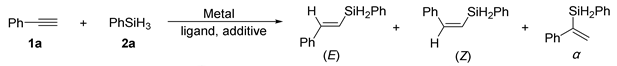
Abstract
Herein, we report the first example of molybdenum-catalyzed (E)-Selective anti-Markovnikov hydrosilylation of alkynes. The reaction operates effectively with the utilization of minute amounts of the inexpensive, bench-stable pre-catalyst and ligand under mild conditions. Moreover, this molybdenum-catalyzed hydrosilylation process features the advantages of simple operation, excellent selectivity, and broad functional groups tolerance.
1. Introduction
Silicon-containing molecules have been widely served as versatile building blocks towards modern organic synthesis and pharmaceutical chemistry [1,2,3]. Among these synthons, vinylsilanes possess the advantages of incomparable reactivity, low toxicity, high stability, and simple operations, and offer ample opportunities for post-synthesis manipulations in synthetic chemistry [4,5]. Therefore, the preparation of these molecules has been thoroughly investigated in recent years [6]. The most straightforward and atom-economic approach for accessing multifunctional vinylsilanes was the transition metal catalyzed hydrosilylation of alkynes [7]. However, the addition products of alkynes with silanes could be Markovnikov type α-vinylsilane isomers, anti-Markovnikov addition type β-(E), or β-(Z) vinylsilane isomers. Hence, the most challenging issue of these methods was the precise controlling of both regioselectivity and stereoselectivity of the desired addition products. Over the past few decades, noble metal catalysts (such as platinum [8,9], rhodium [10,11,12], palladium [13], iridium [14,15], ruthenium [16,17], and gold [18,19]) have been well established to tackle these selectivity challenges. For example, Jiménez’s group reported the complete β-(Z) selectivity synthesis of vinylsilane via the cyclometalated rhodium(III)-triazolylidene homogeneous and heterogeneous terminal alkyne hydrosilylation catalysts [11]. Despite the ubiquitous utilization of these noble metal catalysts, restrictions like elevated and fluctuating catalyst expenses, prevalent side reactions, expensive and complex ligands have limited their applications. On the other hand, concerning the sustainable development of green chemistry, the combination of chemical, economic, and environmental concerns has stimulated the expedition of inexpensive, low-toxic base-metal catalysts, such as cobalt [20,21,22,23,24,25,26,27,28], iron [29,30,31,32], manganese [33], copper [34,35], and nickel catalysts [36].
In the past decades, cobalt catalysts have exhibited exceptional catalytic performance in the precise preparation of the desired isomers. As shown in Scheme 1, Deng and coworkers realized the Markovnikov hydrosilylation of alkynes by dicobalt carbonyl N-heterocyclic carbene catalyst with excellent α/β selectivity and functional group compatibility [26]. In addition, highly Z-selective Co-catalyzed hydrosilylation of alkynes has also been achieved by Ge’ group, and both primary and secondary hydrosilanes were well-tolerated in this transformation [21]. Compared with β-(Z) products, the β-(E) isomers were thermodynamically more stable. As a consequence, effective catalytic systems for the highly regio- and stereoselective construction of β-(E) isomers have been investigated thoroughly in the past decades. Thomas reported a complex of the MesBIPCoCl2 for stereoselective hydrosilylation of for electronically unbiased alkyl alkynes and primary hydrosilanes to synthesize the (E)-β-vinylsilanes with moderate (E)-selectivity [37]. In 2018, Ge and coworkers employed a combination of bench-stable Co(acac)2 and bisphoshpine ligands for the regioselective and stereoselective hydrosilylation of terminal alkynes [38]. This catalytic system was activated by the reaction with hydrosilanes rather than air-sensitive activators, such as Grignard reagents or NaBHEt3. The reaction conditions were extremely mild and practical, providing an effective method for the construction of (E)-β-vinylsilanes. Except for the cobalt catalyst, Zhan reported that iron catalyst also served as an alternative choice for the regio- and stereoselective (E)-β-vinylsilanes synthesis [29]. In 2024, Zhao and coworkers demonstrated significant progress in Cu-catalyzed asymmetric hydrosilylation to construct Si-seterogenic alkenyl silanes [35]. The simple Cu/(S)-Tol-BINAP catalytic system enabled the excellent regio-, stereo-, and enantioselectivities in the reaction. Despite the significant achievements that have been made in this field, considering the environmental and economic aspects, the exploration of new types of non-noble metal catalysts for highly selective hydrosilylation of alkynes remains appealing and desirable.
