Abstract
Electrochemiluminescence (ECL) detection is widely applied in many fields, including chemical measurement, biological analysis, and clinic tests, due to its high sensitivity. Currently, the fast development of many new electrochemical luminophores is continuously improving the ECL-based detection ability. Besides the enhancement of luminescence emission for a high detection sensitivity, minimizing the effect of co-reactants on ECL detection and achieving multiple analysis in one sample are also the main directions in this field. This review focuses on a summary of recently prepared new luminophores to achieve the three aims mentioned above. Especially, the review is composed by three parts, focusing on the luminophores or materials with high ECL efficiency, self-enhancing properties, and multi-color ECL luminophores. The fabrication of biosensors using these molecules is also reviewed to exhibit the advances in biological applications.
1. Introduction
Electrochemiluminescence (ECL) is a light emission process that includes the redox reactions on the electrode surface under a certain voltage. Since the luminescent reagent is excited and emits light without the need for any other light source, ECL has the advantages of a high sensitivity and low background signal [1,2,3]. Accordingly, since its discovery, electrochemiluminescence (ECL) technology has received great attention from the scientific community and has been widely applied in the fields of biological analysis, environmental monitoring, food safety, and medical diagnosis [4,5,6,7,8,9]. Typically, the annihilation and co-reaction pathways are two popular mechanisms for ECL, in which the co-reactive pathway is mostly used [10,11,12,13]. In this pathway, the luminescent reagent (e.g., ruthenium complex) is oxidized or reduced at the electrode, and the co-reactants (e.g., tri-n-propylamine or hydrogen peroxide) help the luminescent reagent to generate excited-state substances and provide additional electrons or reaction intermediates [14,15]. The intermediate is energetically unstable, and thus, it returns to the ground state, releasing energy and emitting visible light in the form of photons. The involvement of co-reactants not only improves the luminescence efficiency but also increases the sensitivity. It is noted that the presence of co-reactants can still lead to some negative effects, such as increased background noise and instability of the ECL signal.
Luminophores and co-reactants, electrode materials, pH, potentials, etc., have a great impact on ECL generation and the performance of ECL analysis [16,17,18,19], among which luminophores are the most important component in ECL detection. These luminophores with different luminescence efficiencies determine the sensitivity and effective detection range of ECL analysis. Therefore, obtaining new luminophores with high ECL efficiency is always the first task in this field. Thanks to the rapid development of materials science, ECL detection technology is experiencing an unprecedented development momentum, with more emerging high-luminescent ECL luminophores [20,21,22]. Due to the continuous enhancement of ECL emission, this method is now being applied for the analysis of living bio-samples, such as single living cells [23,24,25,26]. However, in these studies, toxic co-reactants become a problem, so their concentrations should be minimized. To address this challenge, self-enhancing ECL emission has emerged as a new direction that will push the application of ECL-based analysis into biological study. Furthermore, to achieve detection in complex samples, simultaneous multiplex ECL analysis in a single sample is another important direction, which relies on the preparation of new luminophores to give the spectrum-resolved signals. According to these new directions in ECL-based analysis, this review summarizes the recent development on the ECL luminophores, focusing on high luminescence, the intramolecular ECL process, and multicolor ECL emission. Related applications are also reviewed to exhibit their significant contributions to biological studies.
2. New Luminophores with High ECL Efficiency
In either co-reaction or an annihilation ECL system, the luminescent material with high ECL efficiency plays a core role in the development of ultra-sensitive ECL sensors. The ECL quantum efficiency of classic luminophores such as tris(2,2′-bipyridine)ruthenium(II) (Ru(bpy)32+) and luminol is relatively low, which cannot meet the needs of ultrasensitive ECL analysis. With the development of nanotechnology and quantum technology, many luminophores have been used to achieve enhanced ECL emission. This section focuses on the recent development of luminophores toward achieving high ECL efficiencies.
2.1. Semiconductor Nanocrystal Quantum Dot
Quantum dots (QDs) are a special type of nanomaterial that have a size between 1 and 10 nm and unique optical and electronic properties [27,28]. Traditional QDs usually refer to nanocrystals (NCs) made of semiconductor materials (such as CdSe, PbS, CdTe, etc.), which are used as promising luminescent materials. In 2002, Bard et al. reported the first case of ECL generated from silicon NCs (diameter 2 to 4 nm) [29]. Both annihilation and co-reactant ECL from NCs were observed, which exhibited a peak maximum at 640 nm. This work demonstrated the chemical robustness of Si NCs upon hole and electron injection and the possibility of using NCs as ECL luminophores. Note that the ECL spectrum has a significant red shift of 200 nm from the band-edge photoluminescence (PL), which was later proved to be surface-state emissions (Figure 1A) [30,31]. These surface defects formed by surface atoms result in the quench of band-edge luminescence process and low quantum yield. By passivating the surface states of NCs, the same group observed ECL emissions from both surface states on the NCs and from the bulk in NCs [32]. After this revolutionary work, QDs quickly became an ideal material to achieve high-efficiency ECL since they are efficient emitters with high quantum yields and size-tunable color.
In 2004, Weller et al. reported the first bandgap ECL of CdSe/CdS NCs in aqueous solution, which opens the avenues of QDs applications into practical biosensing [33]. By depositing CdSe NCs on a paraffin-impregnated graphite electrode (PIGE), Ju et al. achieved the first QD ECL sensor for the detection of H2O2 [34]. Based on the electron transfer reaction between electrochemically reduced NC species and hydrogen peroxide, the fabricated ECL sensor could detect the co-reactant H2O2 with a detection limit of 100 nM (Figure 1B). Apart from H2O2, a lot of inorganic and organic molecules and ions, such as Cu2+, dopamine, and glucose (with the help of glucose oxidases), can be detected by directly quenching or enhancing the ECL signals of QDs [35,36,37]. Zhu et al. modified CdS NCs, gold nanoparticles, and a low-density lipoprotein (LDL) ligand on the electrode and generated strong ECL in solution [38]. The LDL concentration can be measured in a label-free manner through the decrease in ECL intensity upon the specific binding of LDL to its ligand. In addition, using QDs as ECL tags in classic ECL assays, proteins, and nucleic acids can also be quantified [39].
Although QDs have been applied in ECL analysis for years, most aqueous QDs generate ECL from surface state, which is indicated by red-shifted wavelengths, broad emission spectrum, and low ECL quantum efficiencies relative to PL [27]. To address this problem, Su, Qin, and Peng et al. prepared CdSe/CdS/ZnS core/shell/shell quantum dots (Figure 1C) [40]. By engineering the interior inorganic structure and the inorganic–organic surfaces, the obtained QD exhibited an absolute ECL efficiency of 99%, which is quite close to its near-unity photoluminescence QY. The ECL intensity was reported to be six orders of magnitude higher than that of Ru(bpy)32+ under the same conditions [41]. Recently, they further modulated the surface ligand of CdSe/CdS/ZnS QDs and observed an increase of ECL intensity by 100 times when changing the ligand from oleate to acetate [42]. These results are expected to provide guidance on the design of QDs for analysis applications.
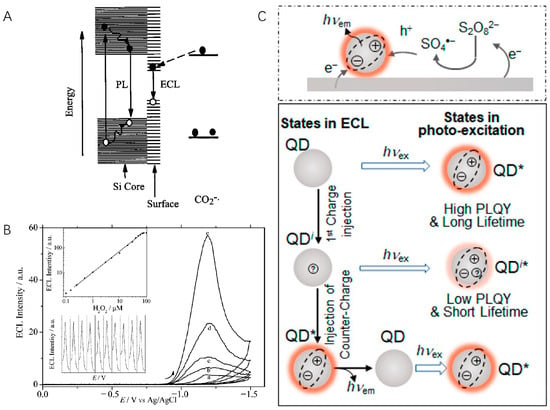
Figure 1.
