Effect of Contrasting Redox Potential Evolutions and Cap Management Techniques on the Chemical Composition of Red Wine
Abstract
1. Introduction
2. Results and Discussion
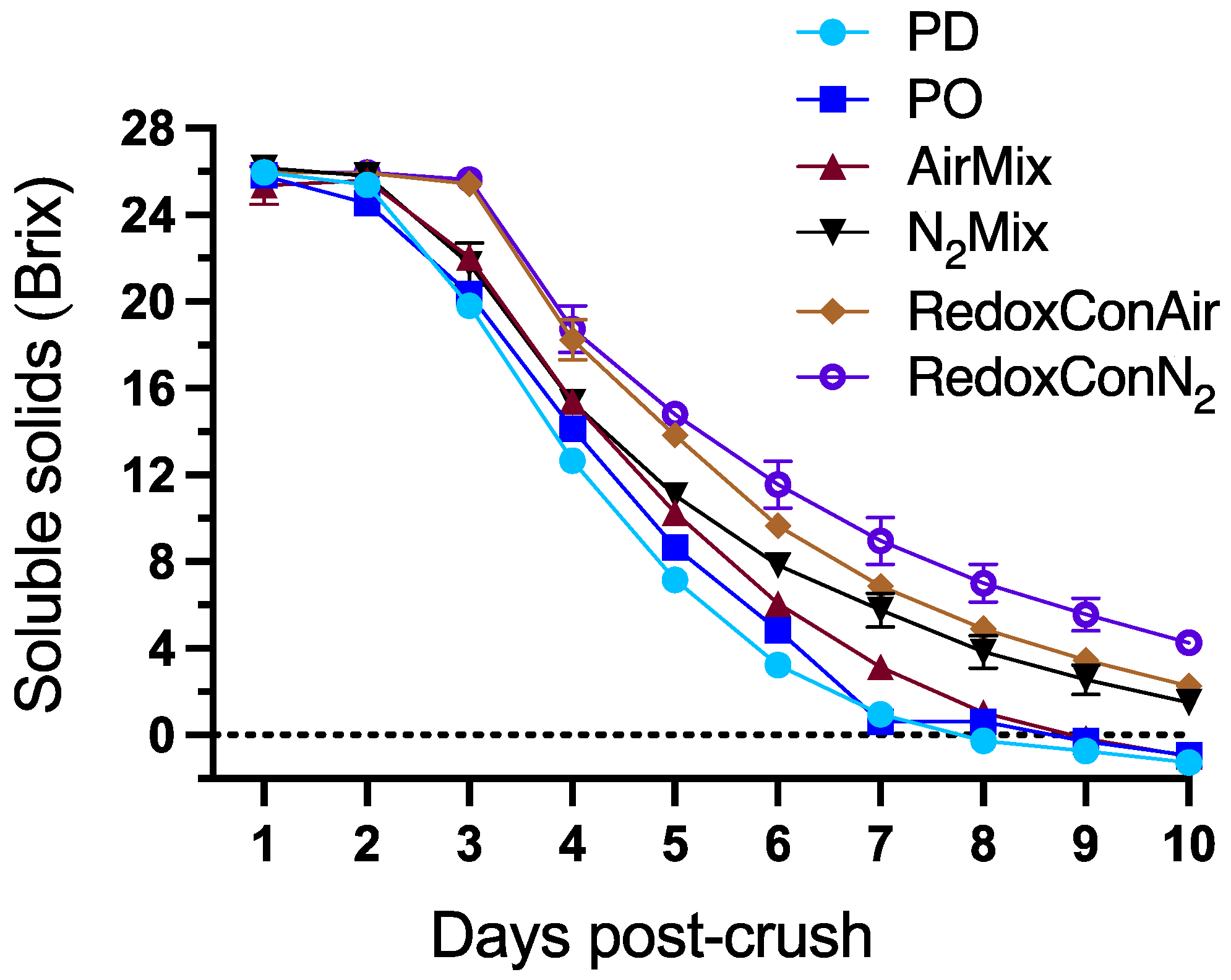
2.1. Alcoholic Fermentation of the Wines
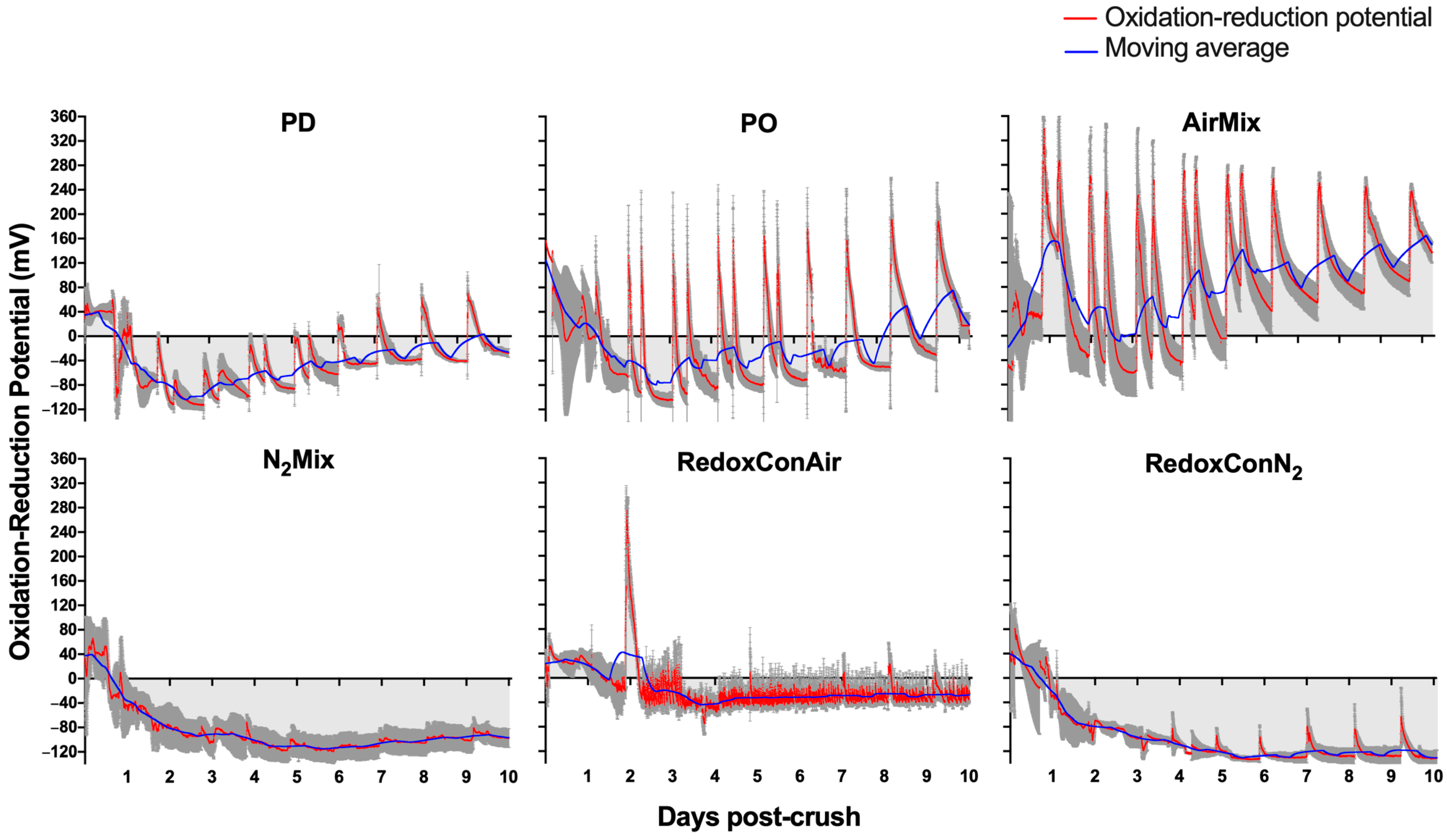
2.2. The Oxidation-Reduction Potential During Alcoholic Fermentation
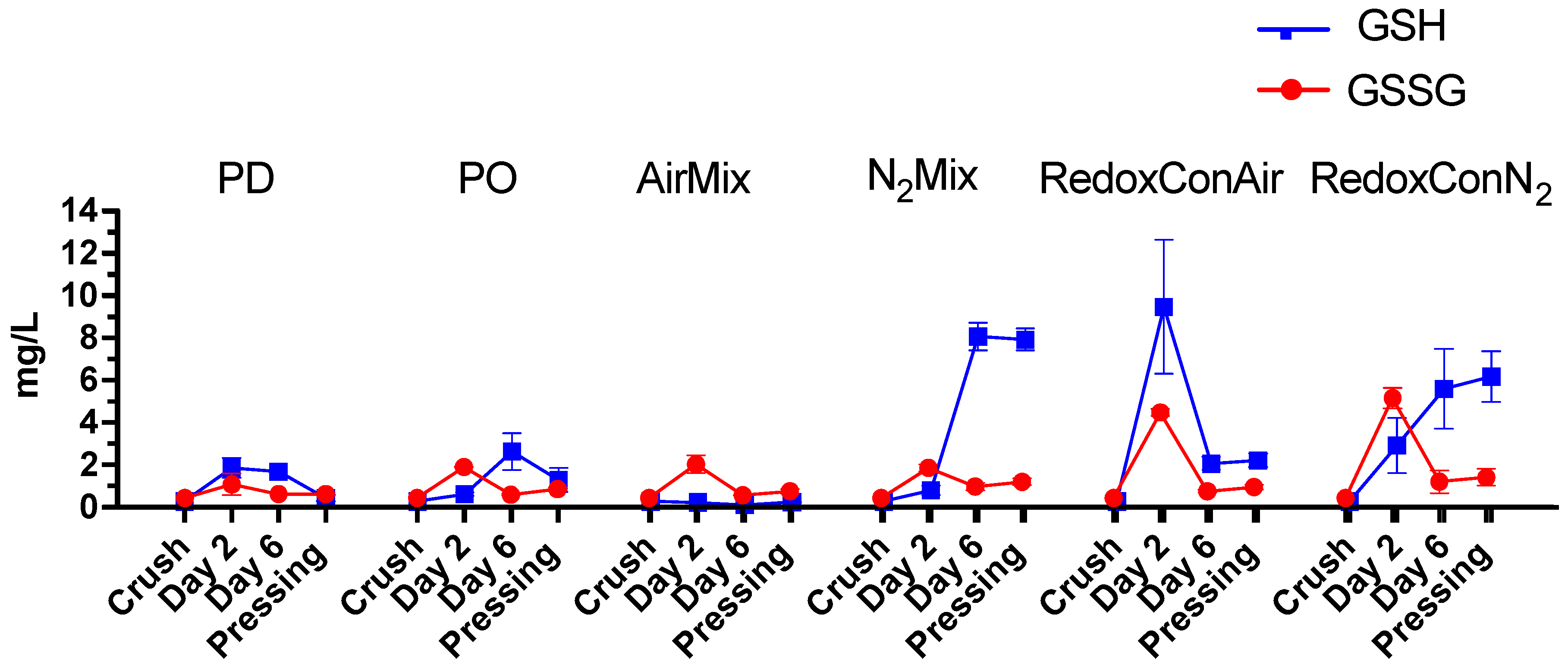
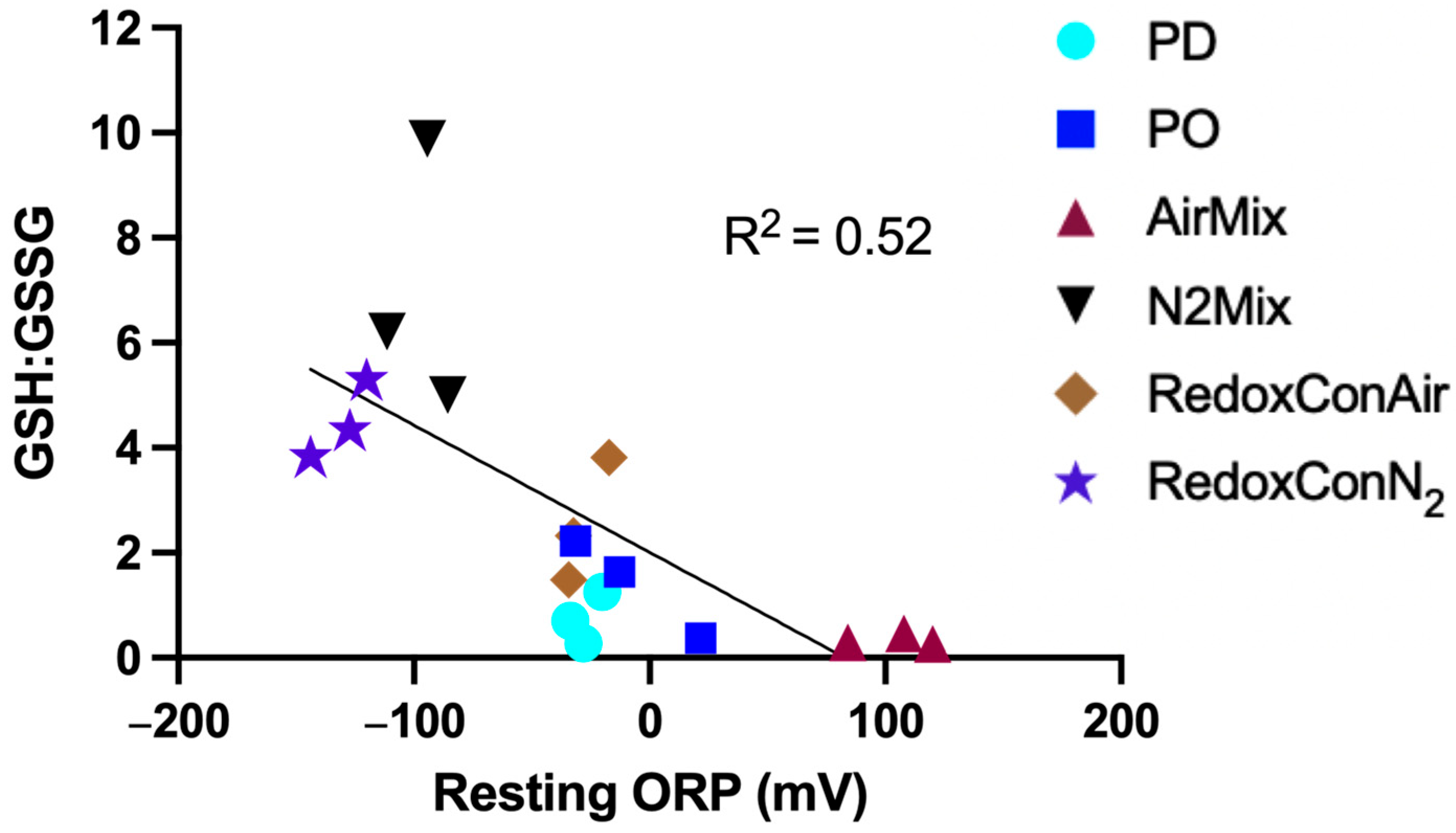
2.3. Glutathione and Glutathione Derivative Composition of the Wines
2.4. Basic Chemistry of the Wines
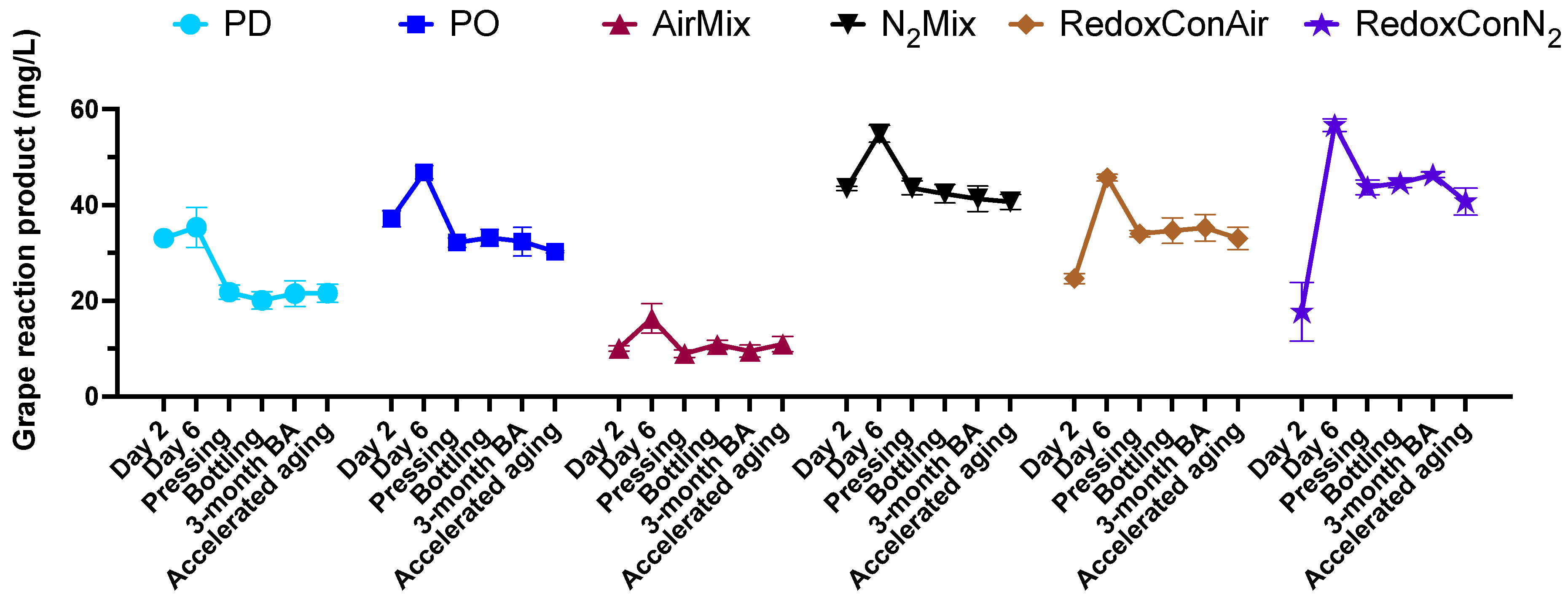
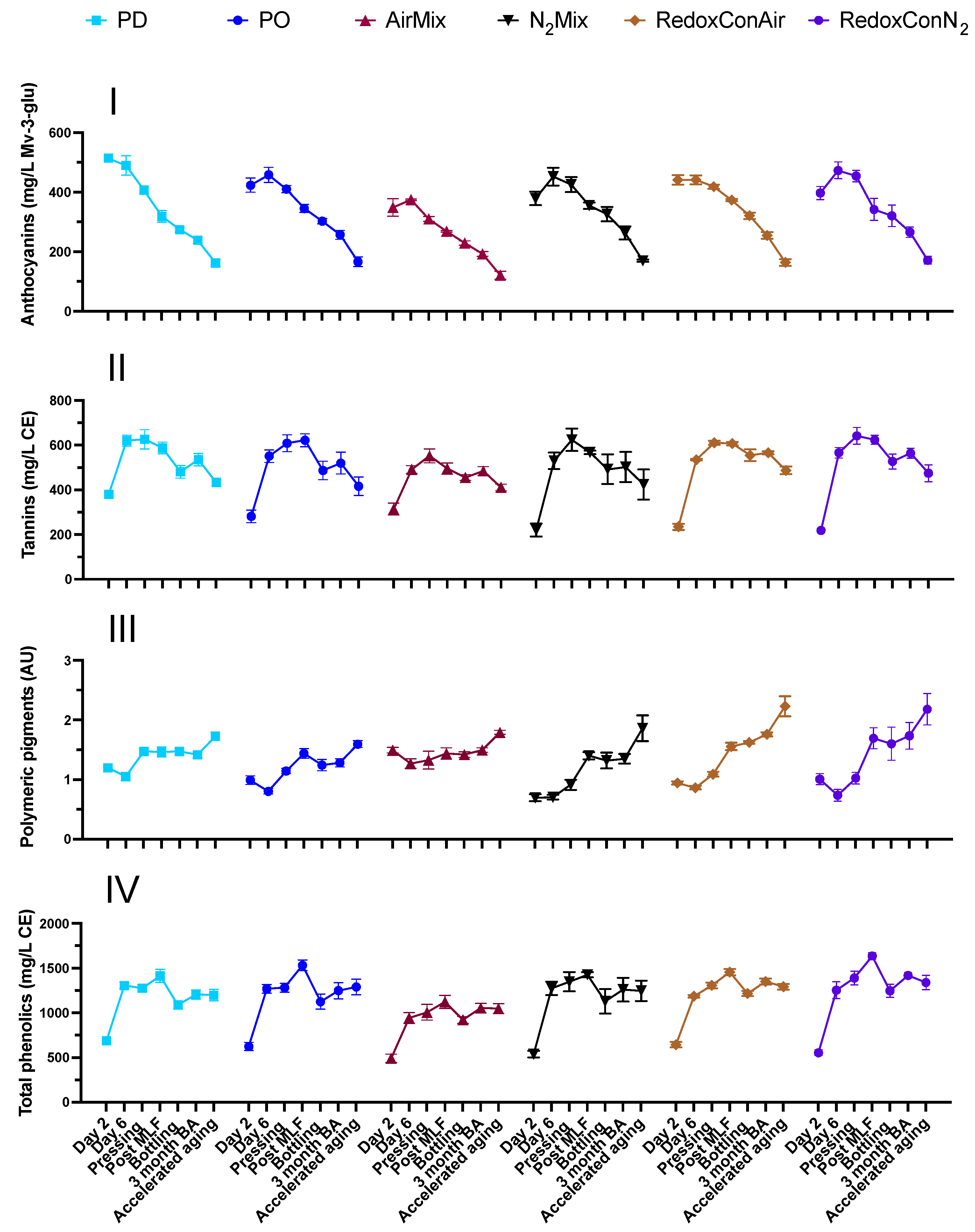
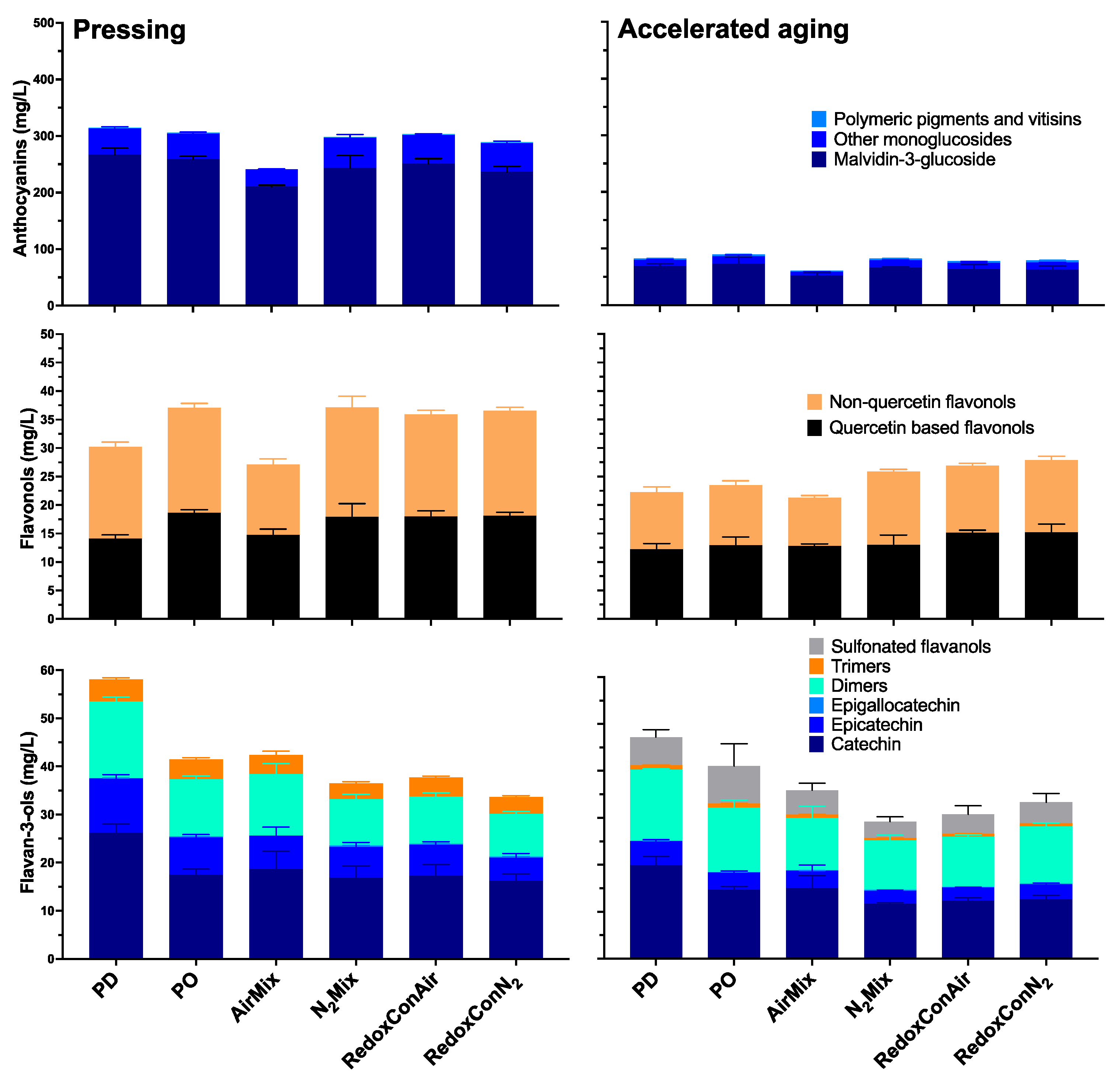
2.5. Phenolic Composition of the Wines
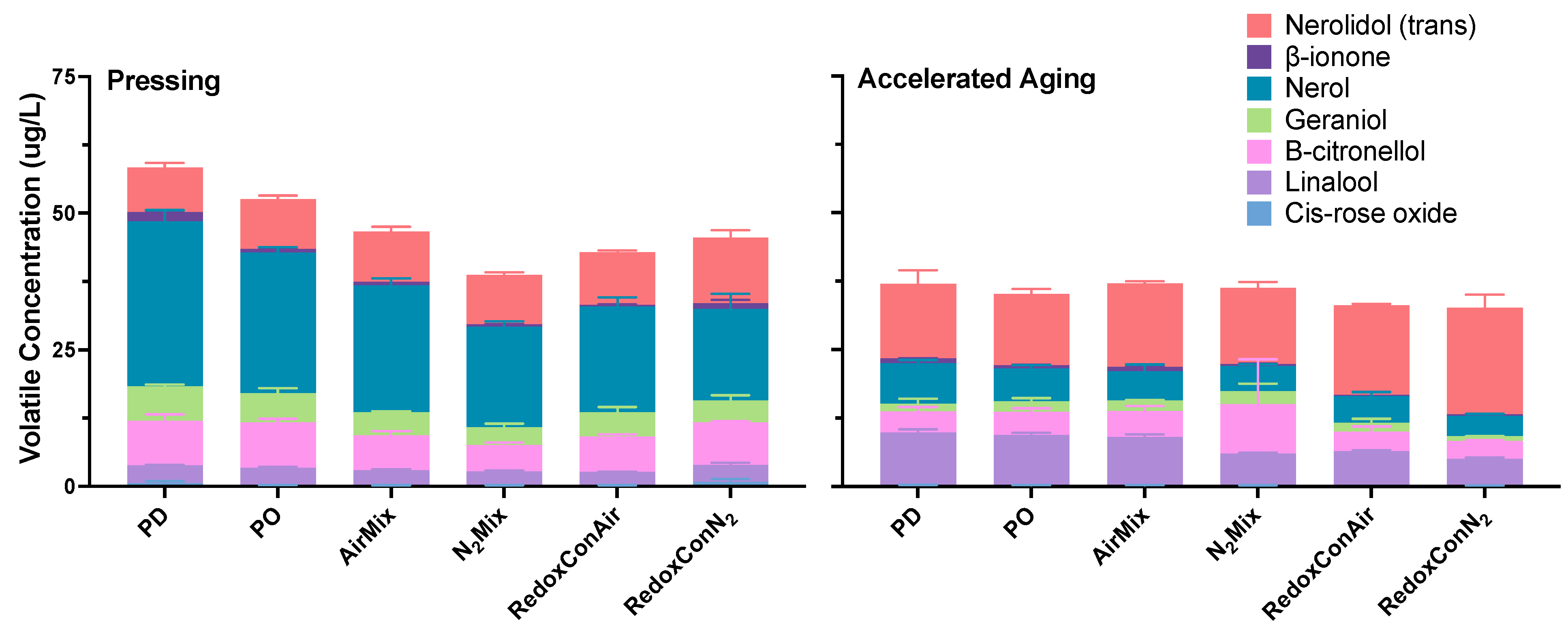
2.6. Volatile Composition of the Wines
3. Materials and Methods
3.1. Grapes
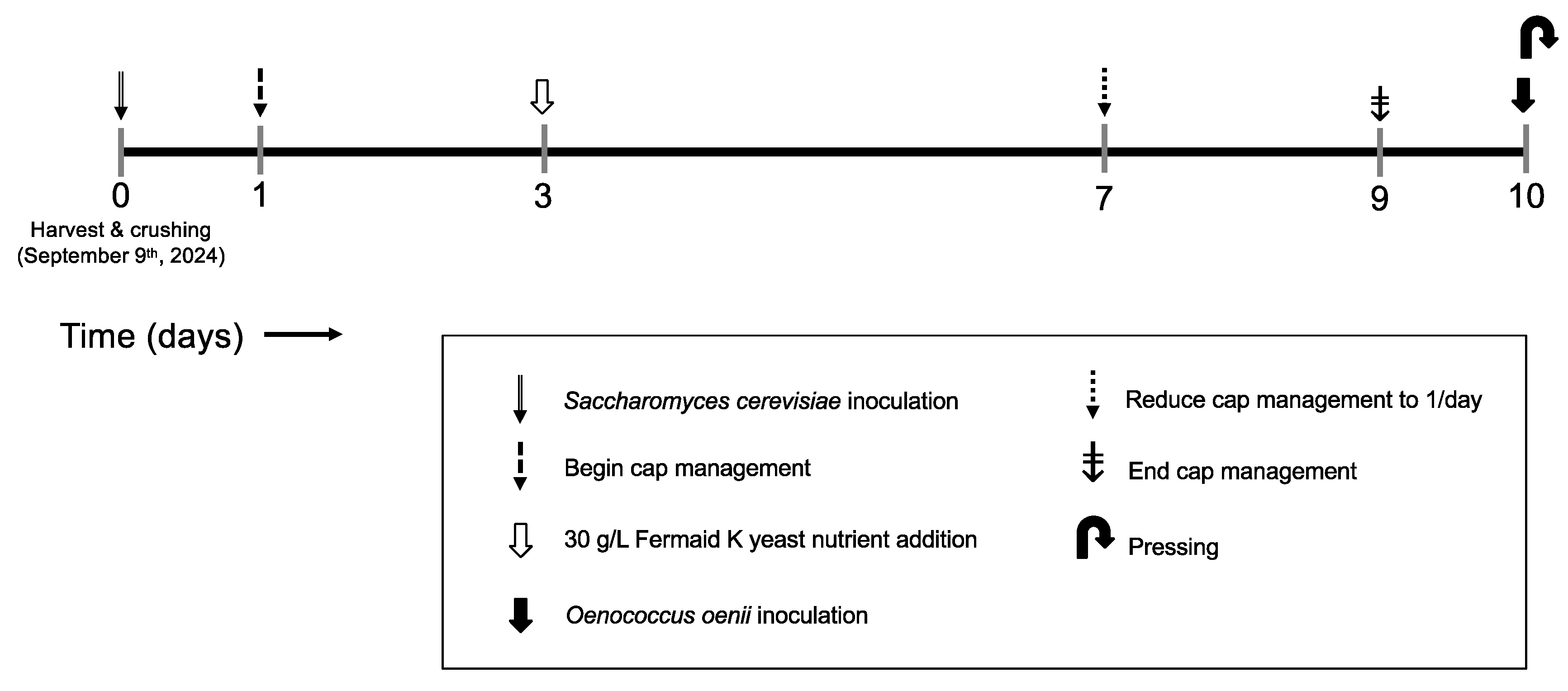
3.2. Winemaking and Experimental Design
Alcoholic Fermentation and Bottling
3.3. Wine Analyses
3.3.1. Chemical Composition
3.3.2. Wine Spectrophotometric Analysis
3.3.3. Anthocyanins, Anthocyanin-Derived Pigments, Flavonols, and the Grape Reaction Product
3.3.4. Monomeric Flavan-3-ols
3.3.5. Glutathione
3.3.6. Volatile Compounds
3.3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ag/AgCl | silver/silver chloride |
| AirMix | air mixing |
| ANOVA | analysis of variance |
| CE | catechin equivalents |
| DO | dissolved oxygen |
| GRP | grape reaction product (C23H27N3O15S) |
| GRP2 | grape reaction product 2 (C33H42N6O21S2) |
| GSH | glutathione |
| GSSG | glutathione disulfide |
| N2Mix | nitrogen mixing |
| OAV | odor activity value |
| ORP | oxidation-reduction potential |
| PD | punch-down |
| PO | pump-over |
| PPO | polyphenol oxidase |
| RedoxConAir | redox control with air |
| RedoxConN2 | nitrogen gas mixing paired to RedoxConAir |
| SHE | standard hydrogen electrode |
| TA | titratable acidity |
| YAN | yeast assimilable nitrogen |
References
- Anderson, M.M.; Smith, R.J.; Williams, M.A.; Wolpert, J.A. Viticultural Evaluation of French and California Pinot Noir Clones Grown for Production of Sparkling Wine. Am. J. Enol. Vitic. 2008, 59, 188–193. [Google Scholar] [CrossRef]
- California Department of Food and Agriculture. California Grape Acreage Report 2023 Crop; California Department of Food and Agriculture: Sacramento, CA, USA, 2024; p. 7.
- Shaw, T.B. A Climatic Analysis of Wine Regions Growing Pinot Noir. J. Wine Res. 2012, 23, 203–228. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Kennedy, J.A.; Adams, D.O. Tannin in Skins and Seeds of Cabernet Sauvignon, Syrah, and Pinot Noir Berries During Ripening. Am. J. Enol. Vitic. 2002, 53, 54–59. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and Astringency of Flavan-3-Ol Monomers, Dimers and Trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Thorngate, J.H.; Singleton, V.L. Reactions of Monomeric and Polymeric Flavan-3-Ols with Monomeric Pigment in Model Wine Solutions. Am. J. Enol. Vitic. 1994, 45, 349–352. [Google Scholar] [CrossRef]
