Abstract
NMR fingerprints are valuable tools for analyzing complex natural product mixtures and identifying incorrectly assigned structures in the literature. Our diagnostic NMR fingerprints for formyl phloroglucinol meroterpenoids revealed discrepancies in the structures reported for eucalyprobusal C (1a) and eucalypcamal K (2a). NMR fingerprinting PCA analyses identified 1a as an oxepine-diformyl phloroglucinol and 2a as an oxepine 3-acyl-1-formyl phloroglucinol, contrary to their initial assignments as pyrano-diformyl and pyrano 3-acyl-1-formyl phloroglucinols, respectively. Extensive reinterpretation of their reported one- and two-dimensional NMR data, coupled with GIAO DFT-calculated 1H and 13C NMR chemical shift and DP4+ analyses, supported the unequivocal reassignment of eucalyprobusal C to 1b and eucalypcamal K to 2b. The absolute configurations of the revised oxepine-containing phloroglucinol meroterpenoids were confirmed via the reinterpretation of their reported ROESY and NOESY NMR data, along with comparative TDDFT-calculated and experimental ECD spectra.
1. Introduction
Accurately establishing the correct molecular structures of complex natural products (NPs) remains crucial for their exploitation by various disciplines, such as biochemistry, drug discovery, agriculture, synthetic biology, and molecular biology. Despite advances in nuclear magnetic resonance spectroscopy (NMR) and associated resources designed to aid NP structure elucidation and dereplication [1,2,3,4], incorrectly assigned compounds continue to permeate the literature and associated databases [5,6,7,8,9,10]. While semi-synthesis or total synthesis and/or single-crystal X-ray diffraction (XRD) provide important methods for confirming the identity of NP structures, difficulties encountered with both approaches, such as synthesizing complex NP scaffolds and inherent complications in crystallization, make computational methods more appealing and cost-effective. Recently, we illustrated the power of principal component analyses (PCA) and machine learning-generated NMR fingerprints for identifying common subclasses of formyl phloroglucinol meroterpenoids (FPCs) in complex NP mixtures [7]. This resulted in the targeted extract selection of Eucalyptus gittinsii subsp. gittinsii and the subsequent isolation and identification of three pyrano-acyl-formyl phloroglucinol NPs [7]. Moreover, utilizing our diagnostic phloroglucinol NMR fingerprint method, 167 inaccurately reported chemical shifts for 44 phloroglucinol-containing NPs were reassigned [7]. In addition, the structures of three erroneously reported NPs—euglobal In-1, psiguadiol E, and psiguadiol G—were revised, corrections of which were validated using gauge, including atomic orbital (GIAO) density functional theory (DFT) NMR calculations [7].
The genus Eucalyptus resides within the Myrtaceae family of flowering plants and comprises more than 800 species, of which >99% are endemic to Australia. The diversity of Eucalyptus is primarily distributed throughout three subgroups: Eucalyptus (717 species), Corymbia (90 species), and Angophora (12 species) [11]. At the chemotaxonomic level, formyl phloroglucinol meroterpenoids (FPCs) isolated from Eucalyptus contrast with the chemical profiles of sister genera Corymbia and Angophora, both of which contain tetramethyl β-triketone acyl phloroglucinol derivatives [12,13,14,15,16,17]. These findings support Hill and Johnson’s (1995) morphological and phylogenetic taxonomic separation of Corymbia (and close taxonomic relationship with Angophora) [18], despite its formal reclassification as a sub-genera of Eucalyptus by Brooker (2000) [19]. Therefore, the presence or absence of FPCs in Eucalypt extracts offers interesting chemotaxonomic data that contribute to an ongoing taxonomic debate within the speciose genus Eucalyptus and the closely related Eucalypt genera, Corymbia and Angophora. The remarkable chemical diversity of Eucalyptus-derived phloroglucinol NPs, encompassing monomers, dimers, trimers, oligomers, polycyclics, meroterpenoids, xanthones, flavonoids, and coumarins, coupled with their demonstrated bioactivities against a broad range of diseases and infection targets, make them attractive targets for biodiscovery efforts [7,20]. Of particular note are the bioactivities demonstrated by FPCs against the pharmacologically relevant infective-disease-causing targets Staphylococcus aureus [21] and Plasmodium falciparum [22].
Machine/deep learning, a subset of artificial intelligence (AI), employs computational algorithms that can be trained to analyze large and high-dimensional datasets without the need for explicit programming. However, the accuracy of machine/deep learning output analyses relies heavily on the precision of the input data under examination [23]. Unfortunately, with incorrectly assigned NP structures continuing to pollute the literature and associated large NP databases, the accuracy of AI-based computational analyses for aiding NP structure elucidation will likely be compromised. While less computationally expensive NMR fact-checking methods are emerging [3,24], solution state GIAO DFT calculations remain best practice for accurately assessing the connectivity and configuration of NP structures [25,26,27]. In addition, comparative metrics commonly employed to compare the accuracy of DFT NMR-calculated chemical shifts with experimental ones, specifically MAE and RMSD, can be expanded upon with DP4+ Bayesian theorem algorithms [28,29]. These probabilistic algorithms analyze and compare scaled and unscaled 1H and 13C chemical shifts with experimental NMR data, facilitating the resolution of multiple candidate structures for a given NP.
Herein, we present a comprehensive approach employing diagnostic NMR fingerprints of FPCs and GIAO DFT NMR analyses. This unified strategy, alongside the reinterpretation of one- and two-dimensional NMR data and comparative time-dependent functional theory (TDDFT) ECD analyses, allowed for the identification and the reassignment of the planar and three-dimensional structures of two misassigned NPs, eucalyprobusal C (1a) [30] and eucalypcamal K (2a) [31], to oxepine FPCs 1b and 2b, respectively (Figure 1).
