Abstract
Four new pyrazolo[3,4-d]pyrimidines (P1–P4) were successfully synthesized in good relative yields by reacting 3-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ol with various alkylating agents (methyl iodide, propargyl bromide, and phenacyl bromide) at room temperature in DMF solvent, employing liquid–solid phase transfer catalysis. The P1–P4 structures were elucidated using NMR spectroscopy and X-ray diffraction. Intermolecular interactions in P1–P4 were analyzed via Hirshfeld surface analysis and 2D fingerprint plots. Geometrical parameters were accurately modeled by DFT calculations using the B3LYP hybrid functional combined with a 6–311++G(d,p) basis set. The antiproliferative activity of P1–P4 towards colorectal carcinoma (HCT 116), human hepatocellular carcinoma (HepG2), and human breast cancer (MCF-7) cell lines, along with one normal cell line (WI38) was investigated using the MTT assay and sunitinib as a reference. Compounds P1 and P2 exhibited antiproliferative activities comparable to the reference drug towards all tested cells, with an IC50 range of 22.7–40.75 µM. Both compounds also showed high selectivity indices and minimal cytotoxic effects on the normal cell line. Molecular docking revealed that the significant antiproliferative activity may attributed to the number and type of intermolecular hydrogen bonding established between pyrazolo[3,4-d]pyrimidines and DNA topoisomerase, a common target for various anticancer agents.
1. Introduction
Cancer is one of the foremost causes of the increase in the number of deaths and morbidity around the globe, mainly in the aging population, and appears to be more likely related to changes in unhealthy lifestyles. Various cancers are categorized according to the damaged tissue or organ. Certain cancers have well-defined mechanisms through which normal cells become cancerous. In the last decades of the 20th century, oncological studies reported that most cancerous cells exhibit a few acquired molecular, biochemical, and cellular characteristics that result from the alteration of key pathways [1]. Heterocycles are the main component of the widely available anti-cancer drugs; the FDA reported that two-thirds of anti-cancer agents contain heterocycle rings. In recent years, there has been increased interest in the chemistry of pyrazolo[3,4-d]pyrimidines owing to their wide-ranging biological activities, such as anticancer [2], antibacterial [3], antitubercular [4], and antitumor effects [5].
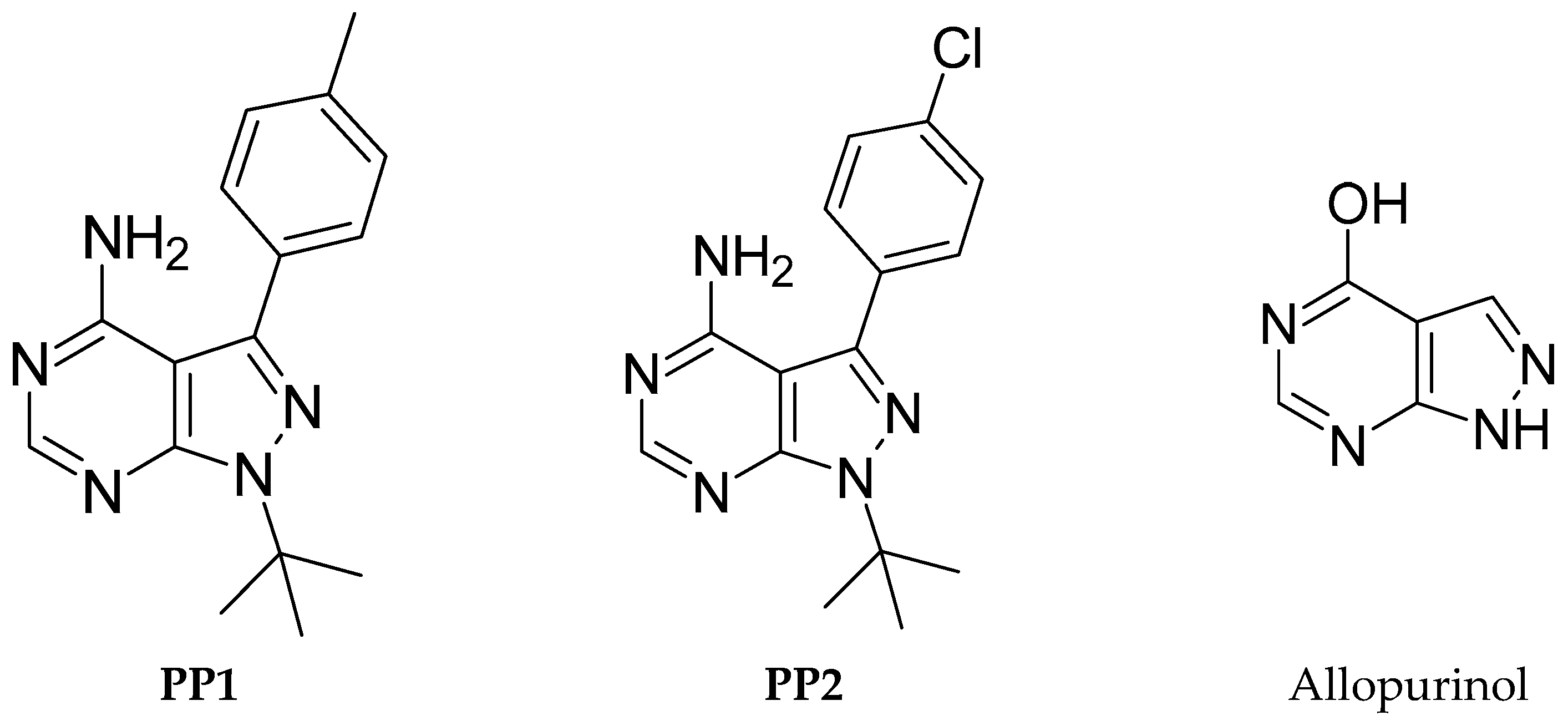
This nucleus constitutes a class of heterocyclic compounds of interest both chemically and pharmaceutically, as they were found to exhibit their antitumor activity by inhibiting different types of enzymes such as Bruton’s tyrosine kinase (BTK) [6], Src tyrosine kinase [7,8], Myt1 kinase [9], and TRAP1 kinase [10]. To the best of our knowledge, the first pyrazolo[3,4-d]pyrimidine kinase inhibitors identified were 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP1) and 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) (Figure 1), which were first discovered to act as kinase inhibitors of the SRC family of non-receptor tyrosine kinases in 1996 [11]. Indeed, pyrazolo[3,4-d]pyrimidines have several active sites, giving them a high reactivity, making these heterocycles excellent precursors in synthesizing new heterocyclic systems likely to present a broad spectrum of activity. For example, Allopurinol (Figure 1), first synthesized by Robins is used in human clinics as a drug in the treatment of gout, a condition characterized by increased levels of uric acid in the blood [12]. Accordingly, synthesizing new pyrazolo[3,4-d]pyrimidines has attracted more attention using various synthetic methods [13,14,15,16,17].
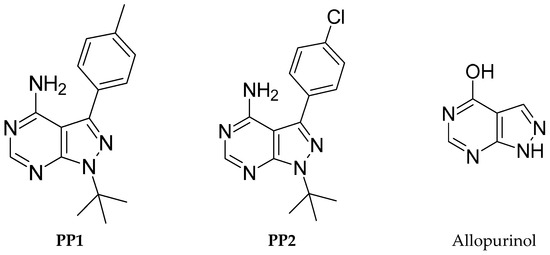
Figure 1.
Chemical structures of reported pyrazolo[3,4-d]pyrimidine derivatives.
Given the importance that pyrazolo[3,4-d]pyrimidine derivatives brought to the biological and therapeutic fields and in continuation of our previous studies [18,19], we report the synthesis and structural elucidation of four new pyrazolo[3,4-d]pyrimidines (P1–P4) that have diverse groups at position 1 of the pyrazolopyrimidine core. Their structures were elucidated by X-ray crystallography and NMR spectroscopy. Additional studies of the new molecules included Hirshfeld surface analysis, DFT calculations, molecular docking, and in vitro antiproliferative investigations.
2. Results and Discussion
2.1. X-Ray Structure Descriptions
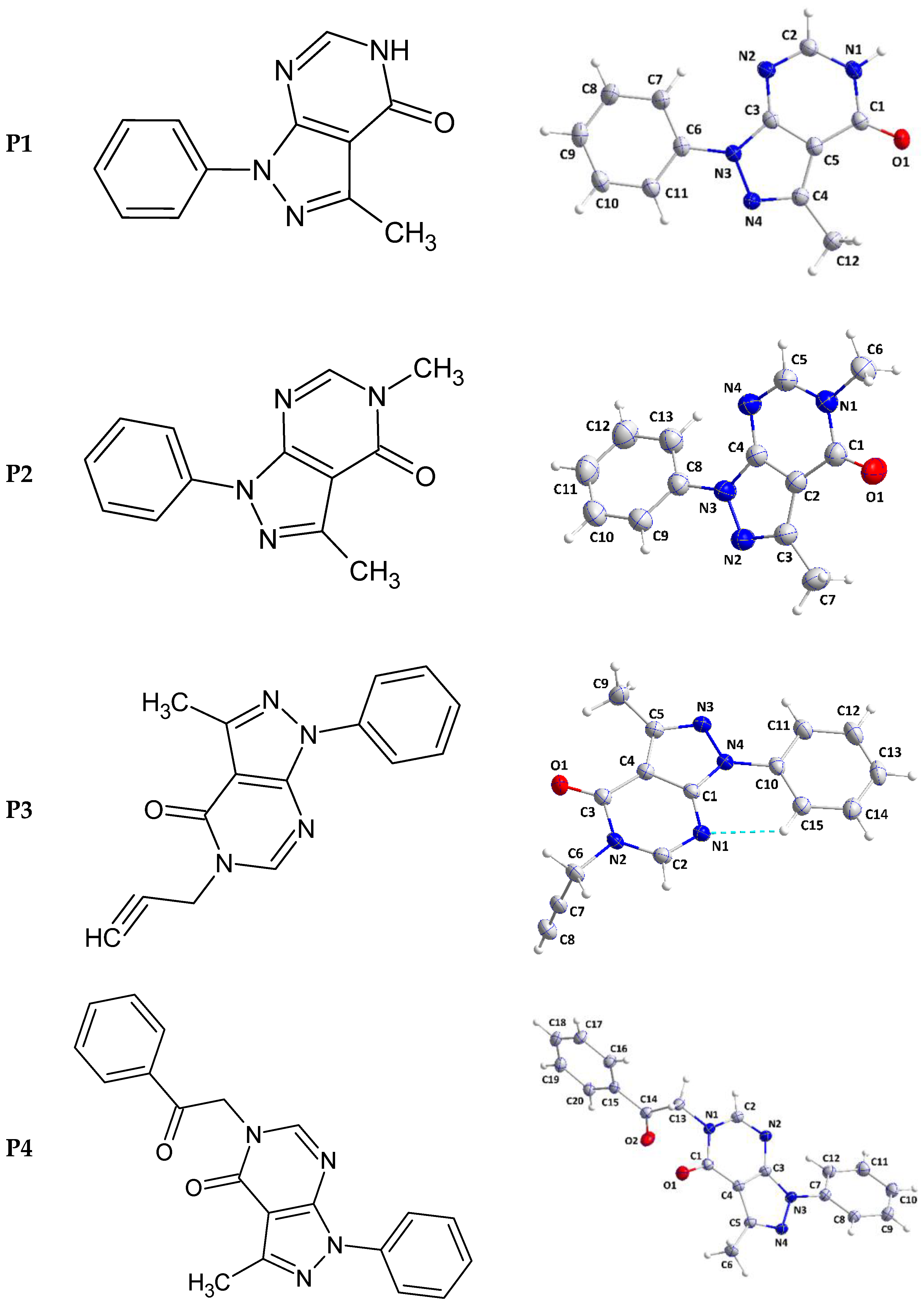
2.1.1. Crystal Structure of 3-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (P1)
The pyrazolopyrimidine unit is slightly non-planar as indicated by the dihedral angle of 1.22(8)° between the mean planes of the constituent rings. The mean plane of the C6···C11 benzene ring is inclined to that of the pyrazole by a dihedral angle of 5.29(9)° so the whole molecular structure is nearly flat (Figure 2).
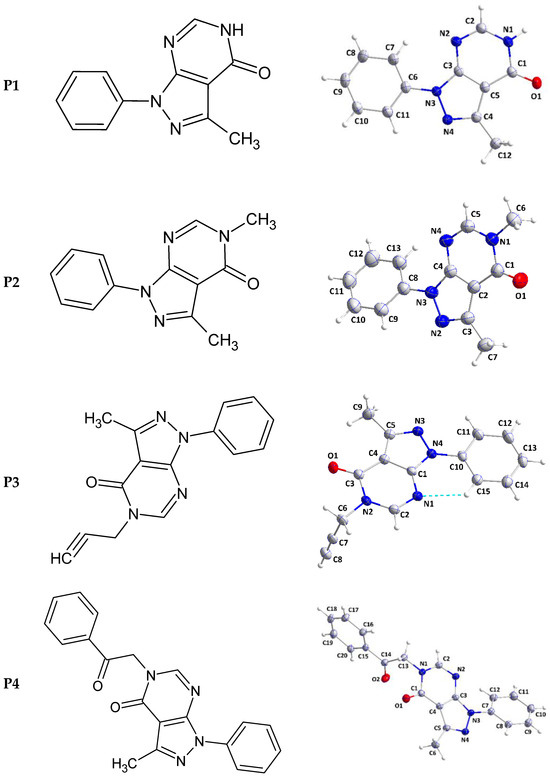
Figure 2.
2D structures (left) and perspective views of P1–P4 (right). The dashed line in P3 represents the intramolecular hydrogen bond.
