The Analgesic Potential of Litsea Species: A Systematic Review
Abstract
1. Introduction
| Species/Vernacular Name | Ethnomedicinal Claims | Plant Parts Used | Preparation/Application | Phytochemical Reported | References |
|---|---|---|---|---|---|
| Litsea cubeba (Lour.) Pers. Mountain Spicy Tree; Tayer (Indian); Cheng Qie Zi/Shan Ji Jiao (Chinese); Siltimur (Nepali) | Analgesic, anti-asthmatic, anti-dysenteric, anti-inflammatory, antiseptic, astringent, carminative, diuretic, expectorant, hair tonic, hypotensive, insecticidal, sedative, stimulant, stomachic, warms body core, moves ‘qi’ and improves blood circulation. Relieves pains including headache, toothache, stomachache and athlete’s foot pain, cough, cold, respiratory diseases, chronic bronchitis, asthma, cholera, food accumulation, ‘qi’ distention, heat stroke, vomiting, diarrhoea, indigestion, inhibited urination, urine opacity, swollen sores, bleeding, skin diseases, worm infection, blood dysentery, bone fracture, arthritis and paralysis. | Whole plant (fruits, bark, leaves, roots and root bark) | Decoction of pounded fresh/dry fruit taken orally to treat cough, diarrhoea, stomachache, toothache, bleeding. Paste of grounded root bark and leaves applied to relieve athlete’s foot pain and other skin diseases. Fresh ripe/unripe fruits consumed as tranquilizer and to relieve cold, cough, respiratory diseases, stomach problems and headache. Fruits oil aids recovery from paralysis. Leaves used to treat cholera. Aqueous mixture of pounded fruits and leaves, taken twice daily to treat blood dysentery, stomach problem and fever. Leaf paste applied on forehead as remedy for headache. Paste of bark applied to heal bone fracture. Seeds chewed to treat thread worm infections. | Alkaloids:
(−)-8-O-Methyloblongine; (−)-Litcubine; (−)-Litcubinine; (−)-Magnocurarine; (−)-Oblongine; (+)-8-methoxyisolaurenine-N-oxide; (+)-Isoboldine β-N-oxide; (+)-N-(Methoxycarbonyl)-N-norboldine; (+)-N-(Methoxycarbonyl)-N-norglaucine; (+)-N-(Methoxycarbonyl)-N-norlauroscholtzine; (+)-N-(methoxylcarbonyl)-N-norglaucine; (+)-N-(methoxylcarbonyl)-N-norbulbodione; (+)-N-(methoxylcarbonyl)-N-nordicentrin; (+)-N-(methoxylcarbonyl)-N-norisocorydione; (+)-N-(methoxylcarbonyl)-N-norpredicentrine; Isoboldine; Atheroline; Boldine; Glaziovine; Isocorydine; Isodomesticine; Laurolitsine; Litebamine; N-Methyllaurotetanine; N-Methyllindcarpine; Norisoboldine; Norisocorydine Xanthoplanine
Monoterpenes: Camphene; 1,8-Cineole; Citronellal; Citronellol; p-Cymene; Geranial (Citral a); Geraniol; Limonene (Cinene); Linalool; Myrcene; Neral (Citral b); β-Phellandrene; α-Pinene; β-Pinene; α-a-Isopulegol; Sabinene; β-Terpenene; α-Terpineol; α-Terpinyl acetate; Litseacubebic acid Sesquiterpenes: β-Caryophyllene; trans-Nerolidol Diterpenoids: Cubelin Flavonoids: Salvigenin Amides: cis-N-Feruloyl-3-methoxytyramine; N-Feruloyl-3-methoxytyramine; 3-Methoxy-N-sinapoyltyramine; N-trans-3,4-methylenecinnamoyl-3-methoxytyramine; Cubebamine A; 1,2-Dihydro-6,8-dimethoxy-7-1-(3,5-dimethoxy-4-hydroxyphenyl)-N1,N2-bis-[2-(4-hydroxyphenyl)ethyl]-2,3-naphthalene dicarboxamide; N-cis-3,4-methylenedioxycinnamoyl-3-methoxytyramine Lignans: Eugenol; Syringaresinol; 9,9′-O-di-(E)-feruloyl-(+)-secoisolariciresinol; 9,9′-O-di-(E)-feruloyl-5,5′-(+)-dimethoxysecoisolariciresinol; Balanophonin B; (+)-Medioresinol Steroids: β-sitostenone; Daucosterol; β-Sitosterol; Capric acid; cis-Dec-4-enoic acid; cis-Dodec-4-enoic acid (Linderic acid); cis-Tetradec-4-enoic acid (Tsuzuic acid); Hexadecenoic acid; Lignoceric acid; Lauric acid; Linoleic acid; Myristic acid; Oleic acid; Palmitic acid; Ethyl palmitate; Stearic acid; Ethyl stearate; Litseacubebic acid; 2,6-Dimethyl-6-hydroxy-2E,4E-hepta-2,4-dienal; 6,7-Dihydroxy-3,7-dimethyl-oct-2-enoic acid Other compounds: 2,5-Dimethoxy-p-benzoquinone; 2,6-Dimethoxy-p-benzoquinone; Vanillic acid; (6R)-3,7-Dimethyl-7-hydroxy-2-octen-6-olide; Cubebanone; Threo-2,3-bis(4-hydroxy-3-methoxyphenyl)-3-ethoxypro-pan-1-ol; Erythro-2,3-bis(4-hydroxy-3-methoxyphenyl)-3-ethoxypropan-1-ol | [42,43,44,45,46,47,48] |
| Litsea glaucescens Kunth Laurel, aguarel, laurelillo (Mexican), Ecapatli (Aztec) | Treats pain, central-nervous-system-related disorders such as epilepsy, depression, anxiety and fright, infections, fever, rheumatism, vomiting, diarrhea, cramps, indigestion, dysmenorrhea, sterility and colic Aids postpartum recovery. | Leaves | Infusion of leaves, scrubbing site with alcoholic extracts of leaves, vapor inhalation of the boiled or burned leaves. Used in baths for postpartum recovery. | Terpenes and phenolic compounds | [49,50] |
| Litsea glutinosa (Lour.) C.B. Rob. Bois d’oiseaux (Mauritian), narra alagi/narra mamidi/Maidalakdi Lenja/Maadho saak/Papal (Indian), Medasak (Sanskrit); Sablot (Philippines); Chan Gao Mu Jiang Zi (Chinese) | Analgesic, aphrodisiac, antiseptic, antispasmodic, demulcent and emollient. Remedy for diarrhoea, dysentery, gastroenteritis, indigestion, rheumatism, arthritis, sprain, bruises, wounds, sore, boil, abscess inflammation, oedema, swelling, backache, rheumatic and gouty joints, bone fracture, traumatic injuries, nervous crisis, haemorrhoids, allergies, colds and asthma. Promotes longevity, semen generation. | Bark, bud, leaves and seeds | Mucilaginous bark or decoction from fresh bark beneficial for diarrhoea, dysentery and rheumatism. Fine paste of ground bark and water is applied warm as a plaster to relieve bruises, sprain, inflammation, wounds, backache, bone fractures and rheumatic and gouty joints. Tea made from powder of 10–15 g dry bark taken at bed time for 2–4 days or mixture of maize flour, Ghee and Gur fried until brown in decoction water used to treat severe backache. Bud used to treat wounds. Leaf poultice as an emollient and for treating haemorrhoids, gastrointestinal disorder, joint pain (rheumatism) and allergies. Seed oil used to treat rheumatism. |
Alkaloids, anthraquinones, cardiac glycosides, flavonoids, glycosides, phenols, saponins, steroids, tannins, terpenoids, volatile compounds, amino acids and carbohydrates Alkaloids: Boldine | [43,45,51,52,53,54,55,56,57] |
| Litsea guatemalensis Mez. Laurel (Mexican), laurelillo, laurel silvestre, arrayán (Spanish) | Treats fevers, headache, arthritis, stomachache, diarrhoea, emesis (vomiting), chills, throat infection, infectious diseases of the digestive system, urinary tract infection, broken bones, gastrointestinal diseases, skin conditions, trauma, muscular pain, rheumatism, stings, cultural affiliated syndromes, renal diseases, colic, swellings and disease of the circulatory and nervous system. | Leaves | Boiled leaf infusion used as remedies for fevers, headache, stomachache, diarrhoea, emesis and chills. Infusion gargled to treat throat infections. Paste of crushed leaves applied to treat arthritis. Used in baths for relieving fevers, chills, urinary tract infections and broken bones. Used as healing tonic for general health. | Monoterpenoids: dl-carvone; Monoterpenes: 1,8-Cineole; Linalool; α-Terpineol; Flavonoids: Pinocembrin; Isoflavones: 5,7,3′,4′-Tetrahydroxy-isoflavone; Coumarins: Scopoletin | [45,50,58] |
| Litsea monopetala (Roxb.) Pers or Litsea polyantha Juss. Menda khal (Bengali); Ngop (Indian) | Stimulant, astringent, spasmolytic, antidiarrheal, analgesic, antiseptic, antidepressant, anti-infertility, cytotoxic, antifungal, insecticide, purgative and laxative. Relieves pains, bruises, contusions, arthritis, stomachache, diarrhoea, dysentery, diabetes, dislocation, bone fractures, gonorrhoea, skin diseases and boils. | Leaves, bark, trunk and roots | Leaves useful against arthritis and bone cracks. Fresh green leaves used to treat diarrhoea and dislocation. Bark used as nerve and bone tonic, stimulant, analgesic and antiseptic and for treating stomachache and arthritis. Bark, leaves and roots used to gonorrhoea, skin diseases, boil, etc. Aqueous bark extract used to treat diarrhoea and dysentery. Pulverised/macerated bark applied to relieve pain due to blows, bruises, strenuous work or fractures. Pulverised roots applied externally for pains, bruises, contusions and swellings. Seed fat used in ointments for relieving rheumatism. |
