Abstract
This study investigates the chemical composition of the essential oil obtained from the leaves of Bocageopsis multiflora (Mart.) R.E.Fr (Annonaceae), examining its effectiveness in combating both the larvae and adult forms of Aedes aegypti mosquitoes. Additionally, for a deeper understanding of the insecticidal activity, toxicity properties and molecular docking calculations were conducted using the main compounds of this essential oil. GC/MS analysis revealed the presence of 26 constituents, representing 95.2% of the essential oil, with the major components identified as the sesquiterpenes α-selinene, β-selinene, and β-elemene. Larvicidal assays demonstrated potent activity of this essential oil with significant LC50 values of 40.8 and 39.4 μg/mL at 24 and 48 h, respectively. Adulticidal assessments highlighted strong efficacy with LC50 of 12.5 µg/mL. Molecular docking analysis identified optimal interaction activities of α-selinene and β-selinene with key Aedes proteins. The in silico studies comparing synthetic insecticides with the major sesquiterpenes of the essential oil revealed that β-selinene exhibited a significantly higher binding affinity compared to the other two sesquiterpenes. Also, ADMET studies of the three main sesquiterpenes indicated acceptable drug-like properties. In these findings, safety evaluations showed low toxicity and skin sensitization for the main sesquiterpenes, contrasting with commercial synthetic insecticides. Therefore, in silico analyses suggest promising interactions with Aedes proteins, indicating its potential as an effective alternative to conventional insecticides These results show the larvicidal and adulticidal potential of the essential oil from Bocageopsis multiflora against Aedes aegypti, supported by its predominant constituents, α-selinene, β-selinene and β-elemene.
Keywords:
adulticidal; Aedes; annonaceae; essential oil; larvicidal; molecular docking; sesquiterpenes 1. Introduction
Mosquitoes are a major global health concern, serving as the primary vector for a range of pathogens that cause diseases, particularly in tropical and subtropical regions. These diseases include malaria, filariasis, yellow fever, dengue, chikungunya, and Zika virus [1]. The World Health Organization (WHO) estimates that vector-borne diseases account for more than 17% of all infectious diseases, causing more than 700,000 deaths annually [2].
Given this context, arboviruses have become important and constant threats in tropical regions due to rapid climate changes, deforestation, population migration, disorderly urbanization, and precarious sanitary conditions that favor viral amplification and transmission [3,4,5]. The Aedes aegypti mosquitoes are efficient transmitters of dengue, chikungunya, and Zika diseases, which significantly impact public health [3]. Zika virus infection is known to lead to Guillain-Barré syndrome and negative pregnancy outcomes. These include a higher likelihood of preterm birth, fetal death, stillbirth, and a group of congenital malformations known as congenital Zika syndrome (CZS) in its most severe form. CZS encompasses microcephaly and other cranial abnormalities [4,5,6]. The number of cases of chikungunya and Zika have been reported in more than 89 countries across Asia, the Americas, and Africa [7,8]. Additionally, it is important to highlight that in the year 2022, 271,176 cases of chikungunya were reported, including 95 deaths in the Americas [9]. As for the epidemiology of dengue in Brazil, it is one of the main endemic diseases in the country. More than 5,867,255 cases were reported between 2014 and 2019, with the highest number of cases (1,696,340) occurring in 2015 [10]. There was a significant growth in the number of dengue fever cases in Brazil in 2019, which represents a public health problem [10]. It is important to mention that according to data from the World Health Organization (WHO), Brazil reported nearly 3 million cases in 2023, out of a global total exceeding 5 million cases [11].
Presently, the primary approach to controlling mosquito populations relies heavily on synthetic insecticides, including chlorinated hydrocarbon compounds, organophosphates, carbamates, pyrethroids, neonicotinoids, formamidines, and other molecules, plus botanical and microbial agents, and insect growth regulators like diflubenzuron and methoprene [12]. However, the continuous and extensive use of these chemicals has led to an increase in resistance among vector populations [13,14]. Furthermore, these compounds can cause adverse effects on non-target organisms and also present risks to human health [1]. In response to these challenges, alternative control methods have been proposed. These include the use of biopesticides, such as Bacillus thuringiensis and B. sphaericus [15,16,17] and, another strategy involves the creation of sterile insects through radiation, a technique that reduces the ability of these insects to reproduce [17,18]. Transgenic technologies, which involve the genetic modification of vectors, are also being explored as potential control methods [17,19].
In response to concerns about chemical resistance and environmental contamination associated with conventional insecticides, plant-derived alternatives such as essential oils (EOs) and their secondary metabolites are established and represent a reasonable framework of scientific research aimed at controlling vector insects [20,21]. EOs offer significant promise to improve insect vector control due to their inherent advantages: low toxicity, multi-target efficacy due to the presence of various bioactive compounds, and relatively low raw material costs [20,22].
In this context, essential oils obtained from plant species of the Annonaceae family exhibit various bioactivities, such as antimicrobial activity [23], cytotoxic activity [24], and activity against Ae. aegypti larvae [25]. Inspired by the biological activity demonstrated by essential oils (EOs) from species of this family, we turned our attention to the genus Bocageopsis. This genus encompasses four species: B. mattogrossensis, B. canescens, B. multiflora, and B. pleiosperma, all restricted to South America, with a presence in the Amazon region of Brazil, specifically in the states of Amazonas and Pará [26].
The chemical exploration of the Bocageopsis genus has involved the characterization and assessment of the essential oils extracted from its leaves. These EOs have been evaluated for their antibacterial properties [23,24] and cytotoxic effects [25]. The chemical composition of the essential oils revealed terpenes such as β-bisabolene, bergamotene, β-farnesene, β-selinene, α-selinene, and farnesol as their main constituents [27,28,29]. In articles specifically addressing B. multiflora (Mart.) R.E.Fr, it has been reported that the essential oils from this species demonstrate activity against the promastigote forms of Leishmania amazonensis [27], as well as exhibiting bactericidal and antimicrobial activity [23,29].
Following on from these findings, the application of molecular docking analysis in assessing the insecticidal or larvicidal potential of essential oils and their key constituents against proteins of Aedes sp. represents a cutting-edge approach to mosquito control [30]. Recent studies have employed molecular docking to uncover the complex interactions between constituents of essential oils and crucial mosquito proteins. Researchers have focused on proteins involved in the nervous system, reproductive processes, and metabolic pathways of insects [31]. A noteworthy example involves the use of molecular docking to assess the binding affinity of specific compounds found in essential oils to the acetylcholinesterase of Aedes aegypti, a vital enzyme in the mosquito’s nervous system [32]. It is also employed to analyze their effects on crucial targets within the mosquito life cycle, such as sterol transporter protein-2 (SCP-2), which is predominantly expressed in the midgut tissue of larvae and plays a crucial role in the absorption of cholesterol during feeding stages, thus being essential for insect metabolism [33,34,35]. In this context, examining the impacts of compounds found in essential oils through molecular docking on key targets within the mosquito life cycle helps in identifying promising compounds for further investigation.
