Abstract
Thio-substituted 2-pyridylferrocenes [CpFe{C5H3(X)(C5H4N)}] (X = SOTol, 3; SMe, 5) were prepared from [CpFe(C5H4R)] (R = SOTol, 1; 2-C5H4N, 2) in moderate yields. The reactions of 3 and 5 with [PtCl2(DMSO)2] yielded the binuclear N, S chelated complexes [CpFe{C5H3(X)(C5H4N)}-(к-N,S)-PtCl2] (X= SOTol, 4, SMe, 6), while the reaction of 5 with Hg(OAc)2/LiCl led to cyclomercuration with generation of [CpFe{C5H2(SMe)(C5H4N)(HgCl)}], 7. The crystal structures of 6·CH2Cl2 and 7 were determined. The structure of 6 showed a weak intramolecular Fe…Pt interaction and several weak intermolecular interactions involving all Cl atoms. Weak intermolecular interactions between Hg and S atoms in the cyclomercurated 7 led to a tetrameric structure involving a Hg2S2 ring.
1. Introduction
Ever since the discovery of the usefulness of ferrocene-based chiral ligands in catalytic dia- and enantioselective catalysis by Hayashi et al. exactly 50 years ago [1], this compound class has been extensively studied and optimized for particular applications. Most of these chiral ferrocene derivatives contain the element of planar chirality, mostly combined with at least one chiral substituent. Thus, many P^P, P^N, P^O, P^S, N^N, N^O, and N^S bidentate donor substituted ferrocenes were developed [2]. We would like to focus here on the N^S type ligands. For the N-donor substituent, either amino-alkyl groups [3], or heterocycles [4], mostly oxazolines [5,6], thiazolines [7,8], or pyridine derivatives [9,10,11] were used. The S-donor component was either an alkyl or aryl thioether [5,6,7,8] or a sulfoxide [9,10,11]. As for dia- or enantioselective catalysis, an enantiopure ligand is essential, and a diastereoselective synthesis of these planar-chiral ferrocenes is highly desirable. For this purpose, either ortho-metalations or C-H activations have been considered [12,13]. Numerous examples exist with metalating agents like lithium alkyls or amides, while for C-H activations Co(II), Cu(I,II), Sc(III), Rh(I,III), Pd(II) and, to a much smaller extent, the 5d metals Ir(III) and Au were used. While Pt(II)-catalyzed C-H functionalizations are well-known [14,15], they have apparently not been used in this context. Similarly, while cyclomercurated ferrocenylamines and their ability to undergo further transmetallation reactions are well-known [16,17], they also have not been used for further functionalization. Our group has been working on the further functionalization, including cycloplatination [18,19] and cyclomercuration [20] of various ferrocenylpyridines. Here, we report our studies on the synthesis of planar-chiral ferrocenylpyridines containing additional sulfenyl and sulfinyl substituents and their reactivity towards [PtCl2(DMSO)2] and Hg(OAc)2/LiCl.
2. Results
2.1. Synthesis
2.1.1. 2-Pyridylferrocenes with Sulphur Containing Substituents
First, we looked at the synthesis of a tolylsulfinyl-substituted pyridylferrocene. Following Jäkle’s synthesis of 2-(3,5-dimethyl-pyridinyl)-1-tolylsulfinylferrocene [9], we treated (S)-tolylsulfinylferrocene 1 with LDA and ZnCl2 followed by Negishi coupling with 2-bromopyridine to obtain the desired 2-(2-pyridyl)-1-p-tolylsulfinylferrocene 3 as an orange powder in ca. 60% yield (Scheme 1).
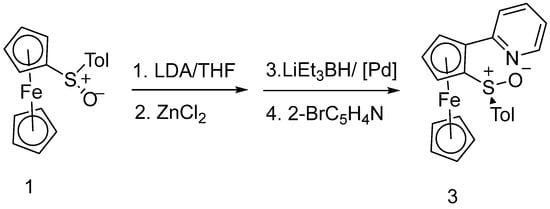
Scheme 1.
Synthesis of 3.
NMR spectroscopic investigation (1H, 13C, see Figures S1 and S2 of the Supporting Information) showed that this product contained an unidentified impurity, which could not be removed by chromatography. Careful examination of the NMR spectra showed that the impurity contained neither a pyridyl nor a tolyl group (no additional signals of significant intensity at δ > 8.5 ppm and around δ = 2.4 ± 0.1 ppm in the 1H NMR spectrum, no additional signals at δ > 145 ppm and around 22 ± 1 ppm in the 13C-NMR spectra). However, the presence of some “aromatic” signals (around δ ≈ 7.5 ppm in the 1H-NMR and δ ≈ 130 ppm in the 13C-NMR spectrum) suggests that a PPh3 containing compound might be present.
Next, we investigated the synthesis of a thioether derivative of pyridylferrocene. Following the introductions of halide or carbonyl substituents described earlier by us [19,21], treatment of 2-pyridylferrocene with n-BuLi and MeSSMe produced the desired racemic 1-methylthio-2-(2-pyridyl)-ferrocene 5 as an orange viscous oil in 68% yield (Scheme 2).
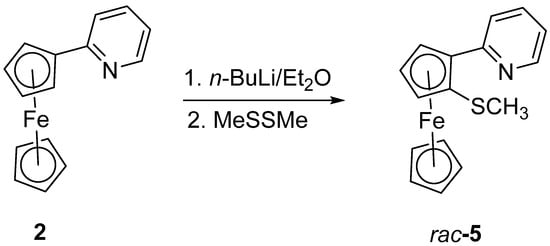
Scheme 2.
