Abstract
Significant interest has emerged for the application of Pd-In2O3 catalysts as high-performance catalysts for CO2 hydrogenation to CH3OH. However, precise active site control in these catalysts and understanding their reaction mechanisms remain major challenges. In this investigation, a series of Pd-InOx catalysts were synthesized, revealing three distinct types of active sites: In-O, Pd-O(H)-In, and Pd2In3. Lower Pd loadings exhibited Pd-O(H)-In sites, while higher loadings resulted in Pd2In3 intermetallic compounds. These variations impacted catalytic performance, with Pd-O(H)-In catalysts showing heightened activity at lower temperatures due to the enhanced CO2 adsorption and H2 activation, and Pd2In3 catalysts performing better at elevated temperatures due to the further enhanced H2 activation. In situ DRIFTS studies revealed an alteration in key intermediates from *HCOO over In-O bonds to *COOH over Pd-O(H)-In and Pd2In3 sites, leading to a shift in the main reaction pathway transition and product distribution. Our findings underscore the importance of active site engineering for optimizing catalytic performance and offer valuable insights for the rational design of efficient CO2 conversion catalysts.
1. Introduction
The hydrogenation of carbon dioxide (CO2) to methanol (CH3OH) represents a crucial catalytic process, pivotal in addressing environmental concerns and fostering sustainable chemical production. This process provides a viable solution for utilizing CO2, a significant greenhouse gas [1], while also contributing to the production of CH3OH, a versatile chemical feedstock with a wide range of industrial applications [2,3]. However, this process presents formidable challenges, considering the ultra-stable C=O bond in CO2 [4,5,6]. Meanwhile, the reverse water–gas shift reaction (RWGS), wherein CO2 reacts with hydrogen for producing water, redirects the reaction towards the undesired production of carbon monoxide (CO) rather than CH3OH.
To address the above issue, indium oxide (In2O3) based catalysts have recently garnered widespread attention due to their exceptional productivity for CH3OH [7]. Nonetheless, pure In2O3 exhibits insufficient adsorption capacity for reaction gases, leading to suboptimal reaction efficiency [2]. Additionally, the instability of the In2O3 structure results in a rapid deactivation of catalysts. Consequently, numerous researchers have explored surface modifications of In2O3 to enhance its activity and stability by altering its active sites. For instance, Liu et al. demonstrated the introduction of new active sites through nitrogen doping, resulting in a great enhancement of reaction activity and catalytic stability of In2O3 [8]. Recent discoveries have revealed that the interface sites between Pd and In2O3 catalysts are more active for the activation of H2 [9]. Ge et al. reported the notable improvement for CO2 hydrogenation to CH3OH by coupling highly dispersed Pd nanoparticles with In2O3, delivering a methanol productivity of 0.89 gMeOH·h−1·gcat−1 [10]. However, it is noteworthy that various catalytic sites may co-exist in addition to the Pd-In2O3 interfaces in the Pd/In2O3 system. For example, metal Pd might tend to form large particles, exacerbating the competitive RWGS reaction, and leading to the increase in CO byproduct [11].
Meanwhile, Pd can also form alloys with other metals, resulting in the tailored active site for CO2 hydrogenation reactions. Bowker et al. reported that ZnO-supported Pd-Zn alloys with optimized particle size and surface structure demonstrated a high performance to activate CO2 with CO2 conversion of 11% and methanol selectivity of 60%, at conditions of 250 °C and 20 bar [12]. Song et al. used SiO2 as support for Pd-Cu alloy particles. With the optimization of Pd/(Pd + Cu) atomic ratios, it was found that Pd(0.34)-Cu/SiO2 was most active with CH3OH yield of 0.31 µmol·gcat−1·s−1 [13]. Additionally, Pd-In alloy may be simultaneously created, which may also shine for CO2 hydrogenation to CH3OH [14]. These complex multi-structures might complicate the reaction route, incubating different intermediates, which makes it difficult to achieve a clear understanding of the reaction mechanism [15,16,17]. Thus, engineering specific active sites and exploring the structure–function relationship is crucial, albeit challenging for the development of advanced Pd/In2O3catalyst [14,18].
In this study, Pd-InOx catalysts with tailor-orchestrated structures were precisely designed and synthesized. The cryogenic activity has great advantages over existing work. Combined (in situ) characterizations were systematically conducted to shed light on the reaction intermediates and reaction pathways. These findings provide valuable insights into the catalytic mechanism of InOx-based catalysts, offering guidance for the rational design and development of catalysts for CO2 hydrogenation to CH3OH.
2. Results and Discussion
2.1. Structure Characterization of Pd-InOx
The synthesis of InOx with varying Pd contents was achieved using a two-step method. Analysis by ICP-AES revealed the actual Pd content in 0.4Pd-InOx and 1.2Pd-InOx are 0.40 wt.% and 1.28 wt.%, respectively (Table S1), consistent with the theoretical values. N2 adsorption–desorption isotherms in Figure 1a indicate that the specific surface area of all samples is below 11 m2·g−1, as summarized in Table S1. XRD analysis depicted the presence of the h-In2O3 phase (JCPDS 04-005-4422) in all InOx and Pd-InOx samples before calcination (Figure 1b). Upon subsequent calcination and reduction of these samples, a coexistence of h-In2O3 and c-In2O3 phases (JCPDS 97-001-4388) was observed over pure InOx and 0.4Pd-InOx samples, indicating a crystal transition of In2O3 (Figure 1c) while only the h-In2O3 phase was observed in 1.2Pd-InOx. Moreover, no diffraction peaks associated with Pd were discernible in the 0.4Pd-InOx sample, indicating highly dispersed Pd on the InOx substrate [19]. In contrast, diffraction peaks corresponding to the Pd2In3 intermetallic compound were evident (JCPDS 97-064-0231) in the 1.2Pd-InOx sample. The interaction between Pd and In in Pd2In3 might prevent the crystal transition of h-In2O3 into the c-In2O3 phase [20,21].