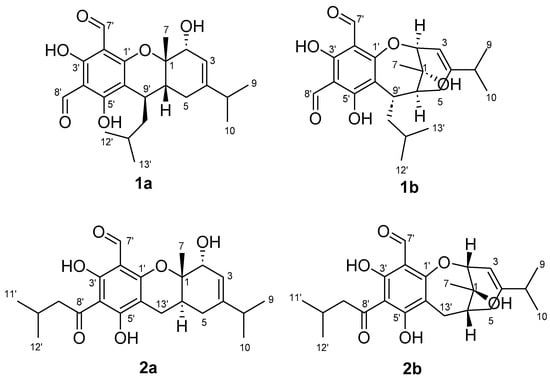
Figure 1.
Incorrectly assigned pyrano formyl phloroglucinol structures reported for eucalyprobusal C (1a) and eucalypcamal K (2a) and their revised oxepine formyl phloroglucinol structures 1b and 2b.
2. Results
2.1. Formyl Phloroglucinol NMR Fingerprinting and PCA Analysis
In a recent publication, we reported the first FPCs containing two spatially separated formyl phloroglucinols conjugated to a terpene core from Eucalyptus camaldulensis [32]. As part of this study, we investigated the structure–activity relationships (SARs) associated with the antibacterial activities of related FPCs. However, during the aforementioned SAR analyses, it became clear that two recently reported FPCs with antibacterial activity, eucalyprobusal C (1a) and eucalypcamal K (2a), were assigned structures inconsistent with their reported NMR data [30,31]. To assess these inconsistencies in more detail, the NMR data (1H and 13C) assigned to the phloroglucinol cores in 1a and 2a were appended to the tabulated NMR data already generated for the 131 FPCs used for our previously reported FPC NMR fingerprints protocol [7]. The tabulated NMR dataset was expanded to include recently published FPCs and now consists of 179 compounds with NMR data reported in CDCl3. The NMR data for 179 FPC’s analyzed via PCA included the six carbons (C-1–C-6) associated with phloroglucinol, aldehyde carbonyl carbons (C-7 and C-9), and associated aldehydic and phenolic protons (Figure 2A). With the PCA output color coded according to the phloroglucinol substructure classes, it was clear that eucalyprobusal C (1a) and eucalypcamal K (2a) occupied regions of PCA space inconsistent with their proposed structure classes (Figure 2B, annotated). Instead, eucalyprobusal C (1a) more closely aligns with oxepine-diformyl phloroglucinols (not pyrano-diformyl phloroglucinols), while eucalypcamal K (2a) is a better match for an oxepine-1-formyl-3-acyl phloroglucinol (not a pyrano 3-acyl-1-formyl phloroglucinol).
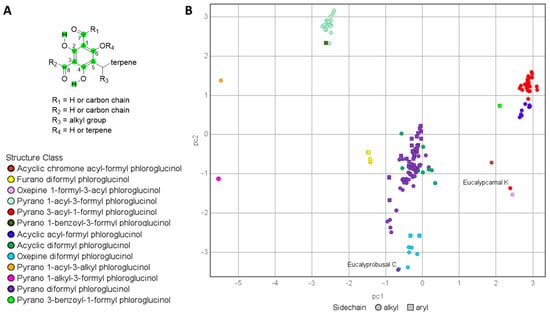
Figure 2.
(A): NMR chemical shifts analyzed via PCA (green = carbon and hydrogen chemical shifts analyzed). (B): PCA analysis of 1H and 13C NMR data for formyl phloroglucinols (n = 179) color coded by sub-structure class. The published structures for eucalyprobusal C and eucalypcamal K (1a and 2a, annotated) do not cluster with other members of their assigned formyl phloroglucinol class, indicative of their structural misassignments.
The 1H and 13C NMR data reported for eucalyprobusal C (1a) and eucalypcamal K (2a) were compared with the diagnostic NMR chemical shifts ranges for subclasses of FPCs reported in the Supplementary Materials of our NMR fingerprinting protocol (adapted Figure S1) [7]. Eucalyprobusal C (1a) displayed markedly better alignment with the NMR fingerprint data ranges associated with oxepine-formyl phloroglucinols in contrast with pyrano-diformyl phloroglucinol NPs (Table S9). Phloroglucinol carbons C-2′ and C-6′ and formyl carbonyl carbon C-7′ in 1a were exceptionally diagnostic and displayed large deviations from the chemical shift ranges for these positions in pyrano-diformyl phloroglucinols (13C = 1.0–12.5 ppm). Moreover, eucalypcamal K (2a) was a more suitable match with the 1H and 13C NMR phloroglucinol fingerprint data for oxepine-formyl phloroglucinols compared with its assignment as a pyrano-3-acyl-1-formyl phloroglucinol (Table S10). Carbons C-1′, 2′, 4′, 6′, and aldehyde C-7′ in 2a contained large chemical shift deviations from the ranges associated with 3-acyl-1-formyl phloroglucinols (13C = 1.4–13.8 ppm). The power and utility of FPC fingerprinting is effectively demonstrated herein, with eucalyprobusal C (1a) and eucalypcamal K (2a) identified as containing structures inconsistent with their proposed structure classes. Moreover, this method contains important predictive capabilities, leading to the re-evaluation of their likely chemical structures as oxepine-diformyl and oxepine-1-formyl-3-acyl phloroglucinols, respectively.
