | Main Aims | Non-Covalent Interactions | Main Conclusions | Ref. Year |
|---|---|---|---|---|---|---|---|
| Dipeptides | Polysaccharide-based | - | Molecular docking | Study of elution order and chiral recognition mechanisms | HB, VDW, and electrostatic | Computational studies explained chromatographic results | [85] 2015 |
| Alcohols | Pirkle-type | NP: Hex/
2-PrOH | Molecular docking and MD | Study of elution order and chiral recognition mechanisms | HB and steric effect | HB and steric hindrance had a key role in
enantioseparation | [86] 2015 |
| Pidotimod | Polysaccharide-based | PI: MTBE/
ACN/TFA | Molecular docking | Study of chiral recognition mechanisms | HB and VDW | VDW interactions had a key role in enantioseparation | [87] 2015 |
| Aromatic amines and α-hydroxy acids | Pirkle-type | NP: Hex/
2-PrOH | Molecular docking | Study of chiral recognition mechanisms | HB, dipole–dipole, π–π stacking, and steric effect | Computational studies explained the interactions responsible for
enantioseparation | [88] 2015 |
| Chiral diarylme-thane | Pirkle-type | NP:
2-PrOH/Hex | QSPR model | Prediction of retention and separation factors | HB, ionic and steric effect | Computational studies predicted separation factors and elution order | [89] 2015 |
| Drugs | Zwitterionic ion-exchange-type | PO: MeOH/THF | MD | Study of chiral recognition mechanisms | HB, π–π stacking,
π-cation and ionic | CSPs acted as cation exchangers, and elution order was interpreted | [90] 2016 |
| Pyrazoles | Polysaccharide-based | NP: Hex/EtOH or 2-PrOH
PO: EtOH, 2-PrOH | MD | Study of solvents and temperature influence on separation | π–π stacking, HB, and hydrophobic | Computational studies predicted elution order and absolute configurations | [91] 2016 |
| Polyhalo-genated 4,4′-bipyridines | Polysaccharide-based | NP: Hex/
2-PrOH | Geometry optimiza-tion, computation of EPSs, and MD | Impact of the halogens on the chiral recognition mechanisms | Dipole–dipole, HB, π–π stacking, and XB | Electrostatic interactions had a key role in enantioseparation | [92] 2016 |
| Drugs | CD-based | RP: H2O/ACN/
AcOH | QM | Investigation of chiral recognition mechanisms | Hydrophobic | Enantioseparation occurred due to different enantiomer binding
affinities | [93] 2016 |
| Drugs | CD-based | RP: H2O/MeOH or ACN | QM | Development, optimization, and validation of an LC-MS/MS method and study of chiral recognition mechanisms | Hydrophobic | The method was linear, accurate, and precise. S-POM established a more stable complex with the chiral selector | [94] 2016 |
| Drugs | Polysaccharide-based | NP: Hex/
2-PrOH or EtOH | Molecular docking | Study of elution order and chiral recognition mechanisms | HB | Elution order was R- prior to S-enantiomer | [95] 2016 |
| Organic
acids | Pirkle-type | NP: Hex/
2-PrOH | Molecular docking, MD, and QM | Study of chiral recognition mechanisms | HB, π–π stacking, VDW, and steric effect | Interactions between the CSP and analytes were identified | [96] 2017 |
| Chiral derivatives of xanthones | Pirkle-type | PO: MeOH/ACN | Molecular docking | Study of chiral recognition mechanisms | HB and
π–π stacking | Good agreement between chromatographic and computational studies | [97] 2017 |
| Dihydro-isoxazole, flavonoids, Troger’s base, amino acids, chromanols | CD-based | RP: H2O/MeOH or ACN | MD | Study of chiral recognition mechanisms | HB and
Hydrophobic | Computational studies precisely predicted the elution order and
resolution | [98] 2017 |
| Butyro-lactones | Polysaccharide-based | NP: Hept/EtOH
PO: MeOH | DFT and molecular docking | Enantiomers isolation and monitorization of elution order | HB | Computational studies confirmed elution order | [99] 2017 |
| Flavonoids | Polysaccharide-based | PO: MeOH
