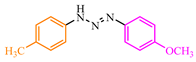
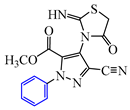
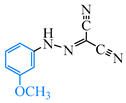
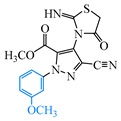
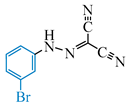
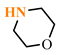
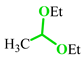
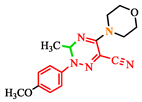
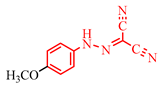
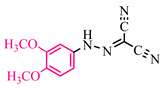
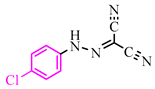
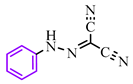
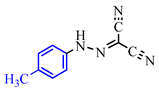
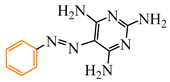
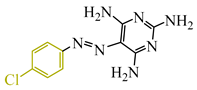
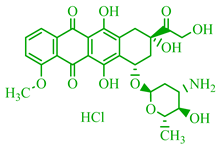
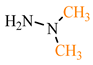Abstract
The significant synthetic potential and reactivity of tetracyanoethylene (TCNE) have captured the interest of numerous chemical communities. One of the most promising, readily achievable, yet least explored pathways for the reactivity of TCNE involves its interaction with arylamines. Typically, the reaction proceeds via tricyanovinylation (TCV); however, deviations from the standard chemical process have been observed in some instances. These include the formation of heterocyclic structures through tricyanovinyl intermediates, aliphatic dicarbonitriles through the cleavage of the C–C bond of a tetracyanoethyl substituent, complexation, and various pericyclic reactions. Therefore, the objective of this study is to review the diverse modes of interaction of TCNE with aromatic nitrogen-containing compounds and to focus the attention of the chemical community on the synthetic capabilities of this reagent, as well as the various biological and optical activities of the structures synthesized based on TCNE.
1. Introduction
TCNE is a promising synthon in organic chemistry, comprising colorless crystals that sublimate at 120 °C and melt at 198–200 °C in a closed capillary [1]. The best yield (85–89%) [2] is achieved by heating brommalonitrile with copper (2:1). It has been 70 years since its discovery, and 65 years since the beginning of its study by Dupont chemists in 1958 [1]. Since then, tens of thousands of articles and reviews have been published on the remarkable synthetic activity of this unique compound. One of the simplest, yet most-promising and least-studied areas of its chemistry is the “tricyanovinylation” of aromatic amines and its potential capabilities. We have compiled all the literature up to 2022 on this subject to draw the attention of the chemical community to its preparative potential and the various types of biological and light-absorbing activities of the carbonitrile organic compounds obtained from it.
Preparatively, highly cost-effective, high-yield syntheses based on tetracyanoethylene (TCNE) and aromatic amines involve tricyanovinylation reactions (TCV). TCV with primary and secondary arylamines presupposes the formation of a tricyanovinyl substituent on the amino group, while with tertiary arylamines, it occurs in pairs with respect to the substituent. These reactions typically proceed smoothly, with high-speed and yield-of-target compounds. TCV yields washing-resistant dyes suitable for hydrophobic fibers [3], components for photo- and electroluminescent devices [4], conductive organic materials [5,6] applicable for TV transmission and recording [6,7], as well as solar batteries [8]. Tricyanovinyl derivatives of aniline also exhibit various types of biological activity, including efficacy against Parkinson’s disease [9], antiviral [9], fungicidal [9,10], antimycotic [9], herbicidal [9], antibacterial [9,10], anthelmintic [10], and antifungal [10,11] activities. Furthermore, nanostructured colored optical films with enhanced electrical conductivity under UV radiation [12] have been developed based on a tricyanovinylated diethylaniline derivative.
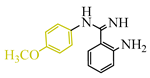
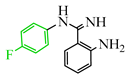
Tricyanilamines are also of interest in medicine. They inhibit oxidative phosphorylation even at a concentration of 10−7 mol−1 and simultaneously reduce the amount of glutathione, a peptide responsible for detoxifying xenobiotics, regulating redox processes in the cell, immune function, and the oxidative state of important sulfhydryl protein groups by up to 30% [13], as confirmed by reaction with thiols [14]. Derivatives of TCNE and arylamines also serve as ideal synthons for the synthesis of biologically active heterocycles. For example, through interaction with arylbenzamidines [15] and 2-aminobenzylamine [16], they can be used in the synthesis of quinazolines [17], which act as kinase inhibitors, promoting the division of cancer cells [18]. However, in some cases, the reaction proceeds differently.
2. Unusual Chemical Reactions with TCNE
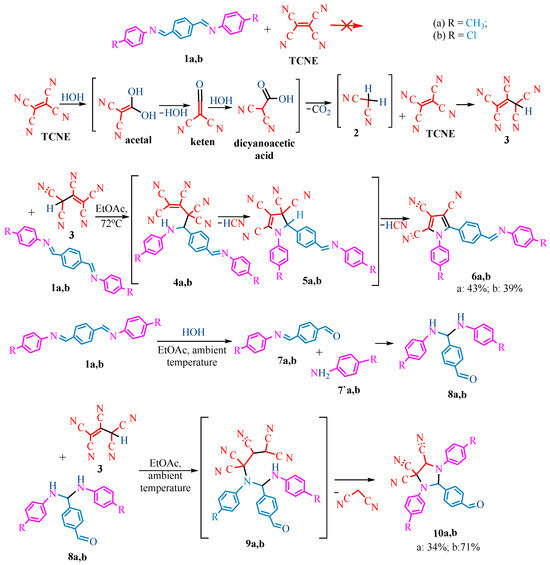
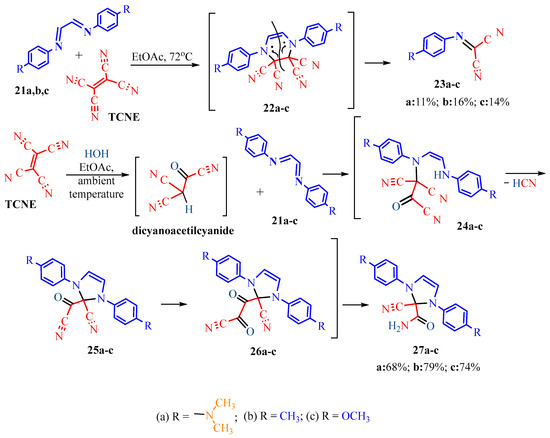
The publication [19] presents interesting reactions of TCNE with nitrogen-containing compounds. The authors conducted TCNE reactions with Schiff bases in ethyl acetate at 72 °C and at room temperature (Scheme 1, Scheme 2 and Scheme 3), leading to different results. For instance, the interaction of TCNE with terephthalaldehyde derivatives 1a,b at high temperature (72 °C) resulted in pyrroles 6a,b, whereas at room temperature, it led to imidazolidines 10a,b. Similarly, the interaction with Schiff’s base 11 at high temperature resulted in imidazolidine 16, while at room temperature, 11 cyclized into pyrrole 20. In the case of glyoxal Schiff’s bases 21a–c, at 72 °C, arylcarbonimidoyldicyanides 23a–c are formed, whereas at 20 °C, dihydroimidazolecarboxamides 27a–c are obtained.
Scheme 1.
Reaction of TCNE with bis-airlmetanimines. Aniline fragments are highlighted in pink color, terephtalic aldehyde—in blue color, TCNE—in red color, water—in turquoise color. Black bonds in the compounds indicate the addition of one molecule to the other, as well as addition of functional groups within the molecule.
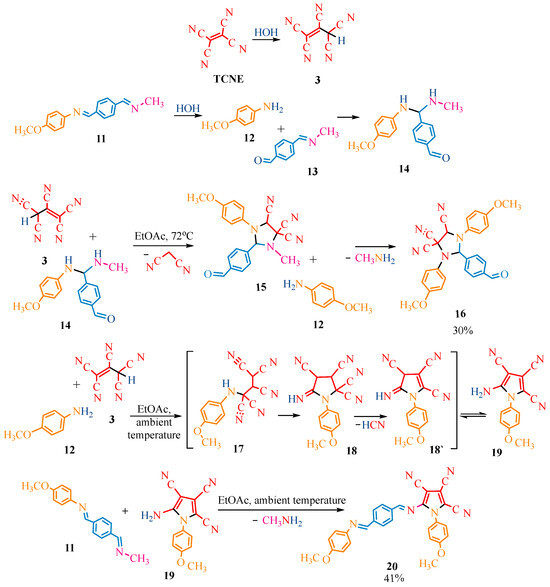
Scheme 2.
Reaction of TCNE with methyl-methoxy phenyl methanimine. Aniline derivative is market in orange color, terephtalic aldehyde—in blue color, methylamine—in crimson color, TCNE in red color. Black bonds in the compounds indicate the addition of one molecule to the other, as well as addition of functional groups within the molecule.
Scheme 3.
Reaction of TCNE with diarylethanediimines. Aniline derivative is market in orange color, terephtalic aldehyde—in blue color, methylamine—in crimson color. Black bonds in the compounds indicate the addition of one molecule to the other, as well as addition of functional groups within the molecule.
Given the availability of the publication in the public domain, we propose our own schemes showing the formation of reaction products in Scheme 1, Scheme 2 and Scheme 3. We proposed that in several cases (Scheme 1, Scheme 2 and Scheme 3), TCNE was hydrolyzed in ethyl acetate containing up to 8% water prior to reaction with Schiff’s bases. This hydrolysis consequently led to acetal, keten, dicyanoacetic acid decarboxylated to malonnitrile 2, which was finally added to TCNE to give pentacyanopropene 3 [20]. Scheme 1 shows the addition of pentacyanopropene 3 to the C=N bond of imines 1a,b by heating at 72 °C. The formed Michael adducts 4a,b underwent intramolecular cyclysis to give dihydropyrroles 5a,b, which were converted to pyrroles 6a,b after prussic acid elimination in yields of 43% and 39%, respectively. Meanwhile, we propose that the original compounds 1a,b were hydrolyzed at room temperature to aldehydes 7a,b and amines 7′a,b. The reaction between them (1a,b and 7a,b) occurred as a Michael-type addition leading to bis(arylamino)methylarylaldehydes 8a,b. The hydrolysis products 8a,b, via intermediates 9a,b, finally formed diarylimidazolidine-tricarbonitriles 10a,b in yields of 34% and 71%, respectively (Scheme 1).
The reaction of TCNE with the methyl derivative of methanimine 11 (Scheme 2) differs from the previous scheme (Scheme 1). Tetracyanoethylene was converted to penthacyanopropene 3, following a similar pattern to the reaction described previously. For products 16 and 20 (Scheme 2), we assumed that Schiff’s base 11 was hydrolyzed to amine 12 and aldehyde 13 at both temperatures (72 °C and ambient). This hydrolysis at 72 °C facilitated the formation of bis(arylamino)methylarylaldehyde 14, and its reaction with penthacyanopropene 3 led to imidazolidine 15 by analogy with Scheme 1 (similar transformations of the original compounds 1a,b and TCNE took place at ambient temperature). The N-methyl of imidazolidine 15 was presumably replaced by residual amino-anisole 12, which is the product of the hydrolysis of Schiff’s base 11, yielding 30% of the final product 16. At room temperature, Schiff’s base 11 underwent partial hydrolysis to amine 12, which probably reacted with pentacyanopropenide 3 to give pentacyanopropane 17. The latter underwent intramolecular cyclization into pyrazolone imine 18. Subsequent elimination of prussic acid 18′ and tautomerization produced pyrrole 19, which then interacted with the original compound 11. The elimination of methylamine resulted in the final pyrrole 20 with a yield of 41% (Scheme 2).