2.2. Reanalysis of One- and Two-Dimensional NMR Data Reported for Eucalyprobusal C and Eucalypcamal K
To confirm our NMR FPC fingerprint analyses and the true structural identities of eucalyprobusal C and eucalypcamal K , their experimental NMR data were thoroughly reanalyzed and compared with the NMR data reported for related FPCs. NMR spectroscopic similarities for the terpenoid sub-structures (MAE = 1.2) of eucalyprobusal C (1a, C-1 to C-10 and C-9′) and eucalypcamal K (2a, C-1 to C-10 and C-13′) advocated for identical terpene substructures, with the exception of an alkyl-substituted methine in 1a instead of a methylene in 2a. In addition, eucalypcamal K (2a) exhibited significant chemical shift differences compared with co-isolated eucalypcamal L (4), a pyrano 3-acyl-1-formyl phloroglucinol and proposed diastereomer of 2a (Figure 3) [31]. Consistent with our PCA and FPC NMR chemical shift analyses above (Figure 2B and Table S10), distinct NMR chemical shift differences between 2a and 4 were evident for phloroglucinol carbons C-2′ (δC 108.4 vs. 103.8), C-4′ (δC 105.6 vs. 103.8), and C-6′ (δC 99.3 vs. 112.3), as well as the aldehyde carbonyl carbon C-7′ (δC 193.4 and 191.8). In addition, terpenoid carbons C-1 (δC 72.4 vs. 81.7), C-2 (δC 77.9 vs. 69.2), C-3 (δC 112.2 vs. 118.8), C-4 (δC 154.7 vs. 144.9), and C-6 (δC 36.7 vs. 32.8) and the methylene C-7′ (δC 24.1 vs. 21.2) also shared large chemical shift deviations, suggesting eucalypcamal K (2a) was indeed not a diastereomer of eucalypcamal L (4).
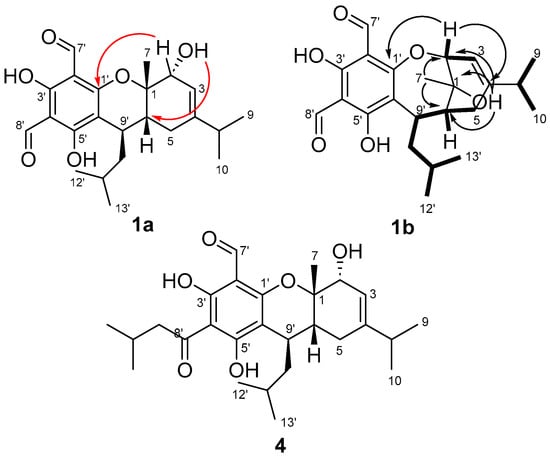
Figure 3.
HMBC (arrows) and COSY (bolded lines) correlations for the structure reassignment of eucalyprobusal C from 1a to 1b. Red arrows represent 4JCH HMBC correlations from 2-OH in 1a, which are more likely 3JCH correlations from 1-OH in revised 1b. Eucalypcamal K (2a) was incorrectly ascribed as the C-6 diastereomer of the co-isolated pyrano 3-acyl-1-formyl phloroglucinol, eucalypcamal L (4).
In addition, closer inspection of the experimental NMR data provided in the supplementary data for eucalyprobusal C (1a) [30] revealed an unassigned oxygenated proton resonance at δH 1.80 consistent with an alcohol group. The oxygenated proton resonance (2-OH in 1a) exhibited three HMBC correlations, two of which should be expected for both structures (1a and 1b) to carbon signals at δC 72.7 (C-1) and 80.2 (C-2). However, a third HMBC correlation was observed to δC 40.2 (C-6), a correlation of which is more likely a 3JCH correlation in 1b than a 4JCH correlation in 1a (Figure 3).
Further, if 1a was indeed a pyran-substituted FPC, a three-bond HMBC correlation would be expected from 1-OH to the sp2 methine C-3 (δC 111.6); however, this correlation was not observed in the reported NMR data. In addition, the HMBC data reported for both eucalyprobusal C and eucalypcamal K clearly displayed 3JCH correlations from H-2 (δH 4.49 and 4.51, respectively) to the oxygenated phloroglucinol carbon C-1′ (δC 165.0 and 164.6, respectively), correlations that could only be assigned as unlikely 4-bond HMBC correlations in the pyrano FPC structures 1a and 2a. These findings clearly suggest that methyl-substituted C-1 in 1a and 2a should be reassigned from an ether to an alcohol in the revised structures 1b and 2b. Moreover, C-2 should also be revised from a secondary alcohol in 1a and 2a to a methine-forming part of an ether linkage to C-1′ of phloroglucinol in 1b and 2b. Reanalysis of the remaining COSY and HMBC NMR data for the terpene substructures for eucalyprobusal C and eucalypcamal K was consistent with ring expansion from a six-membered pyran system to a seven-membered oxepine in the revised structures 1b and 2b. The connectivity of the isopropyl groups to C-4 in both 1b and 2b, as well as the isobutyl to C-9′ in 1b, were consistent with that proposed in their original structure assignments [30,31].
The relative configurations of the revised planar structures 1b and 2b were determined via thorough re-examination of the ROESY NMR spectra for eucalyprobusal C and NOESY NMR spectra for eucalypcamal K, provided in their respective supplementary information [30,31]. Key ROESY correlations from 1-OH to H-5a, as well as from methyl protons H-7 to methylene protons H-10′ and methine H-2, were consistent with *S relative configurations at stereocenters C-2, C-6, and C-7 in 1b (Figure 4). Further, the methine proton H-9′ shared a ROESY correlation with the methylene proton H-5b, suggesting that C-9′ also shared *S relative configuration.
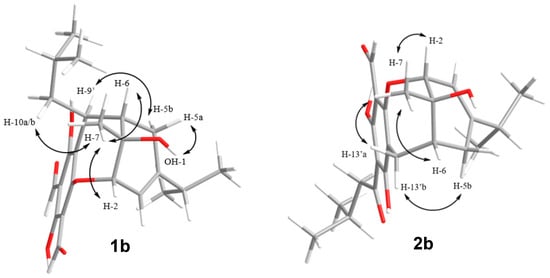
Figure 4.
Key ROESY (eucalyprobusal C) and NOESY (eucalypcamal K) NMR correlations (arrows) used to ascribe the relative configurations for the revised structures 1b and 2b.
