Abstract
Carboxylic acids can be isolated from fermentation broths using reactive liquid-liquid extraction, offering an alternative to the environmentally harmful state-of-the-art process of precipitating calcium lactate. To enhance the sustainability of liquid-liquid extraction processes, greener solvents, such as natural deep eutectic solvents, are investigated. However, fermentation broths often exhibit pH values unsuitable for carboxylic acid extraction, which can be adjusted using mineral acids, though mineral acids may be co-extracted. In this study, we systematically examine the co-extraction of hydrochloric, nitric, sulfuric, and phosphoric acid during extraction and back-extraction of lactic acid. The solvent phase consisted of tri-n-octylamine, trioctylphosphine oxide, or tributyl phosphate diluted in a thymol-menthol deep eutectic solvent. The back-extraction was conducted using a diluent swing with p-cymene as the antisolvent and water as the receiving phase. Tri-n-octylamine showed the highest efficiency for lactic acid (up to 29.8%) but also the highest co-extraction of mineral acids (up to 50.9%). In contrast, trioctylphosphine oxide exhibited a lower but more selective lactic acid extraction (5.94%) with low mineral acids co-extraction (0.135%). Overall, the highest co-extraction was observed for phosphoric acid and the lowest for nitric acid. In conclusion, the selected solvent phase composition and mineral acid influence the co-extraction and, thus, final product purity. The successful application of the natural deep eutectic solvent as the modifier enhances the sustainability of liquid–liquid extraction processes.
1. Introduction
Combating environmental pollution and the associated climate change is a present global challenge. To mitigate this crisis, there is growing emphasis on the transition from a fossil-based industry to a bio-based industry. Biorefineries produce valuable chemicals (e.g., carboxylic acids or phenols), fuels, and other materials from sustainable resources, offering a green alternative to fossil-based refineries.
Lactic acid (LA), or 2-hydroxypropanoic acid according to IUPAC, is a bio-based bulk chemical of great interest because of its various applications, e.g., chemical industry, food industry, cosmetics, or monomer for the production of poly-lactic acid [1]. The global LA market was valued at USD 67.9 billion in 2023, with an expected compound annual growth rate of 10.4% until 2032 [2]. About 90% of the global LA production is carried out via fermentation using microorganisms, such as yeast or bacteria. One advantage of the fermentative production pathway is to yield optically pure L- or D-LA; in contrast, the chemical synthesis of LA leads to a racemic mixture [3]. A second advantage of fermentative LA production is that renewable resources or residues, such as corn stover [4], wheat straw [5], or sweet sorghum [6], can be used. The main cost-driving factor in LA production is downstream processing accounting for up to 60% of the overall production costs [7,8]. The state-of-the-art LA downstream processing involves the precipitation of calcium lactate from the fermentation broth using a calcium base and then converting the calcium lactate to LA through acidification with sulfuric acid. In addition to the high chemical demand, this process generates one mole of gypsum sludge per mole of produced LA, resulting in environmental issues [9]. Therefore, alternative isolation methods for LA, such as reactive liquid-liquid extraction (LLE), are investigated [8,10].
In reactive LLE, LA is transferred from the aqueous fermentation broth into an immiscible, organic solvent phase via a reversible reaction between LA and the reactive extractant in the solvent phase. Commonly applied solvent phases for LA extraction consist of a reactive extractant (e.g., tri-n-octylamine (TOA) or trioctylphosphine oxide (TOPO)), a modifier (e.g., octanol or decanol), and/or a diluent (e.g., octane or undecane) [11]. The modifier increases the solubility of the LA-extractant complex, whereas the diluent influences the properties of the solvent phase, such as density or viscosity [10,12]. Reactive extractants are categorized according to their extraction mechanisms, such as anion exchangers (e.g., secondary or tertiary amines) or solvating agents (e.g., phosphine oxides or alkyl-substituted phosphates) [13]. Besides its advantages, such as large production capacity, low energy consumption, and continuous operation, reactive LLE has the drawback of mainly applying fossil-based chemicals [14]. To enhance the sustainability of the process, alternative solvents, such as natural deep eutectic solvents (NADES), are investigated. NADES are eutectic mixtures consisting of two or more naturally occurring compounds. These mixtures show a negative deviation from the ideal eutectic behavior and, thus, exhibit an even stronger melting point depression compared to ideal eutectic mixtures. The melting point depression results from intermolecular interactions, such as hydrogen bonding or van der Waals interactions [15,16]. As NADES consist of naturally derived compounds, they are considered biocompatible, environmentally friendly, and non-toxic [17]. Nevertheless, toxicological studies are still scarce, and the available literature only investigates the toxicity of hydrophilic NADES. However, the available studies show that the toxicity of hydrophilic NADES depends on their constituents and concentration [18,19,20,21,22]. The variety of possible NADES compounds enables the production of task-specific NADES, which can substitute fossil-based solvents in LLE processes to promote sustainable practices within the chemical processing industry [23].
Depending on the applied reactive extractant, either the protonated or deprotonated form of the LA molecule is preferably extracted. For example, the tertiary amine TOA mainly extracts protonated LA molecules by ion-pair formation [6]. This emphasizes the importance of the pH value of the aqueous feed phase together with the pKa value of LA (see Figure 1).
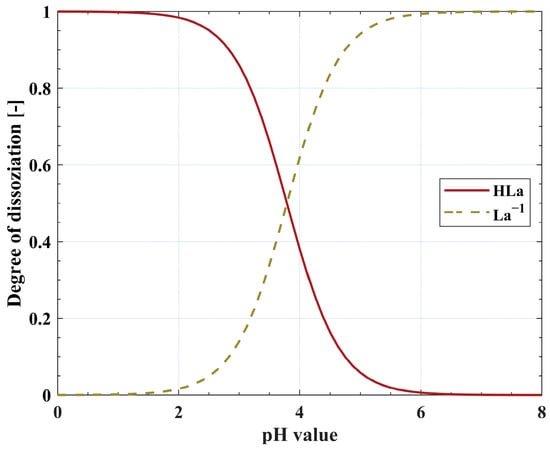
Figure 1.
Dissociation diagram showing the equilibrium distribution between protonated (HLa) and deprotonated LA molecules (La−1) in dependence on the pH value. The pKa value of LA is 3.79 [24].
Industrial LA fermentations are usually carried out at pH values between five and seven, which is well above the pKa value of LA. Thus, the LA molecules are present in their deprotonated form. One exception is the fermentation technology implemented by Cargill in 2008, which uses a genetically modified yeast capable of producing LA at pH values below three [25]. Besides more advanced methods of pH adjustment, such as electrochemical pH swing [26], a common method to adjust the pH value of aqueous solutions is the addition of mineral acids, such as sulfuric acid (H2SO4) [27,28]. Additionally, mineral acids can be found in the aqueous phase, serving as catalysts, for example, in the production of levulinic acid, where H2SO4 is used for the hydrolysis of lignocellulosic materials [29]. Mineral acids are co-extracted to some extent, diminishing the available extractant molecules for the extraction of LA [30].