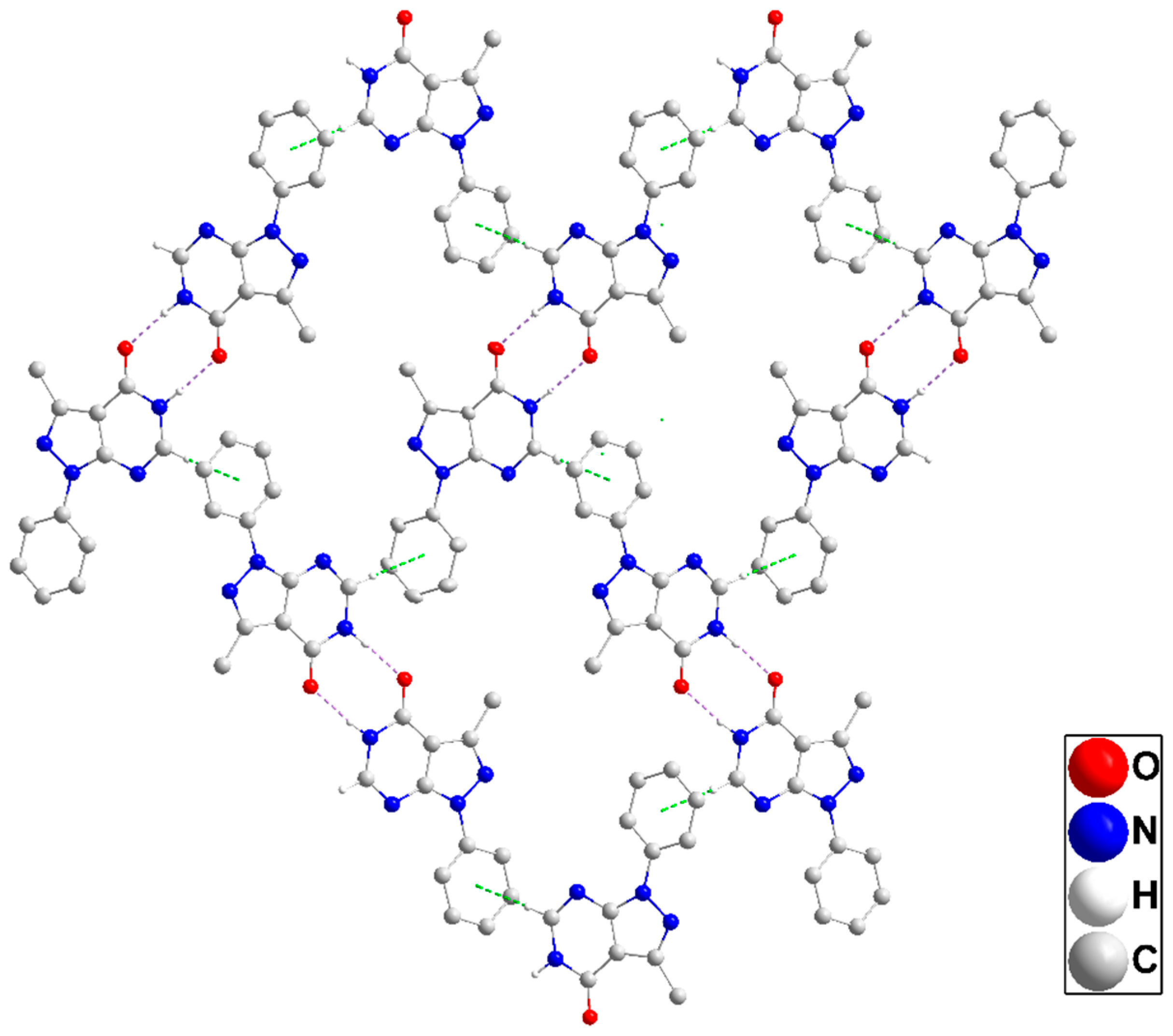
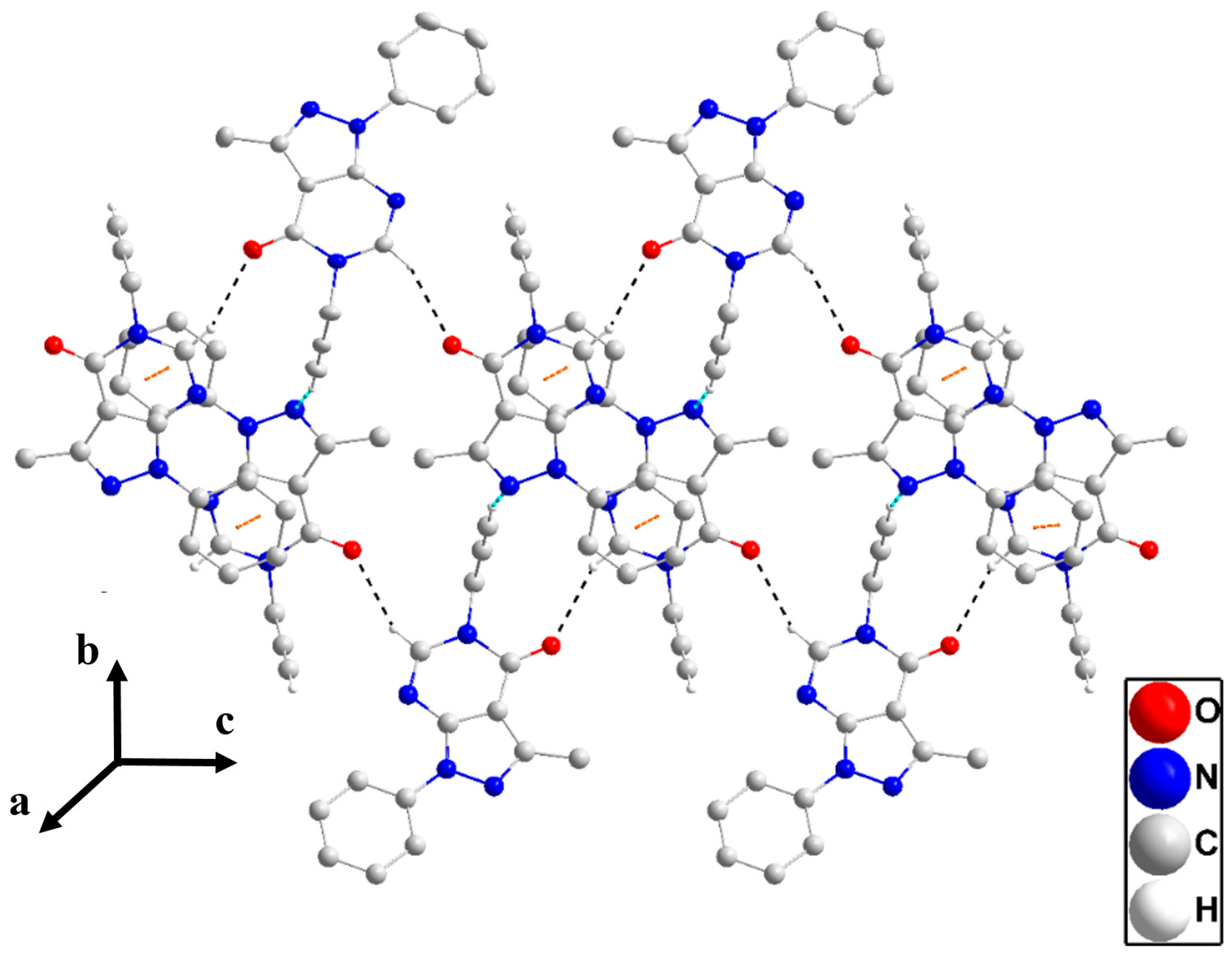
In the crystal, inversion dimers are formed by paired N1—H1···O1 hydrogen bonds (Table 1). The dimers are linked by C2—H2···Cg3 interactions (Table 1) to form corrugated layers of molecules parallel to () (Figure 3). These layers are connected along the a-axis direction through slipped π-stacking interactions between phenyl and pyrazole rings (centroid···centroid = 3.5730(8) Å, dihedral angle = 5.29(7)°) with the slippage alternating between 1.02 and 1.33 Å along the stack (Figure S1). Alternatively, the packing can be described as consisting of oblique stacks of molecules connected by the π-stacking interactions which are then joined by the C2—H2···Cg3 interactions. Crystal, data collection, and refinement details are presented in Table S1.

Table 1.
Hydrogen bond geometry (Å, °) for P1. Cg3 is the centroid of the C6···C11 benzene ring.
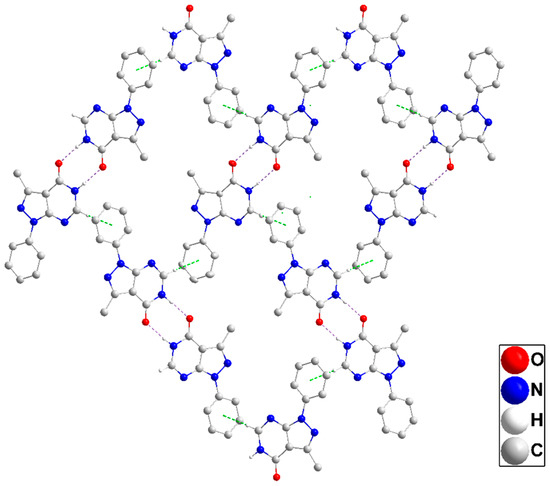
Figure 3.
A portion of one layer in P1 projected onto with the b-axis horizontal and running from left to right. N—H···O hydrogen bonds and C—H···π(ring) interactions are depicted, respectively, by violet and green dashed lines. Non-interacting hydrogen atoms are omitted for clarity.
2.1.2. Crystal Structure of 3,5-dimethyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (P2)
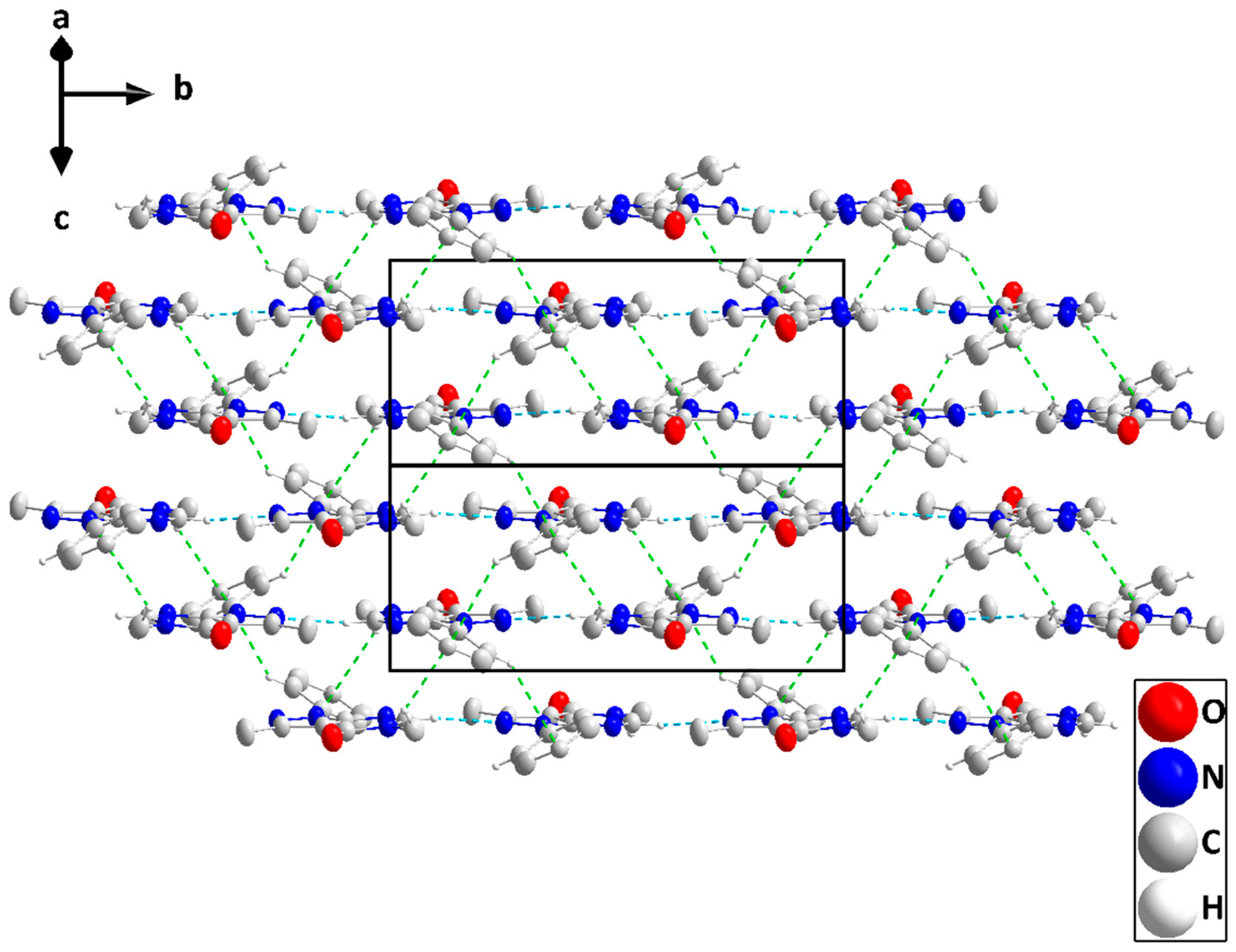
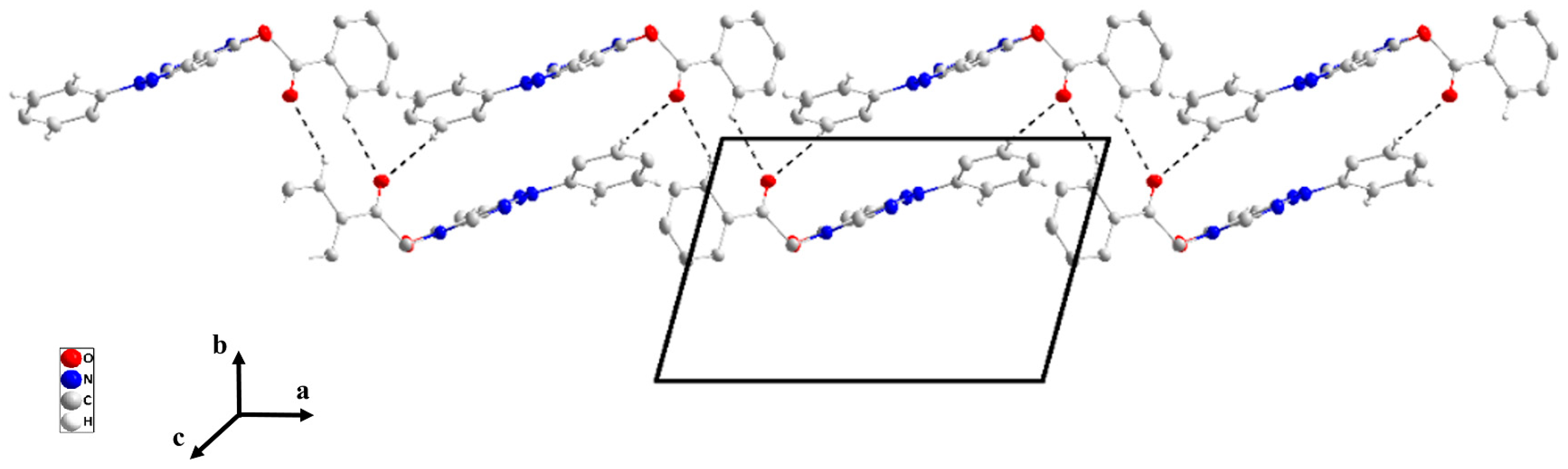
The pyrazolopyrimidine moiety is planar to within 0.0194(7) Å (rmsd = 0.0150 Å) with the plane of the C8···C13 ring inclined to it by 32.38(4)° (Figure 2). In the crystal, C5—H5···N2 hydrogen bonds (Table 2) form chains of molecules extending along the b-axis direction and with the pyrazolopyrimidine moieties approximately parallel to (). These chains stack along the normal to () with C6—H6A···Cg3 and C9—H9···Cg3 interactions (Table 2) holding them together to form layers (Figure 4). Crystal, data collection, and refinement details are presented in Table S2.