Referring to Scheme 3, it was assumed that at 72 °C, TCNE interacts with diarylethanediimines 21a–c via a [4+2] cycloaddition, followed by cleavage of the C=C bond of the adducts 22a–c and acetylene elimination to give imindicarbonitriles 23a–c in yields of 11%, 16%, and 14%, respectively. At room temperature, TCNE is expected to hydrolyze to dicyanoacetyl cyanide, which differs from previous Scheme 1 and Scheme 2. The hydrolysis product is likely to enter the reaction with the original compounds 21a–c as a CH-acid, adding conjugated double bonds 21a–c. Intermediates of 1,4-addition 24a–c consequently underwent intramolecular cyclization to dihydroimidazole 25a–c. Cyano groups of carbonyl cyanide moieties 25a–c were presumably hydrolyzed to α-oxoamides 26a–c. In the final step, we assumed the typical α-oxoacid libation of carbon monoxide to form amides 27a–c in yields of 68%, 79%, and 74%, respectively (Scheme 3).
The tricyanovinylation of dimethylaniline (DMA) is of significant interest. The resulting dye is employed in photovoltaics [5] and as a material for thin films in television and video recording technologies [6] owing to its high electrical conductivity [5]. However, the reaction mechanism between DMA and tetracyanoethylene (TCNE), which yields a compound of practical importance, remains incompletely understood [21].
One of the initial challenges was detecting the light absorption of the π-complex formed between DMA and TCNE using UV–visible spectroscopy, particularly when the reaction was conducted in polar solvents. To decelerate the reaction, isotopic kinetic studies were performed. In this approach, deuterium, being heavier than hydrogen, slows its incorporation into the TCNE multiple bond, thereby extending the lifetime of the π-complex. Through this method, the presence of the TCNE complex was confirmed by observing its decay in dichloromethane [21].
Further efforts were directed toward isolating the intermediates of the DMA-TCNE reaction. Synthesis in a nonpolar solvent—such as dioxane—at room temperature, resulted in the precipitation of the tetracyanoethyl derivative 31 (illustrated in Scheme 4). Compound 31 was recrystallized from benzene [21] and characterized using spectral methods. Based on the structure of compound 31, the structure of its precursor, a σ-complex in the form of a zwitterion, was proposed (Scheme 4).
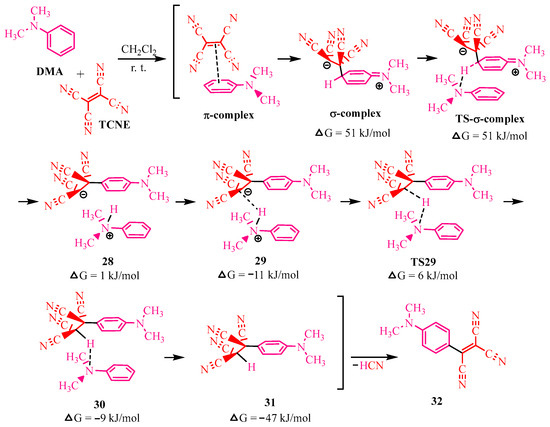
Scheme 4.
Mechanism of reaction of TCNE and DMA. Dimethylaniline is crimson, TCNE is red.
The authors of article [21], utilizing the available literature data, elucidated the intermediate interactions involved in the transformation of the σ-complex into tetracyanoethylated dimethylaniline 3 (Scheme 4). To achieve this, they employed the density functional theory (DFT) method. According to DFT, matter consists of interacting electrons within a lattice of atomic nuclei. The reaction was conducted in dichloromethane at room temperature under conditions identical to those used for the isotopic kinetic method [20].
By measuring electron density throughout the reaction and accounting for harmonic oscillations to capture transition states (TS), seven canonical structures were identified, and their free energies of formation (∆G, kJ/mol) were calculated (Scheme 4). TCNE initially attaches to DMA, forming a zwitterionic σ-complex via a π-complex intermediate. This zwitterion is then deprotonated by a second DMA molecule, yielding zwitterion 28 through the transition state (TS-σ-complex). The DMA cation, exhibiting acidic properties, donates a proton to the aromatic anion. Protonation of the tetracyanoethyl group occurs through the canonical structure 29, the TS29 transition state, and intermediate 30. The final step involves the elimination of cyanohydrogen from tetracyanoethyl dimethylaniline 31, resulting in the formation of tricyanovinyl 32.
The authors of article [22] successfully improved the yield of tricyanovinyl dimethylaniline and identified the most environmentally friendly and economically viable method for its synthesis. The tricyanovinyl derivatives of aniline (34a–h) and 2-methylindole (36i–l) were synthesized using enzyme catalysts and a deep eutectic solvent (DES). The selection of specific enzymatic catalysts, namely lipase [23] and protease [24], was likely due to their high selectivity, reaction rate enhancement, mild operating conditions, and the non-toxic nature of the protein structures.
Deep eutectic solvent (DES) [25] offers several advantages over many low-boiling organic solvents. Besides being non-toxic and non-volatile (facilitating product isolation), DES is non-flammable, biodegradable, safe, inexpensive, and capable of increasing reaction rates [22]. A key advantage, in addition to those mentioned, is the recoverability of both the enzyme catalysts and DES, preserving their activity for reuse [22].
The enzymes were isolated in pure form from commercially available cell lines. Lipase, responsible for fat hydrolysis [23], was derived from the bacterial strain Pseudomonas sp., while protease, which catalyzes the cleavage of peptide bonds in proteins [24], was obtained from a strain of Bacillus subtilis. DES [25] was prepared by mixing choline chloride (ChCl) and urea.
Three different methods were employed for the synthesis of TCNE-based tricyanovinyl derivatives of aniline (34a–h) and 2-methylindole (36i–l), all performed at 35 ± 2 °C, presumably to enhance the solubility of the protein catalysts and the melting of DES. The first method (A) utilized lipase in dichloromethane, while the second method (B) employed protease in the same solvent. Dichloromethane was likely selected due to its low boiling point and sufficiently high polarity, which helped accelerate the reaction and facilitate product isolation. The third method (C) used DES. Scheme 5 outlines the reactions between TCNE and anilines (33a–h) or 2-methylindoles (35i–l) under the corresponding reaction conditions, along with yield ranges for each methodology (A–C).
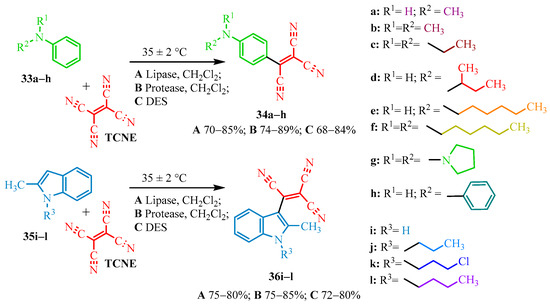
Scheme 5.
Synthesis of tricyanovinilic dyes from the derivatives of aniline and indole.
Table 1 presents the yields of the tricyanovinylated products along with the corresponding reaction times.

Table 1.
Results of tricyanovinylation reaction in three different conditions.
Additionally, the authors of article [22] investigated the reaction of methylaniline (33a) with TCNE. To optimize the yield of tricyanovinylmethylaniline and reduce the reaction time, various deep eutectic solvents (DES), conventional solvents, and solvent-free conditions were tested. The highest yield of 89% and the shortest reaction time of 5 min were achieved using a DES composed of choline chloride and urea. The results are summarized in Table 2.

Table 2.
Optimization of catalysts.
The authors of [22] also investigated the influence of cyano groups on the optical properties of the synthesized compounds (see Table 9, “Absorption maxima for tricyanovinylated compounds”, in Section 6, “Molecular Research” and Figure 1, “Tricyanovinylated dimethylaniline in daylight in various solvents”, below).
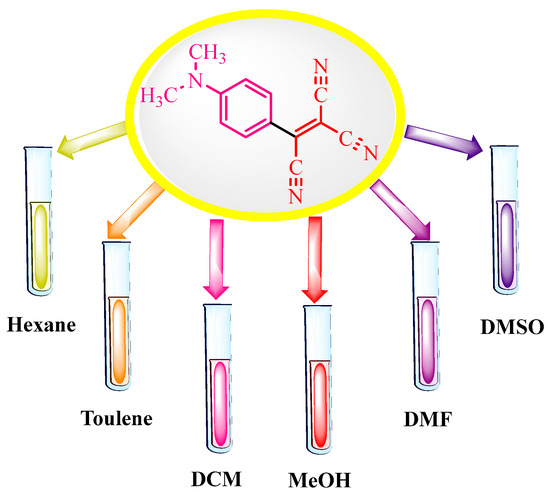
Figure 1.
Tricyanovinilated dimethylaniline in daylight in various solvents.
The interaction of TCNE with p-nitrosodimethylaniline 37 is unusual (Scheme 6). The authors of publication [26] suggest the formation of compound 41 through the intermediate zwitterion 39, with the attachment of a second molecule of arylamine 40, followed by the cleavage of the double bond (Scheme 6).
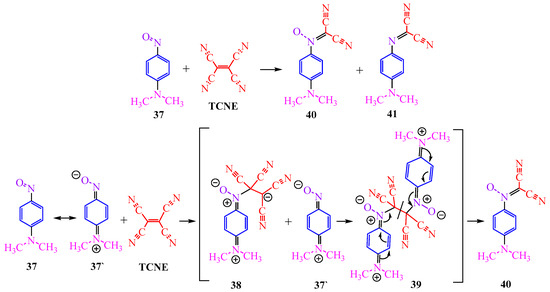
Scheme 6.
Reaction of TCNE with p-nitroso-N,N-dimethylaniline. Dimethilamine (pink) and nitroso-group (violet) in nitrosodimethylamine moiety are marked in individual colors, as they participate in the reaction.
The assumption regarding the intermediate zwitterion 39 is supported by the fact that the reaction with nitrobenzene 37 does not occur under similar conditions (i.e., mixing of reagents in DMF at 20 °C for 30 min) [26].
The reaction of phenylhydrazones 42a–c with TCNE takes an unusual course [8] (Scheme 7) in which the latter compound, rather than undergoing the typical proton substitution reaction at the nitrogen atom of the amino group 42a–c, becomes incorporated at the para-position of the phenyl ring. The authors of [8] synthesized the target compounds 44a–c in two stages: first, phenylhydrazine (PhNHNH2) and the corresponding arylaldehyde 41a–c were refluxed in ethanol under 78–100 °C for 30 min to synthesize the hydrazones, and then, a tricyanovinyl radical was introduced through mixing Schiff’s bases 42a–c with TCNE in DMF at 60–90 °C (Scheme 7).
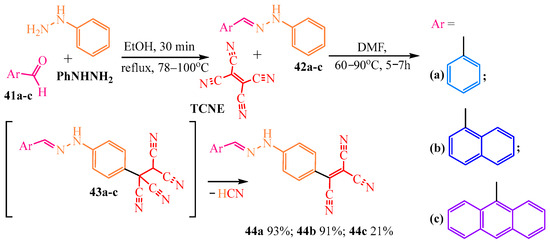
Scheme 7.
Reaction of TCNE with arylhydrazones.
Aromatic amine 45b reacts with the TCNE fragment of butadiene by Diels–Alder, instead of the reaction with amino group ([4+2]-cycloaddition, Scheme 8). In Scheme 8, the yield of aniline derivative 46b, 65%, is provided for comparison with the phenyl derivative 46a, 84% [27].