Phenols, alkaloids, butanolides, amides, butenolactones, steroid fatty acids, lignans, monoterpenes and sesquiterpenes Phenols: Eugenol; Chalcone Sesquiterpenes: Caryophyllene oxide; Humulene oxide Fatty acids: Capric acid; Myristic acid | [45,59,60,61,62,63,64,65] |
| Litsea coreana var. sinensis (C.K. Allen) Yen C. Yang & P.H. Huang | Relieves stomachache and pain and treats traumatic injury. | Roots and leaves | Roots are used to relieve stomach pain, and the leaves are used to treat pain and traumatic injuries | Monoterpenoids: Menthane; Sesquiterpenes: Farnesane; Copaene; Aristolone; Cubebane; Cedrane; α-Patchoulene | [45] |
| Litsea deccanensis Gamble | Alleviates chest pain. | Leaves | - | Alkaloids: Boldine; Corytuberine; Dicentrine; Isocorydine; Laurolitsine; Magnoflorine; Nordicentrine. | [45] |
| Litsea elliptica Blume Medang perawas (Indonesian), Pawas (Bruneian), Tham-mung (Thailand) | Treats headaches, cancer, stomach ulcers and fever. | Leaves | Crushed leaves applied to forehead to treat headaches. | Steroids: Palmitic acid, methyl ester; Linoleic acid, methyl ester; Diterpenoids: Phytol; Other compounds: Catechol; Mono(2-ethylhexyl) phthalate; dl-α-Tocopherol | [66,67,68] |
| Litsea euosma W.W. Smith or Litsea mollis Hemsl. (TBC) Fourflower Litse, Qing Xiang Mu Jiang Zi (Chinese). |
Carminative, diuretic, expectorant, stimulant, stomachic, antiasthmatic, arthritis, sedative, antidysenteric and antiseptic. Treats stomachache, abdominal distention, dyspepsia, spleen dropsy/oedema, arthritis, emesis (vomiting) and diarrhoea. Dispels wind and moves qi, fortifies spleen and disinhibits damp and resolves toxins. | Fruits, roots and leaves | - | Alkaloids: Laurolitsine; Monoterpenes: 1,8-Cineole; Geranial (Citral a); Neral (Citral b); Limonene; Citronellal; Linalool; α-Pinene; β-Pinene; (E)-β-Ocimene; (Z)-β-Ocimene; Germacrene; Sesquiterpenes: Farnesane; Oploanane; Bourbonane; Cedrane; Flavonoids: Astragalin (Kaempferol3-O-β-d-glucopyranoside); Dihydrodehydrodiconiferyl alcohol; Steroids: Stigmasterol; 6-O-Palmitoyl-β-sitosteryl-d-glucoside; Fatty Acids: Docosanoic acid; Hexacosanoic acid; Triacontanoic acid; Other compounds: Euosmoside A; Euosmoside B; 5-Hydroxy-6-methyl-3-(undec-10-enyl)-5,6-dihydropyran-2-one | [43,45] |
| Litsea garciae S. Vidal | Antifungal and antioxidant. Treats caterpillar stings, boils, rectal bleeding, skin infections, diseases and burns, beri-beri, sprains, muscular aches and snake bites. | Leaves and bark | Ground bark used as dressing for treating caterpillar stings and boils. Decoction of bark taken to treat rectal bleeding. Poultice of leaves and young shoots mixed with shallot and fennel seeds applied to treat skin infections, diseases and burns. Warm poultice of leaves applied to treat beri-beri. Poultice of root bark applied to cure the sprains. Pounded and warmed bark used to treat muscular aches and sprains. Combination of L. garciae and durian bark used as antidote for snake bites. | Alkaloids: Actinodaphnine; Boldine; Isodomesticine; Laurolitsine; Reticuline; Monoterpenes: 1,8-Cineole; Geraniol; Sesquiterpenes: γ-Cadinene | [45,69] |
| Litsea. garrettii Gamble |
Heat-clearing, detoxifying, detumescence and analgesic. Relieves jaundice and itching, eliminates parasites, wind and dampness. | Roots | - | - | [45] |
| Litsea lancilimaba | Relieves chest pain, chest tightness, asthma, coronary heart disease and angina pectoris. | - | - | Monoterpenes: Cineole Sesquiterpenes: Copaene; Ylangene; Cubebane; Cedrane; Farnesane | [35,45] |
| Litsea moupinensis var. szechuanica (C. K. Allen) Yen C. Yang et P.H. Huang | Carminative, diuretic, expectorant, stimulant, stomachic, antiasthmatic, antiarthritis, sedative, antidysenteric and antiseptic. | Fruit | - | - | [45] |
| Litsea pedunculata (Diels) Y.C. Yang & P.H. Huang | Relieves gastroenteralgia, edema and rheumatic arthritis. | Stem bark | - | Triterpenoids: Betulin; Flavonoids: Alpinetin; Flavokawin B; Linderol A; Litseaone B; Pinocembrin; Quercetin | [45] |
| Litsea populifolia (Hemsl.) Gamble | Treats stomachache, dyspepsia, relieves pain, indigestion, nausea and emesis (vomiting). | Fruits and leaves | - | Monoterpenes: Citral; Limonene; Nerol; 1,8-Cineole; α-Pinene; β-Pinene; Linalool; Sesquiterpenes: Caryophyllene; Terpenoids: Camphor; Other compounds: Methylheptenone | [45] |
| Litsea pungens Hemsl. Zhen Cai (Chinese) |
Strengthens spleen. Relieves dyspepsia, diarrhea, sunstroke, sore, scab, stomach distension, pain, stomachache, arthralgia, influenza, cough of phlegm-rheum and beri-beri. | Fruits, leaves, stems and roots | - | Alkaloids: Launobine; Laurotetanine Monoterpenes: 1,8-Cineole; (R)-Limonene; Neryl acetate; Sesquiterpenes: Aromadendrene; Monoterpenoids: Carvone; Flavonoids: Pinocembrin; Pinostrobin; 2-(Hexahydro-1,3-benzodioxol-5-yl)-3,4-dihydro-5,7dimethoxy-2H-chromen- 3-ol; 2’,6’-Dihydroxy-4’-methoxychalcone; Steroids: Daucosterol; Fatty acids: cis-4-Decenoic acid, cis-4-Dodecenoic acid; cis-4-Tetradecenoic acid; Other compounds: 5,6-Dehydrokawain; Palmitone | [43,45] |
| Litsea rotundifolia Hemsl. | Treats rheumatic pain. | Roots | - | Alkaloids: Boldine; Laurolitsine; N-Acetyllaurolitsine | [45] |
| Litsea rotundifolia var. oblongifolia (Nees) C.K. Allen | Treats edema, rheumatic arthritis and stomach disorder. | Fruits, leaves, roots and bark | - | Alkaloids: Boldine; Laurolitsine; Butenolactones: Lincomolide A; Lincomolide C; Litsenolide A1; Marliolide; Rotundifolide A; Rotundifolide B; Fatty acids: Undecanoic acid; Lauric acid; Myristic acid; Palmitic acid; 13-Tetradecenoic acid; 11-Dodecynoic acid; 13-Tetradecynoic acid; Other compounds: Oblongifolinol; Rotundifolinol | [45] |
| Litsea rubescens Lecomte | Relieves gastroenteralgia, enterogastritis, edema, rheumatic arthritis, stomachache and dyspepsia. | Stem, bark, roots and fruits | - | Flavonoids: Alpinetin; Flavokawin B; Linderol A; Litseaone A; Pinocembrin; Quercetin | [45] |
| Litsea sebifera Pers. | Treats urinary problems and rheumatic arthritis. | Stem, root and leaves | Extract of stem or root consumes 2–3 times a day to treat urinary problems. Paste made from leaves applied to affected area to treat rheumatic arthritis. | Alkaloids: Boldine; Laurotetanine; Litseglutine A (Litseferine); N-Methyllaurotetanine; Sebiferine | [45,70] |
| Litsea veitchiana Gamble | Treats indigestion, gastroenteralgia and dyspepsia. | Fruits | - | [45] | |
| L. verticillata Hance | Treats rheumatism, dissipates stasis and relieves menstrual cramps, pain, painful swellings from knocks and falls, stomachache, wind-damp impediment pain, soreness, fractured bones and snake bites. | Roots and leaves | - | Butanolides and Butenolactones: 4-Hydroxy-2-methylbut-2-enolide; Hydroxydihydrobovolide; Litseabutenolide; Sesquiterpenes: Aphanamol II; 10-Hydroxy-15-oxo-a-cadinol; Chromolaevanedione; Eudesm-4(15)-ene-1β,6α-diol; 5-epi-Eudesm-4(15)-ene-1β,6β-diol; 7-epi-Eudesm-4(15)-ene-1α,6α-diol; 7-epi-Eudesm-4(15)-ene-1β,6β-diol; Isolitseane A; Isolitseane B; Isolitseane C; Litseachromolaevane A; Litseachromolaevane B; Litseagermacrane; Litseahumulane A; Litseahumulane B; Litseaverticillol A; Litseaverticillol B; Litseaverticillol C; Litseaverticillol D; Litseaverticillol E; Litseaverticillol F; Litseaverticillol G; Litseaverticillol H; 1,2,3,4-Tetrahydro-2,5-dimethyl-8-(1-methylethyl)-naphthalene-1,2-diol; Octahydro-4-hydroxy-3a-methyl-a-(1-methylethyl-7-methylidene-1H-in- dene-1-methanol; Oxyphyllenodiol B; Verticillatol; Lignans: (+)-Epiexcelsin; (+)-5′-Demethoxyepiexcelsin | [43,45,71] |
| Litsea zeylanica Nees and T.Nees | Dispels wind and relieves pain and rheumatism. | Roots | - | Alkaloids: Norisoboldine; Reticuline; Monoterpenes: Linalool; α-Pinene; Terpinen-4-ol; Sesquiterpene: β-Caryophyllene | [45] |
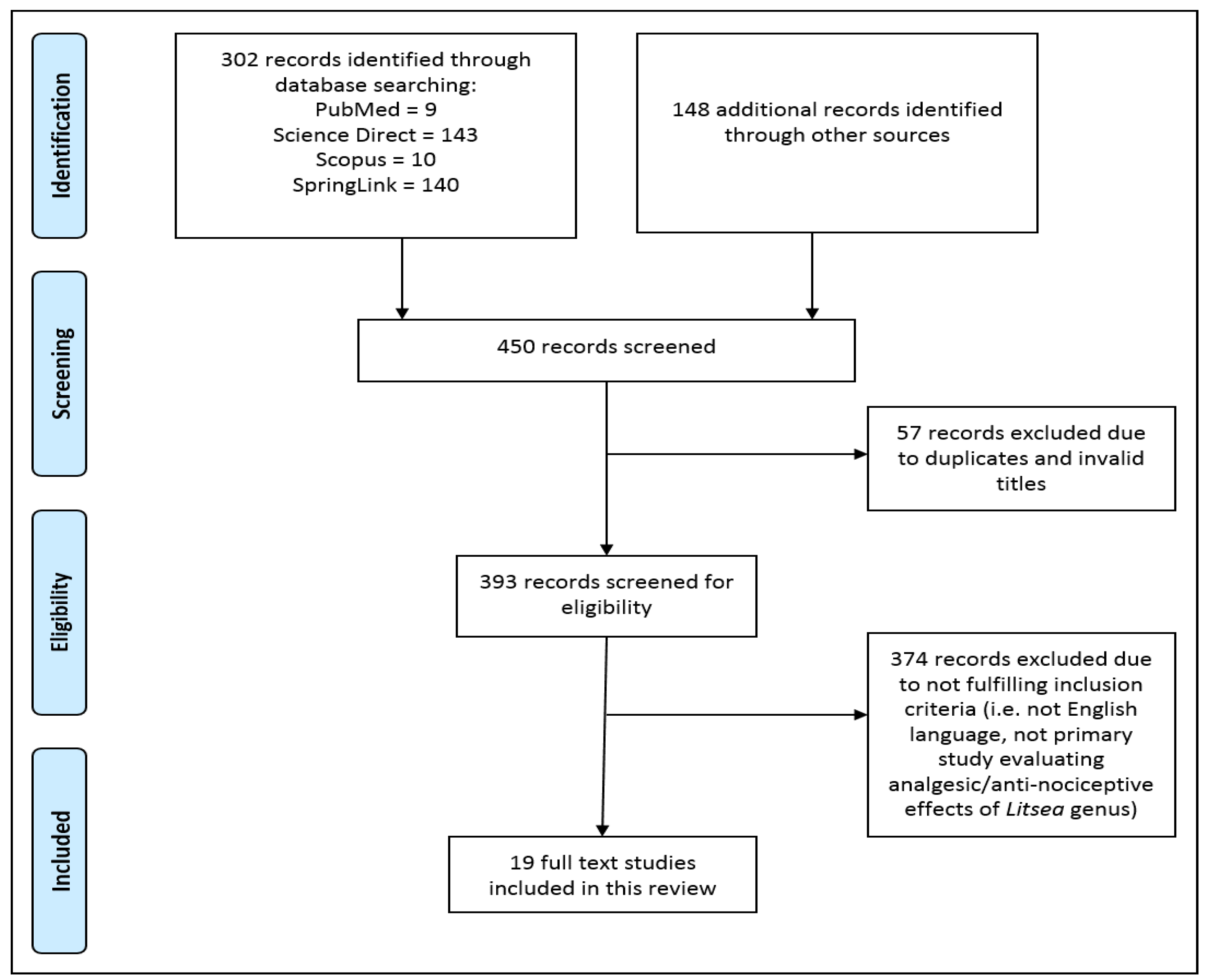
2. Methods
- Articles in the form of research articles, conference proceedings, technical papers or monographs;
- Studies published in the English language only;
- Studies that have demonstrated relevant biological activities pertaining to analgesia, antinociception or pain relief in any Litsea species;
- In vitro laboratory studies, in vivo animal model studies or clinical trials on human candidates.