Eucalypcamal K (2b) displayed NOESY correlations consistent with the ROESY correlations observed for 1b (Figure 4). Key NOESY correlations from methyl protons H-7 to H-2 and alpha methylene proton H-13′a ascribed *R relative configurations at C-1 and C-2, while C-6 was also assigned *R relative configuration with shared NOESY correlations between H-13′b and beta methylene proton H-5b.
2.3. GIAO DFT NMR Chemical Shift Analyses for 1a, 1b, 2a, and 2b with Experimental NMR Data for Eucalyprobusal C and Eucalypcamal K
To confirm our findings from FPC NMR fingerprinting analyses and re-evaluation of the reported NMR data for eucalyprobusal C and eucalypcamal K, DFT GIAO NMR calculations were performed on the incorrectly assigned (1a and 2a) and revised FPC structures (1b and 2b) and compared with their reported experimental NMR data. The experimental 13C NMR chemical shifts were in poor agreement with the DFT-calculated NMR chemical shifts for structures 1a (13C MAE = 3.8, RMSD = 4.96) and 2a (13C MAE = 4.0 and RMSD = 5.29, Figure 5A,B). Notably, and consistent with our FPC NMR fingerprinting analyses outlined above, large deviations in carbon chemical shifts were observed for phloroglucinol carbons C-2′, 4′, and 6′ in 1a, as well as C-1′, 2′ and 6′ in 2a, alongside formyl carbonyl carbons C-7′ in both (Figure 5A,B, Tables S1 and S5). Furthermore, significant chemical shift differences were observed for the terpenoid carbons in 1a (C-1, 2, 3, 6, 7, and 10′) and 2a (C-1, 2, 3, 5, 6, 7, and 11′), consistent with their misassignment as pyran-substituted phloroglucinols. The DFT GIAO-calculated 13C chemical shifts for revised structures 1b (13C MAE = 1.5, RMSD = 1.92) and 2b (13C MAE = 1.4, RMSD = 1.82) were in excellent agreement with the experimental NMR data for eucalyprobusal C and eucalypcamal K (Figure 5A,B, Tables S2 and S6).
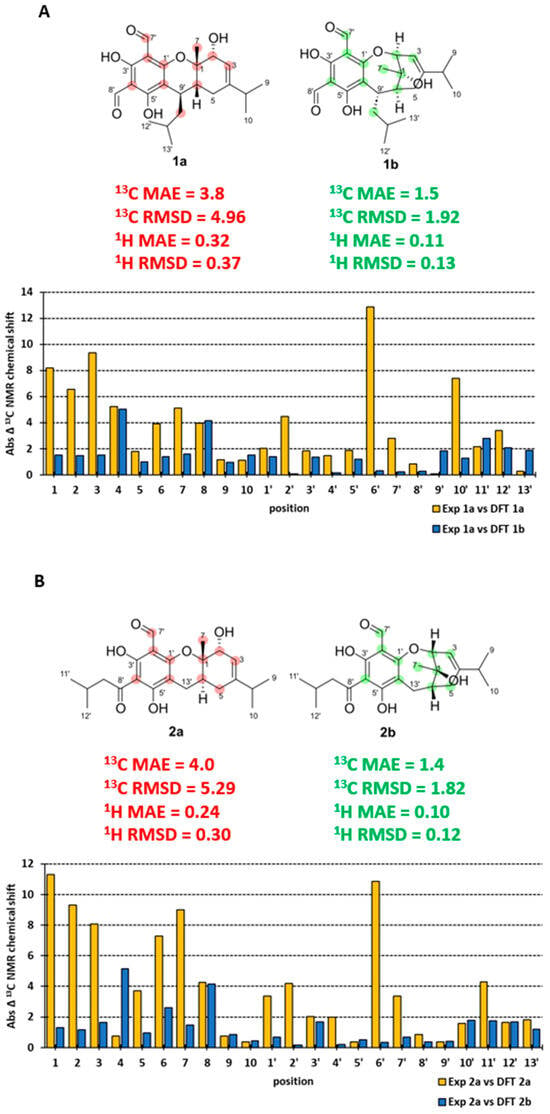
Figure 5.
(A): 13C NMR experimental and DFT-calculated data absolute error (incorrect 1a = yellow and revised 1b = blue) for eucalyprobusal C. (B): 13C NMR experimental and DFT-calculated data absolute error (incorrect 2a = yellow and revised 2b = blue) for eucalypcamal K.
The phloroglucinol carbons (C-1′–C-6′) and the formyl carbon C-7′ for both 1b and 2b were excellent matches with the published experimental 13C NMR data for eucalyprobusal C and eucalypcamal K, respectively. The DFT NMR data for the oxygenated carbons C-1 and C-2 shared minimal deviation (<1.5 ppm) in both oxepine FPCs 1b and 2b, while in 1a and 2a, large errors ranging from 6.5 to 11.3 ppm were observed. These findings substantiate the reassignment of C-1 from an ether to an alcohol, as well as C-2 from an alcohol to an ether, alongside subsequent ring-expansion from pyrano to oxepine FPC structures for both 1b and 2b. Comparative 1H NMR analyses were also performed with the DFT-calculated NMR data for the revised structures, 1b (1H MAE = 0.11, RMSD = 0.13) and 2b (1H MAE = 0.10, RMSD = 0.12), displaying lower errors than those of the incorrectly assigned 1a (1H MAE = 0.32, RMSD = 0.37) and 2b (1H MAE = 0.24; RMSD = 0.30; Figure 5A,B and Tables S3, S4, S7, and S8). Moreover, the DFT-calculated NMR shielding tensors for the incorrect and revised structures of eucalyprobusal C and eucalypcamal K were analyzed using DP4+ Bayesian theorem probability analyses [28]. Unsurprisingly, and consistent with our comparative analyses of the scaled DFT NMR chemical shifts outlined above, DP4+ unequivocally supported the revised structures 1b and 2b with 100% probability over 1a and 2a (Tables S11 and S12).