The investigation of mineral acid co-extraction has been conducted for different reactive LLE systems with fossil-based modifiers and diluents [29,30,31]. However, an open research question is the assessment of mineral acid co-extraction when NADES are applied in reactive LLE. Additionally, research efforts must extend beyond the extraction step to assess the mineral acid co-back-extraction during solvent phase regeneration.
To address this research gap, we investigate the co-extraction of the four mineral acids hydrochloric acid (HCl), nitric acid (HNO3), H2SO4, and phosphoric acid (H3PO4) during reactive LA extraction and back-extraction. As reactive extractants, TOA, TOPO, and tributyl phosphate (TBP) were used, which are commonly applied for carboxylic acid extraction [32,33,34]. The extractants were diluted in a thymol-menthol-based NADES consisting of 60 mol% thymol and 40 mol% menthol (tmDES is further used as a shortcut for this specific NADES). As a benchmark, we employed the frequently used modifier 1-octanol and the green diluent limonene. The back-extraction step was realized by a diluent swing where the extract phase was mixed with an antisolvent, and water was provided as the receiving phase. The antisolvent, a nonpolar compound (e.g., heptane [35]), is not able to accept or donate hydrogen bonds and, thus, reduces the distribution ratio of LA in the solvent phase [36]. As a result, the LA is transferred into the receiving phase. In the present study, we used p-cymene as an antisolvent because it can be produced from renewable resources, e.g., it is a by-product of sulfite pulping [37]. Performing the back-extraction at an elevated temperature of 70 °C further lowers the distribution ratio of LA [34,38,39]. This approach has the advantage, as compared to back-extraction with sodium hydroxide solution [40,41], that the acid is obtained in its protonated form and not in its salt form. Thus, a hydrolysis step to obtain the acid form is not required. The present work provides a systematical approach for the co-extraction of the mineral acids HCl, H2SO4, HNO3, and H3PO4 during both LA extraction and back-extraction, utilizing reactive extractants mixed with a NADES. Substituting the modifier and/or diluent, which account for the major part of the solvent phase, with a NADES enhances the sustainability of the LLE process.
2. Results and Discussion
2.1. Physical Extraction of Mineral Acids
The physical extraction of HCl, HNO3, H2SO4, and H3PO4 by 1-octanol, tmDES, and limonene was investigated using single-acid model solutions containing one of the mentioned mineral acids. The starting concentrations of the single-acid model solutions were 0.449 ± 0.004, 0.438 ± 0.004, 0.443 ± 0.004, and 0.458 ± 0.004 mol·L−1 for HCl, HNO3, H2SO4, and H3PO4, respectively. Table 1 summarizes the initial feed pH value (pHF), raffinate pH value (pHR), and mole-based distribution ratio of the mineral acids for the extraction step (Dextr,mineral).

Table 1.
The feed pH value (pHF), raffinate pH value (pHR), and mole-based distribution ratio (Dextr,mineral) of HCl, HNO3, H2SO4, and H3PO4 for the physical extraction from single-acid model solutions using 1-octanol, tmDES, and limonene at 25 ± 0.5 °C. The volumetric feed-to-solvent phase ratio was one.
The results in Table 1 show that all solvent phases exhibit a low mineral acid extraction with the Dextr,mineral ranging from 0.0359 ± 0.0042 to 0.0877 ± 0.0032. Brouwer et al. [29] reported negligible sulfuric acid extraction for various solvents (e.g., 1-hexanol, 1-octanol, or dodecane) due to the unfavorable solvation of deprotonated acid molecules in the hydrophobic solvents. Despite the presence of hydroxyl groups in 1-octanol and tmDES, no higher Dextr,mineral is observed for these two modifiers as compared to limonene. As a result of the low extraction of mineral acids, the pH value only marginally changes in Table 1.
2.2. Reactive Extraction of Mineral Acids from Single-Acid Model Solutions
To examine the reactive extraction of HCl, HNO3, H2SO4, and H3PO4 from the single-acid model solutions, the reactive extractants TOA, TOPO, and TBP diluted in 1-octanol, tmDES, or limonene were applied. The single-acid model solutions used as the feed phases had the same initial acid concentrations and pHF as in Section 2.1. Table 2 summarizes the results for pHR and Dextr,mineral. The results for extractant loading (zacid) can be found in Table S1.

Table 2.
The raffinate pH value (pHR) and mole-based distribution ratio (Dextr,mineral) for the reactive extraction of HCl, HNO3, H2SO4, and H3PO4 from the single-acid model solutions using TOA, TOPO, or TBP diluted in 1-octanol, tmDES, or limonene at 25 ± 0.5 °C. The volumetric feed-to-solvent phase ratio was one.
Tertiary amines, such as TOA, extract mineral acids via ion pair formation [29]. Furthermore, protonated amine molecules are known to have a higher affinity towards anions of strong acids compared to anions of weak acids [42,43]. Therefore, the highest Dextr,mineral for TOA in Table 2 are attained for HCl and the lowest Dextr,mineral for H3PO4. However, differences between 1-octanol, tmDES, or limonene are observed. The solvent phase TOA:1-octanol shows the highest Dextr,mineral with values of 22.5 ± 0.1, 22.3 ± 0.2, 17.3 ± 0.1, and 16.1 ± 0.0 for HCl, HNO3, H2SO4, and H3PO4, respectively. Besides the polar hydroxyl group in 1-octanol, van der Waals forces between the linear carbon chain of 1-octanol and the octyl chains of TOA result in further stabilization of the acid–amine complex in the extract phase. With tmDES and limonene, a decrease in Dextr,mineral is observed with values of 1.27 ± 0.04 and 1.18 ± 0.01 for HCl, 1.27 ± 0.03 and 1.23 ± 0.01 for HNO3, 0.776 ± 0.008 and 0.686 ± 0.007 for H2SO4, and 0.402 ± 0.009 and 0.386 ± 0.006 for H3PO4, respectively. Interestingly, limonene only shows slightly lower distribution ratios as compared to tmDES although it does not contain hydroxyl groups to stabilize the acid–amine complex. One explanation is that the unsaturated double bonds in the limonene structure contribute to the extraction of mineral acids or improve the stabilization of the acid–amine complex. More detailed investigations regarding the extraction mechanism are required in the future.