(A) ECL and PL mechanisms of Si nanocrystals. Copyright 2002 American Association for the Advancement of Science [29]. (B) ECL detection of H2O2 by using CdSe QDs-modified electrode. The ECL intensity increased with increasing H2O2 concentration from 0 μM to 10 μM (a–e). Inset: calibration curve (top) and reproducibility (down). Copyright 2004 American Chemical Society [34]. (C) Illustration of QD cathodic ECL generation (top) and states (neutral QD (QD), singly charged intermediate (QDi), and excite state (QD*)) involved in ECL and PL (bottom), in which symbols on QDs represent different charged states. hνex: excitation light. hνem: emission light. Copyright 2020 American Chemical Society [40].
2.2. Metal Nanoclusters
Metal nanoclusters (NCs) have become attractive ECL emitters due to their unique compositions with atomic precision, rich luminescence characteristics, and well-defined electrochemical features [43]. Ding and coworkers reported the ECL of a series of gold clusters, including Au25, Au38, and Au144 [44,45,46]. These gold nanoclusters display a near-IR emission, ranging from 800 to 930 nm, that is similar to their PL, indicating that they originate from the same or related excitation states. In addition, smaller NCs (Au25 and Au38) show stronger ECL than the larger one (Au144). A high relative ECL efficiency of >350% in reference to that of Ru(bpy)32+/TPrA was obtained with Au38. By rational structure design, Au21(SR)15 (where SR is H, CH3, C2H5, and C2H7) and bimetallic Au12Ag13 nanoclusters even revealed a relative ECL efficiency of 270 and 400%, respectively [47,48]. In both cases, the improved properties can be attributed to the structure–function correlations. For example, the 13th Ag atom at the central position is the key in Au12Ag13 nanoclusters, which stabilizes charges on the LUMO orbital and makes the cluster core rigid.
Although excellent ECL generation was observed, it should be noted that these works were all performed in organic solutions. For better ECL bioanalysis applications, such as cell and tissue imaging, Wang et al. reported the first intense ECL from aqueous soluble AuNCs nanoclusters [49]. By covalently attaching co-reactant N,N-diethylethylenediamine (DEDA) onto Au clusters, oxidative reduction ECL arises from intracluster reactions with a maximum relative ECL efficiency of 17 compared with that of Ru(bpy)32+. Effective strategies such as pre-oxidation and aggregation-induced emission have been reported to enhance the ECL intensity of Au clusters [50,51,52]. For instance, the ligand-induced assembly of CuNCs simultaneously facilitates electrochemical excitation and radiative transition of NCs, thereby improving their ECL efficiency [52]. However, the intrinsic ECL intensity of nanoclusters in aqueous solution is weak, and therefore, the ECL efficiency of these aqueous soluble nanoclusters cannot outperform Ru(bpy)32+.
2.3. Carbon-Based Nanomaterials
Carbon-based nanomaterials have the advantages of high abundance, low toxicity, and good biocompatibility, making them a highly sought-after ECL luminescent material. Carbon dots, graphene QDs, and graphitic QDs have also been explored [53,54]. Chi et al. studied the ECL behavior and mechanisms of water-soluble carbon nanocrystals (CNCs) for the first time (Figure 2A) [55]. Using S2O82− as the co-reactant, the ECL response of CNCs was observed. In this case, CNCs were prepared via electrochemical oxidation of a graphite working electrode and had abundant -COOH groups at the surfaces, which benefits their labeling. This top-down method possesses advantages including easy operation and mass production but suffers from low product yield.
In contrast, the bottom-up method prepares carbon nanomaterials from molecular precursors, which grow up to form “quantum-sized” particles. This strategy enables the precise control of particle morphology and size, and aggregation can be avoided. Common building blocks include ascorbic acid, glucose, fructose, etc. [56,57,58]. Recently, using hexaethynylbenzene as the precursor, Zhang and Mao et al. fabricated graphdiyne (GDY), a new 2D carbon allotrope for ECL generation (Figure 2B) [59]. Different from other carbon allotropes, GDY comprises both sp2- and sp-hybridized carbon and is an intrinsic semiconductor material. In the presence of K2S2O8, GDY produces strong ECL emission at 705 nm in aqueous media, and the ECL efficiency reaches 424% compared with Ru(bpy)3Cl2/K2S2O8.
Graphitic carbon nitride (CN) is another two-dimensional carbon nanomaterial with adjustable molecular structure and electronic properties [60]. Previously, the poor dispersibility of CN in solvents (similar to nanotubes and graphene), low efficiency of CNs exfoliation, and original chemical inertness limit their practical applications. Therefore, tremendous efforts have been devoted to developing CN-based ECL luminophores. Zhang et al. proposed a non-covalent modification and exfoliation methodology to prepare two-dimensional CNs (Figure 2C) [61]. They mechanically grind bulk CN with aromatic molecules, obtaining CN nanosheets (m-CNNS) noncovalently modified with 1-pyrenebutyrate. DNA and other biological recognition moieties can thus be covalently conjugated onto functionalized CNs via EDC/NHS activation. Moreover, using bottom-up methods such as thermal condensation, a series of CN films were prepared on the electrode surface [62]. A transparent polymeric film on an FTO electrode exhibits more than 2000 times higher ECL intensity than that of the reference Ru(bpy)3Cl2/K2S2O8 system, which enables the preparation of an ultrasensitive visual DNA biosensor using the naked eye [63].
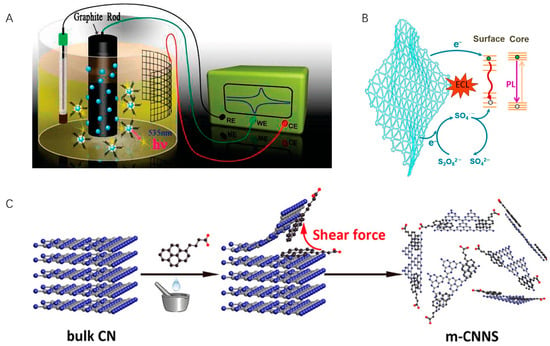
Figure 2.
(A) Schematic illustration of the synthesis of water-soluble CNCs. Copyright 2009 American Chemical Society [55]. (B) ECL mechanism of GDY. Copyright 2022 WILEY-VCH [59]. (C) Exfoliation and modification of 2D carbon nitride. Copyright 2017 American Chemical Society [61].
2.4. Organic Nanoparticles
Organic nanoparticles are also an important type of nanomaterials with ECL signals. Tunable optical properties, easy functionalization, and excellent biocompatibility are the main advantages of organic nanoparticles as ECL luminophores. In 2008, the ECL imaging analysis of single conjugated polymer nanoparticles was achieved after a long integrating time (10–40 s) [64]. Different kinds of polymers have been used to prepare nanoparticles, while their ECL efficiency is lower than that of general metal complexes [65,66]. The poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-(1-cyanovinylene-1,4-phenylene)] Pdots gave a relative ECL efficiency of ca. 12% (vs. Ru(bpy)32+/TPrA) [67,68]. Ju et al. used Pdots as emitters to modify electrodes [68]. Compared with the Ru(bpy)32+-modified electrode, the relative ECL efficiency was calculated to be 120%. Using this Pdot as an ECL tag in immunoassay, the limit of detection for cytokeratin-19-fragment detection was reduced to 0.12 pg mL−1. To date, the research on organic NPs is just beginning. Challenges such as poor water solubility and relatively low ECL efficiency need to be addressed.