2.4. TDDFT ECD Comparison of Revised FPC Structures (1b and 2b) with Experimental ECD Data Reported for Eucalyprobusal C and Eucalypcamal K
With the revised structures for eucalyprobusal C (1b) and eucalypcamal K (2b) affirmed by reinterpretation of their experimental NMR data, alongside comparative and probabilistic DFT NMR analyses, TDDFT ECD calculations were performed to assign their absolute configurations. The TDDFT-calculated ECD spectra for 1b and 2b were compared with the experimental ECD data published for eucalyprobusal C and eucalypcamal K (Figure 6A,B) [30,31]. Both reassigned structures 1b and 2b were found to be excellent matches, with their published experimental ECD spectra confirming the reassignment of absolute configurations.
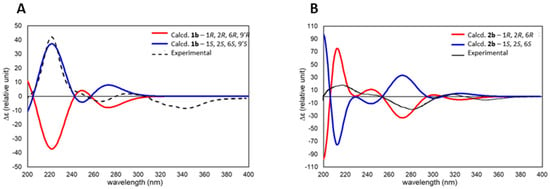
Figure 6.
(A): TDDFT-calculated ECD spectra for eucalyprobusal C (1b—1S, 2S, 6S, and 9′S) overlayed with experimental ECD spectra reported for eucalyprobusal C. (B): TDDFT-calculated ECD spectra for eucalypcamal K (2b—1R, 2R, and 6R) overlayed with experimental ECD spectra reported for eucalypcamal K.
Eucalyprobusal C should be revised to structure 1b with the absolute configuration 1S, 2S, 6S, and 9′S, while eucalypcamal K is revised to 2b with the absolute configuration 1R, 2R, and 6R.
3. Discussion
New diformyl and acyl formyl phloroglucinol NPs continue to be reported from Myrtaceae species on a regular basis; however, approximately 10% of all published FPCs have wrongly assigned structures and/or resonances [7]. FPCs containing oxepine ring systems are rare, yet they contain characteristic phloroglucinol 1H and 13C resonances that differentiate them from the more commonly reported pyrano-containing FPCs. Eucalyprobusal C (1b) is only the ninth oxepine-diformyl phloroglucinol meroterpene reported to date, while eucalypcamal K (2b) is just the second oxepine 1-formyl-3-acyl phloroglucinol meroterpene reported. Interestingly, eucalyprobusal C is the first oxepine-diformyl phloroglucinol conjugated to a monoterpene, with the eight previously reported NPs in this subclass all containing sesquiterpenes conjugated to the phloroglucinol core. The observation that 1b and 2b possess opposite absolute configurations associated with the monoterpene moieties reflects the diversity of terpene building blocks produced by different species of highly speciose genus Eucalyptus. Although both compounds have been isolated from species from the Symphyomyrtus sub-genus E. robusta, the source of eucalyprobusal C is in the section Latoangulatae, while for E. camaldulensis, the source of eucalypcamal K is in the section Exsertaria.
We have previously demonstrated that despite accurate methods to establish correct molecular structures and definitively assign 1H and 13C NMR resonances available to both authors and peer reviewers, wrongly assigned NP structures and/or incorrectly assigned 1H and 13C NMR continue to be published in the literature. Our application of computational pattern recognition of NMR data to propose substructure motifs, followed by the verification of these structures using DFT methods, represents an effective and unique approach that has now resulted in the structure revision of five FPCs [7]. These structure corrections complement an additional thirteen plant and marine NP structures that we have corrected based on the reinterpretation of their reported NMR data [8,10,33,34,35]. It is incumbent upon peer reviewers of NP structures to act as gate keepers in an effort to filter out poor interpretation of NMR spectroscopic data; unfortunately however, there are many instances where this process continues to fail [5,36,37]. The development of more tools, such as our NMR fingerprinting PCA methodology, can support researchers and the peer review process to help to reduce the number of erroneous NP structure assignments and prevent their proliferation throughout the literature. This is particularly important for the current and future development of machine learning and AI tools toward automating the structure analysis of complex NPs. Fast methods to analyze big data sets are also becoming increasingly important. DFT NMR calculation methods that offer more computational efficiency, such as DP4, J-DP4, and DP4+ [27,28,29], are excellent choices over more computationally demanding ones at higher levels of theory.
4. Materials and Methods
4.1. NMR Fingerprint Visualization, Statistical, and Principal Component Analyses
The visualization and analysis of the literature chemical shift data was performed using the same protocol previously reported [7] within the freely available OSIRIS DataWarrior (version 5.2.1) software [38]. The principal component analysis function within DataWarrior was used to analyze the carbon and proton chemical shift data for 179 formyl phloroglucinol NPs reported in the literature with NMR data recorded in CDCl3. PC1 and PC2 were generated with the native visualization function included in the DataWarrior software package (version 5.2.1).
4.2. Computational Methods
Extensive conformer searches were performed on 1a, 1b, 2a, and 2b within the Schrodinger Macromodel (version 10.7) software suite using the Monte Carlo Multiple Minimum (MCMM) method at an energy window of 21.0 kJ/mol and the MMFF forcefield. The step count for Macromodel conformer searches were set so that all low energy conformers were found at least 10 times. The conformer sets for each of the candidate structures (1a, 1b, 2a, and 2b) were subjected to gas-phase geometry optimizations (GO) at the B3LYP/6-31+G(d,p) level of theory within Gaussian 16 (Revision C.01) [39]. The GO sets were filtered for duplicate and high-energy conformers (>3.0 kcal/mol above the energy minimum removed). For NMR calculations, 1H and 13C GIAO NMR DFT chemical shifts were calculated at the mPW1PW91/6-311+G(d,p) level of theory, which included the polarizable continuum PCM solvent model for chloroform [40]. The DFT-calculated NMR isotropic shielding tensors were Boltzmann-averaged across each of the conformational suites (energies < 3.0 kcal/mol) and scaled according to linear regression scaling factors deposited within online resources provided by the Cheshire Chemical Shift Repository (http://cheshirenmr.info/index.htm, accessed 23 October 2023) [41,42].
