Study of Molecular Dimer Morphology Based on Organic Spin Centers: Nitronyl Nitroxide Radicals
Abstract
1. Introduction
2. Results
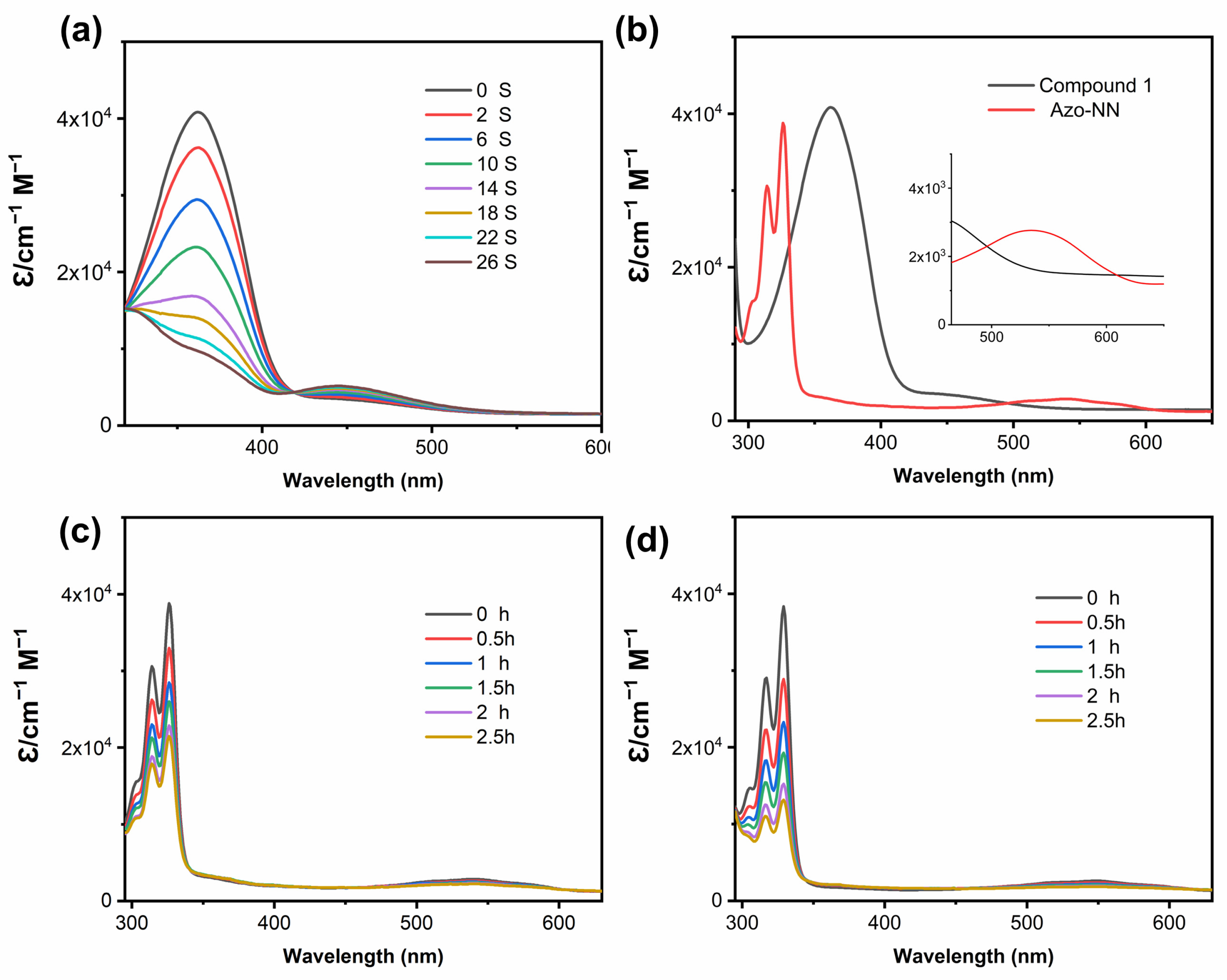
2.1. Optical Properties
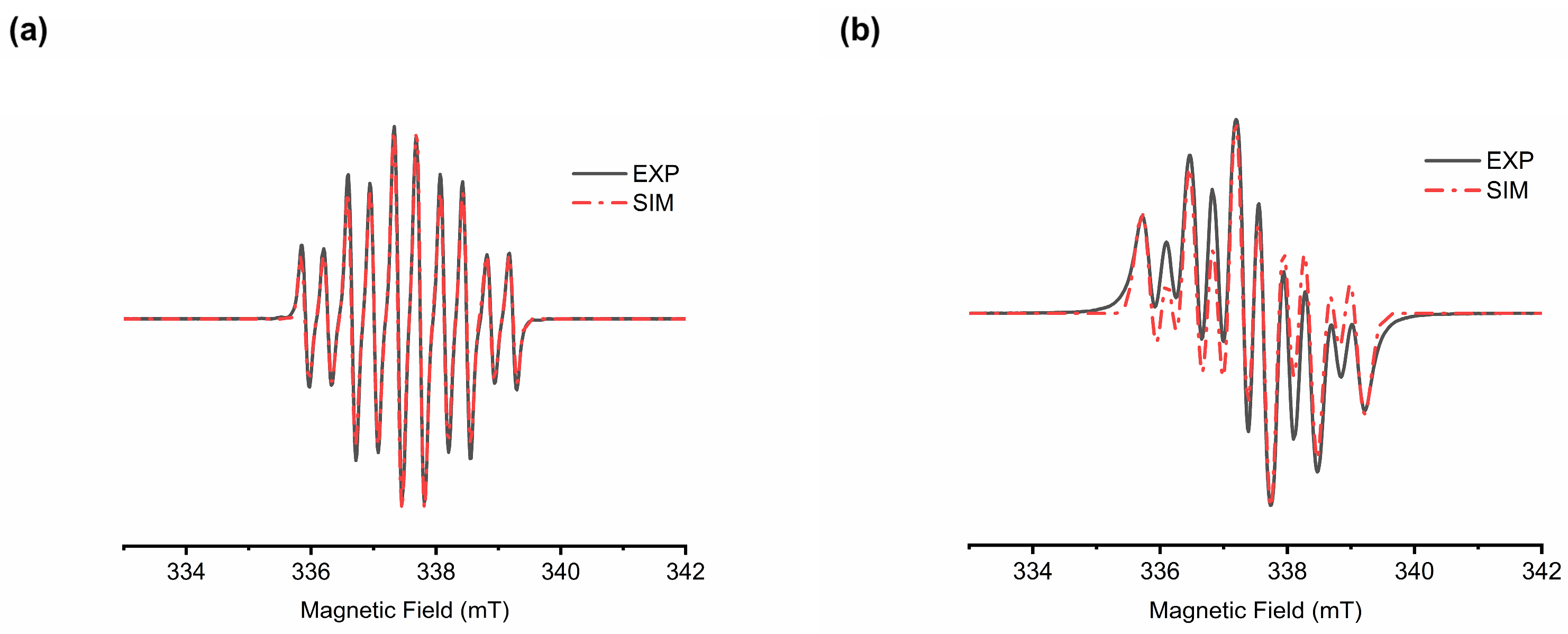
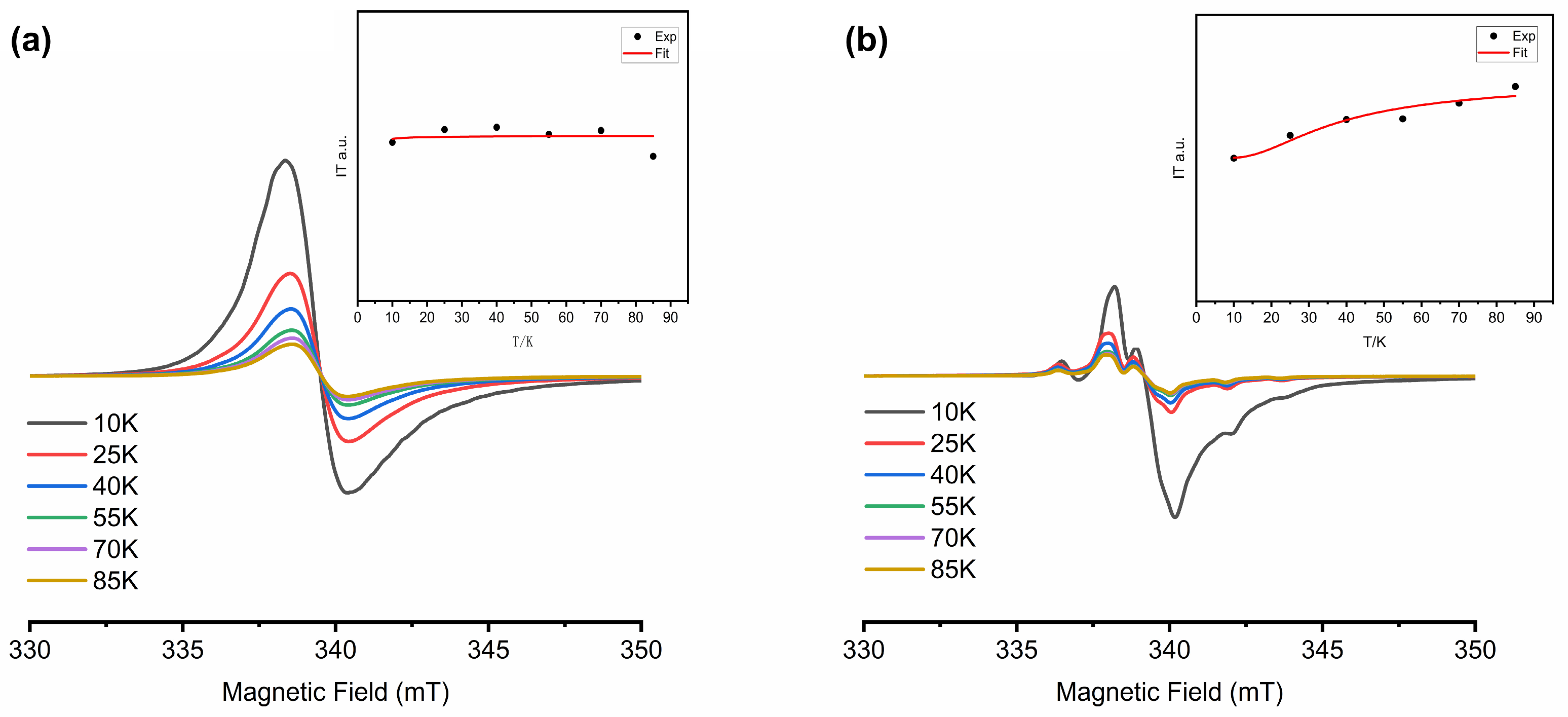
2.2. EPR Spectroscopy
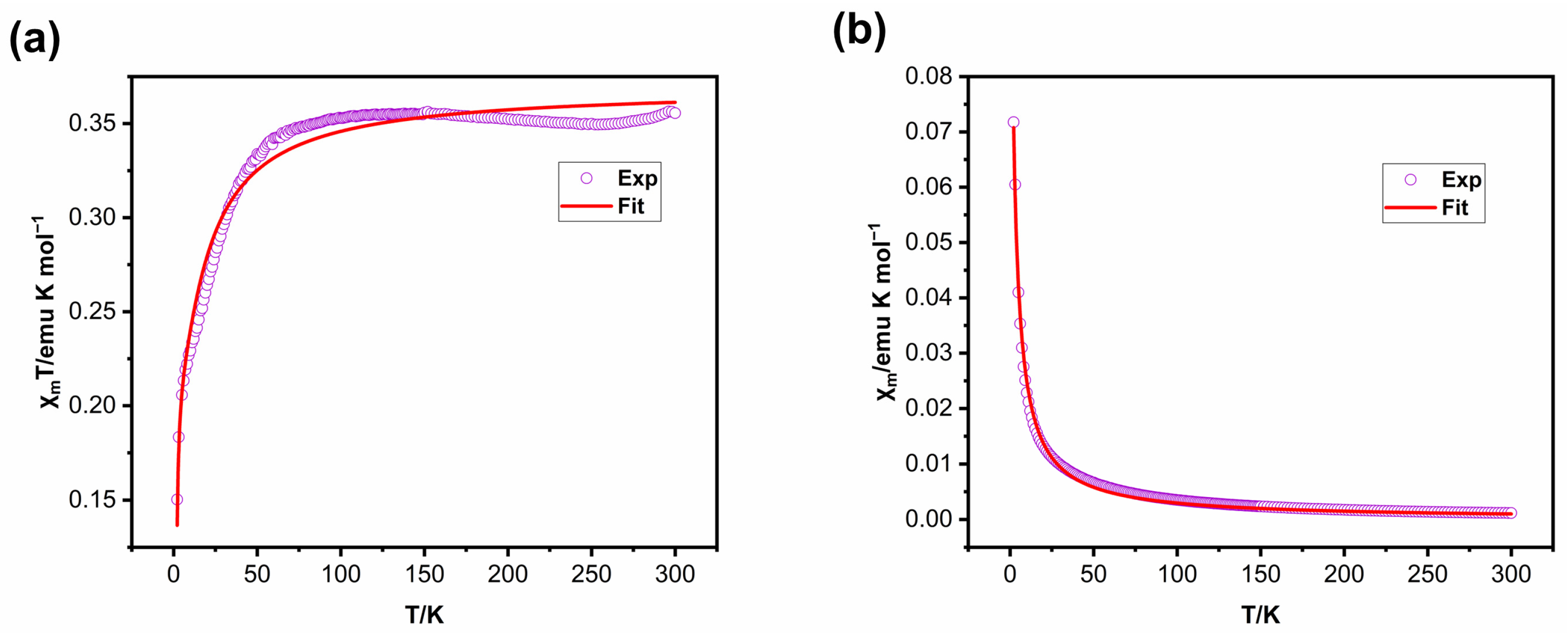
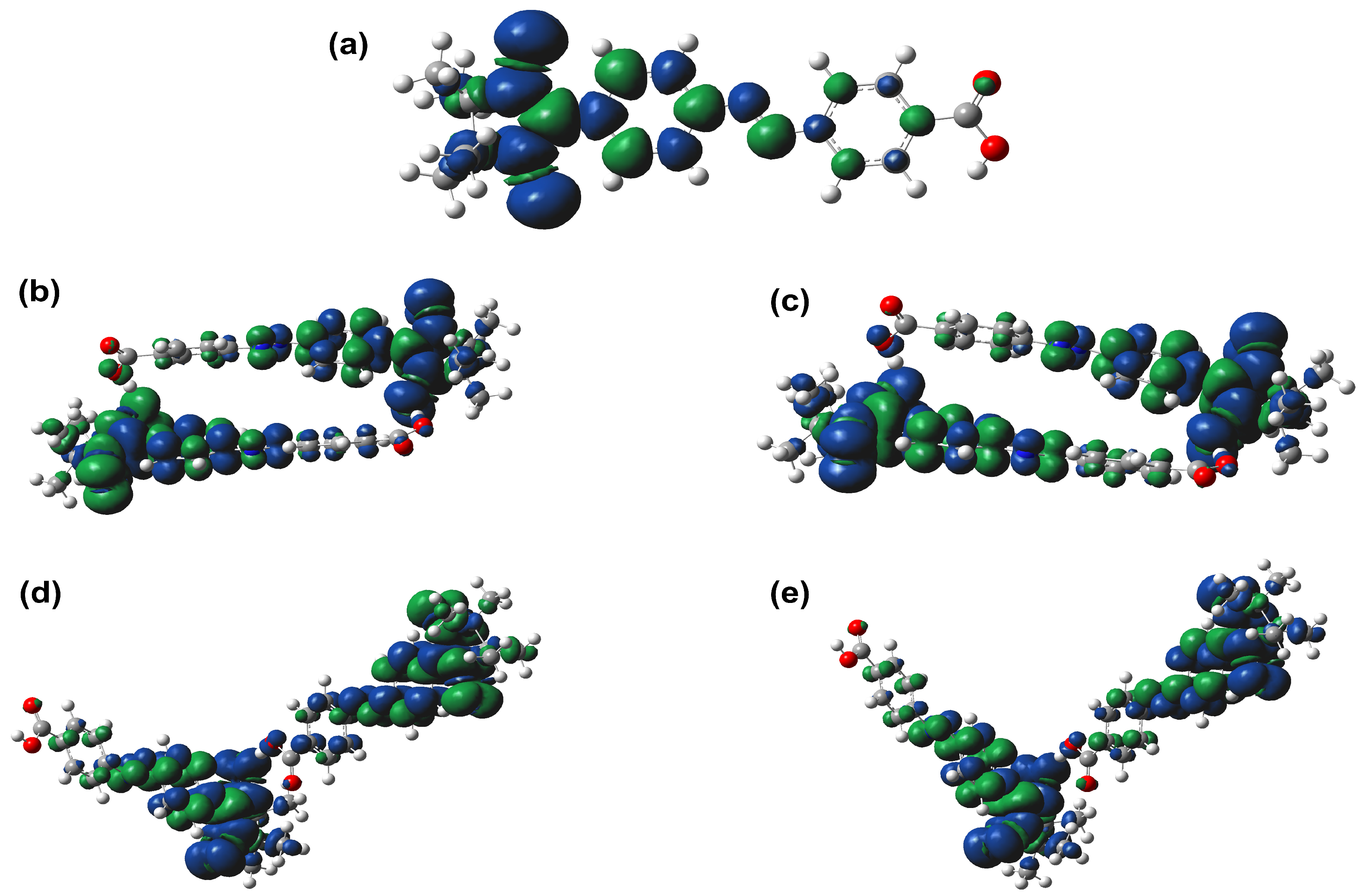
2.3. Magnetic Properties
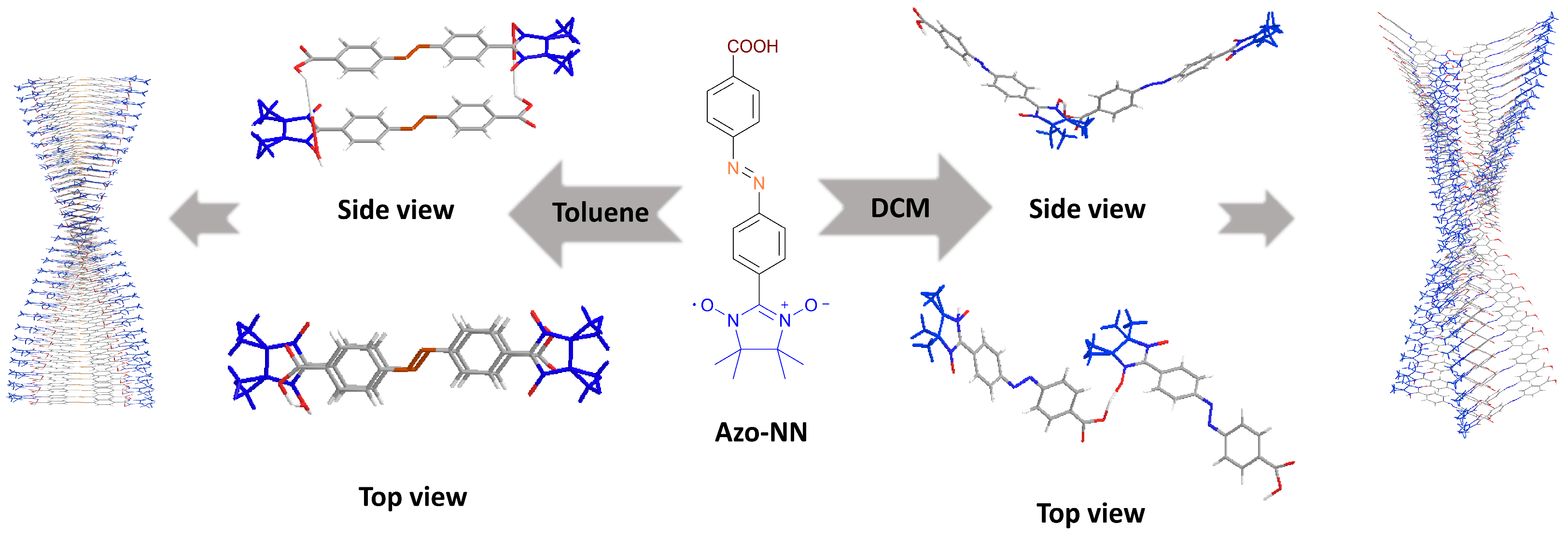
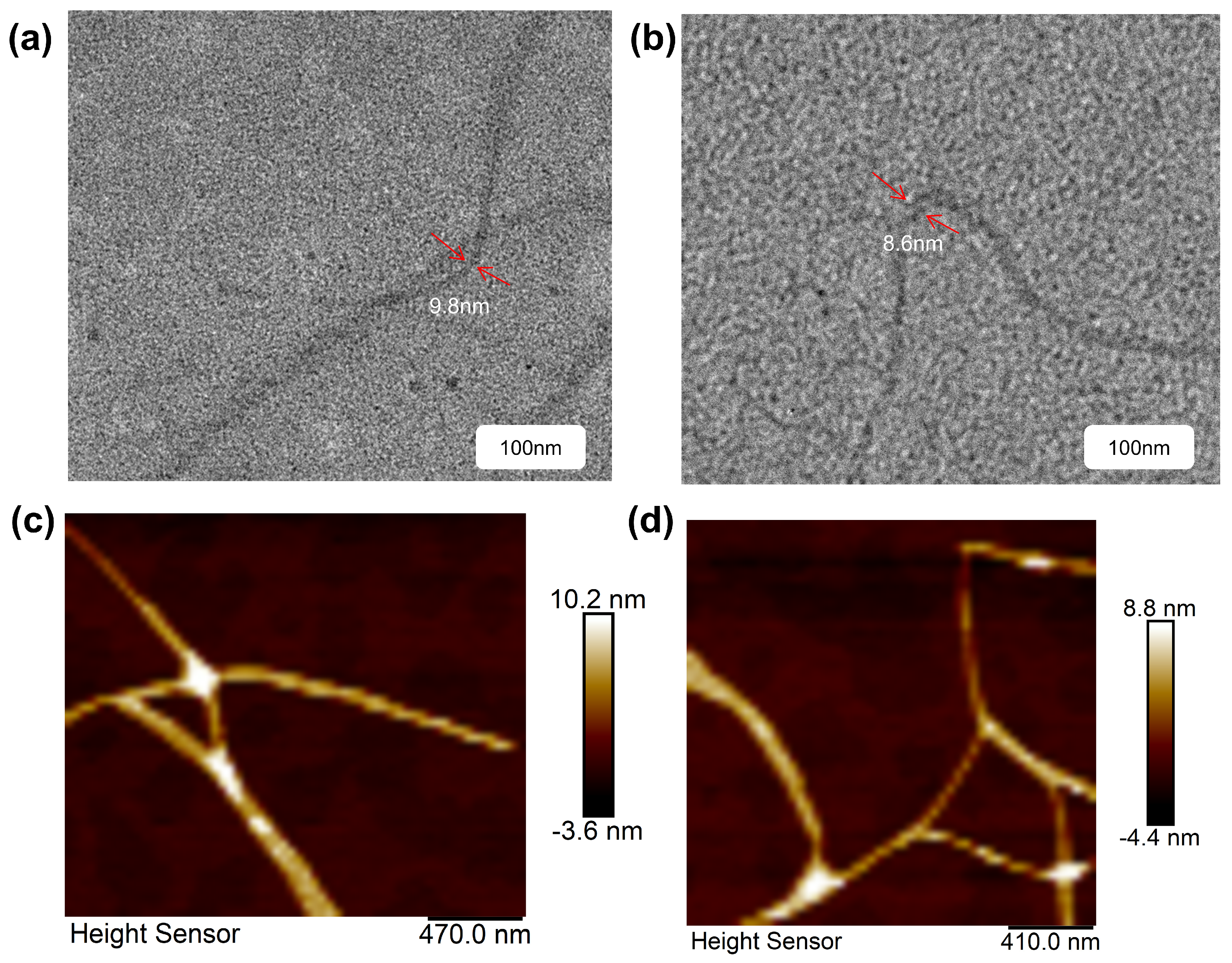
2.4. Characterization of Self-Assembled Structures
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, J.S. Magnetically ordered molecule-based materials. Chem. Soc. Rev. 2011, 40, 3266–3296. [Google Scholar] [CrossRef] [PubMed]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–Organic Framework Magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, K.; Toshimitsu, S.; Kobayashi, Y.; Abe, J. Dynamic Spin–Spin Interaction Observed as Interconversion of Chemical Bonds in Stepwise Two-Photon Induced Photochromic Reaction. J. Am. Chem. Soc. 2021, 143, 13917–13928. [Google Scholar] [CrossRef]
- Abe, M. Diradicals. Chem. Rev. 2013, 113, 7011–7088. [Google Scholar] [CrossRef]
