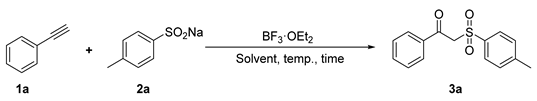
Abstract
An efficient and operationally simple method for the synthesis of β-keto sulfones through the BF3·OEt2-promoted reaction of alkynes and sodium sulfinates is developed. With its facile and selective access to the targets, it features good functional group compatibility, mild conditions, easily available starting materials, and good yields. Notably, the reaction does not require metal catalysts or chemical reagents with pungent odors.
1. Introduction
Sulfone compounds are of considerable importance in synthetic and medicinal chemistry [1,2]. For example, the introduction of sulfonyl groups into medicines can substantially influence their polarity, acidity, aqueous solubility, and other properties [3,4], so the efficient synthesis of sulfone compounds has attracted extensive attention recently [5,6]. Among them, β-keto sulfone as a kind of unique sulfone compound has been extensively used in biomedicine, such as for anti-schistosomal, anti-analgesic, and antibacterial effects (Figure 1) [7,8,9]. At the same time, it can also be used as a synthon and is widely used in synthetic chemistry [10,11,12].
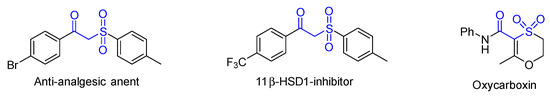
Figure 1.
Some bioactive β-keto sulfone derivatives.
Traditionally, β-keto sulfones can be synthesized through the nucleophilic alkylation of sodium sulfinates with acyl halides [13]. In recent years, with the rise of green synthetic chemistry and the development of various sulfur reagents [14,15,16,17], the coupling reaction of unsaturated bonds (e.g., alkynes) and sulfur reagents has gradually become the main way to synthesize β-keto sulfones [18,19,20]. Among them, being bench-stable, commercially or readily available, and easy to handle [21,22], sodium sulfinates/sulfinic acid is widely used in the synthesis of β-keto sulfones [23,24,25,26].
For instance, Lei’s group [27] reported the oxysulfonylation of terminal alkynes with sulfinic acids catalyzed by pyridine (Scheme 1a). Afterward, Kumar’s group [28] also reported a similar reaction (Scheme 1b). Recently, Sun’s group [29] reported the oxysulfonylation of arylpropiolic acids and sodium sulfnates to generate β-keto sulfones using only hexafluoroisopropanol (HFIP) as a solvent and oxygen as a green oxidant (Scheme 1c).
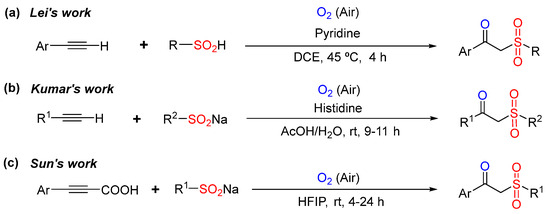
Scheme 1.
Examples of access to β-keto sulfones starting from sodium sulfinates or sulfinic acids [27,28,29].
On the other hand, BF3·OEt2 exhibits strong Lewis acidity [30,31], and its excellent catalytic activity is widely used in various organic synthesis reactions [32,33]. In our previous work [34], we reported a BF3·OEt2-mediated difunctionalization reaction of sodium sulfinates and alkynes to obtain β-sulfinyl alkenylsulfone (Scheme 2b). In fact, during the condition optimization for the above-mentioned work, we also unexpectedly found that if the amount of BF3·OEt2 was below 1.0 equiv., the main product changed from β-sulfinyl alkenylsulfones to β-keto sulfones.
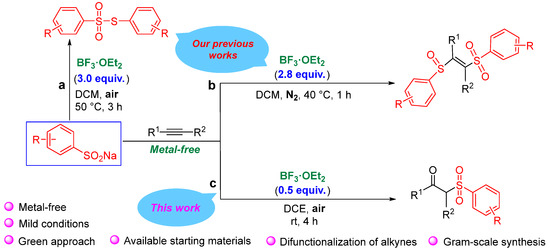
Scheme 2.
Serial synthesis reactions of sodium sulfinates catalyzed by BF3·OEt2 [34,35].
In view of this, based on our previous research on C-S bond construction [21,36], especially the sodium sulfinate reaction with BF3·OEt2 as a catalyst [34,35], herein, we hope to report a new reaction under air atmosphere to efficiently obtain β-keto sulfones via the radical pathway (Scheme 2c). This reaction is designed to synthesize β-keto sulfones without any metal catalysts and does not require the use of chemical reagents with irritating odors such as pyridine and acetic acid. Therefore, this method is more gentle and greener and its successful development is also conducive to expanding the application of BF3·OEt2 as a catalyst. In particular, the regulation and control through this organosulfur reagent and BF3·OEt2 catalyst under different reaction conditions can synthesize different products, such as thiosulfonates, β-sulfinyl alkenylsulfones, and β-keto sulfones. Thus, this strategy is of great significance in the fields of organic sulfur chemistry, BF3 chemistry, and alkyne conversion.
2. Results and Discussion
2.1. Optimization of Reaction Conditions
We attempted the reaction of phenylacetylene (1a) and sodium p-tolylsulfinate (2a) as a model in dichloromethane (DCM) to systematically investigate the influences of different factors to optimize the best conditions. The results are summarized in Table 1.

Table 1.
Optimization of reaction conditions [a].
Initially, we examined the reaction time (Entries 1–3). When the reaction time increased to 6 h, there was no improvement. When the reaction time was shortened, the yield decreased sharply.
In addition, lowering or increasing the reaction temperature did not increase the yield (Entries 4–5 vs. Entry 1).
Similarly, we also examined the amount of BF3·OEt2 used. It was found that increasing or decreasing the amount of Lewis acid did not further increase the yield (Entries 6–7 vs. Entry 1).
At the same time, the effect of solvents on the model reaction was investigated (Entries 1, 8–22). It was found that the solvent plays a significant role in the success of this reaction. Among different solvents, e.g., ethyl acetate (EA), dimethyl sulfoxide (DMSO), tetrahydrofuran (THF), and N,N-dimethylformamide (DMF), using 1,2-dichloroethane (DCE) as the solvent was more favorable to the formation of 3a (Entry 8 vs. Entry 1). Therefore, we decided to choose DCE as the best solvent.
Furthermore, we investigated the ratio of reactants 1a and 2a. When the ratio of 1a:2a was 1:2.4, the best effect was achieved (Entry 8 vs. Entries 23–24).