3. Self-Enhancing ECL Luminophores
To obtain a high ECL signal, most ECL systems require a co-reactant. However, classic co-reactants such as TPrA are toxic, corrosive, and volatile but need to be used in high concentrations. Some co-reactants produce byproducts during the electrochemical reaction process, increasing the background noise and reducing the detection sensitivity. Meanwhile, the selection of co-reactants might change the response of the ECL system to the target, thereby affecting the accuracy of the detection. Many researchers have begun to consider in situ generation of co-reactants to reduce the impact of exogenous co-reactants. Einaga et al. used boron-doped diamond (BDD) electrodes to promote the conversion of inert SO42− into co-reactant S2O82− [69]. In another work, a BDD electrode facilitated the oxidation of carbonate (CO32−) into peroxydicarbonate (C2O62−), which further reacted with water to produce co-reactant H2O2 [70]. Although powerful, the BDD electrode is not a general solution applicable for ECL analysis. To avoid the influence of exogenous co-reactants, more studies should focus on self-enhancing luminophores, whose co-reactant is integrated into the luminophore structures. According to the type of ECL luminescent center (molecules or nanoparticles), we classify these luminophores into two categories.
3.1. Molecules as the Luminescent Center
In 1996, Martin et al. observed that ruthenium-labeled penicillin molecules could generate ECL in the absence of any amine, which results from the intramolecular electron transfer from penicillin to ruthenium [71]. Zysman-Colman and Ding reported for the first time the ECL auto-enhancement phenomenon. They synthesized an iridium(III) complex bearing aryltriazole C^N ligands with two dimethylamino (dma) groups (Figure 3A) [72]. Three emissions were observed in spooling spectrum, correlating to the typical annihilation route (543 nm) and dma co-reactant route (608 and 651 nm). Compared with a structurally similar complex without dma substituents, the ECL intensity and efficiency in the absence of co-reactants were self-enhanced by 16 and 5.5 times, respectively. The strategy of integrating co-reactant and luminophore in a single molecule opens a new avenue for simplifying ECL detection protocols and enhancing detection sensitivity. Two ruthenium (II) complexes carried Schiff base cavities as the luminophore [73]. Thanks to the electrochemical oxidation of phenolic hydroxyl groups and the resonance structure of the imino radicals, electrons can be transferred within the molecule, thereby achieving effective ECL without the addition of co-reactant TPrA. After connecting a dimethylamino moiety to cationic diaza [4] helicene by a short linker, the first self-enhancing organic ECL dye was obtained [74]. In combination with a series of organometallic complexes, self-enhanced multi-color ECL was achieved as well.
Amine-rich polymers and nanomaterial co-reactants can be also used as self-enhancing moieties. Zhuo and Yuan et al. combined the poly(ethylenimine) (PEI) and a derivative of Ru(bpy)32+ bearing -COOH groups to prepare self-enhanced nanocomposites (PEI-Ru) [75,76]. An immunoassay was ultimately devised by using the nanocomposite as ECL tags, with an enhancement in ECL intensity up to 17 times and a detection limit as low as to 0.3 fg/mL. Nitrogen-doped carbon nanodots (NCNDs) have been demonstrated to be powerful alternatives to TPrA. By covalently linking luminophores and NCNDs, De Cola and co-workers synthesized a hybrid of NCNDs and Ru(bpy)32+, in which NCNDs function as both carriers and co-reactants (Figure 3B) [77]. Interestingly, the ECL emission intensity of the Ru-NCND hybrid is not only higher than that obtained for Ru(bpy)32+ but also higher than that of the uncoupled Ru(bpy)32+/NCND system. The reason might be that intramolecular electron transfer is more efficient than the intermolecular transfer, in which the former favors shorter electron transfer distance and less energy loss. A boron carbon nitride nanosheets–Ru nanocomposite (BCN NSs-Ru) was successfully employed to construct an ECL mRNA sensor [78]. No co-reactants were used, and the limit of detection was 32.3 aM.
3.2. Nanomaterials as the Luminescent Center
As described before, amine moieties can be modified onto nanomaterial surfaces or embedded into their structures to obtain self-enhanced ECL. Wang et al. covalently attached the co-reactant N,N-diethylethylenediamine (DEDA) to Au nanoclusters, enabling ECL generation from Au cluster without additional and high-excess co-reactants (Figure 3C) [49]. The intracluster reactions reduce the complication of mass transport between Au cluster and co-reactants and significantly enhance its ECL intensity. By directly embedded tertiary amine groups as co-reactants into the side chain of the polymer unit, a self-enhanced Pdots ECL system (TEA-Pdots) was obtained [79]. The ECL intensity of TEA-Pdots was 132 times higher than that of the mixture of Pdots and TEA at equivalent, and the ECL efficiency waws 4 times higher than that of [Ru(bpy)32+]/TEA system. By using this highly efficient nanoparticle as an ECL tag, ECL microimaging of membrane protein in single living cells without additional co-reactants was achieved. Similar to the design of Pdots, self-enhanced metal–organic frameworks (MOFs) can be facilely prepared by integrating co-reactants as building blocks in MOFs (Figure 3D). Different from the above works, luminophores and co-reactants are not covalently linked in self-enhancing MOFs. The luminophore 9,10-di(pcarboxyphenyl)anthracene (DPA) and co-reactant 1,4-diazabicyclo[2.2.2]octane (D-H2) were integrated as two ligands in Zn2+ MOFs [80]. By shortening the distance between DPA and D-H2 by the frameworks, an efficient charge transfer can be realized between the emitter and co-reactant. Studies have shown a 26.5-fold increase in ECL intensity compared with that of the mixture of DPA and D-H2.
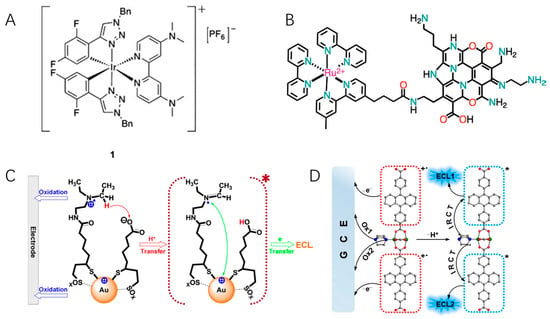
Figure 3.
(A) The self-enhanced iridium(III) complex with two dimethylamino (dma) groups. Copyright 2012 WILEY-VCH [72]. (B) The covalently linked hybrid of NCNDs and Ru(bpy)32+. Copyright 2017 WILEY-VCH [77]. (C) ECL mechanism of self-enhanced Au clusters. Copyright 2016 American Chemical Society [49]. (D) Illustration of the stepwise ECL mechanism of self-enhanced MOFs. Copyright 2021 American Chemical Society [80]. The asterisk * means excited state.
4. Multi-Color ECL Luminophores
Multiplex analysis capable of determining multiple analytes in a single complex sample has become increasingly popular in recent years. The simultaneous detection not only saves detection time and samples but also gives more information about the sample and improves the accuracy of detection. Typically, approaches to achieve parallel ECL detection can be roughly divided into three categories: potential-, spatial-, and spectrum-resolved strategies. Potential-resolved ECL measurements rely on luminophores with potential-controlled switch on/off properties, while only a few luminophores have been reported. Spatial-resolved assays are usually expensive since well-designed spot arrays are required. In comparison, spectrum-resolved ECL can be facilely achieved in one solution with diverse multi-color ECL luminophores, which provides the possibility of multiplex immunoassays. In this part, we mainly focus on the emerging spectrum-resolved ECL systems, some of which may exhibit potential- and spatial-resolved features as well.