- Fischer, U.; Strasser, M.; Gutzler, K. Impact of Fermentation Technology on the Phenolic and Volatile Composition of German Red Wines. Int. J. Food Sci. Technol. 2000, 35, 81–94. [Google Scholar] [CrossRef]
- Parnigoni, D.J.; Kuster, S.; Rivas, G.R.; Putman, G.A.; Stoffel, E.S.; Nelson, J.; Coleman, R.E.; Catania, A.A.; Casassa, L.F. Effect of Contrasting Cap Management Protocols on the Phenolic Composition, Redox Potential, and Sensory Properties of Pinot Noir and Petite Sirah Wines. Aust. J. Grape Wine Res. 2025, 2025, 3732400. [Google Scholar] [CrossRef]
- Nelson, J.; Boulton, R.; Knoesen, A. Redox Potential and Its Control in Research and Commercial Wine Fermentations. Fermentation 2025, 11, 9. [Google Scholar] [CrossRef]
- Casassa, L.F.; Kuster, S.T.; Gannet, P.; Watrelot, A.A. Temperature and Cap Management Effects on the Chemical, Phenolic, and Chromatic Composition of Pinot Noir Wines from the Central Coast of California. Am. J. Enol. Vitic. 2023, 74, 0740031. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Mireles, M.S.; Harwood, E.D.; Weller, K.M.; Ross, C.F. Chemical and Sensory Effects of Saignée, Water Addition, and Extended Maceration on High Brix Must. Am. J. Enol. Vitic. 2009, 60, 450–460. [Google Scholar] [CrossRef]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar] [CrossRef]
- Silva, I.S.; Barros, A.P.A.; Correa, L.C.; De Souza, C.O.; Biasoto, A.C.T. Effect of Thermovinification Temperature on Phenolic Compounds and Colour of Syrah Wine. Beverages 2024, 10, 117. [Google Scholar] [CrossRef]
- Stoffel, E.S.; Kuster, S.T.; Casassa, L.F. The Chemical and Sensory Impact of Cap Management Techniques, Maceration Length, and Ethanol Level in Syrah Wines from the Central Coast of California. Molecules 2025, 30, 1694. [Google Scholar] [CrossRef]
- Casassa, L.F.; LoMonaco, I.; Velasco, M.; Papageorgas, D.D. Effect of Cap Management Frequency on the Phenolic, Chromatic, and Sensory Composition of Cabernet Sauvignon Wines from the Central Coast of California over Two Vintages. Molecules 2024, 29, 2509. [Google Scholar] [CrossRef]
- Lerno, L.A.; Reichwage, M.; Panprivech, S.; Ponangi, R.; Hearne, L.; Oberholster, A.; Block, D.E. Chemical Gradients in Pilot-Scale Cabernet Sauvignon Fermentations and Their Effect on Phenolic Extraction. Am. J. Enol. Vitic. 2017, 68, 401–411. [Google Scholar] [CrossRef]
- Moenne, M.I.; Saa, P.; Laurie, V.F.; Pérez-Correa, J.R.; Agosin, E. Oxygen Incorporation and Dissolution During Industrial-Scale Red Wine Fermentations. Food Bioprocess. Technol. 2014, 7, 2627–2636. [Google Scholar] [CrossRef]
- Lerno, L.A.; Panprivech, S.; Ponangi, R.; Hearne, L.; Blair, T.; Oberholster, A.; Block, D.E. Effect of Pump-Over Conditions on the Extraction of Phenolic Compounds During Cabernet Sauvignon Fermentation. Am. J. Enol. Vitic. 2018, 69, 295–301. [Google Scholar] [CrossRef]