- Hagemann, T.; Winsberg, J.; Häupler, B.; Janoschka, T.; Gruber, J.J.; Wild, A.; Schubert, U.S. A bipolar nitronyl nitroxide small molecule for an all-organic symmetric redox-flow battery. NPG Asia Mater. 2017, 9, e340. [Google Scholar] [CrossRef]
- Borozdina, Y.B.; Mostovich, E.; Enkelmann, V.; Wolf, B.; Cong, P.T.; Tutsch, U.; Lang, M.; Baumgarten, M. Interacting networks of purely organic spin–1/2 dimers. J. Mater. Chem. C 2014, 2, 6618–6629. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, Y.-J.; Chen, K.; Zhou, X.-Y.; Zou, H.; Kang, S.-M.; Liu, N.; Wu, Z.-Q. Reversibly photoswitchable catalysts for high regioselectivity and enantioselectivity in polymerization of allenes. Org. Chem. Front. 2023, 10, 4854–4861. [Google Scholar] [CrossRef]
- Mostovich, E.A.; Borozdina, Y.; Enkelmann, V.; Remović-Langer, K.; Wolf, B.; Lang, M.; Baumgarten, M. Planar Biphenyl-Bridged Biradicals as Building Blocks for the Design of Quantum Magnets. Cryst. Growth Des. 2011, 12, 54–59. [Google Scholar] [CrossRef]
- Yue, Z.; Liu, J.; Baumgarten, M.; Wang, D. Spirobifluorene Mediating the Spin–Spin Coupling of Nitronyl Nitroxide Diradicals. J. Phys. Chem. A 2023, 127, 1565–1575. [Google Scholar] [CrossRef]