Synthesis of 5.
Compound 5 could be characterized by 1H, 13C-NMR (see Figures S3 and S4 in the Supporting Information) as well as by MS (FAB+) and elemental analysis.
2.1.2. Reaction of Compound 3 with [PtCl2(DMSO)2]
With the intention of inducing a cycloplatination reaction, we used the reaction conditions we had successfully applied earlier for the cycloplatinations of other substituted pyridylferrocenes [18,19], for the reaction of (impure) 3 with [PtCl2(DMSO)2]. However, instead of a C-H activation reaction at the ferrocene core, a simple N,S coordination at the substituents occurred (Scheme 3).
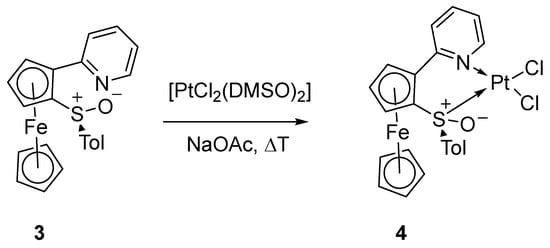
Scheme 3.
Reaction of 3 with [PtCl2(DMSO)2] and NaOAc.
The presumed structure 4 is based mainly on the NMR data (see Figures S5 and S6 in the Supporting Information). The 1H-NMR and 13C spectra show mainly rather large amounts of ethyl acetate (from chromatography) as an impurity. There are some further weak peaks in the 1H-NMR spectrum (there are many “satellite” signals next to the big ones, due to 1H-195Pt coupling), which, however, have no counterparts in the 13C NMR spectrum, and must therefore correspond to rather small and insignificant amounts.
Hoping to be more successful, we studied also the reactivity of the sulfenyl substituted compound 5 towards the same “cycloplatination” conditions. However, again no C-H activation occurred, but only a S,N–chelation by Pt (Scheme 4). The chelate compound 6 could be isolated as an oil in 30% yield and be characterized by 1H and 13C NMR (see Figures S7 and S8 of the Supporting Information) as well as by MS (DEI). After careful recrystallization, a few crystals of 6 could be isolated that were suitable for X-ray diffraction.
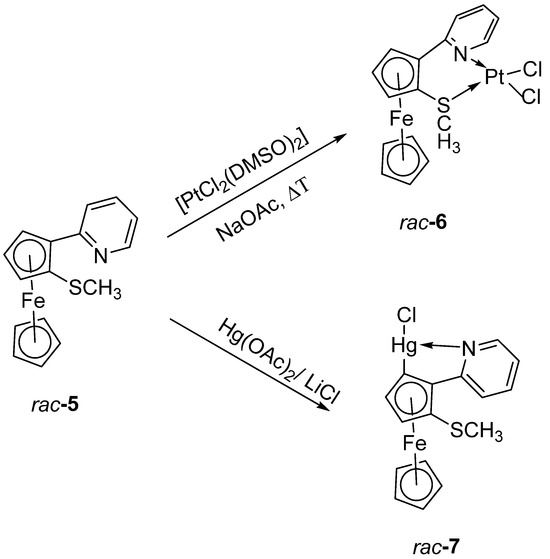
Scheme 4.
Reaction of 5 with [PtCl2(DMSO)2] and Hg(OAc)2/LiCl.
As we had successfully cyclomercurated several pyridylmetallocenes [20,22], we also decided to look at a possible cyclomercuration of 5. To our delight, a C-H activation next to the pyridyl substituent occurred and the cyclomercurated product 7 could be isolated after chromatography and recrystallization in low yield (Scheme 4). Despite the low yield, it was possible to obtain 1H and 13C NMR spectra (see Figure S9 and S10 of the Supporting Information) as well as MS and crystals suitable for X-ray structure determination.
2.2. Crystallographic Studies
2.2.1. Molecular Structures of Compounds 6 and 7
Compound 6 crystallizes together with one molecule of dichloromethane as a twin in the trigonal space group P32. Since this is a polar space group, the selected crystal contains only the Sp enantiomer of this planar-chiral molecule. An ortep3 view is shown in Figure 1, and the most important bond parameters are collected in Table 1.
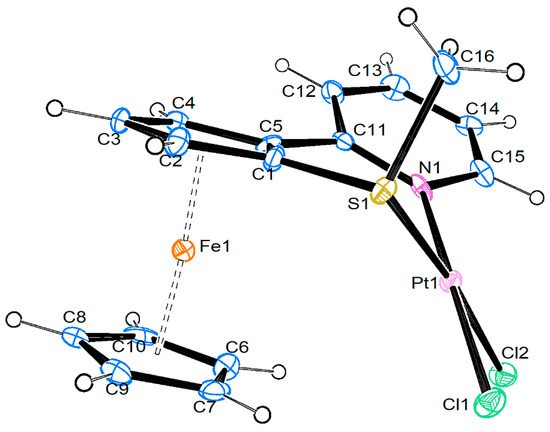
Figure 1.
Molecular structure of compound 6. Displacement ellipsoids at the 50% probability level.

Table 1.
Important bond distances [Å} and angles [°].