In light of the outlined scenario, this study showcases the chromatographic profile of the essential oil obtained from Bocageopsis multiflora. Furthermore, it includes assessments for larvicidal and adulticidal effects against Aedes aegypti. Additionally, the study incorporates molecular docking analyses involving the three main compounds, β-elemene, α-selinene, and β-selinene identified in the essential oil of B. multiflora and three key proteins in the insect’s metabolism. In silico studies were also employed to evaluate ADMET properties, with the aim of elucidating the mode of action and predict the safety profile of these secondary metabolites. The results obtained in this work illustrate the significant potential of this essential oil and its constituents to play a vital role in developing future insecticides and in new strategies for mosquito control.
2. Results and Discussion
2.1. GC/MS Analysis of the Essential Oil of B. multiflora
The extraction of essential oil from B. multiflora represents an expanding area of studies fueled by the diverse chemical composition and biological properties observed in essential oils from species within the Annonaceae family. This interest stems from the remarkable antimicrobial, cytotoxic, and larvicidal actions demonstrated by essential oils of different members of this family against Ae. aegypti larvae [23,24,25,27,29].
In this study, the hydrodistillation of B. multiflora leaves resulted in essential oil with a slightly yellowish hue and a pleasant aroma, showing a yield of 1.3% w/v. Table 1 presents the chemical composition of this essential oil. Gas chromatography/mass spectrometry (GC/MS) analysis of the chromatogram for the EO revealed the presence of 26 constituents, with the main constituents being the sesquiterpenes β-elemene (28.8%), α-selinene (9.8%), and β-selinene (8.6%). The chromatographic profile of an essential oil derived from the same plant species can vary due to various factors, such as abiotic and biotic influences. The chemical composition of essential oils may undergo changes due to environmental factors such as soil type, climate, and altitude [36,37]. These factors can impact the plant’s metabolism, leading to variations in the synthesis of secondary metabolites, including essential oils [36].

Table 1.
Chemical composition of the essential oils obtained from leaves of Bocageopsis multiflora.
In a comparative analysis between the essential oil obtained from B. multiflora leaves collected in Rondônia, Brazil, and the essential oil obtained in our study, significant quantitative and qualitative discrepancies were observed in the main constituents. Particularly, sesquiterpenes were found to be predominant in different essential oils derived from B. multiflora leaves. The essential oil obtained from B. multiflora leaves collected in the city of Porto Velho, State of Rondônia, Brazil, exhibited 1-epi-cubenol (16.6%) as the main sesquiterpene constituent [23]. On the other hand, a study conducted with the EOs from leaves collected in the state of Amazonas during two different climatic seasons showed variations in the sesquiterpene profile. The main constituent during the rainy season was bisabolene (13.2%), while during the dry season, it was spathulenol (16.2%) [27].
2.2. Larvicidal and Adulticidal Activity
The LC50 and LC90 values of B. multiflora EO against Ae. aegypti larvae after 24 and 48 h of application are listed in Table 2.

Table 2.
Larvicidal activity of Bocageopsis multiflora essential oil against Aedes aegypti.
Taking into account the mortality rate for each concentration, the median lethal concentration (LC50) obtained qualifies the essential oils as potential larvicidal agents. Then, the essential oils with an LC50 value below 100 μg/mL are deemed effective larvicidal agents, while those with an LC50 below 50 μg/mL are classified as highly active [38,39]. In this context, the essential oil from B. multiflora leaves demonstrated significant activity against Ae. aegypti larvae in the analyses at 24 and 48 h, with LC50 values of 40.8 μg/mL and 39.4 μg/mL, respectively. The observed activity could be attributed to the synergy among the constituents with higher content: β-selinene (8.6%), α-selinene (9.8%), and β-elemene (28.0%). According to literature data, β-selinene demonstrates an LC50 value of 136.0 μg/mL against Ae. aegypti larvae [40], which is higher than our findings. Moreover, β-elemene exhibits notably superior activity, evidenced by a LC50 of 11.15 μg/mL against Ae. albopictus larvae after 24 h exposure [40].
Many studies in the literature are devoted to investigating the effectiveness of essential oils in the ongoing search for new larvicides. For instance, recently, Luz and coworkers [41] conducted a review encompassing 337 essential oils from 225 plant species with bioactivity against Ae. aegypti larvae. For a better understanding of the connection between essential oils and their larvicidal effects, some studies examine the effectiveness of the main compounds in these oils and show out that the whole essential oil is more active than its main compounds [42]. These reports hold significance and can be correlated with the findings regarding the efficacy of B. multiflora essential oil as a larvicide against Ae. aegypti.
Another interesting approach of the present work was the results regarding the biological activity against adult females of Ae. aegypti that are described in Table 3.

Table 3.
Adulticidal activity of essential oils against Aedes aegypti females after 90 min of exposure.
The EO obtained from B. multiflora leaves exhibited excellent activity against adult Ae. aegypti, with an LC50 of 12.5 µg/mL. This result is noteworthy as it falls below the threshold indicated in the literature for highly active essential oils, i.e., those with LC50 < 50 µg/mL [38,39,43]. This outcome is quite promising and aligns with the ongoing search for plant-based insecticides. Indeed, a number of studies show interest in researching natural products as alternative strategies against insects that are important for public health [22,44,45]. The primary motivation for the interest in natural products, specifically essential oils, lies in their considerable advantages compared to synthetic insecticides. These include low toxicity to mammals, minimal environmental impact, diverse modes of action, and a low likelihood of inducing vector resistance due to the inherent complexity of essential oil composition [22,44]. However, despite their promising activities, establishing a complex mixture of essential oils as a viable insecticide is challenging, making it unlikely, from a commercial standpoint, that any single EO will be utilized for insect control without further research. Several factors must be considered, such as the availability of raw materials, the preparation and formulation of bioinsecticides with controlled chemical compositions, and the identification of active constituents before labeling an EO as a potential agent against mosquitoes [22,45]. Isman [22] recently discussed the development of a bioinsecticide meeting commercial demands, emphasizing the importance of defining procedures for formulating insecticidal products. Nanotechnology holds promise for enhancing the stability and bioavailability of essential oils in these formulations, ensuring the persistence of volatile compounds and facilitating delivery through insect skin. Despite challenges in biomass availability, particularly regarding plant species cultivation, emerging alternatives are being explored to address this issue. However, the selection of an EO or its bioactive components requires further investigation for resolution, given the inherent challenges associated with each option.