NP: Hept/ 2-PrOH | QM/MM and MD | Study of chiral recognition mechanisms | HB, π–π stacking and
steric effect | Computational studies used as a pre-screening tool for choosing enantioseparation
conditions | [100] 2017 |
| Polyhalo-genated
4,4′-bipyri-dines, 2-nitro-1-aryletha-nols | Polysaccharide-based | NP: Hex/
2-PrOH | MM and MD | Study of chiral recognition mechanisms | HB, π–π stacking, XB | Theoretical model allowed one to predict elution order | [101] 2018 |
| Drugs | Polysaccharide-based | NP: Hex/EtOH or 2-PrOH or 1-PrOH | Molecular docking | Study of chiral recognition mechanisms | HB, hydrophobic π–π
stacking | HB and hydrophobic interactions had a key role in enantioseparation | [102] 2018 |
| Fluorinated 3-arylthio-4,4′-bi-pyridines | Polysaccharide-based | NP: Hex/ 2-PrOH, Hex/ 2-PrOH/MeOH PO: MeOH | MM, geometry
Optimiza-tion, computation of EPSs, and MD | Study of chiral recognition mechanisms | Hydrophobic and
π–π stacking | Computational studies designed analytes as probes and clarified
chromatographic behaviors | [103] 2018 |
| Polyhalo-genated
4,4′-bi-pyridines | Polysaccharide-based | NP: Hex/ 2-PrOH, Hex/ 2-PrOH/MeOH | MD | Study of chiral recognition mechanisms and elution order | π–π stacking,
XB and hydrophobic | Computational studies showed higher sensitivity for weak XB
detection and confirmed the elution order | [104] 2018 |
| Coumarins | Polysaccharide-based | PO: MeOH/ACN | Molecular docking | Study of chiral recognition mechanisms | HB, π–π stacking | Computational studies confirmed the importance of HB and π–π
interactions | [105] 2018 |
| Drugs | Zwitterionic ion-exchange-type | PI: MeOH/THF/
DEA/FA | MD | Rationalization of enantiomeric elution order | - | The in silico model provided insights into enantiorecognition | [106] 2018 |
| Drugs | Polysaccharide-based | NP: Hex/EtOH or 2-PrOH, Hex/FA/EtOH | TD-DFT and molecular docking | Study of chiral recognition mechanisms and elution order | HB, dipole–dipole, π–π stacking, and hydrophobic | Computational studies were in
accordance with the experimental elution order | [107] 2018 |
| Imizadoles | Polysaccharide-based | NP: Hex/EtOH or 2-PrOH or n-BuOH | Molecular docking | Study of chiral recognition mechanisms | HB, hydrophobic and π–π stacking | Computational studies predicted enantioseparation | [108] 2018 |
| Alcohol
esters | Polysaccharide-based | NP: Hex/MeOH
PO: MeOH, EtOH, 2-PrOH | Molecular docking and MD | Investigation of solvent, ratio, and enantiomer structure on chiral recognition | HB, dipole–dipole, and π–π stacking | Computational studies predicted elution order and absolute
configuration | [109] 2019 |
| Chiral derivatives of xanthones | Protein-based | RP: ammonium acetate or sodium acetate or potassium phosphate buffers/ACN or MeOH or EtOH or 2-PrOH | Molecular docking | Study of chiral recognition mechanisms | HB and π–π stacking | Computational studies were in
accordance with experimental results | [110] 2019 |
| Triazoles | Polysaccharide-based | PO: ACN | Molecular docking | Study of chiral recognition mechanisms | XB,
anion–π, HB, dipole–dipole, π–π stacking | Computational studies identified the chiral recognition mechanisms | [111] 2019 |
| Drugs | Pirkle-type | NP: Hex/
2-PrOH or 2-PrOH/AcOH | MD | Study of chiral recognition mechanisms | HB | Computational studies were in
accordance with experimental elution order | [112] 2019 |
| Drugs | Macrocyclic antibiotic-based | PI: MeOH/AcOH/TEA | Molecular docking | Identification of thermodynamic properties and study of chiral recognition mechanisms | HB, dipole–dipole, and electrostatic | Good agreement between computational studies and experimental
results | [113] 2019 |
| Indole alkaloids | Polysaccharide-based | NP: Hex/