Table 2.
Hydrogen bond geometry (Å, °) for P2. Cg3 is the centroid of the C8···C13 benzene ring.
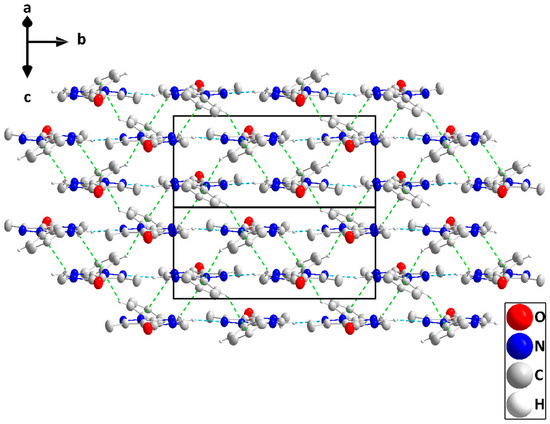
Figure 4.
Elevation view of the packing in P2 seen parallel to () with C—H···N hydrogen bonds and C—H···π(ring) interactions depicted, respectively, by light blue and green dashed lines.
2.1.3. Crystal Structure of 3-methyl-1-phenyl-5-(prop-2-yn-1-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidinone (P3)
The dihydropyrazolopyrimidine moiety is not planar as there is a dihedral angle of 4.38(4)° between the mean planes of its constituent rings. The plane, defined by N2/C6/C7/C8, is inclined to the mean plane of the dihydropyrimidine ring by 84.66(8)° indicating the propynyl group is well out of the plane of the ring. The dihedral angle between the mean planes of the dihydropyrimidine and phenyl rings is only 4.38(5)° and this is likely due to the intramolecular C15—H15···N1 hydrogen bond (Table 3 and Figure 2). In the crystal, zigzag chains of molecules extending along the c-axis direction are formed by C32—H2···O1 hydrogen bonds (Table 3) and are connected into corrugated layers two molecules thick by C8—H8···N3 hydrogen bonds (Table 3) and π-stacking interactions between phenyl and dihydropyrimidine rings in inversion-related molecules (centroid···centroid (at −x + 2, −y + 1, −z + 1) = 3.6770(8) Å, dihedral angle = 4.91(6)°, slippage = 1.19 Å) (Figure 5). The layers stack along the a-axis direction through π-stacking interactions between phenyl and dihydropyrimidine rings in inversion-related molecules (centroid···centroid (at −x + 1, −y + 1, −z + 1) = 3.7177(8) Å, dihedral angle = 4.91(6)°, slippage = 1.66 Å) (Figures S2 and S3). Data collection and refinement details are given in Table S3.

Table 3.
Hydrogen bonding geometry (Å, °) for P3.
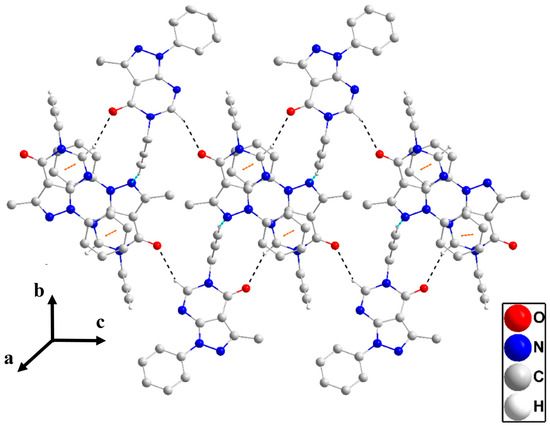
Figure 5.
A portion of two chains in P3 viewed along the a-axis direction with C—H···O and C—H···N hydrogen bonds depicted, respectively, by black and light blue dashed lines. The π-stacking interactions are depicted by orange dashed lines.
2.1.4. Crystal Structure of 3-methyl-5-(2-oxo-2-phenylethyl)-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (P4)
The dihydropyrazolopyrimidine moiety is planar to within 0.0102(6) Å (r.m.s. deviation = 0.0087 Å). The mean planes of the C7···C12 and the C15···C20 benzene rings are inclined to the above plane by 29.25(3) and 85.79(3)°, respectively. The C1—N1—C13—C14 torsion angle of −78.99(9)° indicates that the C14=O2 carbonyl group is also nearly perpendicular to the mean plane of the dihydropyrazolopyrimidine moiety (Figure 2). In the crystal, C11—H11···O2 and C20—H20···O2 hydrogen bonds (Table 4) form chains of molecules extending along the a-axis direction (Figure 6). The chains are linked by C6—H6C···N2 hydrogen bonds and C9—H9···Cg4 interactions (Table 4) forming layers approximately parallel to (011) (Figure S4). Crystal, data collection, and refinement details are presented in Table S4.

Table 4.
Hydrogen bond geometry (Å, °) for P4. Cg4 is the centroid of the C15···C20 benzene ring.
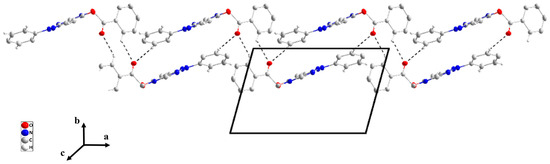
Figure 6.
A portion of one chain of P4 view along the b-axis. Dashed lines C—H···O represent hydrogen intermolecular bonding.
2.1.5. General Structural Comments
In all four structures, the sum of the angles about the nitrogen atom in the pyrazole ring bearing the phenyl substituent is 360° within experimental error, thereby implicating the involvement of the nitrogen lone pair in π bonding with neighboring atoms. As might be expected, this occurs within the five-membered ring. For example, in P3, The endocyclic N4—C1 and N4—N3 distances are, respectively, 1.3591(15) and 1.3805(14) Å, while the exocyclic N4—C10 distance is 1.4236(15) Å. Similar patterns are seen in the other structures. A second feature of note is the extent to which the plane of the pendant phenyl group is rotated from the plane of the five-membered ring to which it is attached. In the case of P3, it was noted that there is an intramolecular C—H···N hydrogen bond which likely constrains the phenyl group to approach co-planarity with the 5-membered ring but in the others, the dihedral angle is 5.29(9)° in P1, 32.38(4)° in P2, and 29.25(3)° in P4. A survey of 13 related molecules found corresponding dihedral angles ranging from 5.72(18)° in 5-(2-chloroethyl)-1-(4-chlorophenyl)-6-(2-chloropyridin-3-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one to 28.96(11)° in 5-(2-chloroethyl)-6-(3-fluorophenyl)-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one [20], with the majority among the 13 structures surveyed being between 20 and 27°. Thus, it seems likely that packing considerations are predominant for determining this dihedral angle. If the strongest intermolecular interactions act to keep the dihydropyrazolopyrimidine cores well-separated, then the phenyl ring can rotate out of the plane of the core moiety to relieve intramolecular steric congestion. If not, π-stacking interactions can be influential which will necessitate the five- and six-membered rings being closer to co-planarity.
2.2. Optimized Geometries
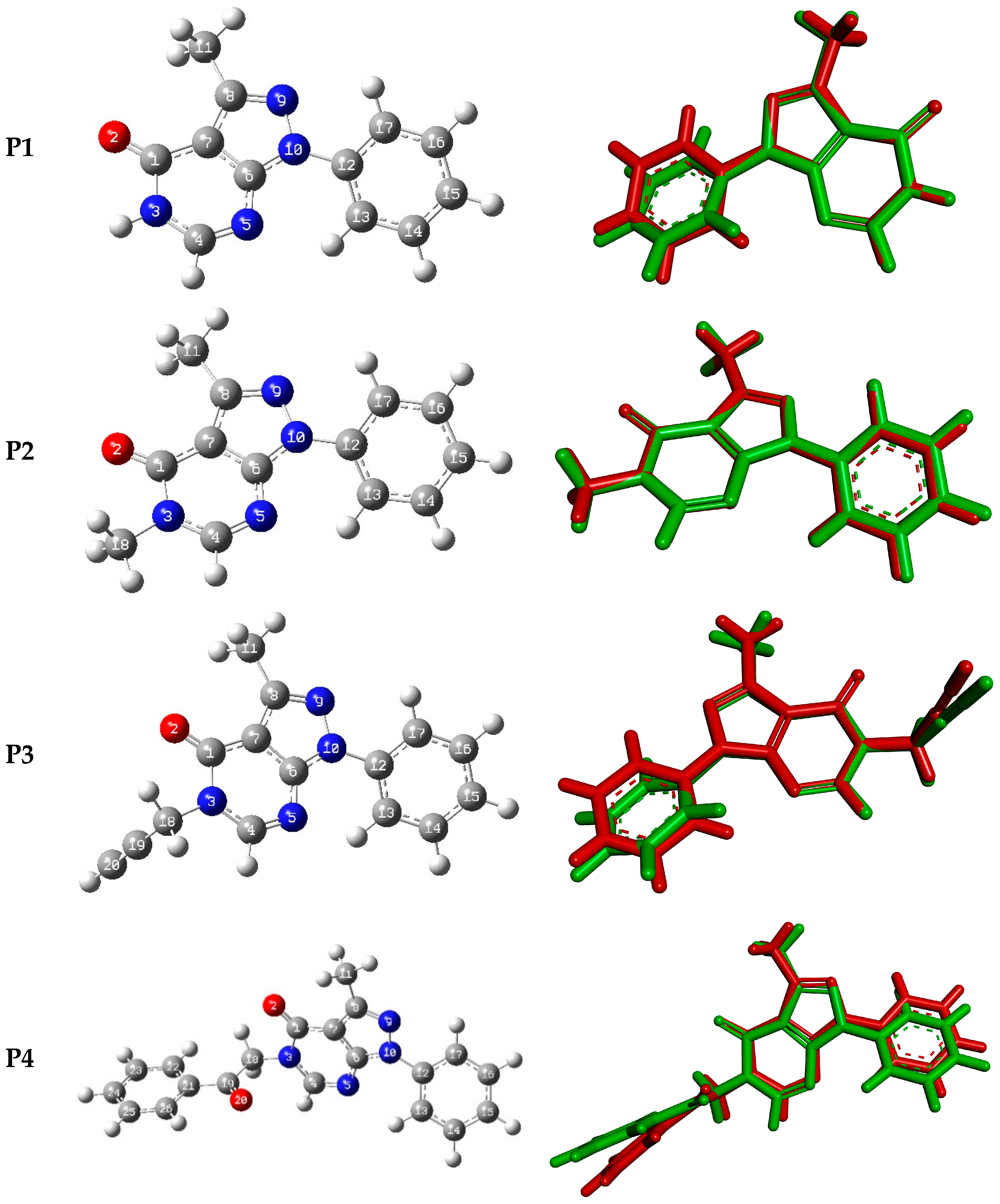
Figure 7 shows the optimized geometries of P1–P4 along with their corresponding numbering and their superpositions upon the experimental structures. Selected experimental and calculated bond lengths, bond angles, and torsion angles for P1–P4 are summarized in Table 5. The experimental bond lengths are relatively well reproduced with variations less than 0.03 Å. Similarly, the bond angles are relatively well reproduced with variations of less than 3 degrees compared to the experimental ones. The agreement between calculated and experimental torsion angles is much poorer, reflecting the presence of packing constraints in the latter which are absent in the former. RMSD values calculated from the superimposed X-ray and optimized geometries of P1–P4 are 0.33, 0.087 Å, 0.43, and 0.63, respectively. Furthermore, the RMSD values were calculated for bond lengths, bond angles, and dihedral angles of P1–P4, and they are found in ranges of 0.08–0.12Å, 0.50–5.00 degrees, and 3.50–13.00 degrees, respectively. Figure S13 displays the linear regression curves between the experimental bond lengths, bond angles, and dihedral angles, and their corresponding predicted ones. The correlation between the experimental bond lengths, bond angles, and torsion angles with their corresponding calculated ones yield linear curves of correlation coefficients in the ranges of 92.00–99.50%, 91.50–99.95%, and 99.00–99.90%, respectively (Figure S13).