In this context, the scarcity of literature data regarding the biological activity of B. multiflora against adult Ae. aegypti underscores the importance of investigating the properties and effectiveness of this plant essential oil for vector control purposes. The results of our study demonstrate that the essential oil derived from this plant is effective in eliminating Ae. aegypti in the larval and adult stages. After obtaining interesting results from experimental analyses on the larvicidal and adulticidal activities of B. multiflora essential oil, a molecular docking analysis was conducted between key enzymes of Aedes species and the three main compounds of the essential oil.
2.3. Molecular Docking
Table 4 displays the findings from the molecular docking analysis of the three key Aedes species related proteins and the three main compounds, β-elemene, α-selinene, and β-selinene, present in the essential oil (EO) obtained from B. multiflora leaves.

Table 4.
Molecular docking scores of five major compounds of essential oil of Bocageopsis multiflora against mosquito proteins (PDB = 1PZ4; 6MFB; 6XYU).
Regarding the insecticidal capability of the compounds identified in the EO, the isomers α-selinene and β-selinene exhibited excellent interaction activities with the three molecular targets of mosquitoes. In other words, the likelihood of action on vital mosquito proteins was more comprehensive, as these sesquiterpenes demonstrated strong interactions with all three receptors. This indicates that these compounds may be responsible for the observed activity. In all three scenarios, these two sesquiterpenes are the ones that interact most with the amino acid residues of the respective proteins, with a predominance of hydrophobic interactions for the three target proteins (Figure 1). This suggests that incorporating these ligands as constituents of a bioinsecticide is likely to impact the vector through diverse mechanisms, leading to insecticidal effects.
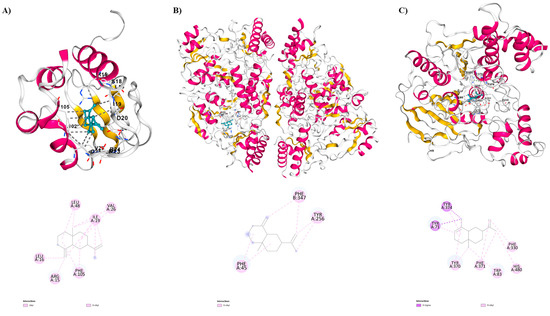
Figure 1.
Two-dimensional and three-dimensional intermolecular contact between EOs compounds: (A) β-selinene against Sterol Carrier Protein-2 (PDB = 1PZ4). (B) β-selinene against Aedes aegypti kynurenine aminotransferase (PDB = 6MFB). (C) α-selinene against Acetylcholinesterase (PDB = 6XYU).
When compared to insecticides such as temephos (−7.8 kcal/mol) and deltamethrin (−8.9 kcal/mol), as well as the other main compounds of the B. multiflora essential oil, β-selinene exhibited a greater binding affinity value (−9.1 kcal/mol) with the target protein Sterol Carrier Protein-2 (PDB = 1PZ4). In this context, the comparison among the sesquiterpenes shows that β-selinene exhibited interactions between the α-helix residues LEU102-PHE105, GLU103 and ARG15, as well as between the β-sheet residues LEU16-SER18, LEU48, ILE19-ASP20, and GLN25-VAL26. The observed interactions are van der Waals, alkyl, and π-alkyl. Additionally, the sites of the interactions observed with the amino acid residues of AeSCP-2 resemble the sites described for the sterol carrier protein inhibitor-1 (SCPI-1; Arg15, Gln25, Val26, Phe32, Leu64, Leu102, Gln103, Phe105) [46] thus providing partial justification for the high affinity observed for this sesquiterpene.
A significant part of the insecticidal capacity of the main compounds presents in the EO obtained from B. multiflora leaves is derived from the mechanism of action on essential molecular targets in the insect life cycle. These ligands can competitively inhibit the cholesterol binding site of SCP-2, occupying the same protein binding region as cholesterol and preventing this binding, blocking the cholesterol metabolic pathway in the insect organism [47].
β-selinene demonstrated a significant binding affinity with a value of −8.0 kcal/mol, surpassing all the main sesquiterpenes found in the EO, as well as when compared to the positive control temephos (−7.4 kcal/mol) in molecular docking studies with 3-hydroxykynurenine transaminase (PDB = 6MFB). Also, β-selinene showed interactions between the α-helix residues PHE45, HIS46, ASP47, in chain A, while in chain B interactions were observed between the α-helix residues ILE103, TRP328, TRP329, SER332, MET336, PHE347, GLY348, and MET351and between the β-sheet residues GLU342, GLN344, GLY345, ARG356. The observed interactions are π-alkyl.
The interactions between the ligand, β-selinene, and the HKT active sites, particularly involving the amino acid residues SER43, ASN44, and PHE45, hold significant importance. Notably, these sites exhibit close proximity to the active residue ARG356, and molecular docking studies have revealed an interaction with the ligand. This finding enables us to delve into a plausible biological mechanism, suggesting the inhibition of HKT enzyme activity. This result is supported by previous research conducted by Rossi and collaborators (2006) as well as by Chen and collaborators (2022) [48,49].
Nature consistently surprises us. The metabolic processes involving tryptophan necessitate mosquitoes to employ biochemical tools, enabling them to host microorganisms harmful to human health, such as Plasmodium falciparum, the causative agent of malaria. In this context, the tryptophan oxidation process produces metabolites that, when accumulated, inflict damage on the insect’s organism [50]. In response to this, the mosquito employs the enzyme 3-hydroxykynurenine transaminase (HKT) to convert 3-hydroxykynurenine (3-HK) into xanthurenic acid [51]. This process not only bolsters the mosquito’s defense, averting adverse effects such as paralysis or death caused by 3-HK [50], but also serves as an antioxidant during the digestion of a blood meal [52]. Another remarkable aspect of this metabolic coordination is the importance of 3-HK in the pigmentation of the mosquito’s eyes. The organism needs to control the quantity of this compound to avoid self-intoxication, underscoring the complexities involved in maintaining a delicate balance [48]. Furthermore, it has been noted that the gut microbiota within Anopheles stephensi contributes to heightened resistance against Plasmodium infection. This resistance is facilitated by the synthesis of the enzyme kynureninase, which leads to the production of 3-HK [48,53]. Considering these findings, the HKT enzyme emerges as a substantial target in the quest for new insecticidal agents against Aedes species.
Acetylcholinesterase (AChE) is a pivotal enzyme in the metabolism of various organisms, including mosquitoes. In insects, AChE’s primary function is to hydrolyze the neurotransmitter acetylcholine, leading to the cessation of neuronal excitation in the postsynaptic membrane, resulting in paralysis and death of the invertebrate [45]. Additionally, other researchers suggest that the neurotoxic mode of action, impacting ion transport and AChE, as well as the disruption of octopamine, a crucial neurotransmitter, neurohormone, and neuromodulator in invertebrate systems, also contribute to the observed effects [54]. Scientific literature reports several studies highlighting the biological activity of natural products, such as essential oils, on AChE [55]. Consequently, these findings may suggest potential pathways for the action of essential oil of B. multiflora on AChE.