The most obvious feature is the bending down of the PtCl2 unit out of the cyclopentadienyl plane towards the iron atom, which also affords a rotation of the pyridine ring out of this Cp plane, and also a slight “tilting” of the Cp-M-Cp unit. A search in the CSD (accessed on 22 September 2024) shows only four compounds that contain a ferrocene-based S,N ligand coordinated to a M′Cl2 fragment, all with M′ = Pd (ceycam, gendae, keyjed, seybaz). Two of them show the same arrangement with the coordinated fragment on the proximal side as the iron atom [7,23], while in the other two the MCl2 group is on the distal side. Still, the Pt…Fe distance of 3.822(3) Å is larger than the sum of van-der-Waals radii (by 0.102 Å), as is true also for the two Pd…C contacts in the mentioned [CpFe{C5H3(SMe)(C3H4NS)}-PdCl2] and [CpFe{C5H3(StBu)(CH2NMe2)}-PdCl2]. The bite angle of the SPtN chelate is 93.8(7)°, compared with 91.02(7) and 100.2(2)° in the two Pd compounds. Further geometrical parameters of the structure can be found in Table 1.
Compound 7 crystallizes in the triclinic space group P-1 with two molecules in the asymmetric unit. Figure 2 shows an ortep3 top view of both molecules. The chlorine atom of molecule B is disordered over two positions. As the space group is centrosymmetric, both enantiomers of this planar-chiral compound are present in the crystal. There are only minor differences between the two independent molecules (see Table 1). The pyridine rings are only slightly rotated out of the plane of the Cp rings, with the N atoms on the distal sides. In the structurally related [CpFe{C5H2(CH3)(C5H4N)(HgCl)}], the pyridyl ring is slightly rotated the other way, while in [CpFe{C5H3(C5H4N)(HgCl)}] it is nearly perfectly coplanar with the Cp ring [20]. The Hg…N distance in molecule A of compound 7 is significantly shorter than in molecule B, and it is also shorter than in the just mentioned literature compounds (2.74 and 2.71 Å, respectively) and other known cyclomercurated ferrocenylimines [17].
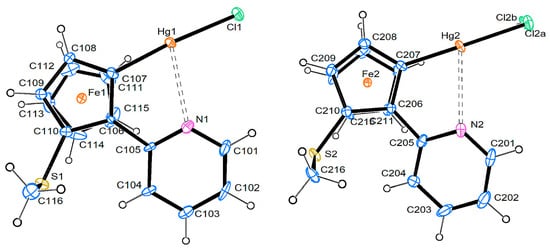
Figure 2.
Molecular structure of compound 7, top views of molecules A and B. Displacement ellipsoids are at the 50% probability level.
2.2.2. Intermolecular Contacts and Packing Diagrams
Both compounds 6 and 7 show non-classical intra- and intermolecular hydrogen bonds. In compound 6, only Cl atoms act as acceptors, both the Pt bound chlorines and the chlorines of the lattice dichloromethane (Figure 3). There are some H…H contacts and C-H…C contacts between pyridine CH bonds and a C-C bond of a neighboring C5H5 ring. In addition, the pyridine ring acts as an acceptor of two C-Cl…π bonds towards the CH2Cl2 molecule and a very weak π-π interaction with the unsubstituted Cp ring.
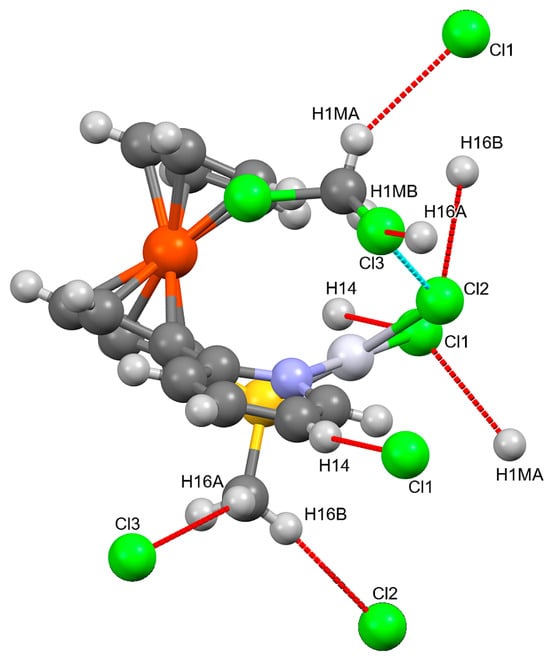
Figure 3.
Hydrogen Bonds in compound 6. mercury standard colouring: Carbon: dark grey; hydrogen: light grey; chlorine: green; sulphur: yellow; iron: orange; nitrogen: blue. Red lines “hanging contacts”, blue lines: both molecules connected are completely in the picture.
In compound 7, chlorine as well as sulphur atoms act as acceptors (Figure 4). Table 2 collects the parameters of the H bonds of compounds 6 and 7. Besides the H bonds, there are also some weak interactions involving Hg1 and S2 atoms, leading to a four-membered Hg2S2 ring formed by two molecules A and two molecules B each. (Figure 5). Quite interestingly, Hg2 and S1 do not take part in similar weak interactions.