Thus, the optimized reaction conditions were identified as using 1a (0.3 mmol), 2a (0.72 mmol), and BF3·OEt2 (0.15 mmol) as the catalyst and 4.0 mL of DCE as the solvent at room temperature (rt) for 4 h.
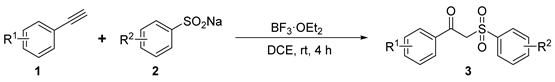
2.2. Scope of Substrates
Under the optimized conditions, a range of alkynes 1 and sodium sulfinates 2 were applied in the transformation to establish the scope and generality of this reaction.
We first investigated various alkyne substrates 1 to explore the scope of this reaction (Table 2).

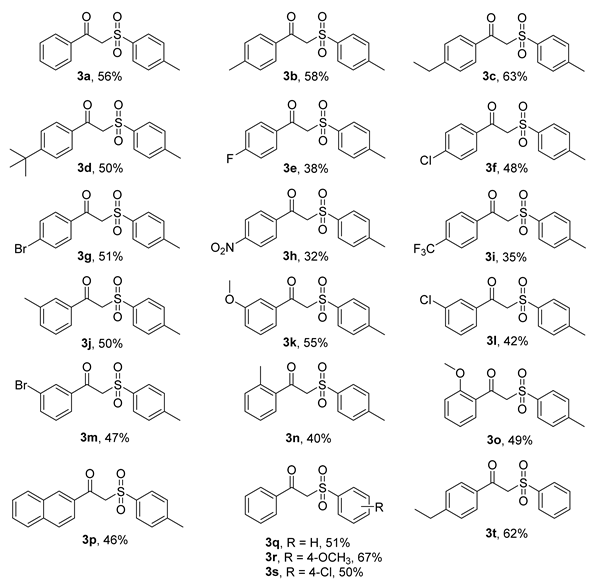
Table 2.
Substrate scope of various alkyne compounds 1 sodium sulfinates 2 [a, b].
Pleasingly, many arylalkynes 1 bearing electron-donating substituents (e.g., methyl, ethyl, and tert-butyl) at the para-position of the aryl group were successfully employed with moderate yields (3a-3d, 50–63%). For other substituents, e.g., halogen, there was a slight decrease in the yield of the desired product (3e-3g, 38–51%). In particular, when the strong electron-withdrawing groups (e.g., -CF3, -NO2) were bearing in 1, the yield decreased further (3h-3i, 32–35%).
In the next step, the effect of different positions of the arylalkyne ring on the yield was investigated. Due to the steric effect, for the same substituent group, when it was a meta-substituted group in the arylalkyne substrate 1, the yield was lower than that of the para-substituted group, such as 3b (58%) vs. 3j (50%), 3f (48%) vs. 3l (42%), and 3g (51%) vs. 3m (47%).
As anticipated, when the arylalkyne substrate 1 was bearing ortho-substituted, the yield was further reduced, such as 3b (58%) vs. 3j (50%) vs. 3n (40%), and 3k (55%) vs. 3o (49%). Furthermore, polycyclic substituted alkynes 1 also can be transformed into the corresponding products (e.g., 3p, 46%).
Importantly, the scope of sodium arylsulfinate substrates 2 was also examined. The results are also summarized in Table 2. Obviously, the substituted 2 was able to react with 1a to produce the corresponding products with moderate yields (3q-3t, 51–67%). Among them, the substrate 2 containing electron-donating groups performed better than those with electron-withdrawing groups, such as 3r (67%) vs. 3a (56%) vs. 3q (51%) vs. 3s (50%), and 3c (63%) vs. 3t (62%).
2.3. Structural Characterization Analysis
From the 1H NMR spectra of the target compounds, it can be seen that the 1H NMR data of the twenty compounds 3a-3t are consistent with the simulated data of the hydrogen atom in the target products. For example, the 1H NMR spectra of the synthesized compounds 3a-3t show that there was a single peak with the integration of two units near 4.72 ppm, which was a characteristic peak caused by methylene hydrogen in the structure of the target products.
At the same time, in the 13C NMR spectra, the single peak of the chemical shift value near 64.0 ppm was also a characteristic peak of methylene carbon. In addition, regardless of whether the product contains one fluorine atom or trifluoromethyl group, the corresponding chemical shift can be found in 19F NMR, as anticipated.
In a word, these test results indicate that the characterization results of NMR are consistent with expectations. The tested data of the synthesized compounds 3a-3t, including the data of the melting point (m.p.), are also consistent with data in the references reported before [17,19,23,25].
Although NMR tests have proven that compounds 3a-3t have the expected structure, in order to further determine the structure of the product, we also conducted single-crystal cultivation of compound 3a. Its crystal resolution data are shown in Table 3, successfully affirming that 3a does have the expected structure [37].

Table 3.
X-ray crystal data and structure refinement for compound 3a.
Accordingly, the X-ray single-crystal diffraction results of compound 3a in Figure 2 indicate that the molecule contains two benzene rings, which are connected to a carbonyl group and a sulfonyl group, respectively. Moreover, the carbonyl and sulfonyl groups are connected by a methylene group. Thus, the structure of compound 3a can also be aptly confirmed through the single crystal structure.
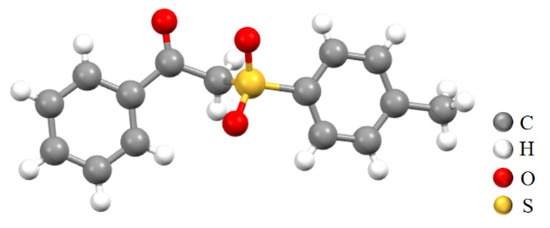
Figure 2.
The crystal structure of 3a (CCDC: 2367445).
On the other hand, the molecular weights of these radicals in control experiments were obtained by high-resolution mass spectrometry (HR-MS), and the error between the tested value and the calculated value was within a reasonable range.
2.4. Mechanism Investigation
In order to understand the reaction mechanism, some control experiments were carried out. The results are shown in Scheme 3.
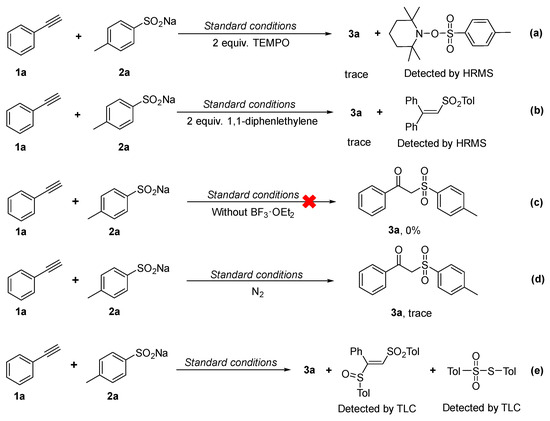
Scheme 3.