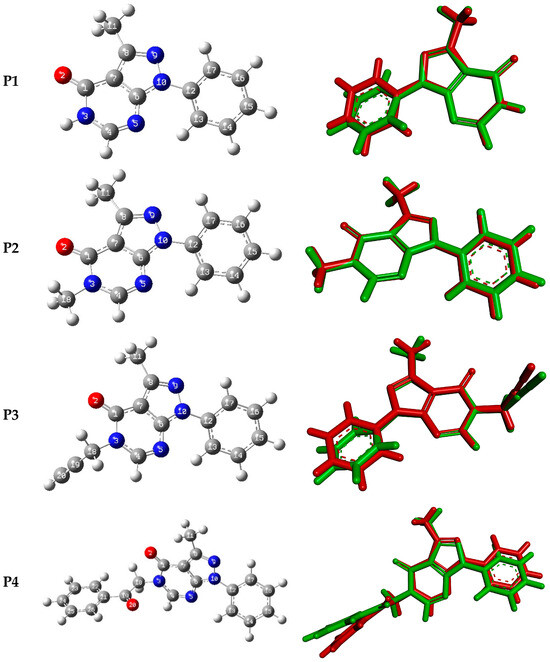
Figure 7.
The optimized geometries of P1–P4 (left) and their superposition with X-ray-generated ones (right, the green color corresponds to the optimized geometry, and the red color corresponds to the X-ray-generated structure).

Table 5.
Selected experimental and calculated geometrical parameters of P1–P4.
2.3. Hirshfeld Surface Analysis
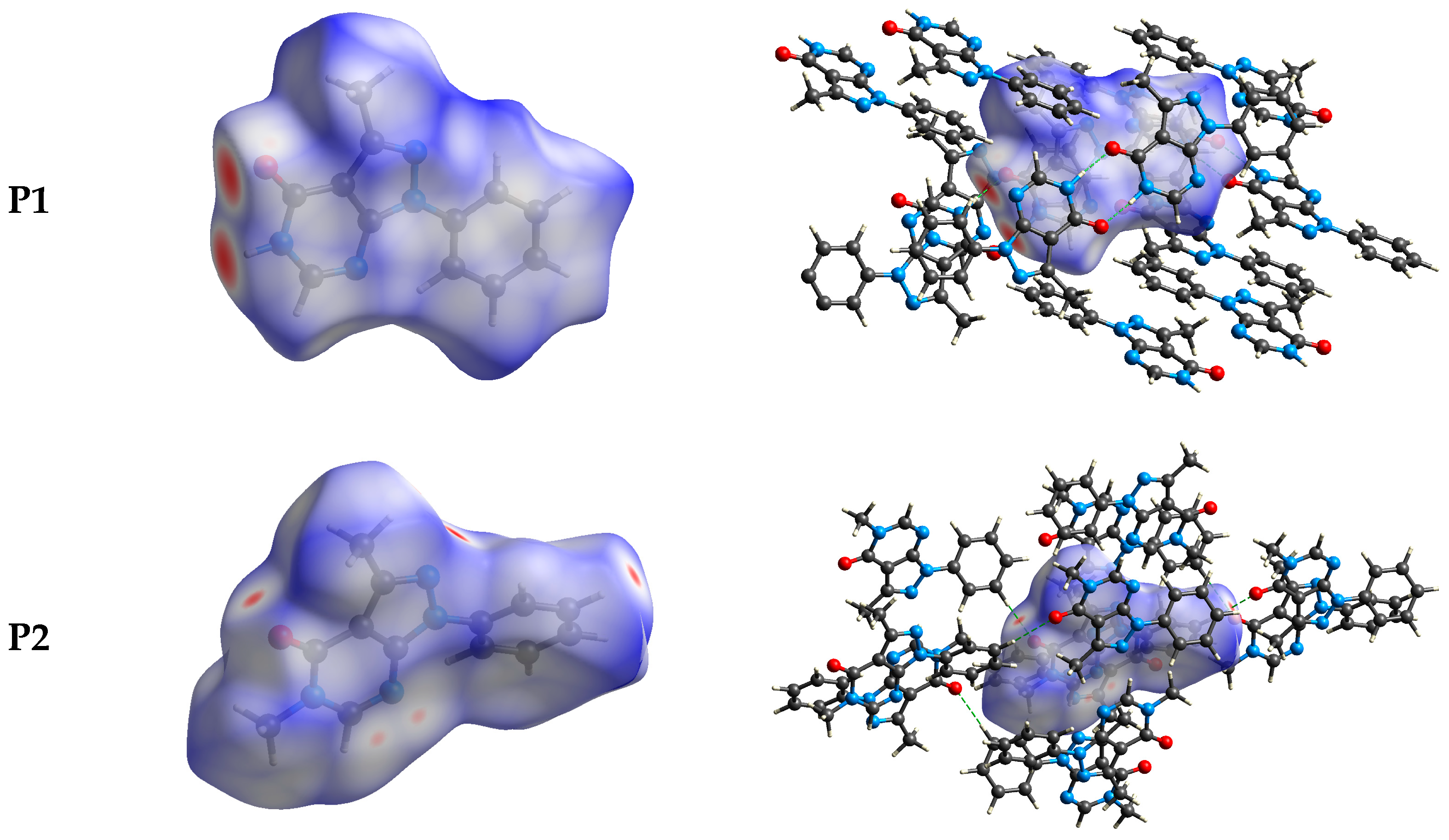
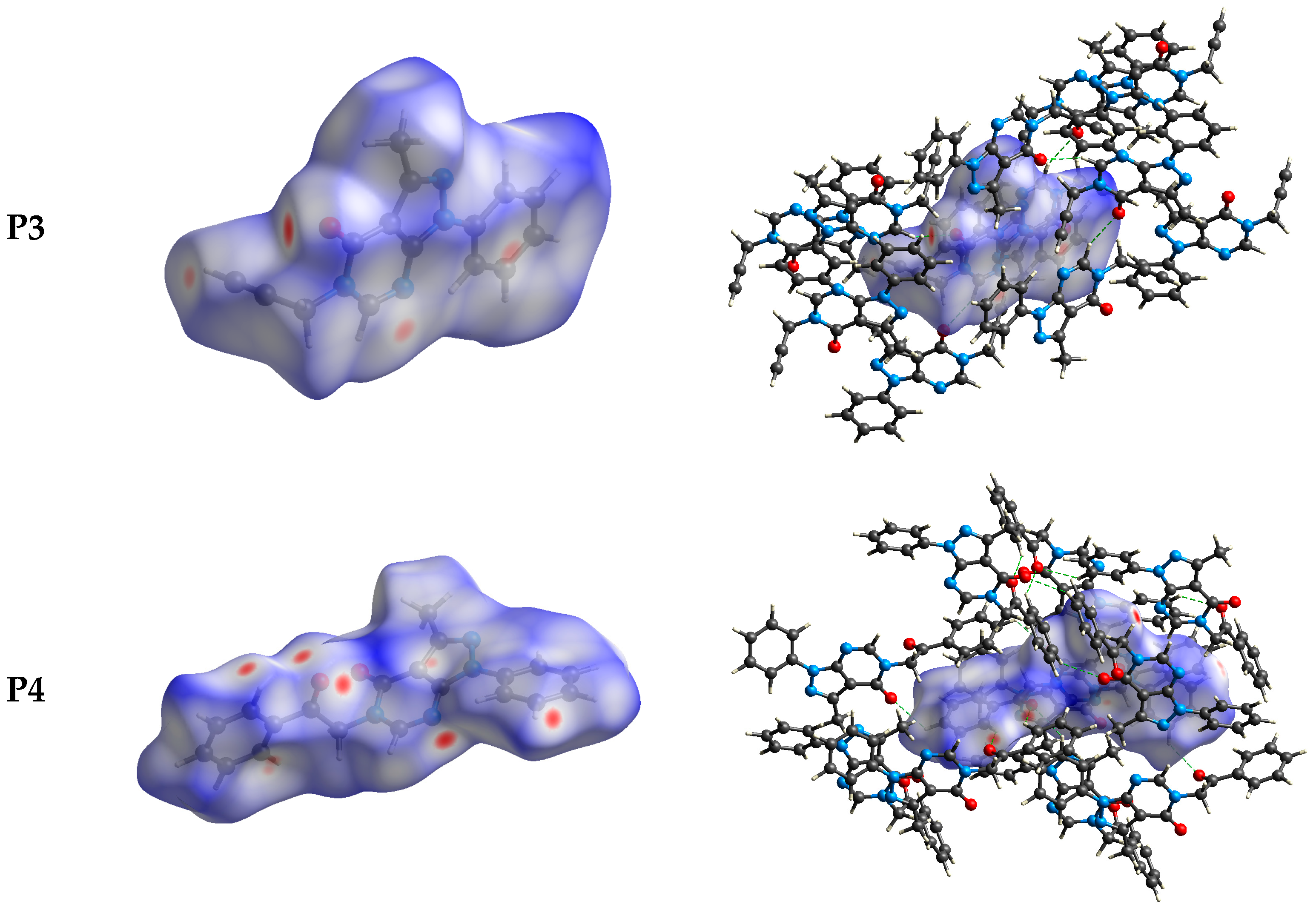
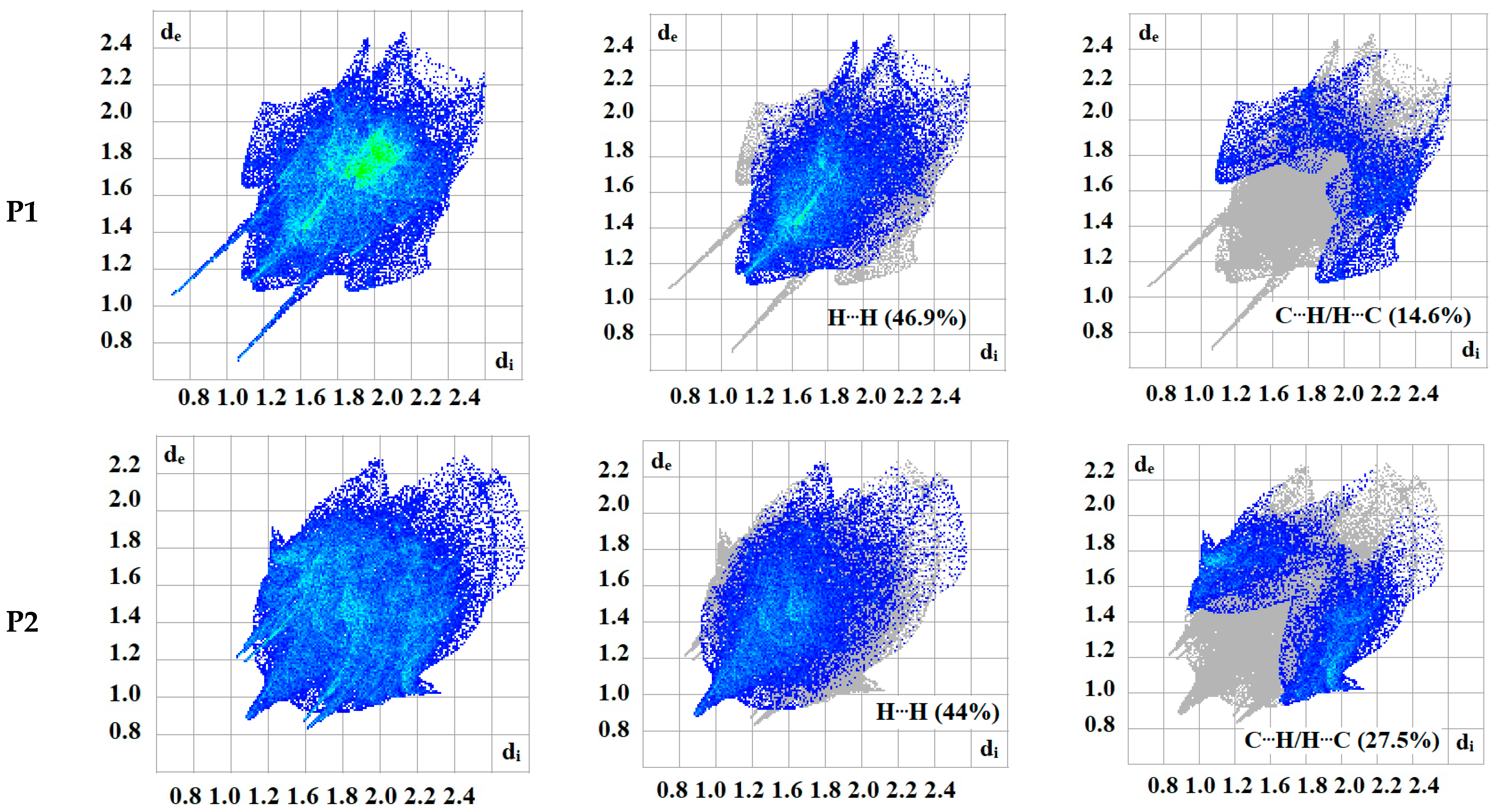
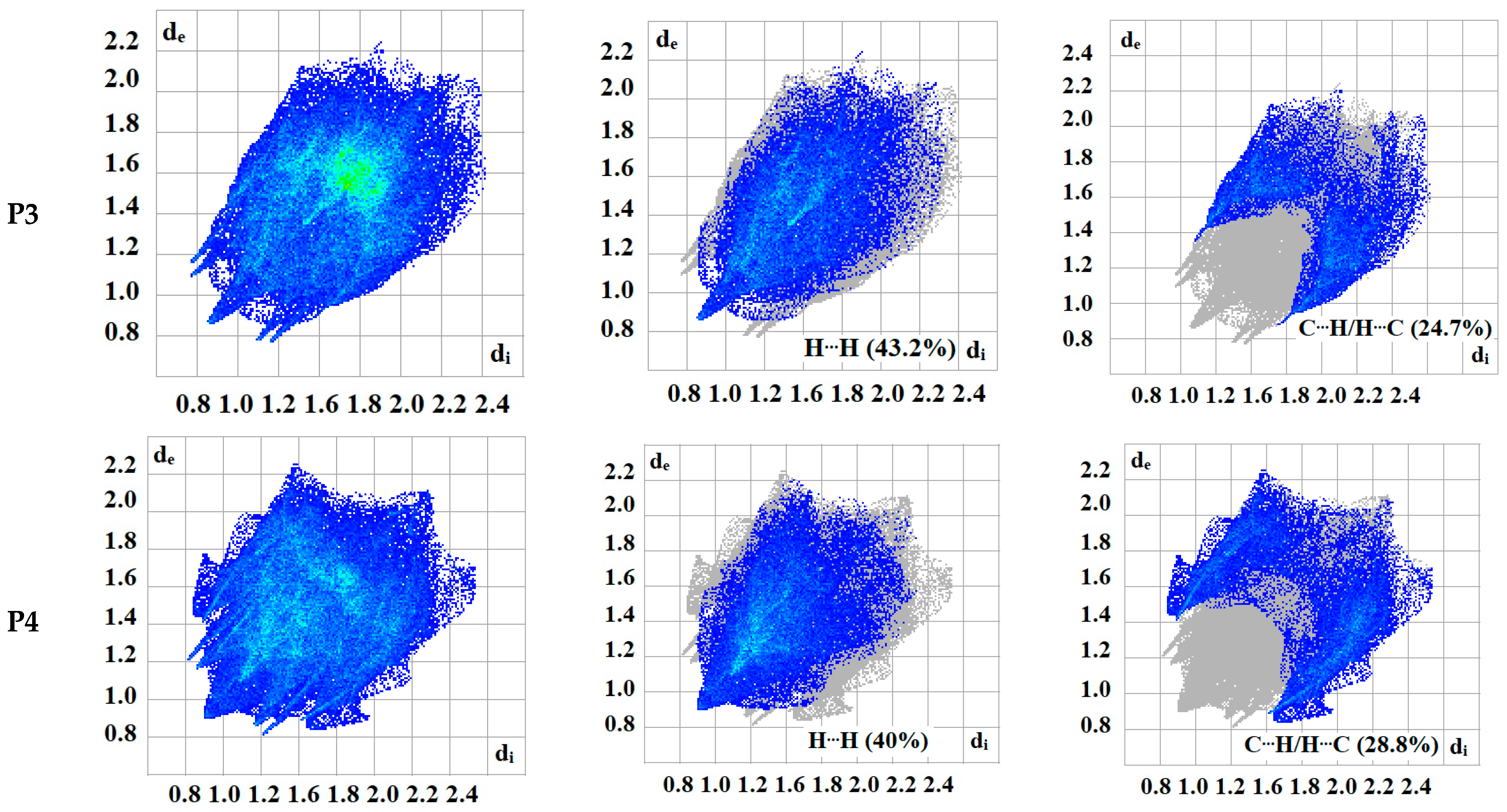
The intermolecular bonding interactions in P1–P4 crystals were investigated by analyzing their corresponding Hirshfeld surfaces (HS). Descriptions and interpretations of the plots obtained from the HS analysis have been published [21]. The red spots on the Hirshfeld surface indicate contacts less than the sum of the van der Waals radii of the respective elements, while those longer than this sum are displayed in blue, and those on the order of this sum are shown in white (Figure 8, left column). The right column of Figure 8 shows each molecule with its HS surrounded by several “next neighbors” that are involved in close contact. The 2D fingerprints giving the contacts contributing most to the overall intermolecular interactions in P1–P4 are displayed in Figure 9. In all crystals, the highest intercontacts are found for H···H, and C···H/H···C interactions (Table 6).
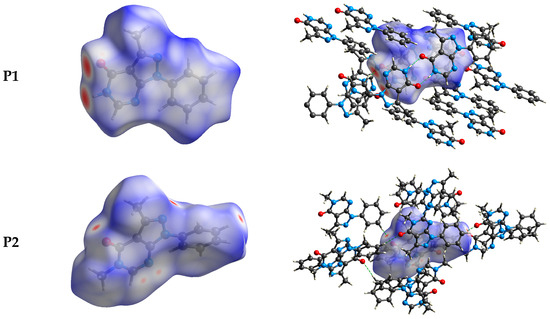
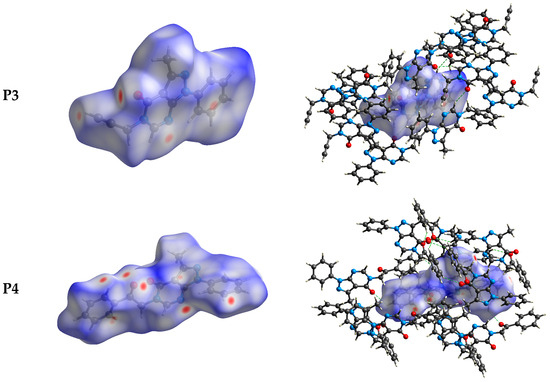
Figure 8.
The dnorm surfaces for viewing hydrogen bonding interactions in P1–P4 crystals.
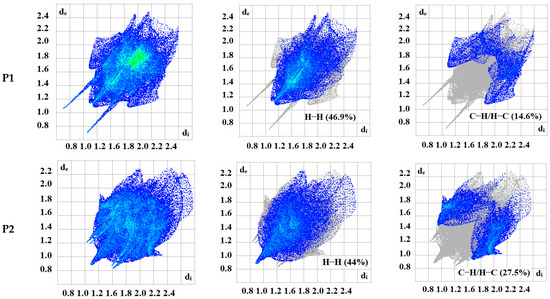
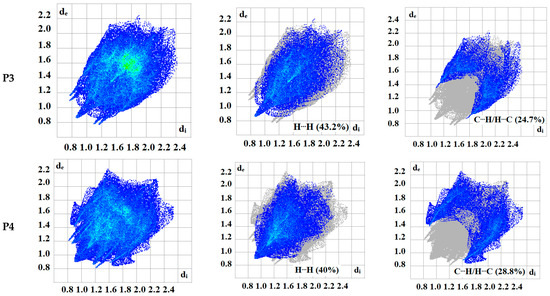
Figure 9.
2D fingerprints of the highest intercontacts in P1–P4.

Table 6.
The largest contributors to intermolecular contacts in P1–P4.
This can be attributed to the significant hydrogen content of the four molecules and the fact that most of the hydrogen atoms are attached to phenyl and methyl groups which protrude out from the centers of the molecules and thus comprise a significant portion of their peripheries. Although not making the largest intermolecular contacts, the fingerprint plot for P1 is notable for the pair of sharp spikes with