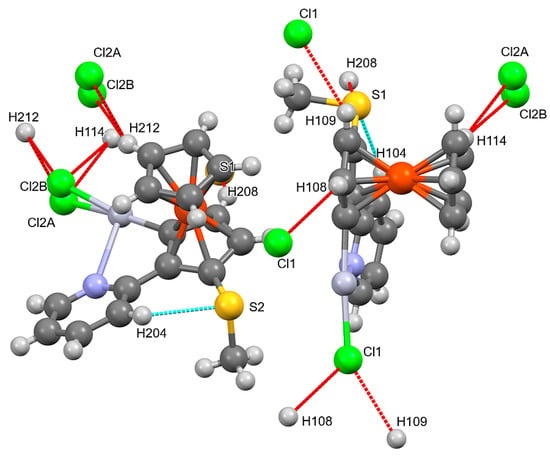
Figure 4.
Hydrogen Bonds in compound 7. Mercury colours as defined in Figure 3.

Table 2.
H bond parameters of compounds 6 and 7.
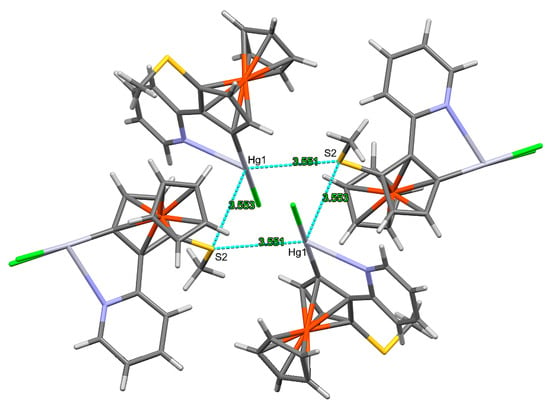
Figure 5.
The formation of four-membered Hg2S2 rings in compound 7. Mercury colours as defined in Figure 3.
The interplay of the different interactions led to the packing diagrams shown in Figure 6 and Figure 7.
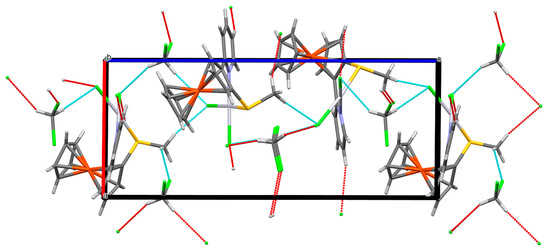
Figure 6.
Packing diagram (mercury) of compound 6, viewed along b. Mercury colours as defined in Figure 3.
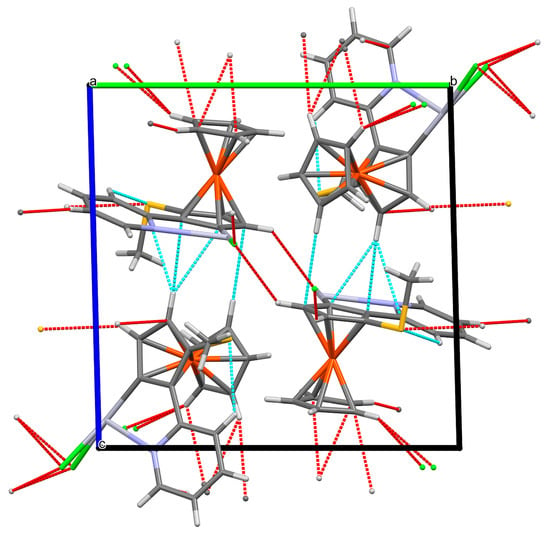
Figure 7.
Packing diagram (mercury) of compound 7, viewed along a. Mercury colours as defined in Figure 3.
3. Discussion
Both synthetic approaches, namely either lithiation of a sulphur containing ferrocene followed by introduction of the pyridinyl substituent, or lithiation of a pyridylferrocene followed by introduction of a sulphur electrophile, led to the synthesis of planar-chiral ferrocenyl N,S-ligands 3 and 5. Unfortunately, these reactions were not 100% chemo-selective, and other products were also formed, which could neither be identified nor be fully separated, without large losses of yield. From our experience with lithiations of both tolylsulfinylferrocene 1 and 2-pyridylferrocene 2, we know that side reactions like mono-lithiation in 3 position or dilithiations either in 2,3 or 2,1’ positions are possible; however, in earlier cases chromatographic separations were possible. Still, characterization by NMR methods, and, in part, by mass spectrometry and elemental analysis was possible; however, no single crystals of 3 or 5 could be obtained.
Reaction of iminoferrocenes with [PtCl2(DMSO)2] under basic conditions are known to yield cycloplatinated complexes with both Pt-C and Pt-N bonds. While there are apparently no examples of ferrocenyl N,S ligands in cycloplatination reactions—at least in the crystallographic literature, there are two examples of cycloplatinated iminobenzenes containing also coordinating S atoms (aboxiy and fugkuo, [24,25]). However, in both cases, the sulphur atom was not directly attached to the benzene ring. When, however, the SCH3 group was bound in ortho-position to the imino group, the Pt atom was only chelating the substituents in a N,S fashion (xeqquh [26]). Therefore, it was not surprising that both compounds 3 and 5 acted only as N,S chelate ligands towards PtCl2 producing the bimetallic complexes 4 and 6. Both compounds could be characterized by NMR methods, and compound 6 also by X-ray diffraction. The crystal of 6 studied contained only the enantiomer with planar S configuration; however, since there were no chiral crystallization conditions, it is most likely that it was only the serendipitous picking of this particular crystal, and not a property of the whole solid product.