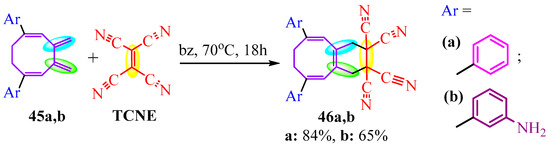
Scheme 8.
The [4+2] cycloaddition.
The authors of publication [28] investigated the capability of TCNE to form complexes with nitrogen- and oxygen-containing compounds, utilizing this property to establish spectroscopic characteristics that elucidate the behavior of drugs in the human body. The identification of TCNE complexes through spectroscopic methods enables the determination of the drug’s mechanism of action, the binding site of the active substance molecule to the biological target, and the physical and thermodynamic properties, allowing for the quantification of drug purity. Additionally, TCNE-based complex compounds exhibit activity against both Gram-positive and Gram-negative bacteria. The main advantage of the method proposed in article [28] is the ability to conduct studies without isolating the active substance from the medicinal compound, which traditionally involves lengthy processes and significant losses of the target product. In the experiment described in article [28], six drugs were utilized (for brief descriptions and molecular weights, see Section 6, “Molecular Research”).
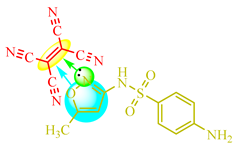
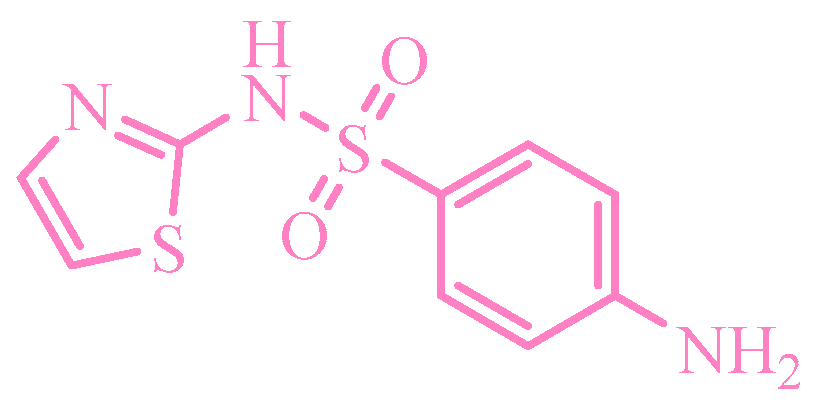
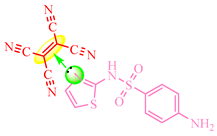
TCNE-based complexes were synthesized by combining a medicinal substance in 20 mL of acetonitrile (resulting in a colorless solution) with TCNE in 20 mL of the same solvent. The reaction mixture was heated and stirred at 0.5 °C, followed by solvent evaporation, yielding a stable yellow complex. Figure 1 illustrates an example of complexation with sulfamethoxazole (Drug 6), where Solution 1 contains TCNE, Solution 2 contains Drug 6, and Solution 3 represents the complex formed with TCNE after brief heating.
Presumably, in this instance, the reaction of the TCV on the amino group did not occur for the following reasons: the use of an aprotic solvent, an insufficiently elevated temperature of the reaction mixture, solvent evaporation during the complexation stage, and the high electron density of the isoxazole ring (Scheme 9).
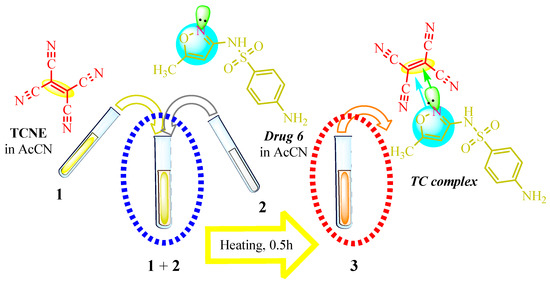
Scheme 9.
Color change in the solution with sulfomethoxazole. Colored circles highlight the centres of complexation between double TCNE bond (yellow), aromatic ring (blue) and nitrogen lone electrone pair (green).
To analyze the obtained complex compounds, the authors of [28] conducted spectrophotometric measurements, stoichiometric titration, and determined the thermodynamic parameters of the compounds under investigation (see Table 11, “(A) Non-splitting and (B) splitting absorption of bonds of synthesized complexes”, in Section 6, “Molecular Research”). The authors of publication [28] also determined the thermodynamic parameters (refer to Table 10, “Thermodynamic parameters of complex compounds”, in Section 6, “Molecular Research”) and unveiled their correlations.
3. Heterocyclic Derivatives via Tricyanovinyl Intermediates
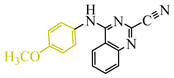
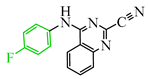
The authors of publication [29] examined the reaction of TCNE with benzamidine 42 (Scheme 10) in an ethyl acetate medium at room temperature for 5 h. This interaction resulted in the formation of three compounds: dihydroimidazole derivative 55 with a yield of 12%, quinazoline 50 with a 37% yield, and iminodihydroquinazoline 52 with 2% yield. The chemical process was presumed to proceed through the tautomeric form 47′ in two pathways. The first involves the attachment of the primary amine to the TCNE in a Michael addition. Subsequently, both prussic acid (compound 49) and malononitrile (compound 51) can be eliminated. Subsequent intramolecular cyclization of the intermediates 49, 51 yield the final products 50, 52 through imine and secondary amine pathways, respectively. In the second pathway, tricyanovinylation of the secondary amine 47′ through the intermediate 53 is followed by intramolecular cyclization 54 according to the Thorpe–Ziegler type, resulting in the formation of the final product 55 (Scheme 10).
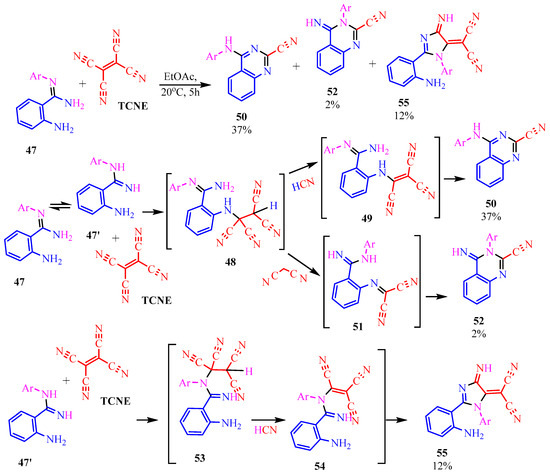
Scheme 10.
Interaction of TCNE with 2-amino-N-arylbenzamidine. In benzamidine 47: anilinecarbonitrile fragment is blue, aromatic amine attaching to triple bond of carbonitrile, is pink.
The authors of [29] achieved an increase in the yield of arylaminoquinazoline 50 from 37% to 93–98% when conducting the same reaction in acetonitrile with the addition of 1 equivalent of acetic acid at room temperature for 7–8 h (Scheme 11).
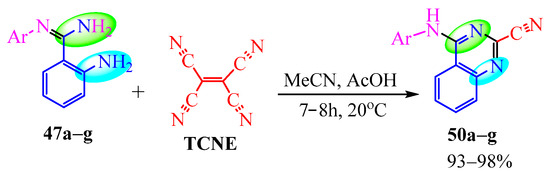
Scheme 11.
Conditions aimed at increasing the yield of the product 50. Colored circles mark the reaction centres in molecules 47a–g.
The results of this synthesis under the conditions described above (Scheme 11) are shown in Table 3.

Table 3.
Yields of arylaminoquinosalines 50a–g.
The authors of publication [30] obtained arylaminoquinazoline through an alternative pathway. In the first stage, they conducted the reaction of quinazoline 56 with aniline derivative 57 in an acidic medium in dioxane at 140 °C, followed by the methylation of the disubstituted amino group of intermediate 58 in dimethylformamide at 0 °C in the presence of methyl iodide and sodium hydride as a strong base for the elimination of hydrogen iodide. Target product 59 resulted in a yield of 61% (Scheme 12). In the second stage, they conducted a cross-coupling of this compound 59 with oxyacrylamide 60. The reaction, catalyzed by a Pd(II) complex, was performed in the polar solvent dimethylformamide at 100 °C in the presence of triethylamine, which is essential for the elimination of the acrylamide vinyl hydrogen from 60. The hydroxyamide group of the cross-coupling product 61 was then hydrolyzed in the presence of hydrochloric acid in dioxane, resulting in the final hydroxyamide 62 with a yield of 30% (Scheme 12).
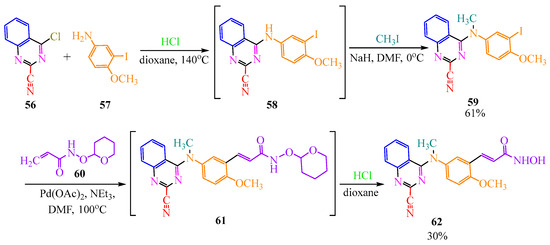
Scheme 12.
Additional method to obtain quinazoline derivative.
The authors of article [30] evaluated the N-methyl derivative of heterocycles 61 and 62 on the HCT116 cell line (refer to the results in Table 12, “Biological activity of arylaminoquinazoline derivatives”, in Section 6, “Molecular Research”).
In addition, a significant number of arylaminoquinazolines demonstrate inhibitory activity against EGFR tyrosine kinase [31,32,33,34,35,36], Hoechst 33,342 [37], PARP [38], HCA [39], NAPE-PLD [40], nsP1 [41], PARP-1 [42], PDE-7 [43], and HFDPS [44]. Moreover, several derivatives also exhibit antibacterial activity [45,46].
The tricyanovinylation of 2-aminobenzylamine (Scheme 13) is carried out through the benzyl nitrogen [16], followed by the addition of an aromatic amino group via a Michael double bond. Compound 66 undergoes further chemical transformations along two pathways: one involving the elimination of malononitrile (compound 67), and the other involving the production of prussic acid (compound 68) (Scheme 13).
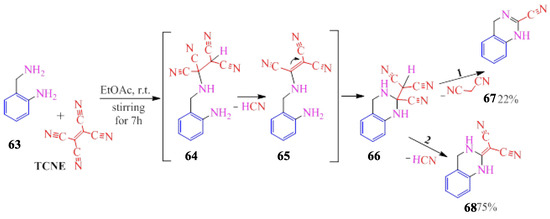
Scheme 13.
Interaction of 2-aminobenzylamine with TCNE.
Additionally, TCNE-based syntheses enable the one-step formation of five-membered heterocycles, including pyrazoles [47,48], triazoles [49], and thiazoles [50].
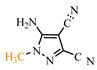
Syntheses involving TCNE and hydrazine derivatives yield N-substituted pyrazoles [47,48]. According to publications [47,48], the cyclization of TCNE with hydrazines proceeds via tricyanovinyl intermediates. The authors of [47] conducted the synthesis using N-methylhydrazine 69a in water, initially stirring for one hour at 0 °C followed by refluxing for 45 min in the temperature range 40–100 °C (see conditions A, Scheme 14). They propose a bifurcated reaction pathway involving the triple bond of the carbonitrile group and the double bond of TCNE. Addition of the N-methyl fragment 69a to the carbonitrile of TCNE forms tricyanovinylimine 70a, which cyclizes to iminopyrazole 71a with elimination of cyanoacetic acid. Zwitterion 71a converts to iminopyrazole 72a, which tautomerizes to aminopyrazole 73a, achieving a yield of 27%. Alternatively, attachment via the double bond of TCNE leads the authors of [47] to suggest the formation of tetracarbonitrile zwitterion 74a, which tautomerizes to ketenimine 75a, followed by elimination of cyanoacetic acid yielding tricyanovinylhydrazine 76a. Intramolecular Thorp–Ziegler cyclization produces 3-iminopyrazole 77a, which tautomerizes to 3-aminopyrazole 78a with a yield of 53%, surpassing the yield of 5-aminopyrazole 73a at 27%.