2-PrOH | Molecular docking | Study of chiral recognition mechanisms | Steric effect, π–π stacking, and HB | HB and π–π interactions were
responsible for enantioseparation | [114] 2019 |
| Chiral derivatives of xanthones, Troger’s base, alcohols, drugs | Pirkle-type | NP: Hex/EtOH or 2-PrOH, Hex/EtOH/ TEA RP:ACN/H2O/ TEA, PO: ACN, ACN/MeOH or EtOH | Molecular docking | Study of chiral recognition mechanisms | HB and π–π stacking | Computational studies identified the structural
requirements for elucidation of chiral recognition | [115] 2019 |
| Epoxide, aromatic ketones, flavonoids, drugs, biaryl compounds, amides, imidazolines | Polysaccharide-based | PO: MeOH, ACN
NP: Hept/ 2-PrOH | MD | Development of a predictive method, considering both the dynamic nature of the process and the role of the solvent | HB, π–π stacking | Developed model was adequate for simulation of
drug-CSP interactions | [59] 2019 |
| Aromatic ketones, biaryl compounds, flavonoids, aromatic alcohols, drugs | CD and polysaccharide-based | RP: H2O/MeOH or ACN | Molecular docking | Study of chiral recognition mechanisms | Hydrophobic, HB, and π–π stacking | Computational studies showed a good agreement with experimental results | [116] 2019 |
| Drugs | Polysaccharide-based | NP: Hex/
EtOH/ DEA | Molecular docking | Study of chiral recognition mechanisms | HB and π–π stacking | Computational studies explained chiral recognition mechanisms | [117] 2019 |
| Troger’s base, epoxide, α-hydroxy ketones, alcohols, metal complexes, flavonoids | Polysaccharide-based | NP: Hex/
2-PrOH | Molecular docking and MD | Study of chiral recognition mechanisms | HB and π-alkyl | Computational studies showed that polymer backbone conformation change was the main
factor for enantioselectivity | [118] 2020 |
| Drugs | Polysaccharide-based | NP: Hex/EtOH or 1-PrOH
or 2-PrOH | Molecular docking | Study of chiral recognition mechanisms | HB, π–π stacking, hydrophobic | Computational studies agreed with experimental | [119] 2020 |
| Indazole derivatives | Pirkle-type | NP: Hex | MD | Study of chiral recognition mechanisms | HB, π-cation and ionic, π–π stacking, and steric effect | In silico computational studies predicted enantioseparation | [120] 2020 |
| Drugs | Polysaccharide-based | NP: Hex/EtOH or 2-PrOH/DEA | Molecular docking | Study of chiral recognition mechanisms | Hydrophobic, HB, π–π stacking, and steric effect | Computational studies agreed with experimental enantioselectivity | [121] 2020 |
| Drugs | Polysaccharide-based | RP: Ammonium acetate buffer/MeOH | Molecular docking | Study of chiral recognition mechanisms | HB, π–π stacking, hydrophobic, and dipole–dipole | Computational studies were in accordance with chromatographic enantioselectivity | [122] 2020 |
| Drugs | Macrocyclic antibiotics-based | RP: Ammonium acetate buffer/MeOH | Molecular docking | Study of chiral recognition mechanisms and elution order | π–π stacking | Computational studies showed that R-enantiomer binds stronger to the CSP, in
accordance with experimental | [123] 2020 |
| Dipeptide | Macrocyclic antibiotic-based | RP: MeOH/H2O | MD | Study of chiral recognition mechanisms | HB, π-cation, ionic, and π–π stacking | Computational studies allowed for the study of chiral recognition
mechanism of teicoplanin-based CSP | [124] 2020 |
| α-Hydroxy acid | Macrocyclic antibiotic-based | NP: Hept/
2-PrOH/TFA | Molecular docking | Develop a vancomycin-based CSP and study of chiral recognition mechanisms | HB, π–π stacking | Computational studies identified the interactions between enantiomers and chiral selector | [125] 2020 |
| α-Hydroxy ketones, aromatic alcohols, oxazolid-ones, alkyl-benzenes, polymers, organo-metallics | Pirkle-type | NP: Hex/
2-PrOH | Molecular docking | Preparation of novel proline-based CSPs and study of chiral recognition mechanisms | HB, π–π stacking, dipole–dipole, and steric effect | HB and π–π interactions were