In this research, the notable binding affinity exhibited by α-selinene (−9.7 kcal/mol) with the protein, approaching that of the deltamethrin (−10.9 kcal/mol), positions this sesquiterpene as a promising candidate for inclusion in a bioinsecticide targeting AChE. The α-selinene showed interactions between the α-helix residues TYR370-PHE371, and TYR374, and between the β-sheet residues TRP83, HIS480, TYR71, and PHE330. The interactions observed are Van der Waals, π-sigma, and π-alkyl.
The binding with the amino acid residues of AChE (PDB = 6XYU) involve a considerable number of active sites for this enzyme, as elucidated by Harel et al. (2000) [56]. This provides a basis for considering an alternative mechanism of action due to the compounds present in the essential oils (EOs) of B. multiflora.
2.4. Prediction of the ADMET Properties of the Main Substances of the EO of B. multiflora
The three main compounds of B. multiflora essential oil were also evaluated through in silico study to predict ADMET properties as listed in Table 5. In this context, all sesquiterpenes were deemed acceptable according to Lipinski’s rules, which establish that a drug-like compound must have a molecular weight (MW) ≤ 500 Da, a logarithm of the n-octanol/water partition coefficient (logP) ≤ 5, a number of hydrogen bond donors (HBD) ≤ 5, and a number of hydrogen bond acceptors (HBA) ≤ 10 [57].

Table 5.
In-silico ADMET profile of the selected ligands.
The plasma protein binding (PPB) of α-selinene exceeded 90%, albeit lower than that of the larvicide temephos and adulticide deltamethrin. This indicates that this sesquiterpene might possess a low therapeutic index as the duration of stay in the body might be low [58].
Concerning metabolism, most of the compounds evaluated inhibit the CYP2C19 and CYP2C9 isoenzymes. These two cytochrome P450 enzymes (CYPs) are estimated to be responsible for roughly 35–40% of drug oxidative metabolism and approximately 25% of overall xenobiotic biotransformation. Xenobiotics encompass a broad range of compounds derived from diverse sources, including environmental contaminants and industrial pollutants [59].
The evaluation of various toxicity parameters, such as AMES toxicity, carcinogenicity, reproductive effects, and hepatotoxicity, confirmed the safety of the main sesquiterpenes found in B. multiflora essential oil. When considering their potential use in products applied to the skin, such as repellent lotions, or bioinsecticides, it is crucial to assess skin sensitization and eye irritation. In this context, all sesquiterpenes assessed through in silico analysis showed skin sensitization values below 0.8, indicating that they are non-sensitizers. Conversely, the insecticides temephos (for larvae) and deltamethrin (for adults) exhibited values of 0.936 and 0.874, respectively, classifying them as sensitizers.
Synthetic insecticides not only have undesired effects on non-target organisms but also lead to the selection of resistant vector insects and pests [60,61], like Ae. aegypti and Ae. albopictus [62,63]. Moreover, these synthetic commercial products can be harmful to human health and the environment (contamination of water, air, and soil resources) due to intensive and prolonged application [64]. Therefore, it is crucial to find environmentally safe alternatives that are potentially more effective and suitable for use in Aedes control programs.
In our in silico study, the aquatic toxicity of the three main sesquiterpenes of EO were characterized by fathead minnow LC50 (96 h), Tetrahymena pyriformis IGC50 (48 h), and Daphnia magna EC50 (48 h). The LC50, IGC50, and LC50DM of α-selinene, β-selinene, and β-elemene are 4.1, 4.7, and 3.6 (μg/mL), 5.6, 6.7, and 5.4 (μg/mL), and 6.2, 6.0, and 6.6 (μg/mL), respectively. In general, when compared to results from other studies, all the sesquiterpenes exhibited low ecotoxicological profiles against the selected organisms [65].
3. Materials and Methods
3.1. Reagent and Chemicals
Temephos and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich Corporation (St. Louis, MO, USA). n-Alkane standard (C7–C30) was purchased from Supelco (Bellefonte, PA, USA). Distilled water was used for general procedures. Solvents (dichloromethane, dimethyl sulfoxide (DMSO), ethanol, and methanol) were of analytical and chromatographic grades (Columbus, OH, USA, TEDIA).
3.2. Plant Material and Extraction of Essential Oil
The leaves of Bocageopsis multiflora were gathered in May 2018 from the Adolpho Ducke Forest Reserve, Km 26 Manaus, Itacoatiara highway, in the State of Amazonas, Brazil. These leaves, weighing 200 g, were chopped and subjected to hydrodistillation for 3 h using a modified Clevenger-type apparatus. The obtained EO was transferred into amber glass flasks and stored in the freezer for future use. Yield was determined based on the weight of the leaves utilized.
3.3. Rearing of Mosquitoes
The insects, Aedes aegypti, were obtained from colonies established in the Laboratory of Malaria and Dengue of the National Institute for Amazonian Research (INPA, Manaus, Brazil). The colonies were maintained without exposure to insecticides, under controlled conditions at room temperature (26 ± 2 °C) and a relative humidity of 70–85%, with a photoperiod of 12:12 h (light/dark). The procedure was performed using the methodology described by Thanigaivel et al. [66]. The eggs of A. aegypti obtained from spawning colonies were placed in containers with water provided for the larvae to hatch. The larvae were raised in a plastic tray that contained distilled water and were fed with a mixture of cat food (Whiskas®) and bovine liver powder in a 1:1 ratio. The larvae were kept until they reached the third larval instar, and subsequently, a number were selected for the larvicidal bioassays. The remaining larvae were left in enameled basins until they reached pupation. The pupae were transferred to round plastic containers (50 mL), containing distilled water, and were placed in breeding cages (dimensions 30 cm × 30 cm × 30 cm) for the emergence of adults. The adults were fed with a 10% sucrose solution and the blood meal, according to the CEUA protocol 054/2018 of the INPA Ethics Committee on Animal Use. Blood-fed females, 2 to 5 days old, were used in the adulticidal bioassays.
3.4. Larvicidal Bioassay
Eggs and adults of Ae. aegypti of Rockefeller strain were acquired at the Insectarium of the Laboratory of Malaria and Dengue of the National Institute for Research in the Amazon (INPA). The eggs were immersed in deionized water for hatching. Fish feed (Whiskas®) was provided ad libitum and the larval water was replaced twice a week. After hatching, the larvae were monitored for 3 to 4 days until they reached the third and fourth stages of development to be used in the larvicidal assays. The mosquito colonies were maintained at 28 ± 2 °C and relative humidity of 80 ± 10% and provided with 10% sucrose solution. According to CEUA Protocol 054/2018 of the Ethics Committee for the Use of Animals, INPA, the animals were anesthetized for 10 min to feed the insects. The females were fed once a week with blood from a hamster to promote egg development. These eggs were collected on wet filter paper surfaces once a week and stored for incubation. The larvicidal assays were performed according to the susceptibility test [67]. Ten larvae at the end of the third to the beginning of the fourth instar were used for the tests. The EO was tested at five different concentrations (100, 75, 50, 25, and 10 μg/mL) diluted in 1 mL of a solution containing 1.0% (v/v) dimethyl sulfoxide (DMSO). All experiments were carried out in quintuplicate with positive control (Temephos® at 0.12 μg/mL), negative control (1.0% (v/v) DMSO in distilled water), and the five different concentrations. After 24 and 48 h, live and dead larvae (immobile individuals and/or those deposited on the bottom of the glass) were counted. Mortality data were corrected, if necessary, using Abbott’s (1925) formula [68].