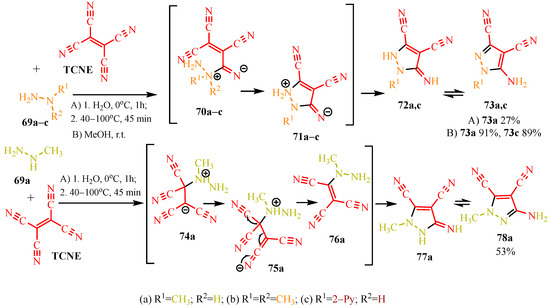
Scheme 14.
N-substituted hydrazines with TCNE.
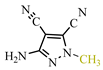
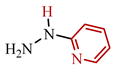
Our synthesis [48] involved TCNE and substituted hydrazines—dimethylhydrazine 69b and pyridyl hydrazine 69c—at room temperature in methanol for one day (see conditions B, Scheme 14). In contrast to the synthesis described above [47], the reaction with dimethylhydrazine 69b [48] proceeds chemoselectively, yielding exclusively 5-aminopyrazole 73a with a yield of 91%. Similarly, under these conditions [48], pyridyl hydrazine 69c also yields pyrazole derivative 73c with a yield of 89%. We hypothesized the formation of pyrazoles based on dimethylhydrazine 73a and pyridyl hydrazine 73c following the same pathway as described in publication [47], involving an addition of the substituted fragment of the corresponding hydrazine to the carbonitrile group of TCNE. Chromatographic methods and qualitative reactions with Prussian blue [48] confirmed that in the case of dimethylhydrazine 69b, during the cyclization of tricyanovinylimine 70b to iminopyrazole 72b, acetonitrile is eliminated, while in the case of pyridyl hydrazine 69c, cyanoacetic acid is eliminated (Scheme 14).
The conditions of syntheses for the original hydrazines 69a–c, pyrazoles 73a, c, and 78a as well as their yields are presented in Table 4.

Table 4.
Conditions of syntheses and yields of N-substituted pyrazoles.
The reaction of TCNE with substituted phenylthiosemicarbazones (79a–c) [49] is quite unusual (Scheme 15). It is proposed that thiocarbazones 79a–c, through zwitterion 80 and its tautomeric form 81, protonate TCNE to form the tetracyanoethyl derivative 82. This derivative undergoes elimination of hydrogen cyanide, yielding a tricyanovinyl intermediate 83, which then undergoes intramolecular cyclization to form triazole 84. Subsequently, a Thorpe–Ziegler-type spiro attachment of the malononitrile fragment to the cyano group in molecule 84 produces dicyanocyclopropanimine 85. A 1,3-hydride transfer occurs, leading to the formation of an unstable zwitterion 86, which opens the cyclopropane during the nucleophilic attack by the malononitrile fragment on the three-membered ring. The resulting iminomalononitrile 87 then tautomerizes to aminomalononitrile 88a–c. The reaction was conducted in ethyl acetate at room temperature for 48 h. Based on the yields presented in Scheme 15, it can be inferred that the formation of the target 1,2,4-triazolium-3-thiolates decreases with increasing multiplicity and aromaticity of the substituents on the thiosemicarbazones.
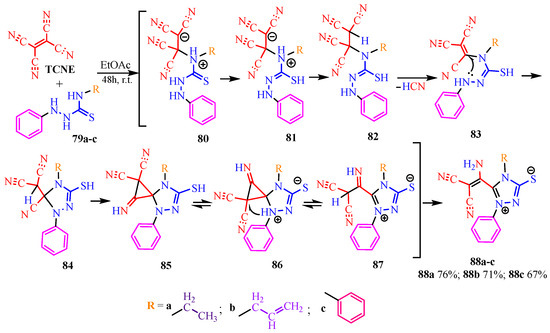
Scheme 15.
Mesoionic 1,2,4-triazolium-3-thiolate derivatives 88a–c.
The reaction of TCNE with disubstituted thiosemicarbazides proceeds differently [50]. The authors of [50] propose that the initial stage involves a complexation reaction (TC–complex). This is followed by the decomposition of the complex into the thiosemicarbazide cation radical (89′) and tetracyanoethane (90). The cation radical 89′ then protonates the anion radical 90, resulting in the formation of two radical species (91 and 92). The addition of the tetracyanoethane radical (92) to the multiple bond of radical 91, accompanied by the redistribution of electron density, yields the tetracyanoethyl derivative 93. This derivative is subsequently protonated by a second thiosemicarbazide molecule (94a–f), leading to the elimination of malononitrile and the formation of anion 95. The anion 95 is again protonated by molecule 94a–f to produce ketenimine 97. The subsequent intramolecular cyclization—via the addition of an amino group to the multiple bond of ketenimine—results in the formation of the target thiazoles (98a–f) (Scheme 16).
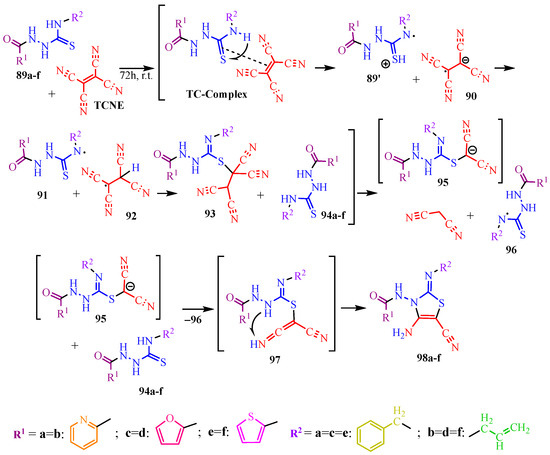
Scheme 16.
Formation of tetra-substituted thiazoles 98a–f.
The reaction of TCNE with thiosemicarbazides (89a–f) was conducted in five different solvents: tetrahydrofuran, dichloromethane, benzene, acetonitrile, and dioxane. The reactions in acetonitrile and dioxane have been previously studied [50], and a notably favorable result was observed in tetrahydrofuran. The results are summarized in Table 5.

Table 5.
Yields of synthetic products 98a–f in different solvents.
The reaction of TCNE with anthranilic (o-aminobenzoic) acid hydrazide 99 offers the opportunity to access a seven-membered cycle that is challenging to obtain [51], holding interest in the realms of organic and pharmaceutical chemistry as a potential antibacterial, antiviral, and psychotropic agent [52,53,54]. The interaction (Scheme 17) takes place through the terminal nitrogen of the hydrazide. Subsequent to the Michael addition (100), the elimination of malononitrile results in the formation of dicyanoazepine 101 (Scheme 17).
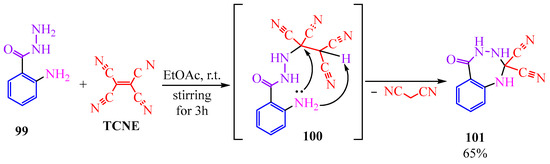
Scheme 17.
Interaction of TCNE with anthranilic acid hydrazide.
4. Heterocyclic Derivatives via Tricyanovinyl Intermediates
The interaction of TCNE with triazenes 102a–c [55] is presumed to proceed through the rearrangement of intermediates 103–106, followed by cleavage of bond 81 resulting in the formation of malonitriles 107a,b and 108a,b (Scheme 18).
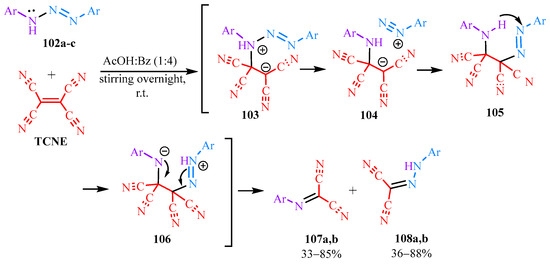
Scheme 18.
Interaction of TCNE with 1,3-aryltriazenes.
The syntheses based on TCNE and triazenes 102a–c have demonstrated that symmetric aryltriazenes 102a,b yield Schiff’s bases 107a,b and hydrazones 108a,b with high yields (85–89%), whereas the same compounds derived from asymmetric aryltriazenes 102c result in relatively lower yields in the range of 33–58%. The original compounds and the products are presented in Table 6.

Table 6.
Yields of Schiff’s bases and hydrazones.
Structures 108a,b were found to affect the oxidative phosphorylation in mitochondria from rat liver, Paracoccus denitrificans bacteria, Candida albicans, yeast, and animal leukemia cells P388 [56].
Additionally, compounds 108a,b exhibited a notably significant MAO inhibitory activity (against monoamine oxidase, which promotes the catabolism of monoamines and catalyzes the synthesis of neurotransmitters and hormones in the body) [57], (refer to IC50 results in Table 13, “MAO inhibitory activity of the compounds”, in Section 6, “Molecular Research”). The authors of publication [57] also constructed a graphical model illustrating the binding sites of dicarbonitriles with the FAD center of monoamine oxidase (see Figure 2, “Binding of dicarbonitriles to the FAD center of monoamine oxidase”, below).
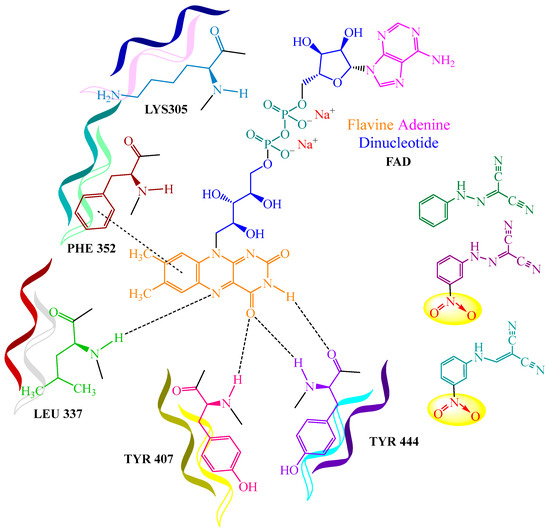
Figure 2.
Binding of dicarbonitriles to the FAD center of monoamine oxidase. Colorful ribbons indicate the protein fragments of monoamineoxidase (MAO); nitro-groups are highlighted in yellow circle.
In publication [58], derivatives of carbonohydrazonoyl dicarbonyl 113 were synthesized from the diazo compound following Scheme 19. Initially, diazonium salt 110 was prepared using standard diazotization conditions, where an aqueous solution of sodium nitrite was slowly added to an aqueous solution of arylamine 109 in hydrochloric acid at 0 °C to prevent decomposition. Subsequently, malononitrile 111 in sodium acetate was added to diazonium chloride 110 at 0 °C to minimize rapid nitrogen release. The resulting azo compounds 112 then underwent tautomerization to yield the final products 113a–e.
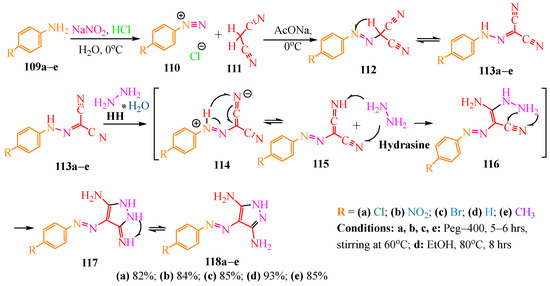
Scheme 19.
Synthesis of pyrazole based on hydrazine hydrate. * indicates binding between water and hydrazine in hydrazine hydrate.