4.1. Transition Metal Complexes
The ECL of Ru(bpy)32+ and its derivatives is generated by the radiative decay of metal-to-ligand charge transfer, and thus, it is difficult to tune their ECL colors for multiplex detection. Over the last few decades, new molecular luminophores have been reported and studied in-depth. The most important one is the iridium(III) complex. Compared with Ru(bpy)32+, iridium complexes generally exhibit much higher PLQY. Thanks to an increased ligand field stabilization energy, the ECL wavelengths of iridium complexes can be facilely modulated in the region from visible to near-UV by changing the substituents on their ligands and coordination modes. These advantages facilitate multianalyte detection and make it a promising option in ECL analysis.
Richter et al. studied the multicolored ECL from a mixture of Ir and Ru complexes (Figure 4A) [81,82]. Ir complexes with blue (Ir(dfppy)2(pic), λECL = 470 nm), green (Ir(ppy)3, λECL = 517 nm), and red (Ir(btp)2(acac), λECL = 600 nm) emissions were synthesized. Either blue or green emitters can be distinguished from Ru(bpy)32+ in ECL spectra, providing the possibility of detecting multiple analytes in a single ECL experiment. Since then, various Ir complexes with different ligands have been prepared, offering more options for spectrum-resolved detections [83,84]. In three-dimensional (intensity, wavelength, and potential) ECL spectra and potential-dependent ECL images, different molecules exploit different ECL onset potentials and wavelengths, which promises to enable multianalyte detection [85,86]. Particularly, Francis and Hogan et al. reported the potential-controlled on/off mechanism of Ir(ppy)3, whose ECL can be “switched-off” at high potentials. This molecular has been widely applied in ECL multiplex analysis [87].
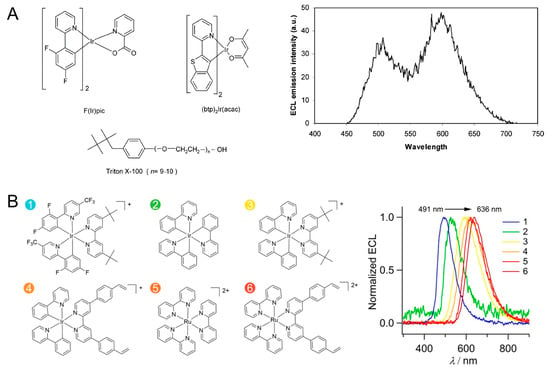
Figure 4.
(A) Structures of multicolor ECL luminophores and the ECL mission spectrum of F(Ir)pic and Ru(bpy)32+ in a same solution. Copyright 2004 American Chemical Society [81]. (B) Molecular structures of six ECL luminophores (1–6) and their ECL spectra. Copyright 2018 American Chemical Society [88].
Su et al. constructed a potential-resolved multicolor ECL system for multiplex immunoassay (Figure 4B) [88]. They synthesized six Ir(III) and Ru(II) complexes, in which the red, green, and cyan luminophores were loaded into polystyrene beads, encoded with three different detection antibodies, and used for the simultaneous detection of three antigens in a sample volume of 300 μL. Xu and coworkers prepared a closed bipolar electrode (BPE) and employed ECL as an optical reporter [89]. The formation of immunocomplex on the BPE modulated the resistance of BPE and the potential in the reporter pole; therefore, the emission color of the Ru(bpy)32+/Ir(ppy)3 mixture was finely tuned. By using Irpic-Ome, Ir(ppy)2(acac), and Ru(bpy)32+ as blue, green, and red ECL emitters to detect three miRNA biomarkers, the ECL emission can cover whole visible regions [90]. It should be noted that iridium complexes suffer from poor water solubility; therefore, the use of polymer beads and bipolar configuration is necessary, in which luminophores are separated from the sensing interfaces. Synthesizing water-soluble iridium complexes and developing new sensing strategies remain hot topics [91,92]. Moreover, although differences in the emission maxima of these metal complexes reach 100–150 nm, there are considerable spectral overlaps. These molecules have a broad spectrum with a full width-at-half-maximum up to ca. 60–80 nm, which limits the throughput of color-resolved ECL analysis in the visible region.
4.2. Multi-Color Nanomaterials
Due to quantum confinement effect, QDs possess size-dependent optical and electronic properties, which inherently favor multi-color ECL analysis [93]. Highly efficient, stable, and spectrally resolved QDs with narrow emission line width are ideal alternatives for developing multiplex ECL assays. CdTe (λECL = 776 nm) and CdSe (λECL = 550 nm) QDs were employed as spectrum-resolved ECL emitters for the simultaneous detection of carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) [94]. By establishing a sandwich-type immunoassay on the glassy carbon electrode, extremely low detection limits of 1 pg/mL and 10 fg/mL for CEA and AFP were achieved. In the immunoassay, some QDs labeled on the secondary antibody might not be electrochemically oxidized or reduced on the electrode surface due to the distance. To promote direct electron transfer, Guo et al. used graphene as a conducting bridge between the electrode and QDs, which led to a 30-fold enhancement in ECL intensity (Figure 5A) [95]. An interesting work reported by Pang and coworkers adopted three QDs with emission wavelengths ranging from 525 nm to 625 nm to visualize biomolecules on single cells [96]. These QDs were decorated with glucose oxidase, perfringolysin O, and lysenin protein to recognize glucose, cholesterol, and sphingomyelin, respectively. Molecular dynamics and inter-molecular interactions can be monitored simultaneously.
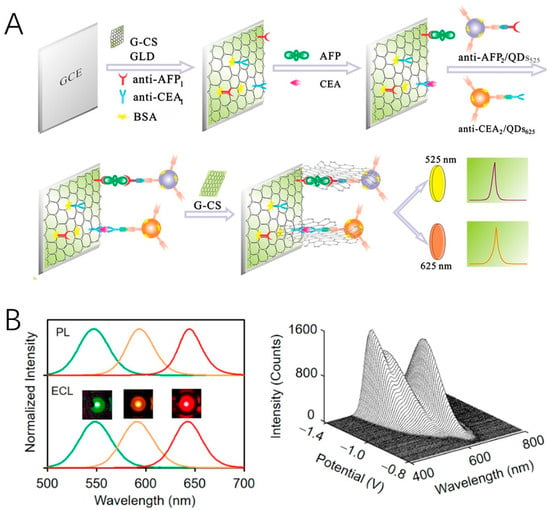
Figure 5.
(A) Fabrication of the ECL immunosensor using QDs as labels and graphene as conducting bridge. Copyright 2013 Elsevier [95]. (B) Multicolor ECL emission from core/shell/shell QDs with different core sizes. Copyright 2020 American Chemical Society [41].
Note that there are two ECL mechanisms for QD ECL, including surface-state and band-edge ECL generation. The latter one is favored in multi-color since it corresponds to size-dependent ECL wavelengths. Su and Peng et al. used an additional wide bandgap ZnS shell to isolate surface traps and a CdS intermediate layer to relieve the lattice strain between ZnS and CdSe (Figure 5B) [41]. By rationally modulating the size of the CdSe core, a group of CdSe/CdS/ZnS core/shell/shell QDs were synthesized, which exhibited multicolor band-edge ECL with K2S2O8 as the co-reactant. In addition to QDs, Pdots allow for multiplex analysis as well. By doping Pdots with luminol and diethylamine, respectively, Pdots emitting light at 450 nm and 675 nm were prepared [97]. Two microRNAs were quantified with the true color ECL imaging.
To better compare the performance of the different luminophores discussed above, here, we summarize typical ECL sensors based on these luminophores in Table 1. Note that some of the emerging luminophores are at an early stage of development without analytical applications, and we also list them in the table to illustrate their unique ECL properties.

Table 1.
Application of different ECL luminophores in ECL sensing *.
