Abstract
The development of earth-abundant nickel-based catalysts is currently one of the greatest challenges for the straightforward synthesis of functionalized polyolefins. With environmental protection concerns, controllable copolymerizations of ethylene with biosourced comonomers derived from castor oil, such as methyl 10-undecenoate (U-COOMe), 10-undecen-1-ol (U-OH), or 10-undecenyl bromide (U-Br), were realized using α-diimine nickel catalyst (Ni-DBB) with dibenzobarrelene backbone. Catalyst Ni-DBB was highly tolerant toward polar comonomers, and functional polyethylenes were successfully prepared. The influences of the polar group, temperature, and comonomer concentration were studied in detail. Catalyst Ni-DBB was able to catalyze the copolymerization of ethylene with U-OH to afford high-molecular-weight (~180 kg/mol) functional polyethylene in a controlled fashion. NMR analysis showed that the produced functional polyethylenes were highly branched and had broad melting peaks ranging from 0 to 30 °C. Water contact angle (WCA) measurements showed that the surface of the obtained hydroxyl-functionalized polyethylene changed from hydrophobic to hydrophilic with the introduction of the comonomer U-OH.
1. Introduction
Polyolefins, especially polyethylene (PE), are commercially the most important family of polymeric materials because of their excellent mechanical, physical, and chemical properties [1]. However, the full non-polar nature of the polyolefins also limits their wider applications. Compared to their non-functionalized analogues, functional polyethylenes exhibit beneficial properties such as adhesion, printability, miscibility, and toughness [2,3]. There are three major approaches for the synthesis of functional polyolefins, including free radical copolymerization of ethylene and polar monomers under harsh conditions [4,5], post-functional modification of polyolefins [6], and direct copolymerization of ethylene and polar monomers through coordination polymerization under mild conditions [7]. Undoubtedly, the direct copolymerization with polar monomers is the most promising approach, which allows the preparation of functional polyolefins with controllable microstructure [8].
Transition metal catalysts play a crucial role in the coordination copolymerization of ethylene with polar comonomers. However, early transition metals, such as those in groups 4–6, are often inactive for the direct copolymerization of ethylene and polar comonomers due to the high oxophilicity of their metal centers [9,10]. In contrast, late transition metal catalysts are ideal candidates for the copolymerization of ethylene and polar monomers because of their low oxophilicity [11,12]. Several late transition metal Ni/Pd catalysts, such as α-diimine [13,14,15], phosphine-sulfonate [16,17,18,19], salicylaldiminate [20,21,22,23,24], and phosphino-phenolate [25,26,27,28,29], have been developed for effectively copolymerizing ethylene and polar comonomers. A notable example is the α-diimine nickel and palladium catalysts (also known as Brookhart-type catalysts), which often produce branched or highly branched copolymers containing a portion of, or all, terminal polar groups due to chain walking [30,31]. Generally, the α-diimine nickel catalysts are more active for ethylene homopolymerization and have lower chain walking ability than palladium analogues. However, nickel catalysts have lower tolerance toward polar comonomers than palladium catalysts [13,32]. Therefore, the copolymerization of ethylene and polar comonomers using α-diimine nickel catalysts remains a significant challenge due to their relatively high metal oxophilicity. This challenge can be addressed by employing: (a) comonomers bearing sterically hindered functional groups or functional groups positioned far from the polymerizable double bond through long spacers [33,34]; (b) comonomers with protected functional groups [35,36]; or (c) catalytic systems with enhanced tolerance toward polar groups [37,38].
Few α-diimine nickel catalysts have been developed to directly copolymerize ethylene and polar comonomers, although Ni-based catalysts are low-cost and abundant in the Earth’s crust [11,39]. Increasing bulky steric hindrance around the metal center is an effective approach to enhance the tolerance of the nickel metal center toward polar groups [40,41]. Introducing bulky anilines such as 2,6-dibenzhydrylanilines and 8-arylnaphthylamine has been widely developed in the design of α-diimine nickel catalysts for copolymerization of ethylene and polar monomers [42,43,44]. Besides, installing bulky backbones such as camphyl [45,46] and dibenzo-/dinaphthobarrelene backbones [47,48,49,50] has also been developed by our group for the copolymerization of ethylene and polar monomers.
With carbon peaking and carbon neutrality goals, as well as environmental protection concerns, the use of biosourced monomers in polymer synthesis is receiving increasing attention [51,52,53]. Biosourced monomers, such as 10-undecenoic acid (U-COOH), derived from the pyrolysis and extraction of castor biomass oil, have attracted considerable interest in the synthesis of functional polymers because of their biosourced character [54]. It has served as a key precursor in the industrial production of pharmaceuticals, consumer goods, and specialty polymers [55,56]. The flexible long-chain alkyl moiety in its molecular structure contributes to improved flexibility of the resultant polymeric materials. Furthermore, a series of 10-undecenoic acid derivatives can be obtained through chemical transformations such as esterification or other derivatization reactions, which function as critical intermediates in developing biodegradable elastomers, hydrogels, and environmentally benign adhesives [57,58].
In this contribution, a dibenzobarrelene α-diimine nickel catalyst was used in the copolymerization of ethylene and biosourced 10-undecenoic acid derivatives (CH₂=CH–(CH₂)ₙ–X, X = COOMe (n = 8), OH, and Br (n = 9)) (Scheme 1). Bulky dibenzobarrelene backbone of the ligand effectively enhanced the tolerance of the nickel catalyst towards polar comonomers, and functional polyethylenes were successfully prepared. More importantly, hydroxyl-functionalized polyethylene with high molecular weight (Mn~180 kg/mol) was synthesized precisely in a controlled fashion.
Scheme 1.
Copolymerization of ethylene and biosourced comonomers derived from castor oil using dibenzobarrelene α-diimine nickel catalyst.