While no cycloplatination could be observed either with 3 or 5, clean cyclomercuration occurred with the latter compound, reacting next to the pyridyl substituent only, giving 7. Cyclomercuration of tolylsulfinylferrocene 1 has been reported to yield a compound, where the oxygen atom of the sulfinyl group was also coordinated to the Hg atom [27]. Although we did not study the analogous reaction of 3, we assume that for steric reasons the mercuration would occur here also next to the pyridinyl substituent. We had already shown earlier that both 2-pyridylferrocene and 2-methyl-1-(2-pyridyl)ferrocene underwent cyclomercuration reaction, yielding products with a T-shaped primary coordination sphere. While the product of the mono-substituted pyridylferrocene had shown no further interactions involving the Hg atom, the methyl-substituted product did: Some kind of “dimerization” had occurred in the crystal, yielding four-membered Hg2Cl2 rings. Our new compound 7 showed a “tetramerization”, this time using the S atoms of neighboring molecules producing a four-membered Hg2S2 ring.
4. Materials and Methods
4.1. Starting Materials and Instrumentation
Reagents LDA solution (1.0 M in THF/hexane), LiEt3BH (1.0 M), n-butyl lithium (2.5 M), ZnCl2, NaOAc, Hg(OAc)2, LiCl, 2-bromopyridine, MeSSMe were obtained commercially and used as provided. Solvents (THF, Et2O, hexane, petroleum ether, dichloromethane, ethyl acetate) were obtained in analytical grade and were saturated with N2. Compounds 1 and 2 as well as [Pd(PPh3)2Cl2] and [PtCl2(DMSO)2] were prepared according to the literature.
NMR spectra were measured on jeol ecp-270 or ex-400 instrument, (both jeol Germany, Freising, Germany) using CD2Cl2 or CDCl3 as solvent. The chemical shifts were obtained relative to the residual solvent signals, as defined by the MestReNova software (Version 14.1.1-24751, Mestrelab Research S.L., Santiago de Compostela, Spain) (δCDCl3 = 7.260 and 77.16 ppm, respectively; δCHDCl2 = 5.320 and 53.84 ppm, respectively). Mass spectra were obtained on Finnigan MAT 90 and JEOL Mstation 700 (jeol Germany, Freising, Germany) instruments, in DEI or FAB mode. Chromatographic separations were performed in Schlenk glass frits of ca. 20 mm diameter with a filling hight of ca. 15 cm of silica gel, which had been suspended in petroleum ether. A slight overpressure of nitrogen was used to promote the migration of the bands through the column.
Crystal structure determinations were performed on an Oxford XCalibur3 diffractometer (Oxford Diffraction Ltd., Oxford, UK). Further details can be found in Table S1 of the Supplementary Information.
4.2. Synthesis of 1-(S)-(P-Tolylsulfinyl)-2-(2-pyridyl)-ferrocene (3)
A solution of 1 (0.65 g, 2.00 mmol) in THF (20 mL) was treated at −78 °C with a 1.0 M solution of LDA in THF/hexane (2.20 mL, 2.20 mmol) with stirring for 1 h. Then, a solution of ZnCl2 (0.30 g, 2.20 mmol) in THF (10 mL) was added and the mixture was warmed to r.t. After stirring for another hour at this temperature, a 1.0 M solution of LiEt3BH (0.20 mL, 0.20 mmol) and solid [PdCl2(PPh3)2] (0.14 g, 0.20 mmol) were added, which led to formation of a black suspension. 2-Bromopyridine (0.20 mL, 2.00 mmol) was added and the suspension was stirred for 20 h. After addition of 1.0 m NaOH solution (20 mL), a two-phase mixture was obtained, which was extracted three times with CH2Cl2 (15 mL each). The combined organic extracts were washed with water and brine and then dried over MgSO4. After complete evaporation of the solvent in vacuo, the residue was taken up in the minimum amount of CH2Cl2 and chromatographed on alumina. A first, yellow fraction, was obtained with PE/EtOAc 4:1 and was discarded. A second yellow fraction eluted with PE/EtOAc 1:1 contained a mixture of unreacted starting material and product, and it was discarded also. Finally, a EtOAc/CH2Cl2 mixture was used as eluent, and after evaporation compound 3 was obtained as orange-yellow powder (0.52 g, 1.30 mmol, 65%).
1H-NMR: (CDCl3, 400 MHz): δ = 8.59 (m, 1H), 7.89 (m, 1H), 7.72 (m, 2H), {7.69–7.60 (m, 2H), 7.54 (m, 0.5H), unidentified impurity}, 7.46 (m, 1H), 7.29 (m, 2H), 7.13 (m, 1H), 5.09 (m, 1H), 4.47 (m, 1H), 4.16 (m, 1H), 4.14 (s, 5H), 2.41 (s, 3H).
13C-NMR (CDCl3, 101 MHz): δ = 156.8, 149.3, 141.3, 140.8, 136.2, 129.4, {132.3, 132.2, 128.7, 128.6; unidentified impurity}, 125.9, 123.0, 121.6, 93.3, 85.9, 71.7, 71.5, 71.4, 70.1, 21.6.