5. Conclusions
In this paper, we summarized the recent advances in ECL luminophores, covering the hot topics including new luminophores with high ECL efficiencies, self-enhancing luminophores, and multi-color ECL systems. Ultrasensitive, co-reactant-free, and high-throughput analyses have been accomplished in these works. However, despite the extensive research into new ECL luminophores, most ECL assays in both fundamental and commercial systems still rely on Ru(bpy)32+. Challenges remain in this field. First, the application of nanomaterials in ECL analysis is still less studied due to the complexity of their structures and relatively large sizes compared with molecular luminophores. Highly efficient luminophores with well-defined structures, clear ECL mechanisms, excellent electrochemical and optical properties, high water solubility, and biological compatibility are still desired. Secondly, most self-enhancing luminophores were constructed by embedding amine moieties into their structures. Since the co-reactant cannot be regenerated in the ECL process, the intensity and stability of these luminophores should be of concern. Breakthroughs in the design principles of self-enhancing luminophores or novel co-reactants are expected. Finally, restricted by the full width-at-half-maximum of ECL spectra, color-resolved ECL analysis is limited to the proof-of-concept experiment, which determines a maximum of two or three biomarkers. The multiplexing capacity should be further elevated. With the combination of new principles and methodologies, we believe ECL analysis could achieve wider and deeper applications in the fields of bioanalysis, environmental detection, and medical diagnosis.
Author Contributions
Writing—original draft preparation, Z.H.; writing—review and editing, H.D. and D.J.; supervision, D.J.; funding acquisition, H.D. and D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (22204074).
Acknowledgments
Z.H. thanks Yindong Ye for the guidance on the literature search and paper writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Richter, M.M. Electrochemiluminescence (ECL). Chem. Rev. 2004, 104, 3003–3036. [Google Scholar] [CrossRef] [PubMed]
- Miao, W. Electrogenerated Chemiluminescence and Its Biorelated Applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qi, W.; Xu, G. Recent advances in electrochemiluminescence. Chem. Soc. Rev. 2015, 44, 3117–3142. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Guo, W.; Su, B. Electrochemiluminescence Single-Cell Analysis: Intensity- and Imaging-Based Methods. ChemPlusChem 2020, 85, 725–733. [Google Scholar] [CrossRef]
- Ding, H.; Zhou, P.; Fu, W.; Ding, L.; Guo, W.; Su, B. Spatially Selective Imaging of Cell-Matrix and Cell-Cell Junctions by Electrochemiluminescence. Angew. Chem. Int. Ed. 2021, 60, 11769–11773. [Google Scholar] [CrossRef]
- Zhang, J.J.; Jin, R.; Jiang, D.C.; Chen, H.Y. Electrochemiluminescence-Based Capacitance Microscopy for Label-Free Imaging of Antigens on the Cellular Plasma Membrane. J. Am. Chem. Soc. 2019, 141, 10294–10299. [Google Scholar] [CrossRef]
- Xu, J.; Huang, P.; Qin, Y.; Jiang, D.; Chen, H.Y. Analysis of Intracellular Glucose at Single Cells Using Electrochemiluminescence Imaging. Anal. Chem. 2016, 88, 4609–4612. [Google Scholar] [CrossRef]
- Hvastkovs, E.G.; Schenkman, J.B.; Rusling, J.F. Metabolic toxicity screening using electrochemiluminescence arrays coupled with enzyme-DNA biocolloid reactors and liquid chromatography-mass spectrometry. Annu. Rev. Anal. Chem. 2012, 5, 79–105. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, C.; Xu, Q.; Zhu, J.-J. Recent progress in electrochemiluminescence microscopy analysis of single cells. Analyst 2022, 147, 2884–2894. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Ma, Q. Nanoparticle-based electrochemiluminescence cytosensors for single cell level detection. TrAC Trends Anal. Chem. 2019, 110, 277–292. [Google Scholar] [CrossRef]
- Ding, H.; Su, B.; Jiang, D. Recent Advances in Single Cell Analysis by Electrochemiluminescence. ChemistryOpen 2023, 12, e202200113. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, Y.; Cao, Z.; Su, B. Imaging Analysis Based on Electrogenerated Chemiluminescence. J. Anal. Test. 2017, 1, 14. [Google Scholar] [CrossRef]
- Zhang, J.; Arbault, S.; Sojic, N.; Jiang, D. Electrochemiluminescence imaging for bioanalysis. Annu. Rev. Anal. Chem. 2019, 12, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, B. Deciphering the Mechanisms of Electrochemiluminescence by Spatially Resolved Measurements. Analysis Sensing 2021, 1, 148–155. [Google Scholar] [CrossRef]
- Mariani, C.; Bogialli, S.; Paolucci, F.; Pastore, P.; Zanut, A.; Valenti, G. Enhancing electrochemiluminescence intensity through emission layer control. Electrochim. Acta 2024, 489, 144256. [Google Scholar] [CrossRef]
- Pruchyathamkorn, J.; Yang, M.; Amin, H.M.A.; Batchelor-McAuley, C.; Compton, R.G. Imaging Electrode Heterogeneity Using Chemically Confined Fluorescence Electrochemical Microscopy. J. Phys. Chem. Lett. 2017, 8, 6124–6127. [Google Scholar] [CrossRef]
- Amin, H.M.A.; El-Kady, M.F.; Atta, N.F.; Galal, A. Gold Nanoparticles Decorated Graphene as a High Performance Sensor for Determination of Trace Hydrazine Levels in Water. Electroanalysis 2018, 30, 1757–1766. [Google Scholar] [CrossRef]
- dos Santos, W.T.P.; Amin, H.M.A.; Compton, R.G. A nano-carbon electrode optimized for adsorptive stripping voltammetry: Application to detection of the stimulant selegiline in authentic saliva. Sens. Actuators, B 2019, 279, 433–439. [Google Scholar] [CrossRef]
- Miao, W.; Choi, J.-P.; Bard, A.J. Electrogenerated Chemiluminescence 69: The Tris(2,2‘-bipyridine)ruthenium(II), (Ru(bpy)32+)/Tri-n-propylamine (TPrA) System RevisitedA New Route Involving TPrA•+ Cation Radicals. J. Am. Chem. Soc. 2002, 124, 14478–14485. [Google Scholar] [CrossRef]
- Wong, J.M.; Zhang, R.; Xie, P.; Yang, L.; Zhang, M.; Zhou, R.; Wang, R.; Shen, Y.; Yang, B.; Wang, H.-B.; et al. Revealing Crystallization-Induced Blue-Shift Emission of a Di-Boron Complex by Enhanced Photoluminescence and Electrochemiluminescence. Angew. Chem. Int. Ed. 2020, 59, 17461–17466. [Google Scholar] [CrossRef]