- 5.
- Studies that reported other pharmacological properties of the Litsea species irrelevant to pain alleviation;
- 6.
- Non-full text articles with minimum information on methodology and results;
- 7.
- Non-English language articles;
- 8.
- Reviews, letters, case studies, opinions, reports or editorial papers.
3. Results
3.1. Litsea cubeba (Lour.) Pers.
3.2. Litsea elliptibacea Merr.
3.3. Litsea japonica (Thunb.) Jussieu
3.4. Litsea glaucescens Kunth
3.5. Litsea glutinosa (Lour.) C.B. Rob.
3.6. Litsea guatemalensis Mez.
3.7. Litsea lancifolia (Roxb.) Hook. F.
3.8. Litsea liyuyingi Liou Ho
3.9. Litsea monopetala Roxb./Litsea polyantha Juss.
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goldberg, D.S.; McGee, S.J. Pain as a global public health priority. BMC Public Health 2011, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Shin, J.I.; Koyanagi, A.; Jacob, L.; Smith, L.; Lee, H.; Chang, Y.; Song, T.J. Global, regional, and national neck pain burden in the general population, 1990–2019: An analysis of the global burden of disease study 2019. Front. Neurol. 2022, 13, 955367. [Google Scholar] [CrossRef]
- Silva-Correa, C.R.; Campos-Reyna, J.L.; Villarreal-La Torre, V.E.; Calderón-Peña, A.A.; Blas, M.V.; Aspajo-Villalaz, C.L.; Cruzado-Razco, J.L.; Sagastegui-Guarniz, W.A.; Guerero-Espino, L.M.; Hilario-Vargas, J. Potential activity of medicinal plants as pain modulators: A review. Pharmacogn. J. 2021, 13, 248–263. [Google Scholar] [CrossRef]
- Parsadaniantz, S.M.; Rivat, C.; Rostène, W.; Goazigo, A.R. Opioid and chemokine receptor crosstalk: A promising target for pain therapy? Nat. Rev. Neurosci. 2015, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, K.; Zhang, F.; Liu, W.; Wang, Y.; Yu, C.; Wang, J.; Zhang, K.; Zhang, C.; Nenadic, G.; et al. Identification of herbal categories active in pain disorder subtypes by machine learning help reveal novel molecular mechanisms of algesia. Pharmacol Res. 2020, 156, 104797. [Google Scholar] [CrossRef] [PubMed]
- McDaid, C.; Maund, E.; Rice, S.; Wright, K.; Jenkins, B.; Woolacott, N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery: A systematic review. Health Technol. Assess. 2010, 14, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J. Prophylactic use of NSAIDs by athletes: A risk/benefit assessment. Phys. Sportsmed. 2010, 38, 132–138. [Google Scholar] [CrossRef]
- Chau, D.L.; Walker, V.; Pai, L.; Cho, L.M. Opiates and elderly: Use and side effects. Clin. Interv. Aging 2008, 3, 273. [Google Scholar] [CrossRef]
- Laine, L. Gastrointestinal effects of NSAIDs and coxibs. J. Pain Symptom. Manag. 2003, 25, 32–40. [Google Scholar] [CrossRef]
- Mahboubi, M. Pepper as analgesic and anti-inflammatory alternative and bio-enhancer agent for treatment of pain. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 487–493. [Google Scholar] [CrossRef]
- Bell, J.E.; Sequeira, S.B.; Chen, D.Q.; Haug, E.C.; Werner, B.C.; Browne, J.A. Preoperative pain management: Is tramadol a safe alternative to traditional opioids before total hip arthroplasty? J. Arthroplast. 2020, 35, 2886–2891. [Google Scholar] [CrossRef]
- Caldera, F.E. Medical cannibus as an alternative for opioids for chronic pain: A case report. SAGE Open Med. Case Rep. 2020, 8, 2050313X20907015. [Google Scholar] [CrossRef] [PubMed]
- Daoust, R.; OPUM Study Group. Another alternative to opioids for acute pain? Can. J. Emerg. Med. 2020, 22, 273–274. [Google Scholar] [CrossRef]
- Najjar, M.; Hall, T.; Estupinan, B. Metoclopramide for acute migraine treatment in the emergency department: An effective alternative to opioids. Cureus 2017, 9, e1181. [Google Scholar] [CrossRef]
- Madhusudana, K.; Shireesha, B.; Naidu, V.G.; Ramakrishna, S.; Narsaiah, B.; Rao, A.R.; Diwan, P.V. Anti-inflammatory potential of thienopyridines as possible alternative to NSAIDs. Eur. J. Pharmacol. 2012, 678, 48–54. [Google Scholar] [CrossRef]
- Treudler, R.; Pohle, K.; Simon, J.C. Flupirtine is a safe alternative drug in patients with hypersensitivity to NSAIDs. Eur. J. Clin. Pharmacol. 2011, 67, 961–963. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arena, A.C.; Leite Kassuya, C.A.; Konkiewitz, E.C.; Ziff, E.B. Natural products as sources of new analgesic drugs. Evid. Based Complement. Altern. Med. 2022, 2022, 9767292. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent. Food Agric. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- Shilpi, J.A.; Uddin, S.J. Chapter Fourteen—Analgesic and antipyretic natural products. Annu. Rep. Med. Chem. 2020, 55, 435–458. [Google Scholar]
- Bulbul, I.J.; Haque, M.R.; Rashid, M.A. Pharmacological investigations of Litsea lancifolia (Roxb.) Hook. F. Bangladesh J. Bot. 2020, 49, 179–183. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Q.; Ma, J. Chemical composition and anti-arthritic activity of the essential oil from Litsea cubeba against Type II collagen rheumatoid arthritis in rat collagen. Trop. J. Pharm. Res. 2020, 19, 645–650. [Google Scholar] [CrossRef]
- Laboni, F.R.; Mahmud, S.; Karim, S.; Das, S.; Shahriar, M. Biological investigations of different leaf extracts of Litsea liyuyingi (Family-Lauraceae). IOSR J. Pharm. Biol. Sci. 2017, 12, 8–17. [Google Scholar] [CrossRef]
- Ferdous, M.R.; Ashrafudolla, M.; Hossain, M.S.; Bellah, S.F. Evaluation of antioxidant, analgesic and antidiarrheal activities of methanolic extract of Litsea monopetala (roxb.) leaves. Clin. Pharmacol. Biopharm. 2018, 7, 185. [Google Scholar] [CrossRef]
- Koo, H.J.; Yoon, W.J.; Sohn, E.H.; Ham, Y.M.; Jang, S.A.; Kwon, J.E.; Jeong, Y.J.; Kwak, J.H.; Sohn, E.; Park, S.Y.; et al. The analgesic and anti-inflammatory effects of Litsea japonica fruit are mediated via suppression of NF-κB and JNK/p38 MAPK activation. Int. Immunopharmacol. 2014, 22, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, R.; Sarwar, M.S.; RahmanDewan, S.M.; Das, A.; Das, B.; NasirUddin, M.M.; Islam, M.S.; Islam, M.S. In vivo analgesic, antipyretic, and anti-inflammatory potential in Swiss albino mice and in vitro thrombolytic activity of hydroalcoholic extract from Litsea glutinosa leaves. Biol. Res. 2014, 47, 56. [Google Scholar] [CrossRef] [PubMed]
- da Silva, K.A.; Klein-Junior, L.C.; Cruz, S.M.; Cáceres, A.; Quintão, N.L.; Delle Monache, F.; Cechinel-Filho, V. Anti-inflammatory and anti-hyperalgesic evaluation of the condiment laurel (Litsea guatemalensis Mez.) and its chemical composition. Food Chem. 2012, 132, 1980–1986. [Google Scholar] [CrossRef]
- Devika, M.; Joshi, H.; Nalini, M.S. Phytochemicals, antioxidative and in vivo hepatoprotective potentials of Litsea floribunda (BL.) Gamble (Lauraceae)-an endemic tree species of the Southern Western Ghats, India. Jordan J. Biol. Sci. 2016, 9, 163–171. [Google Scholar]
- Zhang, Y.; Tian, Y.; Tng, D.Y.; Zhou, J.; Zhang, Y.; Wang, Z.; Li, P.; Wang, Z. Comparative chloroplast genomics of Litsea Lam.(Lauraceae) and its phylogenetic implications. Forests 2021, 12, 744. [Google Scholar] [CrossRef]
- López-Caamal, A.; Reyes-Chilpa, R. The New World Bays (Litsea, Lauraceae). A botanical, chemical, pharmacological and ecological review in relation to their traditional and potential applications as phytomedicines. Bot. Rev. 2021, 87, 392–420. [Google Scholar] [CrossRef]
- Ngearnsaengsaruay, C.; Middleton, D.J.; Chayamarit, K. A revision of the genus Litsea Lam.(Lauraceae) in Thailand. Thai For. Bull. 2014, 39, 40–119. [Google Scholar]
- Ham, Y.M.; Cho, S.H.; Song, S.M.; Yoon, S.A.; Lee, Y.B.; Kim, C.S.; Kwon, S.H.; Jeong, M.S.; Yoon, W.J.; Kim, K.N. Litsenolide A2: The major anti-inflammatory activity compound in Litsea japonica fruit. J. Funct. Foods 2017, 39, 168–174. [Google Scholar] [CrossRef]
- Song, S.M.; Ham, Y.M.; Ko, Y.J.; Ko, E.Y.; Oh, D.J.; Kim, C.S.; Kim, D.; Kim, K.N.; Yoon, W.J. Anti-inflammatory activities of the products of supercritical fluid extraction from Litsea japonica fruit in RAW 264.7 cells. J. Funct. Foods 2016, 22, 44–51. [Google Scholar] [CrossRef]