4.3. Reaction of 3 with [PtCl2(DMSO)2]
A solution of 3 (0.080 g, 0.20 mmol) and [PtCl2(DMSO)2] (0.090 g, 0.20 mmol) in toluene (40 mL) was treated with a solution of NaOAc·3H2O (0.030 g, 0.40 mmol) in MeOH (1 mL), and the mixture was heated to reflux for 3 h. After cooling down to r.t., the solvents were evaporated in vacuo, and the obtained red residue was chromatographed on alumina. A first fraction was obtained with PE/CH2Cl2, and it was discarded. A second fraction was obtained using EtOAc/CH2Cl2 1:2 as eluent. Evaporation left a red solid (0.08 g), presumably [{Fe(C5H5)(C5H3(2-C5H4N)(SO-p-Tol))}-(κ-N,S)-PtCl2] (4) (0.12 mmol, 61%)
1H-NMR (CDCl3, 270 MHz): δ = 9.41 (m, 1H), 8.07 (m, 2H), 7.87 (m, 1H), 7.59 (m, 1H), 7.44 (m, 2H), 7.32 (m, 1H), 5.13 (m, 1H), 4.81 (s, 5H), 4.74 (m, 1H), 4.10 (m, 1H), 2.50 (s, 3H).
13C-NMR (CDCl3, 68 MHz): δ = 156.2, 155.3, 144.6, 139.3, 129.1, 128.7, 128.3, 124.6, 124.4, 89.0, 82.9, 74.2, 71.5, 69.4, 68.7, 21.7.
4.4. Synthesis of Racemic 1-Methylthio-2-(2-pyridyl) Ferrocene (5)
A solution of 2-pyridyl-ferrocene (2, 0.24 g, 0.91 mmol) in Et2O (10 mL) was treated with 2.5 m n-BuLi solution (1.0 mL, 2.50 mmol) at r.t. with stirring for 1 h. Then, the mixture was cooled to −78 °C and MeSSMe (0.27 mL, 3.0 mmol) was added. After stirring for 10 min at this temperature, the cooling bath was removed and stirring was continued for another hour. After evaporation of the solvent in vacuo, the obtained orange-brown residue was chromatographed on alumina and PE/CH2Cl2 1:1 eluted the desired product [Fe(C5H5){C5H3(SMe)(C5H4N-2)-1,2}] (5), obtained as an orange-colored highly viscous oil (0.19g, 0.61 mmol, 68%).
1H-NMR (CDCl3, 270 MHz): δ = 8.54 (m, 1H), 8.17 (m, 1H), 7.63 (m, 1H), 7.11 (m, 1H) 4.97 (m, 1H), 4.49 (m, 1H), 4.37 (m, 1H), 4.09 (s, 5H), 2.27 (s, 3H).
13C{1H}-NMR (CDCl3, 68 MHz): δ = 158.5, 149.0, 135.7, 122.5, 121.0, 85.2, 83.3, 73.3, 71.1, 69.6, 68.6, 20.1.
MS (FAB+): m/z = 307.3 (M+), 263.3 (M+–SCH2).
EA (calc/found): C: 62.15/61.92; H: 4.89/4.97; N: 4.53/4.52; S: 10.37/10.85%.
4.5. Synthesis of [Fe(C5H5){C5H3(SCH3)(C5H4N-2)-1,2}]-(κ-N,S)-PtCl2] (6)
A solution of 5 (0.077 g, 0.25 mmol) and [PtCl2(DMSO)2] (0.11 g, 0.26 mmol) in toluene (50 mL) was treated with a solution of NaOAc·3H2O (0.068 g, 0.50 mmol) in MeOH (1.0 mL). After refluxing this mixture for 5 h and cooling down to r.t., the solvents were evaporated in vacuo. The residue was placed on top of an alumina column. CH2Cl2/EtOAc 2:1 eluted an orange band, which yielded after evaporation 6 (0.043 g, 0.075 mmol, 30%) as an orange-colored oil. Recrystallization from CH2Cl2 at r.t. gave a few single crystals, suitable for X-ray diffraction.
1H-NMR (CDCl3, 270 MHz): δ = 9.39 (m, 1H), 7.81 (m, 1H), 7.50 (m, 1H), 7.26 (m, 1H), 4.99 (m, 1H), 4.95 (m, 1H), 4.77 (s, 5H), 4.71 (m, 1H), 2.16 (s, 3H).
13C{1H}-NMR (CD2Cl2, 68 MHz): δ = 162.6, 156.6, 139.1, 125.7, 124.0, 74.0, 73.6, 71.9, 69.1, 28.2.
MS (DEI): m/z = 575.0 (M+)
4.6. Synthesis of [Fe(C5H5){C5H2(HgCl)(SCH3)(C5H4N-2)-1,3,2}-(κ-C,N)] (7)
A solution of Hg(OAc)2 (0.20 g, 0.63 mmol) in MeOH (25 mL) was added to a solution of 5 (0.19 g, 0.61 mmol) in CH2Cl2 (12 mL). After stirring for 3 h at r.t. a solution of LiCl (0.05 g, 1.2 mmol) in MeOH (19 mL) was added. After stirring for another three days a mixture of CH2Cl2 (15 mL) and water (20 mL) was added. The aqueous phase was separated and extracted with CH2Cl2 (40 mL). The combined organic phases were dried over MgSO4, and after filtering, the solvents were evaporated in vacuo. The obtained orange-colored residue was chromatographed on silicagel. A mixture PE/CH2Cl2 (gradient 2:1 → 1:2) eluted the title compound 7 as orange micro-crystals (0.090 g, 0.16 mmol, 27%). Pure CH2Cl2 eluted another orange fraction (0.080 g after evaporation), which contained 7 besides other unidentified compounds; further elution with CH2Cl2/EtOAc 3:1 yielded after evaporation apparently unreacted 5 (0.080 g, 42% recovery). Recrystallization of the first fraction from CH2Cl2 at r.t. yielded some crystals suitable for X-ray diffraction.