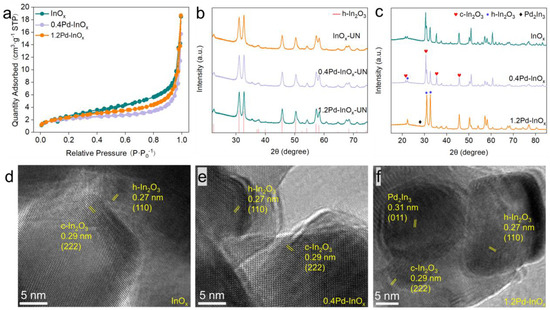
Figure 1.
(a) N2 adsorption–desorption isotherms, XRD patterns of different samples before (b) and after (c) calcination and reduction. HR-TEM images of (d) InOx, (e) 0.4Pd-InOx and (f) 1.2Pd-InOx.
SEM images revealed a bulky shape for the catalysts, while EDS mapping demonstrated the uniform distribution of In, O and Pd atoms in all xPd-InOx samples (Figures S1 and S2). We further investigated the elemental distribution in the material using STEM-EDX. The distribution of elements in InOx (Figure S3) and 0.4Pd-InOx (Figure S4) is uniform, with no large aggregates observed, which confirms the evenly dispersed state of Pd in 0.4Pd-InOx, while for the sample of 1.2Pd-InOx, distinct Pd heterogeneous particles are observed (Figure S5). Combined with the XRD results, it can be inferred that Pd2In3 is formed for the sample of 1.2Pd-InOx. (HR-TEM) analysis revealed inter-planar distances of 0.27 nm and 0.29 nm over InOx (Figure 1d) and 0.4Pd-InOx (Figure 1e), corresponding to the (110) facet of h-In2O3 and (222) facet of c-In2O3, respectively. These characterizations underscore that InOx and 0.4Pd-InOx constitute a homogeneous structure composed of c-In2O3 and h-In2O3. Additionally, a distinct inter-planar distance of 0.31 nm was observed in 1.2Pd-InOx, corresponding to the (011) crystal plane of Pd2In3 (Figure 1f). It suggests that higher loading of Pd leads to some Pd interacting with In to form Pd2In3 intermetallic compounds. The SAED pattern of Pd-InOx is shown in Figure S6, consisting of polycrystalline diffraction rings. From the innermost to the outermost ring, they are indexed as (011), (−130) of Pd2In3, respectively. The SAED values are in good agreement with XRD results, further confirming the presence of Pd2In3 in 1.2Pd-InOx.
2.2. The Structure–Function Relationship
To further investigate the structural states of these catalysts, H2-TPR experiments were conducted on the fresh catalysts. From Figure 2a, peaks at 200–300 °C (as indicated by the red heart) is observed in the curves of all catalysts contributed to the formation of oxygen vacancy on the In2O3 surface, resulting in the reduced state of InOx. Further, compared to InOx, the area of this peak becomes stronger over 0.4Pd-InOx and 1.2Pd-InOx. It suggests the promoted surface reduction of InOx facilitated by the interaction with Pd [22]. Additionally, a new peak at higher temperatures (300–400 °C) over 1.2Pd-InOx is formed. It may be attributed to the presence of Pd2In3 intermetallic compounds [23,24], which also suggested that the Pd2In3 intermetallic compounds significantly benefit the activation of hydrogen.
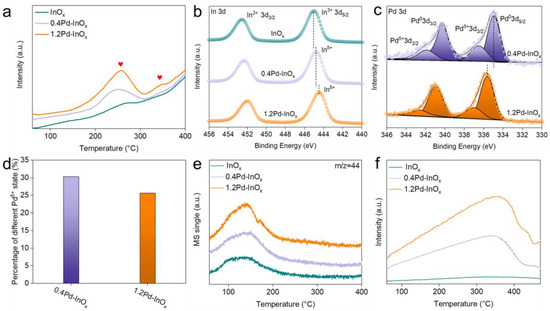
Figure 2.
(a) H2-TPR. The heart symbol represents the peak center. (b) Deconvolution of Pd 3d XPS spectra and (c) In 3d XPS spectra. (d) Percentage of different Pdδ+ states. (e) CO2-TPD and (f) H2-TPD of different samples.
XPS analysis was carried out to characterize the surface chemical information of the catalysts after reduction. The full XPS spectrum proved clearly the presence of In, O, and Pd as displayed in Figure S7. The In 3d spectra exhibit binding energies consistent with In3+ over InOx (Figure 2b) [25,26], while Pd 3d signals could be deconvoluted into four peaks corresponding to Pdδ+ and metallic Pd0 (Figure 2c) [27,28]. Notably, compared to pure In2O3, a slight shift towards lower binding energy for In 3d XPS peak was observed for 0.4Pd/InOx, indicating a reduced valence state [29]. Previous studies have indicated that the incorporation of Pd in oxides may result in the formation of Pd-O(H)-M bonds [30], while the presence of Pdδ+ species in the Pd 3d XPS spectra (Figure 2c) may offer evidence for the existence of Pd-O(H)-In structures for Pd/InOx [31]. It is interesting to find the relative content of Pdδ+ decreased as the content of Pd increased to 1.2 wt.% (Figure 2d); however, the 3d5/2 of In further shifted to a lower binding energy (444.4 eV). This suggests the formation of another chemical bond over 1.2Pd/InOx, possibly attributed to the formation of Pd2In3 intermetallic compound on the surface [18,32]. Thus, Pd species tend to form highly dispersed Pd-O(H)-In sites on the sample surface at a low Pd loading, while partly forming Pd2In3 sites at high Pd loading, resulting in a surface where Pd-O(H)-In and Pd2In3 synergistically co-existence. Moreover, the presence of these active sites may alter the adsorption properties and reaction pathway, influencing the catalytic performance of CO2 hydrogenation. Further detailed investigations are required to elucidate these effects.