critical for chiral discrimination | [126] 2020 |
| Drugs, α-hydroxy ketones, flavonoids | Polysaccharide-based | NP: Hept/
2-PrOH PO: MeOH | MD | Study of solid support role and prediction of enantiomeric elution order | Hydrophobic and HB | Computational studies predicted elution order, outperforming previous models | [127] 2020 |
| Lysine
derivatives | Polysaccharide-based | NP: Hex/
2-PrOH or EtOH | Molecular docking | Study of chiral recognition mechanisms, influence of various alcohol modifiers and column temperature | HB, dipole–dipole and π–π stacking | Chiral separation process was
enthalpy driven and chiral recognition mechanisms were identified | [128] 2020 |
| Bipyridines | Polysaccharide-based | NP: Hex/
2-PrOH | DFT, MD, and molecular docking | Study of chiral recognition mechanisms | HB, π–π stacking, and XB | Computational studies provided elution order in accordance with experimental data | [129] 2021 |
| Drugs | Pirkle-type | NP: Hex/DCM/
MeOH | Molecular docking | Study of chiral recognition mechanisms | HB | Computational studies identified enantiorecognition mechanisms, S-enantiomer presented a better complex stability | [130] 2021 |
| Drugs and α-hydroxy ketones | Protein-based | - | Molecular docking | Study of chiral recognition mechanisms | Hydrophobic, ionic, and HB | The chiral binding sites were located on cAGP | [131] 2021 |
| Biaryl diol | Pirkle-type | NP: Hex/
2-PrOH | MD | Study of chiral recognition mechanisms and elution process simulation | HB, π–π stacking | Computational studies showed that S-enantiomer was more retained | [132] 2021 |
| Drugs | CD-based | RP: ACN/FA buffer | Molecular docking | Study of chiral recognition mechanisms | Hydrophobic and HB | HB interactions and inclusion complexation played a key role in chiral recognition | [133] 2021 |
| Drugs | CD-based | NP: Hex/EtOH or 2-PrOH or
1-PrOH or 1-BuOH PO: MeOH, EtOH, ACN | Molecular docking | Development of a novel MDCPC CSP and
study of chiral recognition mechanisms | HB, π–π stacking | HB, hydrophobic interactions, and inclusion complexation played a crucial role in
enantioseparation | [134] 2021 |
| Drugs | Polysaccharide-based | PO: ACN/MeOH | Molecular docking | Study of chiral recognition mechanisms | HB, π–π stacking, and hydrophobic | π–π, HB, hydrophobic interactions led to
enantioselectivity | [135] 2021 |
| Sulfoxides
derivatives | Polysaccharide-based | PO: 2-PrOH NP: Hex/ 2-PrOH | MMFF, geometry optimiza-tion, conforma-tional and electrostatic potential analysis | Study of chiral recognition mechanisms | Dipole–dipole, π–π stacking, HB, and hydrophobic | Recognition model explained enantioselectivity and several
aspects impacting enantioseparation | [136] 2021 |
| Drugs | Polysaccharide-based | PO: MeOH, ACN | Molecular docking | Study of chiral recognition mechanisms | Electrostatic, HB, and
π-sulfur | Inclusion complexes along with different
interactions led to enantioselectivity | [137] 2021 |
| Alcohols, biaryl diols, metal acetylacetonates,
α-hydroxy ketones | Pirkle-type | NP: Hex/
2-PrOH, Hex | Molecular docking | Study of chiral recognition mechanisms and rationalization of the inductive and steric effects of substituents on chiral discrimination | HB, π–π stacking and dipole–dipole | Computational studies provided a correlation
between polarity, size and position of the substituent on the phenyl ring and chiral recognition | [138] 2021 |
| Sulfoxides | Polysaccharide-based | PO: EtOH,
2-PrOH | TD-DFT and molecular docking | Study of enantiomeric elution order and chiral recognition mechanisms | HB and π–π stacking | Computational studies predicted elution order and established the importance of π–π stacking and