de + di ≈ 2.1 Å. These can be attributed to the N—H···O hydrogen bonds which, in addition to being the shortest intermolecular contact, have essentially the same H···O distance throughout the crystal. For the other three molecules, the fingerprint plots show two pairs of sharp points that extend slightly from either side of the center point. Those in P1 both have de + di ≈ 2.4 Å which is consistent with them representing the C—H···N hydrogen bonds. As the H···N contact distance is longer than the H···O distance in P1, and because it is closer in magnitude to the other intermolecular contacts in P2, these peaks are much less prominent. The same comment applies to the other two overall fingerprint plots with P3 showing peaks at de + di ≈ 2.36 and 2.20 Å and P4 having them at 2.40 and 2.36 Å. In both cases, these can be attributed to C—H···N and C—H···O hydrogen bonds, respectively.
2.4. Antiproliferative Activity
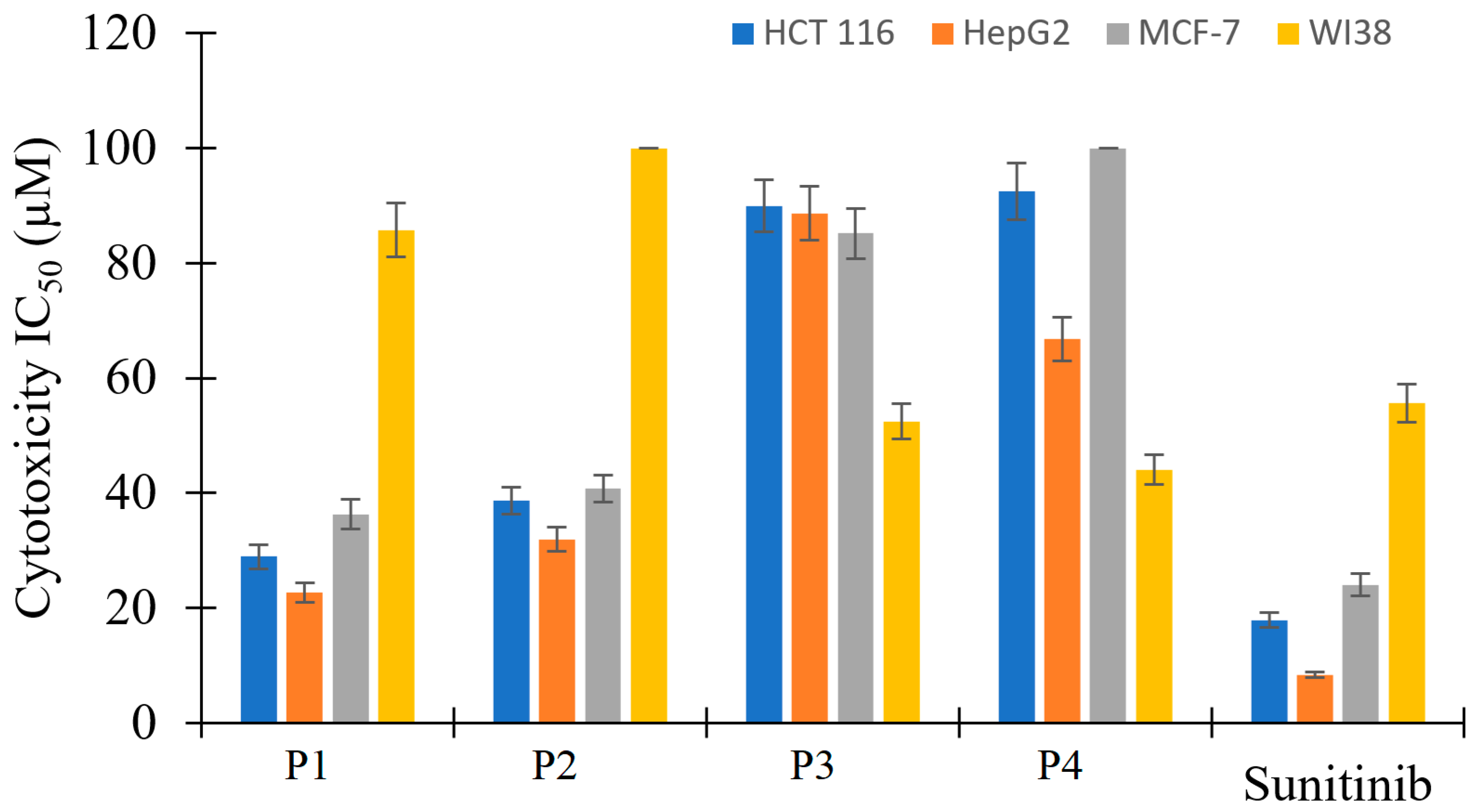
The antiproliferative activity of P1–P4 was evaluated by the MTT standard assay and sunitinib (a well-known FDA-approved multi-kinase inhibitor) as a reference against HCT 116, HepG2, and MCF-7 cell lines, and WI38 normal cell line. Compounds P3 and P4 exhibited very weak anticancer activity with IC50 values above 67 µM (Table 7 and Figure 10); they show more cytotoxicity toward the normal cell line than towards the cancer cell lines. By contrast, P1 and P2 showed an antiproliferative activity comparable to the reference standard anticancer drug against the cancer cell lines as their results have less than three-fold difference compared to sunitinib (IC50 values in 22.7–40.75 µM). P1 and P2 showed excellent selectivity indices as they displayed very weak cytotoxic effects on the normal cell line. The obtained results indicate that biological targets through which the tested compounds exhibit their anticancer effect could not perfectly accommodate relatively huge substitutions on position number three. Therefore, P3 and P4 exhibited weak anticancer effects.

Table 7.
In vitro antiproliferative activity of P1–P4 against HCT 116, HepG2, and MCF-7 cancer cell lines, and a normal cell line WI38.
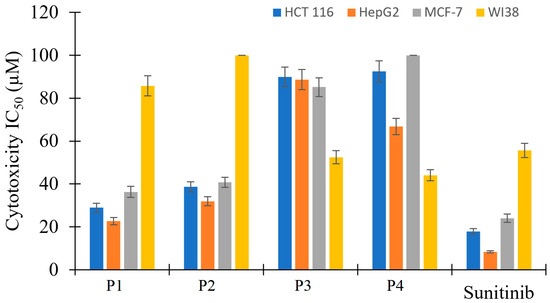
Figure 10.
