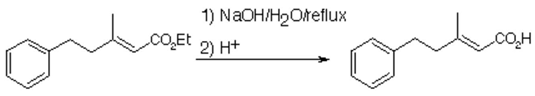
The general part of the experimental section [1] has been presented elsewhere. Ethyl (E)-3-methyl-
5-phenyl-2-pentenoate (3.363 g, 15.4 mmol) was refluxed with sodium hydroxide (2.161 g, 38.5 mmol) in a mixture of water (50 ml) and methanol (10 ml) for 3 hours, cooled and washed with ether (30 ml). The aqueous phase was acidified with concentrated hydrochloric acid to below pH 1. The mixture was extracted with ether (3x30 ml). The combined ether extracts were washed with brine (20 ml), dried (Na2SO4), filtered and evaporated under reduced pressure. (E)-3-Methyl-5-phenyl-2-pentenoic acid (2.79 g, 95%) was obtained as colourless plates from cyclohexane/light petroleum.
M.p. 58°
Anal. calc. for C12H14O2 (190.24): C 75.8, H 7.4; found: C 76.2, H 7.3.
UV (ethanol) 305 (208) nm.
IR (CDCl3) 3500–2800(bs, OH), 3104, 2950, 1694 (s, C=O), 1641, 1260 cm−1.
1H-NMR (90 MHz, CDCl3) 2.16 (3H, d, J 1.1 Hz, CH3), 2.43 (2H, m, =C(CH2), 2.73 (2H, m, Ph-CH2),
5.61 (1H, m, =CH), 6.92–7.40 (5H, m, ArH), 9.98 (1H, bs, COOH).
13C-NMR (15 MHz, CDCl3) 19.22 (CH3), 34.02, 42.85 (CH2), 115.7 (=CH); 126.2, 128.2 128.5 (ArCH),
140.9 (quat, C1’), 161.7 (quat, C3), 171.5 (quat, C1).
EI-MS 190(M+, 3%), 144(10), 91(100).
Supplementary Materials
Acknowledgments
The authors gratefully acknowledge financial support from the Australian Research Council and The University of Sydney.
Reference
- Moloney, M.G.; Pinhey, J.T.; Stoermer, M.J. Vinyl Cation Formation by Decomposition of Vinyl-lead Triacetates. The reactions of Vinylmercury and Vinyltin Compounds with Lead Tetraacetate. J. Chem. Soc. Perkin Trans. 1 1990, 10, 2645. [Google Scholar] [CrossRef]
Sample Availability: No sample available. |
© 1998 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/