2. Results and Discussion
2.1. Copolymerization of Ethylene and Comonomers
Our group has previously developed dibenzobarrelene-based α-diimine nickel and palladium catalysts. Introduction of bulky dibenzobarrelene backbone can enhance thermal stability and controlled fashion in α-diimine nickel-catalyzed ethylene polymerization [47]. Besides, the dibenzobarrelene-based α-diimine palladium catalyst can catalytically prepare functional polyolefins by controlled copolymerization of ethylene and a variety of acrylate comonomers [48,49,51]. Prior to this work, we attempted the copolymerization of ethylene with 10-undecylenic acid (U-COOH). However, only trace amounts of polymer were obtained (entry 13 in Table 1). Herein, the copolymerizations of ethylene with biosourced 10-undecenoic acid derivatives (CH2=CH–(CH2)n–X, X = COOMe (U-COOMe, n = 8), OH, and Br (U-OH or U-Br, n = 9)) using dibenzobarrelene-based α-diimine nickel catalyst Ni-DBB were carried out. In these copolymerization systems, a large amount of AlEt2Cl (Al/Ni = 1600) as a cocatalyst was used compared to ethylene homopolymerization (Al/Ni = 600) [47], because a large amount of alkyl aluminum reagents is helpful for protecting the polar groups for successful copolymerization. [59,60,61,62,63]. These functional groups in the olefins preferentially coordinate to Al, forming stable and protected aluminates that serve as true comonomers for successful copolymerization, and deprotection to the free functionality is accomplished by acid wash upon termination of polymerization [64,65].

Table 1.
Copolymerization results of ethylene and comonomers under various temperatures a.
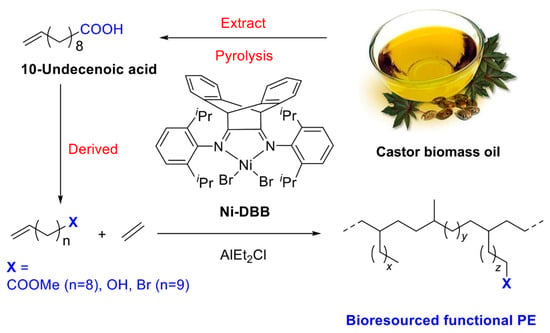
As shown in Table 1, copolymerizations of ethylene and three biosourced comonomers derived from castor oil with different polar groups were conducted in a temperature range from 20 to 65 °C. Excluding the amount of AlEt2Cl used as the activator for ethylene polymerization (Al/Ni = 600, 1.2 mmol), an additional 2.0 mmol of AlEt2Cl was added in the copolymerization system, resulting in an [Al]/[polar monomer] ratio of 1. The polar comonomer incorporation into the copolymer was identified by 1H NMR spectroscopy, and no homopolymer of polar comonomer was present in the copolymerization products. For the ethylene/U-OH copolymer, the signal corresponding to the hydroxyl protons is difficult to observe. Therefore, the incorporation (mol%) of hydroxyl-containing monomers in the copolymer is typically calculated by integrating the methylene proton signals (–CH2OH) relative to the signals of other protons in the 1H NMR spectrum (see Section 3.3) [39,66,67]. As shown in Figure 1, 1H NMR spectra of the obtained functional polyethylene clearly show characteristic peaks for U-COOMe (Ha: 3.60 ppm; Hb: 2.30 ppm; Hc: 1.61 ppm), U-OH (Hd: 3.66 ppm; He: 1.85 ppm), and U-Br (Hf: 3.40 ppm; Hg: 1.85 ppm), respectively, which proves the successful copolymerization of ethylene with U-COOMe, U-OH, or U-Br [47,49,68].
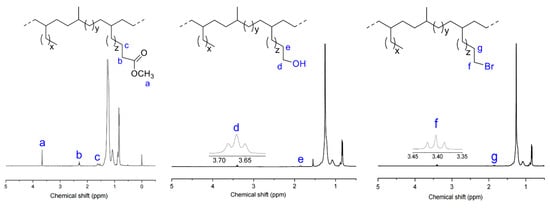
Figure 1.
1H NMR spectra of the obtained functional polyethylenes in CDCl3 (entries 3, 7, and 11 in Table 1).
The effects of polar groups on copolymerization were first examined. As shown in Table 1 and Figure 2, copolymerization activity increased but incorporation of polar comonomers decreased in the order of U-COOMe > U-OH > U-Br under identical reaction conditions, strongly indicating that the ester group -COOMe exhibited the strongest poisoning effect on the nickel metal center. This was attributed to the easiest σ-interaction between the ester group and the nickel metal center [32,69,70]. Additionally, copolymer molecular weight followed the order of U-OH > U-COOMe > U-Br. Although copolymerization of ethylene and U-Br showed the highest activity, the resultant copolymer molecular weight was the lowest. This is attributed to the accelerated chain transfer reaction caused by the electron-withdrawing Br group [71,72].
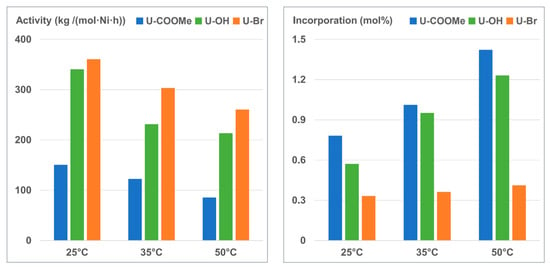
Figure 2.
The effects of polar groups on copolymerization activity and comonomer incorporation at different temperatures.
The influences of reaction parameters on the copolymerization of ethylene and polar comonomers using the catalyst Ni-DBB were further investigated. As shown in Table 1, the copolymerization activity and the molecular weight of copolymers decreased, but the incorporation of the polar monomer increased with an increase in the temperature from 20 to 65 °C. Besides, the copolymers showed relatively narrow distribution (PDI < 1.65), indicating a controlled copolymerization fashion [73]. The molecular weight distribution of the copolymer also became broader with increasing temperatures. These observations suggested that elevated temperature was favorable for the incorporation of polar comonomers and the acceleration of chain transfer. To further illustrate the polar group tolerance of the dibenzobarrelene α-diimine nickel catalyst, copolymerizations were further conducted at various polar monomer concentrations using the catalyst Ni-DBB (Table 2). Increasing the comonomer concentration resulted in a lower [Al]/[polar monomer] ratio (0.3~0.5), which led to an increase in the incorporation of comonomers, whereas the copolymerization activity and the molecular weight of copolymers decreased (entries 3, 6, 8, and 9 in Table 2). Besides, copolymerization of U-Br was conducted at a higher comonomer concentration. Lowering the relative additional Et2AlCl/comonomer ratio to 0.3 still allowed the formation of a large amount of copolymer (entry 9 in Table 2), which was a result of reducing the poisoning effect of Br toward the nickel metal center compared to the ester and hydroxyl groups [69,74].