In article [59], the authors employed carbonohydrazonyl dicarbonitriles 113a–e in the synthesis of pyrazole derivatives 118a–e using hydrazine hydrate (HH) in PEG-400 (polyethylene glycol 400). Conversely, in article [60], the same synthesis was conducted in EtOH according to Scheme 19. It is postulated that the basicity of HH facilitated the transformation of structure 113 into ketenimine 114. Subsequent addition of hydrazine to tautomeric form 115 produced enamine 116, and its intramolecular cyclization led to iminopyrazolone 117. Tautomerization of the latter resulted in the formation of the target pyrazole 118 (Scheme 19).
However, the obtained pyrazoles 118a–e did not exhibit significant MAO inhibitory activity compared to the initial reagents 113a–e used in their synthesis.
The cyclization of dicarbonitriles 119a–h into pyrazoles is also described in publications [61,62]. The synthesis was carried out using bromomethyl acetate 120 in the presence of potassium carbonate—which is essential for the elimination of hydrogen bromide—in a toluene/dioxane system [61] or in toluene [62] with microwave irradiation at 90 W and 110 °C (Scheme 20). In article [61], the authors performed further modifications with heterocycle 122. This was accomplished by adding chloroacetic acid chlorohydride 123 to heterocycle 124 in a sufficiently basic solvent dimethylformamide (DMF), which is necessary for hydrogen elimination from amine 122 to form amide 124. The reaction was conducted at room temperature to prevent the rapid release of hydrogen chloride. Potassium thiocyanate was added to the monosubstituted amide 124 in an acetone solution, leading to the formation of thiocyanoacetamide 125, which cyclized into oxothiazolidines 126a–h (Scheme 20).
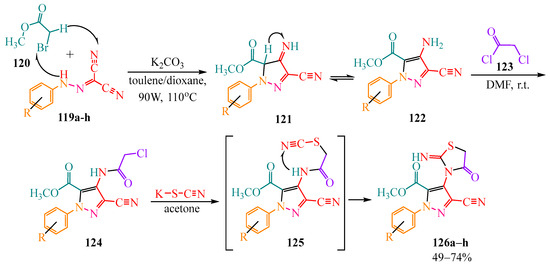
Scheme 20.
Synthesis of pyrazole based on -bromomethyl acetate. In intermediate 121: arrow indicates tautomerization; In intermediate 125: arrow indicates intermolecular cyclization.
The yields of the obtained pyrazoles are shown in Table 7.

Table 7.
Pyrazole 126a–h yields.
Dicarbonitriles 102a–d were also utilized in the two-stage synthesis of triazines with yields ranging from 40 to 94% [62] (Scheme 21). In the first stage, the authors of article [62] conducted the reaction with secondary amines 128a–e in ethanol at 60 °C. It is presumed that the tautomeric form of dicarbonitriles 129, stabilized by the basic amines 128a–e (analogous to Scheme 19), interacted with them to form enamine 131. Acetals 132a,b were added to this compound 131 when heated in toluene in the presence of p-toluenesulfonic acid (TSA) as acetals are hydrolyzed in an acidic medium. This process resulted in the formation of amino-acetal 133, which tautomerized to imino-acetal 134. The intramolecular cyclization of 134 led to the formation of triazines 135a–i (Scheme 21).
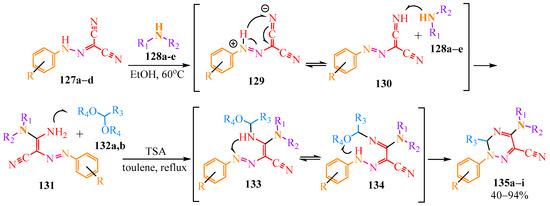
Scheme 21.
Synthesis of triazines 135a–i.
Original compounds: dicyanopyrazoles 127a–d, disubstituted amines 128a–e, target triazines 135a–i, and their yields are presented in Table 8.

Table 8.
Reagents 127a–d, 128a–e, 132a,b, target compounds 135a–i, and their yields.
The authors of publication [63] obtained pyrimidine derivatives 139a,b, which were evaluated for antitumor activity against four cell lines. Doxorubicin was used as a positive control (see Table 14, “Antitumor activity of compounds” in Section 6, “Molecular Research”). Heterocycles 139a,b were synthesized from dicarbonitriles 136a,b and carbamide 137. The latter, 137, reacted with hydrazone dicarbonitriles 136a,b, resulting in the formation of imine 138, which subsequently tautomerized to yield the target compounds 139a,b (Scheme 22).
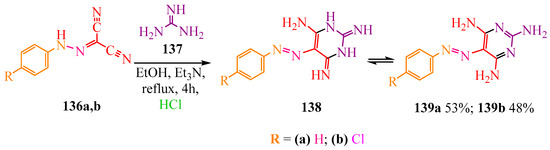
Scheme 22.
Synthesis of pyrimidines 139a,b.
The resulting compound is also the strongest inhibitor of folic acid [64], and its derivatives are inhibitors of dihydrofolate reductase [65,66].
Dicarbonitrile 140 (Scheme 23) is no less interesting to create γ-lactam 143. In publication [67], this heterocycle was derived from vinyl methylketene 141. In diethyl ether the yield was 7.7%, in acetonitrile, 8.1% (Scheme 23).
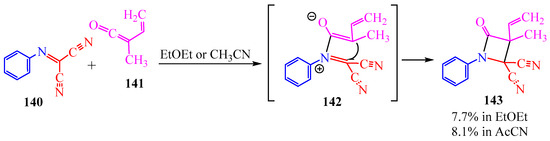
Scheme 23.
Synthesis of γ-lactam.
The synthesis of the azetidine derivative via compound 140 is also described in publication [68]. The authors of [68] initially obtained ketenimine 144′ from N-mesitylcyclopropancarbimidoyl chloride 144 in the presence of the strong base potassium tert-butoxide to eliminate hydrogen chloride. This reaction occurred in tetrahydrofuran at 0 °C to prevent the decomposition of cyclopropane. Cyclopropylidene 144′ then underwent a [2+2]-cycloaddition reaction with carbonimidoyl dicyanide 140, resulting in the formation of azaspirohexane 145 (Scheme 24).
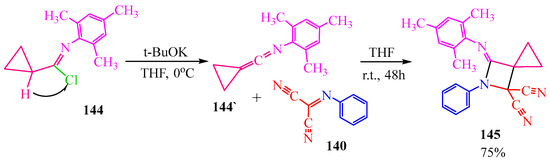
Scheme 24.
Synthesis of azaspiro [2,3]-hexane.
5. Synthesis of Optically Active Compounds via Pericyclic Reactions with TCNE
TCNE-based syntheses of optically active butadiene 1,1,4,4-tetracarbonitriles are utilized to create chromophores [69,70,71] and photosensitizers [72,73] that have widespread applications in organic chemistry [70,71,72,73]. According to publication [73], disubstituted acetylenes 146a–c undergo [2+2]-cycloaddition with TCNE, leading to the formation of cyclobutenes 147 (Scheme 25). In this case, the donor, which is a substituent of the aromatic ring, contributes to the displacement of electron density, resulting in the cleavage of the four-membered cycle 147 into derivatives of butadiene 148a–c (Scheme 25).
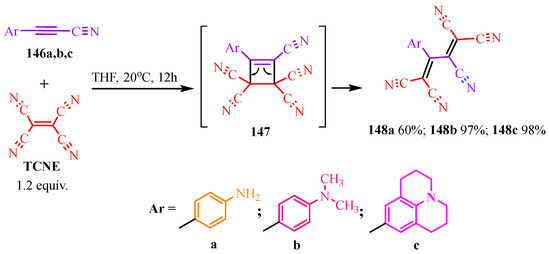
Scheme 25.
The [2+2] cycloaddition.
The synthesized compounds absorb UV radiation within the wavelength range of 300–550 nm. Among them, the hexahydrobenzoquinoline derivative 148c exhibits the most significant bathochromic shift (up to 500 nm) and the highest radiation intensity. Compound 148c proves to be highly effective for use as a photosensitive material due to its strong fluorescent response and high yield (98%) under relatively simple synthesis conditions.
Pericyclic reactions were also employed in the synthesis of calorimetric sensors for sulfide ions [72]. Initially, 4-iodoaminobenzene 149 underwent cross-coupling catalyzed by Pd(II) with trimethylsilyl acetylene 150 in the presence of a disubstituted amine and copper iodide as a buffer. The authors of [72] subsequently removed the trimethylsilyl group from compound 151 using potassium carbonate in a mixture of methanol and dichloromethane for 16 h. Thereafter, the monosubstituted acetylene derivative underwent a cross-coupling reaction with 4-iodoaminobenzene 153 under conditions similar to the preparation of anilyl acetyltrimethylsilane 151. This reaction led to the formation of bis-para-anilyl-acetylene 153. Reaction of compound 153 with TCNE via [2+2]-cycloaddition intermediate 154 resulted in the formation of butadiene-tetracarbonitrile 155 (Scheme 26).
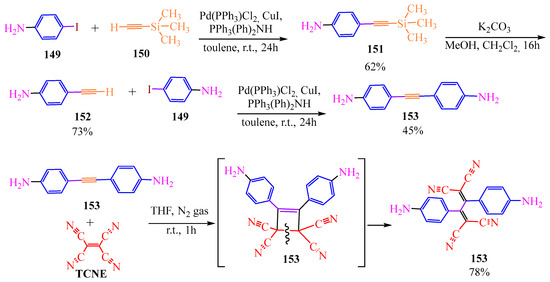
Scheme 26.
Synthesis of calorimetric sensors for sulfide ions.
6. Molecular Research
The authors of article [22] measured the light absorption of tricyanovinyl derivatives derived from aniline and 2-methylindole in six different solvents: hexane, toluene, dichloromethane (DCM), methanol (MeOH), dimethylformamide (DMF), and dimethyl sulfoxide (DMSO). The results indicate a significant correlation between solvent polarity and the observed bathochromic shift. The data are summarized in Table 9.

Table 9.
Absorption maxima for tricyanovinylated compounds.
As an example of the effect of solvent polarity on the shift of light absorption to longer wavelengths, solutions of tricyanovinylated DMA in these solvents are presented in [22] (Figure 1).
The derivatives of reactions between TCNE and aromatic amines considered in this article have a wide range of biological activities and are quite convenient in analytical studies.
Thus, the ability of TCNE to complex with nitrogen- and oxygen-containing compounds was used by the authors of [28] to establish spectrometric characteristics that determine the behavior of drugs in the human body. Identification of TCNE complexes by spectroscopic methods allows us to determine the mechanism of the drug’s action, the binding site of the active ingredient molecule to the biological target, physical and thermodynamic properties, and to quantify the purity of the drug. Moreover, TCNE-based complex compounds are active against both Gram-positive and Gram-negative bacteria. The main advantage of the method proposed by the authors of article [28] is the possibility of conducting the study without isolating the active substance from the drug substance, which is quite time-consuming and accompanied by significant losses of the target product. In the experiment described in article [28], eight drugs were used, as outlined below:
- Glycoside, which is used for the treatment of type 2 diabetes through blood glucose control [74];
- Papaverine hydrochloride, which aims to treat renal colic as well as gastrointestinal, bile duct, and ureteral spasms [75];
- Pilocaprine hydrochloride, which pharmacologically stimulates exocrine glands that promote sweating, salivation, lacrimation, and gastric and pancreatic secretion. Additionally, this drug has been used for a long time to treat glaucoma [76];
- Procaine hydrochloride, which reduces pain from intramuscular injections of penicillin and is used in dentistry [77];
- Aminoantipyrine, which finds application in pharmacological, biological, biochemical, and analytical studies, and can reduce bleeding and denature bovine hemoglobin [78];
- Sulfamethoxazole, which is a cheap and effective synthetic antibiotic used against most Gram-positive and Gram-negative bacteria [79];
- Sulfathiazole, which has the same characteristics as Sulfamethoxazole [80];
- Simvastathathione, which aims to reduce cholesterol levels and the risks of atherosclerosis and myocardial infarction, additionally possessing anti-inflammatory effects on the skin and crack healing [81].