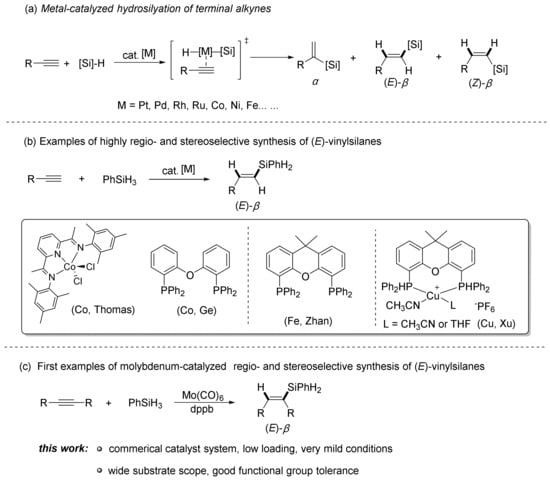
Scheme 1.
Transition-Metal-Catalyzed Hydrosilylation of Terminal Alkynes.
Our group is committed to developing effective and practical organic reactions with excellent regio- and stereoselectivity [39,40,41]. Consequently, we disclose the first example of molybdenum-catalyzed regiodivergent and stereoselective synthesis of (E)-vinylsilanes with both aromatic and aliphatic alkynes after tremendous efforts.
2. Results and Discussion
Initially, our studies commenced with the hydrosilylation of phenylacetylene (1a) and PhSiH3 (2a) as representative substrates to evaluate the optimal reaction conditions. Based on our previous work, we chose non-noble tungsten catalysis, such as W(CO)6, W(CH3CN)3(CO)3, and Mo(CO)6 as catalyst, t-BuOK as additive, PPh3 as ligand, and MeCN as solvent. To our delight, the addition of product 3a was indeed generated as expected with the combination of Mo(CO)6 and PPh3 (entries 1). However, the regioselectivity and stereoselectivity were not satisfied. The Markovnikov addition side product was generated in comparable yields (entries 1). For the purpose of increasing the efficiency and selectivity of this reaction, several commercial phosphine ligands (such as tri-tert-butylphosphine, dppb, dppe, dppbz, xantphos) were screened under identical conditions. These experiments revealed that the bidentate phosphine ligands demonstrated better selectivity than the monodentate phosphine ligands. Among these ligands, dppb displayed optimal regioselectivity and stereoselectivity (entries 3–9). Further investigation was concentrated on different types of solvents, such as THF, Et2O, Toluene, EtOH, and H2O. Fortunately, the implementation of THF was the better solvent both with respect to the reaction yield and the selectivity (entries 10–14). Subsequently, when adjusting the temperature from room temperature to 80 °C, neither the yield nor the selectivity of the reaction was enhanced (entries 15–16). Finally, controlled experiments indicated that the molybdenum catalysis and ligand were essential for this reaction (entries 17–18). Up to this point, the optimal reaction conditions were determined as follows: 1 mol% Mo(CO)6, 1.2 mol% dppb, 5 mol% t-BuOK in 2 mL anhydrous THF at room temperature for 4 h.
Under the identified optimal conditions (entry 10 in Table 1), we turned to study the scope of aromatic terminal alkynes that underwent this molybdenum-catalyzed E-selective hydrosilylation reaction, and the results were presented in Scheme 2. Generally, a diverse array of terminal alkynes with various electronic substituents on the phenyl ring could participate very well to construct the (E)-vinylsilane products in high yield and selectivity. The electronic characteristics of aryl substituents in aromatic alkynes had no significant influence on the regioselectivity of this hydrosilylation process. Diverse substitution patterns (including methyl-, phenyl, trifluoromethyl- and halogen-) were all compatible in this reaction (3a–3o). These halogen substituents (F, Br and Cl) allowed further structural modification of these (E)-vinylsilanes. Moreover, the position of the substituents did not affect the generation of the expected products (3k–3p). Considering the synthetic interests, the fused-ring substrate was examined, the corresponding product obtained only in a slight decrease in the reaction yield (3o).