Table 2.
Copolymerization results of ethylene and comonomers with various concentrations a.
The experimental results, as mentioned above, clearly showed that copolymerization of ethylene and U-OH was the most efficient in producing narrowly dispersed copolymers among the three comonomers. The controlled copolymerization process of ethylene and U-OH using the catalyst Ni-DBB was further studied at 20 °C. Figure 3 shows symmetric GPC traces of the copolymers produced at different reaction times without tailing peaks, which shift to the higher molecular weight region with prolonged reaction time. Plots of number-average molecular weights (Mn) as a function of reaction time (Figure 4) also show that Mn grows linearly with the polymerization time, and Mw/Mn (PDI) values are below 1.20. Further, the incorporation of U-OH into copolymers obtained at different time periods remains nearly constant (∼0.57 mol%), indicating that the comonomer U-OH is uniformly incorporated into polymer chains [48,51]. The hydroxyl functionalized polyethylene with a high molecular weight up to 180 kg/mol can be produced. Therefore, the dibenzobarrelene-based α-diimine nickel catalyst (Ni-DBB) is able to catalyze the copolymerization of ethylene and U-OH in a controlled fashion.
Figure 3.
GPC curves of E/U-OH copolymers obtained at different reaction times.
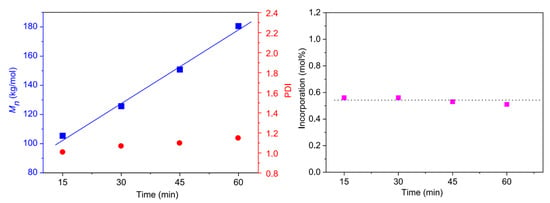
Figure 4.
Plots of Mn, PDI, and incorporation of U-OH as a function of reaction time using Ni-DBB/Et2AlCl at 20 °C.
2.2. Microstructures of Functional Polyethylene
1H NMR spectroscopy analysis (Figure 1) showed that the obtained functional polyethylene was highly branched (75~101/1000 C), which was different from the semi-crystalline polar polyethylene produced by Coates and co-workers [75]. To gain deep insight into the definitive microstructure of the produced functional polyethylene, the functional polyethylenes were analyzed by 13C NMR spectroscopy (Figure 5). 13C NMR spectra of the obtained functional polyethylene show characteristic peaks for U-COOMe (Ca: 174.35 ppm, Cb: 51.49 ppm), U-OH (Cd: 63.38 ppm), and U-Br (Cf: 33.53 ppm), respectively, which prove the successful copolymerization of ethylene and U-OH or U-Br. The branching distribution is quantitatively calculated on the basis of previous resonance assignments [76,77]. The obtained copolymers mostly contain methyl branches and long-chain branches, and the total content of other short branches, such as ethyl, propyl, butyl, and amyl, was very low. This is attributed to the chain walking mechanism previously reported in our work [78].
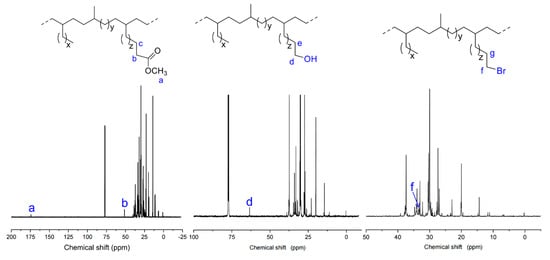
Figure 5.
13C NMR spectra of the obtained functional polyethylenes in CDCl3 (entries 3, 7, and 11 in Table 1).
Differential scanning calorimetry (DSC) analyses were conducted to examine the thermal behavior of the copolymers. As shown in Figure 6, the DSC curves of these copolymers (up to 150 °C) clearly show that no polyethylene homopolymer is present in the copolymerization products. Moreover, the functional polyethylenes have very broad melting endotherms, which are attributed to inhomogeneous branching distribution and different branching lengths. Generally, three kinds of functional polyethylenes have nearly the same DSC profiles, indicating nearly the same branching structure. This observation also indicates that the polar comonomer does not impact the chain walking process during copolymerization.
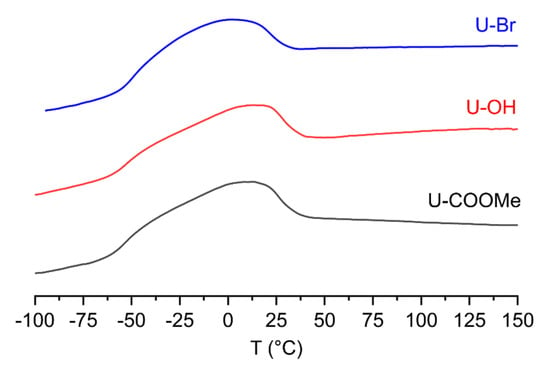
Figure 6.
The DSC curves of functional polyethylenes (entries 3, 7, and 11 in Table 1).
2.3. Hydrophilic Property of Functional Polyethylene
The introduction of a small amount of polar groups in polyolefins can dramatically improve material properties such as compatibility, paintability, printability, and adhesion [79,80]. The surface properties of hydroxyl-functionalized polyethylenes (E/U-OH copolymers) with different -OH incorporations were evaluated by measuring the water contact angle (WCA). As shown in Figure 7, the WCA of pure branched polyethylene is 110° [62,68], while the WCA of copolymer decreased dramatically with the increasing hydroxyl incorporation. The lowest WCA (84°) was obtained when the hydroxyl incorporation was 1.23 mol% [81]. Therefore, the surface of the PE material can be changed from hydrophobic to hydrophilic with the introduction of a small amount of U-OH.
