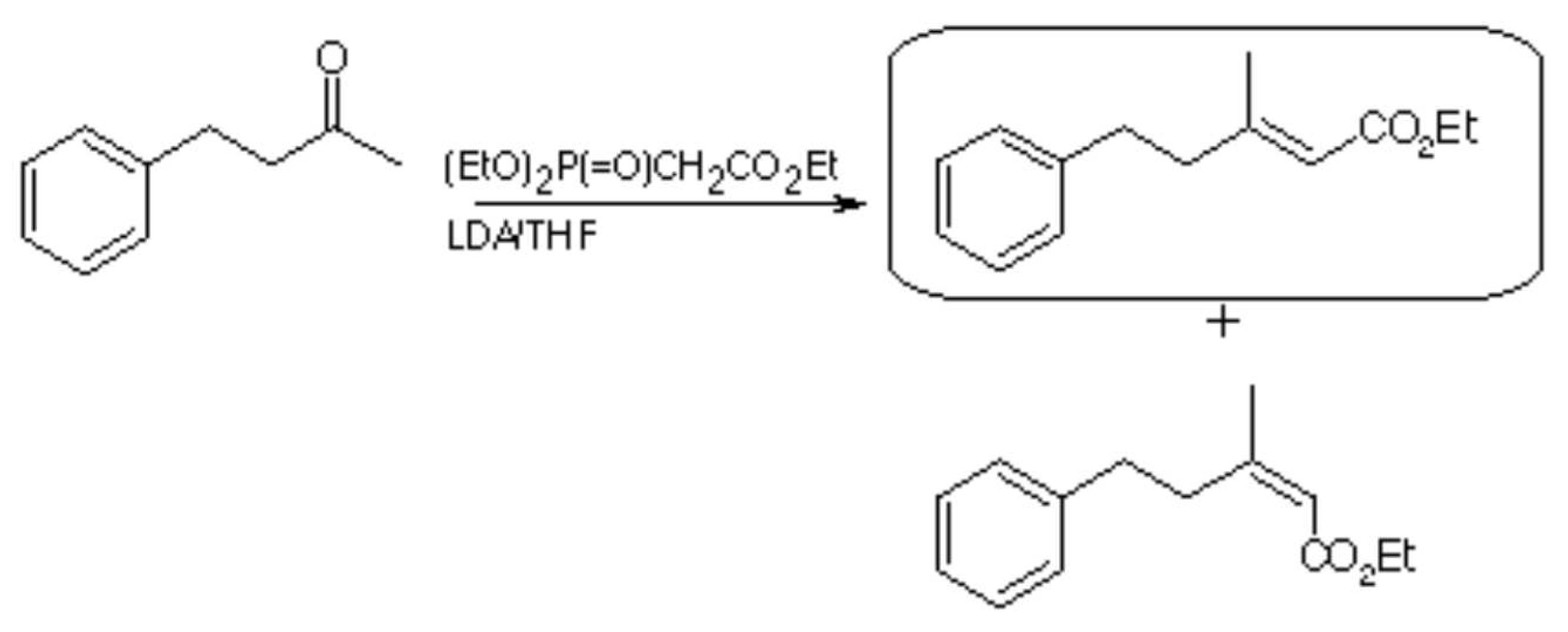
The general part of the experimental section [
1] has been presented elsewhere. To the solution of lithium diisopropylamide prepared from diiisopropylamine (4.46 g, 44 mmol) and n-butyllithium (2.2 M, 20 ml, 44 mmol) in dry tetrahydrofuran (50 ml) at 0° was added triethylphosphonoacetate (8.74 ml, 44 mmol) at 0°. The mixture was stirred at room temperature for 10 minutes and 4-phenyl-2-butanone (6.6 ml, 44 mmol) was added and refluxed for 6 hours. The solvent was removed and the residue partitioned between ether (100 ml) and water (100 ml). The ether extract was washed with hydrochloric acid (3M, 100 ml), brine (50 ml), dried (Na2SO4), filtered and evaporated under reduced pressure. The crude product was purified by flash chromatography (ethyl acetate/light petroleum 1:9), followed by radial chromatography (ethyl acetate/light petroleum 2:98) to yield ethyl (E
)-3-methyl-5-phenyl-2-pentenoate (3.48 g, 36%) as a colourless oil.
B.p. 110°/0.5 mmHg (Kugelrohr)
Anal. calc. for C14H18O2 (218.29): C 77.0, H 8.3; found: C 77.1, H 8.2.
UV (ethanol) 304 (201), 212 (17777) nm.
IR (film) 1716 (s, C=O), 1649, 1224, 1145 cm-1.
1H-NMR (400 MHz, CDCl3) 1.26 (3H, t, J 7.2 Hz, -OCH2CH3), 2.21 (3H, d, J 1.3 Hz, CH3), 2.45 (2H, dt, J 1.2, 8.0 Hz, CH2), 2.79 (2H, bt, J 8.0 Hz, Ph-CH2), 4.17 (2H, q, J 7.2 Hz, -OCH2CH3), 5.69 (1H, tq, J 1.2, 1.3 Hz, =CH), 7.16-7.34 (5H, m, ArH). Stereochemistry confirmed by n.O.e. difference spectroscopy. Irradiation at 5.69 produced no n.O.e. at 2.21 (-23% at 7.19, 6% at 7.26). Irradiation at 2.21 produced no n.O.e. at 5.69 (3% at 2.79, -4% at 2.45).
13C-NMR (15 MHz, CDCl3) 13.96, 18.44 (CH3), 33.70, 42.20, 58.96, (CH2), 115.9 (=CH); 125.7, 127.9, 128.0 (CH), 140.7 (quat, C1'), 158.0 (quat, C3), 166.0 (quat, C1).
EI-MS 218 (M+, 6%), 173(16), 145(13), 144(30), 92(14), 91(100), 65(12).