- Kolanji, K.; Postulka, L.; Wolf, B.; Lang, M.; Schollmeyer, D.; Baumgarten, M. Planar Benzo[1,2-b:4,5-b’]dithiophene Derivatives Decorated with Nitronyl and Imino Nitroxides. J. Org. Chem. 2019, 84, 140–149. [Google Scholar] [CrossRef]
- Borozdina, Y.B.; Mostovich, E.A.; Cong, P.T.; Postulka, L.; Wolf, B.; Lang, M.; Baumgarten, M. Spin-dimer networks: Engineering tools to adjust the magnetic interactions in biradicals. J. Mater. Chem. C 2017, 5, 9053–9065. [Google Scholar] [CrossRef]
- Stroh, C.; Ziessel, R.; Raudaschl-Sieber, G.; Köhler, F.H.; Turek, P. Intramolecular exchange interactions in non-aromatic bis-nitronyl-nitroxides. J. Mater. Chem. 2005, 15, 850–858. [Google Scholar] [CrossRef]
- Shi, C.; Gao, L.; Baumgarten, M.; Wei, D.; Xu, Z.; Wang, W.; Wang, D. Homoconjugation Mediated Spin-Spin Coupling in Triptycene Nitronyl Nitroxide Diradicals. Magnetochemistry 2023, 9, 178. [Google Scholar] [CrossRef]