CO2-TPD analysis reveals a low-temperature desorption peak for all catalysts, with the desorption temperature remaining unchanged but the peak area slightly increasing with more Pd loadings (Figure 2e) [33]. This phenomenon indicates similar adsorption strength but a slightly enhanced adsorption capacity for Pd/InOx [34,35], which may be due to the beneficial effect of Pd-O(H)-In and Pd2In3 for the CO2 adsorption. It can be observed that after the formation of Pd2In3, an overlapping CO2 desorption peak appears at 171 °C, which may be attributed to the change in the CO2 desorption pattern caused by the formation of Pd2In3 [36]. The H2-TPD analysis could demonstrate the catalyst’s capability for dissociating and activating H2. On pure InOx, there is virtually no desorption peak, indicating a weak adsorption and activation capacity for H2 on the pure In-O bonds. As the Pd loading increases, the desorption peak area for xPd-InOx enhances at elevated temperatures (300–400 °C), as depicted in Figure 2f. In comparison, the peak area for 1.2Pd-InOx is greater than that for 0.4Pd-InOx. Considering the formation of Pd-O(H)-In and Pd2In3 active sites within the Pd-InOx catalyst, this finding may corroborate that the Pd-O(H)-In site plays a more significant role in facilitating H2 activation at lower temperature [9], whereas Pd2In3 is more favorable for H2 activation at higher temperatures [14]. This is in agreement with the H2-TPR results. It is to be mentioned that a minor desorption peak observed at approximately 436 °C for 1.2Pd-InOx may be ascribed to the spillover of H2 from Pd2In3 to the support [37].
2.3. Catalytic Performance for CO2 Hydrogenation
Under reaction conditions of 5 MPa and a gas hourly space velocity (WHSV) of 3000 mL·gcat−1·h−1, CO2 hydrogenation experiments were conducted. As shown in Figure 3a and Figure S8, the catalytic performance varies significantly among these three catalysts. At 200 °C, the CO2 conversion rate of InOx is only 1.28%. In contrast, for 0.4Pd-InOx and 1.2Pd-InOx, the CO2 conversion rates at 200 °C are 3.66% and 4.04%, respectively. It implies the promoted role of Pd-O(H)-In sites and Pd2In3 sites in CO2 conversion. Interestingly, the change in the CO2 conversion rate with the increase in reaction temperature differed obviously for these catalysts. For InOx, the CO2 conversion rate improved from 1.28% at 200 °C to 4.20% at 300 °C, while 0.4Pd-InOx demonstrates a slight CO2 conversion rate increase to 4.56% at 300 °C. Prominently, for 1.2Pd-InOx, its CO2 conversion rate increased notably at elevated temperatures, reaching 6.69% at 300 °C. These differences are attributed to the varied active sites over these catalysts [5,33].
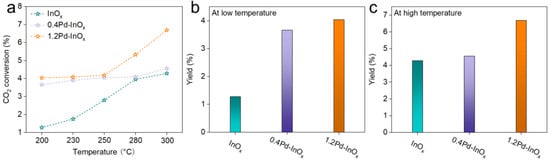
Figure 3.
(a) CO2 conversion. Methanol yield of different samples at (b) 200 °C and (c) 300 °C. Reaction condition: 5 MPa, CO2/H2 = 1/3, and 3000 mL·gcat−1·h−1.
To obtain a more comprehensive understanding of the reaction trend, the yields of CH3OH were calculated. According to the curves, the yield change was divided into the low-temperature region (200–250 °C) and the high-temperature region (250–300 °C). The InOx catalyst, with limited activation capability for H2, yields less than 2% in the lower-temperature region (Figure 3b). With the increase in Pd loading, the yields increase to over 3% for 0.4Pd/InOx and 1.2Pd/InOx catalysts. This might be attributed to the enhanced CO2 and H2 adsorption capability, especially for the H2 adsorption capability at lower temperatures due to the formation of Pd-O(H)-In sites. However, in the higher-temperature region, the yield of 1.2Pd-InOx reached over 6%, much higher than the yields of InOx (4.25%) and 0.4Pd-InOx (4.28%) (Figure 3c). The more favorable reaction of 1.2Pd-InOx at high temperatures may be due to the formation of Pd2In3 which greatly enhances its ability to adsorb and dissociate H2 at high temperatures. These phenomena illustrate that the varied active sites might play different roles in the reaction intermediates and pathways, which will be further discussed. We provide a comparison of the performance of Pd-based catalysts in Table S2.
2.4. Reaction Mechanisms
The activation pathways for CO2 and H2 to produce CH3OH were well established by the previous reports [38]. Initially, CO2 adsorbs onto the sample surface, forming *CO2, which then reacts with lattice oxygen (Olattice) or dissociated hydrogen (*H) to produce either *CO32− or *HCO3−. Subsequently, CH3OH is generated through two possible pathways. The first pathway involves the formation of *CO intermediate, generated via the RWGS routes involving carboxyl (*COOH) species. This intermediate is then hydrogenated to produce CH3OH. The second pathway entails the formation of formate (*HCOO) intermediates through the hydrogenation of CO2, ultimately leading to the production of CH3OH through C-O bond cleavage and *HCO or *H2CO intermediates (referred to as the formate pathway).
To elucidate the effect of varied active sites on intermediate evolution, in situ DRIFTS studies were conducted on InOx (Figure 4a), 0.4Pd-InOx (Figure 4b), and 1.2Pd-InOx (Figure 4c). *CO32− at 1456 and 1520 cm−1 and *HCO3− at 1650 cm−1 are observed on InOx [39,40]. Subsequently, formate at 1559 and 1683 cm−1, as well as *COOH at 1540 and 1637 cm−1, are detected with time on stream [31,38], along with a small amount of *CO at 2077 cm−1 [8,41]. In comparison to InOx, the initial area of CO32− and HCO3− is significantly higher for 0.4Pd-InOx and 1.2Pd-InOx, which may be due to the enhancement of CO2 adsorption caused by the presence of Pd-O(H)-In sites. Furthermore, a new peak corresponding to the adsorption of *COOH at the interface appears at 1758 cm−1, and the proportion of *CO and *COOH intermediates increases, as shown in Figure·5a and Table S3. This suggests that the appearance of Pd-O(H)-In sites alters the main reaction pathway transition, initiating the conversion of the key intermediate in CH3OH synthesis from *HCOO to *COOH and *CO [15,29]. This likely occurs because the formation of Pd-O(H)-In modulates the catalyst’s activation capability concerning *H [42]. H2 undergoes rapid activation at Pd-O(H)-In sites, subsequently converting to *COOH intermediates on the surface [43]. For 1.2Pd-InOx that contained Pd2In3 sites, the peak intensity of *HCO3− initially increases and then decreases. It indicates that the modulation of H2 adsorption and dissociation capability by Pd2In3 renders the catalyst more favorable for the transition from *HCO3− to other intermediates (Figure 5b).