Control experiments.
Initially, once 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) as a free radical scavenger was added under the standard reaction conditions, the formation of 3a was obviously suppressed (Scheme 3a). Importantly, the capture of the sulfonyl radical by TEMPO could be detected by HR-MS (Figure S2), indicating that a free radical pathway may be involved in this process.
Similarly, when using 1,1-diphenylethylene as a free radical scavenger, there was no normal reaction for the formation of 3a (Scheme 3b), and the capture of the sulfonyl radical by 1,1-diphenylethylene was also confirmed by HR-MS. In Figure 3, it can be seen that the theoretical calculated value of [M+H]+ of intermediates is 335.1100, and the actual test value is 335.1096 with an error value of 0.0004, which is indeed within the reasonable range. This also indicates that there is a free radical pathway.
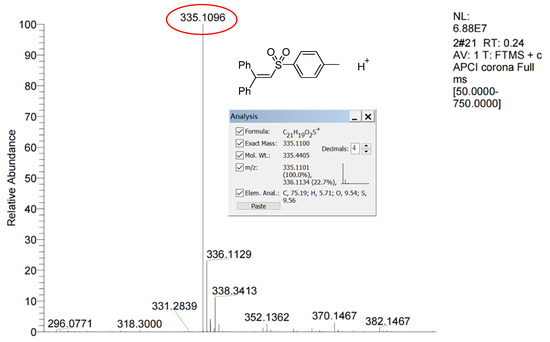
Figure 3.
The HR-MS of the capture of sulfonyl radical by scavenger 1,1-diphenylethylene in the control experiment.
What is more, the control experiment also implies that Lewis acid BF3·OEt2 could be essential for this reaction (Scheme 3c).
At the same time, in order to determine the source of the carboxyl oxygen atom in compound 3, an experiment in an oxygen-free atmosphere was investigated. When the reaction was carried out in a N2 atmosphere, the target product could not be obtained (Scheme 3d). This result shows that O2 in the air may be the source of the carboxyl oxygen atom in the product [28].
Furthermore, when the reaction was carried out under standard conditions, β-sulfinyl alkenylsulfone and thiosulfonate were easily observed by TLC (Scheme 3e), which may be the byproducts of the reaction.
Thus, on the basis of the above control experiments and the previously reported studies [14,16,23,25,27,28,29,34,35,38,39], a possible reaction pathway is proposed as Scheme 4.
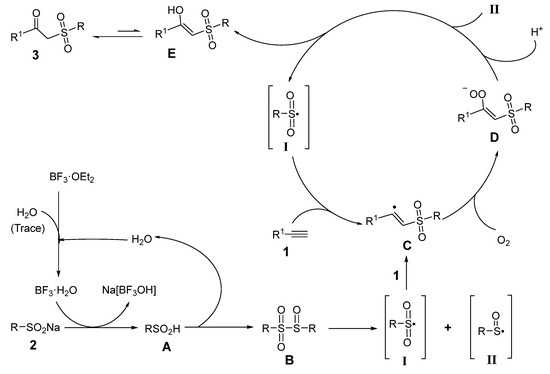
Scheme 4.
Proposed mechanism.
Firstly, BF3·H2O is produced in situ from BF3·OEt2 in the case of a trace amount of water [34,35]. Then, BF3·H2O reacts with sodium sulfinate 2, giving sulfinic acid A and Na[BF3OH]. Subsequently, sulfinyl sulfone B is generated from sulfinic acid A, and it is easy to produce sulfonyl radical I and sulfinyl radical II from intermediate B under heating conditions [38,39].
Secondly, sulfonyl radical I is added to alkyne 1 to give intermediate C [14,23], which is then trapped by oxygen to generate intermediate D [25]. Finally, intermediate D forms intermediate E [16] via the hydrogen ion and radical II, while causing radical II to generate free radical I. Intermediate E is prone to tautomerism [27,28,29], giving product 3 (Scheme 4).
Considering the preliminary experimental results (Scheme 2a and 2b) [34,35], we speculate that the amount of BF3·OEt2 determines the amount of sodium sulfite involved in the reaction and less BF3·OEt2 is beneficial to the reaction of less sodium sulfite (Scheme 2c). At the same time, the reaction atmosphere [35] and the presence [34] or absence [35] of acetylene substrate determine which radicals are easier to generate and react. This is why different products can be produced in similar BF3·OEt2 catalytic systems (Scheme 2).
In addition, as shown in Scheme 5, sulfinyl radicals II are extremely unstable in an air atmosphere and are prone to disproportionation to form sulfonyl radicals I and thiyl radicals III [40,41], which easily couple to form thiosulfonates F (Minor path a from intermediate II) [42,43]. Similarly, a small amount of β-sulfinyl alkenylsulfones G (Minor path b from intermediate II) [34] as a by-product can also be observed, which may be caused by the incomplete disproportionation of sulfinyl radicals [44].
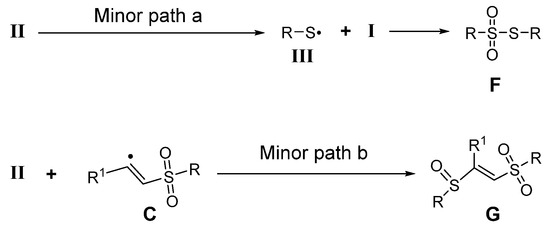
Scheme 5.
Proposed mechanism for the by-products.
2.5. Gram-scale Reaction
Considering that there is a wide application of β-keto sulfones in organic synthesis and biomedicine [45], in order to demonstrate the practicability of this reaction, further gram-scale research was carried out by selecting the synthesis of the target compound 3c as an example (Scheme 6).

Scheme 6.
Gram-scale reaction of 3c.
Obviously, the gram-scale synthesis of 3c is satisfactory. With the increase in the amount of reactant 1c from 0.3 to 6.0 mmol, the yield of 3c is only slightly decreased (60% vs. 63%), and the target compound 3c can be smoothly synthesized in gram-scale with a good yield.
3. Materials and Methods
3.1. General Information
1H and 13C NMR spectra were collected on an AVANCE NEO-600 in CDCl3 using tetramethylsilane (TMS) as an internal standard. Mass spectra were recorded on a Thermo Scientific ISQ gas chromatograph-mass spectrometer. High-resolution mass spectra (HR-MS) were obtained with a MAT 95XP mass spectrometer. The melting point (m.p.) was measured with a WRS-1B melting point instrument. Single-crystal X-ray analysis was performed using Agilent Gemini E. Reactions were monitored using thin-layer chromatography (TLC) and visualized with a UV light at 254 nm.