Table 1.
Optimization of the reaction conditions a.
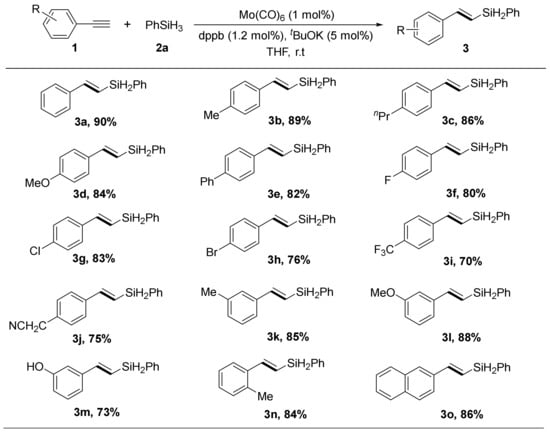
Scheme 2.
Scope of terminal alkynes for the Mo/dppb catalyzed anti-Markovnikov hydrosilylation with PhSiH3. Alkynes 1 (0.5 mmol), PhSiH3 2a (0.75 mmol), Mo(CO)6 (1 mol%), dppb (1.2 mol%), t-BuOK (5 mol %), anhydrous THF (2 mL), r. t., 4 h. Yields of isolated products. The selectivity for product (β-E-vinylsilane product: α-isomers) was ≥20:1, unless otherwise noted, determined by 1H NMR spectroscopy.
The catalytic efficiency of the Mo(CO)6/dppb system was further explored by using a variety of electronically unbiased alkyl alkynes. Pleasingly, all substrates transformed into the corresponding (E)-vinylsilane in good yields and excellent selectivity (both high stereoselectivity and regioselectivity) (Scheme 3). The halogen-substituted alkyl alkyne substrates were well-tolerated in this reaction with no dehalogenation observing. These halogen substituents undoubtedly allowed further structural modification of the vinylsilane. Except for the terminal alkynes, the internal substrates hex-3-yne (2s) could proceed this anti-Markovnikov hydrosilylation with lower yields and comparable selectivity. Unfortunately, secondary hydrosilane (Ph2SiH2) and tertiary hydrosilanes ((EtO)3SiH) turned out to be inactive in this reaction.
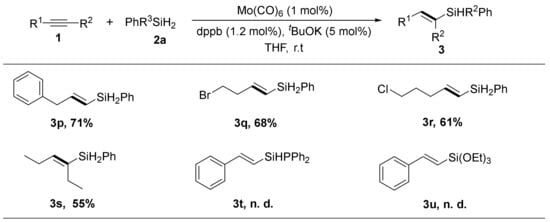
Scheme 3.
Scope of alkynes for the Mo/dppb catalyzed anti-Markovnikov hydrosilylation with silanes. Alkynes 1 (0.5 mmol), PhSiH3 2a (0.75 mmol), Mo(CO)6 (1 mol%), dppb (1.2 mol%), t-BuOK (5 mol %), anhydrous THF (2 mL), r. t., 4 h, n.d. = not detected. Yields of isolated products. The selectivity for product (β-E-vinylsilane product: α-isomers) was ≥20:1, unless otherwise noted, determined by 1H NMR spectroscopy.
To emphasize the usefulness of this practical procedure for the construction of (E)-vinylsilane, phenylacetylene (1a) and PhSiH3 (3a), in the presence of Mo(CO)6 and dppb, could be reacted to yield 87% at room temperature in 4 h on a 10 mmol scale. In addition, we also conducted further conversion of vinylsilanes via the Hiyama–Denmark coupling reaction to synthesize internal (E)-vinylarenes in 80% yield (Scheme 4).
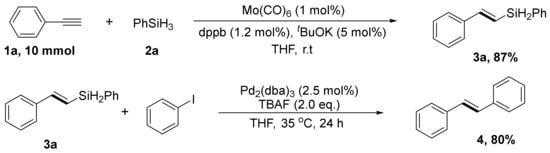
Scheme 4.