- Gallagher, N.M.; Bauer, J.J.; Pink, M.; Rajca, S.; Rajca, A. High-Spin Organic Diradical with Robust Stability. J. Am. Chem. Soc. 2016, 138, 9377–9380. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Pink, M.; Junghoefer, T.; Nadler, E.; Rajca, S.; Casu, M.B.; Rajca, A. Synthesis and Thin Films of Thermally Robust Quartet (S = 3/2) Ground State Triradical. J. Am Chem. Soc. 2021, 143, 5508–5518. [Google Scholar] [CrossRef] [PubMed]
- Tretyakov, E.V.; Zhivetyeva, S.I.; Petunin, P.V.; Gorbunov, D.E.; Gritsan, N.P.; Bagryanskaya, I.Y.; Bogomyakov, A.S.; Postnikov, P.S.; Kazantsev, M.S.; Trusova, M.E.; et al. Ferromagnetically Coupled S=1 Chains in Crystals of Verdazyl-Nitronyl Nitroxide Diradicals. Angew. Chem. Int. Ed. 2020, 59, 20704–20710. [Google Scholar] [CrossRef] [PubMed]
- Vuong, W.; Mosquera-Guagua, F.; Sanichar, R.; McDonald, T.R.; Ernst, O.P.; Wang, L.; Vederas, J.C. Synthesis of Chiral Spin-Labeled Amino Acids. Org. Lett. 2019, 21, 10149–10153. [Google Scholar] [CrossRef] [PubMed]
- Steenbock, T.; Shultz, D.A.; Kirk, M.L.; Herrmann, C. Influence of Radical Bridges on Electron Spin Coupling. J. Phys. Chem. A 2017, 121, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Danila, I.; Riobe, F.; Piron, F.; Puigmarti-Luis, J.; Wallis, J.D.; Linares, M.; Agren, H.; Beljonne, D.; Amabilino, D.B.; Avarvari, N. Hierarchical