enantiomer inclusion | [139] 2021 |
| α-Hydroxy acids | Zwitterionic ion-exchange-type | PI: MeOH/AcOH or ACN/AcOH, ACN/AcOH or MeOH/AcOH or FA | TD-DFT and MD | Development of an LC-MS method and evaluate the enantiomeric elution order | HB | Computational studies predicted the elution order and unveiled the role of phenolic group in retention mechanism | [140] 2021 |
| Epoxides, amines, flavonoids | Polysaccharide-based | NP: Hex/
2-PrOH | MD | Elution order and separation factors prediction | HB, hydrophobic and π–π stacking | Computational studies predicted elution order and enantioselectivity (except for trans-stilbene) | [141] 2021 |
| 3-Arylthio-4,4′-bi-pyridines derivatives | Macrocyclic antibiotic-based | PO: MeOH, EtOH, MeOH/ACN | Molecular docking and MD | Study of chiral recognition mechanisms | Steric effect, HB, and π–π stacking | Computational studies provided insights into recognition
mechanisms on TAG-based CSPs | [142] 2022 |
| Drugs | Polysaccharide-based | NP:Hex/EtOH/AcOH or AcOH/DEA or AcOH/TEA | Molecular docking | Study of chiral recognition mechanisms | HB, hydrophobic and π–π stacking | Computational studies revealed the stereoselective interactions | [143] 2022 |
| Drugs | Polysaccharide-based | RP: ACN/H2O/FA | Molecular docking | Study of chiral recognition mechanisms | HB, hydrophobic and π–π stacking | HB, hydrophobic interactions, and π–π stacking played a key role in chiral recognition | [144] 2022 |
| 4,4′-Bi-pyridines | Polysaccharide-based | NP: Hex/ 2-PrOH PO: MeOH | DFT and MD | Study of chiral recognition mechanisms | HB, π–π stacking, and hydrophobic | Computational studies identified the interactions responsible for
enantioseparation | [145] 2022 |
| Troger’s base, epoxides, α-hydroxy ketones, alcohols, flavonoids, sulfoxides, and drugs | Polysaccharide-based | NP: Hex/
2-PrOH | Molecular docking | Study of chiral recognition mechanisms | HB, π–π stacking | Computational studies showed the contribution of different
chitosan derivatives for enantioresolution | [146] 2022 |
| Ferrocenes | Polysaccharide-based | NP: Hex/ 2-PrOH or 2-PrOH/MeOH PO: MeOH RP: MeOH/H2O | Molecular electron density
isosurfaces and MD | Study of chiral recognition mechanisms | HB, hydrophobic | Analyte confinement in a hydrophobic cavity and HB interaction were essential for complex
stabilization | [147] 2022 |
| Esters | Polysaccharide-based | NP: MeOH/Hex | MD | Study of chiral recognition mechanisms | HB, π–π stacking and
hydrophobic | HB interactions were the most important for
enantioseparation | [148] 2022 |
| Drugs and α-hydroxy acids | Zwitterionic ion-exchange-type | RP: MeOH/H2O/
TEA | Molecular docking | Study of chiral recognition mechanisms | HB, π–π stacking | Good agreement between computational and
experimental | [149] 2022 |
| Drugs | Polysaccharide-based | PO: ACN, MeOH | Molecular docking | Study of chiral recognition mechanisms | HB, hydrophobic
and π–π stacking | Computational studies suggested that chiral recognition is due to different enantiomer binding poses | [150] 2022 |
| Aromatic ketones, naphthols, indanol, cyclohexa-nols, esters, alcohols, sulfonami-des, oxazo-lidones, binaphthol | Synthetic polymer-based | NP: Hex/
2-PrOH | Molecular docking | Study of chiral recognition mechanisms and correlation between stereocenters configuration/
position and chiral resolution | HB and π–π stacking | Computational studies showed that the strength and number of
intermolecular HB played a key role in chiral discrimination | [151] 2022 |
| Carboxami-de derivatives | Polysaccharide-based | - | Molecular docking | Computational studies of new carboxamide derivatives | - | All compounds presented good docking score | [152] 2022 |