All reagents and solvents were purchased from commercial sources and used without further purification.
3.2. Experimental Procedure for Sodium Sulfinates 2
Different sodium sulfinates 2 were synthesized as Scheme 7 according to the procedure in the literature [22,26,46].
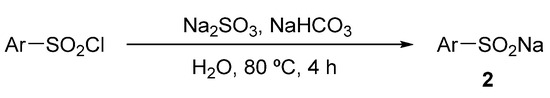
Scheme 7.
Synthesis route of different sodium sulfinates 2.
According to the literature [22,26,46], the mixture of arylsulfonyl chloride (10 mmol), sodium sulfite 2 (20 mmol), and sodium bicarbonate (20 mmol) in H2O (15 mL) was stirred at 80 °C for 4 h. Water was removed by a rotary evaporator. Then, the remaining solid was extracted and recrystallized by ethanol to obtain the required compound 2.
3.3. Experimental Procedure for Compounds 3a-3t
As shown in Scheme 8, the mixture of alkyne compound 1 (0.30 mmol, 1.0 equiv.), sodium sulfinate 2 (0.72 mmol, 2.4 equiv.), and BF3·OEt2 (0.15 mmol, 0.5 equiv.) in DCE (4 mL) under an air atmosphere was stirred at room temperature for 4 h. After the completion of the reaction, ethyl acetate (EA) (15 mL) was poured into the reaction mixture. The organic layers were extracted with a saturated sodium chloride solution (3 × 15 mL). Then, the organic layer was dried over anhydrous Na2SO4. Finally, after the filtration and evaporation of the solvents under reduced pressure, the crude product was purified by column chromatography on silica gel to afford the desired product 3.
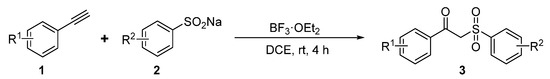
Scheme 8.
Synthesis route for Compounds 3a-3t.
3.4. Characterization Data for All Products 3a-3t
The structures of the serial compounds 3a-3t were systematically characterized via NMR, m.p., etc., and the corresponding data are summarized as follows:
(1) 1-Phenyl-2-tosylethan-1-one (3a), white solid (43.0 mg, 56%); m.p.: 106–108 °C (103–105 °C [23]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.44 (s, 3H, CH3), 4.72 (s, 2H, CH2), 7.33 (d, J = 8.4 Hz, 2H, ArH), 7.46–7.49 (m, 2H, ArH), 7.61 (t, J = 7.2 Hz, 1H, ArH), 7.76 (d, J = 8.4 Hz, 2H, ArH), 7.94 (d, J = 7.2 Hz, 2H); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.9, 63.8, 128.8, 129.0, 129.5, 130.0, 134.5, 135.8, 135.9, 145.5, 188.3.
(2) 1-(p-Tolyl)-2-tosylethan-1-one (3b), white solid (50.1 mg, 58%); m.p.: 109–111 °C (119–121 °C [47]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.42 (s, 3H, CH3), 2.44 (s, 3H, CH3), 4.69 (s, 2H, CH2), 7.27 (d, J = 8.4 Hz, 2H, ArH), 7.33 (d, J = 8.4 Hz, 2H, ArH), 7.75 (d, J = 8.4 Hz, 2H, ArH), 7.85 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.9, 21.9, 63.7, 128.7, 129.6, 129.7, 129.9, 133.5, 135.9, 145.4, 145.7, 187.8.
(3) 1-(4-Ethylphenyl)-2-tosylethan-1-one (3c), white solid (57.1 mg, 63%); m.p.: 95–97 °C (84% [48]); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.26 (t, J = 7.2 Hz, 3H, CH3), 2.44 (s, 3H, CH3), 2.72 (q, J = 7.2 Hz, 2H, CH2), 4.69 (s, 2H, CH2), 7.30 (d, J = 8.4 Hz, 2H, ArH), 7.33 (d, J = 8.4 Hz, 2H, ArH), 7.76 (d, J = 8.4 Hz, 2H, ArH), 7.87 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 15.2, 21.8, 29.2, 63.7, 128.5, 129.7, 129.7, 129.9, 133.7, 135.9, 145.4, 151.8, 187.8.
(4) 1-(4-(tert-Butyl)phenyl)-2-tosylethan-1-one (3d), white solid (49.5 mg, 50%); m.p.: 93–94 °C (95–97 °C [49]); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.34 (s, 9H, CH3), 2.43 (s, 3H CH3), 4.69 (s, 2H, CH2), 7.32 (d, J = 8.4 Hz, 2H, ArH), 7.48 (d, J = 8.4 Hz, 2H, ArH), 7.76 (d, J = 8.4 Hz, 2H, ArH), 7.88 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.8, 31.1, 35.4, 63.6, 126.0, 128.7, 129.5, 129.9, 133.4, 135.9, 145.4, 158.5, 187.8.
(5) 1-(4-Fluorophenyl)-2-tosylethan-1-one (3e), white solid (33.3 mg, 38%); m.p.: 134–135 °C (125–127 °C [47]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.45 (s, 3H, CH3), 4.68 (s, 2H CH2), 7.14–7.17 (m, 2H, ArH), 7.34 (d, J = 8.4 Hz, 2H, ArH), 7.75 (d, J = 8.4 Hz, 2H, ArH), 7.99–8.01 (m, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.9, 63.9, 116.2 (d, J = 22.5 Hz) 128.7, 130.0, 132.3, 132.4 (d, J = 10.5 Hz), 135.7, 145.7, 166.6 (d, J = 256.5 Hz), 186.7; 19F NMR (564 MHz, CDCl3), δ, ppm: -102.4.
(6) 1-(4-Chlorophenyl)-2-tosylethan-1-one (3f), white solid (44.4 mg, 48%); m.p.: 132–134 °C (138–139 °C [46]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.45 (s, 3H, CH3), 4.68 (s, 2H, CH2), 7.34 (d, J = 7.8 Hz, 2H, ArH), 7.46 (d, J = 8.4 Hz, 2H, ArH), 7.74 (d, J = 7.8 Hz, 2H, ArH), 7.90 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.9, 63.9, 128.7, 129.3, 130.0, 130.9, 134.2, 135.7, 141.2, 145.7, 187.2.
(7) 1-(4-Bromophenyl)-2-tosylethan-1-one (3g), white solid (53.9 mg, 51%); m.p.: 140–141 °C (143–145 °C [23]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.45 (s, 3H, CH3), 4.67 (s, 2H, CH2), 7.34 (d, J = 8.4 Hz, 2H, ArH), 7.63 (d, J = 9.0 Hz, 2H, ArH), 7.74 (d, J = 8.4 Hz, 2H, ArH), 7.82 (d, J = 9.0 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.9, 63.9, 128.7, 130.0, 130.1, 131.0, 132.4, 134.6, 135.7, 145.7, 187.4.