- Kong, D.G.; Zhao, Y.; Li, G.H.; Chen, B.J.; Wang, X.N.; Zhou, H.L.; Lou, H.X.; Ren, D.M.; Shen, T. The genus Litsea in traditional Chinese medicine: An ethnomedical, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 164, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Mia, M.M.; Kadir, M.F.; Hossan, M.S.; Rahmatullah, M. Medicinal plants of the Garo tribe inhabiting the Madhupur forest region of Bangladesh. Am. Eurasian J. Sustain. Agric. 2009, 3, 165–171. [Google Scholar]
- Muhammad, I.; Xiao, Y.Z.; Hassan, S.S.; Xiao, X.; Yan, S.K.; Guo, Y.Q.; Ma, X.P.; Jin, H.Z. Three new guaiane-type sesquiterpenoids and a monoterpenoid from Litsea lancilimba Merr. Nat. Prod. Res. 2022, 36, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Dalimunthe, A.; Hasibuan, P.A.; Silalahi, J.; Sinaga, S.F.; Satria, D. Antioxidant activity of alkaloid compounds from Litsea cubeba Lour. Orient. J. Chem. 2018, 34, 1149. [Google Scholar] [CrossRef]
- Devika, M.; Nalini, M.S. Evaluation of antidepressant activity of Litsea floribunda (bl.) Gamble-lauraceae using animal models. Int. J. Pharm. Sci. Res. 2018, 9, 3427–3432. [Google Scholar]
- Pérez, F.; Santizo, A.; Cáceres, A.; Apel, M.; Henriquez, A.; Cruz, S.M.; Mérida, M. Chemical composition of essential oil of Litsea guatemalensis (Mexican bay) from different provenances of Guatemala. In Proceedings of the International Symposium on Medicinal and Aromatic Plants IMAPS2010 and History of Mayan Ethnopharmacology IMAPS2011, Shiraz, Iran, 21–24 June 2010–20–23 November 2011; Ghaemghami, J., Khosh-Khui, M., Omidbaigi, R., Eds.; ISHS Acta Horticulturae 964. International Society for Horticultural Science: Leuven, Belgium, 2011; pp. 47–57. [Google Scholar]
- Takaishi, M.; Fujita, F.; Uchida, K.; Yamamoto, S.; Sawada, M.; Hatai, C.; Shimizu, M.; Tominaga, M. 1,8-cineole, a TRPM8 agonist, is a novel natural antagonist of human TRPA1. Mol. Pain. 2012, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Estrella, G.R.; Eva, G.T.; Alberto, H.L.; Guadalupe, V.D.; Azucena, C.V.; Sandra, O.S.; Noé, A.V.; Javier, L.M.F. Limonene from Agastache mexicana essential oil produces antinociceptive effects, gastrointestinal protection and improves experimental ulcerative colitis. J. Ethnopharmacol. 2021, 280, 114462. [Google Scholar] [CrossRef]
- Peana, A.T.; Paolo, S.D.; Chessa, M.L.; Moretti, M.D.; Serra, G.; Pippia, P. (−)-Linalool produces antinociception in two experimental models of pain. Eur. J. Pharmacol. 2003, 460, 37–41. [Google Scholar] [CrossRef]
- Perme, N.; Choudhury, S.N.; Choudhury, R.; Natung, T.; De, B. Medicinal plants in traditional use at Arunachal Pradesh, India. Int. J. Phytopharm. 2015, 5, 86–98. [Google Scholar]
- Zhou, J.; Xie, G.; Yan, X. Volume 5 Isolated Compounds (TZ) References TCM Plants and Congeners. In Encyclopedia of Traditional Chinese Medicines-Molecular Structures, Pharmacological Activities, Natural Sources and Applications; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–601. [Google Scholar]
- Hong, L.; Guo, Z.; Huang, K.; Wei, S.; Liu, B.; Meng, S.; Long, C. Ethnobotanical study on medicinal plants used by Maonan people in China. J. Ethnobiol. Ethnomed. 2015, 11, 32. [Google Scholar] [CrossRef]
- Wang, Y.S.; Wen, Z.Q.; Li, B.T.; Zhang, H.B.; Yang, J.H. Ethnobotany, phytochemistry, and pharmacology of the genus Litsea: An update. J. Ethnopharmacol. 2016, 181, 66–107. [Google Scholar] [CrossRef] [PubMed]
- Tamang, S.; Singh, A.; Bussmann, R.W.; Shukla, V.; Nautiyal, M.C. Ethno-medicinal plants of tribal people: A case study in Pakyong subdivision of East Sikkim, India. Acta Ecologica. Sin. 2021, 43, 34–46. [Google Scholar] [CrossRef]
- Malla, B.; Gauchan, D.P.; Chhetri, R.B. An ethnobotanical study of medicinal plants used by ethnic people in Parbat district of western Nepal. J. Ethnopharmacol. 2015, 165, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.C. Traditional knowledge of Adi tribe of Arunachal Pradesh on plants. Indian J. Tradit. Knowl. 2009, 8, 146–153. [Google Scholar]
- López-Romero, J.C.; González-Ríos, H.; Peña-Ramos, A.; Velazquez, C.; Navarro, M.; Robles-Zepeda, R.; Martínez-Benavidez, E.; Higuera-Ciapara, I.; Virués, C.; Olivares, J.L.; et al. Seasonal effect on the biological activities of Litsea glaucescens Kunth extracts. Evid. Based Complement. Altern. Med. 2018, 2018, 2738489. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pérez, N.D.; Lorea-Hernández, F.G. Identity and delimitation of the American species of Litsea Lam.(Lauraceae): A morphological approach. Plant Syst. Evol. 2009, 283, 19–32. [Google Scholar] [CrossRef]
- Owk, A.K.; Lagudu, M.N. Litsea glutinosa (Lauraceae): Evaluation of its foliar phytochemical constituents for antimicrobial activity. Not Sci. Biol. 2018, 10, 21–25. [Google Scholar]
- Jain, B.; Rawat, A.; Mariyam, A.; Parkhe, G. Phytochemical screening and thin-layer chromatographic studies of Litsea glutinosa (lour.) bark extract. Asian J. Pharm. Clin. Res. 2017, 6, 18–23. [Google Scholar]
- Chawra, H.S.; Gupta, G.; Singh, S.K.; Pathak, S.; Rawat, S.; Mishra, A.; Gilhotra, R.M. Phytochemical constituents, ethno medicinal properties and applications of plant: Litsea glutinosa (lour.) CB robinson (Lauraceae). Res. J. Pharm. Technol. 2021, 14, 6113–6118. [Google Scholar] [CrossRef]
- Suroowan, S.; Pynee, K.B.; Mahomoodally, M.F. A comprehensive review of ethnopharmacologically important medicinal plant species from Mauritius. S. Afr. J. Bot. 2019, 122, 189–213. [Google Scholar] [CrossRef]
- Rathi, B.; Rathi, R. Quantitative analysis of Medicinal plants used by the traditional healers of Karanja block of Wardha district for treating musculoskeletal disorders. Int. J. Ayurveda Med. 2020, 11, 175–183. [Google Scholar] [CrossRef]
- Shah, A.; Bharati, K.A.; Ahmad, J.; Sharma, M.P. New ethnomedicinal claims from Gujjar and Bakerwals tribes of Rajouri and Poonch districts of Jammu and Kashmir, India. J. Ethnopharmacol. 2015, 166, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.; Bhat, J.A.; Chakravarty, S. Species richness and folk therapeutic uses of ethnomedicinal plants in West Bengal, India–A meta-analysis. Phytomed. Plus 2022, 2, 100158. [Google Scholar] [CrossRef]
- Cates, R.G.; Thompson, A.; Brabazon, H.; McDonald, S.; Lawrence, M.; Williams, S.; Peniallilo, P.; Soria, J.A.F.; Espinoza, L.V.; Martinez, J.E.V.; et al. Activities of Guatemalan medicinal plants against cancer cell lines and selected microbes: Evidence for their conservation. J. Med. Plant Res. 2014, 8, 1040–1050. [Google Scholar]
- Hasan, H.; Al Azad, M.S.; Islam, M.Z.; Rahman, S.M.; Islam, M.R.; Rahman, S.; Rahmatullah, M. Antihyperglycemic activity of methanolic extract of Litsea monopetala (Roxb.) Pers. leaves. Adv. Nat. Appl. Sci. 2014, 8, 51–55. [Google Scholar]
- Mohammad, N.; Kobir, E.; Rahman, M.; Babu, H.; Islam, J. A study on Litsea monopetala for evaluating its pharmacological properties. Discov. Phytomed. 2021, 8, 43–49. [Google Scholar] [CrossRef]
- Arfan, M.; Amin, H.; Kosinska, A.; Karamac, M.; Amarowicz, R. Antioxidant activity of phenolic fractions of Litsea monopetala [Persimon-leaved Litsea] bark extract. Pol. J. Food Nutr. Sci. 2008, 58, 229–233. [Google Scholar]
- Rahmatullah, M.; Ayman, U.; Akter, F.; Sarker, M.; Sifa, R.; Sarker, B.; Chyti, H.N.; Jahan, F.I.; Chowdhury, M.H.; Chowdhury, S.A. Medicinal formulations of a Kanda tribal healer–a tribe on the verge of disappearance in Bangladesh. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 213–222. [Google Scholar] [CrossRef]
- Amin, F.; Ali, H.; Sher, H. Conservation issues of Litsea monopetala (ROXB.) persoon (Lauracae) in Pakistan. Pak. J. Bot. 2020, 52, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Biswas, N.N.; Acharzo, A.K.; Anamika, S.; Khushi, S.; Bokshi, B. Screening of natural bioactive metabolites and investigation of antioxidant, antimicrobial, antihyperglycemic, neuropharmacological, and cytotoxicity potentials of Litsea polyantha Juss. ethanolic root extract. Evid. Based Complement. Altern. Med. 2017, 2017, 3701349. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Sinha, B.N. Central analgesic activity of Litsea polyantha Juss. bark extract. In Proceedings of the 19th International Electronic Conference on Synthetic Organic Chemistry, Online, 1–30 November 2015; MDPI: Basel, Switzerland, 2015; p. 1. [Google Scholar]