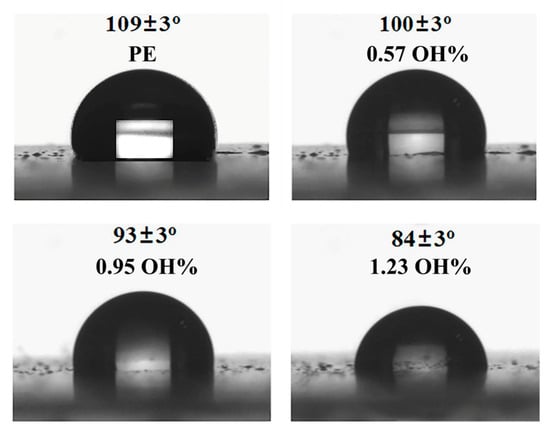
Figure 7.
The water contact angle of hydroxyl-functionalized polyethylenes (entries 5–7 in Table 1).
3. Materials and Methods
3.1. General Procedures
All manipulations involving air- and moisture-sensitive compounds were carried out under an atmosphere of dried and purified nitrogen using standard vacuum-line, Schlenk, or glovebox techniques.
3.2. Materials
Toluene was refluxed over Na/K alloy before use, and dichloromethane was dried over P2O5 and was distilled under nitrogen. Et2AlCl (0.9 M in Toluene) was purchased from Acros. Ethylene (99.99%) was purified by passing through Agilent moisture and oxygen traps. Methyl 10-undecenoate (U-COOMe), 10-undecen-1-ol (U-OH), and 10-undecenyl bromide (U-Br) were purchased from Aldrich. Other commercially available reagents were purchased and used without purification.
The dibenzobarrelene α-diimine nickel complex, Ni-DBB, was prepared according to our previous work [47]. The α-diimine ligand DBB-L was first synthesized and fully confirmed by 1H and 13C NMR. 1H NMR (CDCl3, 400 MHz), δ (ppm): 7.25–7.02 (m, 14H, Ar-H), 4.98 (s, 2H, CH), 2.49 (m, 4H, CH(CH3)2), 1.15 (d, 12H, CH3), 1.02 (d, 12H, CH3). 13C NMR (CDCl3, 100 MHz), δ (ppm): 158.46, 145.56, 138.54, 136.36, 127.29, 125.38, 124.14, 122.81, 51.12, 23.29, 22.48. Nickel complex DBB-Ni was obtained by the addition of the ligand DBB-L to a stirring suspension of (dimethoxyethane)NiBr2 in CH2Cl2 and confirmed by elemental analysis. Anal. Calcd for C40H44Br2N2Ni: C, 62.29; H, 5.75; N, 3.63; Found: C, 62.36; H, 5.66; N, 3.51.
3.3. Measurements
The NMR analysis of the copolymers was performed on a Bruker Avance III HD 400 MHz spectrometer. The incorporation (mol%) of U-COOMe in the copolymer was calculated by integrating methyl proton signals (-OCH3) of inserted U-COOMe with respect to signals of other protons in the 1H NMR spectrum (incorporation mol% = 4IOCH3/3(ICH3 + ICH2 + ICH) × 100%) [48]. The incorporation (mol%) of U-OH and U-Br in the copolymer was calculated by integrating methylene proton signals (-CH2-) of inserted U-OH/U-Br with respect to signals of other protons in the 1H NMR spectrum (incorporation mol% = 2ICH2/(ICH3 + ICH2 + ICH) × 100%) [66,67]. The branching density per 1000 carbon atoms of copolymers was determined by 1H NMR spectrum. The branching distribution was quantitatively calculated based on previously reported resonance assignments [76,77]. For each distinguishable type of branch in the ¹³C NMR spectrum, a representative signal was selected, and the content of each branch type was determined by integrating the area under the corresponding peak. Differential scanning calorimeter (DSC) analyses of PEs were conducted with a PerkinElmer DSC-4000 system. The DSC curves were recorded as second heating curves from −100 °C to +150 °C at a heating rate of 10 °C/min and a cooling rate of 10 °C/min. Recorded at second heating curves to examine the thermal behavior of the copolymers. GPC analyses of the molecular weights and molecular weight distributions (PDI = Mw/Mn) of the PE samples at 150 °C were performed on a PL-GPC 220 high-temperature chromatograph equipped with two GPC columns (PLgel 10 μm MIXED-B, Santa Clara, CA, USA), (Agilent Technologies, Palo Alto, CA, USA) and a differential refractive-index detector. 1,2,4-Trichlorobenzene (TCB) (Agilent Technologies, Palo Alto, CA, USA) was used as the eluent at a flow rate of 1.0 mL/min. The molecular weight data were analyzed using narrow polystyrene standards (Polymer Laboratories, Long Beach, CA, USA) and were corre cted for polymer samples by universal calibration. The water contact angle (WCA) test was conducted on a goniometer (DSA100S) (KRÜSS Scientific, Hamburg, Germany). Elemental analyses were performed on a Vario EL microanalyzer.
3.4. Procedure for the Copolymerization of Ethylene and Comonomers
A round-bottom Schlenk flask with stirring bar was heated for 2 h at 150 °C under vacuum and then cooled to room temperature. The flask was pressurized to 1.2 atm of ethylene and vented three times. Then the glass reactor was charged with the required amount of freshly distilled toluene, Et2AlCl, and polar monomer in sequence under 1.2 atm of ethylene. The system was continuously stirred for 5 min at the desired temperature, and then 1 mL of a solution of nickel complex in CH2Cl2 was added. The total reaction volume was kept at 21 mL. The ethylene pressure was kept constant at 1.2 atm. Polymerization was terminated by the addition of acidic methanol after releasing ethylene pressure. The resulting precipitated polymers were collected and treated by filtering and washed with methanol several times. The final product was dried under vacuum at 40 °C to a constant weight.