The authors of [28] also determined the thermodynamic parameters (Table 10) and found their correlations. The enthalpy value is strongly correlated with entropy and Gibbs energy. The correlation between entropy and Gibbs energy is especially pronounced.

Table 10.
Thermodynamic parameters of complex compounds.
To investigate the obtained complex compounds, the authors of [28] performed spectrophotometric measurements, stoichiometric titration, and determined the thermodynamic parameters of the studied compounds. During light absorption measurements, the authors of [28] noticed an interesting phenomenon: the peaks of TCNE complexes with Drugs 1, 4, 5, and 7 have one absorption maximum, while the Drug 2, 3, 6, and 8 complexes split into two peaks. The authors believe that one maximum of light absorption corresponds to the n→π* transition, further corresponding to the interaction of the TCNE double bond with the drug by one reaction center—the unshared electron pair of the N or O atom with (type A interaction, Table 11) two maximums—the π→π* transition, implying the interaction of the TCNE double bond by two reaction centers—the unshared electron pair of the N or O atom and the electron density of the aromatic ring or C=O (type B interaction, Table 11).

Table 11.
(A) Non-splitting and (B) splitting absorption of bonds of synthesized complexes.
The authors of article [30] evaluated the derivatives of compound 56 for its cytotoxic activity against the HCT116 colon cancer cell line (Table 12). The HCT116 cells were cultured in RPMI-1640 medium, which lacks growth factors, necessitating supplementation with 10% fetal calf serum (FCS) and 1% glutamine. The FCS was pretreated with the antitumor agent mitomycin C and irradiated with ultraviolet light to inhibit cell division, thereby allowing it to serve only a metabolic function to support HCT116 cell growth. The colon cancer cells were incubated at 37 °C in a humidified atmosphere with 5% CO2, which likely helped neutralize ammonia generated from the decomposition of glutamine. After 24 h, the medium containing the cells was treated with the test compounds at various concentrations. The cells, along with the quinozaline derivatives, were incubated for an additional 72 h. Subsequently, 100 µL of CellTiter-Glo Reagent was added to each well to assess the biological activity of the compounds via luminescence and spectrophotometric analysis. The CellTiter-Glo Reagent produces a luminescent signal through its interaction with adenosine triphosphate (ATP) molecules, the intensity of which is directly proportional to the number of viable HCT116 cells. A reduction in luminescence indicates the inhibitory effect of the tested compounds on tumor cell proliferation.

Table 12.
Biological activity of arylaminoquinazoline derivatives.
The compounds tested demonstrated significant antitumor activity against the HCT116 cell line, with the results summarized in Table 12.
TCNE and triazene derivatives, specifically hydrazonemalononitriles, have demonstrated significant MAO inhibitory activity (monoamine oxidase inhibition), which plays a crucial role in the catabolism of monoamines and the regulation of neurotransmitter and hormone synthesis in the body [57]. MAO inhibitors are widely used in the treatment of neurodegenerative diseases such as Alzheimer’s and Parkinson’s, as well as in managing conditions like anxiety, panic attacks, social phobia, and post-traumatic stress disorder.
The MAO inhibitory activity of hydrazonemalononitriles was assessed through fluorometric detection of hydrogen peroxide (H2O2), a by-product generated during the oxidative deamination of amines by monoamine oxidase (MAO). In this assay, horseradish peroxidase (acting as a model enzyme), tyramine (the substrate to be deaminated), resazurin (a fluorescent indicator for H2O2), and the test compound were used. Resazurin and tyramine, both dissolved in phosphate buffer solution, along with the test compound dissolved in DMSO, were sequentially added to a solution of horseradish peroxidase in phosphate buffer. The mixture was incubated in a microplate well at 37 °C for 30 min, after which fluorometric measurements were performed.
Upon interaction between horseradish peroxidase and tyramine, H2O2 is produced [82], which subsequently oxidizes resazurin to resorufin. This reaction is accompanied by a color change from blue to fluorescent pink, with an excitation maximum at 530–570 nm and an emission maximum at 580–590 nm [83]. In the presence of an active test compound, the formation of H2O2 is inhibited, resulting in a cessation of the color change and a decrease in both excitation and emission intensity.
The results of these assays are summarized in Table 13. These data indicate that the nitrobenzene derivative exhibit the most potent inhibitory effect with the lowest measurement error.

Table 13.
MAO inhibitory activity of the compounds.
The authors of publication [57] also developed a graphical model illustrating the binding sites of dicarbonitriles to the flavin adenine dinucleotide (FAD) center of monoamine oxidase (Figure 2). The study revealed that the nitro group within the dicarbonitrile structures plays a crucial role in binding to this coenzyme (FAD).
TCNE-based pyrimidines [63] were evaluated for their antitumor activity against four cell lines: MCF-7 (breast cancer), NCI-H460 (lung cancer), SF-268 (brain tumor), and WI-38 (normal pulmonary fibroblasts) (Table 14). These cell cultures were maintained in RPMI-1640 medium, supplemented with glutamine, heat-inactivated fetal bovine serum (FBS)—which supports cell viability and division—and antibiotics (penicillin and streptomycin) to prevent contamination from FBS. The cultures were incubated for 24 h at 37 °C in a humidified atmosphere with CO2 to neutralize the ammonia (NH3) produced during glutamine degradation.

Table 14.
Antitumor activity of compounds.
Following incubation, the cells were stained with sulforhodamine B, a dye that binds to cellular proteins and forms a red fluorescent complex upon laser excitation, enabling the quantification of cell numbers [84]. Each test compound was added to the cultured and stained cells at five different concentrations, with a maximum concentration of 150 μM. After 48 h, the cells treated with the test compounds were fixed, washed with 0.5% DMSO, and stained. The stained cells were then dissolved in DMSO for subsequent measurement of light absorption at 492 nm (noting that sulforhodamine B exhibits maximum absorption at 565 nm and maximum fluorescence emission at 568 nm [84]).
A reduction in light absorption intensity indicated a decrease in cancer cell viability. Doxorubicin, tested under similar conditions, served as a reference compound. TCNE-derived pyrimidines exhibited moderate antitumor activity against the tested cell lines. The results are summarized in Table 14.
7. Conclusions
The products resulting from the reaction of tetracyanoethylene (TCNE) with arylamines have attracted significant attention across diverse scientific disciplines, including optics, pharmacology, and organic chemistry. Moreover, this reaction follows alternative pathways in several notable cases:
- -
- TCNE undergoes a [3+2]-cycloaddition with subsequent rearrangement and C-C bond cleavage when reacting with triazenes;
- -
- Addition of TCNE to 2-amino-N-benzamidine occurs through multiple reaction centers, followed by intramolecular cyclization. One instance suggests the elimination of both hydrogen cyanide and malononitrile;
- -
- Tricyanovinylation selectively proceeds with primary arylamines such as 2-aminobenzylamine and anthranilic acid hydrazide. In these cases, TCNE adds to benzylamine and to the terminal nitrogen of the hydrazide, respectively;
- -
- Aromatic amino groups in compounds containing alkynes, butadiene moieties, and nitroso groups do not participate in the reaction with TCNE uniformly. For instance, disubstituted alkyne derivatives undergo [2+2]-cycloaddition followed by cyclobutene cleavage, whereas compounds with butadiene moieties undergo [2+4]-cycloaddition to form stable six-membered cycles. Certain arylamine molecules containing both amino and nitroso groups add to TCNE, leading to N-N bond cleavage and formation of malononitrile derivatives.
The deviations from the standard tricyanovinylation scheme in these reactions warrant further investigation into the chemistry of TCNE with arylamines. TCNE-based syntheses show promising applications for the future, including the non-destructive analysis of drugs in pharmaceutical laboratories. Additionally, the facile synthesis of electrically conductive butadienetetracarbonitriles with high luminescent efficiency (97–98% yield via straightforward mixing in tetrahydrofuran at room temperature) holds potential for the development of photoelectronic and photosensitive materials. TCNE’s reactivity also facilitates the production of complex biologically active heterocyclic structures with high yields (95–98%) using a relatively simple technique (synthesis of arylaminoquinazolines by mixing TCNE with benzamidine in acetonitrile at room temperature), making it suitable for adoption in various pharmaceutical applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29194727/s1, Supplementary File S1: Spectral data of the synthetysed compounds.
Author Contributions
Conceptualization, O.N.; investigation, M.O., Y.K., Y.M., S.K., T.V. and L.U.; writing—review and editing, O.N., E.I., M.O., S.K., E.Z., S.M., L.U. and Y.M.; visualization, E.I., S.M., S.K., E.Z., Y.K. and Y.S.; supervision, O.N.; funding acquisition, O.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 23-23-00656.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Ulyanov Chuvash State University, Moskovsky pr., 15, Cheboksary 428015, Russia (protocol code XIX 7.03.2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data on Spectra and High-Pressure Liquid Chromatography (HPLC) supporting reported results, can be found at “Supplementary Matherials”.