- Mayer, M.; Takegami, S.; Neumeier, M.; Rink, S.; Jacobi von Wangelin, A.; Schulte, S.; Vollmer, M.; Griesbeck, A.G.; Duerkop, A.; Baeumner, A.J. Electrochemiluminescence Bioassays with a Water-Soluble Luminol Derivative Can Outperform Fluorescence Assays. Angew. Chem. Int. Ed. 2018, 57, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Ren, G.; Zhang, M.; Mao, L. Electroless Deposition of Palladium Nanoparticles on Graphdiyne Boosts Electrochemiluminescence. J. Am. Chem. Soc. 2024, 146, 3836–3843. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Li, B.; Liu, J.; Jiang, D.; Liu, B.; Sojic, N. Single Biomolecule Imaging by Electrochemiluminescence. J. Am. Chem. Soc. 2021, 143, 17910–17914. [Google Scholar] [CrossRef]
- Han, D.; Goudeau, B.; Manojlovic, D.; Jiang, D.; Fang, D.; Sojic, N. Electrochemiluminescence Loss in Photobleaching. Angew. Chem. Int. Ed. 2021, 60, 7686–7690. [Google Scholar] [CrossRef]
- Ding, H.; Guo, W.; Su, B. Imaging Cell-Matrix Adhesions and Collective Migration of Living Cells by Electrochemiluminescence Microscopy. Angew. Chem. Int. Ed. 2020, 59, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.; Scarabino, S.; Goudeau, B.; Lesch, A.; Jovic, M.; Villani, E.; Sentic, M.; Rapino, S.; Arbault, S.; Paolucci, F.; et al. Single Cell Electrochemiluminescence Imaging: From the Proof-of-Concept to Disposable Device-Based Analysis. J. Am. Chem. Soc. 2017, 139, 16830–16837. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, P.; Su, B. Electrochemiluminescence of Semiconductor Quantum Dots and Its Biosensing Applications: A Comprehensive Review. Biosensors 2023, 13, 708. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Zhu, J.-J. Electrochemiluminescence based on quantum dots and their analytical application. Anal. Methods 2011, 3, 33–42. [Google Scholar] [CrossRef]
- Ding, Z.; Quinn, B.M.; Haram, S.K.; Pell, L.E.; Korgel, B.A.; Bard, A.J. Electrochemistry and Electrogenerated Chemiluminescence from Silicon Nanocrystal Quantum Dots. Science 2002, 296, 1293–1297. [Google Scholar] [CrossRef]
- Myung, N.; Ding, Z.; Bard, A.J. Electrogenerated Chemiluminescence of CdSe Nanocrystals. Nano Lett. 2002, 2, 1315–1319. [Google Scholar] [CrossRef]
- Bae, Y.; Myung, N.; Bard, A.J. Electrochemistry and Electrogenerated Chemiluminescence of CdTe Nanoparticles. Nano Lett. 2004, 4, 1153–1161. [Google Scholar] [CrossRef]
- Myung, N.; Bae, Y.; Bard, A.J. Effect of Surface Passivation on the Electrogenerated Chemiluminescence of CdSe/ZnSe Nanocrystals. Nano Lett. 2003, 3, 1053–1055. [Google Scholar] [CrossRef]
- Poznyak, S.K.; Talapin, D.V.; Shevchenko, E.V.; Weller, H. Quantum Dot Chemiluminescence. Nano Lett. 2004, 4, 693–698. [Google Scholar] [CrossRef]
- Zou, G.; Ju, H. Electrogenerated Chemiluminescence from a CdSe Nanocrystal Film and Its Sensing Application in Aqueous Solution. Anal. Chem. 2004, 76, 6871–6876. [Google Scholar] [CrossRef]
- Zhang, L.; Shang, L.; Dong, S. Sensitive and selective determination of Cu2+ by electrochemiluminescence of CdTe quantum dots. Electrochem. Commun. 2008, 10, 1452–1454. [Google Scholar] [CrossRef]
- Stewart, A.J.; Hendry, J.; Dennany, L. Whole Blood Electrochemiluminescent Detection of Dopamine. Anal. Chem. 2015, 87, 11847–11853. [Google Scholar] [CrossRef]
- Jiang, H.; Ju, H. Enzyme–quantum dots architecture for highly sensitive electrochemiluminescence biosensing of oxidase substrates. Chem. Commun. 2007, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Jie, G.; Liu, B.; Pan, H.; Zhu, J.-J.; Chen, H.-Y. CdS Nanocrystal-Based Electrochemiluminescence Biosensor for the Detection of Low-Density Lipoprotein by Increasing Sensitivity with Gold Nanoparticle Amplification. Anal. Chem. 2007, 79, 5574–5581. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, Y.; Liang, Z.; Wang, Z.; Xu, S.; Ma, Q. DNA-Mediated Au–Au Dimer-Based Surface Plasmon Coupling Electrochemiluminescence Sensor for BRCA1 Gene Detection. Anal. Chem. 2021, 93, 3308–3314. [Google Scholar] [CrossRef]
- Cao, Z.; Li, C.; Shu, Y.; Zhu, M.; Su, B.; Qin, H.; Peng, X. Unraveling Mechanisms of Highly Efficient Yet Stable Electrochemiluminescence from Quantum Dots. J. Am. Chem. Soc. 2023, 145, 26425–26434. [Google Scholar] [CrossRef]
- Cao, Z.; Shu, Y.; Qin, H.; Su, B.; Peng, X. Quantum Dots with Highly Efficient, Stable, and Multicolor Electrochemiluminescence. ACS Cent. Sci. 2020, 6, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Cao, Z.Y.; Qin, H.Y.; Peng, X.G.; Su, B. Ligand-controlled electrochemiluminescence generation from CdSe/CdS/ZnS core/shell/shell quantum dots. Nano Res. 2024, 17, 7776–7785. [Google Scholar] [CrossRef]
- Hesari, M.; Ding, Z. A Grand Avenue to Au Nanocluster Electrochemiluminescence. Acc. Chem. Res. 2017, 50, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Swanick, K.N.; Hesari, M.; Workentin, M.S.; Ding, Z. Interrogating Near-Infrared Electrogenerated Chemiluminescence of Au25(SC2H4Ph)18+ Clusters. J. Am. Chem. Soc. 2012, 134, 15205–15208. [Google Scholar] [CrossRef]
- Hesari, M.; Workentin, M.S.; Ding, Z. Highly Efficient Electrogenerated Chemiluminescence of Au-38 Nanoclusters. ACS Nano 2014, 8, 8543–8553. [Google Scholar] [CrossRef] [PubMed]
- Hesari, M.; Ding, Z.; Workentin, M.S. Electrogenerated Chemiluminescence of Monodisperse Au144(SC2H4Ph)60 Clusters. Organometallics 2014, 33, 4888–4892. [Google Scholar] [CrossRef]
- Hesari, M.; Ding, Z. Identifying Highly Photoelectrochemical Active Sites of Two Au21 Nanocluster Isomers toward Bright Near-Infrared Electrochemiluminescence. J. Am. Chem. Soc. 2021, 143, 19474–19485. [Google Scholar] [CrossRef]
- Chen, S.; Ma, H.; Padelford, J.W.; Qinchen, W.; Yu, W.; Wang, S.; Zhu, M.; Wang, G. Near Infrared Electrochemiluminescence of Rod-Shape 25-Atom AuAg Nanoclusters That Is Hundreds-Fold Stronger Than That of Ru(bpy)3 Standard. J. Am. Chem. Soc. 2019, 141, 9603–9609. [Google Scholar] [CrossRef]
- Wang, T.; Wang, D.; Padelford, J.W.; Jiang, J.; Wang, G. Near-Infrared Electrogenerated Chemiluminescence from Aqueous Soluble Lipoic Acid Au Nanoclusters. J. Am. Chem. Soc. 2016, 138, 6380–6383. [Google Scholar] [CrossRef]
- Peng, H.; Huang, Z.; Sheng, Y.; Zhang, X.; Deng, H.; Chen, W.; Liu, J. Pre-oxidation of Gold Nanoclusters Results in a 66 % Anodic Electrochemiluminescence Yield and Drives Mechanistic Insights. Angew. Chem. Int. Ed. 2019, 58, 11691–11694. [Google Scholar] [CrossRef]