4. Conclusions
In summary, an α-diimine nickel catalyst with a bulky dibenzobarrelene backbone was able to catalyze the copolymerization of ethylene and biosourced comonomers derived from castor oil, such as methyl 10-undecenoate (U-COOMe), 10-undecen-1-ol (U-OH), or 10-undecenyl bromide (U-Br). Copolymerization activity increased, but the incorporation of polar comonomers decreased in the order of U-COOMe > U-OH > U-Br. Besides, the copolymer molecular weight followed the order of U-OH > U-COOMe > U-Br. The copolymerization activity and the molecular weight of copolymers decreased, but the incorporation of monomer increased with an increase in temperature. Increasing the comonomer concentration led to an increase in the incorporation of comonomers, whereas the copolymerization activity and the molecular weight of copolymers decreased. Controlled copolymerization of ethylene and U-OH was realized at 20 °C to afford a narrowly dispersed copolymer. The produced functional polyethylenes are highly branched, and their hydrophilic properties improve with increasing incorporation of polar groups. This study provides access to synthesize bio-based functional polyolefins by copolymerization of ethylene and biosourced comonomers using earth-abundant nickel catalysts.
Author Contributions
Conceptualization and writing—original draft preparation, and funding acquisition, H.Z. (Handou Zheng); conceptualization and writing—review and editing, J.W.; data curation, Z.Q.; methodology, C.F.; data curation, H.Z. (Haotian Zhou); formal analysis, G.T.; funding acquisition and supervision, H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by National Natural Science Foundation of China (NSFC) (52173016), the State Key Research Development Program of China (Grant No. 2021YFB3800701), Guangdong Basic and Applied Basic Research Foundation (2024A1515012784, 2024A1515011102, and 2023A1515110549), Fundamental Research Funds for the Central Universities, Sun Yat-sen University (24qnpy047).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From Multisite Polymerization Catalysis to Sustainable Materials and All-Polyolefin Composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zou, C.; Chen, C. Material Properties of Functional Polyethylenes from Transition-Metal-Catalyzed Ethylene-Polar Monomer Copolymerization. Macromolecules 2022, 55, 1910–1922. [Google Scholar] [CrossRef]
- Tan, C.; Si, G.; Zou, C.; Chen, C. Functional Polyolefins and Composites. Angew. Chem. Int. Ed. 2025, 64, e202424529. [Google Scholar] [CrossRef]
- Ghiass, M.; Hutchinson, R.A. Simulation of Free Radical High-Pressure Copolymerization in a Multizone Autoclave: Model Development and Application. Polym. React. Eng. 2003, 11, 989–1015. [Google Scholar] [CrossRef]
- Franssen, N.M.G.; Reek, J.N.H.; de Bruin, B. Synthesis of Functional ‘Polyolefins’: State of the Art and Remaining Challenges. Chem. Soc. Rev. 2013, 42, 5809–5832. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, J.; Xu, X. Studies on High Density Polyethylene (HDPE) Functionalized by Ultraviolet Irradiation and its Application. Polym. Int. 2003, 52, 1527–1530. [Google Scholar] [CrossRef]
- Berkefeld, A.; Mecking, S. Coordination Copolymerization of Polar Vinyl Monomers H2C=CHX. Angew. Chem. Int. Ed. 2008, 47, 2538–2542. [Google Scholar] [CrossRef]
- Guo, L.; Dai, S.; Sui, X.; Chen, C. Palladium and Nickel Catalyzed Chain Walking Olefin Polymerization and Copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Y.; Marks, T.J. Early Transition Metal Catalysis for Olefin–Polar Monomer Copolymerization. Angew. Chem. Int. Ed. 2020, 59, 14726–14735. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Xin, S.; Li, H.; Luo, Y.; He, S. DFT Studies on the Early-Transition-Metal-Catalyzed Polymerization of Polar Monomers with a Methylene Spacer between Vinyl and Functional Groups. Organometallics 2022, 41, 3514–3521. [Google Scholar] [CrossRef]
- Mu, H.; Zhou, G.; Hu, X.; Jian, Z. Recent Advances in Nickel Mediated Copolymerization of Olefin with Polar Monomers. Coord. Chem. Rev. 2021, 435, 213802. [Google Scholar] [CrossRef]
- Zheng, H.; Qiu, Z.; Li, D.; Pei, L.; Gao, H. Advance on Nickel- and Palladium-Catalyzed Insertion Copolymerization of Ethylene and Acrylate Monomers. J. Polym. Sci. 2023, 61, 2987–3021. [Google Scholar] [CrossRef]
- Chen, Z.; Brookhart, M. Exploring Ethylene/Polar Vinyl Monomer Copolymerizations Using Ni and Pd α-Diimine Catalysts. Acc. Chem. Res. 2018, 51, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, C. A continuing legend: The Brookhart-type α-Diimine Nickel and Palladium Catalysts. Polym. Chem. 2019, 10, 2354–2369. [Google Scholar] [CrossRef]
- Wang, X.; Ma, L.; Dong, B.; Zhang, C.; Zhang, X.; Liu, H. Axial Anagostic Interaction in α-Diimine Nickel Catalysts: An Ultraefficient Occupation Strategy in Suppressing Associative Chain Transfers to Achieve UHMWPEs. Macromolecules 2025, 58, 1888–1897. [Google Scholar] [CrossRef]