- Grosvenor, P.W.; Gothard, P.K.; McWilliam, N.C.; Supriono, A.; Gray, D.O. Medicinal plants from riau province, sumatra, Indonesia. Part 1: Uses. J. Ethnopharmacol. 1995, 45, 75–95. [Google Scholar] [CrossRef]
- Taib, I.S.; Budin, S.B.; Siti Nor Ain, S.M.; Mohamed, J.; Louis, S.R.; Das, S.; Sallehudin, S.; Rajab, N.F.; Hidayatulfathi, O. Toxic effects of Litsea elliptica Blume essential oil on red blood cells of Sprague-Dawley rats. J. Zhejiang Univ. Sci. B 2009, 10, 813–819. [Google Scholar] [CrossRef]
- Goh, M.P.; Kamaluddin, A.F.; Tan, T.J.; Yasin, H.; Taha, H.; Jama, A.; Ahmad, N. An evaluation of the phytochemical composition, antioxidant and cytotoxicity of the leaves of Litsea elliptica Blume—An ethnomedicinal plant from Brunei Darussalam. Saudi J. Biol. Sci. 2022, 29, 304–317. [Google Scholar] [CrossRef]
- Amit, Z.; Zinyin, L. A mini review on the nutritional compositions and pharmacological properties of Litsea garciae. Malays. Appl. Biol. 2021, 50, 29–39. [Google Scholar] [CrossRef]
- Rahman, M.A.; Uddin, S.B.; Wilcock, C.C. Medicinal plants used by Chakma tribe in Hill Tracts districts of Bangladesh. Indian J. Tradit. Knowl. 2007, 6, 508–517. [Google Scholar]
- Prinsloo, G.; Marokane, C.K.; Street, R.A. Anti-HIV activity of southern African plants: Current developments, phytochemistry and future research. J. Ethnopharmacol. 2018, 210, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Tseng, Y.H.; Chu, F.H.; Wen, T.Y.; Cheng, W.W.; Chen, Y.T.; Tsao, N.W.; Wang, S.Y. Neuropharmacological activities of fruit essential oil from Litsea cubeba Persoon. J. Wood Sci. 2012, 58, 538–543. [Google Scholar] [CrossRef]
- Chung, L.Y.; Goh, S.H.; Imiyabir, Z. Central nervous system receptor activities of some Malaysian plant species. Pharm. Biol. 2005, 43, 280–288. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, O.; Kim, E.A.; Yoon, W.J.; Kim, J.H.; Kim, J.Y. Randomized double-blind placebo-controlled study of the efficacy of Litsea japonica fruit extract in subjects with mild to moderate knee osteoarthritis. J. Funct. Foods 2017, 34, 304–310. [Google Scholar] [CrossRef]
- Guzmán-Gutiérrez, S.L.; Bonilla-Jaime, H.; Gómez-Cansino, R.; Reyes-Chilpa, R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015, 128, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Rumzhum, N.N.; Rahman, M.M.; Sharukh, A.A.; Chowdhury, S.A.; Pervin, M.N. In vitro antioxidant and antinociceptive potentialities of methanolic extract of Litsea glutinosa. Bangladesh J. Sci. Ind. Res. 2012, 47, 401–406. [Google Scholar] [CrossRef]
- Mozaffar, M. Biological Investigation of the Ethanolic Extract of Polygonum Hydropiper (L), Coccinia Grandis (L) and Litsea Glutinosa (Lour.). Ph.D. Thesis, East West University, Dhaka, Bangladesh, 2012. [Google Scholar]
- Pradeepa, K.; Krishna, V.; Santosh, K.; Girish, K.K. Antinociceptive property of leaves extract of Litsea glutinosa. Asian J. Pharm. Clin. Res. 2013, 6, 182–184. [Google Scholar]
- Lohitha, P.; Muchandi, I.S.; Haricharan, K.; Himabindu, K.N.; Mamatha, G.; Tejaswi, C.H.; Ramanjaneyulu, K.; Sagar, S.V. Study of analgesic activity of Litsea glutinosa (L.) ethanolic extract on Swiss Albino mice. Int. J. Pharm. Sci. Res. 2010, 1, 93–97. [Google Scholar]
- Hossen, M.F.; Hossain, S.; Ahamed, M.I.; Patwary, M.S.; Imtiaz, O.; Hasan, M.; Al Mahmud, A. Evaluation of in vivo analgesic, antiemetic and anxiolytic effect of methanolic extract of Litsea monopetala in animal model. Discov. Phytomed. 2019, 6, 126–129. [Google Scholar]
- Bulbul, I.J.; Rashid, M.A.; Haque, M.R. Pharmacological studies of different fractions of Litsea monopetala Roxb. Bangladesh Pharm. J. 2020, 23, 61–64. [Google Scholar] [CrossRef]
- Manik, G.; Sinha, B.N.; Sasmal, D. Antinociceptive potential of Litsea polyantha Juss. bark extract. In Proceedings of the Pharmacon-2010 Eastern Regional Conference of Indian Pharmacological Society, Mesra, Ranchi, India, 9–10 April 2010; PS-OP05: 12. Birla Institute of Technology: Ranchi, India, 2010. [Google Scholar]
- Yang, K.; Wang, C.F.; You, C.X.; Geng, Z.F.; Sun, R.Q.; Guo, S.S.; Du, S.S.; Liu, Z.L.; Deng, Z.W. Bioactivity of essential oil of Litsea cubeba from China and its main compounds against two stored product insects. J. Asia Pac. Entomol. 2014, 17, 459–466. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The composition, pharmacological and economic importance of essential oil of Litsea cubeba (Lour.) Pers. Food Sci. Technol. 2020, 42, e35720. [Google Scholar] [CrossRef]
- Maswal, M.; Dar, A.A. Formulation challenges in encapsulation and delivery of citral for improved food quality. Food Hydrocoll. 2014, 37, 182–195. [Google Scholar] [CrossRef]
- Sattayakhom, A.; Songsamoe, S.; Yusakul, G.; Kalarat, K.; Matan, N.; Koomhin, P. Effects of Thai local ingredient odorants, Litsea cubeba and garlic essential oils, on brainwaves and moods. Molecules 2021, 26, 2939. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, Y.J.; Li, Y.S.; Zhang, W.K.; Tang, H.B. α-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. J. Ethnopharmacol. 2016, 179, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Q.; Guo, Q.; Zhao, Y.; Gao, X.; Chai, X.; Tu, P. Characterization and simultaneous quantification of biological aporphine alkaloids in Litsea cubeba by HPLC with hybrid ion trap time-of-flight mass spectrometry and HPLC with diode array detection. J. Sep. Sci. 2015, 38, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Silva, D.O.; Bello, F.; Alviano, C.S.; Alviano, D.S.; Matheus, M.E.; Fernandes, P.D. Characterization of the antinociceptive and anti-inflammatory activities from Cocos nucifera L. (Palmae). J. Ethnopharmacol. 2009, 122, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Rezaee-Asl, M.; Sabour, M.; Nikoui, V.; Ostadhadi, S.; Bakhtiarian, A. The study of analgesic effects of Leonurus cardiaca L. in mice by formalin, tail flick and hot plate tests. Int. Sch. Res. Not. 2014, 2014, 687697. [Google Scholar] [CrossRef] [PubMed]
- Khozirah, S.; Noor Rain, A.; Siti Najila, M.J.; Imiyabir, Z.; Madani, L.; Rodaya, C. In vitro antiplasmodial properties of selected plants of Sabah. Pertanika J. Sci. Technol. 2011, 19, 11–17. [Google Scholar]
- Said, I.M.; Din, L.B.; Samsudin, M.W.; Yusoff, N.I.; Latif, A.; Mat-Ali, R.; Hadi, H.A. A phytochemical survey of Sayap-Kinabalu park, Sabah. ASEAN Rev. Biodivers. Environ. Conserv. 1998, 137–144. [Google Scholar]
- Gomes, N.G.; Campos, M.G.; Órfão, J.M.; Ribeiro, C.A. Plants with neurobiological activity as potential targets for drug discovery. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1372–1389. [Google Scholar] [CrossRef] [PubMed]
- Linciano, P.; Sorbi, C.; Comitato, A.; Lesniak, A.; Bujalska-Zadrożny, M.; Pawłowska, A.; Bielenica, A.; Orzelska-Górka, J.; Kędzierska, E.; Biała, G.; et al. Identification of a potent and selective 5-HT1A receptor agonist with in vitro and in vivo antinociceptive activity. ACS Chem. Neurosci. 2020, 11, 4111–4127. [Google Scholar] [CrossRef]
- Fiorino, F.; Severino, B.; Magli, E.; Ciano, A.; Caliendo, G.; Santagada, V.; Frecentese, F.; Perissutti, E. 5-HT1A receptor: An old target as a new attractive tool in drug discovery from central nervous system to cancer. J. Med. Chem. 2014, 57, 4407–4426. [Google Scholar] [CrossRef]
- Bardoni, R. Serotonergic modulation of nociceptive circuits in spinal cord dorsal horn. Curr. Neuropharmacol. 2019, 17, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, L.D.; Ghelardini, C.; Micheli, L.; Del Bello, F.; Giannella, M.; Piergentili, A.; Pigini, M.; Quaglia, W. Synergic stimulation of serotonin 5-HT1A receptor and α2-adrenoceptors for neuropathic pain relief: Preclinical effects of 2-substituted imidazoline derivatives. Eur. J. Pharmacol. 2017, 810, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Sałat, K.; Kołaczkowski, M.; Furgała, A.; Rojek, A.; Śniecikowska, J.; Varney, M.A.; Newman-Tancredi, A. Antinociceptive, antiallodynic and antihyperalgesic effects of the 5-HT1A receptor selective agonist, NLX-112 in mouse models of pain. Neuropharmacology 2017, 125, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Deseure, K.; Bréand, S.; Colpaert, F.C. Curative-like analgesia in a neuropathic pain model: Parametric analysis of the dose and the duration of treatment with a high-efficacy 5-HT1A receptor agonist. Eur. J. Pharmacol. 2007, 568, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Colpaert, F.C. 5-HT (1A) receptor activation: New molecular and neuroadaptive mechanisms of pain relief. Curr. Opin. Investig. Drugs 2006, 7, 40–47. [Google Scholar]