(8) 1-(4-Nitrophenyl)-2-tosylethan-1-one (3h), white solid (30.6 mg, 32%); m.p.: 140–142 °C (145–147 °C [19]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.47 (s, 3H, CH3), 4.75 (s, 2H, CH2), 7.37 (d, J = 8.4 Hz, 2H, ArH), 7.74 (d, J = 8.4 Hz, 2H, ArH), 8.15 (d, J = 9.0 Hz, 2H, ArH), 8.33 (d, J = 9.0 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.9, 64.3, 124.1, 128.7, 130.2, 130.7, 135.5, 140.1, 146.1, 151.0, 187.1.
(9) 2-Tosyl-1-(4-trifluoromethylphenyl)ethan-1-one (3i), white solid (35.9 mg, 35%); m.p.: 134–136 °C (129–131 °C [23]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.45 (s, 3H, CH3), 4.73 (s, 2H, CH2), 7.34 (d, J = 8.4 Hz, 2H, ArH), 7.73–7.76 (m, 4H, ArH), 8.07 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.9, 64.0, 123.5 (q, J = 271.5 Hz), 126.0 (q, J = 4.5 Hz128.7, 129.9, 130.1, 135.5 (q, J = 33.0 Hz), 135.6, 138.4, 145.9, 187.6; 19F NMR (564 MHz, CDCl3), δ, ppm: -63.3.
(10) 1-(m-Tolyl)-2-tosylethan-1-one (3j), white solid (43.2 mg, 50%); m.p.: 97–98 °C (97–99 °C [47]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.40 (s, 3H, CH3), 2.44 (s, 3H, CH3), 4.70 (s, 2H, CH2), 7.33 (d, J = 8.4 Hz, 2H, ArH), 7.35–7.38 (m, 1H, ArH), 7.42 (d, J = 7.8 Hz, 1H, ArH), 7.71 (s, 1H, ArH), 7.74 (d, J = 7.8 Hz, 1H, ArH), 7.76 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.4, 21.9, 63.7, 126.8, 128.8, 128.9, 129.8, 129.9, 135.3, 136.0, 138.9, 145.5, 188.4.
(11) 1-(3-Methoxyphenyl)-2-tosylethan-1-one (3k), colorless oil (50.2 mg, 55%) ( 54% [47]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.44 (s, 3H, CH3), 3.84 (s, 3H, OCH3), 4.70 (s, 2H, CH2), 7.16 (d, J = 7.8 Hz, 1H, ArH), 7.34 (d, J = 8.4 Hz, 2H, ArH), 7.37–7.40 (m, 1H, ArH), 7.43 (s, 1H, ArH), 7.52 (d, J = 7.8 Hz, 1H, ArH), 7.77 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.9, 55.6, 63.8, 113.1, 121.3, 122.3, 128.8, 130.0, 135.9, 137.2, 145.5, 160.1, 188.4.
(12) 1-(3-Chlorophenyl)-2-tosylethan-1-one (3l), white solid (38.8 mg, 42%); m.p.: 71–73 °C (71–73 °C [47]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.45 (s, 3H, CH3), 4.68 (s, 2H, CH2), 7.34 (d, J = 7.8 Hz, 2H, ArH), 7.42–7.46 (m, 1H, ArH), 7.58 (d, J = 7.2 Hz, 1H, ArH), 7.75 (d, J = 7.8 Hz, 2H, ArH), 7.84–7.86 (m, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.9, 63.9, 127.7, 128.7, 129.3, 130.1, 130.3, 134.4, 135.4, 135.7, 137.4, 145.8, 187.2.
(13) 1-(3-Bromophenyl)-2-tosylethan-1-one (3m), white solid (49.6 mg, 47%); m.p.: 140–142 °C (140–141 °C [50]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.45 (s, 3H, CH3), 4.68 (s, 2H, CH2), 7.34 (d, J = 8.4 Hz, 2H, ArH), 7.36–7.39 (m, 1H, ArH), 7.72–7.75 (m, 3H, ArH), 7.89 (d, J = 7.8 Hz, 1H, ArH), 8.00 (s, 1H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.9, 63.8, 123.3, 128.2, 128.7, 130.1, 130.5, 132.2, 135.6, 137.3, 137.5, 145.8, 187.1.
(14) 1-(o-Tolyl)-2-tosylethan-1-one (3n), white solid (34.6 mg, 40%); m.p.: 99–101 °C (95–97 °C [51]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.43 (s, 3H, CH3), 2.44 (s, 3H, CH3), 4.69 (s, 2H, CH2), 7.24–7.26 (m, 1H, ArH), 7.28 (d, J = 7.8 Hz, 1H, ArH), 7.33 (d, J = 8.4 Hz, 2H, ArH), 7.41–7.43 (m, 1H, ArH), 7.73 (d, J = 7.8 Hz, 1H, ArH), 7.75 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.7, 21.9, 65.7, 126.0, 128.6, 130.0, 130.6, 132.4, 132.9, 135.8, 136.1, 140.2, 145.4, 190.6.
(15) 1-(2-Methoxyphenyl)-2-tosylethan-1-one (3o), white solid (44.7 mg, 49%); m.p.: 124–126 °C (128–130 °C [51]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.42 (s, 3H, CH3), 3.88 (s, 3H, OCH3), 4.92 (s, 2H, CH2), 6.90 (d, J = 8.4 Hz, 1H, ArH), 6.97–7.00 (m, 1H, ArH), 7.29 (d, J = 8.4 Hz, 2H, ArH), 7.47–7.50 (m, 1H, ArH), 7.65 (d, 1H, J = 7.8 Hz, ArH), 7.74 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.7, 55.7, 67.5, 111.7, 121.0, 126.3, 128.5, 129.5, 131.3, 135.2, 136.7, 144.8, 159.0, 189.2.