For ECD calculations, the filtered GO conformers used for GIAO NMR calculations (B3LYP/6-31+G(d,p)) were promoted to TDDFT rotational strength and electronic transition calculations using the CAM-B3LYP/6-311+G(d,p) level of theory, with D3 empirical dispersion and the PCM solvent model for chloroform included. The resultant TDDFT-calculated UV and ECD spectra were Boltzmann-weighted and matched with experimental UV and ECD data using the freely available SpecDis (1.71) software [43]. A Gaussian band shape of (eV) of 0.23 and UV corrections of −8 and +7 were applied to 1b and 2b, respectively, to match with the published ECD spectra reported for eucalyprobusal C and eucalypcamal K [30,31]. Automation processes with the high-performance computing cluster (‘Gowonda’) were carried out using customized Python scripts [44].
5. Conclusions
In conclusion, the incorrectly assigned structures for two FPCs isolated from Eucalyptus species, eucalyprobusal C (1a) and eucalypcamal K (2a), were unequivocally revised to 1b and 2b, respectively. Utilizing our previously established NMR fingerprinting method, now expanded to include diagnostic NMR data for 179 FPCs, we identified eucalyprobusal C (1a) and eucalypcamal K (2a) as having structures inconsistent with their assigned structure classes. Specifically, 1a, originally identified as a diformyl-pyrano phloroglucinol, and 2a, designated as a 3-acyl-1-formyl pyrano phloroglucinol, were found to be better matched with NMR fingerprints associated with oxepine-formyl phloroglucinols. After the extensive reanalysis of their reported experimental NMR data and comparison with similar FPC structures in the primary literature, we revised their structures to oxepine-formyl phloroglucinol structures 1b and 2b. Subsequent GIAO DFT 1H and 13C NMR calculations were performed on both the incorrectly assigned structures (1a and 2a) and the revised structures (1b and 2b), followed by extensive comparative analyses using their respective experimental NMR data. The DFT-calculated NMR data for the revised structures 1b and 2b were found to be in excellent agreement with the reported experimental NMR data for eucalyprobusal C and eucalypcamal K, respectively. In addition, their absolute configurations were determined by comparing the TDDFT-calculated ECD spectra of the revised structures (1b and 2b) with their published experimental ECD data. By extension, DP4+ Bayesian probability analyses showed 100% probability for the revised structures of eucalyprobusal C (1b) and eucalypcamal K (2b) over 1a and 2a. These structure corrections helped us to refine the data that are publicly available for accurate applications of NMR data for machine learning to aid structure determination of unknown FPCs that might be identified in the future.
The workflow presented herein further outlines the utility of NMR fingerprinting for identifying incorrectly assigned NPs in the literature and associated databases. In combination with computational DFT NMR calculations, we have provided a powerful method for revising the structures of complex NPs. The broad scope of our FPC NMR fingerprinting method also has other demonstrated uses, including the targeting of extracts that contain FPCs and/or identifying subclasses of FPCs within complex NP mixtures [7]. Future applications for NMR fingerprinting should extend to mining subclasses of FPCs from complex NP extracts, particularly efforts targeting specific biological activities such as those currently prioritized for drug resistance (anti-infective ones). Moreover, extending NMR fingerprinting analyses to other valuable subclasses of NPs would provide valuable tools for the many diverse research areas where NPs are of central importance and should decrease the number of incorrect NP structures reported in the literature.
Supplementary Materials
The following supporting information can be downloaded via this link: https://www.mdpi.com/article/10.3390/molecules29030594/s1, Table S1–S8; Comparative experimental (eucalyprobusal C and eucalypcamal K) and DFT calculated NMR data and MAE/RMSD for 1a, 1b, 2a, and 2b, Table S9; Comparison of eucalyprobusal C (1a) experimental NMR data with diagnostic 1H and 13C NMR data fingerprint data for pyrano-diformyl phloroglucinols and oxepine-formyl phloroglucinols, Table S10; Comparison of eucalypcamal K (2a) experimental NMR data with diagnostic 1H and 13C NMR data fingerprint data for pyrano-3-acyl-1-formyl phloroglucinols and oxepine-formyl phloroglucinols, Table S11; Eucalyprobusal C incorrect (1a = Isomer 1 − 0.00%) versus revised (1b = Isomer 2 – 100.00%) DP4+ output, Table S12; Eucalypcamal K incorrect (2a = Isomer 1 − 0.00%) versus revised (2b = Isomer 2 − 100.00%) DP4+ output, Figure S1; Classification of subclasses for formyl phloroglucinol meroterpenes (image with adapted from Baxter et al.), Tables S13–S16; Eucalyprobusal C (1a and 1b) and eucalypcamal K (2a and 2b) conformational sets, energies, and distributions for GIAO-DFT NMR and TDDFT-ECD calculations, and Tables S17–20; Eucalyprobusal C (1a and 1b) and eucalypcamal K (2a and 2b) DFT geometry optimized conformers calculated at the B3LYP/6-31+G(d,p) level of theory.
Author Contributions
Conceptualization, Investigation, Methodology, and Writing—draft, review, and editing, D.C.H. and A.R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Supporting data are available in the Electronic Supporting Information (ESI) provided.