Acknowledgments
The authors gratefully acknowledge the Russian Science Foundation, number 23-23-00656, for support for this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cairns, T.L.; Carboni, R.A.; Coffman, D.D.; Engelhardt, V.A.; Heckert, R.E.; Little, E.L.; McGeer Edith, G.; Kusick, B.C.; Middleton, W.J.; Scribner, R.M.; et al. Cyanocarbon Chemistry. I. Preparation and Reactions of Tetracyanoethylene. J. Am. Chem. Soc. 1958, 80, 2775–2778. [Google Scholar] [CrossRef]
- Carboni, R.A. Tetracyanoethylene. Org. Synth. 1959, 39, 64. [Google Scholar]
- McKusick, B.C.; Heckert, R.E.; Cairns, T.L.; Coffman, D.D.; Mower, H.F. Cyanocarbon Chemistry. VI.1 Tricyanovinylamines. J. Am. Chem. Soc. 1958, 80, 2806–2815. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Li, Y.; Zhang, B.; Du, C.; Wan, X.; Chen, Y. Synthesis, characterization, and electroluminescent properties of star shaped donor–acceptor dendrimers with carbazole dendrons as peripheral branches and heterotriangulene as central core. Tetrahedron 2009, 65, 4455–4463. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Zeyada, H.M.; Abd-El-Rahman, K.F.; Farag, A.A.M.; Darwish, A.A.A. Fourier-transform infrared and optical absorption spectra of 4-tricyanovinyl-N,N-diethylaniline thin films. Spectrochim. Acta Part A 2008, 69, 205–210. [Google Scholar] [CrossRef] [PubMed]
- El-Nahass, M.M.; Abd-El-Rahman, K.F.; Darwish, A.A.A. Optical properties of organic thin films of 4-tricyanovinyl-N, N-diethylaniline. Eur. Phys. J. Appl. Phys. 2009, 48, 20402. [Google Scholar] [CrossRef]
- Deshpande, A.V.; Beidoun, A.; Penzkofer, A.; Wagenblast, G. Absorption and emission spectroscopic investigation of cyanovinyldiethylaniline dye vapors. Chem. Phys. 1990, 142, 123–131. [Google Scholar] [CrossRef]
- Al-Sehemi, G.A.; Irfan, A.; Asiri, M.A.; Ammar, A.Y. Synthesis, characterization and density functional theory study of low cost hydrazone sensitizers. Bull. Chem. Soc. Ethiop. 2015, 29, 137. [Google Scholar] [CrossRef]
- Kreutzberger, A.; Daus, S. Antivirale Wirkstoffe, 30. Mitt. (Halogenanilino)ethentricarbonitrile. Arch. Pharm. 1987, 320, 37–42. [Google Scholar] [CrossRef]
- Kreutzberger, A.; Daus, S. Antibakterielle wirkstoffe, XII [1] (trifluormethylanilino)ethentricarbonitrile. J. Fluor. Chem. 1987, 36, 461–470. [Google Scholar] [CrossRef]
- Kreutzberger, A.; Daus, S. Trichomonazide Wirkstoffe, 5. Mitt. (Dichloranilino)ethentricarbonitrile. Arch. Pharm. 1986, 319, 1143–1145. [Google Scholar] [CrossRef] [PubMed]
- AlGarni, S.E.; Darwish, A. Nanostructured dye films of 4-tricyanvinyl-N,N-diethylaniline (TCVA) for optoelectronic applications: Changing the microstructure and improving electrical conductivity under the influence of UV radiation. Phys. Scr. 2019, 95, 045806. [Google Scholar] [CrossRef]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef] [PubMed]
- Podhradský, D.; Paulíková, H.; Imrich, J. Reactions of N-tricyanovinylamines with thiols in aqueous solutions. Collect. Czech. Chem. Commun. 1990, 55, 1630–1634. [Google Scholar] [CrossRef]
- El-Shayeb, K.M.; Hopf, H.; Jones, P.G.Z. Synthesis of 2-(2-Aminophenyl)-4-arylquinazoline Derivatives by Reaction of 2-Aminoarylbenzimidamides with Isatoic Anhydride. Naturforsch. B J. Chem. Sci. 2009, 64, 858–864. [Google Scholar] [CrossRef]
- El-Shayeb, K.M.; Jones, P.G. Chemical and structural properties of 2-aminobenzylamine derivatives. Z. Med. Phys. 2013, 68, 913–923. [Google Scholar] [CrossRef][Green Version]
- Rewcastle, G.W. Pyrimidines and their Benzo Derivatives. Compr. Heterocycl. Chem. III 2008, 8, 117–272. [Google Scholar]
- Wissner, A.; Fraser, H.L.; Ingalls, C.L.; Dushin, R.G.; Floyd, M.B.; Cheung, K.; Loganzo, F. Dual irreversible kinase inhibitors: Quinazoline-based inhibitors incorporating two independent reactive centers with each targeting different cysteine residues in the kinase domains of EGFR and VEGFR-2. Bioorg. Med. Chem. 2007, 15, 3635–3648. [Google Scholar] [CrossRef]
- Hassan, A.A.; Aly, A.A.; Mohamed, N.K.; Mourad, A.-F.E. Diimine-Tetracyanoethylene Donor-Acceptor Interactions: Synthesis of Pyrroles, Imidazolidines and Quinolines. J. Chem. Res. 1996, 4, 208–209. [Google Scholar] [CrossRef]
- Middleton, W.J.; Little, E.L.; Coffman, D.D.; Engelhardt, V.A. Cyanocarbon Chemistry. V.1 Cyanocarbon Acids and their Salts. J. Am. Chem. Soc. 1958, 80, 2795–2806. [Google Scholar] [CrossRef]
- Menezes da Silva, V.H.; Monezi, N.M.; Ando, R.A.; Braga, A.A.C. New insights into the electrophilic aromatic substitution mechanism of tricyanovinylation reaction involving tetracyanoethylene and N,N-dimethylaniline: An interpretation based on density functional theory calculations. J. Mol. Struct. 2017, 1142, 58–65. [Google Scholar] [CrossRef]
- Sanap, A.K.; Shankarling, G.S. Eco-friendly and recyclable media for rapid synthesis of tricyanovinylated aromatics using biocatalyst and deep eutectic solvent. Catal. Commun. 2014, 49, 58–62. [Google Scholar] [CrossRef]
- Sharma, R.; Chisti, Y.; Banerjee, U.C. Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 2001, 19, 627–662. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Bond, J.S. Proteases: Multifunctional Enzymes in Life and Disease. J. Biol. Chem. 2008, 283, 30433–30437. [Google Scholar] [CrossRef]
- Gurkan, B.; Squire, H.; Pentzer, E.B. Metal-Free Deep Eutectic Solvents: Preparation, Physical Properties, and Significance. J. Phys. Chem. Lett. 2019, 10, 7956–7964. [Google Scholar] [CrossRef]
- Lipilin, D.L.; Churakov, A.M.; Ioffe, S.L.; Strelenko, Y.A.; Tartakovsky, V.A. Formation of nitron in the reaction of para-nitroso-N,N-dimethylaniline with tetracyanoethylene. Russ. Chem. Bull. 1997, 46, 596–598. [Google Scholar] [CrossRef]
- Lee, P.H.; Lee, K. Intermolecular tandem Pd-catalyzed cross-coupling/[4+4] and [4+2] Cycloaddition: One-component five-component assembly of bicyclos [6.4.0] dodecans. Angew. Chem. Int. Ed. 2005, 44, 3253–3256. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.M.A.; Refat, M.S.; Hegab, M.S.; Saad, H.A. Spectrophotometric and thermodynamic studies on the 1:1 charge transfer interaction of several clinically important drugs with tetracyanoethylene in solution-state: Part one. J. Mol. Liq. 2016, 224, 311–321. [Google Scholar] [CrossRef]
- Mirallai, S.I.; Manoli, M.; Koutentis, P.A. The reaction of 2-amino-N′-arylbenzamidines with tetracyanoethene reinvestigated: Routes to imidazoles, quinazolines and quinolino [2′,3′:4,5]imidazo [1,2-c]quinazoline-8-carbonitrile. Tetrahedron 2015, 71, 8766–8780. [Google Scholar] [CrossRef]
- Hauguel, C.; Ducellier, S.; Provot, O.; Ibrahim, N.; Lamaa, D.; Balcerowiak, C.; Letribot, B.; Nascimento, M.; Blanchard, V.; Askenatzis, L.; et al. Design, synthesis and biological evaluation of quinoline-2-carbonitrile-based hydroxamic acids as dual tubulin polymerization and histone deacetylases inhibitors. Eur. J. Med. Chem. 2022, 240, 114573. [Google Scholar] [CrossRef] [PubMed]
- Vincent, P.W.; Bridges, A.J.; Dykes, D.J.; Fry, D.W.; Leopold, W.R.; Patmore, S.J.; Elliott, W.L. Anticancer efficacy of the irreversible EGFr tyrosine kinase inhibitor PD 0169414 against human tumor xenografts. Cancer Chemother. Pharmacol. 2000, 45, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Bridges, A.J.; Zhou, H.; Cody, D.R.; Rewcastle, G.W.; McMichael, A.; Showalter, H.D.H.; Denny, W.A. Tyrosine Kinase Inhibitors. 8. An Unusually Steep Structure−Activity Relationship for Analogues of 4-(3-Bromoanilino)-6,7-dimethoxyquinazoline (PD 153035), a Potent Inhibitor of the Epidermal Growth Factor Receptor. J. Med. Chem. 1996, 39, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Rewcastle, G.W.; Denny, W.A.; Bridges, A.J.; Zhou, H.; Cody, D.R.; McMichael, A.; Fry, D.W. Tyrosine kinase inhibitors. 5. Synthesis and structure-activity relationships for 4-[(phenylmethyl)amino]- and 4-(phenylamino)quinazolines as potent adenosine 5′-triphosphate binding site inhibitors of the tyrosine kinase domain of the epidermal growth factor receptor. J. Med. Chem. 1995, 38, 3482–3487. [Google Scholar] [PubMed]
- Palmer, B.D.; Trumpp-Kallmeyer, S.; Fry, D.W.; Nelson, J.M.; Showalter, H.D.H.; Denny, W.A. Tyrosine Kinase Inhibitors. 11. Soluble Analogues of Pyrrolo- and Pyrazoloquinazolines as Epidermal Growth Factor Receptor Inhibitors: Synthesis, Biological Evaluation, and Modeling of the Mode of Binding. J. Med. Chem. 1997, 40, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Shen, Q.; Kong, W.; Ye, B. QSAR analysis of tyrosine kinase inhibitor using modified ant colony optimization and multiple linear regression. Eur. J. Med. Chem. 2007, 42, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kurup, A.; Garg, R.; Hansch, C. Comparative QSAR Study of Tyrosine Kinase Inhibitors. Chem. Rev. 2001, 101, 2573–2600. [Google Scholar] [CrossRef]
- Krapf, M.K.; Gallus, J.; Spindler, A.; Wiese, M. Synthesis and biological evaluation of quinazoline derivatives—A SAR study of novel inhibitors of ABCG2. Eur. J. Med. Chem. 2018, 161, 506–525. [Google Scholar] [CrossRef] [PubMed]
- Costantino, G.; Macchiarulo, A.; Camaioni, E.; Pellicciari, R. Modeling of Poly(ADP-ribose)polymerase (PARP) Inhibitors. Docking of Ligands and Quantitative Structure−Activity Relationship Analysis. J. Med. Chem. 2001, 44, 3786–3794. [Google Scholar] [CrossRef]
- Bozdag, M.; Alafeefy, A.M.; Altamimi, A.M.; Vullo, D.; Carta, F.; Supuran, C.T. Coumarins and other fused bicyclic heterocycles with selective tumor-associated carbonic anhydrase isoforms inhibitory activity. Bioorg. Med. Chem. 2017, 25, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Castellani, B.; Diamanti, E.; Pizzirani, D.; Tardia, P.; Maccesi, M.; Realini, N.; Piomelli, D. Synthesis and characterization of the first inhibitor of N-acylphosphatidylethanolamine phospholipase D (NAPE-PLD). Chem. Commun. 2017, 53, 12814–12817. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Ramos, A.S.; Li, C.; Eydoux, C.; Contreras, J.M.; Morice, C.; Quérat, G.; Coutard, B. Approved drugs screening against the nsP1 capping enzyme of Venezuelan equine encephalitis virus using an immuno-based assay. Antivir. Res. 2019, 163, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Bellocchi, D.; Macchiarulo, A.; Costantino, G.; Pellicciari, R. Docking studies on PARP-1 inhibitors: Insights into the role of a binding pocket water molecule. Bioorg. Med. Chem. 2005, 13, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Jerez, M.J.; Gil, C.; Calderón, F.; Doménech, T.; Nueda, A.; Martínez, A. CODES, a novel procedure for ligand-based virtual screening: PDE7 inhibitors as an application example. Eur. J. Med. Chem. 2008, 43, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Manse, Y.; Luo, F.; Fukui, H.; Inoue, Y.; Kaieda, T.; Ninomiya, K.; Muraoka, O.; Yoshikawa, M. Indole Glycosides from Calanthe discolor with Proliferative Activity on Human Hair Follicle Dermal Papilla Cells. Chem. Pharm. Bull. 2021, 69, 464–471. [Google Scholar] [CrossRef]