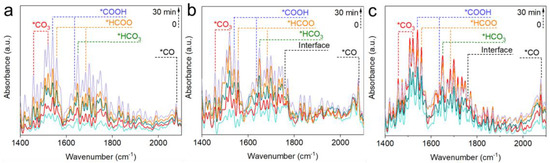
Figure 4.
(a) In situ DRIFT spectra of InOx, (b) 0.4Pd-InOx, and (c) 1.2Pd-InOx. Reaction conditions: 200 °C, CO2/H2 = 1/3, 10 mL·min−1, and 0.1 MPa.
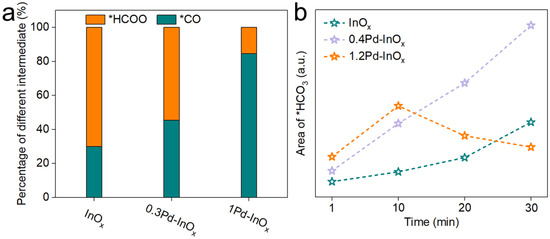
Figure 5.
(a) Percentage of different intermediates over the xPd/InOx catalysts calculated by in situ DRIFT. (b) The trend of in situ DRIFT peak area changes for *HCO3−.
Based on the phenomenon, distinct active sites might induce different reaction pathways, as summarized in Figure 6 [24]. On the InOx surface, CO2 and H2 were activated on In-O, generating small amounts of *CO32− and *HCO3−, which are then hydrogenated to form *HCOO, leading to CH3OH production primarily through the formate pathway [2,44]. With the formation of Pd-O(H)-In interfacial sites on 0.4Pd-InOx, the initial peak area of *HCO3− and *CO32− increases. Subsequently, a larger number of *COOH and *CO intermediates are formed as H2 is more readily activated over Pd-O(H)-In sites, leading to CH3OH synthesis through the reverse water–gas pathway [45]. After the formation of Pd2In3 sites over 1.2Pd-InOx, H2 becomes more accessible to be activated compared to when only Pd-O(H)-In sites are present [42]. It could be further observed that the content of *HCO3− intermediates shows a trend of initially increasing and then decreasing, which aids in the continued conversion of *HCO3− to *COOH and *CO, further hydrogenating to produce CH3OH.
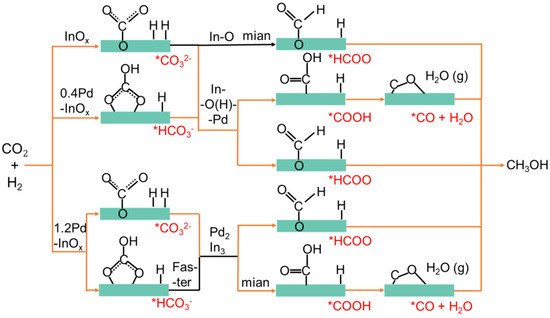
Figure 6.
Possible reaction pathways corresponding to different active sites.
3. Experimental Section
3.1. Preparation of InOx Catalysts
Firstly, 2.0 g of indium nitrate (99.99%, Macklin, Shanghai, China) was dissolved in a solution comprising 30 mL deionized (DI) water and 60 mL ethanol (AR, Sinopharm, Shanghai, China). Subsequently, 0.1 mL of nitric acid (AR, Sinopharm, Shanghai, China) was added. After complete dissolution, the obtained homogeneous solution was transferred into a Teflon-lined autoclave with a capacity of 200 mL and placed in an oven at 150 °C for 12 h. Upon naturally cooling down to room temperature, the resulting product was collected by centrifugation, washed with plenty of DI water and ethanol, and then dried at 60 °C overnight to obtain the InOx-UN. The InOx-UN was calcinated at 450 °C for 3 h in air to obtain the InOx. Before characterizations and the catalytic applications, InOx was reduced by H2 at 380 °C for 120 min, except for H2-TPR.
3.2. Preparation of Pd-InOx Catalysts
The Pd-InOx samples were synthesized using the impregnation method. The palladium nitrate solution (Pd 18.09 wt.% in nitric acid, Macklin, Shanghai, China) was diluted with DI water to a concentration of 5.04 g·L−1, designated as solution A. Subsequently, 0.8 g of InOx-UN (the precursor before reduction and roasting) was dispersed in 100 mL of ethanol under stirring, followed by the addition of 0.48 mL of solution A, which was stirred to dry at 70 °C. The resulting product was further dried overnight at 60 °C to obtain the 0.4Pd-InOx-UN. The samples were calcinated at 450 °C for 3 h in air, denoted as 0.4Pd-InOx (the number represented the weight percentage of Pd). 1.2Pd-InOx was also prepared with a similar procedure to that of 0.4 Pd-InOx but adding 1.60 mL of palladium nitrate solution. Before characterizations and the catalytic applications, the catalysts were reduced by H2 at 380 °C for 120 min, except for H2-TPR.