chiral expression from the nano- to mesoscale in synthetic supramolecular helical fibers of a nonamphiphilic C3-symmetrical pi-functional molecule. J. Am. Chem. Soc. 2011, 133, 8344–8353. [Google Scholar] [CrossRef]
- Gillissen, M.A.; Koenigs, M.M.; Spiering, J.J.; Vekemans, J.A.; Palmans, A.R.; Voets, I.K.; Meijer, E.W. Triple helix formation in amphiphilic discotics: Demystifying solvent effects in supramolecular self-assembly. J. Am. Chem. Soc. 2014, 136, 336–343. [Google Scholar] [CrossRef]
- Gong, Y.; Jiao, T.; Hu, Q.; Cheng, N.; Xu, W.; Bi, Y.; Yu, L. Rational Design of Multiple-Stimulus-Responsive Materials via Supramolecular Self-Assembly. J. Phys. Chem. C 2015, 119, 16349–16357. [Google Scholar] [CrossRef]
- Zhang, C.; Jiao, N. Copper-catalyzed aerobic oxidative dehydrogenative coupling of anilines leading to aromatic azo compounds using dioxygen as an oxidant. Angew. Chem. Int. Ed. 2010, 49, 6174–6177. [Google Scholar] [CrossRef]
- Han, M.R.; Hara, M. Chain length-dependent photoinduced formation of azobenzene aggregates. New J. Chem. 2006, 30, 223–227. [Google Scholar] [CrossRef]