In contrast to TOA, the protonation of the two phosphorous-based extractants TOPO [44] and TBP [45,46] is only possible at a high proton activity. The pKa value of TBPH+ is reported to be below one, e.g., Lommelen and Binnemans [46] used a pKa of −0.5 for TBPH+ in their simulations. With the starting pH value of 0.46 ± 0.01 to 1.15 ± 0.01 of the single-acid model solutions, the protonation of TOPO and TBP can be neglected. Consequently, the extraction of mineral acids by these two extractants mainly takes place via hydrogen bonding of the protonated mineral acid molecules to the oxygen atom at the phosphorus atom [29,46,47]. This explains the low Dextr,mineral in the case of HCl, HNO3, and H2SO4 as these acids are mainly present in their deprotonated form at the investigated pH values. When using TOPO or TBP as an extractant, the Dextr,mineral ranges from 0.0100 ± 0.0018 to 0.0746 ± 0.0029 for HCl, from 0.0239 ± 0.0069 to 0.0853 ± 0.0010 in the case of HNO3, and from 0.0332 ± 0.0049 to 0.0888 ± 0.0016 for H2SO4. Slightly higher Dextr,mineral are obtained for H3PO4 (0.114 ± 0.015 to 0.130 ± 0.011), which can be attributed to its pKa value of the first dissociation step of 2.16 [48] and the starting pH value of the H3PO4 single-acid model solution of 1.15 ± 0.01. Based on the dissociation of H3PO4, about 90% of the acid molecules are protonated and, hence, can be extracted by TOPO and TBP.
2.3. Reactive Extraction of Mineral Acids from a Multi-Acid Model Solution
To investigate the effect of the presence of multiple mineral acids in the feed solution on the distribution coefficient, the same solvent phases as in Section 2.2 were used with a model solution containing the four mineral acids HCl, HNO3, H2SO4, and H3PO4. Initial acid concentrations were 0.101 ± 0.001, 0.103 ± 0.001, 0.104 ± 0.001, and 0.105 ± 0.001 mol·L−1 for HCl, HNO3, H2SO4, and H3PO4, respectively. The pHF of the multi-acid model solution was 0.597 ± 0.006. Results for the pHR, total extractant loading (ztot), and Dextr,mineral are summarized in Table 3. The results for zacid are summarized in Table S2.

Table 3.
Raffinate pH value (pHR), total extractant loading (ztot), and mole-based distribution ratio (Dextr,mineral) of HCl, HNO3, H2SO4, and H3PO4 for the reactive extraction from the multi-acid model solution using TOA, TOPO, or TBP diluted in 1-octanol, tmDES, or limonene at 25 ± 0.5 °C. The volumetric feed-to-solvent phase ratio was one.
The comparison of the Dextr,mineral of the four mineral acids when using TOA as an extractant reveals that the highest Dextr,mineral are obtained for the strongest acids HCl and HNO3, followed by H2SO4. H3PO4, the weakest acid, exhibits the lowest Dextr,mineral when using TOA as reactive extractant. Moreover, Dextr,mineral decreases from 1-octanol to tmDES to limonene. Using the solvent phase TOA:1-octanol, Dextr,mineral is 2.06 ± 0.01, 3.26 ± 0.03, 1.09 ± 0.01, and 0.176 ± 0.002 for HCl, HNO3, H2SO4, and H3PO4, respectively, at a ztot of 1.07 ± 0.02. In contrast, the zacid in Table S1, which corresponds to ztot as only one acid is present in the feed phase, for TOA:1-octanol with the single-acid model solutions in Section 2.2 is 2.07 ± 0.00, 2.01 ± 0.00, 2.02 ± 0.00, and 2.09 ± 0.00 for HCl, HNO3, H2SO4, and H3PO4, respectively. This shows that the presence of multiple acids in the feed phase lowers the extractant loading. When diluting TOA in the tmDES or limonene, the Dextr,mineral is 1.82 ± 0.02 and 1.19 ± 0.01 for HCl, 1.87 ± 0.02 and 1.28 ± 0.01 for HNO3, 0.573 ± 0.010 and 0.392 ± 0.002 for H2SO4, and 0.171 ± 0.002 and 0.150 ± 0.019 for H3PO4, respectively. The ztot of 0.933 ± 0.002 for TOA:tmDES and 0.783 ± 0.008 for TOA:limonene shows that also for tmDES and limonene, lower extractant loadings are obtained for the multi-acid solution as compared to the single-acid solutions; zacid for the single-acid solutions in Table S1 ranges from 0.624 ± 0.008 to 1.31 ± 0.01.
The phosphorous-based extractants TOPO and TBP diluted in 1-octanol, tmDES, or limonene show similar Dextr,mineral for all four mineral acids with the Dextr,mineral ranging from 0.0118 ± 0.0037 to 0.0497 ± 0.0019 for HCl, from 0.0209 ± 0.0066 to 0.0551 ± 0.0067 in the case of HNO3, from 0.0231 ± 0.0063 to 0.0487 ± 0.0056 for H2SO4, and from 0.0154 ± 0.0053 to 0.0417 ± 0.0055 for H3PO4. As a result of the low Dextr,mineral, TOPO and TBP exhibit a low ztot with values of 0.0486 ± 0.0062 to 0.0840 ± 0.0096.
In conclusion, Section 2.2 and Section 2.3 show that the tertiary amine TOA shows the highest extraction of the mineral acids HCl, HNO3, H2SO4, and H3PO4. However, TOA diluted in 1-octanol, tmDES, or limonene results in different Dextr,mineral with 1-octanol showing higher Dextr,mineral as compared to tmDES and limonene. In contrast to TOA, the extractants TOPO and TBP show a low mineral acid extraction for 1-octanol, tmDES, and limonene.
2.4. Selectivity of Reactive Lactic Acid Extraction in the Presence of Mineral Acids
To adjust the feed pH value in carboxylic acid extraction, mineral acids, such as H2SO4 [6,49], are commonly applied. However, as shown in Section 2.2 and Section 2.3, especially strong mineral acids are extracted by tertiary amines. This suggests that mineral acid molecules used for pH adjustment in carboxylic acid extraction are co-extracted and, thus, reduce the number of extractant molecules in the solvent phase available for carboxylic acid extraction. As a result, the carboxylic acid extraction is limited, while the optimum pH value for carboxylic acid extraction prevails. To quantify the co-extraction of the mineral acids HCl, HNO3, H2SO4, and H3PO4 during reactive extraction of LA, the extractant TOA diluted in 1-octanol, tmDES, or limonene was applied. In addition, the solvent phase TOPO:tmDES was investigated. The starting concentrations of the model solutions were 0.196 ± 0.003, 0.209 ± 0.002, 0.206 ± 0.002, 0.201 ± 0.002, and 0.203 ± 0.002 mol·L−1 for LA, HCl, HNO3, H2SO4, and H3PO4, respectively. Table 4 summarizes the pHF, pHR, ztot, mole-based distribution ratio of LA for the extraction step (Dextr,LA), Dextr,mineral, and selectivity of the LA extraction (Sextr,LA) for the conducted experiments. The results for zacid can be found in the Supplementary Materials Table S3.

Table 4.