- Schmid, F.; Schadt, J.; Jiranek, V.; Block, D.E. Formation of Temperature Gradients in Large- and Small-Scale Red Wine Fermentations During Cap Management. Aust. J. Grape Wine Res. 2009, 15, 249–255. [Google Scholar] [CrossRef]
- Fornairon-Bonnefond, C.; Aguera, E.; Deytieux, C.; Sablayrolles, J.-M.; Salmon, J.-M. Impact of Oxygen Addition During Enological Fermentation on Sterol Contents in Yeast Lees and Their Reactivity towards Oxygen. J. Biosci. Bioeng. 2003, 95, 496–503. [Google Scholar] [CrossRef]
- Rosenfeld, E.; Beauvoit, B.; Blondin, B.; Salmon, J.-M. Oxygen Consumption by Anaerobic Saccharomyces Cerevisiae Under Enological Conditions: Effect on Fermentation Kinetics. Appl. Environ. Microbiol. 2003, 69, 113–121. [Google Scholar] [CrossRef]
- Benucci, I.; Cerreti, M.; Esti, M. Dosing Oxygen from the Early Stages of White Winemaking: Effect on Oxidation–Reduction Potential, Browning Stability, Volatile Composition, and Sensory Properties. Food Chem. 2024, 432, 137243. [Google Scholar] [CrossRef] [PubMed]
- Killeen, D.J.; Boulton, R.; Knoesen, A. Advanced Monitoring and Control of Redox Potential in Wine Fermentation. Am. J. Enol. Vitic. 2018, 69, 394–399. [Google Scholar] [CrossRef]
- Nelson, J.; Coleman, R.; Chacón-Rodríguez, L.; Runnebaum, R.; Boulton, R.; Knoesen, A. Advanced Monitoring and Control of Redox Potential in Wine Fermentation across Scales. Fermentation 2022, 9, 7. [Google Scholar] [CrossRef]
- Xue, S.J.; Wang, L.; Chen, S.; Liu, X.H.; Hu, K.; Jiang, J.; Tao, Y.S.; Jin, G.J. Controlled Aeration Driven by Oxidation-Reduction Potential Affects Ester Profile in Wine Alcohol Fermentation with Different Starter Cultures. Aust. J. Grape Wine Res. 2023, 2023, 5667458. [Google Scholar] [CrossRef]
- Dahod, S.K. Redox Potential as a Better Substitute for Dissolved Oxygen in Fermentation Process Control. Biotechnol. Bioeng. 1982, 24, 2123–2125. [Google Scholar] [CrossRef]
- Liu, C.-G.; Hao, X.-M.; Lin, Y.-H.; Bai, F.-W. Redox Potential Driven Aeration During Very-High-Gravity Ethanol Fermentation by Using Flocculating Yeast. Sci. Rep. 2016, 6, 25763. [Google Scholar] [CrossRef]
- Inzelt, G.; Lewenstam, A.; Scholz, F. (Eds.) Handbook of Reference Electrodes; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kilmartin, P.A. Uncovering the Influence of Antioxidants on Polyphenol Oxidation in Wines Using an Electrochemical Method: Cyclic Voltammetry. J. Electroanal. Chem. 2009, 633, 165–174. [Google Scholar] [CrossRef]
- Čakar, U.; Čolović, M.; Milenković, D.; Pagnacco, M.; Maksimović, J.; Krstić, D.; Đorđević, B. Strawberry and Drupe Fruit Wines Antioxidant Activity and Protective Effect Against Induced Oxidative Stress in Rat Synaptosomes. Antioxidants 2025, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Fenton, H.J.H. LXXIII.—Oxidation of Tartaric Acid in Presence of Iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Berovic, M. The Role and Application of Redox Potential in Wine Technology. Fermentation 2024, 10, 312. [Google Scholar] [CrossRef]