(16) 1-(Naphthalen-2-yl)-2-tosylethan-1-one (3p), white solid (44.7 mg, 46%); m.p.: 150–151 °C (150–152 °C [17]); 1H NMR (600 MHz, CDCl3), δ, ppm: 2.43 (s, 3H, CH3), 4.86 (s, 2H, CH3), 7.34 (d, J = 8.4 Hz, 2H, ArH), 7.59–7.62 (m, 1H, ArH), 7.65–7.68 (m, 1H, ArH), 7.80 (d, J = 8.4 Hz, 2H, ArH), 7.90–7.93 (m, 2H, ArH), 7.98–8.00 (m, 2H, ArH), 8.48 (s, 1H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 21.8, 64.0, 124.1, 127.3, 128.0, 128.8, 129.0, 129.5, 130.0, 130.1, 132.3, 132.4, 133.3, 135.8, 135.2, 145.6, 188.4
(17) 1-Phenyl-2-(phenylsulfonyl)ethan-1-one (3q), white solid (39.8 mg, 51%); m.p.: 86–88 °C (85–87 °C [23]); 1H NMR (600 MHz, CDCl3), δ, ppm: 4.74 (s, 2H, CH2), 6.46–7.49 (m, 2H, ArH), 7.53–7.56 (m, 2H, ArH-11,13), 7.62 (t, J = 7.8 Hz, 1H, ArH), 7.66 (t, J = 7.8 Hz, 1H, ArH), 7.90 (d, J = 7.2 Hz, 2H, ArH), 7.93 (d, J = 7.2 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 63.6, 128.7, 129.0, 129.3, 129.4, 134.4, 134.5, 135.8, 138.8, 188.1.
(18) 2-(4-Methoxypheny)sulfonyl)-1-phenylethan-1-one (3r), white solid (50.6 mg, 53%); m.p.: 100–102 °C (100–102 °C [25]); 1H NMR (600 MHz, CDCl3), δ, ppm: 3.86 (s, 3H, OCH3), 4.71 (s, 2H, CH2), 6.97 (d, J = 9.0 Hz, 2H, ArH), 7.46–7.48 (m, 2H, ArH), 7.61 (t, J = 7.8 Hz, 1H, ArH), 7.79 (d, J = 9.0 Hz, 2H, ArH), 7.93 (d, J = 7.2 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 55.8, 63.8, 114.5, 128.9, 129.4, 130.3, 131.0, 134.4, 135.9, 164.2, 188.4.
(19) 2-(4-Chlorophenylsulfonyl)-1-phenylethan-1-one (3s), white solid (44.1 mg, 50%); m.p.: 131–132 °C (132–134 °C [17]); 1H NMR (600 MHz, CDCl3), δ, ppm: 4.74 (s, 2H, CH2), 7.48–7.51 (m, 2H, ArH), 7.52 (d, J = 8.4 Hz, 2H, ArH), 7.64 (t, J = 7.8 Hz, 1H, ArH), 7.83 (d, J = 8.4 Hz, 2H, ArH), 7.94 (d, J = 7.8 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 63.5, 129.1, 129.4, 129.7, 130.3, 134.7, 135.7, 137.2, 141.3, 188.0.
(20) 1-(4-Ethylphenyl)-2-phenylsulfonylethan-1-one (3t), white solid (53.6 mg, 62%); m.p.: 128–129 °C (127–129 °C [25]); 1H NMR (600 MHz, CDCl3), δ, ppm: 1.25 (t, J = 7.8 Hz, 3H, CH3), 2.71 (q, J = 7.8 Hz, 2H, CH2), 4.72 (s, 2H, CH2), 7.29 (d, J = 8.4 Hz, ArH), 7.52–7.55 (m, 2H, ArH), 7.65 (t, J = 7.8 Hz, 1H, ArH), 7.86 (d, J = 8.4 Hz, 2H, ArH), 7.89 (d, J = 7.2 Hz, 2H, ArH); 13C NMR (150 MHz, CDCl3), δ, ppm: 15.1, 29.1, 63.5, 128.5, 128.7, 129.3, 129.7, 133.6, 134.3, 138.9, 151.8, 187.6.
The detailed 1H, 13C, and 19F NMR spectra for all compounds 3a-3t are provided in the Supplementary Materials.
4. Conclusions
In summary, we successfully found a BF3·OEt2-mediated oxysulfonylation reaction of sodium sulfinates and alkynes. The reaction does not need any metal catalysts, and the simple and mild conditions make it a convenient procedure for the synthesis of β-keto sulfones. A possible radical mechanism was also proposed on the basis of control studies. Importantly, this reaction uses oxygen in the air as an oxidant and does not need to use chemical reagents with pungent odors such as pyridine and acetic acid. It meets the requirements of the development of green chemistry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29153559/s1, and contains the 1H, 13C, and 19F NMR spectra for all compounds 3a-3t.
Author Contributions
Conceptualization, Z.-Y.W.; methodology, S.-W.Y. and Z.-J.C.; formal analysis, S.-W.Y. and H.-Q.L.; data curation, Y.L. and W.-X.L.; writing—original draft preparation, S.-W.Y.; writing—review and editing, S.-W.Y., Z.L. and Z.-Y.W.; project administration, Z.-Y.W.; funding acquisition, Z.-Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Guangdong Basic and Applied Basic Research Foundation (no. 2021A1515012342).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the findings of this study are available within the paper and within its Supplementary Materials published online.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Corpas, J.; Kim-Lee, S.-H.; Mauleón, P.; Arrayás, R.G.; Carretero, J.C. Beyond classical sulfone chemistry: Metal- and photocatalytic approaches for C-S bond functionalization of sulfones. Chem. Soc. Rev. 2022, 51, 6774–6823. [Google Scholar] [CrossRef]
- Wu, H.; Chen, S.G.; Liu, C.N.; Zhao, Q.S.; Wang, Z.; Jin, Q.R.; Sun, S.J.; Guo, J.; He, X.W.; Walsh, P.J.; et al. Construction of C-S and C-Se bonds from unstrained ketone precursors under photoredox catalysis. Angew. Chem. Int. Ed. 2024, 63, e202314790. [Google Scholar] [CrossRef]
- Markham, A.; Keam, S.J. Danoprevir: First global approval. Drugs 2018, 78, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.R.; Chen, J.W.; Li, L.; Wen, K.M.; Yao, X.G.; Pang, J.X.; Wu, T.; Tang, X.D. Copper-mediated decarboxylative sulfonylation of arylacetic acids with sodium sulfinates. Org. Lett. 2020, 22, 7164–7168. [Google Scholar] [CrossRef]
- Wasilewska-Rosa, A.; Kisiel, K.; Tkaczyk, A.; Loska, R. Stereospecific synthesis of allenes from β-ketosulfones. Adv. Synth. Catal. 2023, 365, 704–708. [Google Scholar] [CrossRef]
- Lu, L.J.; Luo, J.; Milstein, D. Manganese(I)-pincer catalyzed α-alkylation of sulfones by alcohols. ACS Catal. 2023, 13, 5949–5954. [Google Scholar] [CrossRef]