The feed pH value (pHF), raffinate pH value (pHR), total extractant loading (ztot), mole-based distribution ratio of LA (Dextr,LA), mole-based distribution ratio of mineral acids (Dextr,mineral), and selectivity of LA extraction (Sextr,LA) for the reactive extraction of LA from a model solution using TOA, TOPO, or TBP diluted in 1-octanol, tmDES, or limonene at 25 ± 0.5 °C. The model solutions contained LA and one mineral acid, and the volumetric feed-to-solvent phase ratio was one.
For all four mineral acids, the solvent phase TOA:1-octanol exhibits the highest LA extraction. The values for Dextr,LA decrease in the order H3PO4 > HCl > H2SO4 > HNO3. The solvent phase TOA:tmDES exhibits a similar trend as the Dextr,LA decreases in the order H3PO4 > HCl > HNO3 > H2SO4. In terms of mineral acid co-extraction, TOA:1-octanol and TOA:tmDES show similar results. The highest Dextr,mineral is observed for HNO3 with values of 21.5 ± 0.3 and 13.0 ± 0.1 for TOA:1-octanol and TOA:tmDES, respectively. For the other mineral acids, Dextr,mineral is 18.9 ± 0.2 and 7.42 ± 0.37 for HCl, 1.56 ± 0.02 and 1.27 ± 0.01 for H2SO4, and 1.18 ± 0.04 and 0.836 ± 0.008 for H3PO4 when using TOA:1-octanol and TOA:tmDES, respectively. The high Dextr,LA when using H3PO4 for pH adjustment can be explained by the low Dextr,mineral of H3PO4. As a result, fewer TOA molecules are occupied by H3PO4 molecules and, thus, are available for LA extraction. The second highest Dextr,LA is obtained with HCl, although HCl exhibits the second highest Dextr,mineral. This might be explained by the chlorine anion being the smallest anion of the four mineral acids. As a result, the TOA molecule is sterically easier to access by an LA molecule as in the case of the other mineral acids. This makes the overloading of a TOA molecule more likely. Overloading is the extraction of more than one acid molecule per extractant molecule, indicated by an extractant loading higher than one. Interestingly, no clear difference in Dextr,LA between HNO3 and H2SO4 can be observed for TOA:1-octanol and TOA:tmDES regardless of the higher Dextr,mineral of HNO3 as compared to H2SO4. The solvent phase TOA:limonene results in similar Dextr,LA for H2SO4 (0.145 ± 0.002), H3PO4 (0.128 ± 0.012), HCl (0.116 ± 0.003), and HNO3 (0.106 ± 0.006). For the mineral acids, TOA:limonene shows the same trend as the solvent phases TOA:1-octanol and TOA:tmDES with Dextr,mineral decreasing in the order HNO3 > HCl > H2SO4 > H3PO4.
In contrast to the solvent phases containing TOA as the reactive extractant, TOPO:tmDES shows low Dextr,LA (0.0728 ± 0.0036 to 0.0828 ± 0.0023) and low Dextr,mineral (0.0207 ± 0.0004 to 0.0261 ± 0.0002). Brouwer et al. [29] explained the low co-extraction of H2SO4 with TOPO by the fact that TOPO is a solvating extractant as compared to TOA which is an anion-active extractant. The low Dextr,LA, in combination with the even lower values for Dextr,mineral, results in higher Sextr,LA for TOPO:tmDES as compared to the solvent phases containing TOA. Sextr,LA with TOPO:tmDES is 3.64 ± 0.04 for HNO3, 3.56 ± 0.03 for HCl, 3.29 ± 0.03 for H3PO4, and 2.63 ± 0.03 for H2SO4. For solvent phases containing TOA, the highest Sextr,LA are achieved with H3PO4 (0.527 ± 0.005 to 0.948 ± 0.009), followed by H2SO4 (0.122 ± 0.001 to 0.181 ± 0.002), HCl (0.0201 ± 0.0002 to 0.0295 ± 0.0003), and HNO3 (0.0113 ± 0.0001 to 0.0126 ± 0.0001).
The results of this section show that the solvent phase TOPO:tmDES shows a higher selectivity towards LA as compared to solvent phases containing TOA as the reactive extractant but suffer from low Dextr,LA. In contrast, solvent phases with TOA exhibit higher Dextr,LA as compared to TOPO:tmDES but result in high mineral acid co-extraction.
2.5. Back-Extraction of Lactic Acid
The final section of this study examines the back-extraction of LA from the extract phase. This also includes the co-back-extraction of the mineral acids. For the back-extraction experiments, p-cymene was applied as an antisolvent, and distilled water was used for the receiving phase. The experiments were conducted using the extract phases from the extraction experiments in Section 2.4; the acid concentrations in the extract phase are provided in the Supplementary Materials in Table S4. Table 5 summarizes the pH value of the loaded receiving phase (pHLR), mole-based distribution ratio of LA for the back-extraction step (Dback,LA), mole-based distribution ratio of the mineral acids for the back-extraction step (Dback,mineral), and selectivity of the LA back-extraction (Sback,LA).

Table 5.
The pH value of the loaded receiving phase (pHLR), mole-based distribution ratio of LA (Dback,LA), mole-based distribution ratio of the mineral acids (Dback,mineral), and the selectivity of LA back-extraction (Sback,LA) for back-extraction experiments. The extract phases were from experiments performed in Section 2.4, the back-extraction temperature was 70 ± 0.5 °C, and the volumetric phase ratio was 1:2:3 (extract phase:receiving phase:antisolvent).
The Dback,LA in Table 5 ranges from 1.10 ± 0.04 to 4.71 ± 0.14 for the different solvent phases and mineral acids. The comparison of the Dback,LA obtained with TOA:1-octanol (1.77 ± 0.04 to 4.71 ± 0.14) and TOA:tmDES (1.10 ± 0.04 to 3.07 ± 0.11) reveals that Dback,LA is always higher for TOA:1-octanol. This might be explained by tmDES having stronger interactions, such as hydrogen bonding, with LA molecules as compared to 1-octanol, which reduces the back-extractability of LA. The high Dback,LA (2.49 ± 0.20 to 3.79 ± 0.18) obtained with the solvent phase TOA:limonene can be attributed to the hydrophobic nature of limonene leading to a lower stabilization of the acid–amine complex in the extract phase. Thereby, the release of LA into the receiving phase is facilitated. The solvent phase TOPO:tmDES shows good LA back-extraction with a Dback,LA of 2.22 ± 0.15 to 2.68 ± 0.01. However, in this case, the low Dextr,LA of 0.0728 ± 0.0036 to 0.0828 ± 0.0023 in Table 4 must be considered resulting in low LA concentrations in the extract phases.