3.5. Adulticidal Bioassay
The adulticidal bioassay was carried out according to the guidelines of Brogdon and Chan [69]. The essential oil was solubilized in acetone to obtain five concentrations (50, 20, 15, 10, and 7 μg/mL). Glass bottles of 295 mL capacity were used for the test and 1.0 mL of each concentration was added to the flask. Acetone and deltamethrin (0.60 μg/mL) were used as negative and positive controls, respectively. The contents of each bottle were gently agitated by turning to coat all sides of the bottle. Then the caps were removed and the bottles were continuously rolled on their sides until all the liquid disappeared. The bottles were left horizontally for 12 h. Using a mouth aspirator, 15 female mosquitoes were introduced into each test bottle, and the control. The number of alive and dead mosquitoes was registered after 90 min., with readings every 15 min. In the test, a mosquito is classified as alive if it is able to fly regardless of the number of legs still intact; and it is considered dead or knocked over if it is immobile, unable to fly, or stand in a coordinated manner. All bioassays were performed at 28 ± 2 °C and relative humidity of 80 ± 10%. The experiments were performed in three replications, together with the control. Mortality data were corrected, if necessary, using Abbott’s (1925) formula [68].
3.6. Essential Oil Analysis Using Gas Chromatography/Mass Spectrometry (GC/MS)
The identification of the constituents of the essential oil was performed by comparison of their retention indices and mass spectra against those reported in the literature [70] or those presented in the Wiley library (version 7.0) for the GC/MS equipment. The retention indices were calculated for all volatile constituents using n-alkane homologous series. GC/MS analyses were performed using a Shimadzu gas chromatograph interfaced with a HP 5973N Mass Selective Detector (ionization voltage 70 eV), equipped with a DB-5MS capillary column (30 m × 0.25 mm, film thickness 0.25 μm), using helium as carrier gas (1.0 mL min−1). The oven temperature was programmed from 50 to 290 °C at a rate of 3 °C min−1, then isothermal heating at 290 °C for 10 min, using He as the carrier gas (1.0 mL min−1). Injector and detector temperatures were 230 °C and 290 °C, respectively. Injection volume was 1.0 μL (2 mg of the sample in 1 mL of CH2Cl2), in splitless mode. Linear velocity (ū) was 14 cm s−1. MS interface temperature was 280 °C; mass range was 40–700 μ; scan speed was 150 μs−1; interval was 0.50 s (2 Hz).
3.7. Acquiring Protein Structures
The AeSCP-2 protein (PDB = 1PZ4), 3-hydroxykynurenine transaminase (HKT) (PDB = 6MFB), and the crystallographic structure of Drosophila melanogaster acetylcholinesterase (AChE) in complex with the tacrine derivative, 9-(3-iodobenzylamino)-1,2,3,4-tetrahydroacridine (PDB = 6XYU), were obtained from the database https://www.rcsb.org/, accessed on 15 January 2024. All structures were downloaded in PDB format.
3.8. Selection and Construction of 3D Ligand Structures
The three main compounds identified in the essential oil obtained from Bocageopsis multiflora were selected for molecular docking: α-selinene ((3R,4aR,8aR)-5,8a-dimethyl-3-prop-1-en-2-yl-2,3,4,4a,7,8-hexahydro-1H-naphthalene); β-selinene ((3R,4aS,8aR)-8a-methyl-5-methylidene-3-prop-1-en-2-yl-1,2,3,4,4a,6,7,8 octahydronaphthalene); β-Elemene ((1S,2S,4R)-1-ethenyl-1-methyl-2,4-bis(prop-1-en-2-yl)cyclohexane). Temephos ([4-(4-dimethoxyphosphinothioyloxyphenyl)sulfanylphenoxy]-dimethoxy-sulfanylidene-λ5-phosphane), and deltamethrin ((S)-cyano (3-phenoxyphenyl)methyl (1R,3R)-3-(2,2-dibromoethen-1-yl)-2,2-dimethylcyclopropane-1-carboxylate) were used for comparison purposes in the docking score.
The acquisition of isomeric smiles of the ligands was performed from the database: https://pubchem.ncbi.nlm.nih.gov/, accessed on 15 January 2024. Subsequently, the structures were drawn using the ChemDraw Professional 12.0 molecules software, and through the software extension, Chem3D 12.0, the conversion and visualization of the structures in 3D were performed. With the obtained three-dimensional structures of the ligands, calculations were carried out in MM2 for energy minimization. Once the 3D molecules with minimized energy were obtained, they were saved in mol2 format in a single folder for docking analysis.
3.9. Molecular Docking
Molecular docking simulations were conducted through blind docking procedures using the online server CB-Dock2 [71,72]. CB-Dock is a ligand-protein blind docking server that employs a cavity detection approach based on protein surface curvature to guide molecular docking with AutoDock Vina. The enhanced version, utilized in this study, CB-Dock2, integrates cavity detection, docking, and homology model fitting to increase accuracy in identifying the binding site and predicting the binding pose.
Analysis of Intermolecular Interactions and Figure Construction
The results of molecular docking, i.e., the generated poses of interactions between receptor and ligand, were assessed using the Discovery Studio Visualizer v21.1.0.20298 molecular visualization software.
3.10. Prediction of Properties of ADMET
ADMET filtering analysis of all ligands selected for docking was predicted using the ADMETlab 2.0 web server [https://admetmesh.scbdd.com/service/evaluation/index (accessed on 15 January 2024)] and a SwissADME® [http://www.swissadme.ch (accessed on 16 January 2024)]. The isomeric SMILES of the ligands downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/, accessed on 15 January 2024) were used to calculate the ADME/tox parameters in standard mode.
3.11. Statistical Analysis
The lethal concentration that kills 50% of larvae or adults (LC50) and the lethal concentration that kills 90% of larvae or adults (LC90) were determined by probit regression with 95% fiducial limits using the POLO PLUS® program (LeOra Software version 1.0, Berkeley, CA, USA) [73,74]. Significant differences were determined by analysis of variance (bidirectional ANOVA) followed by Tukey’s test (p < 0.01 and p < 0.05) using BioEstat 5.0 Windows software (Belém, PA, Brazil).