- Inanaga, K.; Fukuyama, T.; Kubota, M.; Komatsu, Y.; Chiba, H.; Kayano, A.; Tagami, K. Novel and efficient chromium(II)-mediated desulfonylation of α-sulfonyl ketone. Org. Lett. 2015, 17, 3158–3161. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Samanta, S.; Ghosh, A.K.; Neogi, S.; Hajra, A. Advances in oxosulfonylation reaction. Adv. Synth. Catal. 2020, 362, 4552–4578. [Google Scholar] [CrossRef]
- Reddy, R.J.; Kumari, A.H.; Kumar, J.J. Recent advances in the synthesis and applications of β-keto sulfones: New prospects for the synthesis of β-keto thiosulfones. Org. Biomol. Chem. 2021, 19, 3087–3118. [Google Scholar] [CrossRef]
- Mulina, O.M.; Ilovaisky, A.I.; Parshin, V.D.; Terent’ev, A.O. Oxidative sulfonylation of multiple carbon-carbon bonds with sulfonyl hydrazides, sulfinic acids and their salts. Adv. Synth. Catal. 2020, 362, 4579–4654. [Google Scholar] [CrossRef]
- Yang, W.H.; Zhou, Y.; Tong, Y.; Wang, Z.Y.; Wu, H.L.; Mei, L.; Li, Q.; Xie, L.Y.; Zhang, J.Q.; Wei, W.T. Nickel-catalyzed reaction between vinyl azides and an alkyl sulfonyl radical generated from DMSO: Rapid access to β-keto sulfones. ACS Sustainable Chem. Eng. 2024, 12, 5046–5051. [Google Scholar] [CrossRef]
- Abdukerem, D.; Chen, H.; Mao, Z.; Xia, K.; Zhu, W.; Liu, C.; Yu, Y.; Abdukader, A. Transition metal-free C(sp3)-H selenation of β-ketosulfones. Org. Biomol. Chem. 2024, 22, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Pospísil, J.; Sato, H. Practical synthesis of β-acyl and β-alkoxycarbonyl heterocyclic sulfones. J. Org. Chem. 2011, 76, 2269–2272. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.Q.; Zhang, B.B.; Han, J.E.; Peng, B.H.; Shan, Y.L.; Niu, T.F. Heterogeneous carbon nitrides photocatalysis multicomponent hydrosulfonylation of alkynes to access β-keto sulfones with the insertion of sulfur dioxide in aerobic aqueous medium. Org. Lett. 2020, 22, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Pampana, V.K.K.; Charpe, V.P.; Sagadevan, A.; Das, D.K.; Lin, C.C.; Hwu, J.R.; Hwang, K.C. Oxy-sulfonylation of terminal alkynes via C-S coupling enabled by copper photoredox catalysis. Green Chem. 2021, 23, 3569–3574. [Google Scholar] [CrossRef]
- Song, T.; Zhang, Y.P.; Wang, C.; Li, Y.F.; Yang, Y. Photocatalytic aerobic oxysulfonylation of alkynes to access β-keto sulfones catalyzed by OVs-N-Nb2O5. Chin. J. Chem. 2022, 40, 2618–2624. [Google Scholar] [CrossRef]
- Dam, B.; Sahoo, A.K.; Patel, B.K. Visible-light-mediated synthesis of β-keto sulfones using g-C3N4 as a recyclable photocatalyst under sustainable conditions. Green Chem. 2022, 24, 7122–7130. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhao, Y.H.; Cai, C.Q.; Wang, L.Y.; Gong, H. Dioxygen-triggered oxosulfonylation/sulfonylation of terminal olefins toward β-keto sulfones/sulfones. Org. Lett. 2021, 23, 8296–8301. [Google Scholar] [CrossRef]
- Chikunova, E.I.; Kukushkin, V.Y.; Dubovtsev, A.Y. Atom-economic synthesis of β-ketosulfones based on gold-catalyzed highly regioselective hydration of alkynylsulfones. Green Chem. 2022, 24, 3314–3320. [Google Scholar] [CrossRef]
- Tyagi, A.; Taneja, N.; Khan, J.; Hazra, C.K. I2-Catalyzed/mediated C-S and C-I bond formation: Solvent- and metal-free approach for the synthesis of β-keto sulfones and branched sulfones. Adv. Synth. Catal. 2023, 365, 1247–1254. [Google Scholar] [CrossRef]
- Wang, B.-W.; Jiang, K.; Li, J.-X.; Luo, S.-H.; Wang, Z.-Y.; Jiang, H.-F. 1,1-Diphenylvinylsulfide as functional AIEgen derived from the aggregation-caused-quenching molecule 1,1-diphenylvinylsulfide through simple thioetherification. Angew. Chem. Int. Ed. 2020, 59, 2338–2343. [Google Scholar] [CrossRef]
- Tian, Z.B.; Gong, Q.H.; Huang, T.Z.; Liu, L.; Chen, T.Q. Practical electro-oxidative sulfonylation of phenols with sodium arenesulfinates generating arylsulfonate esters. J. Org. Chem. 2021, 86, 15914–15926. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.; Fennewald, J.C.; Lipshutz, B.H. Aerobic oxidation in nanomicelles of aryl alkynes, in water at room temperature. Angew. Chem. Int. Ed. 2014, 53, 3432–3435. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Hong, Y.Y.; Peng, S.; Xu, X.Q.; Tang, S.S.; Yang, L.H.; Xie, L.Y. Photo-sensitizer-free synthesis of β-keto sulfones via visible-light-induced oxysulfonylation of alkenes with sulfonic acids. Org. Biomol. Chem. 2021, 19, 4537–4541. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Lu, S.X.; Zheng, Y.Y.; Wang, J.G.; Yang, L.; Sun, P. Synthesis of β-keto sulfones by oxy-sulfonylation of alkynes in HFIP. Adv. Synth. Catal. 2022, 364, 1305–1312. [Google Scholar] [CrossRef]
- Saxena, B.; Patel, R.I.; Sharma, S.; Sharma, A. Mechanochemical-assisted decarboxylative sulfonylation of α,β-unsaturated carboxylic acids with sodium sulfinate salts. Green Chem. 2024, 26, 2721–2729. [Google Scholar] [CrossRef]
- Lu, Q.Q.; Zhang, J.; Zhao, G.L.; Qi, Y.; Wang, H.M.; Lei, A.W. Dioxygen-triggered oxidative radical reaction: Direct aerobic difunctionalization of terminal alkynes toward β-keto sulfones. J. Am. Chem. Soc. 2013, 135, 11481–11484. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kumar, A. Amino acid-catalyzed direct synthesis of β-keto sulfones via aerobic difunctionalization of terminal alkynes in an aqueous medium. ACS Sustain. Chem. Eng. 2019, 7, 9182–9188. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chang, X.Q.; Zhang, S.C.; Lu, S.X.; Yang, L.; Sun, P. Air-triggered, catalyst-free decarboxylative oxysulfonylation of arylpropiolic acids with sodium sulfinates. Environ. Chem. Lett. 2022, 20, 2773–2779. [Google Scholar] [CrossRef]