Dback,mineral for the different solvent phases in Table 5 varies between 0.0263 ± 0.0034 and 53.1 ± 0.5. The lowest Dback,mineral are achieved for the strongest mineral acids HCl (0.0597 ± 0.0039 to 0.668 ± 0.026) and HNO3 (0.0463 ± 0.0019 to 0.451 ± 0.003) followed by H2SO4 (0.0263 ± 0.0034 to 0.896 ± 0.015). In contrast, the weakest acid, OA, exhibits higher Dback,mineral of 0.0624 ± 0.0040 to 53.1 ± 0.5. This leads to the conclusion that the back-extractability of mineral acids increases with decreasing acid strength. Upon comparing Dback,mineral for the different solvent phases, the solvent phase TOPO:tmDES shows the lowest Dback,mineral (0.0263 ± 0.0034 to 0.0624 ± 0.0040), followed by TOA:tmDES (0.0846 ± 0.0010 to 4.07 ± 0.07) and TOA:1-octanol (0.131 ± 0.003 to 15.5 ± 0.4). The solvent phase TOA:limonene shows a higher back-extractability of the mineral acids with a Dback,mineral ranging from 0.451 ± 0.003 to 53.1 ± 0.5. High variations in Dback,LA and Dback,mineral among the solvent phases lead to considerable differences in the Sback,LA, which ranges from 0.0715 ± 0.0026 to 102 ± 10. The lowest Sback,LA (0.0715 ± 0.0026 to 5.53 ± 0.48) is attained with TOA:limonene due to its high Dback,mineral, whereas the solvent phases TOA:1-octanol and TOA:tmDES exhibit a Sback,LA of 0.114 ± 0.001 to 35.9 ± 0.3. The highest Sback,LA (37.6 ± 4.9 to 102 ± 10) is observed with the solvent phase TOPO:tmDES, however, at the same time TOPO:tmDES shows the lowest Dextr,LA (Table 4).
The Dback,mineral and Dback,LA indicate good back-extractability of the mineral acids and LA from the extract phases suggesting good solvent phase recyclability. However, a more detailed investigation of the recyclability of the solvent phase is required in future work.
For a clearer assessment of the extraction and back-extraction of LA and mineral acids, Figure 2 shows the total efficiency for LA (Etot,LA) and the mineral acids (Etot,mineral), which measures the percentages of moles extracted from the feed phase in the extraction step to the receiving phase in the back-extraction step.
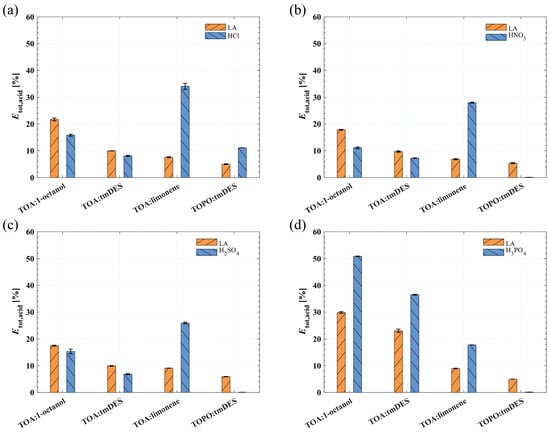
Figure 2.
The total efficiency (Etot,acid) for LA and the mineral acids (a) HCl, (b) HNO3, (c) H2SO4, and (d) H3PO4. The extraction was performed at 25.0 ± 0.5 °C at a volumetric phase ratio of one. The back-extraction was examined at 70.0 ± 0.5 °C at a volumetric phase ratio of 1:2:3 (extract phase:receiving phase:antisolvent).
The results for Etot,LA in Figure 2 show a similar trend for HCl, HNO3, H2SO4, and H3PO4. The Etot,LA decreases from TOA:1-octanol to TOA:tmDES to TOA:limonene to TOPO:tmDES. The highest Etot,LA is achieved with the mineral acid H3PO4 and the solvent phases TOA:1-octanol (29.8 ± 0.3%) and TOA:tmDES (23.1 ± 0.6%). This might lead to the conclusion that H3PO4 is the right choice for pH adjustment for LA extraction. However, the Etot,mineral for H3PO4 in Figure 2d is 50.9 ± 0.1% with TOA:1-octanol and 36.5 ± 0.2% with TOA:tmDES indicating high mineral acid co-extraction. All solvent phases containing TOA as the reactive extractant show a significant co-extraction of HCl, HNO3, H2SO4, and H3PO4. Especially TOA:limonene exhibits high Etot,mineral of 34.1 ± 1.1, 28.0 ± 0.2, 25.9 ± 0.3, and 17.8 ± 0.0 for HCl, HNO3, H2SO4, and H3PO4, respectively. In contrast, the solvent phase TOPO:tmDES shows a lower but more selective LA extraction when using HNO3, H2SO4, or H3PO4 for pH adjustment. An Etot,LA of 5.37 ± 0.18, 5.94 ± 0.08, and 5.02 ± 0.01 with an Etot,mineral of 0.0990 ± 0.0054, 0.0652 ± 0.0117, and 0.135 ± 0.011 is achieved for HNO3, H2SO4, and H3PO4.
3. Materials and Methods
This section summarizes the utilized materials and chemicals. Additionally, the employed analytical techniques and the protocols for conducting extraction and back-extraction experiments are outlined.
3.1. Materials and Chemicals
Table 6 lists all chemicals used in this study. The tmDES consisting of 60 mol% thymol and 40 mol% L-menthol was prepared as described in our previous work [6]. Model solutions were prepared with distilled water.

Table 6.
Chemicals employed in this study.
3.2. Analytical Methods
3.2.1. High-Performance Liquid Chromatography
The LA concentrations in aqueous samples were analyzed using a high-performance liquid chromatography (HPLC) system (Dionex UltiMate 3000 from Thermo Fisher Scientific, Waltham, MA, USA) with a UV–Vis detector (operated at 210 nm) and a REZEX-ROA column (Rezex™ ROA-Organic Acid H+ 8%, LC Column 300 × 7.8 mm, Ea from Phenomenex). The mobile phase (flow rate 0.5 mL·min−1) was a 0.0025 M H2SO4 solution prepared with a 1 N H2SO4 solution (Carl Roth, Karlsruhe, Germany) and ultrapure water (CB1703, Adrona SIA, Riga, Latvia). The column oven temperature (Dionex STH 585 from Thermo Fisher Scientific, Waltham, Massachusetts, USA) was 40 °C. All HPLC samples were diluted with 0.0025 M H2SO4 solution, and DMSO was added as an internal standard.