- Drent, E.; van Dijk, R.; van Ginkel, R.; van Oort, B.; Pugh, R.I. Palladium Catalysed Copolymerisation of Ethene with Alkylacrylates: Polar Comonomer Built into the Linear Polymer Chain. Chem. Commun. 2002, 7, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Guironnet, D.; Roesle, P.; Rünzi, T.; Göttker-Schnetmann, I.; Mecking, S. Insertion Polymerization of Acrylate. J. Am. Chem. Soc. 2009, 131, 422–423. [Google Scholar] [CrossRef]
- Ota, Y.; Ito, S.; Kuroda, J.-I.; Okumura, Y.; Nozaki, K. Quantification of the Steric Influence of Alkylphosphine–Sulfonate Ligands on Polymerization, Leading to High-Molecular-Weight Copolymers of Ethylene and Polar Monomers. J. Am. Chem. Soc. 2014, 136, 11898–11901. [Google Scholar] [CrossRef]
- Iberl, S.; Voccia, M.; Ritacco, I.; Odenwald, L.; Baur, M.; Falivene, L.; Caporaso, L.; Mecking, S. Keto-Polyethylene Material from Pd(II)-Catalyzed Copolymerization with Continuous Carbon Monoxide Feed. ACS Catal. 2025, 15, 8259–8267. [Google Scholar] [CrossRef]
- Younkin Todd, R.; Connor Eric, F.; Henderson Jason, I.; Friedrich Stefan, K.; Grubbs Robert, H.; Bansleben Donald, A. Neutral, Single-Component Nickel (II) Polyolefin Catalysts That Tolerate Heteroatoms. Science 2000, 287, 460–462. [Google Scholar] [CrossRef]
- Mu, H.; Pan, L.; Song, D.; Li, Y. Neutral Nickel Catalysts for Olefin Homo- and Copolymerization: Relationships between Catalyst Structures and Catalytic Properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, P.; Wörner, M.; Mecking, S. Controlled Polymerization in Polar Solvents to Ultrahigh Molecular Weight Polyethylene. J. Am. Chem. Soc. 2018, 140, 6685–6689. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, D.; Chiba, Y.; Takano, S.; Osakada, K. Double-Decker-Type Dinuclear Nickel Catalyst for Olefin Polymerization: Efficient Incorporation of Functional Co-monomers. Angew. Chem. Int. Ed. 2013, 52, 12536–12540. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yao, E.; Wang, J.; Gong, X.; Ma, Y. Ethylene (Co)polymerization by Binuclear Nickel Phenoxyiminato Catalysts with Cofacial Orientation. Macromolecules 2016, 49, 8848–8854. [Google Scholar] [CrossRef]
- Xin, B.S.; Sato, N.; Tanna, A.; Oishi, Y.; Konishi, Y.; Shimizu, F. Nickel Catalyzed Copolymerization of Ethylene and Alkyl Acrylates. J. Am. Chem. Soc. 2017, 139, 3611–3614. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, H.; Pan, L.; Wang, X.; Li, Y. Robust Bulky [P,O] Neutral Nickel Catalysts for Copolymerization of Ethylene with Polar Vinyl Monomers. ACS Catal. 2018, 8, 5963–5976. [Google Scholar] [CrossRef]
- Xiong, S.; Shoshani, M.M.; Zhang, X.; Spinney, H.A.; Nett, A.J.; Henderson, B.S.; Miller, T.F., III; Agapie, T. Efficient Copolymerization of Acrylate and Ethylene with Neutral P, O-Chelated Nickel Catalysts: Mechanistic Investigations of Monomer Insertion and Chelate Formation. J. Am. Chem. Soc. 2021, 143, 6516–6527. [Google Scholar] [CrossRef]
- Baur, M.; Lin, F.; Morgen, T.O.; Odenwald, L.; Mecking, S. Polyethylene Materials with In-Chain Ketones from Nonalternating Catalytic Copolymerization. Science 2021, 374, 604–607. [Google Scholar] [CrossRef]
- Yang, Q.; Kang, X.; Liu, Y.; Mu, H.; Jian, Z. Ultrahigh Molecular Weight Ethylene–Acrylate Copolymers Synthesized with Highly Active Neutral Nickel Catalysts. Angew. Chem. Int. Ed. 2025, 64, e202421904. [Google Scholar] [CrossRef]
- Johnson, L.K.; Mecking, S.; Brookhart, M. Copolymerization of Ethylene and Propylene with Functionalized Vinyl Monomers by Palladium(II) Catalysts. J. Am. Chem. Soc. 1996, 118, 267–268. [Google Scholar] [CrossRef]
- Mecking, S.; Johnson, L.K.; Wang, L.; Brookhart, M. Mechanistic Studies of the Palladium-Catalyzed Copolymerization of Ethylene and α-Olefins with Methyl Acrylate. J. Am. Chem. Soc. 1998, 120, 888–899. [Google Scholar] [CrossRef]
- Keyes, A.; Basbug Alhan, H.E.; Ordonez, E.; Ha, U.; Beezer, D.B.; Dau, H.; Liu, Y.-S.; Tsogtgerel, E.; Jones, G.R.; Harth, E. Olefins and Vinyl Polar Monomers: Bridging the Gap for Next Generation Materials. Angew. Chem. Int. Ed. 2019, 58, 12370–12391. [Google Scholar] [CrossRef] [PubMed]
- Kesti, M.R.; Coates, G.W.; Waymouth, R.M. Homogeneous Ziegler-Natta Polymerization of Functionalized Monomers Catalyzed by Cationic Group IV Metallocenes. J. Am. Chem. Soc. 1992, 114, 9679–9680. [Google Scholar] [CrossRef]
- Sampson, J.; Bruening, M.; Akhtar, M.N.; Jaseer, E.A.; Theravalappil, R.; Garcia, N.; Agapie, T. Copolymerization of Ethylene and Long-Chain Functional α-Olefins by Dinuclear Zirconium Catalysts. Organometallics 2021, 40, 1854–1858. [Google Scholar] [CrossRef]
- Boffa, L.S.; Novak, B.M. Copolymerization of Polar Monomers with Olefins Using Transition-Metal Complexes. Chem. Rev. 2000, 100, 1479–1494. [Google Scholar] [CrossRef]
- Kotzabasakis, V.; Petzetakis, N.; Pitsikalis, M.; Hadjichristidis, N.; Lohse, D.J. Copolymerization of Tetradecene-1 and Octene-1 with Silyl-Protected 10-Undecen-1-ol Using a Cs-Symmetry Hafnium Metallocene Catalyst. A Route to Functionalized Poly(α-olefins). J. Polym. Sci. Part A Polym. Chem. 2009, 47, 876–886. [Google Scholar] [CrossRef]