Gram-Scale Synthesis and further transformations.
Based on mechanical experiments and prior studies [29,31,38], the plausible mechanism accounting for the syhthesis of (E)-vinylsilane was discussed in Scheme 5. Firstly, in the presence of dppb ligand and the base additive tBuOK, the molybdenum catalysis could react with PhSiH3 to form the monohydrido species LnMo(H)(SiH2Ph) (B) through the oxidative addition process. Subsequently, the key intermediate (C) was generated after the coordination of the alkyne and migratory insertion into the Mo-H bond. Finally, the expected product, (E)-vinylsilane, was afforded via the reductive elimination procedure.
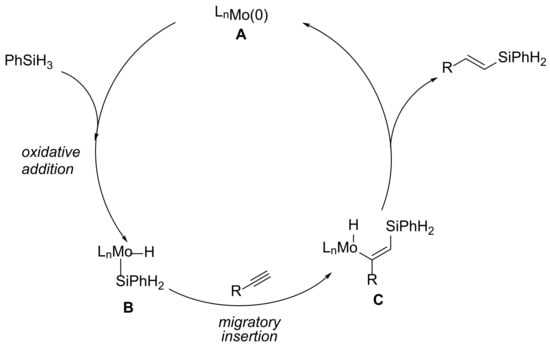
Scheme 5.
Plausible Mechanistic Pathway.
3. Materials and Methods
3.1. Materials
All the reagents were obtained from commercial sources and used directly without further purification, unless otherwise noted. 1H NMR and 13C NMR spectra were recorded on a Bruker AVANCE III 400 MHz. 1H NMR and 13C NMR chemical shifts were determined relative to the internal standard TMS at δ 0.0. Chemical shifts (δ) are reported in ppm, and coupling constants (J) are reported in Hertz (Hz).
3.2. General Methods for the Preparation of (E)-Vinylsilanes
A 25 mL Schlenk tube was charged with Mo(CO)6 (1 mol%) and was mixed with anhydrous THF (2 mL) in a glass vial equipped with a magnetic stirring bar. Then, dppb (1.2 mol%), tBuOK (5 mol%), phenylacetylene (1a; 0.5 mmol), and PhSiH3 (2a, 0.75 mmol) were added. The mixture was stirred vigorously at room temperature for 4–6 h. After completing the reaction, then cooling to room temperature, the reaction mixture was washed with 10 mL H2O, then diluted with EtOAc (10 mL). The organic phase was dried over MgSO4 and concentrated in vacuo. The residue was purified by flash chromatography on silica gel using petroleum ether/ethyl acetate as the eluent to give the desired product (E)-phenyl(styryl)silane (3a).
4. Conclusions
In summary, a convenient and efficient molybdenum-catalyzed (E)-selective anti-Markovnikov hydrosilylation of alkynes and PhSiH3 is disclosed. Compared with the reported works, we cannot claim that we possess the discernible superior advantages over the selectivity, scope, and efficiency. But considering the importance of the (E)-vinylsilanes, our reaction could provide alternatives for the construction of these compounds. This catalytic system relies on the natural abundance of molybdenum and the commercial, inexpensive dppb ligand. Furthermore, the advantages of readily available reagents, mild reaction conditions (room temperature, short reaction time and low catalyst and ligand loading), and good functional-group tolerance make this protocol practical for (E)-selective vinylsilane synthesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29245952/s1, the NMR data and spectra of the catalytic products.