- Peng, H.; Huang, Z.; Deng, H.; Wu, W.; Huang, K.; Li, Z.; Chen, W.; Liu, J. Dual Enhancement of Gold Nanocluster Electrochemiluminescence: Electrocatalytic Excitation and Aggregation-Induced Emission. Angew. Chem. Int. Ed. 2020, 59, 9982–9985. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ning, Z.; Yang, E.; Yin, F.; Wu, G.; Zhang, Y.; Shen, Y. Ligand-induced Assembly of Copper Nanoclusters with Enhanced Electrochemical Excitation and Radiative Transition for Electrochemiluminescence. Angew. Chem. Int. Ed. 2023, 62, e202312053. [Google Scholar] [CrossRef]
- Fu, Y.; Ma, Q. Recent developments in electrochemiluminescence nanosensors for cancer diagnosis applications. Nanoscale 2020, 12, 13879–13898. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, J.; Gao, C.; Wang, E. Applications of carbon quantum dots in electrochemiluminescence: A mini review. Electrochem. Commun. 2014, 48, 151–154. [Google Scholar] [CrossRef]
- Zheng, L.; Chi, Y.; Dong, Y.; Lin, J.; Wang, B. Electrochemiluminescence of Water-Soluble Carbon Nanocrystals Released Electrochemically from Graphite. J. Am. Chem. Soc. 2009, 131, 4564–4565. [Google Scholar] [CrossRef]
- Dai, H.; Yang, C.; Tong, Y.; Xu, G.; Ma, X.; Lin, Y.; Chen, G. Label-free electrochemiluminescent immunosensor for α-fetoprotein: Performance of Nafion–carbon nanodots nanocomposite films as antibody carriers. Chem. Commun. 2012, 48, 3055–3057. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-X.; Zheng, C.-L.; Li, Q.-L.; Ding, S.-N. Electrochemiluminescence of a nanoAg–carbon nanodot composite and its application to detect sulfide ions. Analyst 2014, 139, 1751–1755. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, Z.; Luo, D.; Yu, W.; Guo, Z.; Wang, T. Dual-Peak Electrogenerated Chemiluminescence of Carbon Dots for Iron Ions Detection. Anal. Chem. 2014, 86, 5620–5623. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Zeng, H.; Wang, X.; Zhang, Y.; Zhang, S.; Cui, R.; Zhang, M.; Mao, L. Graphdiyne: A New Carbon Allotrope for Electrochemiluminescence. Angew. Chem. Int. Ed. 2022, 61, e202204485. [Google Scholar] [CrossRef]
- Chen, L.; Huang, D.; Ren, S.; Dong, T.; Chi, Y.; Chen, G. Preparation of graphite-like carbon nitride nanoflake film with strong fluorescent and electrochemiluminescent activity. Nanoscale 2013, 5, 225–230. [Google Scholar] [CrossRef]
- Ji, J.; Wen, J.; Shen, Y.; Lv, Y.; Chen, Y.; Liu, S.; Ma, H.; Zhang, Y. Simultaneous Noncovalent Modification and Exfoliation of 2D Carbon Nitride for Enhanced Electrochemiluminescent Biosensing. J. Am. Chem. Soc. 2017, 139, 11698–11701. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhou, Q.; Lv, Y.; Han, D.; Wu, K.; Zhao, L.; Shen, Y.; Liu, S.; Zhang, Y. Ultrafast Condensation of Carbon Nitride on Electrodes with Exceptional Boosted Photocurrent and Electrochemiluminescence. Angew. Chem. Int. Ed. 2020, 59, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Fang, Y.; Zhou, Z.; Hong, Q.; Li, W.; Yang, H.; Wu, K.; Xu, Y.; Cao, X.; Han, D.; et al. Growth of Robust Carbon Nitride Films by Double Crystallization with Exceptionally Boosted Electrochemiluminescence for Visual DNA Detection. Adv. Opt. Mater. 2023, 11, 2202737. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Palacios, R.E.; Fan, F.-R.F.; Bard, A.J.; Barbara, P.F. Electrogenerated Chemiluminescence of Single Conjugated Polymer Nanoparticles. J. Am. Chem. Soc. 2008, 130, 8906–8907. [Google Scholar] [CrossRef]
- Liu, J.-L.; Zhuo, Y.; Chai, Y.-Q.; Yuan, R. BSA stabilized tetraphenylethylene nanocrystals as aggregation-induced enhanced electrochemiluminescence emitters for ultrasensitive microRNA assay. Chem. Commun. 2019, 55, 9959–9962. [Google Scholar] [CrossRef]
- Feng, Y.; Dai, C.; Lei, J.; Ju, H.; Cheng, Y. Silole-Containing Polymer Nanodot: An Aqueous Low-Potential Electrochemiluminescence Emitter for Biosensing. Anal. Chem. 2016, 88, 845–850. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.; Wang, Y.; Ju, H.; Yan, F. Electrochemiluminescent Imaging for Multi-immunoassay Sensitized by Dual DNA Amplification of Polymer Dot Signal. Anal. Chem. 2018, 90, 7708–7714. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, N.; Ju, H. Highly Efficient Electrochemiluminescence of Cyanovinylene-Contained Polymer Dots in Aqueous Medium and Its Application in Imaging Analysis. Anal. Chem. 2018, 90, 1202–1208. [Google Scholar] [CrossRef]
- Irkham; Watanabe, T.; Fiorani, A.; Valenti, G.; Paolucci, F.; Einaga, Y. Co-reactant-on-Demand ECL: Electrogenerated Chemiluminescence by the in Situ Production of S2O82– at Boron-Doped Diamond Electrodes. J. Am. Chem. Soc. 2016, 138, 15636–15641. [Google Scholar] [CrossRef]
- Irkham; Fiorani, A.; Valenti, G.; Kamoshida, N.; Paolucci, F.; Einaga, Y. Electrogenerated Chemiluminescence by in Situ Production of Coreactant Hydrogen Peroxide in Carbonate Aqueous Solution at a Boron-Doped Diamond Electrode. J. Am. Chem. Soc. 2020, 142, 1518–1525. [Google Scholar] [CrossRef]
- Liang, P.; Dong, L.; Martin, M.T. Light Emission from Ruthenium-Labeled Penicillins Signaling Their Hydrolysis by β-Lactamase. J. Am. Chem. Soc. 1996, 118, 9198–9199. [Google Scholar] [CrossRef]
- Swanick, K.N.; Ladouceur, S.; Zysman-Colman, E.; Ding, Z. Self-Enhanced Electrochemiluminescence of an Iridium(III) Complex: Mechanistic Insight. Angew. Chem. Int. Ed. 2012, 51, 11079–11082. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jin, Z.; Zhao, M.; Xu, Y.; Guo, Y.; Xiao, D. Self-enhanced electrogenerated chemiluminescence of ruthenium(ii) complexes conjugated with Schiff bases. Dalton Trans. 2015, 44, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Voci, S.; Duwald, R.; Grass, S.; Hayne, D.J.; Bouffier, L.; Francis, P.S.; Lacour, J.; Sojic, N. Self-enhanced multicolor electrochemiluminescence by competitive electron-transfer processes. Chem. Sci. 2020, 11, 4508–4515. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Liao, N.; Chai, Y.-Q.; Gui, G.-F.; Zhao, M.; Han, J.; Xiang, Y.; Yuan, R. Ultrasensitive Apurinic/Apyrimidinic Endonuclease 1 Immunosensing Based on Self-Enhanced Electrochemiluminescence of a Ru(II) Complex. Anal. Chem. 2014, 86, 1053–1060. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Wang, J.-P.; Zhao, M.; Hong, L.-R.; Chai, Y.-Q.; Yuan, R.; Zhuo, Y. Target-catalyzed hairpin assembly and intramolecular/intermolecular co-reaction for signal amplified electrochemiluminescent detection of microRNA. Biosens. Bioelectron. 2016, 77, 442–450. [Google Scholar] [CrossRef]