Bar representation of the in vitro antiproliferative activity of P1–P4 and sunitinib against HCT 116, HepG2, and MCF-7 cancer cell lines, and one normal cell line WI38.
2.5. Molecular Docking Results
Since P1–P4 compounds have no better antiproliferative activity than the reference, their binding affinity within the binding site of the human DNA topoisomerase was explored using molecular docking to better understand their modest activity. The calculated binding energies of the stable complexes Pi=1–4-DNA topoisomerase, the number of intermolecular hydrogen bonds, and the number of closest amino acids that interact with P1–P4 are summarized in Table 8.

Table 8.
Calculated binding energies (BE), number of hydrogen bonds (HBs), and the number of closest residues (NR) to the docked P1–P4 into DNA topoisomerase binding site.
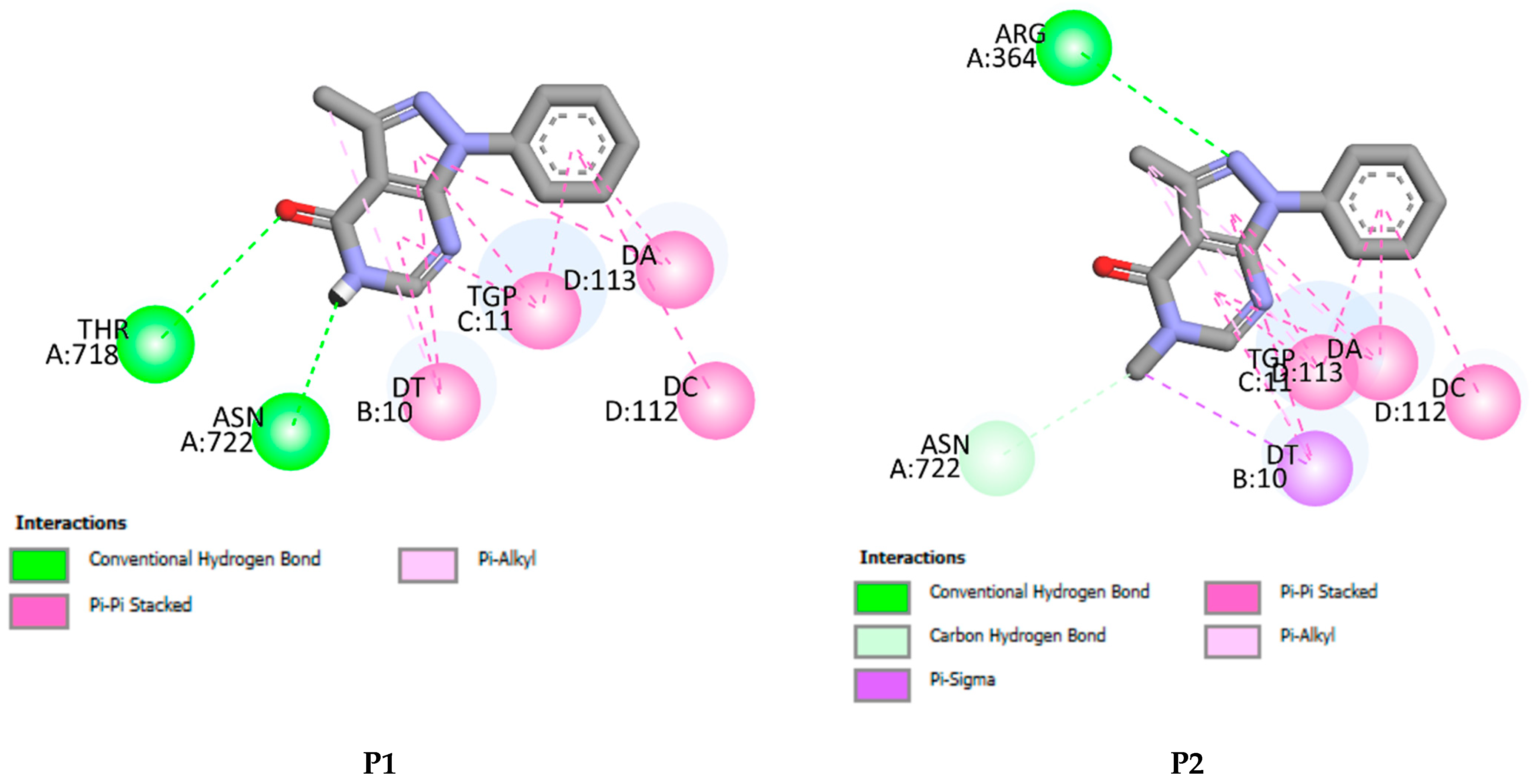
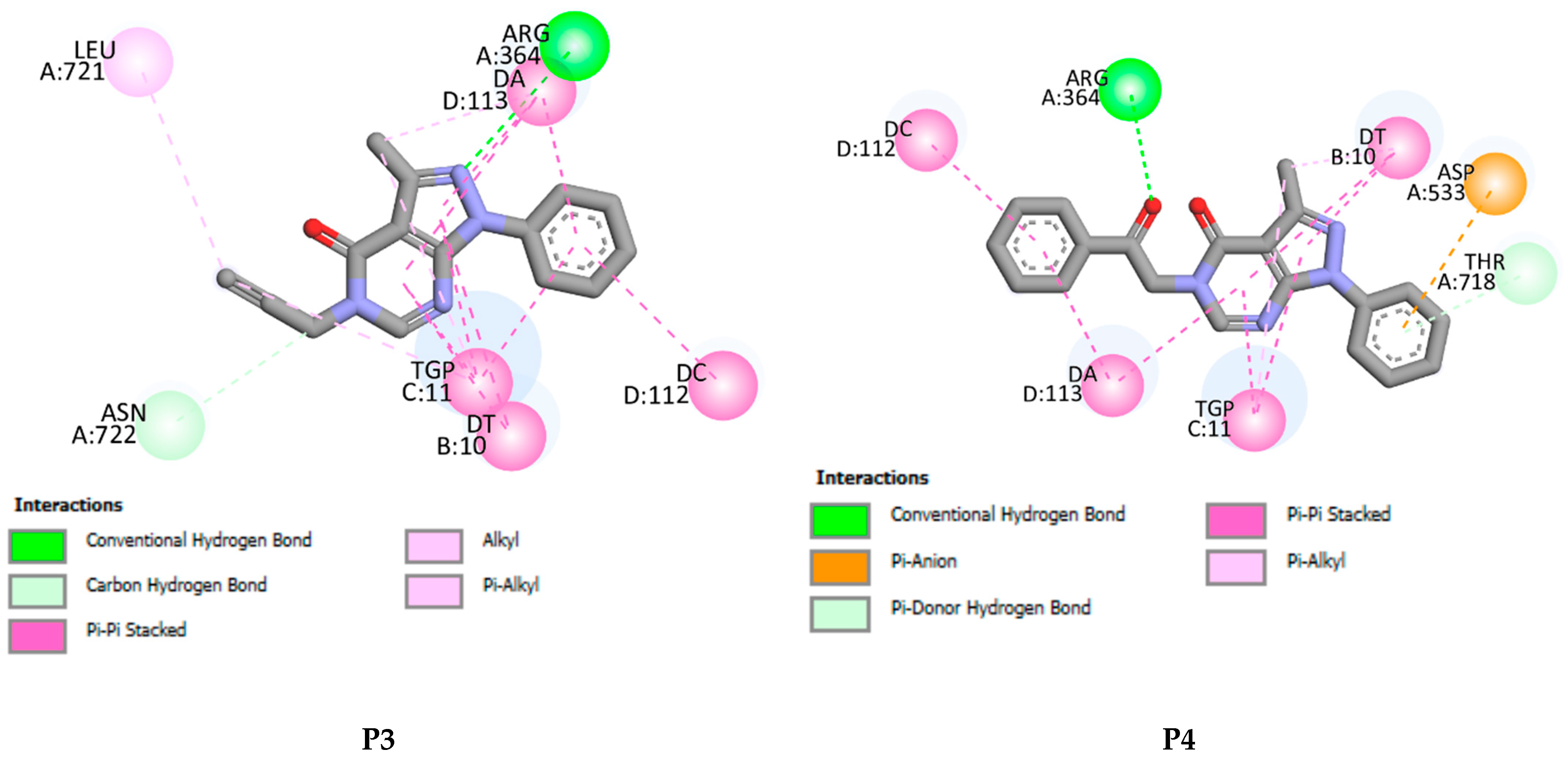
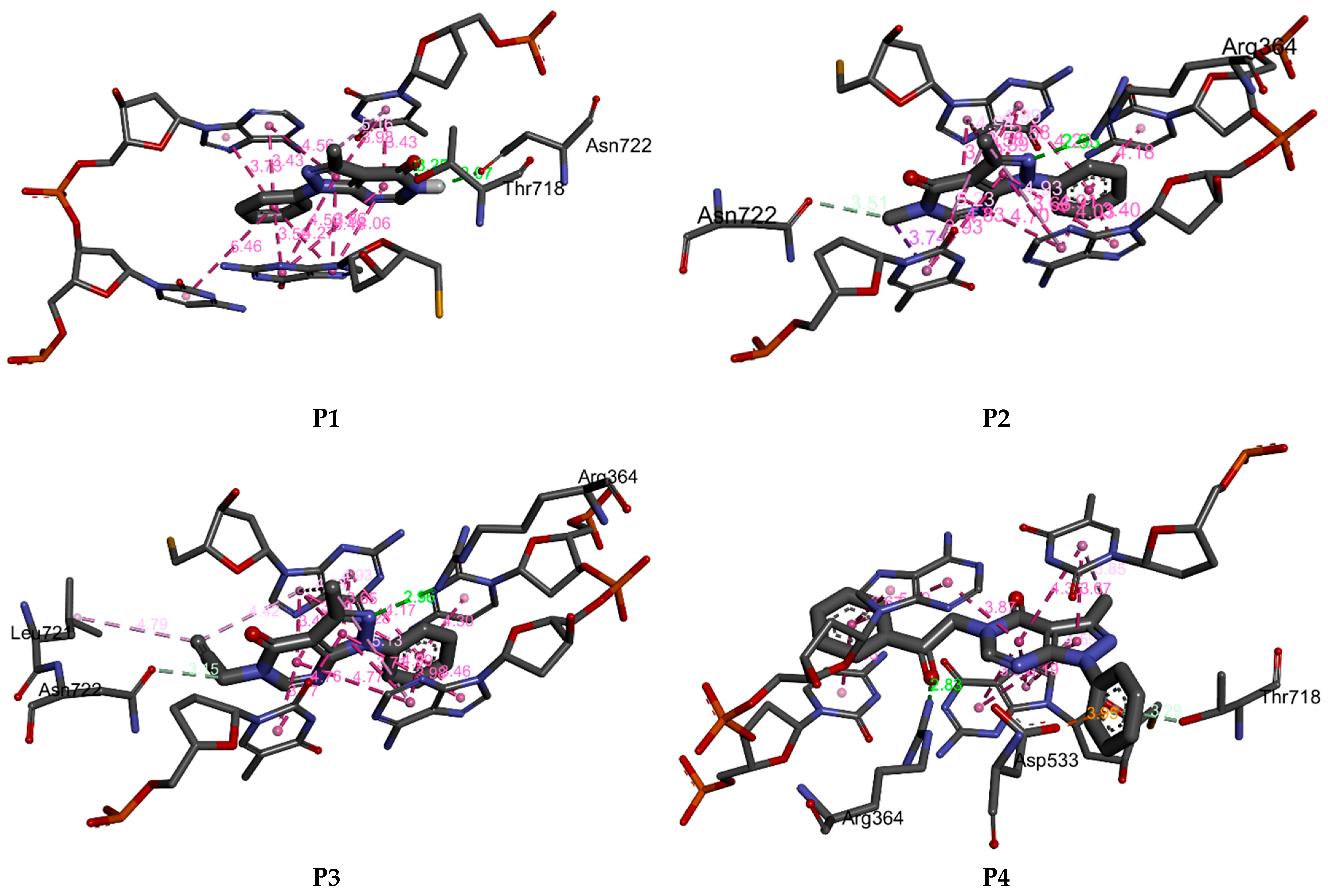
P1–P4 fit well into the DNA topoisomerase binding site. They lead to the formation of stable complexes with binding energies in the range of −7 to −8.52 kcal.mol−1. The negative binding energies may indicate that the inhibition of DNA topoisomerase by P1–P4 is thermodynamically favorable and spontaneous. Figure 11 and Figure 12 display, respectively, the 2D and 3D binding interaction modes established in stable Pi=1–4-DNA topoisomerase complexes. These interactions involve (i) conventional hydrogen bond interactions (ii) π-π stacked interactions, (iii) π-alkyl, (iv) π-σ, and (v-carbon hydrogen bond interactions (Figure 11 and Figure 12). Binding energies of stable Pi=1–4-DNA topoisomerase complexes vary slightly (less than 1.5 kcal-mol−1) so the binding energy may not be a significant factor in explaining the observed antiproliferative activity of P1–P4 against the three cancer cell lines. Among P1–P4, P1 shows the best activity against the three cell lines and it may be that the higher activity of P1 relates to the number of hydrogen bonds it forms with the amino acids of DNA topoisomerase and the types of amino acids involved. In P2–P4, a hydrogen bond is established with ARG A364. However, in P1, its keto and amine functional groups form two hydrogen bonds with THR A178 and ASN A722 of DNA topoisomerase of distances 3.25 and 2.07 Å, respectively (Figure 11 and Figure 12). Thus, one may conclude that the higher inhibition of P1 may return to hydrogen binding established between ASN A722 and the amine of pyrazolo[3,4-d]pyrimidine.