Author Contributions
Writing—review and editing, F.Y.; investigation and methodology, Z.H., Q.W. and J.L.; data curation, Z.H. and L.W.; funding acquisition, F.Y. and X.L.; conceptualization, L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Zhaoqing University Science Fund (ZD202413), College Students’ innovation and entrepreneurship training program (S202410580062, S202410580066), Zhaoqing University High-Level Project Training Programme Project (GCCZK202406), Zhaoqing University Innovation Research Team Project (TD202413), Guangdong Provincial Key Laboratory of Eco-environmental Studies and Low-carbon Agriculture in Peri-urban Areas (2020B121201014).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Langkopf, E.; Schinzer, D. Uses of Silicon-Containing Compounds in the Synthesis of Natural Products. Chem. Rev. 1995, 95, 1375–1408. [Google Scholar] [CrossRef]
- Bracegirdle, S.; Anderson, E.A. Recent advances in the use of temporary silicon tethers in metal-mediated reactions. Chem. Soc. Rev. 2010, 39, 4114–4129. [Google Scholar] [CrossRef] [PubMed]
- Rémond, E.; Martin, C.; Martinez, J.; Cavelier, F. Siliconcontaining amino acids: Synthetic aspects, conformational studies, and applications to bioactive peptides. Chem. Rev. 2016, 116, 11654–11684. [Google Scholar] [CrossRef] [PubMed]
- Blumenkopf, T.A.; Overman, L.E. Vinylsilane- and Alkynylsilane-Terminated Cyclization Reactions. Chem. Rev. 1986, 86, 857. [Google Scholar] [CrossRef]
- Komiyama, T.; Minami, Y.; Hiyama, T. Recent Advances in Transition-Metal-Catalyzed Synthetic Transformations of Organosilicon Reagents. ACS Catal. 2017, 7, 631–651. [Google Scholar] [CrossRef]
- Roy, A.K. A review of recent progress in catalyzed homogeneous hydrosilation (hydrosilylation). Adv. Organomet. Chem. 2007, 55, 1–59. [Google Scholar]
- Anderson, E.; Lim, D. Synthesis of Vinylsilanes. Synthesis 2012, 44, 983–1010. [Google Scholar] [CrossRef]
- Shvydkiy, N.V.; Rimskiy, K.V.; Perekalin, D.S. Cyclobutadiene platinum complex as a new type of precatalyst for hydrosilylation of alkenes and alkynes. Appl. Organomet. Chem. 2023, 33, e7008. [Google Scholar] [CrossRef]
- Ibáñez-Ibáñez, K.; Lázaro, A.; Mejuto, C.; Crespo, M.; Vicent, C.; Rodríguez, L.; Mata, J.A. Visible light harvesting alkyne hydrosilylation mediated by pincer platinum complexes. J. Catal. 2023, 428, 115155. [Google Scholar] [CrossRef]
- Puerta-Oteo, R.; Munarriz, J.; Polo, V.; Jiménez, M.V.; Pérez-Torrente, J.J. Carboxylate-Assisted β-(Z) Stereoselective Hydrosilylation of Terminal Alkynes Catalyzed by a Zwitterionic Bis-NHC Rhodium(III) Complex. ACS Catal. 2020, 10, 7367–7380. [Google Scholar] [CrossRef]
- Sánchez-Page, B.; Munarriz, J.; Jiménez, M.V.; Pérez-Torrente, J.J.; Blasco, J.; Subias, G.; Passarelli, V.; Álvarez, P. β-(Z) Selectivity Control by Cyclometalated Rhodium(III)-Triazolylidene Homogeneous and Heterogeneous Terminal Alkyne Hydrosilylation Catalysts. ACS Catal. 2020, 10, 13334–13351. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Yi, M.; Wu, X.; Lu, Y. Dirhodium(II)/XantPhos Catalyzed Synthesis of β-(E)-Vinylsilanes via Hydrosilylation and Isomerization from Alkynes. Chem. Eur. J. 2024, 30, e202402406. [Google Scholar]