| Amines and alcohols | Polysaccharide-based | NP: PrOH/Hex | Molecular docking | Study of chiral recognition mechanisms | HB, π–π stacking, and dipole–dipole | Computational studies supported the experimental results | [153] 2023 |
| Drugs | Polysaccharide-based | NP: Hex/EtOH/FA, Hex/
2-PrOH/FA | Molecular docking | Study of chiral recognition mechanisms | HB, hydrophobic and steric effect | Computational methods showed that chiral recognition on CDCPC is an exothermic process driven by HB, hydrophobic interactions, and steric effects | [154] 2023 |
| Ferrocenes | Polysaccharide-based | NP: Hex/
2-PrOH | Electrostatic potential and MD | Study of chiral recognition mechanisms | HB, π–π stacking, dipole–dipole and XB | Computational studies showed that XBs participate in recognition mechanisms | [155] 2023 |
| Drugs | CD-based | PO: MeOH, ACN | Molecular docking | Study of chiral recognition mechanisms | HB, hydrophobic and π–π stacking | Computational studies predicted elution order in several cases | [156] 2023 |
| Drugs | Protein-based | RP: phosphate buffer and MeOH, EtOH,
1-PrOH, 2-PrOH, or ACN | Molecular docking | Study of chiral recognition mechanisms | π–π stacking | Computational studies detailed the characterization of the complex
APR-HSA | [157] 2023 |
| Isoxazolines, dansyl amino acids, flavonoids | CD-based | PO: MeOH, RP: MeOH/H2O, MeOH/TEAA | Molecular docking | Preparation of alkylinidazole CD-based CSPs, and study of chiral recognition mechanisms | HB, steric effects, and hydrophobic | Secondary hydroxyl groups, cavity size of CDs, hydrophobic and steric effects had a key role in chiral recognition | [158] 2023 |
| Isoxazolines, dansyl amino
acids, and flavonoids | CD-based | PO: MeOH, MeOH/TEAA RP: MeOH/H2O | Molecular docking | Preparation of CD-based CSPs and study of chiral recognition mechanisms | Inclusion complexation, HB, electrostatic, hydrophobic, and steric effects | Synergistic inclusion effect and rich electrostatic interaction sites were essential for chiral recognition | [159] 2023 |
| Coumarins | Polysaccharide-based | PO: EtOH, 2-PrOH, BuOH | Molecular docking | Study of chiral recognition mechanisms | HB, π–π stacking, and hydrophobic | Computational studies predicted elution order and chiral recognition mechanisms | [160] 2023 |
| Standard analytes | Metal–organic framework-based | PO:ACN
NP: Hex/EtOH | QM, DFT, and geometry optimization | Prediction of the enantioseparation | - | Experimental data confirmed the models’ validity and the performance of TAMOF-1
columns | [161] 2023 |
| Phenethyl-amines, tryptamines, cathinones | Crown ether-based | RP: MeOH/ H2O/AcOH PI: MeOH/ ACN, TEA, AcOH | QM and DFT | Development of enantioselective methods and investigation of absolute stereochemistry | - | Computational studies predicted elution order and chiral recognition mechanisms | [162] 2023 |
| Drugs | Polysaccharide-based | NP: Hex/EtOH/
DEA | Molecular docking and MD | Study of chiral recognition mechanisms | HB and π–π stacking | Computational studies were in
accordance with experimental | [163] 2023 |
| Bipyridines
derivatives | Polysaccharide-based | NP: Hex/2-PrOH, Hex/2-PrOH/MeOH | Electrostatic potential analysis and MD | Study of chiral recognition mechanisms | HB, π–π stacking, and VDW | HB, π–π, and VDW interactions had a key role in enantioselectivity | [164] 2023 |
| Pidotimod | Polysaccharide-based | PI: ACN/FA/ MeOH/TFA, ACN/TFA/ 2-PrOH/FA | QM | Development and validation of an HPLC-MS method and study of chiral recognition mechanisms | HB | Validated method showed high sensitivity, and computational studies predicted
enantioseparation | [165] 2023 |
| Amino acid derivatives | Zwitterionic ion-exchange-type | RP: MeOH/AcOH/Ammonium acetate | MD | Study of chiral recognition mechanisms | HB, π–π stacking, and π-cation | Computational studies were in