4. Conclusions
The study of the essential oil (EO) obtained from the leaves of Bocageopsis multiflora indicates the predominance of sesquiterpenes, which could be associated with the biological activities observed against the larvae and adults of Aedes aegypti. Molecular docking analysis revealed that the three sesquiterpenes, α-selinene, β-selinene and β-elemene. Specifically, α-selinene and β-selinene, exhibited optimal interaction activities with mosquito proteins, suggesting their potential role in insecticidal action. Notably, β-selinene showed higher binding affinity than conventional insecticides, indicating its promising use in bioinsecticide formulations. According to docking and ADMET results, the three main sesquiterpenes appear to be safe for use. This study emphasizes their safety compared to commercial insecticides, stressing the importance of environmentally friendly alternatives for effective Aedes control. Overall, the essential oil from B. multiflora shows promise as an eco-friendly option for managing Aedes aegypti populations, supported by its chemical composition, insecticidal effects, favorable ADMET properties, and minimal ecotoxicological impact. However, further research is needed to assess its practical integration into mosquito vector control programs.
Author Contributions
Conceptualization, A.A.d.O., A.C.F.A. and J.R.d.A.S.; methodology, A.A.d.O., J.D.d.C. and J.R.d.A.S.; software, A.A.d.O., J.D.d.C. and L.P.F.; formal analysis, A.A.d.O., J.D.d.C. and L.P.F.; investigation, A.A.d.O., J.D.d.C. and L.P.F.; data curation, A.A.d.O., J.D.d.C. and L.P.F.; supervision, J.R.d.A.S.; project administration, J.R.d.A.S. and A.C.F.A.; funding acquisition, J.R.d.A.S. and A.C.F.A.; resources, J.R.d.A.S. and A.C.F.A.; writing—original draft preparation, J.R.d.A.S. and A.C.F.A.; writing—review and editing, J.R.d.A.S. and A.C.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) (Edital N. 006/2019–Universal Amazonas Program); PROEP 440011/2022-1-CNPq; MCTI-CNPq 406238/2022-7. Universidade Federal do Amazonas (UFAM) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). FAPERJ E-26/211.083/19.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of Instituto Nacional de Pesquisa da Amazônia (INPA) (protocol code 054/2018, approved on 30 October 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors express their gratitude to Wanderli Pedro Tadei (in memoriam) for the invaluable contribution of the INPA malaria-dengue laboratory in conducting this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jobe, N.B.; Huijben, S.; Paaijmans, K.P. Non-target effects of chemical malaria vector control on other biological and mechanical infectious disease vectors. Lancet Planet. Health 2023, 7, e706–e717. [Google Scholar] [CrossRef] [PubMed]
- WHO. Vector-Borne Diseases. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 16 February 2024).
- Laporta, G.Z.; Potter, A.M.; Oliveira, J.F.A.; Bourke, B.P.; Pecor, D.B.; Linton, Y.M. Global Distribution of Aedes Aegypti and Aedes Albopictus in a Climate Change Scenario of Regional Rivalry. Insects 2023, 14, 49. [Google Scholar] [CrossRef]
- World Health Organization—WHO. Available online: https://www.who.int/publications/m/item/zika-epidemiology-update---february-2022 (accessed on 20 February 2024).
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Halani, S.; Tombindo, P.E.; O’reilly, R.; Miranda, R.N.; Erdman, L.K.; Whitehead, C.; Bielecki, J.M.; Ramsay, L.; Ximenes, R.; Boyle, J.; et al. Clinical manifestations and health outcomes associated with Zika virus infections in adults: A Systematic Review. PLoS Negl. Trop. Dis. 2021, 15, e0009516. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization—WHO. Available online: https://cdn.who.int/media/docs/default-source/documents/emergencies/zika/zika-epidemiology-update_february-2022_clean-version.pdf?sfvrsn=c4cec7b7_13&download=true (accessed on 20 February 2024).
- World Health Organization—WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/chikungunya (accessed on 20 February 2024).
- Epidemiological Alert: Chikungunya Increase in the Region of the Americas. Available online: https://www.paho.org/en/documents/epidemiological-alert-chikungunya-increase-region-americas#:~:text=In%202022%2C%20the%20number%20of,and%20deaths%20becoming%20more%20evident (accessed on 20 February 2024).
- Oneda, R.M.; Basso, S.R.; Frasson, L.R.; Mottecy, N.M.; Saraiva, L.; Bassani, C. Epidemiological profile of dengue in Brazil between the years 2014 and 2019. Rev. Assoc. Med. Bras. 2021, 67, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Alves, L. Brazil to Start Widespread Dengue Vaccinations. Lancet 2024, 403, 133. [Google Scholar] [CrossRef] [PubMed]
- Juache-Villagrana, A.E.; Pando-Robles, V.; Garcia-Luna, S.M.; Ponce-Garcia, G.; Fernandez-Salas, I.; Lopez-Monroy, B.; Rodriguez-Sanchez, I.P.; Flores, A.E. Assessing the impact of insecticide resistance on vector competence: A review. Insects 2022, 13, 377. [Google Scholar] [CrossRef]
- Rivero, A.; Vézilier, J.; Weill, M.; Read, A.F.; Gandon, S. Insecticide control of vector-borne diseases: When is insecticide resistance a problem? PLoS Pathog. 2010, 6, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Asgarian, T.S.; Vatandoost, H.; Hanafi-Bojd, A.A.; Nikpoor, F. Worldwide status of insecticide resistance of Aedes Aegypti and Ae. Albopictus, vectors of arboviruses of Chikungunya, Dengue, Zika and Yellow Fever. J. Arthropod Borne Dis. 2023, 17, 1–27. [Google Scholar] [CrossRef]
- Silva-Filha, M.H.N.L.; Romão, T.P.; Rezende, T.M.T.; Carvalho, K.D.S.; de Menezes, H.S.G.; Do Nascimento, N.A.; Soberón, M.; Bravo, A. Bacterial toxins active against mosquitoes: Mode of action and resistance. Toxins 2021, 13, 523. [Google Scholar] [CrossRef]
- Pitton, S.; Negri, A.; Pezzali, G.; Piazzoni, M.; Locarno, S.; Gabrieli, P.; Quadri, R.; Mastrantonio, V.; Urbanelli, S.; Porretta, D.; et al. MosChito Rafts as Effective and eco-friendly tool for the delivery of a Bacillus thuringiensis-based insecticide to Aedes albopictus Larvae. Sci. Rep. 2023, 13, 3041. [Google Scholar] [CrossRef] [PubMed]