- Ren, S.; Ye, B.; Li, S.; Pang, L.; Pan, Y.; Tang, H. Well-defined coordination environment breaks the bottleneck of organic synthesis: Single-atom palladium catalyzed hydrosilylation of internal alkynes. Nano Res. 2022, 15, 1500–1508. [Google Scholar] [CrossRef]
- Roemer, M.; Gonçales, V.R.; Keaveney, S.T.; Pernik, I.; Lian, J.; Downes, J.; Gooding, J.J.; Messerle, B.A. Carbon supported hybrid catalysts for controlled product selectivity in the hydrosilylation of alkynes. Catal. Sci. Technol. 2021, 11, 1888–1898. [Google Scholar] [CrossRef]
- Li, Q.; Gu, D.; Yu, D.; Liu, Y. Caged Iridium Catalyst for Hydrosilylation of Alkynes with High Site Selectivity. ChemCatChem 2022, 14, e202101727. [Google Scholar] [CrossRef]
- Dai, W.; Wu, X.; Li, C.; Zhang, R.; Wang, J.; Liu, H. Regio-selective and stereo-selective hydrosilylation of internal alkynes catalyzed by ruthenium complexes. RSC Adv. 2018, 8, 28261–28265. [Google Scholar] [CrossRef]
- Kanno, K.; Noguchi, S.; Ono, Y.; Egawa, S.; Otsuka, N.; Mita, M.; Kyushin, S. Ruthenium-catalyzed hydrosilylation of alkynes with preservation of the Si–Si bond of hydrooligosilanes: Regio- and stereoselective synthesis of (Z)-alkenyloligosilanes and carbonyl-functionalized alkenyldisilanes. J. Organomet. Chem. 2022, 961, 122234. [Google Scholar] [CrossRef]
- Saridakis, I.; Kidonakis, M.; Stratakis, M. Unique Reactivity of Dihydrosilanes under Catalysis by Supported Gold Nanoparticles: cis-1,2-Dehydrogenative Disilylation of Alkynes. ChemCatChem 2018, 10, 980–983. [Google Scholar] [CrossRef]
- Feng, X.; Guo, J.; Wang, S.; Wu, Q.; Chen, Z. Atomically dispersed gold anchored on carbon nitride nanosheets as effective catalyst for regioselective hydrosilylation of alkynes. J. Mater. Chem. A 2021, 9, 17885–17892. [Google Scholar] [CrossRef]
- Lu, Z.; Guo, J. Highly Chemo-, Regio-, and Stereoselective Cobalt-Catalyzed Markovnikov Hydrosilylation of Alkynes. Angew. Chem. Int. Ed. 2016, 55, 10835–10838. [Google Scholar]
- Teo, W.; Wang, C.; Tan, Y.; Ge, S. Cobalt-Catalyzed Z-Selective Hydrosilylation of Terminal Alkynes. Angew. Chem. Int. Ed. 2017, 56, 4328–4332. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Hou, W.; Zhang, Y.; Huang, Z. Pincer cobalt complex-catalyzed Z-selective hydrosilylation of terminal alkyne. Org. Chem. Front. 2017, 4, 1517–1521. [Google Scholar] [CrossRef]
- Wu, G.; Chakraborty, U.; Wangelin, A.J. Regiocontrol in the cobalt-catalyzed hydrosilylation of alkynes. Chem. Commun. 2018, 54, 12322–12325. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-H.; An, X.-M.; Yang, M.; Li, D.-C.; Hu, Z.-L.; Zhan, Z.-P. Highly Regio- and Stereoselective Heterogeneous Hydrosilylation of Terminal Alkynes over Cobalt-Metalated Porous Organic Polymer. Org. Lett. 2018, 20, 5023–5026. [Google Scholar] [CrossRef]
- Skrodzki, M.; Patroniak, V.; Pawluć, P. Schiff Base Cobalt(II) Complex-Catalyzed Highly Markovnikov-Selective Hydrosilylation of Alkynes. Org. Lett. 2021, 23, 663–667. [Google Scholar] [CrossRef]
- Wang, D.; Lai, Y.; Wang, P.; Leng, X.; Xiao, J.; Deng, L. Markovnikov Hydrosilylation of Alkynes with Tertiary Silanes Catalyzed by Dinuclear Cobalt Carbonyl Complexes with NHC Ligation. J. Am. Chem. Soc. 2021, 143, 12847–12856. [Google Scholar] [CrossRef]