- Carrara, S.; Arcudi, F.; Prato, M.; De Cola, L. Amine-Rich Nitrogen-Doped Carbon Nanodots as a Platform for Self-Enhancing Electrochemiluminescence. Angew. Chem. Int. Ed. 2017, 56, 4757–4761. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Chai, Y.; Yuan, R. Boron Carbon Nitride Nanosheets-Ru Nanocomposite Self-Enhancement Electrochemiluminescence Emitter with a Three-Dimensional DNA Network Structure as a Signal Amplifier for Ultrasensitive Detection of TK1 mRNA. Anal. Chem. 2022, 94, 11345–11351. [Google Scholar] [CrossRef]
- Wang, N.; Gao, H.; Li, Y.; Li, G.; Chen, W.; Jin, Z.; Lei, J.; Wei, Q.; Ju, H. Dual Intramolecular Electron Transfer for In Situ Coreactant-Embedded Electrochemiluminescence Microimaging of Membrane Protein. Angew. Chem. Int. Ed. 2021, 60, 197–201. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, Y.; Bao, S.; Wang, N.; Yu, S.; Luo, R.; Ma, J.; Ju, H.; Lei, J. Dual Intrareticular Oxidation of Mixed-Ligand Metal–Organic Frameworks for Stepwise Electrochemiluminescence. J. Am. Chem. Soc. 2021, 143, 3049–3053. [Google Scholar] [CrossRef]
- Muegge, B.D.; Richter, M.M. Multicolored Electrogenerated Chemiluminescence from Ortho-Metalated Iridium(III) Systems. Anal. Chem. 2004, 76, 73–77. [Google Scholar] [CrossRef]
- Bruce, D.; Richter, M.M. Green Electrochemiluminescence from Ortho-Metalated Tris(2-phenylpyridine)iridium(III). Anal. Chem. 2002, 74, 1340–1342. [Google Scholar] [CrossRef] [PubMed]
- Kerr, E.; Doeven, E.H.; Barbante, G.J.; Hogan, C.F.; Bower, D.J.; Donnelly, P.S.; Connell, T.U.; Francis, P.S. Annihilation electrogenerated chemiluminescence of mixed metal chelates in solution: Modulating emission colour by manipulating the energetics. Chem. Sci. 2015, 6, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, H.; Wang, X.; Qi, H. Electrogenerated Chemiluminescence from Heteroleptic Iridium(III) Complexes with Multicolor Emission. Inorg. Chem. 2015, 54, 1446–1453. [Google Scholar] [CrossRef]
- Doeven, E.H.; Zammit, E.M.; Barbante, G.J.; Hogan, C.F.; Barnett, N.W.; Francis, P.S. Selective Excitation of Concomitant Electrochemiluminophores: Tuning Emission Color by Electrode Potential. Angew. Chem. Int. Ed. 2012, 51, 4354–4357. [Google Scholar] [CrossRef]
- Doeven, E.H.; Barbante, G.J.; Kerr, E.; Hogan, C.F.; Endler, J.A.; Francis, P.S. Red–Green–Blue Electrogenerated Chemiluminescence Utilizing a Digital Camera as Detector. Anal. Chem. 2014, 86, 2727–2732. [Google Scholar] [CrossRef]
- Doeven, E.H.; Zammit, E.M.; Barbante, G.J.; Francis, P.S.; Barnett, N.W.; Hogan, C.F. A potential-controlled switch on/off mechanism for selective excitation in mixed electrochemiluminescent systems. Chem. Sci. 2013, 4, 977–982. [Google Scholar] [CrossRef]
- Guo, W.; Ding, H.; Gu, C.; Liu, Y.; Jiang, X.; Su, B.; Shao, Y. Potential-Resolved Multicolor Electrochemiluminescence for Multiplex Immunoassay in a Single Sample. J. Am. Chem. Soc. 2018, 140, 15904–15915. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Xu, C.-H.; Zhao, W.; Guan, Q.-Y.; Chen, H.-Y.; Xu, J.-J. Bipolar Electrode Based Multicolor Electrochemiluminescence Biosensor. Anal. Chem. 2017, 89, 8050–8056. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Zhang, Y.-Q.; Liu, Y.; Li, Y.-R.; Li, M.-L.; Meng, G.-R.; Mi, L.; Hu, Y.-H.; Xu, J.-J. Tripedal DNA Walker as a Signal Amplifier Combined with a Potential-Resolved Multicolor Electrochemiluminescence Strategy for Ultrasensitive Detection of Prostate Cancer Staging Indicators. Anal. Chem. 2024, 96, 5852–5859. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Y.; Huang, X.; Hang, J.; Guo, W.; Dai, Z. Multicolor Iridium(III) Complexes with Host–Guest Recognition Motifs for Enhanced Electrochemiluminescence and Modular Labeling. Anal. Chem. 2023, 95, 4543–4549. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hayne, D.J.; Doeven, E.H.; Agugiaro, J.; Wilson, D.J.D.; Henderson, L.C.; Connell, T.U.; Nai, Y.H.; Alexander, R.; Carrara, S.; et al. A conceptual framework for the development of iridium(iii) complex-based electrogenerated chemiluminescence labels. Chem. Sci. 2019, 10, 8654–8667. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-L.; Ding, S.-N. Multicolor electrochemiluminescence of core-shell CdSe@ZnS quantum dots based on the size effect. Sci. China: Chem. 2016, 59, 1508–1512. [Google Scholar] [CrossRef]
- Zou, G.; Tan, X.; Long, X.; He, Y.; Miao, W. Spectrum-Resolved Dual-Color Electrochemiluminescence Immunoassay for Simultaneous Detection of Two Targets with Nanocrystals as Tags. Anal. Chem. 2017, 89, 13024–13029. [Google Scholar] [CrossRef]
- Guo, Z.; Hao, T.; Du, S.; Chen, B.; Wang, Z.; Li, X.; Wang, S. Multiplex electrochemiluminescence immunoassay of two tumor markers using multicolor quantum dots as labels and graphene asconductingbridge. Biosens. Bioelectron. 2013, 44, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.-G.; Ning, D.; Hu, Y.; Liu, S.-L.; Pang, D.-W. Real-time monitoring of biomolecular dynamics on cell membranes by quantum dot-based multicolor electrochemiluminescence. Nano Today 2023, 50, 101855. [Google Scholar] [CrossRef]
- Wang, N.; Chen, L.; Chen, W.; Ju, H. Potential- and Color-Resolved Electrochemiluminescence of Polymer Dots for Array Imaging of Multiplex MicroRNAs. Anal. Chem. 2021, 93, 5327–5333. [Google Scholar] [CrossRef]
- Cui, R.; Gu, Y.-P.; Bao, L.; Zhao, J.-Y.; Qi, B.-P.; Zhang, Z.-L.; Xie, Z.-X.; Pang, D.-W. Near-Infrared Electrogenerated Chemiluminescence of Ultrasmall Ag2Se Quantum Dots for the Detection of Dopamine. Anal. Chem. 2012, 84, 8932–8935. [Google Scholar] [CrossRef]
- Zhu, L.; Ye, J.; Yan, M.; Yu, L.; Peng, Y.; Huang, J.; Yang, X. Sensitive and Programmable “Signal-Off” Electrochemiluminescence Sensing Platform Based on Cascade Amplification and Multiple Quenching Mechanisms. Anal. Chem. 2021, 93, 2644–2651. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Shen, Y.; Zhang, J.; Zhu, J.-J. Electrogenerated Chemiluminescence of Au Nanoclusters for the Detection of Dopamine. Anal. Chem. 2011, 83, 661–665. [Google Scholar] [CrossRef]
- Fang, Y.-M.; Song, J.; Li, J.; Wang, Y.-W.; Yang, H.-H.; Sun, J.-J.; Chen, G.-N. Electrogenerated chemiluminescence from Au nanoclusters. Chem. Commun. 2011, 47, 2369–2371. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).