- da Silva Sá, G.C.; Bezerra, P.V.V.; da Silva, M.F.A.; da Silva, L.B.; Barra, P.B.; de Fátima Freire de Melo Ximenes, M.; Uchôa, A.F. Arbovirus vectors insects: Are botanical insecticides an alternative for its management? J. Pest Sci. 2023, 96, 1–20. [Google Scholar] [CrossRef]
- Oliva, C.F.; Benedict, M.Q.; Matilda Collins, C.; Baldet, T.; Bellini, R.; Bossin, H.; Bouyer, J.; Corbel, V.; Facchinelli, L.; Fouque, F.; et al. Sterile Insect Technique (SIT) against Aedes Species Mosquitoes: A Roadmap and Good Practice Framework for Designing, Implementing and Evaluating Pilot Field Trials. Insects 2021, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- St. Leger, R.J. From the lab to the last mile: Deploying transgenic approaches against mosquitoes. Front. Trop. Dis. 2021, 2, 804066. [Google Scholar] [CrossRef]
- Dunan, L.; Malanga, T.; Benhamou, S.; Papaiconomou, N.; Desneux, N.; Lavoir, A.V.; Michel, T. Effects of essential oil-based formulation on biopesticide activity. Ind. Crops Prod. 2023, 202, 117006. [Google Scholar] [CrossRef]
- Vanegas-Estévez, T.; Duque, F.M.; Urbina, D.L.; Vesga, L.C.; Mendez-Sanchez, S.C.; Duque, J.E. Design and elucidation of an insecticide from natural compounds targeting mitochondrial proteins of Aedes Aegypti. Pestic. Biochem. Physiol. 2024, 198, 105721. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Bioinsecticides based on plant essential oils: A short overview. Z. Naturforschung C 2020, 75, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Bay, M.; Souza de Oliveira, J.V.; Sales Junior, P.A.; Fonseca Murta, S.M.; Rogério dos Santos, A.; dos Santos Bastos, I.; Puccinelli Orlandi, P.; Teixeira de Sousa Junior, P. In vitro trypanocidal and antibacterial activities of essential oils from four species of the family Annonaceae. Chem. Biodivers. 2019, 16, e1900359. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, L.M.; Menezes, L.R.A.; Rodrigues, A.C.B.C.; Dias, R.B.; Gurgel Rocha, C.A.; Soares, M.B.P.; Neto, A.F.S.; Nascimento, M.P.; Campos, A.F.; Silva, L.C.R.C.E.; et al. Antitumour activity of the microencapsulation of Annona vepretorum essential oil. Basic. Clin. Pharmacol. Toxicol. 2016, 118, 208–213. [Google Scholar] [CrossRef]
- de Lima Barros, A.; de Lima, E.J.S.P.; Faria, J.V.; Acho, L.R.D.; Lima, E.S.; Bezerra, D.P.; Soares, E.R.; de Lima, B.R.; Costa, E.V.; Pinheiro, M.L.B.; et al. Cytotoxicity and lipase inhibition of essential oils from Amazon Annonaceae species. Chemistry 2022, 4, 1208–1225. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2024. Available online: http://www.plantsoftheworldonline.org/ (accessed on 20 February 2024).
- Oliveira, E.S.C.; Amaral, A.C.F.; Lima, E.S.; Jefferson, J.R. Chemical composition and biological activities of Bocageopsis multiflora Essential Oil. J. Essent. Oil Res. 2014, 26, 161–165. [Google Scholar] [CrossRef]
- Soares, E.R.; da Silva, F.M.A.; de Almeida, R.A.; de Lima, B.R.; Koolen, H.H.F.; Lourenço, C.C.; Salvador, M.J.; Flach, A.; da Costa, L.A.M.A.; de Souza, A.Q.L.; et al. Chemical composition and antimicrobial evaluation of the essential oils of Bocageopsis Pleiosperma Maas. Nat. Prod. Res. 2015, 29, 1285–1288. [Google Scholar] [CrossRef]
- da Paz Lima, M.; De Lucena, J.M.V.M.; Alcântara, J.M.; Soares, P.I.L.; Marques, M.O.M. Essential oils from branches of Bocageopsis, Guatteria and Unonopsis species: Chemical composition and antibacterial activity. Concilium 2023, 23, 355–362. [Google Scholar] [CrossRef]
- Duque, J.E.; Urbina, D.L.; Vesga, L.C.; Ortiz-Rodríguez, L.A.; Vanegas, T.S.; Stashenko, E.E.; Mendez-Sanchez, S.C. Insecticidal activity of essential oils from American native plants against Aedes aegypti (Diptera: Culicidae): An introduction to their possible mechanism of action. Sci. Rep. 2023, 13, 2989. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, E.J.A.; Carvalho, F.C.; de Castro Oliveira, J.A.; Bertolucci, S.K.V.; Scotti, M.T.; Silveira, C.H.; Guedes, F.C.; Melo, J.O.F.; de Melo-Minardi, R.C.; de Lima, L.H.F. Elucidating the molecular mechanisms of essential oils’ insecticidal action using a novel cheminformatics protocol. Sci. Rep. 2023, 13, 4598. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, W.; Jian, R.; Ren, X.; Chen, X.; Hong, W.D.; Wu, M.; Cai, J.; Lao, C.; Xu, X.; et al. Larvicidal, acetylcholinesterase inhibitory activities of four essential oils and their constituents against Aedes Albopictus, and nanoemulsion preparation. J. Pest Sci. 2023, 96, 961–971. [Google Scholar] [CrossRef]
- Chaudhary, A.; Gupta, K.K. Potentials of plant-derived sterol carrier protein inhibitors in insect management. Acta Ecol. Sin. 2023, 43, 925–932. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Barros, R.P.C.; Teles, Y.C.F.; Oliveira, L.H.G.; Lima, J.B.; Scotti, M.T.; Nunes, F.C.; Conceição, A.S.; Vanderlei de Souza, M.D.F. Larvicidal compounds extracted from Helicteres velutina K. Schum (Sterculiaceae) evaluated against Aedes aegypti L. Molecules 2019, 24, 2315. [Google Scholar] [CrossRef]
- Fu, Q.; Inankur, B.; Yin, J.; Striker, R.; Lan, Q. Sterol Carrier Protein 2, a Critical host factor for dengue virus infection, alters the cholesterol distribution in mosquito Aag2 Cells. J. Med. Entomol 2015, 52, 1124–1134. [Google Scholar] [CrossRef]
- Németh-Zámboriné, É. Natural variability of essential oil components. In Handbook of Essential Oils, 2nd ed.; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press-Taylor and Francis Group LLC: Boca Raton, FL, USA, 2016. [Google Scholar]
- Medbouhi, A.; Benbelaïd, F.; Djabou, N.; Beaufay, C.; Bendahou, M.; Quetin-Leclercq, J.; Tintaru, A.; Costa, J.; Muselli, A. Essential Oil of Algerian Eryngium campestre: Chemical variability and evaluation of biological activities. Molecules 2019, 24, 2575. [Google Scholar] [CrossRef]