- Chuah, C.H.; Lee, K.H.; Goh, S.H. Alkaloids from Litsea elliptibacea (Lauraccae). Malays. J. Sci. 1999, 18, 63–66. [Google Scholar]
- Yun, I.G.; Ahn, S.H.; Yoon, W.J.; Kim, C.S.; Lim, Y.K.; Kook, J.K.; Jung, S.; Choi, C.H.; Lee, T.H. Litsea japonica leaf extract suppresses proinflammatory cytokine production in periodontal ligament fibroblasts Stimulated with oral pathogenic bacteria or interleukin-1β. Int. J. Mol. Sci. 2018, 19, 2494. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Q.M.; Cao, T.Q.; Tran, P.L.; Kim, J.A.; Seo, S.T.; Kim, J.C.; Woo, M.H.; Lee, J.H.; Min, B.S. Lactones from the pericarps of Litsea japonica and their anti-inflammatory activities. Bioorg Med. Chem. Lett. 2018, 28, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Min, B.S.; Kim, J.H.; Lee, J.; Kim, T.J.; Kim, C.S.; Kim, Y.H.; Lee, H.K. Flavonoids from the leaves of Litsea japonica and their anti-complement activity. Phytother. Res. 2005, 19, 273–276. [Google Scholar] [CrossRef]
- Sohn, E.; Kim, J.; Kim, C.S.; Lee, Y.M.; Jo, K.; Shin, S.D.; Kim, J.H.; Kim, J.S. The extract of Litsea japonica reduced the development of diabetic nephropathy via the inhibition of advanced glycation end products accumulation in db/db mice. Evid. Based Complement. Altern. Med. 2013, 2013, 769416. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.S.; Lee, I.S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.H.; Kim, J.S. Extract of Litsea japonica ameliorates blood–retinal barrier breakdown in db/db mice. Endocrine 2014, 46, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.A.; Salleh, W.M. Chemical composition and biological activities of essential oils of the genus Litsea (Lauraceae)—A review. Poljopr. Znan. Smotra 2020, 85, 97–103. [Google Scholar]
- Min, B.S.; Lee, S.Y.; Kim, J.H.; Kwon, O.K.; Park, B.Y.; An, R.B.; Lee, J.K.; Moon, H.I.; Kim, T.J.; Kim, Y.H.; et al. Lactones from the leaves of Litsea japonica and their anti-complement activity. J. Nat. Prod. 2003, 66, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Nakamura, T.; Ichino, K.; Ito, K.; Tanaka, T. Butanolides from Litsea japonica. Phytochemistry 1990, 29, 857–859. [Google Scholar] [CrossRef]
- Takeda, K.I.; Sakurawi, K.; Ishii, H. Components of the Lauraceae family—I: New lactonic compounds from Litsea japoncia. Tetrahedron 1972, 28, 3757–3766. [Google Scholar] [CrossRef]
- Rengel, Y.; Ospelt, C.; Gay, S. Proteinases in the joint: Clinical relevance of proteinases in joint destruction. Arthritis Res. Ther. 2007, 9, 221. [Google Scholar] [CrossRef]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef]
- Bonnet, C.S.; Walsh, D.A. Osteoarthritis, angiogenesis and inflammation. Rheumatology 2005, 44, 7–16. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, H.J.; Yang, W.K.; Lee, J.E.; Cho, J.H.; Park, I.J.; Park, S.; Park, B.K.; Jin, M. Suppressive effect of the n-hexane extract of Litsea japonica fruit flesh on monosodium-iodoacetate-induced osteoarthritis in rats. Evid. Based Complement. Altern. Med. 2017, 2017, 1791403. [Google Scholar] [CrossRef]
- César, L.R.; Javier, H.; Fernando, A.Z.; Carlos, V.; Enrique, R.Z.; Efrain, A.; Evelin, M.B.; Inocencio, H.C.; Luis, O.J.; Zaira, D. Identification of the main phenolic compounds responsible for the antioxidant activity of Litsea glaucescens Kunth. S. Afr. J. Bot. 2022, 147, 208–214. [Google Scholar] [CrossRef]
- Guzmán-Gutiérrez, S.L.; Gómez-Cansino, R.; García-Zebadúa, J.C.; Jiménez-Pérez, N.C.; Reyes-Chilpa, R. Antidepressant activity of Litsea glaucescens essential oil: Identification of β-pinene and linalool as active principles. J. Ethnopharmacol. 2012, 143, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.A.; Rao, V.S. Antiinflammatory and antinociceptive effects of 1, 8-cineole a terpenoid oxide present in many plant essential oils. Phytother. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Rabena, A.R. Propagation techniques of endangered Sablot (Litsea glutinosa) Lour. CB Rob. JPAIR Multidiscip. Res. J. 2010, 5, 1. [Google Scholar]
- Reddy, K.N.; Reddy, C.S. First red list of medicinal plants of Andhra Pradesh, India-conservation assessment and management planning. Ethnobot. Leafl. 2008, 12, 103–107. [Google Scholar]
- Ndi, C.P.; Sykes, M.J.; Claudie, D.J.; McKinnon, R.A.; Semple, S.J.; Simpson, B.S. Antiproliferative aporphine alkaloids from Litsea glutinosa and ethnopharmacological relevance to kuuku i’yu traditional medicine. Aust. J. Chem. 2015, 69, 145–151. [Google Scholar] [CrossRef]
- Haque, T.; Uddin, M.Z.; Saha, M.L.; Mazid, M.A.; Hassan, M.A. Propagation, antibacterial activity and phytochemical profiles of Litsea glutinosa (Lour.) CB Robinson. Dhaka Univ. J. Biol. Sci. 2014, 23, 165–171. [Google Scholar] [CrossRef]
- Parikh, P.H.; Rangrez, A.Y. Extraction and phytochemical evaluation of Litsea glutinosa bark methanolic extract. J. Appl. Pharm. Sci. 2012, 2, 71–78. [Google Scholar]
- Devi, P.; Meera, R. Study of antioxdant, antiinflammatory and woundhealing activity of extracts of Litsea glutinosa. J. Pharm. Sci. Res. 2010, 2, 155–163. [Google Scholar]
- Lohitha, P.; Shivsagar, K.; Charan, V.N.; Priya, U.P.; Sagar, S.V.; Ramanjaneyulu, K.; Verma, V.H.K. Phytochemical screening and evaluation of in vitro antibacterial activity of Litsea glutinosa (L) bark ethanol extract. Pharmacologyonline 2010, 1, 618–623. [Google Scholar]
- Mandal, S.C.; Kumar, C.A.; Majumder, A.; Majumder, R.; Maity, B.C. Antibacterial activity of Litsea glutinosa bark. Fitoterapia 2000, 71, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Pareek, D.; Dobhal, S.; Sharma, M.C.; Joshi, Y.C.; Dobhal, M.P. Butanolides from methanolic extract of Litsea glutinosa. Chem. Biodivers. 2013, 10, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Arunodaya, H.S.; Krishna, V.; Shashikumar, R.; Girish Kumar, K. Antibacterial and antioxidant activities of stem bark essential oil constituents of Litsea glutinosa CB Rob. Int. J. Pharm. Pharm. Sci. 2016, 8, 258–264. [Google Scholar]
- Benhassine, I.; Ouafi, S.; Eras, J.; Harrat, Z.; Bouslama, Z.; Canela-Garayoa, R. Anti-inflammatory, analgesic activities and phytochemical study of Traganum nudatum Delile. Iran J. Pharm. Sci. 2021, 17, 1–22. [Google Scholar]
- Wong, K.L.; Cheung, C.W.; So, E.C.; Huang, B.M.; Leung, Y.M. Abstract PR635: Palmitic Acid-Induced Cytotoxicity in Human Alveolar A549 Cells Involved Endoplasmic Reticulum (Er) Stress And Reactive Oxygen Species Production. Anesth. Analg. 2016, 123, 818. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.P.; Kumaravel, S.; Lalitha, C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr. J. Biochem. Res. 2010, 4, 191–195. [Google Scholar]
- Déciga-Campos, M.; Montiel-Ruiz, R.M.; Navarrete-Vázquez, G.; López-Muñoz, F.J. Palmitic acid analogues exhibiting antinociceptive activity in mice. Proc. West Pharmacol. Soc. 2007, 50, 75–77. [Google Scholar] [PubMed]
- Villaseñor, I.M.; Angelada, J.; Canlas, A.P.; Echegoyen, D. Bioactivity studies on β-sitosterol and its glucoside. Phytother. Res. 2002, 16, 417–421. [Google Scholar] [CrossRef]
- da Fonseca Pacheco, D.; Romero, T.R.; Duarte, I.D. Central antinociception induced by ketamine is mediated by endogenous opioids and μ-and δ-opioid receptors. Brain Res. 2014, 1562, 69–75. [Google Scholar] [CrossRef]
- Vallverdú, C.; Vila, R.; Cruz, S.M.; Cáceres, A.; Cañigueral, S. Composition of the essential oil from leaves of Litsea guatemalensis. Flavour. Fragr. J. 2005, 20, 415–418. [Google Scholar] [CrossRef]