accordance with the experimental elution order and elucidate the chiral recognition mechanisms | [166] 2024 |
| Dipeptides | Macrocyclic antibiotic-based | RP: MeOH/
Sodium acetate buffer | Molecular docking | Study of chiral recognition mechanisms | π–π stacking and hydrophobic | Computational studies not
predicted elution order for all stereoisomers | [167] 2024 |
| Amino acids | Macrocyclic antibiotic-based | RP: H2O/MeOH or EtOH or CH3CN | Molecular docking | Study of chiral recognition mechanisms | HB and π–π stacking | Computational studies elucidated selector and analyte interactions | [168] 2024 |
| Drugs | Polysaccharide-based | NP: Hex/EtOH/
DEA | Molecular docking | Study of chiral recognition mechanisms | HB and π–π stacking | Computational studies allowed understanding the chiral recognition mechanisms | [169] 2024 |
| Drugs | Pillar [5] arene-based mesoporous silica | NP: Hex/
2-PrOH RP: MeOH or ACN/H2O | Molecular docking | Preparation of new CSPs and study of chiral recognition mechanisms | HB and π–π stacking | Computational studies allowed understanding the chiral recognition mechanisms | [170] 2024 |
| Allantoin | Zwitterionic ion-exchange-type | RP: ACN/MeOH/ H2O/AcOH | MD | Study of the chiral recognition mechanisms | HB and π–π stacking | Computational studies
demonstrated HB was the key interaction for enantioselectivity | [171] 2024 |
| Drugs and synthetic
products | Protein-based | RP: Potassium phosphate buffer/ACN or 2-PrOH | Molecular docking | Study of the chiral recognition mechanisms | HB and π–π stacking | Computational studies suggested that drugs
competition occurred in both HSA sites I and II | [172] 2024 |
| 1,4-Dihydro-pyrimidine derivatives | Polysaccharide-based | NP: Hex/
2-PrOH | Molecular docking | Determination of kinetic properties and recognition mechanisms | π–π, π-alkyl, and π-halogen | Computational studies predicted enantiomers
elution order | [173] 2024 |
| Quinoline alkaloid isomers | CD-based | RP: MeOH/ACN/
2-PrOH-H2O or ACN-ammonium formate buffer | Molecular docking | Synthesis of novel CSPs and study of chiral recognition mechanisms | HB and hydrophobic | Computational studies confirmed the advantages of the CSP supramolecular structure | [174] 2024 |
| Drugs | Protein-based | RP: Phosphate buffer/MeOH or EtOH or
2-PrOH | Molecular docking | Study of the chiral recognition mechanisms | HB, π–π, and alkyl-π | Computational studies allowed understanding the chiral recognition mechanisms | [175] 2024 |
| Standard compounds | Pirkle-type | NP: Hex/CHCl3 | Molecular docking | Study of the chiral recognition mechanisms | HB and π–π stacking | Computational studies revealed the relevance
chiral selector bent structure and cleft-like cavity for chiral recognition | [176] 2024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, R.; Neves, R.P.P.; Fernandes, P.A.; Silva, A.M.S.; Fernandes, C. Unveiling the Power of Computational Tools in Chiral Liquid Chromatography. Molecules 2025, 30, 3218. https://doi.org/10.3390/molecules30153218
Lima R, Neves RPP, Fernandes PA, Silva AMS, Fernandes C. Unveiling the Power of Computational Tools in Chiral Liquid Chromatography. Molecules. 2025; 30(15):3218. https://doi.org/10.3390/molecules30153218
Chicago/Turabian StyleLima, Rita, Rui P. P. Neves, Pedro A. Fernandes, Artur M. S. Silva, and Carla Fernandes. 2025. "Unveiling the Power of Computational Tools in Chiral Liquid Chromatography" Molecules 30, no. 15: 3218. https://doi.org/10.3390/molecules30153218
APA StyleLima, R., Neves, R. P. P., Fernandes, P. A., Silva, A. M. S., & Fernandes, C. (2025). Unveiling the Power of Computational Tools in Chiral Liquid Chromatography. Molecules, 30(15), 3218. https://doi.org/10.3390/molecules30153218