- Huang, X.; Li, T. Recent progress in the development of molecular-scale electronics based on photoswitchable molecules. J. Mater. Chem. C 2020, 8, 821–848. [Google Scholar] [CrossRef]
- Qin, C.; Feng, Y.; An, H.; Han, J.; Cao, C.; Feng, W. Tetracarboxylated Azobenzene/Polymer Supramolecular Assemblies as High-Performance Multiresponsive Actuators. ACS Appl. Mater. Interfaces 2017, 9, 4066–4073. [Google Scholar] [CrossRef]
- Calbo, J.; Weston, C.E.; White, A.J.; Rzepa, H.S.; Contreras-Garcia, J.; Fuchter, M.J. Tuning Azoheteroarene Photoswitch Performance through Heteroaryl Design. J. Am. Chem. Soc. 2017, 139, 1261–1274. [Google Scholar] [CrossRef]
- Lou, X.-Y.; Zhang, S.; Wang, Y.; Yang, Y.-W. Smart organic materials based on macrocycle hosts. Chem. Soc. Rev. 2023, 52, 6644–6663. [Google Scholar] [CrossRef]
- Zhang, X.; Suzuki, S.; Kozaki, M.; Okada, K. NCN Pincer–Pt Complexes Coordinated by (Nitronyl Nitroxide)-2-ide Radical Anion. J. Am. Chem. Soc. 2012, 134, 17866–17868. [Google Scholar] [CrossRef]