- Xing, B.; Feng, N.; Zhang, J.; Li, Y.; Hou, X.; Wu, H.; Liu, W.; Han, G. Pinocembrin relieves hip fracture-induced pain by repressing spinal substance P signaling in aged rats. J. Neurophysiol. 2022, 127, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Mahattanadul, S.; Ridtitid, W.; Nima, S.; Phdoongsombut, N.; Ratanasuwon, P.; Kasiwong, S. Effects of Morinda citrifolia aqueous fruit extract and its biomarker scopoletin on reflux esophagitis and gastric ulcer in rats. J. Ethnopharmacol. 2011, 134, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ribas, C.M.; Meotti, F.C.; Nascimento, F.P.; Jacques, A.V.; Dafre, A.L.; Rodrigues, A.L.; Farina, M.; Soldi, C.; Mendes, B.G.; Pizzolatti, M.G. Antinociceptive effect of the Polygala sabulosa hydroalcoholic extract in mice: Evidence for the involvement of glutamatergic receptors and cytokine pathways. Basic Clin. Pharmacol. Toxicol. 2008, 103, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Recio, M.C.; Schinella, G.R.; Máñez, S.; Giner, R.M.; Cerdá-Nicolás, M.; Ríos, J.L. Assessment of the anti-inflammatory activity and free radical scavenger activity of tiliroside. Eur. J. Pharmacol. 2003, 461, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, A.; Cruz, S.M. Contributions of natural ingredients from the Mesoamerican biodiversity for the phytocosmetic industry. Int. J. Phytocosme. Nat. Ingred. 2015, 2, 2. [Google Scholar] [CrossRef][Green Version]
- Cruz, S.M.; Marroquín, M.N.; Gaitán, I.C.; Cáceres, A. Antimicrobial activity of essential oils and ethanolic extracts of three species of laurel (Litsea spp.) from Guatemala. In Proceedings of the International Symposium on Medicinal Plants and Natural Products 1030, Quito, Ecuador, 3–6 December 2012; Ghaemghami, J., Alarcón Gallegos, R., Navarrete, H., Eds.; ISHS Acta Horticulturae 1030. ISHS: Leuven, Belgium, 2014; pp. 23–29. [Google Scholar]
- Cáceres, A.; Lange, K.; Cruz, S.M.; Velásquez, R.; Lima, S.; Menéndez, M.C.; Dardón, R.; Córdova, D.; González, J. Assessment of antioxidant activity of 24 native plants used in Guatemala for their potential application in natural product industry. In Proceedings of the International Symposium on Medicinal and Aromatic Plants IMAPS2010 and History of Mayan Ethnopharmacology IMAPS2011, Shiraz, Iran, 21–24 June 2010; Antigua, Guatemala, 20–23 November 2011. Ghaemghami, J., Khosh-Khui, M., Omidbaigi, R., Eds.; ISHS Acta Horticulturae 964. ISHS: Leuven, Belgium, 2011; pp. 85–92. [Google Scholar]
- Komatsu, T.; Katsuyama, S.; Uezono, Y.; Sakurada, C.; Tsuzuki, M.; Hamamura, K.; Bagetta, G.; Sakurada, S.; Sakurada, T. Possible involvement of the peripheral Mu-opioid system in antinociception induced by bergamot essential oil to allodynia after peripheral nerve injury. Neurosci. Lett. 2018, 686, 127–132. [Google Scholar] [CrossRef]
- Xie, H.T.; Xia, Z.Y.; Pan, X.; Zhao, B.; Liu, Z.G. Puerarin ameliorates allodynia and hyperalgesia in rats with peripheral nerve injury. Neural Regen. Res. 2018, 13, 1263–1268. [Google Scholar] [CrossRef]
- Kim, C.F.; Moalem-Taylor, G. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J. Pain. 2011, 12, 370–383. [Google Scholar] [CrossRef]
- Toriyabe, M.; Omote, K.; Kawamata, T.; Namiki, A. Contribution of interaction between nitric oxide and cyclooxygenases to the production of prostaglandins in carrageenan-induced inflammation. Anesthesiology 2004, 101, 983–990. [Google Scholar] [CrossRef]
- Chong, K.Y.; Neo, L.; Tan, S.Y.; Koh, C.Y.; Lim, R.C.; Loh, J.W.; Ng, W.Q.; Seah, W.W.; Yee, A.T.K.; Tan, H.T.W. Towards a field guide to the trees of the Nee Soon Swamp Forest (I): Lauraceae. Nat. Singapore 2016, 9, 1–28. [Google Scholar]
- Sulaiman, S.N. Alkaloids Isolated from Litsea grandis and Litsea lancifolia (Lauraceae). Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2012. [Google Scholar]
- Nelson, J.; Noweg, T. Assessment of forest regeneration following a series of disturbances in two types of primary forest at Bungo Range, Bau, Sarawak. J. Trop. For. Sci. 2021, 33, 126–136. [Google Scholar] [CrossRef]
- Allen, C.K. Studies in the Lauraceae. I. Chinese and Indo-Chinese species of Litsea, Neolitsea, and Actinodaphne. Ann. Mo. Bot. Gard. 1938, 25, 361–434. [Google Scholar] [CrossRef]
- Bulbul, I.J.; Uddin, M.E.; Nahar, N.; Kuddus, M.R.; Haque, M.R. Antidiarrheal activity of four different species of Litsea available in Bangladesh. Biomed. Pharmacol. J. 2021, 14, 1259–1266. [Google Scholar] [CrossRef]
- Bhatt, B.P.; Lemtur, M.; Changkija, S.; Sarkar, B. Fuelwood characteristics of important trees and shrubs of Eastern Himalaya. Energy Source Part A 2016, 39, 47–50. [Google Scholar] [CrossRef]
- Eswani, N.; Abd Kudus, K.; Nazre, M.; Noor, A.A.; Ali, M. Medicinal plant diversity and vegetation analysis of logged over hill forest of Tekai Tembeling Forest Reserve, Jerantut, Pahang. J. Agric. Sci. 2010, 2, 189–210. [Google Scholar] [CrossRef]
- Yang, S.; Li, L.W.; Yang, X.D.; Zhao, J.F.; Li, L. Studies on the chemical constituents of Litsea lancifolia. Zhong Yao Cai 2008, 31, 985–987. [Google Scholar] [PubMed]
- Sulaiman, S.N.; Mukhtar, M.R.; Hadi, A.H.; Awang, K.; Hazni, H.; Zahari, A.; Litaudon, M.; Zaima, K.; Morita, H. Lancifoliaine, a new bisbenzylisoquinoline from the bark of Litsea lancifolia. Molecules 2011, 16, 3119–3127. [Google Scholar] [CrossRef]
- Alsawalha, M.; Al-Subaie, A.M.; Al-Jindan, R.Y.; Bolla, S.R.; Balakrishna, J.P.; Ravi, P.K.; Gollapalli, S.S.R.; Veeraraghavan, V.P.; Pillai, A.A.; Joseph, J.P.; et al. Effect of Litsea lancifolia leaf extract on glucose transporter 4 translocation and glucose uptake in 3T3L1 cell line. J. Pharm. Bioallied. Sci. 2019, 11, 240–247. [Google Scholar]
- Hossan, S.; Agarwala, B.; Sarwar, S.; Karim, M.; Jahan, R.; Rahmatullah, M. Traditional use of medicinal plants in Bangladesh to treat urinary tract infections and sexually transmitted diseases. Ethnobot. Res. Appl. 2010, 8, 61–74. [Google Scholar] [CrossRef]
- Mollik, M.A. 715 use of medicinal plants for gestational diabetes in Bangladesh: A pragmatic randomized ethnopharmacological survey in Narail District. Pediatr. Res. 2010, 68, 362. [Google Scholar] [CrossRef][Green Version]
- Rahmatullah, M.; Mollik, M.A.H.; Harun-or-Rashid, M.; Tanzin, R.; Ghosh, K.C.; Rahman, H.; Alam, J.; Faruque, M.O.; Hasan, M.M.; Jahan, R.; et al. A comparative analysis of medicinal plants used by folk medicinal healers in villages adjoining the Ghaghot, Bangali and Padma Rivers of Bangladesh. Am. Eurasian J. Sustain. Agric. 2010, 4, 70–85. [Google Scholar]
- Rahmatullah, M.; Mollik, M.A.; Khatun, M.A.; Jahan, R.; Chowdhury, A.R.; Seraj, S.; Hossain, M.S.; Nasrin, D.; Khatun, Z. A survey on the use of medicinal plants by folk medicinal practitioners in five villages of Boalia sub-district, Rajshahi district, Bangladesh. Adv. Nat. Appl. Sci. 2010, 4, 39–44. [Google Scholar]
- Cadar, E. The impact of alkaloids structures from naturalcompounds on public health. Eur. J. Soc. Sci. Educ. Res. 2015, 2, 34–39. [Google Scholar] [CrossRef]
- Ahmmad, A.; Islam, M.T.; Sultana, I.; Mahmood, A.; Hossain, J.A.; Homa, Z.; Ibrahim, M.; Chowdhury, M.M.U. Pharmacological and phytochemical screening of ethanol extract of Litsea monopetala (Roxb.) Pers. IOSR J. Pharm. 2012, 2, 398–402. [Google Scholar]
- Devi, B.; Chutia, M.; Bhattacharyya, N. Food plant diversity, distribution, and nutritional aspects of the endemic golden silk producing silkworm, Antheraea assamensis—A review. Entomol. Exp. Appl. 2021, 169, 237–248. [Google Scholar] [CrossRef]
- Bhuinya, T.; Mukherjee, S.K. Role of four species of Litsea Lam. In Muga Silk Industry. J. Interacademicia 2011, 15, 198–201. [Google Scholar]
- Ghosh, M.; Sinha, B.N. GC-MS studies on the bark extracts of Litsea polyantha Juss. Middle-East J. Sci. Res. 2010, 5, 441–444. [Google Scholar]
- Dzoyem, J.P.; McGaw, L.J.; Kuete, V.; Bakowsky, U. Chapter 9—Anti-inflammatory and anti-nociceptive activities of African medicinal spices and vegetables. In Medicinal Spices and Vegetables from Africa; Kuete, V., Ed.; Academic Press: Cambridge, MA, USA, 2 017; pp. 239–270.