3.2.2. Ion Chromatography
Chlorine, nitrate, sulfate, and phosphate concentrations were measured in a Dionex Integrion ion chromatography system (IC) with a continuously regenerated anion trap column Dionex CR ATC 600 and a Dionex As-DV autosampler (Thermo Fisher Scientific, Waltham, MA, USA)). For eluent generation, potassium hydroxide from a Dionex EGC 500 KOH eluent generator cartridge and ultra-pure water were used. The system was equipped with a Dionex IonPac AG11-HC (2 × 50 mm) guard column, a Dionex IonPac AS11 HC (2 × 250 mm) analytical column, and a Thermo Scientific (Waltham, MA, USA) conductivity cell with a Dionex ASRS 300 2 mm electrolytically self-regenerating suppressor. Sample dilution was conducted with ultra-pure water. To increase the accuracy of the measurement, formate (TraceCERT® 1000 mg·L−1 formate IC standard, Merck, Darmstadt, Germany) was added as an internal standard.
3.2.3. pH Value, Density, and Water Content Measurement
The pH value of the aqueous samples was measured using a WTW SenTix® 41 electrode with an integrated temperature sensor and a Knick (Berlin, Germany) Portavo® 904(X) PH pH meter. The density of aqueous and organic phases was measured in an Anton Paar (Graz, Austria) DMA 45 density meter connected to a thermostat (±0.5 °C). The water content of organic phases was analyzed in an SI Analytics Titrator TitroLine® 7500 KF with an Aquastar® solvent for volumetric Karl Fischer titration (Supelco®, Merck, Darmstadt, Germany). Hydranal®-Titrant 5 (Honeywell Fluka, Charlotte, NC, USA) was used as a titrant.
3.3. Single-Stage Phase Equilibrium Measurements
Single-stage phase equilibrium measurements were performed in temperature-controlled separatory funnels connected to a thermostat (M3 MS, Lauda, Lauda-Königshofen, Germany) and mounted on a laboratory shaker (SM 25, Edmund Bühler, Bodelshausen, Germany). The phases were mixed for 60 min at 170 rpm, followed by 60 min of gravitational settling. All experiments were performed in duplicate to ensure reproducibility.
3.3.1. Extraction
Three types of feed phases were used in the extraction experiments: a single-acid model solution containing one mineral acid, a multi-acid model solution comprising four mineral acids, and an LA model solution containing LA and one mineral acid. The organic solvent phases consisted of 0.2 mol·L−1 reactive extractant (TOA, TOPO, or TBP) in 1-octanol, tmDES, or limonene. The experiments were conducted with a volumetric phase ratio between the aqueous feed phase and the solvent phase of one (10 mL of each phase), and the temperature was set to 25 ± 0.5 °C. The evaluation of the extraction experiments was conducted using the mole-based distribution ratio Dextr,acid according to Equation (1).
Dextr,acid = (cacid,E · VE)/(cacid,R · VR)
The extract phase volume VE [L] and the raffinate phase volume VR [L] were calculated using mass balances accounting for the volume change due to water transport from the aqueous feed phase into the extract phase, e.g., hydrogen-bonded to the extractant molecules or extracted acid molecules (see Equation (S1) to Equation (S6) in the Supplementary Materials). The solvent phases were assumed to have negligible solubility in the aqueous phase [6]. The acid concentration in the raffinate phase cacid,R [mol·L−1] was measured using HPLC, and the acid concentration in the extract phase cacid,E [mol·L−1] was determined using mass balances (see Equation (S1) to Equation (S6) in the Supplementary Materials) [6]. To evaluate the selectivity of the LA extraction over the extraction of the mineral acids, the selectivity Sextr,LA, as defined by Equation (2), was calculated using the mole-based distribution ratio of LA and the mineral acid, denoted by Dextr,LA and Dextr,mineral, respectively.
Sextr,LA = Dextr,LA/Dextr,mineral
Moreover, the extractant loading zacid and the total extractant loading ztot were calculated according to Equations (3) and (4) using the moles of the respective acid in the extract phase nacid,E [mol] and the moles of extractant in the extract phase nextractant,E [mol].
zacid = nacid,E/nextractant,E
ztot = (Σnacid,E)/nextractant,E
3.3.2. Back-Extraction
The back-extraction experiments were conducted by mixing the extract phase with the antisolvent p-cymene. Distilled water was used as the receiving phase. To improve the recovery of the acids from the extract phase, the back-extraction was performed at an elevated temperature of 70 ± 0.5 °C. The volumetric extract phase-to-antisolvent phase ratio was three, and the volumetric extract phase-to-receiving phase ratio was two (5 mL extract phase, 15 mL antisolvent, and 10 mL receiving phase). The evaluation of the back-extraction experiments was conducted using the mole-based distribution ratio Dback,acid according to Equation (5).
Dback,acid = (cacid,LR · VLR)/(cacid,ASEX · VASEX)
The loaded receiving phase volume VLR [L] and volume of the mixture of extract phase and antisolvent after the back-extraction VASEX [L] were determined by mass balances accounting for the water transport between the phases according to the same principle as for the extraction step. The acid concentration in the loaded receiving phase cacid,LR [mol·L−1] was measured by HPLC, and the acid concentration in the extract phase-antisolvent mixture cacid,ASEX [mol·L−1] was determined by mass balances. The selectivity of the LA back-extraction over the back-extraction of the mineral acid Sback,LA was calculated according to Equation (6), where Dback,LA denotes the mole-based distribution ratio of LA and Dback,mineral denotes the mole-based distribution ration of the respective mineral acid.
Sback,LA = Dback,LA/Dback,mineral
To assess the efficiency of the extraction and back-extraction, the total efficiency Etot [%] was calculated according to Equation (7) using the moles of acid in the loaded receiving phase and the feed phase, denoted by nacid,LR [mol] and nacid,F [mol], respectively.
Etot,acid = nacid,LR/nacid,F
4. Conclusions
In this study, we evaluated the co-extraction of hydrochloric, nitric, sulfuric, and phosphoric acid during reactive extraction of lactic acid from model solutions. The solvent phase consisted of a reactive extractant (tri-n-octylamine, trioctylphosphine oxide, or tributyl phosphate) diluted in a thymol-menthol-based deep eutectic solvent, limonene, or 1-octanol. Moreover, the co-back-extraction of the mineral acids was investigated for the back-extraction of lactic acid using a diluent swing with p-cymene as the antisolvent and water as the receiving phase. The tertiary amine tri-n-octylamine exhibited the highest total lactic acid efficiency (summarizing the extraction and back-extraction) of up to 29.8% but resulted in the highest mineral acid co-extraction of up to 50.9% in single-stage extractions. With trioctylphosphine oxide, a lower but more selective lactic acid extraction (5.94%) was achieved, with a lower mineral acid co-extraction (0.135%). The highest mineral acid co-extraction was observed for phosphoric acid and the lowest for nitric acid. In conclusion, the results show that the solvent phase composition and mineral acid influence the co-extraction and, hence, the final product purity. Moreover, the successful application of the natural deep eutectic solvent as a modifier enhances the sustainability of liquid-liquid extraction processes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29081722/s1, Table S1: Extractant loading (zacid) for HCl, HNO3, H2SO4, and H3PO4 for the reactive extraction from the single-acid model solutions using TOA, TOPO, or TBP diluted in 1-octanol, tmDES, or limonene at 25 ± 0.5 °C. Table S2: Extractant loading (zacid) for HCl, HNO3, H2SO4, and H3PO4 for the reactive extraction from the multi-acid model solution using TOA, TOPO, or TBP diluted in 1-octanol, tmDES, or limonene at 25 ± 0.5 °C. Table S3: Extractant loading for LA (zLA) and the mineral acid (zmineral) for the reactive extraction of LA from a model solution using TOA, TOPO, or TBP diluted in 1-octanol, tmDES, or limonene at 25 ± 0.5 °C. Table S4: LA concentration in the extract phase (cLA,E) and mineral acid concentration in the extract (cmineral,E) for back-extraction experiments.