1H-NMR (CD2Cl2, 270 MHz): δ = 8.66 (m, 1H), 8.45 (m, 1H), 7.71 (m, 1H), 7.22 (m, 1H), 4.73 (m, 1H), 4.38 (m, 1H), 4.08 (s, 5H) [* actually this signal overlapped with the signal of an impurity, which is most likely derived from metalation of compound 2], 2.34 (s, 3H).
13C{1H}-NMR (CD2Cl2, 68 MHz): δ = 158.4, 148.4, 137.6, 122.1, 121.9, 86.6. 86.5, 83.9, 79.0, 75.5, 71.9, 21.0.
MS (DEI+). m/z = 545.3 (M+) {among others, in F2: 591.3 (M+, 8)}.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29204884/s1, Figures S1–S10: 1H and 13C{1H} NMR spectra of compounds 3, 5, 4, 6, 7 (in this sequence). Table S1: Experimental Details for the Crystal Structure determinations.
Author Contributions
S.W. designed and performed the reactions, purifications, and crystallizations. K.S. performed the crystal structure determinations, supervised the experiments, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Copies of all NMR spectra relevant to this study are collected in the supporting Information. The experimental details of the crystal structure determinations can be found in Table S1 of the Supporting Information. The crystallographic cif files have been deposited with the Cambridge Structural database. CCDC 2386172–2386173 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223336033.
Acknowledgments
We want to express our special thanks to Christian Maier for help with the experimental work for the preparation of compounds 1, 3, and 4, and to Harald Budde for help with the synthesis of compounds 2, 5 and 6. We also thank Peter Mayer for performing the measurements on the diffractometer.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hayashi, T.; Yamamaoto, K.; Kumada, M. Asymmetric catalytic Hydrosilylation of ketones. Preparation of chiral ferrocenylphosphines as chiral ligands. Tetr. Lett. 1974, 49–50, 4405–4408. [Google Scholar] [CrossRef]
- Cunningham, L.; Benson, A.; Guiry, P.J. Recent developments in the synthesis andapplications of chiral ferrocene ligands and organocatalysts in asymmetric catalysis. Org. Biomol. Chem. 2020, 18, 9329–9370. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.L.; Au-Yeung, T.T.L.; Cheung, H.Y.; Kok, S.H.L.; Lam, W.S.; Wong, K.Y.; Chan, A.S.C. Easily accessible ferrocenyl N-P/S type ligands and their applications in asymmetric allylic substitutions. Tetrahedron Asymmetry 2006, 17, 497–499. [Google Scholar] [CrossRef]
- Musikhina, A.A.; Serebrennikova, P.O.; Zabelina, O.N.; Utepova, I.A.; Chupakhin, O.N. Advanced Application of Planar Chiral Heterocyclic Ferrocenes. Inorganics 2022, 10, 152. [Google Scholar] [CrossRef]
- Arthur, R.A.; Richards, C.J. Deuterium as a Stereochemically Invisible Blocking Group for Chiral Ligand Synthesis. Org. Lett. 2017, 19, 702–705. [Google Scholar] [CrossRef]
- Dai, L.; Yang, M.J. Synthesis of 2-oxazoline ferrocenes: Towards high-efficient chiral ligands and catalysts. J. Organomet. Chem. 2023, 999, 122831. [Google Scholar] [CrossRef]
- Corona-Sanchez, R.; Toscano, R.A.; Ortega-Alfaro, M.C.; Sandoval-Chavez, C.; Lopez-Cortes, J.G. 2-Ferrocenyl-2-thiazoline as a building block of novel phosphine-free ligands. Dalton Trans. 2013, 42, 11992–12004. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.P.; Hochberger-Roa, F.; Corona-Sanchez, R.; Barquera-Lozada, J.E.; Toscano, R.A.; Urrutigoity, M.; Gouygou, M.; Ortega-Alfaro, M.C.; Lopez-Cortes, J.G. Chiral bidentate [N,S]-ferrocene ligands based on a thiazoline framework. Synthesis and use in palladium-catalyzed asymmetric allylic alkylation. Dalton Trans. 2017, 46, 1510–1518. [Google Scholar] [CrossRef]
- Chen, J.; Lalancette, R.A.; Jäkle, F. Stereoselective Ortho Borylation of Pyridylferrocenes. Organometallics 2013, 32, 5843–5851. [Google Scholar] [CrossRef]
- Musikhina, A.A.; Utepova, I.A.; Serebrennikova, P.O.; Chupakhin, O.N.; Charushin, V.N. Synthesis of chiral Ferrocenylazines. Negishi Cross-Coupling or SNH reactions. Russ. J. Org. Chem. 2013, 49, 1191–1194. [Google Scholar] [CrossRef]