- Bueno, L.; Fioramonti, J. Effects of Inflammatory Mediators on Gut Sensititvity. Can. J. Gastroenterol. 1999, 13, 42A–46A. [Google Scholar] [CrossRef] [PubMed]
- Kissel, C.L.; Kovács, K.J.; Larson, A.A. Evidence for the modulation of nociception in mice by central mast cells. Eur. J. Pain. 2017, 21, 1743–1755. [Google Scholar] [CrossRef]
- Carter, M.; Shieh, J. Chapter 2—Animal behavior. In Guide to Research Techniques in Neuroscience; Academic Press: Cambridge, MA, USA, 2015; pp. 39–71. [Google Scholar]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Dogrul, A.; Gülmez, S.E.; Deveci, M.S.; Gul, H.; Ossipov, M.H.; Porreca, F.; Tulunay, F.C. The local antinociceptive actions of nonsteroidal antiinflammatory drugs in the mouse radiant heat tail-flick test. Anesth. Analg. 2007, 104, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Gouin, O.; L’herondelle, K.; Lebonvallet, N.; Gall-Ianotto, L.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Jara-Oseguera, A.; Simon, S.A.; Rosenbaum, T. TRPV1: On the road to pain relief. Curr. Mol. Pharmacol. 2008, 1, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Quirion, R. Partial sciatic nerve ligation induces increase in the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) in astrocytes in the lumbar spinal dorsal horn and the gracile nucleus. Pain 2002, 99, 175–184. [Google Scholar] [CrossRef]
- Ma, W.; Eisenach, J.C. Four PGE2 EP receptors are up-regulated in injured nerve following partial sciatic nerve ligation. Exp. Neurol. 2003, 183, 581–592. [Google Scholar] [CrossRef]
- Stotz, S.C.; Vriens, J.; Martyn, D.; Clardy, J.; Clapham, D.E. Citral sensing by transient receptor potential channels in dorsal root ganglion neurons. PLoS ONE 2008, 3, e2082. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; Guimarães, A.G.; Santana, M.T.; Araújo, B.E.; Moreira, F.V.; Bonjardim, L.R.; Araújo, A.A.; Siqueira, J.S.; Antoniolli, Â.R.; Botelho, M.A.; et al. Citral reduces nociceptive and inflammatory response in rodents. Rev. Bras. Farmacogn. 2011, 21, 497–502. [Google Scholar] [CrossRef]
- Katsukawa, M.; Nakata, R.; Takizawa, Y.; Hori, K.; Takahashi, S.; Inoue, H. Citral, a component of lemongrass oil, activates PPARα and γ and suppresses COX-2 expression. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 1214–1220. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, H.S.; Kim, D.J.; Noh, Y.H.; Yuk, D.Y.; Hong, J.T. Inhibitory effect of citral on NO production by suppression of iNOS expression and NF-κB activation in RAW264. 7 cells. Arch. Pharm. Res. 2008, 31, 342–349. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- de Cássia da Silveira e Sá, R.; Lima, T.C.; da Nobrega, F.R.; de Brito, A.E.; de Sousa, D.P. Analgesic-like activity of essential oil constituents: An update. Int. J. Mol. Sci. 2017, 18, 2392. [Google Scholar] [CrossRef]
- Liapi, C.; Anifantis, G.; Chinou, I.; Kourounakis, A.P.; Theodosopoulos, S.; Galanopoulou, P. Antinociceptive properties of 1, 8-cineole and β-pinene, from the essential oil of Eucalyptus camaldulensis leaves, in rodents. Planta Med. 2007, 73, 1247–1254. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of (+)-α-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, H.J.; Jeon, Y.D.; Han, Y.H.; Kee, J.Y.; Kim, H.J.; Shin, H.J.; Kang, J.; Lee, B.S.; Kim, S.H.; et al. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef] [PubMed]
| Species/Vernacular Name | Extract | Analgesic/Antinociceptive Study | Other Related Pharmacological Activities Reported | Phytochemicals Reported in the Study | Reference |
|---|---|---|---|---|---|
| Litsea cubeba (Lour.) Pers. | Fruit oil | 50, 100 and 200 mg/kg of L. cubeba fruit oil exerted varying inhibitory effects on acetic-acid-induced torsion but not on thermal stimulation-induced pain. | Anti-inflammatory—symptoms of rheumatoid arthritis ameliorated via regulation of inflammatory cytokines (decreased TNF-α, IL-1β, -6, -8 and -17A levels; increased IL-10 level). | Monoterpenes: α-Citral (26.42%); β-Citral (21.94%); α-Pinene; Limonene (12.79%); β-Myrcene; Eucalyptol; Linalool; Geraniol Sesquiterpenes: γ-Elemene; Cis-nerolidol; Caryophyllene oxide Fatty acids: dodecanoic acid ethyl ester | [21] |
| Fruit oil | 500 mg/kg of L. cubeba fruit oil exhibited antinociceptive activity via prolongation of pain response in tail-flick test (maximum activity at 60 min post-treatment). 100 and 300 mg/kg of fruit essential oil did not show significant effects. | Other neuropharmaological effects—anxiolytic and prolonged pentobarbital-induced sleeping time. | Major compounds: Monoterpenes: α-Citral (geranial) (37.16%); β-Citral (neral) (28.29%); d-Limonene (22.90%) Minor compounds: Monoterpenes: α-Pinene; Camphene; Sabinene; β-Pinene; β-Myrcene; p-Cymene; 1,8-Cineol; Terpinolene; Linalool; Citronellal; α-Terpinyl acetate; Neryl acetate; Geranyl acetate; Sesquiterpenes: α-Copaene; β-Caryophyllene; β-Copaene; Elixene; α-Caryophyllene; Caryophyllene oxide; Other compounds: 6-Methyl-5-hepten-2-one | [72] | |
| Litsea elliptibacea Merr. | Methanolic bark extract | Active against the 5-hydroxytryptamine 1a (5HT1a) CNS receptor (84 ± 1% inhibition), agonism of which inhibits transmission of nociceptive signals and thus induces effective pain relief. Not active against the GABA (GABAB) and dopamine (D2S) receptors. | - | - | [73] |
| Litsea japonica (Thunb.) Jussieu | Fruit extract | Improved MMP-9 levels, joint pain, stiffness and function as shown in a randomized, double-blind, placebo-controlled study. | - | - | [74] |
| 30% ethanolic fruit extract and CH2Cl2 fraction | 50 and 100 mg/kg by weight of the extract, fraction and active compounds each significantly reduced writhing frequency in a dose dependent manner in the acetic-acid-induced writhing test. The extract, fraction and active compounds also increased the tail-flick latency and latency period in the tail flick and hot plate test. | Suppressed inflammatory mediators, including PGE2/COX-2, NO/iNOS and pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α. Inhibited IκB phosphorylation and subsequent nuclear translocation of NF-κB (p65/p50) and suppressed JNK/p38 MAPKs phosphorylation—indicates inhibition of LPS-induced inflammatory responses. | Butenolactones: Hamabiwalactone A and B | [24] | |
| Litsea glaucescens Kunth | Essential oil | Linalool was reported to interact with 5HT1a receptors which inhibits the transmission of nociceptive signals and thus induces effective pain relief. | Showed antidepressant activity in mice subjected to the forced swimming test (FST). | Monoterpenes: Linalool; β-pinene | [75] |
| Litsea glutinosa (Lour.) C.B. Rob. | Methanolic leaf extracts and n-hexane, ethyl acetate and chloroform soluble fractions | 500 mg/kg of the crude methanolic extract exerted maximum pain inhibitory activity in both hotplate (15.54 ± 0.37 sec latency) and acetic-acid-induced writhing test (56.32%). | The methanolic extract demonstrated significant thrombolytic, anti-inflammatory and anti-pyretic activities. | - | [25] |
| 90% methanolic leaf extract | 250 mg/kg and 500 mg/kg body weight of the extract produced 69.57% and 86.96% writhing inhibition respectively in the acetic-acid-induced writhing test. | - | - | [76] | |
| Methanolic leaf extract | 100 mg/L showed 76.65% writhing inhibition in the acetic-acid-induced writhing test | The extract showed low antimicrobial activity against Vibrio mimicus and good antioxidant activity. | - | [77] | |
| Ethanolic leaf extract | 300 mg/kg inhibited the nociception induced by acetic acid by 65% and showed highly significant anti-nociceptive activity in the tail-flick test. | 2000 mg/kg did not show any sign of mortality in the acute toxicity study. | - | [78] | |
| Ethanolic bark extract | 100 and 300 mg/kg of the extract significantly increased the pain threshold in hot plate test. | - | - | [79] | |
| Litsea guatemalensis Mez. | 50% ethanolic leaf extract | Intraperitoneal treatment with 30 mg/kg of extract and 1 mg/kg of isolated isoflavone exhibited prominent anti-hyperalgesic properties in the partial sciatic nerve ligation (PSNL) test model for persistent pain. | Extract and compound showed potent anti-inflammatory action via paw oedema inhibition and inhibition of lymphocyte (mainly neutrophile) influx to the pleural cavity. | Flavonoids: Pinocembrin; Isoflavones: 5,7,3′,4′-Tetrahydroxy-isoflavone; Coumarins: Scopoletin | [26] |
| Litsea lancifolia (Roxb.) Hook. F. | Methanolic leaf extract and petroleum ether, chloroform and ethyl acetate soluble fractions | 100 and 200 mg/kg body weight of the methanolic extract demonstrated significant peripheral analgesic activity with acetic-acid-induced writhing inhibition of 69.45 and 77.96%, respectively. | Ethyl acetate fraction possessed highest total phenolic content and free radical scavenging activity. The fractions showed potential antimicrobial activities against P. aeruginosa, E. coli, B. cereus and S. paratyphi. Methanolic extract showed significant hypoglycemic activity. All fractions exhibited CNS depressant activity. | - | [20] |
| Litsea liyuyingi Liou | Methanolic leaf extract and petroleum-ether, carbon tetrachloride and aqueous soluble fractions | 400 mg/kg methanol extract demonstrated significant in vivo antinociceptive activity in the acetic-acid-induced writhing and tail immersion test. | Methanol extract and its pet-ether fraction exhibited and good thrombolytic and membrane stabilizing effects. Carbon tetrachloride and aqueous fractions showed good membrane stabilizing effects, significant phenolic content and free radical scavenging activities. Extract and fractions showed inhibitory activities against gram positive and Gram-negative bacteria. Methanol extract possessed significant anti-diarrheal and hypoglycemic activity at 400 mg/kg. | Flavonoids, saponins, alkaloids, phenols and tannins | [22] |
| Litsea monopetala (Roxb.) Pers or Litsea polyantha Juss. | Methanolic leaf extract | 500 mg/kg extract significantly inhibited acetic-acid-induced writhing by 68.75%. | Extract showed antioxidant activity (IC50 = 223.22 µg/mL). Extract significantly reduced frequency of castor oil-induced diarrhea in mice. | - | [23] |
| Methanolic leaf extract | 400 mg/kg of extract exhibited 66.67% inhibition of paw licking in mice. | 200 mg/kg and 400 mg/kg extract showed dose-dependent and statistically significant antiemetic activity and excellent CNS depressant activity in both elevated plus maze (EPM) and hole board method. | Terpenes, flavonoids, tannins, saponin and sterols | [80] | |
| Methanolic extract of leaves and petroleum, chloroform and ethyl acetate soluble fractions | 100 and 200 mg/kg b.w. of methanolic extract of L. monopetala showed significant peripheral analgesic activity with writhing inhibition of 33.89 and 38.98%, respectively. | Petroleum ether fraction showed maximum free radical scavenging activity (IC50 = 59.76 ± 0.71 μg/mL). Fractions showed varying antimicrobial activities. 300 mg/kg/day and 500 mg/kg/day doses of the extract significantly decreased blood glucose level on the 5th and 7th day of treatment. 500 mg/kg dose of extract decreased in locomotion of test animals in CNS depressant activity test. | Alkaloids, tannins, saponins, cardiac glycosides and anthraquinone glycosides | [81] | |
| 90% methanolic extract | 50, 75 and 100 mg/kg b.w. of the extract showed significant and dose-dependent central analgesic activity in the tail-flick (22.2–60.4% pain inhibition percentage (PIP)), tail immersion (21.2–67.9% PIP) and hot plate (39.9–100% PIP) tests. | - | Alkaloids and flavonoids | [65] | |
| 90% methanolic extract | 50, 75 and 100 mg/kg b.w. of the extract demonstrated dose-dependent anti-nociceptive activity in acetic-acid-induced writhing tests (34.2–56.5% reduction). | - | - | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, M.P.Y.; Samsul, R.N.; Mohaimin, A.W.; Goh, H.P.; Zaini, N.H.; Kifli, N.; Ahmad, N. The Analgesic Potential of Litsea Species: A Systematic Review. Molecules 2024, 29, 2079. https://doi.org/10.3390/molecules29092079
Goh MPY, Samsul RN, Mohaimin AW, Goh HP, Zaini NH, Kifli N, Ahmad N. The Analgesic Potential of Litsea Species: A Systematic Review. Molecules. 2024; 29(9):2079. https://doi.org/10.3390/molecules29092079
Chicago/Turabian StyleGoh, May Poh Yik, Raudhatun Na’emah Samsul, Amal Widaad Mohaimin, Hui Poh Goh, Nurul Hazlina Zaini, Nurolaini Kifli, and Norhayati Ahmad. 2024. "The Analgesic Potential of Litsea Species: A Systematic Review" Molecules 29, no. 9: 2079. https://doi.org/10.3390/molecules29092079
APA StyleGoh, M. P. Y., Samsul, R. N., Mohaimin, A. W., Goh, H. P., Zaini, N. H., Kifli, N., & Ahmad, N. (2024). The Analgesic Potential of Litsea Species: A Systematic Review. Molecules, 29(9), 2079. https://doi.org/10.3390/molecules29092079