Author Contributions
Conceptualization, P.D. and M.K.; methodology, P.D. and M.K.; validation, P.D., M.Ć. and M.K.; formal analysis, P.D. and M.Ć.; investigation, M.Ć.; resources, M.K.; data curation, P.D. and M.K.; writing—original draft preparation, P.D.; writing—review and editing, P.D. and M.K.; visualization, P.D.; supervision, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
The work received funding from the Open Access Funding by the Graz University of Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors thank Noé Kautzmann for support in the back-extraction experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Polaris Market Research. Global Bio-Based Chemicals Market Share, Size, Trends, Industry Analysis Report, By Type (Bio-Lubricants, Bio-Solvents, Bioplastics, Bio-Alcohols, Bio-Surfactants, Bio-Based Acids); By End-Use; By Region; Segment Forecast, 2023–2032, New York City, NY, USA, 2023. Available online: https://www.polarismarketresearch.com/industry-analysis/bio-based-chemicals-market (accessed on 13 November 2023).
- Kim, J.; Kim, Y.-M.; Lebaka, V.R.; Wee, Y.-J. Lactic Acid for Green Chemical Industry: Recent Advances in and Future Prospects for Production Technology, Recovery, and Applications. Fermentation 2022, 8, 609. [Google Scholar] [CrossRef]
- Ahring, B.K.; Traverso, J.J.; Murali, N.; Srinivas, K. Continuous fermentation of clarified corn stover hydrolysate for the production of lactic acid at high yield and productivity. Biochem. Eng. J. 2016, 109, 162–169. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Juodeikiene, G.; Damasius, J. Use of wheat straw biomass in production of L-lactic acid applying biocatalysis and combined lactic acid bacteria strains belonging to the genus Lactobacillus. Biocatal. Agric. Biotechnol. 2018, 15, 185–191. [Google Scholar] [CrossRef]
- Demmelmayer, P.; Steiner, L.; Weber, H.; Kienberger, M. Thymol-menthol-based deep eutectic solvent as a modifier in reactive liquid-liquid extraction of carboxylic acids from pretreated sweet sorghum silage press juice. Sep. Purif. Technol. 2023, 310, 123060. [Google Scholar] [CrossRef]
- Komesu, A.; Wolf Maciel, M.R.; Maciel Filho, R. Separation and Purification Technologies for Lactic Acid—A Brief Review. BioResources 2017, 12, 6885–6901. [Google Scholar] [CrossRef]
- Kocks, C.; Görtz, J.; Holtz, A.; Gausmann, M.; Jupke, A. Electrochemical Crystallization Concept for Succinic Acid Reduces Waste Salt Production. Chemie Ingenieur Technik. Chem. Ing. Tech. 2020, 92, 221–228. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Alexandri, M.; Komesu, A.; Venus, J.; Vaz Rossell, C.E.; Maciel Filho, R. Current Advances in Separation and Purification of Second-Generation Lactic Acid. Sep. Purif. Rev. 2020, 49, 159–175. [Google Scholar] [CrossRef]
- Chakraborty, D.; Palani, S.G.; Ghangrekar, M.M.; Wong, J.W.C. Reactive extraction of lactic and acetic acids from leached bed reactor leachate and process optimization by response surface methodology. Environ. Technol. 2023, 1–16. [Google Scholar] [CrossRef]
- Thakre, N. Reactive Extraction of Citric Acid Using Different Extractants: Equilibrium, Kinetics and Modeling. Chem. Biochem. Eng. Q. 2018, 31, 437–446. [Google Scholar] [CrossRef]
- Stas, J.; Alsawaf, H. Liquid—Liquid Extraction of Hydrochloric Acid from Aqueous Solutions by Tri-n-dodecylamine and Tri-n-octylamine/diluents. Period. Polytech. Chem. Eng. 2015, 60, 130–135. [Google Scholar] [CrossRef]
- Bart, H.-J. Reactive Extraction; Springer: Berlin/Heidelberg, Germany, 2001; ISBN 9783642074301. [Google Scholar]
- Chen, H.; Wang, L. Posttreatment Strategies for Biomass Conversion. In Technologies for Biochemical Conversion of Biomass; Chen, H., Wang, L., Eds.; Metallurgical Industry Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK; Beijing, China, 2017; pp. 197–217. ISBN 9780128024171. [Google Scholar]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- van Osch, D.J.G.P.; Dietz, C.H.J.T.; van Spronsen, J.; Kroon, M.C.; Gallucci, F.; van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Vieira Sanches, M.; Freitas, R.; Oliva, M.; Mero, A.; de Marchi, L.; Cuccaro, A.; Fumagalli, G.; Mezzetta, A.; Colombo Dugoni, G.; Ferro, M.; et al. Are natural deep eutectic solvents always a sustainable option? A bioassay-based study. Environ. Sci. Pollut. Res. Int. 2022, 30, 17268–17279. [Google Scholar] [CrossRef]
- Zaib, Q.; Eckelman, M.J.; Yang, Y.; Kyung, D. Are deep eutectic solvents really green?: A life-cycle perspective. Green Chem. 2022, 24, 7924–7930. [Google Scholar] [CrossRef]
- Khorsandi, M.; Shekaari, H.; Mokhtarpour, M.; Hamishehkar, H. Cytotoxicity of some choline-based deep eutectic solvents and their effect on solubility of coumarin drug. Eur. J. Pharm. Sci. 2021, 167, 106022. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Čanak, I.; Panić, M.; Markov, K.; Bubalo, M.C.; Frece, J.; Srček, V.G.; Redovniković, I.R. Antimicrobial, cytotoxic and antioxidative evaluation of natural deep eutectic solvents. Environ. Sci. Pollut. Res. Int. 2018, 25, 14188–14196. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, J.-X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef]
- Dwamena, A.K. Recent Advances in Hydrophobic Deep Eutectic Solvents for Extraction. Separations 2019, 6, 9. [Google Scholar] [CrossRef]
- Novalin, S.; Zweckmair, T. Renewable resources—Green biorefinery: Separation of valuable substances from fluid-fractions by means of membrane technology. Biofuels Bioprod. Bioref. 2009, 3, 20–27. [Google Scholar] [CrossRef]
- Miller, C.; Fosmer, A.; Rush, B.; McMullin, T.; Beacom, D.; Suominen, P. 3.17—Industrial Production of Lactic Acid. In Comprehensive Biotechnology: Principles and Practices in Industry, Agcriculture, Medicine and the Environment, 2nd ed.; Moo-Young, M., Ed.; Elsevier: Amsterdam, The Netherlands; Heidelberg, Germany, 2011; pp. 179–188. ISBN 978-0-08-088504-9. [Google Scholar]
- Gausmann, M.; Gössi, A.; Bertram, F.; Riedl, W.; Schuur, B.; Jupke, A. Electrochemical membrane-assisted pH-swing extraction and back-extraction of lactic acid. Sep. Purif. Technol. 2022, 289, 120702. [Google Scholar] [CrossRef]
- Lan, K.; Xu, S.; Li, J.; Hu, C. Recovery of Lactic Acid from Corn Stover Hemicellulose-Derived Liquor. ACS Omega 2019, 4, 10571–10579. [Google Scholar] [CrossRef] [PubMed]
- Kloetzer, L.; Tucaliuc, A.; Galaction, A.-I.; Caşcaval, D. Fractionation of dicarboxylic acids produced by Rhizopus oryzae using reactive extraction. Sci. Rep. 2022, 12, 2020. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, T.; Blahusiak, M.; Babic, K.; Schuur, B. Reactive extraction and recovery of levulinic acid, formic acid and furfural from aqueous solutions containing sulphuric acid. Sep. Purif. Technol. 2017, 185, 186–195. [Google Scholar] [CrossRef]
- Kyuchoukov, G.; Yankov, D. Theoretical and Experimental Study of Lactic Acid Stripping from Loaded Organic Phase. Ind. Eng. Chem. Res. 2010, 49, 8238–8243. [Google Scholar] [CrossRef]
- Gausmann, M.; Wall, D.; Jupke, A. Reliable Identification of Relevant Factors for the Reactive Extraction of Succinic Acid from Electrolyte Containing Solutions. Solvent Extr. Ion Exch. 2023, 41, 826–853. [Google Scholar] [CrossRef]
- Aimer, M.; Klemm, E.; Langanke, B.; Gehrke, H.; Stubenrauch, C. Reactive Extraction of Lactic Acid by Using Tri-n-octylamine: Structure of the Ionic Phase. Chemistry 2016, 22, 3268–3272. [Google Scholar] [CrossRef] [PubMed]
- Belova, V.V.; Zakhodyaeva, Y.A.; Voshkin, A.A. Extraction of carboxylic acids with neutral extractants. Theor. Found. Chem. Eng. 2017, 51, 786–794. [Google Scholar] [CrossRef]
- Lux, S.; Siebenhofer, M. Investigation of liquid-liquid phase equilibria for reactive extraction of lactic acid with organophosphorus solvents. J. Chem. Technol. Biotechnol. 2013, 88, 462–467. [Google Scholar] [CrossRef]
- Gössi, A.; Burgener, F.; Kohler, D.; Urso, A.; Kolvenbach, B.A.; Riedl, W.; Schuur, B. In-situ recovery of carboxylic acids from fermentation broths through membrane supported reactive extraction using membrane modules with improved stability. Sep. Purif. Technol. 2020, 241, 116694. [Google Scholar] [CrossRef]
- Sprakel, L.; Schuur, B. Solvent developments for liquid-liquid extraction of carboxylic acids in perspective. Sep. Purif. Technol. 2019, 211, 935–957. [Google Scholar] [CrossRef]
- Strömvall, A.-M.; Petersson, G. Terpenes Emitted to Air from TMP and Sulphite Pulp Mills. Holzforschung 1992, 46, 99–102. [Google Scholar] [CrossRef]
- Hong, Y.K.; Hong, W.H.; Han, D.H. Application of reactive extraction to recovery of carboxylic acids. Biotechnol. Bioprocess Eng. 2001, 6, 386–394. [Google Scholar] [CrossRef]
- Liu, L.; Su, B.; Wei, Q.; Ren, X. Selective separation of lactic, malic, and tartaric acids based on the hydrophobic deep eutectic solvents of terpenes and amides. Green Chem. 2021, 23, 5866–5874. [Google Scholar] [CrossRef]
- Demmelmayer, P.; Kienberger, M. Reactive extraction of lactic acid from sweet sorghum silage press juice. Sep. Purif. Technol. 2022, 282, 120090. [Google Scholar] [CrossRef]
- Choudhury, B.; Swaminathan, T. Lactic acid extraction with trioctyl amine. Bioprocess Biosyst. Eng. 1998, 19, 317. [Google Scholar] [CrossRef]
- Brandani, S.; Brandani, V.; Vegliò, F. Extraction of Anions from Aqueous Solutions Using Secondary Amines. Ind. Eng. Chem. Res. 1998, 37, 292–295. [Google Scholar] [CrossRef]
- Genov, L.; Dukov, I. Extraktion von starken einwertigen Suren mit Trioctylamin. Monatsh. Chem. 1972, 103, 1552–1559. [Google Scholar] [CrossRef]
- de Keukeleere, K.; Coucke, S.; de Canck, E.; van der Voort, P.; Delpech, F.; Coppel, Y.; Hens, Z.; van Driessche, I.; Owen, J.S.; De Roo, J. Stabilization of Colloidal Ti, Zr, and Hf Oxide Nanocrystals by Protonated Tri- n -octylphosphine Oxide (TOPO) and Its Decomposition Products. Chem. Mater. 2017, 29, 10233–10242. [Google Scholar] [CrossRef]
- Baaden, M.; Burgard, M.; Wipff, G. TBP at the Water−Oil Interface: The Effect of TBP Concentration and Water Acidity Investigated by Molecular Dynamics Simulations. J. Phys. Chem. B 2001, 105, 11131–11141. [Google Scholar] [CrossRef]
- Lommelen, R.; Binnemans, K. Molecular thermodynamic model for solvent extraction of mineral acids by tri-n-butyl phosphate (TBP). Sep. Purif. Technol. 2023, 313, 123475. [Google Scholar] [CrossRef]
- Dhouib-Sahnoun, R.; Feki, M.; Ayedi, H.F. Liquid−Liquid Equilibria of the Ternary System Water + Phosphoric Acid + Tributyl Phosphate at 298.15 K and 323.15 K. J. Chem. Eng. Data 2002, 47, 861–866. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. (Eds.) CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781498754293. [Google Scholar]
- Caşcaval, D.; Kloetzer, L.; Galaction, A.-I. Influence of Organic Phase Polarity on Interfacial Mechanism and Efficiency of Reactive Extraction of Acetic Acid with Tri- n -octylamine. J. Chem. Eng. Data 2011, 56, 2521–2526. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).