3.3. Catalyst Characterizations
The Inductively coupled plasma-atomic emission spectroscopy (ICP-AES) measurements were carried out on an Avio 200 instrument (Thermo Electron Corporation, Massachusetts, USA). N2 adsorption–desorption isotherms were measured on a Micromeritics ASAP 2460 (Micromeritics, Shanghai, China) at -196 °C. All the samples were outgassed at 150 °C overnight before the measurement. The specific surface area was calculated using the Brunauer-Emmett-Teller (BET) method. Scanning and transmission analytical electron microscopy (STEM-EDX) was performed using the FEI Talos F200X (FEI, Hillsboro, UAS). High-resolution transmission electron microscopy (HR-TEM) and selected area electron diffraction (SAED) was performed using the JEM 2100F (JEOL, Japan). X-ray diffraction (XRD) patterns were collected on a Japanese Science Ultima IV (Rigaku Corporation, Japan) diffractometer equipped with a Cu Kα radiation source, operated at 40 kV and 40 mA. The data were collected at a scan speed of 5°·min−1 in the 2θ range of 15–85°.
X-ray photoelectron spectroscopy (XPS) experiments were carried out on a Thermo Scientific ESCALAB Xi+ XPS instrument (Thermo, Massachusetts, USA). Spectra of In 3d, C 1s, and Pd 3d were obtained, which were calibrated with the C 1s peak at 284.8 eV. To avoid the influence of air for the XPS test, the sample was protected by Ar gas first and then was vacuum packaged before transferring to the chamber of the XPS equipment. Hydrogen temperature-programmed reduction (H2-TPR) was performed on a ChemBET Pulsar (Quantachrome, Florida, USA). Typically, 50 mg of sample was placed in a U-pipe and purged with an argon flow (30 mL·min−1) at 260 °C for 60 min. The temperature was then decreased to 50 °C. Meanwhile, the gas flow was switched to a 10% H2/Ar mixture (30 mL·min−1) and the temperature was increased from 50 to 400 °C at a rate of 10 °C·min−1. The effluent gas was monitored in real time via a thermal conductivity detector (TCD). CO2 temperature-programmed desorption (CO2-TPD-MS) was performed on a lab-made reactor, and the outlet gases were analyzed by mass spectrum (HPR-20 EGA, HIDEN, Warrington, UK). In particular, the catalysts were flushed with He gas flow (30 mL·min−1) at room temperature for 30 min. Next, the catalysts were saturated with 10% CO2/He (30 mL·min−1) at room temperature for 30 min, followed by purging with He flow (30 mL·min−1) for 30 min. Then, the CO2-TPD experiment was measured from room temperature to 400 °C with a ramping rate of 10 °C·min−1 under He gas flow (30 mL·min−1). H2 temperature-programmed desorption (H2-TPD) was also performed on a ChemBET Pulsar (Quantachrome, FL, USA). The catalysts were flushed with He gas flow (30 mL·min−1) at room temperature for 30 min. Next, the catalysts were saturated with 10% H2/He (30 mL·min−1) at room temperature for 30 min, followed by purging with He flow (30 mL·min−1) for 30 min. Then, the H2-TPD experiment was measured from room temperature to 450 °C with a ramping rate of 10 °C·min−1 under He gas flow (30 mL·min−1).
In situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFTS) spectra were collected using a Thermo IS10 FTIR (Massachusetts, USA) spectrometer with a mercury cadmium telluride (MCT) detector cooled with liquid nitrogen. The CO2 and H2 treatment on the xPd-InOx catalyst was investigated at 200 °C. Before the measurement, 30 mg of the sample was pretreated at 380 °C for 30 min under 10% H2/Ar mixed gas. Then, the gas was changed to Ar for 30 min. After background acquisition, the reaction gas with H2/CO2/Ar = 69/23/8 was introduced into the chamber. All DRIFTS results were analyzed by using OPUS software (OMMIC 9.2).
3.4. Catalytic Performance Evaluation
The catalytic performance of CO2 hydrogenation was evaluated using a vertical fixed-bed reactor. Before the reaction, the catalyst was in situ reduced by H2 (99.999%) at 380 °C for 120 min. After cooling to room temperature, the feed reactants (H2/CO2/Ar = 69/23/8) were introduced at a weight hourly velocity (WHSV) of 3000 mL·gcat−1·h−1 under 5 MPa with the reaction temperature ranging from 200 to 300 °C. Effluent analysis was performed by an online gas chromatograph (Agilent 8890, Beijing, China) equipped with a flame-ionized detector (FID) and a thermal conductivity detector (TCD). To prevent CH3OH condensation, all lines and valves post the reactor were maintained at 170 °C.
4. Conclusions
In this study, the investigation focused on Pd-containing InOx catalysts for CO2 hydrogenation, aiming to uncover the structure–function relationships. A comprehensive suite of characterization techniques revealed distinct structural disparities among the catalysts. Lower Pd loadings exhibited Pd-O(H)-In sites, while higher loadings resulted in Pd2In3 intermetallic compounds. Catalysts with Pd-O(H)-In sites exhibit heightened activity at lower temperatures, attributed to enhanced CO2 adsorption and H2 activation capabilities. Conversely, catalysts featuring Pd2In3 showed enhanced performance at elevated temperatures, facilitated by the strengthened H2 activation. In situ DRIFTS studies revealed a shift in the main reaction pathway, altering key intermediates from *HCOO over In-O bonds to *COOH over Pd-O(H)-In and Pd2In3 sites. Moreover, Pd2In3 significantly enhanced the conversion capability of *HCO3−. The alteration led to a change in product distribution. These findings contribute to the understanding of InOx-based catalysts and offer insights into the design of catalysts for CO2 hydrogenation to CH3OH.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29163715/s1, Figure S1: SEM image and EDS elemental mapping of InOx; Figure S2: SEM images and EDS elemental mapping of (a) 0.4Pd-InOx and (b) 1.2Pd-InOx; Figure S3: STEM-EDX image of InOx; Figure S4: STEM-EDX image of 0.4Pd-InOx; Figure S5: STEM-EDX image of 1.2Pd-InOx; Figure S6: SAED patterns showing polycrystalline diffraction rings acquired of 1.2Pd-InOx; Figure S7: XPS full spectrum of different samples; Figure S8: CH3OH selectivity versus temperature. Reaction condition: 5 MPa, CO2/H2 = 1/3, 3000 mL·gcat−1·h−1; Table S1: Composition and porous properties of the samples with different Pd contents; Table S2: Comparison of catalytic performance between Pd-InOx and other representative catalysts; Table S3: The assignment of in-situ DRIFT peaks. references [10,12,13,17,46,47,48,49,50] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, X.Y.; Formal analysis, Y.L.; Resources, G.L. and C.Z.; Writing—original draft, F.Z.; Writing—review & editing, F.J. and X.L.; Supervision, L.X. and W.J.; Project administration, H.B. All authors have read and agreed to the published version of the manuscript.