- Matsushita, M.M.; Izuoka, A.; Sugawara, T.; Kobayashi, T.; Wada, N.; Takeda, N.; Ishikawa, M. Hydrogen-Bonded Organic Ferromagnet. J. Am. Chem. Soc. 1997, 119, 4369–4379. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, S.; Song, D.; Ye, Z.; Xu, R.; Luo, Y.; Xu, Q. Lipidoid Artificial Compartments for Bidirectional Regulation of Enzyme Activity through Nanomechanical Action. J. Am. Chem. Soc. 2022, 145, 551–559. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, L.; Chen, H.; Ding, Y.; Zheng, Y.-Z.; Miao, M.-s.; Zheng, Y. Air stable high-spin blatter diradicals: Non-Kekulé versus Kekulé structures. J. Mater. Chem. C 2019, 7, 6559–6563. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Jensen, F.; Dorigo, A.; Houk, K.N. A spin correction procedure for unrestricted Hartree-Fock and Møller-Plesset wavefunctions for singlet diradicals and polyradicals. Chem. Phys. Lett. 1988, 149, 537–542. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Y.; Wolf, B.; Kokorin, A.I.; Baumgarten, M. Temperature-Dependent Intramolecular Spin Coupling Interactions of a Flexible Bridged Nitronyl Nitroxide Biradical in Solution. J. Phys. Chem. A 2018, 122, 574–581. [Google Scholar] [CrossRef]

| Dimers | J/kB (K) a | Jab/kB (K) b | JDFT/kB (K) c | ΔES−T (kcal/mol) a | ΔES−T (kcal/mol) b | ΔES−T (kcal/mol) c |