- Tan, C.; Chen, C. Emerging Palladium and Nickel Catalysts for Copolymerization of Olefins with Polar Monomers. Angew. Chem. Int. Ed. 2019, 58, 7192–7200. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, H. Noncovalent Interactions in Late Transition Metal-Catalyzed Polymerization of Olefins. Macromolecules 2024, 57, 6899–6913. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Luo, Y.; Chen, C. A Second-Coordination-Sphere Strategy to Modulate Nickel- and Palladium-Catalyzed Olefin Polymerization and Copolymerization. Angew. Chem. Int. Ed. 2017, 56, 11604–11609. [Google Scholar] [CrossRef]
- Mitchell, N.E.; Long, B.K. Recent Advances in Thermally Robust, Late Transition Metal-Catalyzed Olefin Polymerization. Polym. Int. 2019, 68, 14–26. [Google Scholar] [CrossRef]
- Muhammad, Q.; Tan, C.; Chen, C. Concerted Steric and Electronic Effects on α-Diimine Nickel- and Palladium-Catalyzed Ethylene Polymerization and Copolymerization. Sci. Bull. 2020, 65, 300–307. [Google Scholar] [CrossRef]
- Dai, S.; Sui, X.; Chen, C. Highly Robust Palladium(II) α-Diimine Catalysts for Slow-Chain-Walking Polymerization of Ethylene and Copolymerization with Methyl Acrylate. Angew. Chem. Int. Ed. 2015, 54, 9948–9953. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, Y.; Du, W.; Cheung, C.S.; Li, D.; Gao, H.; Deng, H.; Gao, H. Unprecedented Square-Planar α-Diimine Dibromonickel Complexes and Their Ethylene Polymerizations Modulated by Ni–Phenyl Interactions. Macromolecules 2022, 55, 3533–3540. [Google Scholar] [CrossRef]
- Zheng, H.; Qiu, Z.; Gao, H.; Li, D.; Cheng, Z.; Tu, G.; Gao, H. Noncovalent Ni–Phenyl Interactions Promoted α-Diimine Nickel-Catalyzed Copolymerization of Ethylene and Methyl Acrylate. Macromolecules 2024, 57, 5279–5288. [Google Scholar] [CrossRef]
- Liu, F.S.; Hu, H.-B.; Xu, Y.; Guo, L.-H.; Zai, S.-B.; Song, K.-M.; Gao, H.-Y.; Zhang, L.; Zhu, F.-M.; Wu, Q. Thermostable α-Diimine Nickel(II) Catalyst for Ethylene Polymerization: Effects of the Substituted Backbone Structure on Catalytic Properties and Branching Structure of Polyethylene. Macromolecules 2009, 42, 7789–7796. [Google Scholar] [CrossRef]
- Guo, L.; Gao, H.; Guan, Q.; Hu, H.; Deng, J.; Liu, J.; Liu, F.; Wu, Q. Substituent Effects of the Backbone in α-Diimine Palladium Catalysts on Homo- and Copolymerization of Ethylene with Methyl Acrylate. Organometallics 2012, 31, 6054–6062. [Google Scholar] [CrossRef]
- Zhong, L.; Li, G.; Liang, G.; Gao, H.; Wu, Q. Enhancing Thermal Stability and Living Fashion in α-Diimine–Nickel-Catalyzed (Co)polymerization of Ethylene and Polar Monomer by Increasing the Steric Bulk of Ligand Backbone. Macromolecules 2017, 50, 2675–2682. [Google Scholar] [CrossRef]
- Zhong, S.; Tan, Y.; Zhong, L.; Gao, J.; Liao, H.; Jiang, L.; Gao, H.; Wu, Q. Precision Synthesis of Ethylene and Polar Monomer Copolymers by Palladium-Catalyzed Living Coordination Copolymerization. Macromolecules 2017, 50, 5661–5669. [Google Scholar] [CrossRef]
- Zhong, L.; Zheng, H.; Du, C.; Du, W.; Liao, G.; Cheung, C.; Gao, H. Thermally Robust α-Diimine Nickel and Palladium Catalysts with Constrained Space for Ethylene (Co)Polymerizations. J. Catal. 2020, 384, 208–217. [Google Scholar] [CrossRef]
- Zheng, H.; Zhong, L.; Du, C.; Du, W.; Cheung, C.S.; Ruan, J.; Gao, H. Combining Hydrogen Bonding Interactions with Steric and Electronic Modifications for Thermally Robust α-Diimine Palladium Catalysts Toward Ethylene (Co)Polymerization. Catal. Sci. Technol. 2021, 11, 124–135. [Google Scholar] [CrossRef]
- Du, C.; Zhong, L.; Gao, J.; Zhong, S.H.; Liao, H.; Gao, H.Y.; Wu, Q. Living (Co)polymerization of Ethylene and Bio-based Furfuryl Acrylate Using Dibenzobarrelene Derived -Diimine Palladium Catalysts. Polym. Chem. 2019, 10, 2029–2038. [Google Scholar] [CrossRef]
- Xu, M.; Chen, A.; Li, W.; Li, Y.; Zou, C.; Chen, C. Efficient Synthesis of Polar Functionalized Polyolefins with High Biomass Content. Macromolecules 2023, 56, 1372–1378. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. From Monomers to Polymers from Renewable Resources: Recent Advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Dai, S.; Li, S.; Xu, G.; Chen, C. Direct Synthesis of Polar Functionalized Polyethylene Thermoplastic Elastomer. Macromolecules 2020, 53, 2539–2546. [Google Scholar] [CrossRef]
- Biermann, U.; Metzger, J.O. Synthesis of Alkyl-Branched Fatty Acids. Eur. J. Lipid Sci. Technol. 2008, 110, 805–811. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P.; Zeng, J.; Li, J.; Gao, W.; Wang, B.; Xu, J.; Chen, K. Acetylated Cellulose Nanofibers Enhanced Bio-Based Polyesters Derived from 10-Undecanoic Acid toward Recyclable and Degradable Plastics. Chem. Eng. J. 2024, 479, 147797. [Google Scholar] [CrossRef]
- Quinzler, D.; Mecking, S. Renewable Resource-Based Poly(Dodecyloate) by Carbonylation Polymerization. Chem. Commun. 2009, 36, 5400–5402. [Google Scholar] [CrossRef]
- Liu, Y.; Mecking, S. A Synthetic Polyester from Plant Oil Feedstock by Functionalizing Polymerization. Angew. Chem., Int. Ed. 2019, 58, 3346–3350. [Google Scholar] [CrossRef]