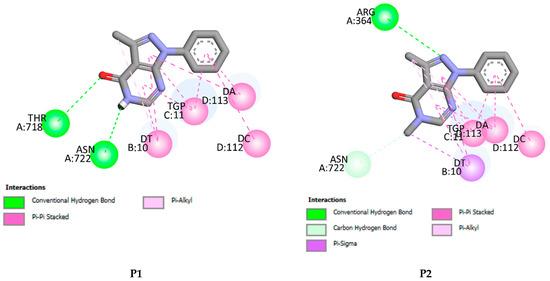
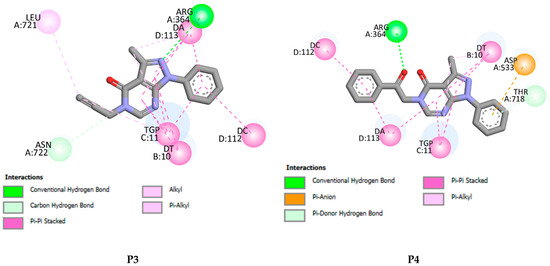
Figure 11.
2D binding affinities of P1–P4 into the DNA topoisomerase binding site.
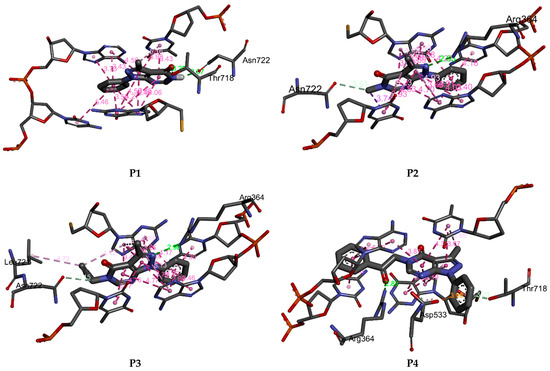
Figure 12.
3D binding affinities of P1–P4 into the DNA topoisomerase binding site.
3. Materials and Methods
3.1. Reagents and Instruments
All chemicals used in this work were purchased from commercial sources (Sigma-Aldrich, Fluka, Saint Louis, MO, USA). They were used without further purification. Mel-Temp 3.0 apparatus was used to determine the melting points. NMR spectra were generated using Bruker Advance/DPX 250 (250 MHz 1H, 62.9MHz 13C) and Bruker (Billerica, MA, USA) Advance II/DPX 400 (400 MHz 1H, 100 MHz 13C) spectrometers.
3.2. Synthesis
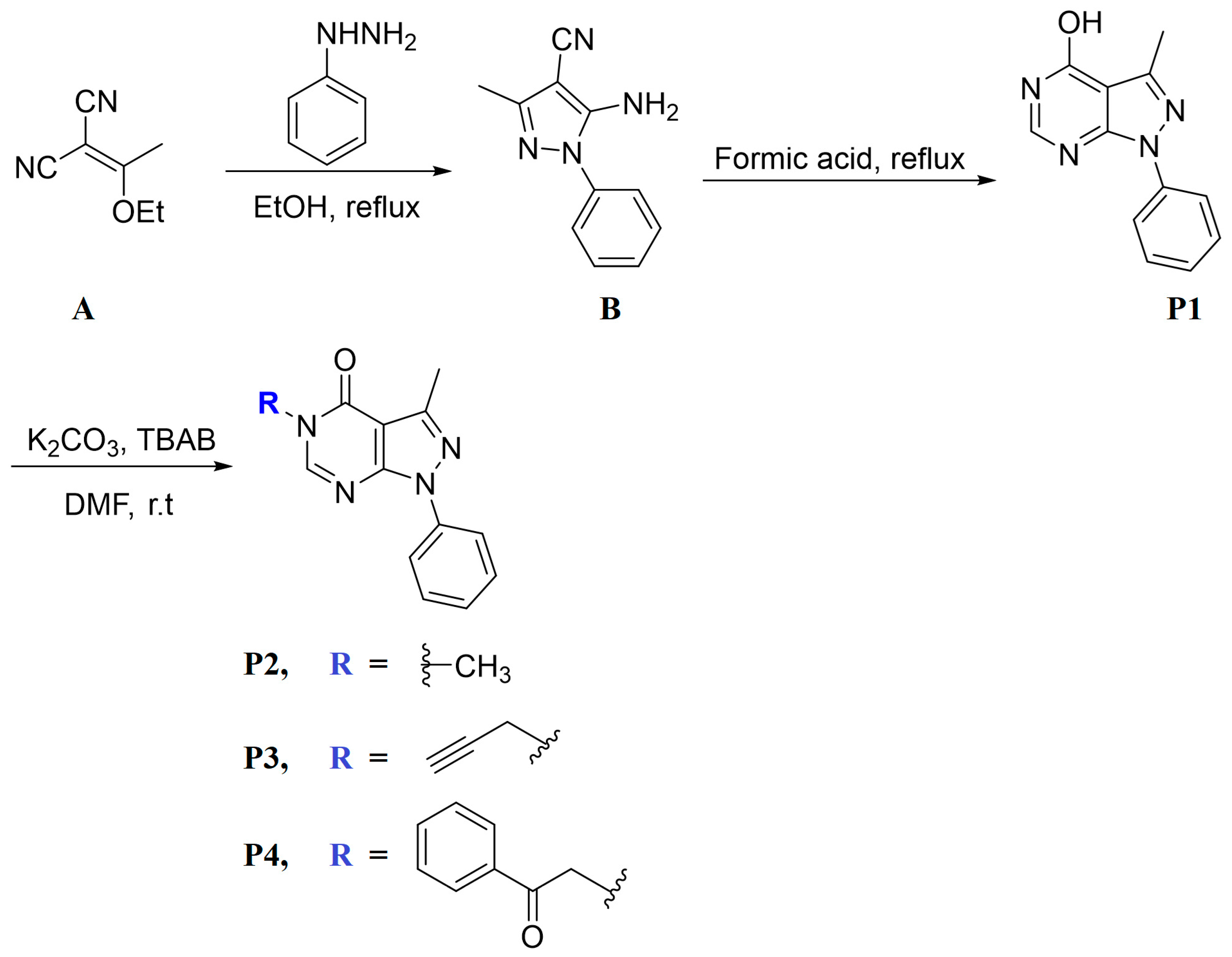
Pyrazolo [3,4-d] pyrimidine derivatives (P1–P4) were synthesized by following the procedure mentioned under Scheme 1. Based on the literature, 2-(1-ethoxyethylidene)malononitrile (A) and phenyl hydrazine were refluxed in ethanol for 2 h to afford 5-amino-3-methyl-1-phenyl-1H-pyrazole-4-carbonitrile (B) [20]. The latter was reacted with formic acid under reflux for 7 h to give compound P1 in good yield, which was reacted with the selected alkylating agent: methyl iodide, propargyl bromide, phenacyl bromide to give the final products P2, P3, and P4, respectively, in good yield.
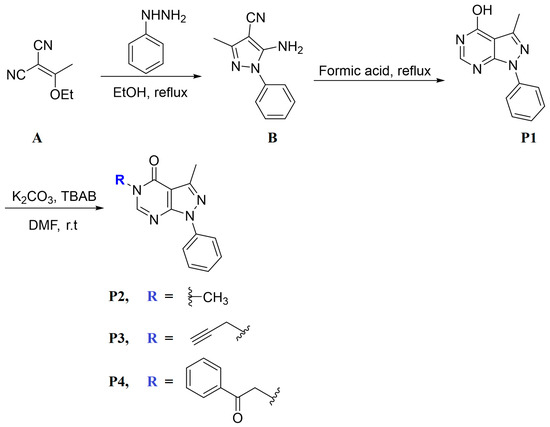
Scheme 1.
Synthesis path of P1–P4.
3.2.1. Synthesis of P1
A solution of 5-amino-3-methyl-1-phenyl-1H-pyrazole-4-carbonitrile (B) (1.0 g, 5.04 mmol) in formic acid (30 mL) was refluxed for 7 h. The final mixture was poured into ice water and the resulting precipitate was filtered off, dried, and recrystallized from ethanol (Scheme 1).
3.2.2. Synthesis of P2–P4
A DMF (5 mL) solution containing P1 (0.1 g, 0.44 mmol), K2CO3 (1.2 equiv.), and tetra n-butylammonium bromide (TBAB) catalyst was stirred for 10 min. Then, the selected alkylating agent (1.2 equiv.) was added to the solution, and the mixture was stirred overnight. The mixture was then poured into ice water and the resulting precipitate was filtered off, dried, and recrystallized from ethanol yielding the final product (Scheme 1).
3,5-Dimethyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (P2)
Yield: 70%. mp: 143–145 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.40 (s, 1H), 8.02–7.99 (m, 2H), 7.54–7.50 (m, 2H), 7.37–7.33 (m, 1H), 3.47 (s, 3H), 2.52 (s, 3H) (Figure S7).13C NMR (101 MHz, DMSO-d6) δ 157.60, 151.90, 151.70, 145.86, 138.27, 129.24, 126.66, 121.20, 104.94, 33.15, 13.41 (Figure S8).
3-Methyl-1-phenyl-5-(prop-2-yn-1-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (P3)
Yield 65%, mp = 155–157 °C. 1H NMR (250 MHz, DMSO-d6) δ 8.54 (s, 1H), 8.02–7.99 (m, 2H), 7.54 (m, 2H), 7.39 (m, 1H), 4.82 (d, J = 2.5 Hz, 2H), 3.43 (t, J = 2.5 Hz, 1H), 2.55 (s, 3H) (Figure S9).13C NMR (63 MHz, DMSO-d6) δ 156.31, 151.44, 150.71, 146.10, 138.02, 129.21, 126.87, 121.47, 104.84, 78.65, 75.75, 34.63, 13.34 (Figure S10).
3-Methyl-5-(2-oxo-2-phenylethyl)-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (P4)
Yield 69%, mp = 1170–172 °C. 1H NMR (250 MHz, DMSO-d6) δ 8.43 (s, 1H), 8.12–8.03 (m, 4H), 7.75–7.73 (m, 1H), 7.65–7.53 (m, 4H), 7.42–7.35 (m, 1H), 5.64 (s, 2H), 2.53 (s, 3H) (Figure S11).13C NMR (63 MHz, DMSO-d6) δ 192.84, 156.91, 151.84, 151.71, 146.07, 138.12, 134.27, 129.23, 129.07, 128.10, 126.82, 121.44, 104.85, 51.48, 13.32 (Figure S12).
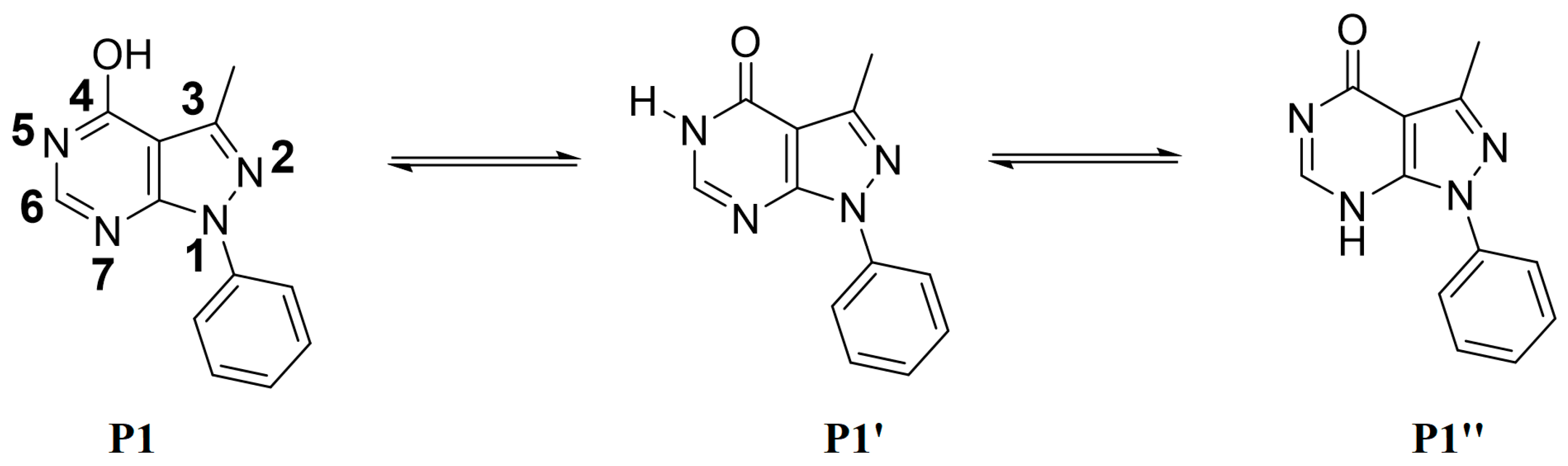
The possible tautomeric forms of P1 (P1–P1″) are displayed in Scheme 2. It possesses three reactive sites towards the alkylating agents N5, N7, and O of OH at C4. The alkylation reactions carried out under liquid–solid phase transfer catalysis lead in each case to only one alkylated product, P2–P4, showing that N5 of the pyrazolopyrimidine is the most reactive site of the heterocyclic compound P1.
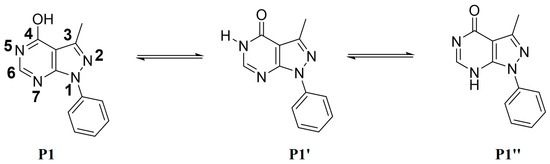
Scheme 2.
Tautomerism of compound P1.