- Coleman, R.; Stuchebrukhov, A.; Boulton, R. The Kinetics of Autoxidation in Wine. In Recent Advances in Chemical Kinetics; Farrukh, M.A., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Danilewicz, J.C. Role of Tartaric and Malic Acids in Wine Oxidation. J. Agric. Food Chem. 2014, 62, 5149–5155. [Google Scholar] [CrossRef] [PubMed]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Oxygen, Carbon Dioxide, and Nitrogen. In Wine Analysis and Production; Springer: Boston, MA, USA, 1995; pp. 216–227. [Google Scholar] [CrossRef]
- Cheynier, V.; Souquet, J.M.; Moutounet, M. Glutathione Content and Glutathione to Hydroxycinnamic Acid Ratio in Vitis vinifera Grapes and Musts. Am. J. Enol. Vitic. 1989, 40, 320–324. [Google Scholar] [CrossRef]
- Santos, L.O.; Silva, P.G.P.; Lemos Junior, W.J.F.; De Oliveira, V.S.; Anschau, A. Glutathione Production by Saccharomyces Cerevisiae: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 1879–1894. [Google Scholar] [CrossRef]
- Jones, D.P. [11] Redox Potential of GSH/GSSG Couple: Assay and Biological Significance. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2002; Volume 348, pp. 93–112. [Google Scholar] [CrossRef]
- Gajewska, J.; Chełchowska, M.; Rychłowska-Pruszyńska, M.; Klepacka, T.; Ambroszkiewicz, J. Oxidative and Antioxidative Status Expressed as OSI Index and GSH/GSSG Ratio in Children with Bone Tumors after Anticancer Therapy Completion. J. Clin. Med. 2022, 11, 1663. [Google Scholar] [CrossRef] [PubMed]
- Webber, V.; Dutra, S.V.; Spinelli, F.R.; Carnieli, G.J.; Cardozo, A.; Vanderlinde, R. Effect of Glutathione During Bottle Storage of Sparkling Wine. Food Chem. 2017, 216, 254–259. [Google Scholar] [CrossRef]
- Dubourdieu, D.; Lavigne-Cruege, V. The Role of Glutathione on the Aromatic Evolution of Dry White Wine. Vinidea Net 2004, 2, 1–9. [Google Scholar]
- Roussis, I.G.; Lambropoulos, I.; Tzimas, P. Protection of Volatiles in a Wine with Low Sulfur Dioxide by Caffeic Acid or Glutathione. Am. J. Enol. Vitic. 2007, 58, 274–278. [Google Scholar] [CrossRef]
- Frost, S.C.; Blackman, J.W.; Hjelmeland, A.K.; Ebeler, S.E.; Heymann, H. Extended Maceration and Cap Management Impacts on the Phenolic, Volatile, and Sensory Profiles of Merlot Wine. Am. J. Enol. Vitic. 2018, 69, 360–370. [Google Scholar] [CrossRef]
- Bisson, L.F.; Butzke, C.E. Diagnosis and Rectification of Stuck and Sluggish Fermentations. Am. J. Enol. Vitic. 2000, 51, 168–177. [Google Scholar] [CrossRef]
- Valero, E.; Millán, C.; Ortega, J.M. Influence of Oxygen Addition During Growth Phase on the Biosynthesis of Lipids in Saccharomyces Cerevisiae (M330-9) in Enological Fermentations. J. Biosci. Bioeng. 2001, 92, 33–38. [Google Scholar] [CrossRef]