- Park, J.-W. Cobalt-catalyzed alkyne hydrosilylation as a new frontier to selectively access silyl-hydrocarbons. Chem. Commun. 2022, 58, 491–504. [Google Scholar] [CrossRef]
- Stachowiak-Dłużyńska, H.; Kuciński, K.; Wyrzykiewicz, B.; Kempe, R.; Hreczycho, G. Co-catalyzed Selective syn-Hydrosilylation of Internal Alkynes. ChemCatChem 2023, 15, e202300592. [Google Scholar] [CrossRef]
- Liu, Z.-K.; Zhang, G.-L.; Li, D.-C.; Yang, Y.; Chen, L.; Zhan, Z.-P. Iron-Catalyzed Synthesis of (E)-β-Vinylsilanes via a Regio- and Stereoselective Hydrosilylation from Terminal Alkynes. Synlett 2019, 30, 235–239. [Google Scholar]
- Guo, Z.; Wen, H.; Liu, G.; Huang, Z. Iron-Catalyzed Regio- and Stereoselective Hydrosilylation of 1,3-Enynes To Access 1,3-Dienylsilanes. Org. Lett. 2021, 23, 2375–2379. [Google Scholar] [CrossRef]
- Hu, W.-Y.; He, P.; Qiao, T.-Z.; Sun, W.; Li, W.-T.; Lian, J.; Li, J.-H.; Zhu, S.-F. Iron-Catalyzed Regiodivergent Alkyne Hydrosilylation. J. Am. Chem. Soc. 2020, 142, 16894–16902. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Sen, T.; Kumar, R.; Kumar, C.P.; Kumar, H.; Kumar, K.; Kumar, S.H. Iron-catalyzed (E)-selective hydrosilylation of alkynes: Scope and mechanistic insights. Catal. Sci. Technol. 2024, 14, 2752–2760. [Google Scholar] [CrossRef]
- Behera, R.R.; Saha, R.; Kumar, A.A.; Sethi, S.; Jana, N.C.; Bagh, B. Hydrosilylation of Terminal Alkynes Catalyzed by an Air-Stable Manganese-NHC Complex. J. Org. Chem. 2023, 88, 8133–8149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; Zhang, F.-L.; Xu, J.-L.; Shan, C.-C.; Zhao, M.; Xu, Y.-H. Copper-Catalyzed Anti-Markovnikov Hydrosilylation of Terminal Alkynes. Org. Lett. 2020, 22, 7735–7742. [Google Scholar] [CrossRef]
- Xu, J.-L.; Wang, Z.-L.; Zhao, J.-B.; Xu, Y.-H. Enantioselective construction of Si-stereogenic linear alkenylhydrosilanes via copper-catalyzed hydrosilylation of alkynes. Chem Catal. 2024, 4, 100887. [Google Scholar] [CrossRef]
- Bai, D.; Cheng, R.; Yang, J.; Xu, W.; Chen, X.; Chang, J. Regiodivergent hydrosilylation in the nickel(0)-catalyzed cyclization of 1,6-enynes. Org. Chem. Front. 2022, 9, 5285–5291. [Google Scholar] [CrossRef]
- Docherty, J.H.; Peng, J.; Dominey, A.P.; Thomas, S.P. Activation and discovery of earth-abundant metal catalysts using sodium tert-butoxide. Nat. Chem. 2017, 9, 595–600. [Google Scholar] [CrossRef]
- Wu, C.; Teo, W.; Ge, S. Cobalt-Catalyzed (E)-Selective anti-Markovnikov Hydrosilylation of Terminal Alkynes. ACS Catal. 2018, 8, 5896–5900. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Tan, C.; Wu, W.; Jiang, H. DDQ-mediated regioselective C-S bond formation: Efficient access to allylic sulfides. Org. Chem. Front. 2018, 5, 3158–3162. [Google Scholar] [CrossRef]
- Li, C.; Li, M.; Zhong, W.; Jin, Y.; Li, J.; Wu, W.; Jiang, H. Palladium-Catalyzed Oxidative Allylation of Sulfoxonium Ylides: Regioselective Synthesis of Conjugated Dienones. Org. Lett. 2019, 21, 872–875. [Google Scholar] [CrossRef]
- Li, C.; Liao, M.; He, Z.; Chen, Y.; Mai, J.; Dong, R.; Chen, J.; Chen, L. Metal-Free Oxidative Cross-Dehydrogenative Coupling of Alkenes with Thiophenols. ChemistrySelect 2023, 8, e202300845. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).