|---|---|---|---|---|---|---|
| Ring-closed dimer | f | 0.18 | 0 | f | 0.0071 | 0 |
| “L”-type dimer | 12.86 | 9.26 | 50.39 | 0.051 | 0.037 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, D.; Qin, Y.; Xu, Z.; Liu, H.; Chen, R.; Yu, Y.; Wang, D. Study of Molecular Dimer Morphology Based on Organic Spin Centers: Nitronyl Nitroxide Radicals. Molecules 2024, 29, 2042. https://doi.org/10.3390/molecules29092042
Wei D, Qin Y, Xu Z, Liu H, Chen R, Yu Y, Wang D. Study of Molecular Dimer Morphology Based on Organic Spin Centers: Nitronyl Nitroxide Radicals. Molecules. 2024; 29(9):2042. https://doi.org/10.3390/molecules29092042
Chicago/Turabian StyleWei, Dongdong, Yongliang Qin, Zhipeng Xu, Hui Liu, Ranran Chen, Yang Yu, and Di Wang. 2024. "Study of Molecular Dimer Morphology Based on Organic Spin Centers: Nitronyl Nitroxide Radicals" Molecules 29, no. 9: 2042. https://doi.org/10.3390/molecules29092042
APA StyleWei, D., Qin, Y., Xu, Z., Liu, H., Chen, R., Yu, Y., & Wang, D. (2024). Study of Molecular Dimer Morphology Based on Organic Spin Centers: Nitronyl Nitroxide Radicals. Molecules, 29(9), 2042. https://doi.org/10.3390/molecules29092042