- Binati, R.L.; Larini, I.; Salvetti, E.; Torriani, S. Glutathione Production by Non-Saccharomyces Yeasts and Its Impact on Winemaking: A Review. Food Res. Int. 2022, 156, 111333. [Google Scholar] [CrossRef]
- Rost, J.; Rapoport, S. Reduction-Potential of Glutathione. Nature 1964, 201, 185. [Google Scholar] [CrossRef]
- Cheynier, V.; Rigaud, J.; Moutounet, M. Oxidation Kinetics of Trans-Caffeoyltartrate and Its Glutathione Derivatives in Grape Musts. Phytochemistry 1990, 29, 1751–1753. [Google Scholar] [CrossRef]
- Cheynier, V.F.; Trousdale, E.K.; Singleton, V.L.; Salgues, M.J.; Wylde, R. Characterization of 2-S-Glutathionyl Caftaric Acid and Its Hydrolysis in Relation to Grape Wines. J. Agric. Food Chem. 1986, 34, 217–221. [Google Scholar] [CrossRef]
- Kritzinger, E.C.; Bauer, F.F.; Du Toit, W.J. Role of Glutathione in Winemaking: A Review. J. Agric. Food Chem. 2013, 61, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Sonni, F.; Clark, A.C.; Prenzler, P.D.; Riponi, C.; Scollary, G.R. Antioxidant Action of Glutathione and the Ascorbic Acid/Glutathione Pair in a Model White Wine. J. Agric. Food Chem. 2011, 59, 3940–3949. [Google Scholar] [CrossRef]
- Penninckx, M. A Short Review on the Role of Glutathione in the Response of Yeasts to Nutritional, Environmental, and Oxidative Stresses. Enzyme Microb. Technol. 2000, 26, 737–742. [Google Scholar] [CrossRef]
- Salgues, M.; Cheynier, V.; Gunata, Z.; Wylde, R. Oxidation of Grape Juice 2-S-Glutathionyl Caffeoyl Tartaric Acid by Botrytis Cinerea Laccase and Characterization of a New Substance: 2,5-di-S-Glutathionyl Caffeoyl Tartaric Acid. J. Food Sci. 1986, 51, 1191–1194. [Google Scholar] [CrossRef]
- Newair, E.F.; Al-Anazi, A.; Garcia, F. Oxidation of Wine Polyphenols by Electrochemical Means in the Presence of Glutathione. Antioxidants 2023, 12, 1891. [Google Scholar] [CrossRef]
- Singleton, V.L.; Salgues, M.; Zaya, J.; Trousdale, E. Caftaric Acid Disappearance and Conversion to Products of Enzymic Oxidation in Grape Must and Wine. Am. J. Enol. Vitic. 1985, 36, 50–56. [Google Scholar] [CrossRef]
- Casassa, L.F.; Kuster, S.; Perlette, D.J.; Bargetto, K.L. Chemical and Chromatic Composition of Wines Produced with Reduced Cap Management at Industrial Scale on Two Clones of Pinot Noir from California. J. Food Comp. Anal. 2024, 125, 105728. [Google Scholar] [CrossRef]
- Guymon, J.F.; Crowell, E.A. The Nature and Cause of Cap-Liquid Temperature Differences During Wine Fermentation. Am. J. Enol. Vitic. 1977, 28, 74–78. [Google Scholar] [CrossRef]
- Bustamante, M.; Gil-Cortiella, M.; Peña-Neira, Á.; Gombau, J.; García-Roldán, A.; Cisterna, M.; Montané, X.; Fort, F.; Rozès, N.; Canals, J.M.; et al. Oxygen-Induced Enzymatic and Chemical Degradation Kinetics in Wine Model Solution of Selected Phenolic Compounds Involved in Browning. Food Chem. 2025, 484, 144421. [Google Scholar] [CrossRef] [PubMed]