- Gong, Y.; Li, S.; Gong, Q.; Zhang, S.; Liu, B.; Dai, S. Systematic Investigations of Ligand Steric Effects on α-Diimine Nickel Catalyzed Olefin Polymerization and Copolymerization. Organometallics 2019, 38, 2919–2926. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.; Jian, Z. Comprehensive Studies of the Ligand Electronic Effect on Unsymmetrical α-Diimine Nickel(II) Promoted Ethylene (Co)Polymerizations. Polym. Chem. 2020, 11, 4005–4012. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Zhang, Y.; Jian, Z. Unsymmetrical Strategy Makes Significant Differences in α-Diimine Nickel and Palladium Catalyzed Ethylene (Co)Polymerizations. ChemCatChem 2020, 12, 2497–2505. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Li, B.; Jian, Z. Fluorinated α-Diimine Nickel Mediated Ethylene (Co)Polymerization. Chem.-Eur. J. 2021, 27, 11935–11942. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Y.; Li, B.; Jian, Z. Horizontally and Vertically Concerted Steric Strategy in α-Diimine Nickel Promoted Ethylene (Co)Polymerization. Chin. J. Chem. 2021, 39, 2829–2836. [Google Scholar] [CrossRef]
- Clark, K.J.; Powell, T. Polymers of Halogen-Substituted 1-Olefins. Polymer 1965, 6, 531–534. [Google Scholar] [CrossRef]
- Hakala, K.; Löfgren, B.; Helaja, T. Copolymerizations of Oxygen-Functionalized Olefins with Propylene Using Metallocene/Methylaluminoxane Catalyst. Eur. Polym. J. 1998, 34, 1093–1097. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Li, H.; Zhang, Z.; Lu, Y.; Wu, C.; Hu, Y. Highly Active Copolymerization of Ethylene with 10-Undecen-1-ol Using Phenoxy-Based Zirconium/Methylaluminoxane Catalysts. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5944–5952. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C. Rational Design of High-Performance Phosphine Sulfonate Nickel Catalysts for Ethylene Polymerization and Copolymerization with Polar Monomers. ACS Catal. 2017, 7, 1308–1312. [Google Scholar] [CrossRef]
- Dai, S.; Chen, C. Direct Synthesis of Functionalized High-Molecular-Weight Polyethylene by Copolymerization of Ethylene with Polar Monomers. Angew. Chem. Int. Ed. 2016, 55, 13281–13285. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Z.; Zhao, Y.; Luo, Y.; He, S. Multivariate Linear Regression Models to Predict Monomer Poisoning Effect in Ethylene/Polar Monomer Copolymerization Catalyzed by Late Transition Metals. Inorganics 2022, 10, 26. [Google Scholar] [CrossRef]
- Michalak, A.; Ziegler, T. A Comparison of Ni- and Pd-Diimine Complexes as Catalysts for Ethylene/Methyl Acrylate Copolymerization. A Static and Dynamic Density Functional Theory Study. Organometallics 2003, 22, 2660–2669. [Google Scholar] [CrossRef]
- Popeney, C.S.; Guan, Z. Effect of Ligand Electronics on the Stability and Chain Transfer Rates of Substituted Pd(II) α-Diimine Catalysts. Macromolecules 2010, 43, 4091–4097. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhong, L.; Du, C.; Du, W.; Zheng, H.; Cheung, C.S.; Wang, L.; Gao, H. Unprecedented Steric and Positioning Effects of Comonomer Substituents on α-Diimine Palladium-Catalyzed Vinyl Arene/CO Copolymerization. Macromolecules 2021, 54, 687–695. [Google Scholar] [CrossRef]
- Gao, H.; Hu, H.; Zhu, F.; Wu, Q. A Thermally Robust Amine-Imine Nickel Catalyst Precursor for Living Polymerization of Ethylene above Room Temperature. Chem. Commun. 2012, 48, 3312–3314. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Foro, S.; Plenio, H. Bispentiptycenyl-Diimine-Nickel Complexes for Ethene Polymerization and Copolymerization with Polar Monomers. Organometallics 2019, 38, 544–551. [Google Scholar] [CrossRef]
- Long, B.K.; Eagan, J.M.; Mulzer, M.; Coates, G.W. Semi-Crystalline Polar Polyethylene: Ester-Functionalized Linear Polyolefins Enabled by a Functional-Group-Tolerant, Cationic Nickel Catalyst. Angew. Chem. Int. Ed. 2016, 55, 7106–7110. [Google Scholar] [CrossRef]
- Usami, T.; Takayama, S. Fine-Branching Structure in High-Pressure, Low-Density Polyethylenes by 50.10-MHz Carbon-13 NMR Analysis. Macromolecules 1984, 17, 1756–1761. [Google Scholar] [CrossRef]
- Galland, G.B.; de Souza, R.F.; Mauler, R.S.; Nunes, F.F. 13C NMR Determination of the Composition of Linear Low-Density Polyethylene Obtained with [η3-Methallyl-nickel-diimine]PF6 Complex. Macromolecules 1999, 32, 1620–1625. [Google Scholar] [CrossRef]
- Pei, L.; Liu, F.; Liao, H.; Gao, J.; Zhong, L.; Gao, H.; Wu, Q. Synthesis of Polyethylenes with Controlled Branching with α-Diimine Nickel Catalysts and Revisiting Formation of Long-Chain Branching. ACS Catal. 2018, 8, 1104–1113. [Google Scholar] [CrossRef]
- Nakamura, A.; Ito, S.; Nozaki, K. Coordination−Insertion Copolymerization of Fundamental Polar Monomers. Chem. Rev. 2009, 109, 5215–5244. [Google Scholar] [CrossRef]
- Dong, J.; Hu, Y. Design and Synthesis of Structurally Well-Defined Functional Polyolefins via Transition Metal-Mediated Olefin Polymerization Chemistry. Coord. Chem. Rev. 2006, 250, 47–65. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, Y.X.; Huang, S.M.; Lin, Z.Y. Effect of the Surface-modifying Macromolecules on the Duration of the Surface Functionalization. Appl. Surf. Sci. 2007, 253, 4659–4667. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).