Acknowledgments
We gratefully acknowledge Griffith University and the university’s eResearch team for the use of the ‘Gowonda’ high-performance computing cluster for DFT NMR and TDDFT ECD calculations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, C.; Idelbayev, Y.; Roberts, N.; Tao, Y.; Nannapaneni, Y.; Duggan, B.M.; Min, J.; Lin, E.C.; Gerwick, E.C.; Cottrell, G.W.; et al. Small Molecule Accurate Recognition Technology (SMART) to Enhance Natural Products Research. Sci. Rep. 2017, 7, 14243. [Google Scholar] [CrossRef]
- Guan, Y.; Shree Sowndarya, S.V.; Gallegos, L.C.; St. John, P.C.; Paton, R.S. Real-time prediction of 1H and 13C chemical shifts with DFT accuracy using a 3D graph neural network. Chem. Sci. 2021, 12, 12012–12026. [Google Scholar] [CrossRef]
- Robien, W. Computer-Assisted Peer Reviewing of Spectral Data: The CSEARCH Protocol. Monatsh. Chem. 2019, 150, 927–932. [Google Scholar] [CrossRef]
- Kleks, G.; Holland, D.C.; Porter, J.; Carroll, A.R. Natural Products Dereplication by Diffusion Ordered NMR Spectroscopy (DOSY). Chem. Sci. 2021, 12, 10930–10943. [Google Scholar] [CrossRef]
- Chhetri, B.K.; Lavoie, S.; Sweeney-Jones, A.M.; Kubanek, J. Recent Trends in the Structural Revision of Natural Products. Nat. Prod. Rep. 2018, 35, 514–531. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.M.; Appendino, G.; Guo, Y.W. Pitfalls in the Structural Elucidation of Small Molecules. A Critical Analysis of a Decade of Structural Misassignments of Marine Natural Products. Nat. Prod. Rep. 2022, 39, 1803–1832. [Google Scholar] [CrossRef]
- Baxter, J.R.; Holland, D.C.; Gavranich, B.; Nicolle, D.; Hayton, J.B.; Avery, V.M.; Carroll, A.R. NMR Fingerprints of Formyl Phloroglucinol Meroterpenoids and Their Application to the Investigation of Eucalyptus gittinsii subsp. gittinsii. J. Nat. Prod. 2023, 86, 1317–1334. [Google Scholar] [CrossRef]
- Holland, D.C.; Kiefel, M.J.; Carroll, A.R. Structure Revisions of the Sponge-Derived Dibrominated Bis-Indole Alkaloids, Echinosulfone A and the Echinosulfonic Acids A to D. J. Org. Chem. 2020, 85, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.P.; Moodie, L.W.K.; Holland, D.C.; Jandér, K.C.; Göransson, U. Sulfadiazine Masquerading as a Natural Product from Scilla Madeirensis (Scilloideae). J. Nat. Prod. 2020, 83, 1305–1308. [Google Scholar] [CrossRef]
- Carroll, A.R. Structure Revision of Pilidiostigmin from the Leaves of Pilidiostigma Glabrum. Tetrahedron Lett. 2016, 57, 281–284. [Google Scholar] [CrossRef]
- Prebble, D.W.; Holland, D.C.; Ferretti, F.; Hayton, J.B.; Avery, V.M.; Mellick, G.D.; Carroll, A.R. α-Synuclein Aggregation Inhibitory and Antiplasmodial Activity of Constituents from the Australian Tree Eucalyptus Cloeziana. J. Nat. Prod. 2023, 86, 2171–2184. [Google Scholar] [CrossRef]
- Senadeera, S.P.D.; Duffy, S.; Avery, V.M.; Carroll, A.R. Antiplasmodial β-Triketones from the Flowers of the Australian Tree Angophora Woodsiana. Bioorg. Med. Chem. Lett. 2017, 27, 2602–2607. [Google Scholar] [CrossRef]
- Senadeera, S.P.D.; Lucantoni, L.; Duffy, S.; Avery, V.M.; Carroll, A.R. Antiplasmodial β-Triketone-Flavanone Hybrids from the Flowers of the Australian Tree Corymbia Torelliana. J. Nat. Prod. 2018, 81, 1588–1597. [Google Scholar] [CrossRef]
- Senadeera, S.P.D.; Robertson, L.P.; Duffy, S.; Wang, Y.; Avery, V.M.; Carroll, A.R. β-Triketone-Monoterpene Hybrids from the Flowers of the Australian Tree Corymbia Intermedia. J. Nat. Prod. 2018, 81, 2455–2461. [Google Scholar] [CrossRef]
- Carroll, A.R.; Urban, S.; Lamb, J.; Moni, R.; Guymer, G.P.; Forster, P.I.; Quinn, R.J. Corymbones A and B, Phloroglucinols with Thyrotropin Releasing Hormone Receptor 2 Binding Affinity from the Flowers of Corymbia Peltata. J. Nat. Prod. 2008, 71, 881–883. [Google Scholar] [CrossRef]
- Carroll, A.R.; Avery, V.M.; Duffy, S.; Forster, P.I.; Guymer, G.P. Watsonianone A-C, Anti-Plasmodial β-Triketones from the Australian Tree, Corymbia Watsoniana. Org. Biomol. Chem. 2013, 11, 453–458. [Google Scholar] [CrossRef]
- Carroll, A.R.; Lamb, J.; Moni, R.; Guymer, G.P.; Forster, P.I.; Quinn, R.J. Myrtucommulones F-I, Phloroglucinols with Thyrotropin-Releasing Hormone Receptor-2 Binding Affinity from the Seeds of Corymbia Scabrida. J. Nat. Prod. 2008, 71, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.D.; Johnson, L.A.S. Sytematic Studies in the Eucalypts 7. A Revision of the Bloodwoods, Genus Corymbia (Mytraceae). Telopea 1995, 6, 185–504. [Google Scholar] [CrossRef]
- Brooker, M.I.H. A New Classification of the Genus Eucalyptus L’Her. (Myrtaceae). Aust. Syst. Bot. 2000, 13, 79–148. [Google Scholar] [CrossRef]
- Phang, Y.L.; Liu, S.; Zheng, C.; Xu, H. Recent Advances in the Synthesis of Natural Products Containing the Phloroglucinol Motif. Nat. Prod. Rep. 2022, 39, 1766–1802. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, Y.; Murata, M.; Shimizu, A.; Homma, S. Isolation and Characterization of Macrocarpals B-G Antibacterial Compounds from Eucalyptus macrocarpa. Biosci. Biotechnol. Biochem. 1992, 56, 1570–1576. [Google Scholar] [CrossRef]