Funding
The National Natural Science Foundation of China (No. 22002066), the Shandong provincial colleges and Universities youth innovation technology support program (No.2023KJ356), the State Key Laboratory of Bio-Fibers and Eco-Textiles in Qingdao University (No. G2RC202021), the Key Laboratory of Hubei Province for Coal Conversion and New Carbon Materials (Wuhan University of Science and Technology No.WKDM202303), the Open Research Fund of Key Laboratory of the Ministry of Education for Advanced Catalysis Materials and Zhejiang Key Laboratory for Reactive Chemistry on Solid Surfaces, Zhejiang Normal University (KLMEACM202203) and the Opening Project of Jiangsu Key Laboratory of Atmospheric Environment Monitoring and Pollution Control.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors gratefully acknowledge the National Natural Science Foundation of China (No. 22002066), the Shandong provincial colleges and Universities youth innovation technology support program (No.2023KJ356), the State Key Laboratory of Bio-Fibers and Eco-Textiles in Qingdao University (No. G2RC202021), the Key Laboratory of Hubei Province for Coal Conversion and New Carbon Materials (Wuhan University of Science and Technology No.WKDM202303), the Open Research Fund of Key Laboratory of the Ministry of Education for Advanced Catalysis Materials and Zhejiang Key Laboratory for Reactive Chemistry on Solid Surfaces, Zhejiang Normal University (KLMEACM202203) and the Opening Project of Jiangsu Key Laboratory of Atmospheric Environment Monitoring and Pollution Control.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- Zhang, W.; Yang, Y.; Li, Y.; Li, F.; Luo, M. Recent progress on integrated CO2 capture and electrochemical upgrading. Mater. Today Catal. 2023, 2, 100006. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, R.; Ma, S.; Xu, K.; Chen, Y.; Jiang, K.; Fang, Y.; Zhu, C.; Liu, X.; Tang, Y.; et al. The role of Cu1–O3 species in single-atom Cu/ZrO2 catalyst for CO2 hydrogenation. Nat. Catal. 2022, 5, 818–831. [Google Scholar] [CrossRef]
- Tebar-Soler, C.; Martin-Diaconescu, V.; Simonelli, L.; Missyul, A.; Perez-Dieste, V.; Villar-Garcia, I.J.; Brubach, J.B.; Roy, P.; Haro, M.L.; Calvino, J.J.; et al. Low-oxidation-state Ru sites stabilized in carbon-doped RuO2 with low-temperature CO2 activation to yield methane. Nat. Mater. 2023, 22, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Claver, C.; Yeamin, M.B.; Reguero, M.; Masdeu-Bultó, A.M. Recent advances in the use of catalysts based on natural products for the conversion of CO2 into cyclic carbonates. Green Chem. 2020, 22, 7665–7706. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Luis, P. Top-Down Polyelectrolytes for Membrane-Based Post-Combustion CO2 Capture. Molecules 2020, 25, 323. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.; Martin, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferre, D.; Perez-Ramirez, J. Indium Oxide as a Superior Catalyst for Methanol Synthesis by CO2 Hydrogenation. Angew. Chem. Int. Ed. 2016, 55, 6261–6265. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, C.; Sun, K.; Mei, D.; Liu, C.-j. Enhanced Surface Charge Localization Over Nitrogen-Doped In2O3 for CO2 Hydrogenation to Methanol with Improved Stability. ACS Catal. 2023, 13, 6154–6168. [Google Scholar] [CrossRef]
- Li, Y.; Fei, N.; Li, W.; Cao, Y.; Ge, X.; Dai, S.; Yan, K.; Yuwen, Q.; Zhou, X.; Yuan, W.; et al. H2 activation on metal oxides promoted by highly dispersed Pd. Catal. Commun. 2023, 177, 106645. [Google Scholar] [CrossRef]
- Rui, N.; Wang, Z.; Sun, K.; Ye, J.; Ge, Q.; Liu, C.-J. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy. Appl. Catal. B Environ. 2017, 218, 488–497. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, H.; Wang, J.; Miao, S.; Yang, F.; Wang, G.; Wang, J.; Bao, X. Size-dependent electrocatalytic reduction of CO2 over Pd nanoparticles. J. Am. Chem. Soc. 2015, 137, 4288–4291. [Google Scholar] [CrossRef] [PubMed]
- Bahruji, H.; Bowker, M.; Hutchings, G.; Dimitratos, N.; Wells, P.; Gibson, E.; Jones, W.; Brookes, C.; Morgan, D.; Lalev, G. Pd/ZnO catalysts for direct CO2 hydrogenation to methanol. J. Catal. 2016, 343, 133–146. [Google Scholar] [CrossRef]
- Jiang, X.; Koizumi, N.; Guo, X.; Song, C. Bimetallic Pd–Cu catalysts for selective CO2 hydrogenation to methanol. Appl. Catal. B Environ. 2015, 170–171, 173–185. [Google Scholar] [CrossRef]