- Utepova, I.A.; Chupakhin, O.N.; Serebrennikova, P.O.; Musikhina, A.A.; Charushin, V.N. Two Approaches in the Synthesis of Planar Chiral Azinylferrocenes. J. Org. Chem. 2014, 79, 8659–8667. [Google Scholar] [CrossRef] [PubMed]
- Čarný, T.; Šebesta, R. Comparison of Diastereoselective ortho-Metalations and C–H Activations of Chiral Ferrocenes. Synlett 2024, 35, 165–182. [Google Scholar]
- Zhang, Z.Z.; Huang, D.Y.; Shi, B.F. Recent advances in the synthesis of ferrocene derivatives via 3d transition metal-catalyzed C–H functionalization. Org. Biomol. Chem. 2022, 20, 4061–4073. [Google Scholar] [CrossRef]
- Barham, A.; Neu, J.; Canter, C.L.; Pike, R.D.; Li, Y.; Huo, S. Isomerization-Induced Multiple Reaction Pathways in Platinum-Catalyzed C–H Acylation Reaction of 2-Aryloxypyridines. Organometallics 2021, 40, 3158–3169. [Google Scholar] [CrossRef]
- Labinger, J.A. Platinum-Catalyzed C-H functionalization. Chem. Rev. 2017, 117, 8483–8496. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, X.; Zhou, N.; Song, M.; Yun, H.; Du, C.; Zhu, Y. Synthesis, isolation and structural characterization of optically active planar chiral cyclomercurated ferrocenylimines. Tetrahedron Asymmetry 2000, 11, 4877–4883. [Google Scholar] [CrossRef]
- Thiesen, K.E.; Maitra, K.; Olmstead, M.M.; Attar, S. A new synthetic route to optically active cyclomercurated ferrocenylimines. J. Organomet. Chem. 2011, 696, 1355–1358. [Google Scholar] [CrossRef]
- Sünkel, K.; Branzan, R.; Weigand, S. 2-Pyridylmetallocenes, part IV. Cycloplatination of 2-pyridyl-ferrocene, –ruthenocene and –cymantrene. Molecular structures of σ-Pt{CpM[C5H3(2-C5H4N)]}Cl (DMSO) (M= Fe, Ru) and σ-Pt{CpFe[C5H3(2-C5H4N)]}(acac). Inorg. Chim. Acta 2013, 399, 193–199. [Google Scholar] [CrossRef]
- Sünkel, K.; Budde, H.; Weigand, S. 2-Pyridylmetallocenes, Part V. Synthesis of Hydroxymethyl-, Formyl-, and Carboxy-pyridylferrocene and Study of Their Cycloplatination Reactions. Molecular Structures of [{C5H3R(2-C5H4N)-1, 2}FeCp]-(R = CHO, COOH) and σ-Pt[(C5H5)Fe{C5H2(2-C5H4N)-(CH2OH)}]Cl (DMSO). Z. Anorg. Allg. Chem. 2013, 639, 1242–1247. [Google Scholar]
- Sünkel, K.; Weigand, S. 2-Pyridylmetallocenes: Part 3. Cyclomercuration of 2-pyridyl-ferrocene and1-(2-pyridyl)-2-methyl-ferrocene. Molecular structures of [1-(2-C5H4N)-2-(CH3)C5H3]Fe(C5H5), [1-(ClHg)-2-(2-C5H4N)C5H3]Fe(C5H5) and [1-(ClHg)-2-(2-C5H4N)-3-(CH3)C5H2]Fe(C5H5). Polyhedron 2012, 44, 133–137. [Google Scholar] [CrossRef]
- Sünkel, K.; Weigand, S. 2-Pyridylmetallocenes, Part I. Electrophilic Halogenation of 2-pyridylferrocene. Molecular Structures of 2-pyridylferrocene and its α-brominated and -fluorinated derivatives. Synthesis of 2-pyridylruthenocene and 2-pyridylcymantrene. Inorg. Chim. Acta 2011, 370, 224–229. [Google Scholar] [CrossRef]
- Sünkel, K.; Weigand, S. 2-Pyridylmetallocenes: Part 2. Cyclomercuration of 2-pyridyl-cyrhetrene. Molecular structures of 2-pyridyl-cyrhetrene and 1-chloromercurio-2-(2-pyridyl)-cyrhetrene. Inorg. Chem. Comm. 2012, 21, 24–27. [Google Scholar] [CrossRef]
- Ward, D.L.; Naiini, A.A.; Okoroafor, M.O.; Brubaker, C.H. Dichloro[1-(dimethylamino)methyl-2-(t-butylthio)ferrocene]-palladium(II), C5H5FeC5H3[CH2NMe2][S-t-Bu][PdCl2]. J. Cryst. Spectr. Res. 1990, 20, 193. [Google Scholar] [CrossRef]
- Riera, X.; Lopez, C.; Caubet, A.; Moreno, V.; Solans, X.; Font-Bardia, M. Platinum(II) and Palladium(II) Compounds Containing Chiral Thioimines. Eur. J. Inorg. Chem. 2001, 2001, 2135–2141. [Google Scholar] [CrossRef]
- Isozaki, K.; Sato, A.; Miki, K. Racemic (RSC,SRS)-(2-{[1-allyl-oxy-carbonyl-3-(methyl-sulfanyl)prop-yl]iminometh-yl}phenyl-κ3S,N,C1)chloride-platinum(II). Acta. Cryst. 2009, 65E, m1401. [Google Scholar]
- Dutta, P.K.; Panda, S.; Krishna, G.R.; Reddy, C.M.; Zade, S.S. Reaction time dependent formation of Pd(II) and Pt(II) complexes of bis(methyl)thiasalen podand. Dalton Trans. 2013, 42, 476. [Google Scholar] [CrossRef]
- Sathesh, V.; Chinta, R.V.R.N.; Mamidala, R.; Mukundam, V.; Dhanunjayarao, K.; Venkatasubbaiah, K. Mercuration of ferrocenyl-p-tolyl sulfoxide and its conversion to 1,2-disubstituted ferrocenes. J. Organomet. Chem. 2017, 853, 74–80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).