3.3. Single Crystal X-Ray Diffraction
The crystal structures of P1–P4 were generated from X-ray intensity data collected on a Bruker D8 QUEST PHOTON 3 diffractometer. More details on the X-ray diffraction methodology may be found in our previous studies [21].
3.4. Hirshfeld Surface Study
Hirshfeld surfaces and fingerprint plots of P1–P4 were generated with Crystal Explorer 3.0 [22,23]. dnorm was mapped from −0.60 a.u (blue) to 1.25 a.u (red), while 2D fingerprints were plotted and displayed on a 0.8–2.4 Å expanded scale.
3.5. DFT Calculations
The stable geometries of P1–P4 were optimized in solvent using the B3LYP hybrid functional along with the basis set 6–311++G(d,p). The frequency calculations confirm that the optimized geometries of P1–P4 are true minima. The experimental atom coordinates of P1–P4 were used as starting inputs for the optimization. The solvent was modulated using the implicit polarizable continuum model (PCM) and chloroform as solvent [24]. Theoretical calculations were accomplished using the Gaussian 16 software [25].
3.6. Molecular Docking Study
The binding affinities of P1–P4 into the human DNA topoisomerase binding site as the target of anticancer agents were explored with the Autodock package [26]. The structural geometries of the human topoisomerase I-DNA and its EHD ligand were downloaded from the data website RCSB (PDB file 1T8I) [27]. The re-docking of EHD is relatively well reproduced, with an RMSD value of 0.30 Å and binding energy of −10.50 kcal-mol−1. Details of the docking calculations may be found in our previous study [21].
3.7. Cytotoxicity Assay
The antiproliferative activity of P1–P4 was assessed by using an MTT standard assay and sunitinib drug towards HCT 116, HepG2, MCF-7 cells, and WI38 normal cell line using the previously described methodology [21].
4. Summary
Four new pyrazolo[3,4-d]pyrimidines (P1–P4) have been synthesized and their structures determined using NMR spectroscopy and single crystal X-ray diffraction. Hirshfeld surfaces and 2D fingerprints revealed that H···H and C···H/H···C are the major intermolecular contacts in P1–P4. Bond lengths, bond angles, and torsion angles of P1–P4 are relatively well reproduced at the B3LYP level of theory with correlation coefficients higher than 90%. The antiproliferative activity of P1–P4 is weak compared to the antiproliferative activity of the reference standard anticancer drug against the cancer cells with IC50 of 22.7–40.75 µM. Molecular docking reveals that DNA topoisomerase inhibition by P1–P4 is a spontaneous, thermodynamically favorable process and is influenced by the number of hydrogen bonds and the types of amino acids involved in the formation of hydrogen bonds between P1–P4 and the amino acids of DNA topoisomerase.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29215020/s1, Figure S1: Perspective view of the packing with N—H···O hydrogen bonds and C—H···π(ring) and π-stacking interactions depicted, respectively, by violet, green, and orange dashed lines. Non-interacting hydrogen atoms are omitted for clarity; Figure S2: Packing viewed along the a-axis direction with intermolecular interactions depicted as in Figure 4; Figure S3: Packing viewed along the c-axis direction with intermolecular interactions depicted as in Figure 4; Figure S4: Packing viewed along the b-axis direction. C—H···O and C—H···N hydrogen bonds are depicted, respectively, by black and light blue dashed lines while C—H···π(ring) interactions are depicted by green dashed lines. Non-interacting hydrogen atoms are omitted for clarity; Figure S5: 1H NMR spectrum of compound D; Figure S6: 13C NMR spectrum of compound D; Figure S7: 1H NMR spectrum of compound D3; Figure S8: 13C NMR spectrum of compound D3; Figure S9: 1H NMR spectrum of compound D4; Figure S10: 13C NMR spectrum of compound D4; Figure S11: 1H NMR spectrum of compound D5; Figure S12: 13C NMR spectrum of compound D5; Figure S13: Correlation curves between experimental and calculated bond lengths, bond angles, and dihedral angles of P1-P4; Table S1: The crystal data and structure refinement details of compound D; Table S2: The crystal data and structure refinement details of compound D3; Table S3: The crystal data and structure refinement details of compound D4; Table S4: The crystal data and structure refinement details of compound D5.
Author Contributions
Conceptualization, M.E.H., E.H.A., E.M.E. and A.S.A.; methodology, M.E.H., E.H.A., S.L., I.F., A.S.A. and J.T.M.; software, E.H.A., I.F., A.S.A. and J.T.M.; validation, M.E.H., E.H.A., M.H., S.L., M.B., M.L., A.S.A., I.F., M.H. and J.T.M.; formal analysis, M.E.H., I.F., L.E.G., M.H. and A.S.A.; investigation, M.E.H., E.H.A., S.L., A.S.A., I.F., M.L., M.B., L.E.G., M.H. and J.T.M.; resources, E.H.A., E.M.E., J.T.M. and A.S.A.; data curation, M.E.H., L.E.G., M.L. and J.T.M.; writing—original draft preparation, M.E.H., E.H.A., J.T.M. and S.L.; writing—review and editing, E.H.A., J.T.M., A.S.A. and E.M.E.; visualization, E.H.A., S.L., I.F. and J.T.M.; supervision, E.M.E. and J.T.M.; project administration, M.E.H., E.H.A., A.S.A. and E.M.E.; funding acquisition, A.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R342), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. This work was also funded by the Researchers Supporting Project number (RSPD2024R754), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
J.T.M. thanks Tulane University for its support of the Tulane Crystallography Laboratory.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kreeger, P.K.; Lauffenburger, D.A. Cancer systems biology: A network modeling perspective. Carcinogenesis 2010, 31, 2–8. [Google Scholar] [CrossRef]
- Gaber, A.A.; Sobhy, M.; Turky, A.; Abdulwahab, H.G.; Al-Karmalawy, A.A.; Elhendawy, M.A.; Radwan, M.M.; Elkaeed, E.B.; Ibrahim, I.M.; Elzahabi, H.S. Discovery of new 1 H-pyrazolo [3,4-d] pyrimidine derivatives as anticancer agents targeting EGFRWT and EGFRT790M. J. Enzym. Inhib. Med. Chem. 2022, 37, 2283–2303. [Google Scholar] [CrossRef]
- Bondock, S.; Rabie, R.; Etman, H.A.; Fadda, A.A. Synthesis and antimicrobial activity of some new heterocycles incorporating antipyrine moiety. Eur. J. Med. Chem. 2008, 43, 2122–2129. [Google Scholar] [CrossRef]
- Trivedi, A.R.; Dholariya, B.H.; Vakhariya, C.P.; Dodiya, D.K.; Ram, H.K.; Kataria, V.B.; Siddiqui, A.B.; Shah, V.H. Synthesis and anti-tubercular evaluation of some novel pyrazolo [3,4-d] pyrimidine derivatives. Med. Chem. Res. 2012, 21, 1887–1891. [Google Scholar] [CrossRef]
- Tintori, C.; Fallacara, A.L.; Radi, M.; Zamperini, C.; Dreassi, E.; Crespan, E.; Maga, G.; Schenone, S.; Musumeci, F.; Brullo, C. Combining X-ray crystallography and molecular modeling toward the optimization of pyrazolo [3,4-d] pyrimidines as potent c-Src inhibitors active in vivo against neuroblastoma. J. Med. Chem. 2015, 58, 347–361. [Google Scholar] [CrossRef]
- Yeom, H.; Achary, R.; Choi, Y.; Park, C.H.; Lee, J.Y.; Lee, H.K.; Kim, P.; Cho, S.Y. Pyrazolo [3,4-d] pyrimidine derivatives as irreversible Bruton’s tyrosine kinase inhibitors. Bull. Korean Chem. Soc. 2022, 43, 577–584. [Google Scholar] [CrossRef]
- Podolski-Renić, A.; Dinić, J.; Stanković, T.; Tsakovska, I.; Pajeva, I.; Tuccinardi, T.; Botta, L.; Schenone, S.; Pešić, M. New therapeutic strategy for overcoming multidrug resistance in cancer cells with pyrazolo [3,4-d] pyrimidine tyrosine kinase inhibitors. Cancers 2021, 13, 5308. [Google Scholar] [CrossRef]
- Di Maria, S.; Picarazzi, F.; Mori, M.; Cianciusi, A.; Carbone, A.; Crespan, E.; Perini, C.; Sabetta, S.; Deplano, S.; Poggialini, F. Novel pyrazolo [3,4-d] pyrimidines as dual Src/Bcr-Abl kinase inhibitors: Synthesis and biological evaluation for chronic myeloid leukemia treatment. Bioorganic Chem. 2022, 128, 106071. [Google Scholar] [CrossRef]
- Pavlović, K.T.; Kocić, G.; Šmelcerović, A. Myt1 kinase inhibitors-Insight into structural features, offering potential frameworks. Chem.-Biol. Interact. 2024, 391, 110901. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Abdellattif, M.H.; Ali, A.; AbuAli, O.; Shahbaaz, M.; Ahsan, M.J.; Hussien, M.A. Computational approaches for the design of novel anticancer compounds based on Pyrazolo [3,4-d] pyrimidine derivatives as TRAP1 inhibitor. Molecules 2021, 26, 5932. [Google Scholar] [CrossRef] [PubMed]
- Hanke, J.H.; Gardner, J.P.; Dow, R.L.; Changelian, P.S.; Brissette, W.H.; Weringer, E.J.; Pollok, B.A.; Connelly, P.A. Discovery of a Novel, Potent, and Src Family-selective Tyrosine Kinase Inhibitor: STUDY OF Lck-AND FynT-DEPENDENT T CELL ACTIVATION (∗). J. Biol. Chem. 1996, 271, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Robins, R.K. Potential purine antagonists. I. Synthesis of some 4, 6-substituted pyrazolo [3,4-d] pyrimidines1. J. Am. Chem. Soc. 1956, 78, 784–790. [Google Scholar] [CrossRef]
- Smyth, L.A.; Matthews, T.P.; Horton, P.N.; Hursthouse, M.B.; Collins, I. Divergent cyclisations of 2-(5-amino-4-carbamoyl-1H-pyrazol-3-yl) acetic acids with formyl and acetyl electrophiles. Tetrahedron 2007, 63, 9627–9634. [Google Scholar] [CrossRef]
- Abd El Hamid, M.K.; Mihovilovic, M.D.; El-Nassan, H.B. Synthesis of novel pyrazolo [3,4-d] pyrimidine derivatives as potential anti-breast cancer agents. Eur. J. Med. Chem. 2012, 57, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, J.; Chai, H.; Zhang, K.; Yang, D.; Zhang, Q.; Shi, D. A convenient four-component one-pot strategy toward the synthesis of pyrazolo [3,4-d] pyrimidines. Beilstein J. Org. Chem. 2015, 11, 2125–2131. [Google Scholar] [CrossRef]
- Gaonkar, S.; Savanur, M.A.; Nadaf, A.A.; Najare, M.S.; Mantur, S.; Garbhagudi, M.; Mulla, S.I.; Khazi, I.A.M. Novel pyrazolo [3,4-d] pyrimidine derivatives inhibit human cancer cell proliferation and induce apoptosis by ROS generation. Arch. Der Pharm. 2020, 353, 1900296. [Google Scholar] [CrossRef]
- Salem, I.M.; Mostafa, S.M.; Salama, I.; El-Sabbagh, O.I.; Hegazy, W.A.; Ibrahim, T.S. Design, synthesis and antitumor evaluation of novel pyrazolo [3,4-d] pyrimidines incorporating different amino acid conjugates as potential DHFR inhibitors. J. Enzym. Inhib. Med. Chem. 2023, 38, 203–215. [Google Scholar] [CrossRef]
- Bassoude, I.; Tber, Z.; Essassi, E.M.; Guillaumet, G.; Berteina-Raboin, S. A one-pot process for the microwave-assisted synthesis of 7-substituted pyrazolo [1, 5-a] pyrimidine. RSC Adv. 2016, 6, 3301–3306. [Google Scholar] [CrossRef]
- El Hafi, M.; Kansiz, S.; Lahmidi, S.; Boulhaoua, M.; Ramli, Y.; Dege, N.; Essassi, E.M.; Mague, J.T. Crystal structure and Hirshfeld surface analysis of 3-(4-methoxyphenyl)-1-methyl-4-phenyl-1H-pyrazolo [3,4-d] pyrimidine. Acta Crystallogr. Sect. E Crystallogr. Commun. 2019, 75, 638–641. [Google Scholar] [CrossRef]
- Somakala, K.; Tariq, S.; Amir, M. Synthesis, evaluation and docking of novel pyrazolo pyrimidines as potent p38α MAP kinase inhibitors with improved anti-inflammatory, ulcerogenic and TNF-α inhibitory properties. Bioorganic Chem. 2019, 87, 550–559. [Google Scholar] [CrossRef]
- Lazrak, F.; Lahmidi, S.; Anouar, E.H.; Alanazi, M.M.; Alanazi, A.S.; Essassi, E.M.; Mague, J.T. Synthesis, X-ray Crystal Structure, Anticancer, Hirshfeld Surface Analysis, DFT, TD-DFT, ADMET, and Molecular Docking of 3-Phenyl-1,2,4-triazolo[3,4-h]-13,4-thiaza-11-crown-4. Molecules 2023, 28, 3166. [Google Scholar] [CrossRef] [PubMed]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; McKinnon, J.; Wolff, S.; Grimwood, D.; Spackman, P.; Jayatilaka, D.; Spackman, M. CrystalExplorer17; University of Western Australia: Crawley, WA, Australia, 2017. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Staker, B.L.; Feese, M.D.; Cushman, M.; Pommier, Y.; Zembower, D.; Stewart, L.; Burgin, A.B. Structures of three classes of anticancer agents bound to the human topoisomerase I−DNA covalent complex. J. Med. Chem. 2005, 48, 2336–2345. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).