- Cheng, S.S.; Chang, H.T.; Chang, S.T.; Tsai, K.H.; Chen, W.J. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti Larvae. Bioresour. Technol. 2003, 89, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.N.; Moraes, D.F.C. Essential oils and their compounds as Aedes aegypti L. (Diptera: Culicidae) larvicides: Review. Parasitol. Res. 2014, 113, 565–592. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Benelli, G. α-Humulene and β-Elemene from Syzygium zeylanicum (Myrtaceae) essential oil: Highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus (Diptera: Culicidae). Parasitol. Res. 2016, 115, 2771–2778. [Google Scholar] [CrossRef] [PubMed]
- Luz, T.R.S.A.; de Mesquita, L.S.S.; do Amaral, F.M.M.; Coutinho, D.F. Essential oils and their chemical constituents against Aedes aegypti L. (Diptera: Culicidae) Larvae. Acta Trop 2020, 212, 105705. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Jian, R.; Xie, F.; Chen, B.; Zhang, K.; Li, D.; Chen, W.; Huang, C.; Zhang, Y.; Hu, L.; et al. Screening of larvicidal activity of 53 essential oils and their synergistic effect for the improvement of deltamethrin efficacy against Aedes albopictus. Ind. Crops Prod. 2020, 145, 112131. [Google Scholar] [CrossRef]
- Gomes, P.R.B.; Silva, A.L.S.; Pinheiro, H.A.; Carvalho, L.L.; Lima, H.S.; Silva, E.F.; Silva, R.P.; Louzeiro, C.H.; Oliveira, M.B.; Filho, V.E.M. Avaliação da atividade larvicida do óleo essencial do Zingiber officinale Roscoe (Gengibre) frente ao mosquito Aedes aegypti. Rev. Bras. Plantas Med. 2016, 18, 597–604. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? challenges and constraints. Trends Plant Sci 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Hematpoor, A.; Liew, S.Y.; Azirun, M.S.; Awang, K. Insecticidal activity and the mechanism of action of three phenylpropanoids isolated from the roots of Piper sarmentosum Roxb. Sci. Rep. 2017, 7, 12576. [Google Scholar] [CrossRef]
- Priya, D.D.; Surendra, T.V.; Shajahan, S.; Muthuraja, S.; Roopan, S.M. Design and sustainable production of natural carbon incorporated CuO/C nanocomposite using Cyperus rotundus biomass. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Perera, H.; Wijerathna, T. Sterol Carrier Protein Inhibition-Based control of mosquito vectors: Current knowledge and future perspectives. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 7240356. [Google Scholar] [CrossRef]
- Chen, H.; Bhowmick, B.; Tang, Y.; Lozano-Fernandez, J.; Han, Q. Biochemical evolution of a potent target of mosquito larvicide, 3-Hydroxykynurenine Transaminase. Molecules 2022, 27, 4929. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Garavaglia, S.; Battista Giovenzana, G.; Arcà, B.; Li, J.; Rizzi, M. Crystal structure of the Anopheles gambiae 3-hydroxykynurenine transaminase. Proc. Natl. Acad. Sci. USA 2006, 103, 5711–5716. [Google Scholar] [CrossRef] [PubMed]
- Maciel, L.G.; Ferraz, M.V.F.; Oliveira, A.A.; Lins, R.D.; dos Anjos, J.V.; Guido, R.V.C.; Soares, T.A. Inhibition of 3-Hydroxykynurenine Transaminase from Aedes aegypti and Anopheles gambiae: A mosquito-specific target to combat the transmission of arboviruses. ACS Bio. Med. Chem. Au 2023, 3, 211–222. [Google Scholar] [CrossRef]
- Han, Q.; Beerntsen, B.T.; Li, J. The tryptophan oxidation pathway in mosquitoes with emphasis on xanthurenic acid biosynthesis. J. Insect Physiol. 2007, 53, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.L.A.; Dias, F.; Nunes, R.D.; Pereira, L.O.; Santos, T.S.R.; Chiarini, L.B.; Ramos, T.D.; Silva-Mendes, B.J.; Perales, J.; Valente, R.H.; et al. The antioxidant role of xanthurenic acid in the Aedes aegypti midgut during digestion of a blood meal. PLoS ONE 2012, 7, e38349. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Peng, Y.; Song, X.; Wen, H.; An, Y.; Tang, H.; Wang, J. Anopheline mosquitoes are protected against parasite infection by tryptophan catabolism in gut microbiota. Nat. Microbiol. 2022, 7, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Rajeswary, M.; Govindarajan, M. Towards green oviposition deterrents? effectiveness of Syzygium lanceolatum (Myrtaceae) essential oil against six mosquito vectors and impact on four aquatic biological control agents. Environ. Sci. Pollut. Res. 2018, 25, 10218–10227. [Google Scholar] [CrossRef] [PubMed]
- França, L.P.; Amaral, A.C.F.; Ramos, A.D.S.; Ferreira, J.L.P.; Maria, A.C.B.; Oliveira, K.M.T.; Araujo, E.S.; Branches, A.D.S.; Silva, J.N.; Silva, N.G.; et al. Piper capitarianum essential oil: A promising insecticidal agent for the management of Aedes aegypti and Aedes albopictus. Environ. Sci. Pollut. Res. 2021, 28, 9760–9776. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Kryger, G.; Rosenberry, T.L.; Mallender, W.D.; Lewis, T.; Fletcher, R.J.; Guss, J.M.; Silman, I.; Sussman, J.L. Three-dimensional structures of Drosophila melanogaster acetylcholinesterase and of its complexes with two potent inhibitors. Protein Sci. 2000, 9, 1063–1072. [Google Scholar] [CrossRef]
- Protti, Í.F.; Rodrigues, D.R.; Fonseca, S.K.; Alves, R.J.; de Oliveira, R.B.; Maltarollo, V.G. Do drug-likeness rules apply to oral prodrugs? ChemMedChem 2021, 16, 1446–1456. [Google Scholar] [CrossRef]
- Qiu, F.; Dziegielewska, K.M.; Huang, Y.; Habgood, M.D.; Fitzpatrick, G.; Saunders, N.R. Developmental changes in the extent of drug binding to rat plasma proteins. Sci. Rep. 2023, 13, 1266. [Google Scholar] [CrossRef] [PubMed]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central role of cytochrome P450 in xenobiotic metabolism—A brief review on a fascinating enzyme family. J. Xenobiot 2021, 11, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Gress, B.E.; Zalom, F.G. Identification and risk assessment of Spinosad resistance in a California population of Drosophila suzukii. Pest Manag. Sci. 2019, 75, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.P.; Favero, S. Assessment of the insecticidal potential of Eucalyptus urograndis essential oil against Rhodnius neglectus Lent (Hemiptera: Reduviidae). