Adsorption of O2 on the Preferred -O-Au Sites of Small Gold Oxide Clusters: Charge-dependent Interaction and Activation
Abstract
1. Introduction
2. Results and Discussion
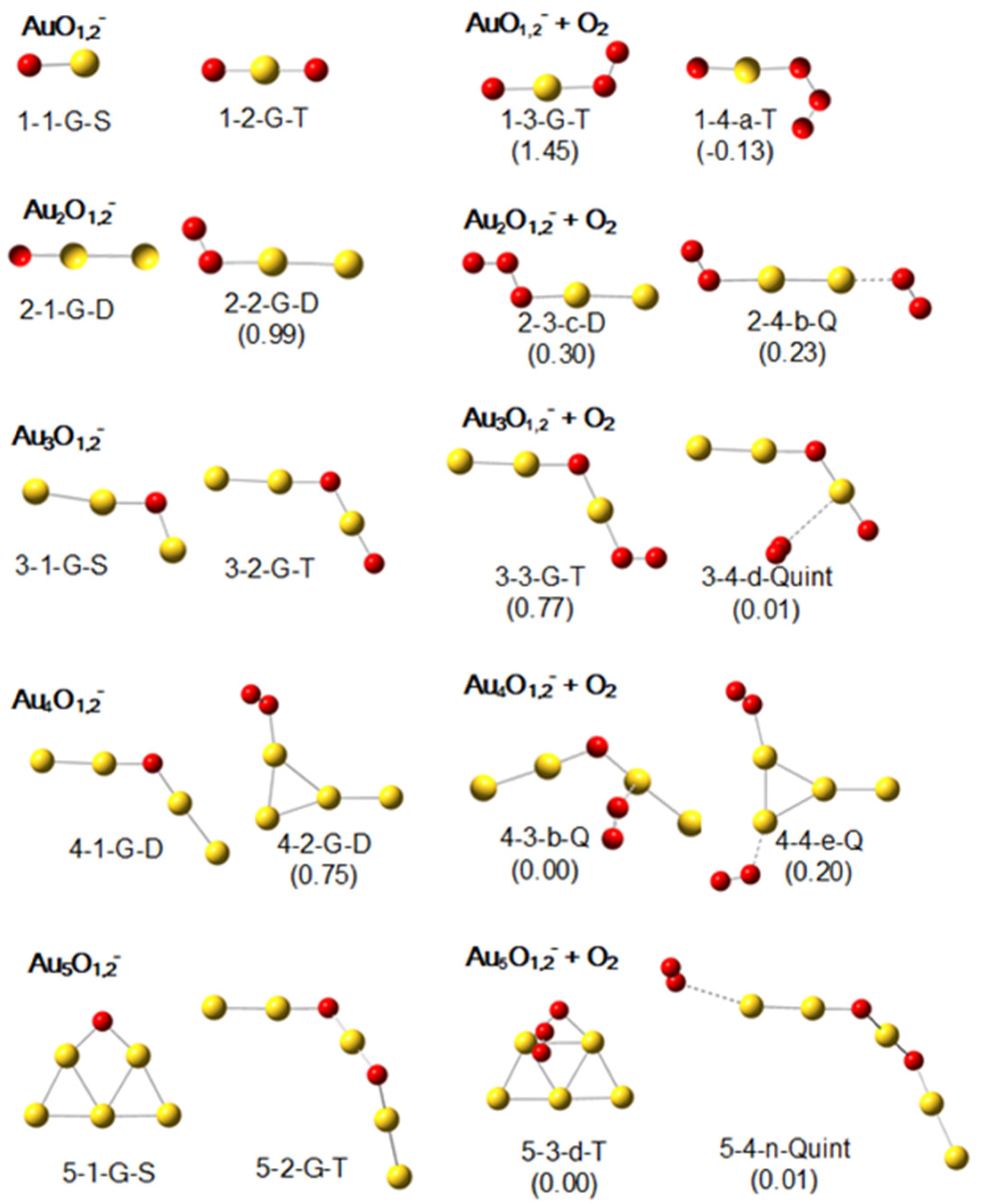
2.1. Geometric Structures of Au1–5O1,2− and Their Products with an O2
2.2. Geometric Structures of Au1–5O1,2+ and Their Products with an O2
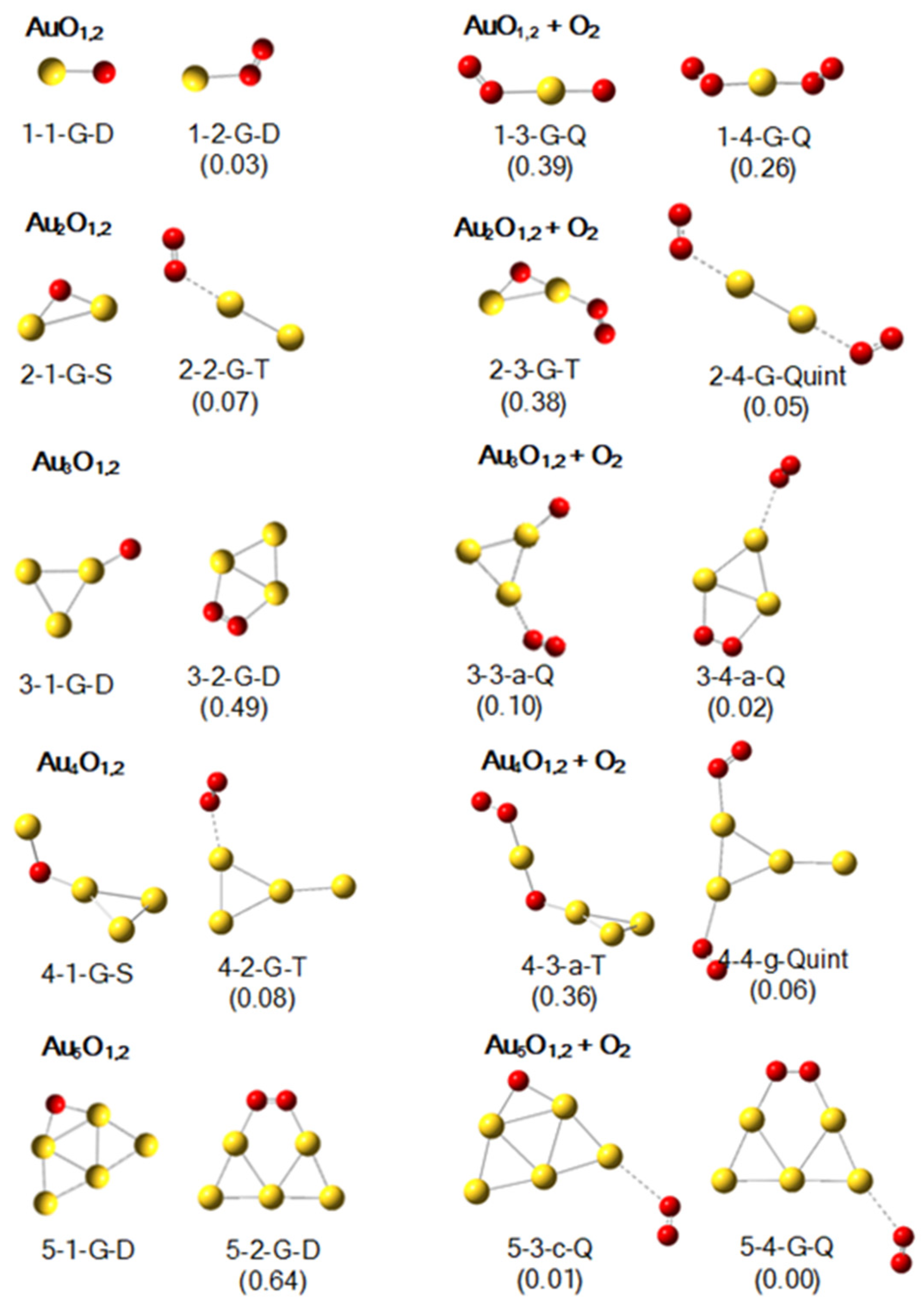
2.3. Geometric Structures of Au1–5O1,20 and Their Products with an O2
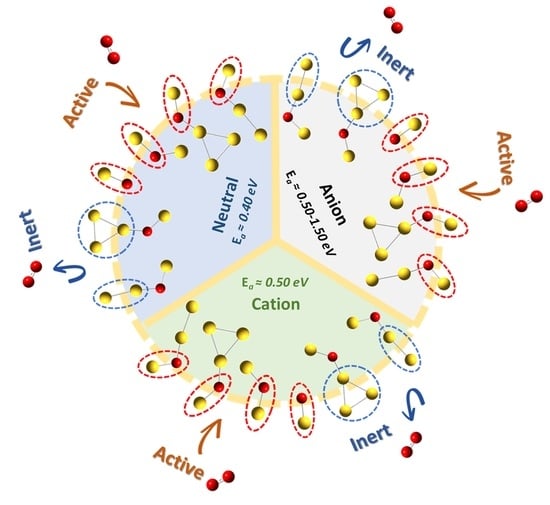
2.4. Charge-Dependent Bonding Strengths and Activation Degrees
2.5. Analyses on the Bonding Patterns
3. Methods
- (1)
- In our search program, we specify the number of gold and oxygen atoms and the multiplicity of the clusters. Based on the complexity of cluster searching, we determine the type and number of initial structures as initial random structures with diverse motifs. We have designed a module capable of generating seven typical motifs for a defined cluster size: the space-free motif, the close packing motif, the simple cubic packing motif, the cage motif, the solid sphere motif, the ring motif, and the specially defined motif through atomic coordinates. The latter allows users to input specially defined or previously reported structures.
- (2)
- The initial random structures undergo relaxation using an incomplete optimization approach and are screened using the competition method under the small basis set we specify. The surviving structures become the offspring of the first generation.
- (3)
- The first-generation results undergo multiple iterations of crossover and mutation under the genetic algorithm framework, generating a substantial number of offspring. After deduplication and competition, the next generation of structures is produced. This cycle continues until a global minimum is attained under the specified convergence limit. The structure optimizations at this stage were performed using a relatively coarse DFT method. Specifically, the B3LYP hybrid functional [80,81] with the LANL2DZ basis set [82] for Au and the 6–31+G* basis set [83,84,85] for O were utilized. For each Au1–5Ox−/+/0 (x = 1–2), the program explored structure candidates in the two lowest-lying spin multiplicities, and for each Au1–5Ox−/+/0 (x = 3–4), the program explored structure candidates in the three lowest-lying spin multiplicities. When conducting a structural search for the system containing three to four O atoms, the randomly generated structures consist of either all the O atoms being randomly dispersed or two of the O atoms combined as an O2 unit being adsorbed on the remaining gold oxide clusters containing a single O atom or two O atoms.
- (4)
- All structures that were relatively stable (within approximately 1.0 eV of the lowest-lying one) underwent further optimization and scrutiny at a more sophisticated theory level, in which the B3LYP hybrid functional in combination with the def2-SVP basis set for Au and the def2-TZVP basis sets for O [86,87] was utilized. Scalar and spin-orbital relativistic effects of Au were addressed through energy consistent relativistic pseudopotentials. The ultimate global minima were validated via vibrational mode analysis, confirming the absence of imaginary frequencies.
- (5)
- The adsorption energies of O2 on specific structures were calculated based on the Hartree–Fock energies corrected by the zero-point energies from frequency analyses. The formula for calculating the adsorption energy is the sum of the energies of the gold oxide cluster and O2, minus the energy of the compound after adsorption. The distribution of charges localized on the adsorbed O2 and the Au atom of the -O-Au sites were examined using the Natural Bond Analysis method [88]. The density of state (DOS) spectrum was obtained by broadening the calculated Kohn–Sham (KS) orbitals from the more sophisticated theory level using the Gaussian function with a FWHM of 0.1 eV. The position of HOMO in the DOS spectrum has been corrected using the clusters’ vertical detachment energy (VDE) values. All DFT calculations were performed using the Gaussian 09 program [89], and the DOS spectra were generated from the calculation results using the Multiwfn software [90].
4. Conclusions
- Regardless of the charge states of gold oxide clusters, the -O-Au sites are inevitably the primary sites for O2 adsorption.
- The charge states of gold oxide clusters determine the bonding strengths and the activation degrees of the adsorbed O2. For anionic gold oxide clusters, the occurrence of electron transfer from the -O-Au sites to the adsorbed O2 leads to the formation of typical chemical bonds and high activation degrees of O2. For both cationic and neutral gold oxide clusters, their interactions with O2 are predominantly electrostatic. More positive charges on the Au atom of -O-Au sites in the cationic clusters lead to stronger binding energies than those of corresponding neutral ones. Meanwhile, the lower electron densities around the Au atom of -O-Au sites in the cationic clusters make electron transfer to O2 more unlikely, and O2 activation on the cationic gold oxide clusters is less effective than those in neutral species.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, A.T. The Impact of Nanoscience on Heterogeneous Catalysis. Science 2003, 299, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Pacchioni, G.; Freund, H.-J. Controlling the Charge State of Supported Nanoparticles in Catalysis: Lessons from Model Systems. Chem. Soc. Rev. 2018, 47, 8474–8502. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Xu, J.; Zhang, Y.; Pan, C.; Dong, Y.; Zhu, Y. Metal-support Interaction for Heterogeneous Catalysis: From Nanoparticles to Single Atoms. Mater. Today Nano 2020, 12, 100093. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Mejia, C.H.; de Jong, K.P. Control of Metal-support Interactions in Heterogeneous Catalysts to Enhance Activity and Selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold Catalysts Prepared by Coprecipitation for Low-temperature Oxidation of Hydrogen and of Carbon Monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Haruta, M. Size- and Support-dependency in the Catalysis of Gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Cunningham, D.A.H.; Vogel, W.; Kageyama, H.; Tsubota, S.; Haruta, M. The Relationship between the Structure and Activity of Nanometer Size Gold When Supported on Mg(OH)2. J. Catal. 1998, 177, 1–10. [Google Scholar] [CrossRef]
- Blick, K.; Mitrelias, T.D.; Hargreaves, J.S.J.; Hutchings, G.J.; Joyner, R.W.; Kiely, C.J.; Wagner, F.E. Methane Oxidation Using Au/MgO Catalysts. Catal. Lett. 1998, 50, 211–218. [Google Scholar] [CrossRef]
- Finch, R.M.; Hodge, N.A.; Hutchings, G.J.; Meagher, A.; Pankhurst, Q.A.; Siddiqui, M.R.H.; Wagner, F.E.; Whyman, R. Identification of Active Phases in Au-Fe Catalysts for Low-temperature CO Oxidation. Phys. Chem. Chem. Phys. 1999, 1, 485–489. [Google Scholar] [CrossRef]
- Widmann, D.; Behm, R.J. Activation of Molecular Oxygen and the Nature of the Active Oxygen Species for CO Oxidation on Oxide Supported Au Catalysts. Acc. Chem. Res. 2014, 47, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Montemore, M.M.; van Spronsen, M.A.; Madix, R.J.; Friend, C.M. O2 Activation by Metal Surfaces: Implications for Bonding and Reactivity on Heterogeneous Catalysts. Chem. Rev. 2018, 118, 2816–2862. [Google Scholar] [CrossRef] [PubMed]
- Staykov, A.; Miwa, T.; Yoshizawa, K. Aerobic Oxidation of Alkanes on Icosahedron Gold Nanoparticle Au55. J. Catal. 2018, 364, 141–153. [Google Scholar] [CrossRef]
- Ke, Y.; Li, X.; Li, J.; Liu, C.-L.; Xu, C.; Dong, W.-S. Conversion of Glycerol to Dihydroxyacetone over Au Catalysts on Various Supports. J. Chem. Technol. Biotechnol. 2020, 95, 1153–1162. [Google Scholar] [CrossRef]
- Shcherbakov, V.; Denisov, S.A.; Mostafavi, M. Selective Oxidation of Transient Organic Radicals in the Presence of Gold Nanoparticles. Nanomaterials 2021, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Deka, R.C.; Bhattacharjee, D.; Chakrabartty, A.K.; Mishra, B.K. Catalytic Oxidation of NO by Au2− Dimers: A DFT Study. Rsc. Adv. 2014, 4, 5399–5404. [Google Scholar] [CrossRef]
- Pal, R.; Wang, L.M.; Pei, Y.; Wang, L.S.; Zeng, X.C. Unraveling the Mechanisms of O2 Activation by Size-selected Gold Clusters: Transition from Superoxo To Peroxo Chemisorption. J. Am. Chem. Soc. 2012, 134, 9438–9445. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Guan, P.; McKenna, K.; Lang, X.; Hirata, A.; Zhang, L.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; Tanaka, N.; et al. Atomic Origins of the High Catalytic Activity of Nanoporous Gold. Nat. Mater. 2012, 11, 775–780. [Google Scholar] [CrossRef]
- Roldan, A.; Manel Ricart, J.; Illas, F.; Pacchioni, G. O2 Adsorption and Dissociation on Neutral, Positively and Negatively Charged Aun(n = 5–79) Clusters. Phys. Chem. Chem. Phys. 2010, 12, 10723–10729. [Google Scholar] [CrossRef]
- Woodham, A.P.; Fielicke, A. Superoxide Formation on Isolated Cationic Gold Clusters. Angew. Chem. Int. Ed. 2014, 53, 6554–6557. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Golovko, V.B.; Vaughan, O.P.H.; Abdulkin, P.; Berenguer-Murcia, A.; Tikhov, M.S.; Johnson, B.F.G.; Lambert, R.M. Selective Oxidation with Dioxygen by Gold Nanoparticle Catalysts Derived from 55-atom Clusters. Nature 2008, 454, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Pacchioni, G. Electronic Interactions and Charge Transfers of Metal Atoms and Clusters on Oxide Surfaces. Phys. Chem. Chem. Phys. 2013, 15, 1737–1757. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.M.; Bernhardt, T.M.; Barnett, R.N.; Landman, U. Temperature-Tunable Selective Methane Catalysis on Au2+: From Cryogenic Partial Oxidation Yielding Formaldehyde to Cold Ethylene Production. J. Phys. Chem. C 2011, 115, 6788–6795. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, X.-N.; He, S.-G. CO Oxidation Promoted by Gold Atoms Loosely Attached in AuFeO3− Cluster Anions. J. Phys. Chem. Lett. 2014, 5, 1585–1590. [Google Scholar] [CrossRef]
- Baishya, S.; Deka, R.C. Catalytic Activities of Au6, Au6−, and Au6+ Clusters for CO Oxidation: A Density Functional Study. Int. J. Quantum. Chem. 2014, 114, 1559–1566. [Google Scholar] [CrossRef]
- Woodham, A.P.; Meijer, G.; Fielicke, A. Charge Separation Promoted Activation of Molecular Oxygen by Neutral Gold Clusters. J. Am. Chem. Soc. 2013, 135, 1727–1730. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, Q.; Yuan, K.; Yang, W.; Chen, Q.; Geng, Z.; Zhang, J.; Shao, X.; Chen, W.; Xu, G.; et al. Unraveling Charge State of Supported Au Single-Atoms during CO Oxidation. J. Am. Chem. Soc. 2018, 140, 554–557. [Google Scholar] [CrossRef]
- Camellone, M.F.; Fabris, S. Reaction Mechanisms for the CO Oxidation on Au/CeO2 Catalysts: Activity of Substitutional Au3+/Au+ Cations and Deactivation of Supported Au+ Adatoms. J. Am. Chem. Soc. 2009, 131, 10473–10483. [Google Scholar] [CrossRef]
- Yoon, B.; Hakkinen, H.; Landman, U.; Worz, A.S.; Antonietti, J.M.; Abbet, S.; Judai, K.; Heiz, U. Charging Effects on Bonding and Catalyzed Oxidation of CO on Au8 Clusters on MgO. Science 2005, 307, 403–407. [Google Scholar] [CrossRef]
- Burgel, C.; Reilly, N.M.; Johnson, G.E.; Mitric, R.; Kimble, M.L.; Castleman, A.W., Jr.; Bonacic-Koutecky, V. Influence of Charge State on the Mechanism of CO Oxidation on Gold Clusters. J. Am. Chem. Soc. 2008, 130, 1694–1698. [Google Scholar] [CrossRef] [PubMed]
- Min, B.K.; Friend, C.M. Heterogeneous Gold-based Catalysis for Green Chemistry: Low-temperature CO Oxidation and Propene Oxidation. Chem. Rev. 2007, 107, 2709–2724. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Choi, E.-A.; Ikoma, Y.; Yu, S.M.; Bae, J.-S.; Lee, S.-G.; Han, S.Z.; Lee, G.-H.; Yun, J. An Unexpected Role of Atomic Oxygen Dopants in Au Evolution from Clusters to A Layer. Acta Mater. 2021, 202, 277–289. [Google Scholar] [CrossRef]
- Li, Y.; Dononelli, W.; Moreira, R.; Risse, T.; Baeumer, M.; Kluener, T.; Moskaleva, L.V. Oxygen-Driven Surface Evolution of Nanoporous Gold: Insights from Ab Initio Molecular Dynamics and Auger Electron Spectroscopy. J. Phys. Chem. C 2018, 122, 5349–5357. [Google Scholar] [CrossRef]
- Baber, A.E.; Torres, D.; Muller, K.; Nazzarro, M.; Liu, P.; Starr, D.E.; Stacchiola, D.J. Reactivity and Morphology of Oxygen-Modified Au Surfaces. J. Phys. Chem. C 2012, 116, 18292–18299. [Google Scholar] [CrossRef]
- Xu, B.; Haubrich, J.; Baker, T.A.; Kaxiras, E.; Friend, C.M. Theoretical Study of O-Assisted Selective Coupling of Methanol on Au(111). J. Phys. Chem. C 2011, 115, 3703–3708. [Google Scholar] [CrossRef]
- Alves, L.; Ballesteros, B.; Boronat, M.; Cabrero-Antonino, J.R.; Concepcion, P.; Corma, A.; Correa-Duarte, M.A.; Mendoza, E. Synthesis and Stabilization of Subnanometric Gold Oxide Nanoparticles on Multiwalled Carbon Nanotubes and Their Catalytic Activity. J. Am. Chem. Soc. 2011, 133, 10251–10261. [Google Scholar] [CrossRef] [PubMed]
- Biener, J.; Biener, M.M.; Nowitzki, T.; Hamza, A.V.; Friend, C.M.; Zielasek, V.; Baeumer, M. On the Role of Oxygen in Stabilizing Low-coordinated Au Atoms. Chemphyschem 2006, 7, 1906–1908. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.A.; Liu, X.; Friend, C.M. The mystery of gold’s chemical activity: Local bonding, morphology and reactivity of atomic oxygen. Phys. Chem. Chem. Phys. 2011, 13, 34–46. [Google Scholar] [CrossRef]
- Qin, R.; Liu, K.; Wu, Q.; Zheng, N. Surface Coordination Chemistry of Atomically Dispersed Metal Catalysts. Chem. Rev. 2020, 120, 11810–11899. [Google Scholar] [CrossRef]
- Zhou, Y.; Perket, J.M.; Zhou, J. Growth of Pt Nanoparticles on Reducible CeO2 (111) Thin Films: Effect of Nanostructures and Redox Properties of Ceria. J. Phys. Chem. C 2010, 114, 11853–11860. [Google Scholar] [CrossRef]
- Tanaka, H.; Neukermans, S.; Janssens, E.; Silverans, R.E.; Lievens, P. Sigma Aromaticity of the Bimetallic Au5Zn+ Cluster. J. Am. Chem. Soc. 2003, 125, 2862–2863. [Google Scholar] [CrossRef]
- Sergeeva, A.P.; Popov, I.A.; Piazza, Z.A.; Li, W.-L.; Romanescu, C.; Wang, L.-S.; Boldyrev, A.I. Understanding Boron through Size-Selected Clusters: Structure, Chemical Bonding, and Fluxionality. Acc. Chem. Res. 2014, 47, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kiran, B.; Cui, L.F.; Wang, L.S. Magnetic Properties in Transition-metal-doped Gold Clusters: M@Au6 (M = Ti, V, Cr). Phys. Rev. Lett. 2005, 95, 253401. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.M.; Bernhardt, T.M. Gas Phase Metal Cluster Model Systems for Heterogeneous Catalysis. Phys. Chem. Chem. Phys. 2012, 14, 9255–9269. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ma, J.; Yin, B.; Xing, X. Adsorption of O2 on Anionic Gold Clusters in the 0–1 nm Size Range: An Insight into the Electron Transfer Dynamics from Kinetic Measurements. J. Phys. Chem. A 2018, 122, 3346–3352. [Google Scholar] [CrossRef]
- Salisbury, B.E.; Wallace, W.T.; Whetten, R.L. Low-temperature Activation of Molecular Oxygen by Gold Clusters: A Stoichiometric Process Correlated to Electron Affinity. Chem. Phys. 2000, 262, 131–141. [Google Scholar] [CrossRef]
- Huang, W.; Zhai, H.-J.; Wang, L.-S. Probing the Interactions of O2 with Small Gold Cluster Anions (Aun−, n = 1–7): Chemisorption vs Physisorption. J. Am. Chem. Soc. 2010, 132, 4344–4351. [Google Scholar] [CrossRef]
- Woodham, A.P.; Meijer, G.; Fielicke, A. Activation of Molecular Oxygen by Anionic Gold Clusters. Angew. Chem. Int. Ed. Engl. 2012, 51, 4444–4447. [Google Scholar] [CrossRef]
- Mills, G.; Gordon, M.S.; Metiu, H. The Adsorption of Molecular Oxygen on Neutral and Negative AunClusters (n = 2–5). Chem. Phys. Lett. 2002, 359, 493–499. [Google Scholar] [CrossRef]
- Yoon, B.; Hakkinen, H.; Landman, U. Interaction of O2 with Gold Clusters: Molecular and Dissociative Adsorption. J. Phys. Chem. A 2003, 107, 4066–4071. [Google Scholar] [CrossRef]
- Sun, Q.; Jena, P.; Kim, Y.D.; Fischer, M.; Gantefor, G. Interactions of Au Cluster Anions with Oxygen. J. Chem. Phys. 2004, 120, 6510–6515. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Lee, K.H.; Lee, G.; Kim, K.S. Geometrical and Electronic Characteristics of AunO2− (n = 2–7). J. Phys. Chem. C 2015, 119, 14383–14391. [Google Scholar] [CrossRef]
- Okumura, M.; Kitagawa, Y.; Haruta, M.; Yamaguchi, K. DFT Studies of Interaction between O2 and Au Clusters. The Role of Anionic Surface Au Atoms on Au Clusters for Catalyzed Oxygenation. Chem. Phys. Lett. 2001, 346, 163–168. [Google Scholar] [CrossRef]
- Shi, H.X.; Sun, W.G.; Kuang, X.Y.; Lu, C.; Xia, X.X.; Chen, B.L.; Hermann, A. Probing the Interactions of O2 with Small Gold Cluster AunQ (n = 2–10, Q = 0, −1): A Neutral Chemisorbed Complex Au5O2 Cluster Predicted. J. Phys. Chem. C 2017, 121, 24886–24893. [Google Scholar] [CrossRef]
- Ding, X.-L.; Liao, H.-L.; Zhang, Y.; Chen, Y.-M.; Wang, D.; Wang, Y.-Y.; Zhang, H.-Y. Geometric and Electronic Properties of Gold Clusters Doped with A Single Oxygen Atom. Phys. Chem. Chem. Phys. 2016, 18, 28960–28972. [Google Scholar] [CrossRef]
- Zhai, H.-J.; Buergel, C.; Bonacic-Koutecky, V.; Wang, L.-S. Probing the Electronic Structure and Chemical Bonding of Gold Oxides and Sulfides in AuOn− and AuSn− (n = 1, 2). J. Am. Chem. Soc. 2008, 130, 9156–9167. [Google Scholar] [CrossRef]
- Kimble, M.L.; Castleman, A.W. Gas-phase Studies of AunOm+ Interacting with Carbon Monoxide. Int. J. Mass Spectrom. 2004, 233, 99–101. [Google Scholar] [CrossRef]
- Kimble, M.L.; Castleman, A.W.; Mitric, R.; Burgel, C.; Bonacic-Koutecky, V. Reactivity of Atomic Gold Anions toward Oxygen and the Oxidation of CO: Experiment and Theory. J. Am. Chem. Soc. 2004, 126, 2526–2535. [Google Scholar] [CrossRef]
- Kimble, M.L.; Castleman, A.W.; Bürgel, C.; Bonačić-Koutecký, V. Interactions of CO with AunOm− (n ≥ 4). Int. J. Mass Spectrom. 2006, 254, 163–167. [Google Scholar] [CrossRef]
- Kimble, M.L.; Moore, N.A.; Johnson, G.E.; Castleman, A.W.; Burgel, C.; Mitric, R.; Bonacic-Koutecky, V. Joint Experimental and Theoretical Investigations of the Reactivity of Au2On− and Au3On− (n = 1–5) with Carbon Monoxide. J. Chem. Phys. 2006, 125, 240311. [Google Scholar] [CrossRef] [PubMed]
- Kimble, M.L.; Moore, N.A.; Castleman, A.W., Jr.; Buergel, C.; Mitric, R.; Bonacic-Koutecky, V. Reactivity of Anionic Gold Oxide Clusters Towards CO: Experiment and Theory. Eur. Phys. J. D. 2007, 43, 205–208. [Google Scholar] [CrossRef]
- Yin, B.; Wang, T.; Chen, Y.; Yang, J.; Wang, G.; Xing, X. Cooperative Bonding Interactions of Various Oxygen Species on the IB Group Metal Anions. Int. J. Mass Spectrom. 2020, 451, 116312. [Google Scholar] [CrossRef]
- Huang, L.; Liu, W.; Hu, J.; Xing, X. Adsorption and Activation of O2 on Small Gold Oxide Clusters: The Reactivity Dominated by Site-Specific Factors. J. Phys. Chem. A 2022, 126, 5594–5603. [Google Scholar] [CrossRef] [PubMed]
- Ricci, D.; Bongiorno, A.; Pacchioni, G.; Landman, U. Bonding Trends and Dimensionality Crossover of Gold Nanoclusters on Metal-Supported MgO Thin Films. Phys. Rev. Lett. 2006, 97, 036106. [Google Scholar] [CrossRef]
- Mäkinen, M.; Laasonen, K. Density Functional Theory Study of Trends in Water Dissociation on Oxygen-preadsorbed and Pure Transition Metal Surfaces. Sur. Sci. 2023, 734, 122305. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Shen, G.; Shen, M.; Zhao, Y.; Cho, K.; Shan, B.; Chen, R. Tuning the Structure of Bifunctional Pt/SmMn2O5 Interfaces for Promoted Low-temperature CO oxidation Activity. Nanoscale 2019, 11, 8150–8159. [Google Scholar] [CrossRef]
- Savchenko, V.I.; Boreskov, G.K.; Kalinkin, A.V.; Salanov, A.N. State of Oxygen on Metal Surfaces and Catalytic Activity for the Oxidation of Carbon Monoxide. Kinet. Catal. 1983, 24, 1154–1161. [Google Scholar]
- Liao, M.-S.; Watts, J.D.; Huang, M.-J. Theoretical Comparative Study of Oxygen Adsorption on Neutral and Anionic Agnand AunClusters (n = 2–25). J. Phys. Chem. C 2014, 118, 21911–21927. [Google Scholar] [CrossRef]
- Ding, X.; Li, Z.; Yang, J.; Hou, J.G.; Zhu, Q. Adsorption Energies of Molecular Oxygen on Au Clusters. J. Chem. Phys. 2004, 120, 9594–9600. [Google Scholar] [CrossRef]
- Ferrari, P.; Janssens, E. Argon Adsorption on Cationic Gold Clusters Aun+ (n ≤ 20). Molecules 2021, 26, 4082. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.; Hou, G.-L.; Lushchikova, O.V.; Calvo, F.; Bakker, J.M.; Janssens, E. The Structures of Cationic Gold Clusters Probed by Far-infrared Spectroscopy. Phys. Chem. Chem. Phys. 2020, 22, 11572–11577. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.; Hussein, H.A.; Heard, C.J.; Vanbuel, J.; Johnston, R.L.; Lievens, P.; Janssens, E. Effect of Palladium Doping on the Stability and Fragmentation Patterns of Cationic Gold Clusters. Phys. Rev. A 2018, 97, 052508. [Google Scholar] [CrossRef]
- Computational Chemistry Comparison and Benchmark DataBase. Available online: https://cccbdb.nist.gov/exp2x.asp?casno=7782447&charge=0 (accessed on 22 May 2022).
- Deaven, D.M.; Ho, K.M. Molecular-Geometry Optimization with a Genetic Algorithm. Phys. Rev. Lett. 1995, 75, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Deaven, D.M.; Tit, N.; Morris, J.R.; Ho, K.M. Structural Optimization of Lennard-Jones Clusters by A Genetic Algorithm. Chem. Phys. Lett. 1996, 256, 195–200. [Google Scholar] [CrossRef]
- Stowers, K.J.; Madix, R.J.; Friend, C.M. From Model Studies on Au(111) to Working Conditions with Unsupported Nanoporous Gold Catalysts: Oxygen-assisted Coupling Reactions. J. Catal. 2013, 308, 131–141. [Google Scholar] [CrossRef]
- Liu, W.; Huang, L.; Meng, L.; Hu, J.; Xing, X. The Global Minimum of Ag30: A Prolate Spheroidal Structure Predicted by the Genetic Algorithm with Incomplete Local Optimizations at a DFT Level. Phys. Chem. Chem. Phys. 2023, 25, 14303–14310. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, W.; Hu, J.; Xing, X. Exploring the Effects of a Doping Silver Atom on Anionic Gold Clusters’ Reactivity with O2. J. Phys. Chem. A 2021, 125, 9995–10005. [Google Scholar] [CrossRef]
- Becke, A. Becke’s Three Parameter Hybrid Method Using the LYP Correlation Functional. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Abinitio Effective Core Potentials for Molecular Calculations—Potentials for K to Au Including the Outermost Core Orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular-Orbital Methods.20. Basis Set for Correlated Wave-Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-Consistent Molecular-Orbital Methods.25. Supplementary Functions for Gaussian-Basis Sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Schafer, A.; Huber, C.; Ahlrichs, R. Fully Optimized Contracted Gaussian-Basis Sets of Triple Zeta Valence Quality for Atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta V alence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural-Population Analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, J.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Schlegel, H.B.; Scalmani, G.; Barone, V.; Mennucci, B.; et al. Gaussian 09, Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]

 ) and other structures (the cross symbols +) with the -O-Au sites in the Supplementary Materials. The results of the anionic, the neutral, and the cationic species are shown by the black, the blue, and the green symbols, respectively.
) and other structures (the cross symbols +) with the -O-Au sites in the Supplementary Materials. The results of the anionic, the neutral, and the cationic species are shown by the black, the blue, and the green symbols, respectively.
 ) and other structures (the cross symbols +) with the -O-Au sites in the Supplementary Materials. The results of the anionic, the neutral, and the cationic species are shown by the black, the blue, and the green symbols, respectively.
) and other structures (the cross symbols +) with the -O-Au sites in the Supplementary Materials. The results of the anionic, the neutral, and the cationic species are shown by the black, the blue, and the green symbols, respectively.

| AunO−/+/0 + O2 Corresponding Pro | Ea (eV) | BLO-O (Å) | ChargeO-O (a.u.) | SpinO-O (a.u.) (a.u.) | |
|---|---|---|---|---|---|
| Anions | 1-3-G-T | 1.45 | 1.329 | −0.720 | 0.986 |
| 3-3-G-T | 0.77 | 1.321 | −0.626 | 1.038 | |
| Cations | 1-3-G-Quint | 0.78 | 1.207 | +0.129 | 1.937 |
| 2-3-G-D | 0.64 | 1.207 | +0.101 | 1.984 | |
| 3-3-G-T | 0.56 | 1.211 | +0.080 | 1.888 | |
| 4-3-G-Q | 0.53 | 1.210 | +0.076 | 1.901 | |
| 5-3-G-T | 0.51 | 1.212 | +0.061 | 1.928 | |
| 1-4-G-Quint | 0.60 | 1.207 | +0.096 | 1.896 | |
| Neutrals | 1-3-G-Q | 0.39 | 1.225 | −0.065 | 1.670 |
| 2-3-G-T | 0.38 | 1.225 | −0.071 | 1.729 | |
| 4-3-a-T | 0.36 | 1.226 | −0.099 | 1.732 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Liu, W.; Xing, X. Adsorption of O2 on the Preferred -O-Au Sites of Small Gold Oxide Clusters: Charge-dependent Interaction and Activation. Molecules 2024, 29, 1645. https://doi.org/10.3390/molecules29071645
Huang L, Liu W, Xing X. Adsorption of O2 on the Preferred -O-Au Sites of Small Gold Oxide Clusters: Charge-dependent Interaction and Activation. Molecules. 2024; 29(7):1645. https://doi.org/10.3390/molecules29071645
Chicago/Turabian StyleHuang, Lulu, Wen Liu, and Xiaopeng Xing. 2024. "Adsorption of O2 on the Preferred -O-Au Sites of Small Gold Oxide Clusters: Charge-dependent Interaction and Activation" Molecules 29, no. 7: 1645. https://doi.org/10.3390/molecules29071645
APA StyleHuang, L., Liu, W., & Xing, X. (2024). Adsorption of O2 on the Preferred -O-Au Sites of Small Gold Oxide Clusters: Charge-dependent Interaction and Activation. Molecules, 29(7), 1645. https://doi.org/10.3390/molecules29071645