- Wissemann, K.W.; Lee, C.Y. Polyphenoloxidase Activity During Grape Maturation and Wine Production. Am. J. Enol. Vitic. 1980, 31, 206–211. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Le Quéré, J.-M.; Renard, C.M.G.C.; Cheynier, V. Inhibition of apple polyphenol oxidase activity by procyanidins and polyphenol oxidation products. J. Agric. Food Chem. 2005, 53, 926–934. [Google Scholar] [CrossRef]
- Vernhet, A.; Dubascoux, S.; Cabane, B.; Fulcrand, H.; Dubreucq, E.; Poncet-Legrand, C. Characterization of Oxidized Tannins: Comparison of Depolymerization Methods, Asymmetric Flow Field-Flow Fractionation and Small-Angle X-Ray Scattering. Anal. Bioanal. Chem. 2011, 401, 1559–1569. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Cabane, B.; Bautista-Ortín, A.-B.; Carrillo, S.; Fulcrand, H.; Pérez, J.; Vernhet, A. Tannin Oxidation: Intra- versus Intermolecular Reactions. Biomacromolecules 2010, 11, 2376–2386. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Non-Covalent Interaction Between Procyanidins and Apple Cell Wall Material. Biochim. Biophys. Acta Gen. Subj. 2004, 1672, 192–202. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Interactions Between Apple (Malus × Domestica Borkh.) Polyphenols and Cell Walls Modulate the Extractability of Polysaccharides. Carbohydr. Polym. 2009, 75, 251–261. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Jiang, X.; Wang, H.; Wang, Z.; Bai, Z.; Ocone, R. Effects of Pressure and Particle Size on Bubble Behaviors in a Pseudo 2D Pressured Fluidized Bed with Geldart A/B, B and D Particles. Chem. Eng. J. 2023, 470, 143904. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Waterhouse, A.L. Redox Cycling of Iron: Effects of Chemical Composition on Reaction Rates with Phenols and Oxygen in Model Wine. Am. J. Enol. Vitic. 2021, 72, ajev.2021.20024-OA. [Google Scholar] [CrossRef]
- Saa, P.A.; Moenne, M.I.; Pérez-Correa, J.R.; Agosin, E. Modeling Oxygen Dissolution and Biological Uptake During Pulse Oxygen Additions in Oenological Fermentations. Bioprocess. Biosyst. Eng. 2012, 35, 1167–1178. [Google Scholar] [CrossRef]
- Bai, X.; Chen, X.; Li, X.; Tan, F.; Sam, F.E.; Tao, Y. Wine Polyphenol Oxidation Mechanism and the Effects on Wine Quality: A Review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70035. [Google Scholar] [CrossRef] [PubMed]
- Casassa, L.F.; Harbertson, J.F. Extraction, Evolution, and Sensory Impact of Phenolic Compounds During Red Wine Maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Ma, L.; Watrelot, A.A.; Addison, B.; Waterhouse, A.L. Condensed Tannin Reacts with SO2 During Wine Aging, Yielding Flavan-3-Ol Sulfonates. J. Agric. Food Chem. 2018, 66, 9259–9268. [Google Scholar] [CrossRef]