- Osawa, K.; Yasuda, H.; Morita, H.; Takeya, K.; Itokawa, H. Macrocarpals H, I, and J from the Leaves of Eucalyptus globulus. J. Nat. Prod. 1996, 59, 823–827. Available online: https://pubs.acs.org/sharingguidelines (accessed on 14 January 2021). [CrossRef]
- Cobas, C. NMR Signal Processing, Prediction, and Structure Verification with Machine Learning Techniques. Magn. Reson. Chem. 2020, 58, 512–519. [Google Scholar] [CrossRef]
- Elyashberg, M.; Argyropoulos, D. Computer Assisted Structure Elucidation (CASE): Current and Future Perspectives. Magn. Reson. Chem. 2021, 59, 669–690. [Google Scholar] [CrossRef]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational Prediction of 1H and 13C Chemical Shifts: A Useful Tool for Natural Product, Mechanistic, and Synthetic Organic Chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef]
- Hiranrat, A.; Holland, D.C.; Mahabusarakam, W.; Hooper, J.N.A.; Avery, V.M.; Carroll, A.R. Tedaniophorbasins A and B—Novel Fluorescent Pteridine Alkaloids Incorporating a Thiomorpholine from the Sponge Tedaniophorbas ceratosis. Mar. Drugs 2021, 19, 95. [Google Scholar] [CrossRef]
- Sarotti, A.M. Successful Combination of Computationally Inexpensive GIAO 13C NMR Calculations and Artificial Neural Network Pattern Recognition: A New Strategy for Simple and Rapid Detection of Structural Misassignments. Org. Biomol. Chem. 2013, 11, 4847–4859. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An Improved Probability for the Stereochemical Assignment of Isomeric Compounds Using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Grimblat, N.; Gavín, J.A.; Hernández Daranas, A.; Sarotti, A.M. Combining the Power of J Coupling and DP4 Analysis on Stereochemical Assignments: The J-DP4 Methods. Org. Lett. 2019, 21, 4003–4007. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; He, X.Z.; Feng, M.Y.; Yuan-Zeng; Rauwolf, T.J.; Shao, L.D.; Ni, W.; Yan, H.; Porco, J.A.; Hao, X.J.; et al. Acylphloroglucinols with Acetylcholinesterase Inhibitory Effects from the Fruits of Eucalyptus robusta. Bioorg. Chem. 2020, 103, 104127. [Google Scholar] [CrossRef] [PubMed]
- Daus, M.; Wunnoo, S.; Voravuthikunchai, S.P.; Saithong, S.; Poldorn, P.; Jungsuttiwong, S.; Chomlamay, N.; Yangok, K.; Watanapokasin, R.; Chakthong, S. Phloroglucinol–Meroterpenoids from the Leaves of Eucalyptus camaldulensis Dehnh. Phytochemistry 2022, 200, 113179. [Google Scholar] [CrossRef]
- Daus, M.; Hayton, J.B.; Holland, D.C.; Voravuthikunchai, S.P.; Carroll, A.R.; Chakthong, S. Camaldulensals A-C, the First Meroterpenoids Possessing Two Spatially Separated Formyl Phloroglucinols Conjugated to a Terpene Core from the Leaves of Eucalyptus Camaldulensis Dehnh. J. Nat. Prod. 2023, 86, 1994–2005. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A. Structure Revision of Four Acylphloroglucinols Isolated from the Leaves of Syzygium Polyanthum. Planta Med. Lett. 2016, 3, e8–e9. [Google Scholar] [CrossRef][Green Version]
- Carroll, A.R.; Duffy, S.; Sykes, M.; Avery, V.M. Wilsoniamines A and B: Novel Alkaloids from the Temperate Australian Bryozoan, Amathia Wilsoni. Org. Biomol. Chem. 2011, 9, 604–609. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Quinn, R.J. Revised Structure of Palau’amine. Tetrahedron Lett. 2007, 48, 4573–4574. [Google Scholar] [CrossRef]
- Burns, D.C.; Reynolds, W.F. Minimizing the Risk of Deducing Wrong Natural Product Structures from NMR Data. Magn. Reson. Chem. 2021, 59, 500–533. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Product. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An Open-Source Program for Chemistry Aware Data Visualization and Analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 (Revision C.01); Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF Version of the PCM Solvation Method: An Overview of a New Method Addressed to Study Molecular Solutes at the QM Ab Initio Level. J. Mol. Struct. THEOCHEM 1999, 464, 211–226. [Google Scholar] [CrossRef]
- CHESHIRE Chemical Shift Repository. Available online: http://cheshirenmr.info/ScalingFactors.htm#table5dimethylsulfoxideheading (accessed on 14 January 2021).
- Pierens, G.K. 1H and 13C NMR Scaling Factors for the Calculation of Chemical Shifts in Commonly Used Solvents Using Density Functional Theory. J. Comput. Chem. 2014, 35, 1388–1394. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumloffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, P.H.; Jansma, M.J.; Hoye, T.R. A Guide to Small-Molecule Structure Assignment through Computation of (1H and 13C) NMR Chemical Shifts. Nat. Protoc. 2014, 9, 643–660. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).