- Snider, J.L.; Streibel, V.; Hubert, M.A.; Choksi, T.S.; Valle, E.; Upham, D.C.; Schumann, J.; Duyar, M.S.; Gallo, A.; Abild-Pedersen, F.; et al. Revealing the Synergy between Oxide and Alloy Phases on the Performance of Bimetallic In–Pd Catalysts for CO2 Hydrogenation to Methanol. ACS Catal. 2019, 9, 3399–3412. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Dou, M.; Yu, Y.; Chen, Y. The synergetic effect of Pd, In and Zr on the mechanism of Pd/In2O3–ZrO2 for CO2 hydrogenation to methanol. React. Chem. Eng. 2022, 7, 2433–2444. [Google Scholar] [CrossRef]
- Bi, F.; Feng, X.; Zhou, Z.; Zhang, Y.; Wei, J.; Yuan, L.; Liu, B.; Huang, Y.; Zhang, X. Mn-based catalysts derived from the non-thermal treatment of Mn-MIL-100 to enhance its water-resistance for toluene oxidation: Mechanism study. Chem. Eng. J. 2024, 485, 149776. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Pei, G.; Yang, J.; Liu, J.; Zhao, F.; Jin, F.; Jiang, W.; Ben, H.; Zhang, L. Strong metal–support interaction boosts the electrocatalytic hydrogen evolution capability of Ru nanoparticles supported on titanium nitride. Carbon Energy 2023, 6, e391. [Google Scholar] [CrossRef]
- García-Trenco, A.; Regoutz, A.; White, E.R.; Payne, D.J.; Shaffer, M.S.P.; Williams, C.K. PdIn intermetallic nanoparticles for the Hydrogenation of CO2 to Methanol. Appl. Catal. B Environ. 2018, 220, 9–18. [Google Scholar] [CrossRef]
- Wang, Z.; Men, G.; Zhang, R.; Gu, F.; Han, D. Pd loading induced excellent NO2 gas sensing of 3DOM In2O3 at room temperature. Sens. Actuators B Chem. 2018, 263, 218–228. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Fu, H.; Wan, H.; Wan, Y.; Qu, X.; Xu, Z.; Yin, D.; Zheng, S. Enhanced catalytic hydrogenation reduction of bromate on Pd catalyst supported on CeO2 modified SBA-15 prepared by strong electrostatic adsorption. Appl. Catal. B Environ. 2018, 229, 32–40. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, X.; Cui, P.; Jia, W.; Zhou, J.; Wang, Y.; Zahid, H.; Wu, Y.; Rafique, M.U.; Yin, X.; et al. Enhancing alkyne semi-hydrogenation through engineering metal–support interactions of Pd on oxides. Nano Res. 2023, 17, 3707–3713. [Google Scholar] [CrossRef]
- Tian, G.; Wu, Y.; Wu, S.; Huang, S.; Gao, J. Solid-State Synthesis of Pd/In2O3 Catalysts for CO2 Hydrogenation to Methanol. Catal. Lett. 2022, 153, 903–910. [Google Scholar] [CrossRef]
- Neumann, M.; Teschner, D.; Knop-Gericke, A.; Reschetilowski, W.; Armbrüster, M. Controlled synthesis and catalytic properties of supported In–Pd intermetallic compounds. J. Catal. 2016, 340, 49–59. [Google Scholar] [CrossRef]
- Ojelade, O.A.; Zaman, S.F. A Review on Pd Based Catalysts for CO2 Hydrogenation to Methanol: In-Depth Activity and DRIFTS Mechanistic Study. Catal. Surv. Asia 2019, 24, 11–37. [Google Scholar] [CrossRef]
- Shen, C.; Sun, K.; Zhang, Z.; Rui, N.; Jia, X.; Mei, D.; Liu, C.-J. Highly Active Ir/In2O3 Catalysts for Selective Hydrogenation of CO2 to Methanol: Experimental and Theoretical Studies. ACS Catal. 2021, 11, 4036–4046. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, M.; Zhao, F. Cr2O3 Promoted In2O3 Catalysts for CO2 Hydrogenation to Methanol. Chemphyschem 2024, 25, e202300530. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Dai, J.; Li, W.; Tan, K.B.; Huang, Z.; Zhan, G.; Huang, J.; Li, Q. Pd Supported on MIL-68(In)-Derived In2O3 Nanotubes as Superior Catalysts to Boost CO2 Hydrogenation to Methanol. ACS Catal. 2020, 10, 13275–13289. [Google Scholar] [CrossRef]
- Furukawa, S.; Endo, M.; Komatsu, T. Bifunctional Catalytic System Effective for Oxidative Dehydrogenation of 1-Butene and n-Butane Using Pd-Based Intermetallic Compounds. ACS Catal. 2014, 4, 3533–3542. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, D.; Zhang, J.; Wu, D. Regulating the crystal structure of layered double hydroxide-derived Co-In catalysts for highly selective CO2 hydrogenation to methanol. Chem. Eng. J. 2023, 452, 139144. [Google Scholar] [CrossRef]
- Chen, J.; Zha, Y.; Liu, B.; Li, Y.; Xu, Y.; Liu, X. Rationally Designed Water Enriched Nano Reactor for Stable CO2 Hydrogenation with Near 100% Ethanol Selectivity over Diatomic Palladium Active Sites. ACS Catal. 2023, 13, 7110–7121. [Google Scholar] [CrossRef]
- Yang, X.; Duan, H.; Wang, R.; Zhao, F.; Jin, F.; Jiang, W.; Han, G.; Guan, Q.; Ben, H. Tailoring Zeolite L-Supported-Cu Catalysts for CO2 Hydrogenation: Insights into the Mechanism of CH3OH and CO Formation. Inorg. Chem. 2023, 62, 13419–13427. [Google Scholar] [CrossRef] [PubMed]