- Lorrain, B.; Tempere, S.; Iturmendi, N.; Moine, V.; De Revel, G.; Teissedre, P.-L. Influence of Phenolic Compounds on the Sensorial Perception and Volatility of Red Wine Esters in Model Solution: An Insight at the Molecular Level. Food Chem. 2013, 140, 76–82. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative Determination of the Odorants of Young Red Wines from Different Grape Varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Slaghenaufi, D.; Ugliano, M. Norisoprenoids, Sesquiterpenes and Terpenoids Content of Valpolicella Wines During Aging: Investigating Aroma Potential in Relationship to Evolution of Tobacco and Balsamic Aroma in Aged Wine. Front. Chem. 2018, 6, 66. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Bogaki, T. Mechanisms of Production and Control of Acetate Esters in Yeasts. J. Biosci. Bioeng. 2023, 136, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.R.; Carter, P.; Cowan, R.S.; Wallace, G.G. Impact of protein fouling on the charge injection capacity, impedance, and effective electrode area of platinum electrodes for bionic devices. ChemElectroChem 2021, 8, 1078–1090. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Picciotto, E.A.; Adams, D.O. Measurement of Polymeric Pigments in Grape Berry Extract Sand Wines Using a Protein Precipitation Assay Combined with Bisulfite Bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar] [CrossRef]
- Downey, M.O.; Rochfort, S. Simultaneous Separation by Reversed-Phase High-Performance Liquid Chromatography and Mass Spectral Identification of Anthocyanins and Flavonols in Shiraz Grape Skin. J. Chromatogr. A 2008, 1201, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Dienes-Nagy, Á.; Vuichard, F.; Belcher, S.; Blackford, M.; Rösti, J.; Lorenzini, F. Simultaneous Quantification of Glutathione, Glutathione Disulfide and Glutathione-S-Sulfonate in Grape and Wine Using LC-MS/MS. Food Chem. 2022, 386, 132756. [Google Scholar] [CrossRef]
- Marín-San Román, S.; Carot-Sierra, J.M.; Sáenz De Urturi, I.; Rubio-Bretón, P.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Optimization of Stir Bar Sorptive Extraction (SBSE) and Multi-Stir Bar Sorptive Extraction (mSBSE) to Improve Must Volatile Compounds Extraction. LWT 2022, 172, 114182. [Google Scholar] [CrossRef]
- Cebrián-Tarancón, C.; Sánchez-Gómez, R.; Cabrita, M.J.; García, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Winemaking with Vine-Shoots. Modulating the Composition of Wines by Using Their Own Resources. Food Res. Int. 2019, 121, 117–126. [Google Scholar] [CrossRef]
- Elsharif, S.A.; Buettner, A. Structure–Odor Relationship Study on Geraniol, Nerol, and Their Synthesized Oxygenated Derivatives. J. Agric. Food Chem. 2018, 66, 2324–2333. [Google Scholar] [CrossRef]
- Ferreira, V.; Peña, C.; Escudero, A.; Cacho, J. Losses of Volatile Compounds During Fermentation. Z. Für Lebensm.-Unters. Und Forsch. 1996, 202, 318–323. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Padrayuttawat, A.; Yoshizawa, T.; Tamura, H.; Tokunaga, T. Optical Isomers and Odor Thresholds of Volatile Constituents in Citrus Sudachi. Food Sci. Technol. Int. 1997, 3, 402–408. [Google Scholar] [CrossRef]