- Chen, W.; Ma, Y.H.; He, W.Y.; Wu, Y.X.; Huang, Y.C.; Zhang, Y.P.; Tian, H.C.; Wei, K.; Yang, X.D.; Zhang, H.B. Structure units oriented approach towards collective synthesis of sarpagine-ajmaline-koumine type alkaloids. Nat. Commun. 2022, 13, 908. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Yu, S.-W.; Zhou, Y.-J.; Li, H.-Q.; Qiu, Q.-W.; Li, M.-X.; Wang, Z.-Y. Application of BF3·OEt2 in organic synthesis as a catalyst or synthon. Chin. J. Org. Chem. 2023, 43, 3107–3118. [Google Scholar] [CrossRef]
- Li, A.Q.; Zhao, J.; Zhang, C.; Jiang, Q.X.; Zhu, B.F.; Cao, H. Lewis acid-promoted three-component cyclization for the construction of functionalized oxazoles. J. Org. Chem. 2023, 88, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.L.; Hu, J.W.; Cao, J.; Xu, L.W. Intramolecular Hosomi-Sakurai reaction for the synthesis of benzoxasiloles. J. Org. Chem. 2024, 89, 9027–9030. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-W.; Chen, Z.-J.; Chen, Z.-H.; Chen, S.-H.; Yang, K.; Xu, W.-J.; Wang, Z.-Y. Trace water in a BF3·OEt2 system: A facile access to sulfinyl alkenylsulfones from alkynes and sodium sulfinates. Org. Biomol. Chem. 2023, 21, 7776–7781. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Luo, S.-H.; Jiang, K.; Hao, Z.-F.; Wang, B.-W.; Pang, C.-M.; Wang, Z.-Y. Disproportionate coupling reaction of sodium sulfinates mediated by BF3∙OEt2: An approach to symmetrical/unsymmetrical thiosulfonates. Org. Lett. 2018, 20, 4754–4758. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Q.; Yang, K.; Chen, X.-Y.; Arulkumar, M.; Wang, N.; Chen, S.-H.; Wang, Z.-Y. A 3,4-dihalo-2(5H)-furanone initiated ring-opening reaction of DABCO in the absence of a metal catalyst and additive and its application in a one-pot two-step reaction. Green Chem. 2019, 21, 3782–3788. [Google Scholar] [CrossRef]
- CCDC 2367445 (for 3a) Contains the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge from the Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 3 July 2024).
- Wang, Z.K.; Zhang, Z.S.; Zhao, W.J.; Sivaguru, P.; Zanoni, G.; Wang, Y.Y.; Anderson, E.A.; Bi, X.H. Synthetic exploration of sulfinyl radicals using sulfinyl sulfones. Nat. Commun. 2021, 12, 5244. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Q.; Feng, C.; Wang, Z.; Zhao, W.; Ning, Y.; Wu, Y. The sulfinylsulfonation of alkynes for β-sulfinyl alkenylsulfone. Chem. –Asian J. 2022, 17, e202200299. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F. vic-Disulfoxides and OS-sulfenyl sulfinates. Chem. Rev. 1984, 84, 117–135. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Haug, G.C.; Greco, S.G.; Trevino, R.; Karki, G.B.; Arman, H.D.; Larionov, O.V. Decarboxylative sulfinylation enables a direct, metal-free access to sulfoxides from carboxylic acids. Angew. Chem. Int. Ed. 2022, 61, e202210525. [Google Scholar] [CrossRef]
- Mampuys, P.; McElroy, C.R.; Clark, J.H.; Orru, R.V.A.; Maes, B.U.W. Thiosulfonates as emerging reactants: Synthesis and applications. Adv. Synth. Catal. 2020, 362, 3–64. [Google Scholar] [CrossRef]
- Lv, Y.F.; Luo, J.Y.; Ma, Y.C.; Dong, Q.; He, L. Visible-light-promoted sulfonylation of thiols with aryldiazonium and sodium metabisulphite leading to unsymmetrical thiosulfonates. Org. Chem. Front. 2021, 8, 2461–2467. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wang, X.R.; Sivaguru, P.; Wang, Z.K. Exploring the synthetic application of sulfinyl radicals. Org. Chem. Front. 2022, 9, 6063–6076. [Google Scholar] [CrossRef]
- Chen, X.W.; Luo, Z.L.; Hu, Z.J.; Sun, D.H.; He, Y.Y.; Lu, J.N.; Chen, L.L.; Liu, S.Y. Discovery of potent thiazolidin-4-one sulfone derivatives for inhibition of proliferation of osteosarcoma in vitro and in vivo. Eur. J. Med. Chem. 2024, 266, 116082. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Qian, P.; Wang, Y.; Mei, H.; Han, J.; Pan, Y. Cu-Catalyzed deoxygenative C2-sulfonylation reaction of quinoline N-Oxides with sodium sulfinate. Org. Lett. 2016, 18, 4144–4147. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Liang, Z.; Jialingbieke, A.; Gao, J.; Du, D. NHC-Catalyzed Synthesis of α-sulfonyl ketones via radical- mediated sulfonyl methylation of aldehydes. Org. Lett. 2023, 25, 2657–2662. [Google Scholar] [CrossRef] [PubMed]
- Jadhao, A.R.; Gaikwad, S.S.; Patil, L.R.; Waghmode, S.B. Sonication-assisted one pot, metal-free synthesis of β-keto sulfones from styrenes, NBS and aromatic sodium sulfinate salts. Chem. Pap. 2021, 75, 4959–4968. [Google Scholar] [CrossRef]
- Deng, S.Q.; Liang, E.; Wu, Y.R.; Tang, X.D. Efficient sulfonylation of ketones with sodium sulfinates for the synthesis of β-keto sulfones. Tetrahedron Lett. 2018, 59, 3955–3957. [Google Scholar] [CrossRef]
- Chen, W.T.; Liu, X.Y.; Chen, E.; Chen, B.H.; Shao, J.A.; Yu, Y.P. KI-Mediated radical multi-functionalization of vinyl azides: A one-pot and efficient approach to β-keto sulfones and α-halo-β-keto sulfones. Org. Chem. Front. 2017, 4, 1162–1166. [Google Scholar] [CrossRef]
- Yavari, I.; Shaabanzadeh, S. Electrochemical synthesis of β-ketosulfones from switchable starting materials. Org. Lett. 2020, 22, 464–467. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).