- Rameshan, C.; Lorenz, H.; Mayr, L.; Penner, S.; Zemlyanov, D.; Arrigo, R.; Haevecker, M.; Blume, R.; Knop-Gericke, A.; Schlogl, R.; et al. CO2-selective methanol steam reforming on In-doped Pd studied by in situ X-ray photoelectron spectroscopy. J. Catal. 2012, 295, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Rocha, R.; Mercado-Sánchez, I.; Vargas-Rodriguez, I.; Hernández-Lima, J.; Bazán-Jimínez, A.; Robles, J.; García-Revilla, M.A. Modeling Adsorption of CO2 in Rutile Metallic Oxide Surfaces: Implications in CO2 Catalysis. Molecules 2023, 28, 1776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, G.; Bing, H.; Zhong, J.; Yuan, H.; Xie, J.; Chen, Y. High-performance Ni–Ce1-xZrxO2 nanoparticles for biogas reforming: Enhanced CO2 activation and stability. Sustain. Energy Fuels 2021, 5, 6449–6459. [Google Scholar] [CrossRef]
- Li, K.; Chang, X.; Pei, C.; Li, X.; Chen, S.; Zhang, X.; Assabumrungrat, S.; Zhao, Z.-J.; Zeng, L.; Gong, J. Ordered mesoporous Ni/La2O3 catalysts with interfacial synergism towards CO2 activation in dry reforming of methane. Appl. Catal. B Environ. 2019, 259, 118092. [Google Scholar] [CrossRef]
- Malik, A.S.; Zaman, S.F.; Al-Zahrani, A.A.; Daous, M.A.; Driss, H.; Petrov, L.A. Development of highly selective PdZn/CeO2 and Ca-doped PdZn/CeO2 catalysts for methanol synthesis from CO2 hydrogenation. Appl. Catal. A Gen. 2018, 560, 42–53. [Google Scholar] [CrossRef]
- Ma, J.; Xu, L.; Xu, L.; Wang, H.; Xu, S.; Li, H.; Xie, S.; Li, H. Highly Dispersed Pd on Co–B Amorphous Alloy: Facile Synthesis via Galvanic Replacement Reaction and Synergetic Effect between Pd and Co. ACS Catal. 2013, 3, 985–992. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.; Yang, Y.; Chen, J.G.; Liu, P. Optimizing Binding Energies of Key Intermediates for CO2 Hydrogenation to Methanol over Oxide-Supported Copper. J. Am. Chem. Soc. 2016, 138, 12440–12450. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qu, Z.; Song, L.; Fu, Q. CO2 hydrogenation to methanol over Cu/CeO2 and Cu/ZrO2 catalysts: Tuning methanol selectivity via metal-support interaction. J. Energy Chem. 2020, 40, 22–30. [Google Scholar] [CrossRef]
- Daifeng, L.; Zhen, Z.; Yinye, C.; Lingxing, Z.; Xiaochuan, C.; Xuhui, Y.; Baoquan, H.; Yongjin, L.; Qingrong, Q.; Qinghua, C. The Co-In2O3 interaction concerning the effect of amorphous Co metal on CO2 hydrogenation to methanol. J. CO2 Util. 2022, 65, 102209. [Google Scholar] [CrossRef]
- Jiao, J.; Yuan, Q.; Tan, M.; Han, X.; Gao, M.; Zhang, C.; Yang, X.; Shi, Z.; Ma, Y.; Xiao, H.; et al. Constructing asymmetric double-atomic sites for synergistic catalysis of electrochemical CO2 reduction. Nat. Commun. 2023, 14, 6164. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, G.; Zhu, J.; Zhang, X.; Ding, F.; Zhang, A.; Guo, X.; Song, C. CO2 Hydrogenation to Methanol over In2O3-Based Catalysts: From Mechanism to Catalyst Development. ACS Catal. 2021, 11, 1406–1423. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Li, M.; Meng, Y.; Wang, X.; Yan, T.; Fan, Q.; Lou, S.N.; Cui, W.; Zhang, S. Pd-doped In2O3 for CO2 Electroreduction to Ethanol through CO Binding Regulation. ChemCatChem 2024, 16, e202301700. [Google Scholar] [CrossRef]
- Ning, H.H.; Li, Y.D.; Zhang, C.J. Recent Progress in the Integration of CO2 Capture and Utilization. Molecules 2023, 28, 4500. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.Y.; Liu, C.J.; Ge, Q. DFT Study of CO2 Adsorption and Hydrogenation on the In2O3 Surface. J. Phys. Chem. C 2012, 116, 7817–7825. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Liu, X.; Pan, X.; Pei, G.; Huang, Y.; Wang, X.; Zhang, T.; Geng, H. Methanol synthesis from CO2 and H2 over Pd/ZnO/Al2O3: Catalyst structure dependence of methanol selectivity. Appl. Catal. A Gen. 2016, 514, 51–59. [Google Scholar] [CrossRef]
- Khobragade, R.; Roškarič, M.; Žerjav, G.; Košiček, M.; Zavašnik, J.; Van de Velde, N.; Jerman, I.; Tušar, N.N.; Pintar, A. Exploring the effect of morphology and surface properties of nanoshaped Pd/CeO2 catalysts on CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2021, 627, 118394. [Google Scholar] [CrossRef]
- Lawes, N.; Aggett, K.J.; Smith, L.R.; Slater, T.J.A.; Dearg, M.; Morgan, D.J.; Dummer, N.F.; Taylor, S.H.; Hutchings, G.J.; Bowker, M. Zn Loading Effects on the Selectivity of PdZn Catalysts for CO2 Hydrogenation to Methanol. Catal. Lett. 2023, 154, 1603–1610. [Google Scholar] [CrossRef]
- Pan, H.; Ma, B.; Zhou, L.; Hu, Y.; Shakouri, M.; Guo, Y.; Liu, X.; Wang, Y. Highly Efficient CuPd0.1/γ-Al2O3 Catalyst with Isolated Pd Species for CO2 Hydrogenation to Methanol. ACS Sustain. Chem. Eng. 2023, 11, 7489–7499. [Google Scholar] [CrossRef]
- Song, J.; Liu, S.; Yang, C.; Wang, G.; Tian, H.; Zhao, Z.-J.; Mu, R.; Gong, J. The role of Al doping in Pd/ZnO catalyst for CO2 hydrogenation to methanol. Appl. Catal. B Environ. 2020, 263, 118367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).