- Liao, B.-L.; Pan, Y.-J.; Zhang, W.; Pan, L.-W. Four Natural Compounds Separated from Folium Isatidis: Crystal Structures and Antibacterial Activity. Chem. Biodivers. 2018, 15, e1800152. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.-B.; Liang, Y.-Q.; Lin, J.-L.; Liao, X.-J.; Xu, S.-H.; Zhao, B.-X. Two new pyrrolidine alkaloids from the red alga Acanthophora spicifera. Nat. Prod. Res. 2020, 35, 3824–3829. [Google Scholar] [CrossRef]
- Hecht, S.M.; Werner, D.; Traficante, D.D.; Sundaralingam, M.; Prusiner, P.; Ito, T.; Sakurai, T. Structure determination of the N-methyl isomers of 5-amino-3,4-dicyanopyrazole and certain related pyrazolo [3,4-d]pyrimidines. J. Org. Chem. 1975, 40, 1815–1822. [Google Scholar] [CrossRef]
- Ivanova, E.S.; Nasakin, O.E.; Maryasov, M.A.; Andreeva, V.V.; Romashov, N.P.; Lodochnikova, O.A. Reactions of tetracyanoethylene with dimethyl/arylhydrazines and arylamines. Mendeleev Commun. 2023, 33, 853–855. [Google Scholar] [CrossRef]
- Hassan, A.A.; El-Shaieb, K.M.A.; Mohamed, N.K.; Tawfeek, H.N.; Bräse, S.; Nieger, M. A novel and facile synthesis of mesoionic 1,2,4-triazolium-3-thiolate derivatives. Tetrahedron Lett. 2014, 55, 2385–2388. [Google Scholar] [CrossRef]
- Hassan, A.A.; Aly, A.A.; Mohamed, N.K.; El-Haleem, L.E.A.; Bräse, S.; Nieger, M. Tetracyanoethylene as a building block in the facile synthesis of heteroyl-tetrasubstituted thiazoles. Monatsh. Chem. 2020, 151, 1425–1431. [Google Scholar] [CrossRef]
- El-Shayeb, K.M.; Amen, M.A.; Abdel-Latif, F.F.; Mohamed, A.H. Synthesis of 1,2,4-triazepine and 1,2,5-triazocine derivatives from the reaction of 2-aminobenzohydrazide with π-acceptors. J. Chem. Res. 2012, 36, 528–531. [Google Scholar] [CrossRef]
- Verardo, G.; Toniutti, N.; Giumanini, A.G. 1-Substituted 1,4,5,6-tetrahydropyridazines and 1-(N-substituted)-aminopyrrolidines from hydrazines and 2, 5-dimethoxytetrahydrofuran. Tetrahedron 1997, 53, 3707–3722. [Google Scholar]
- Elattar, K.M.; Abozeid, M.A.; Mousa, I.A.; El-Mekabaty, A. ChemInform Abstract: Advances in 1,2,4-Triazepines Chemistry. Chem. Inform. 2016, 47, 106710–106753. [Google Scholar] [CrossRef]
- Sladowska, H.; Bodetko, M.; Sieklucka-Dziuba, M.; Rajtar, G.; Zółkowska, D.; Kleinrok, Z. Transformation of some pyrido [2,3-d]pyrimidine derivatives into other di- and triheterocyclic systems. Farmaco 1997, 52, 657–662. Available online: https://pubmed.ncbi.nlm.nih.gov/9550090/ (accessed on 1 November 1997). [CrossRef] [PubMed]
- Mitsuhashi, T. Mechanism of reaction of 1,3-diaryltriazenes with tetracyanoethylene in the presence of acetic acid. J. Chem. Soc. Perkin Trans. 2 1986, 2, 1495–1499. [Google Scholar] [CrossRef]
- Antalik, M.; Sturdík, E.; Sulo, P.; Propperová, A.; Mihalovová, E.; Podhradský, D.; Dzurila, M. Uncoupling effect of protonophoric and nonprotonophoric analogs of carbonyl cyanide phenylhydrazone on mitochondrial oxidative phosphorylation. Gen. Physiol. Biophys. 1988, 7, 517–528. [Google Scholar] [PubMed]
- Silva, D.; Mendes, E.; Summers, E.J.; Neca, A.; Jacinto, A.C.; Reis, T.; Carreiras, M.C. Synthesis, biological evaluation, and molecular modeling of nitrile-containing compounds: Exploring multiple activities as anti-Alzheimer agents. Drug Dev. Res. 2019, 1–17. [Google Scholar] [CrossRef]
- Devidas, P.; Sunil, G.; Sonali, K.; Priya, G.; Milind, G.; Bhaskar, D. Design, Synthesis, Docking and Biological Study of Pyrazole-3,5-diamine Derivatives with Potent Antitubercular Activity. Chem. Methodol. 2022, 6, 677–690. [Google Scholar]
- Elnagdy, H.M.F.; Chetia, T.; Dehingia, N.; Chetia, B.; Dutta, P.; Sarma, D. Sensing and optical activities of new pyrazole containing polymeric analogues. Bull. Mater. Sci. 2022, 45, 86. [Google Scholar] [CrossRef]
- Braud, E.; Le Corre, L.; Dasso Lang, M.; Garbay, C.; Gravier-Pelletier, C.; Busca, P.; Ethève-Quelquejeu, M. Synthesis of Multifunctionalized 2-Iminothiazolidin-4-ones and Their 2-Arylimino Derivatives. Synthesis 2016, 48, 4569–4579. [Google Scholar] [CrossRef]
- Fichez, J.; Soulie, C.; Le Corre, L.; Sayon, S.; Priet, S.; Alvarez, K.; Busca, P. Discovery, SAR study and ADME properties of methyl 4-amino-3-cyano-1-(2-benzyloxyphenyl)-1H-pyrazole-5-carboxylate as an HIV-1 replication inhibitor. RSC Med. Chem. 2020, 11, 577–582. [Google Scholar] [CrossRef]
- Belskaya, N.P.; Gavlik, K.D.; Naumenkova, P.O. Reaction of arylhydrazonoacetamidines with carbonyl compounds, Novel synthetic route to 2,3-dihydro-1,2,4-triazines. Russ. Chem. Bull. 2014, 63, 1584–1589. [Google Scholar] [CrossRef]
- AlHazmi, H.A.; Albratty, M.M.; El-Sharkawy, K.A. Design, synthesis and biological evaluation of pyrimidine-based derivatives as antitumor agents. Rev. Roum. Chim. 2020, 65, 227–238. [Google Scholar]
- Hampshire, J.; Hebborn, P.; Triggle, A.M.; Triggle, D.J.; Vickers, S. Potential Folic Acid Antagonists. I. The Antitumor and Folic Acid Reductase Inhibitory Properties of 6-Substituted 2,4-Diamino-5-arylazopyrimidines1. J. Med. Chem. 1965, 8, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, J.J.; McCormack, J.J., Jr. Dihydrofolate reductase from Trypanosoma equiperdum. I. Isolation, partial purification, and properties. Mol. Pharmacol. 1967, 3, 359–369. [Google Scholar] [PubMed]
- Bowden, K.; Harris, N.V.; Watson, C.A. Structure-Activity Relationships of Dihydrofolate Reductase Inhibitors. J. Chemother. 1993, 5, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, G.; Battaglia, A.; Giorgianni, P. Periselectivity in cycloadditions to vinylmethylketene and structurally related vinylketene imines. Am. J. Org. Chem. 1987, 52, 3289–3296. [Google Scholar] [CrossRef]
- Battaglia, A.; Barbaro, G.; Giorgianni, P. Synthesis and Reactivity of N-Mesitylcyclopropylideneazomethine. J. Org. Chem. 1985, 50, 5368–5370. [Google Scholar] [CrossRef]
- Lacy, A.R.; Vogt, A.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W.B.; Diederich, F. Post-Cycloaddition-Retroelectrocyclization Transformations of Polycyanobutadienes. Eur. J. Org. Chem. 2012, 2013, 869–879. [Google Scholar] [CrossRef]
- Michinobu, T. Click-Type Reaction of Aromatic Polyamines for Improvement of Thermal and Optoelectronic Properties. J. Am. Chem. Soc. 2008, 130, 14074–14075. [Google Scholar] [CrossRef]
- Shoji, T.; Higashi, J.; Ito, S.; Okujima, T.; Yasunami, M.; Morita, N. Synthesis of Redox-Active, Intramolecular Charge-Transfer Chromophores by the [2+2] Cycloaddition of Ethynylated 2H-Cyclohepta[b]furan-2-ones with Tetracyanoethylene. Chem.-Eur. J. 2011, 17, 5116–5129. [Google Scholar] [CrossRef] [PubMed]
- Nhu Pham, Q.N.; Silpcharu, K.; Vchirawongkwin, V.; Sukwattanasinitt, M.; Rashatasakhon, P. 2,3-Diaryl-1,1,4,4-tetracyanobutadienes as Colorimetric Sensors for Sulfide Ion in Aqueous Media. Synlett 2022, 33, 1335–1340. [Google Scholar]
- Reutenauer, P.; Kivala, M.; Jarowski, P.D.; Boudon, C.; Gisselbrecht, J.-P.; Gross, M.; Diederich, F. New strong organic acceptors by cycloaddition of TCNE and TCNQ to donor-substituted cyanoalkines. Chem. Commun. 2007, 46, 4898. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, T.; Yamaguchi, S.; Takahashi, K.; Katsuta, H.; Ito, E.; Seki, H.; Ishida, H. Gliclazide protects 3T3L1 adipocytes against insulin resistance induced by hydrogen peroxide with restoration of GLUT4 translocation. Metabolism 2006, 55, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.; Couldwell, W.T. Intra-Arterial Papaverine Infusions for the Treatment of Cerebral Vasospasm Induced by Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2005, 2, 124–132. [Google Scholar] [CrossRef]
- Mian, P.; Maurer, J.M.; Touw, D.J.; Vos, M.J.; Rottier, B.L. Pharmacy compounded pilocarpine: An adequate solution to overcome shortage of pilogel discs for sweat testing in patients with cystic fibrosis. J. Cyst. Fibros. 2024, 23, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Ruetsch, Y.; Boni, T.; Borgeat, A. From Cocaine to Ropivacaine: The History of Local Anesthetic Drugs. Curr. Top. Med. Chem. 2001, 1, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.N. The Unusual Occurrence of 4-Aminoantipyrine (4-Aminophenazone) in Human Biological Fluids. J. Anal. Toxicol. 1983, 7, 76–78. [Google Scholar] [CrossRef]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Photochemical fate of sulfa drugs in the aquatic environment: Sulfa drugs containing five-membered heterocyclic groups. Environ. Sci. Technol. 2004, 38, 3933–3940. [Google Scholar] [CrossRef]
- Rouf, A.; Tanyeli, C. Bioactive thiazole and benzothiazole derivatives. Eur. J. Med. Chem. 2015, 97, 911–927. [Google Scholar] [CrossRef]
- Caldwell, S.H.; Hespenheide, E.E.; van Bortsel, G. Myositis, microvesicular hepatitis and progression to cirrhosis from troglitazone added to simvastatin. Dig. Dis. Sci. 2001, 46, 376–378. [Google Scholar] [CrossRef]
- Sağlık, B.N.; Kaya Çavuşoğlu, B.; Osmaniye, D.; Levent, S.; Acar Çevik, U.; Ilgın, S.; Öztürk, Y. In Vitro and in silico evaluation of new thiazole compounds as monoamine oxidase inhibitors. Bioorg. Chem. 2019, 85, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Steele, T.W.J.; Stuckey, D.C. Modeling and Application of a Rapid Fluorescence-Based Assay for Biotoxicity in Anaerobic Digestion. Environ. Sci. Technol. 2015, 49, 13463–13471. [Google Scholar] [CrossRef] [PubMed]
- Coppeta, J.; Rogers, C. Dual emission laser induced fluorescence for direct planar scalar behavior measurements. Exp. Fluids 1998, 25, 1–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).