- Takeoka, G.; Buttery, R.G.; Ling, L. Odour Thresholds of Various Branched and Straight Chain Acetates. LWT-Food Sci. Technol. 1996, 29, 677–680. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Buttery, R.G.; Turnbaugh, J.G.; Benson, M. Odor Thresholds of Various Branched Esters. LWT-Food Sci. Technol. 1995, 28, 153–156. [Google Scholar] [CrossRef]
- Kritzinger, E.C. Winemaking Practices Affecting Glutathione Concentrations in White Wine. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2012. [Google Scholar]
- Nelson, J.; Boulton, R. Models for Wine Fermentation and Their Suitability for Commercial Applications. Fermentation 2024, 10, 269. [Google Scholar] [CrossRef]
- Cano-López, M.; Pardo-Mínguez, F.; Schmauch, G.; Saucier, C.; Teissedre, P.-L.; López-Roca, J.M.; Gómez-Plaza, E. Effect of Micro-Oxygenation on Color and Anthocyanin-Related Compounds of Wines with Different Phenolic Contents. J. Agric. Food Chem. 2008, 56, 5932–5941. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen Llaudy, M.; Canals, R.; González-Manzano, S.; Canals, J.M.; Santos-Buelga, C.; Zamora, F. Influence of Micro-Oxygenation Treatment before Oak Aging on Phenolic Compounds Composition, Astringency, and Color of Red Wine. J. Agric. Food Chem. 2006, 54, 4246–4252. [Google Scholar] [CrossRef]
- Yang, Y.; Deed, R.C.; Araujo, L.D.; Waterhouse, A.L.; Kilmartin, P.A. Effect of Microoxygenation on Acetaldehyde, Yeast and Colour Before and After Malolactic Fermentation on Pinot Noir Wine. Aust. J. Grape Wine Res. 2022, 28, 50–60. [Google Scholar] [CrossRef]
- Malfeito-Ferreira, M. Spoilage Yeasts in Red Wines. In Red Wine Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–235. [Google Scholar] [CrossRef]
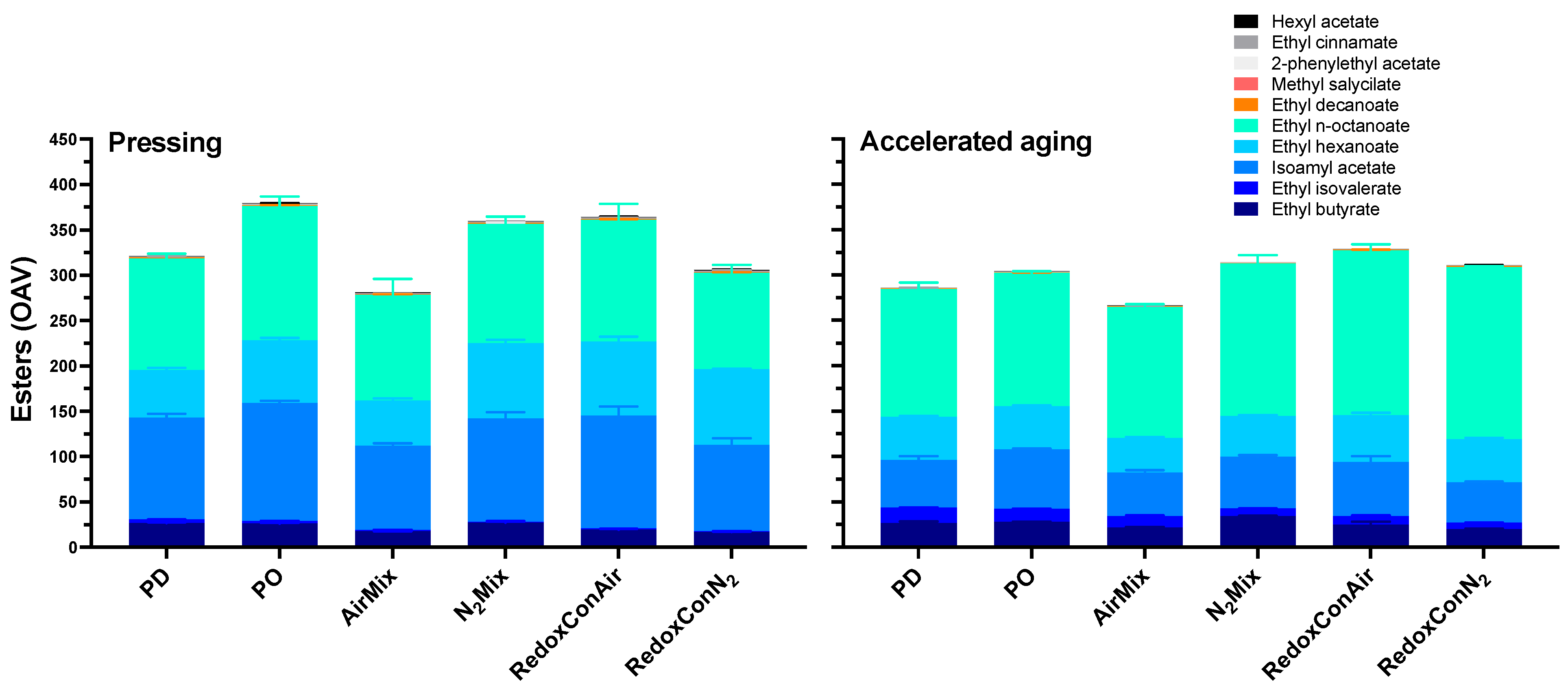
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parnigoni, D.J.; Kuster, S.T.; Villalobos, J.; Nelson, J.; Coleman, R.E.; Casassa, L.F. Effect of Contrasting Redox Potential Evolutions and Cap Management Techniques on the Chemical Composition of Red Wine. Molecules 2025, 30, 3172. https://doi.org/10.3390/molecules30153172
Parnigoni DJ, Kuster ST, Villalobos J, Nelson J, Coleman RE, Casassa LF. Effect of Contrasting Redox Potential Evolutions and Cap Management Techniques on the Chemical Composition of Red Wine. Molecules. 2025; 30(15):3172. https://doi.org/10.3390/molecules30153172
Chicago/Turabian StyleParnigoni, Dallas J., Sean T. Kuster, Jesus Villalobos, James Nelson, Robert E. Coleman, and L. Federico Casassa. 2025. "Effect of Contrasting Redox Potential Evolutions and Cap Management Techniques on the Chemical Composition of Red Wine" Molecules 30, no. 15: 3172. https://doi.org/10.3390/molecules30153172
APA StyleParnigoni, D. J., Kuster, S. T., Villalobos, J., Nelson, J., Coleman, R. E., & Casassa, L. F. (2025). Effect of Contrasting Redox Potential Evolutions and Cap Management Techniques on the Chemical Composition of Red Wine. Molecules, 30(15), 3172. https://doi.org/10.3390/molecules30153172







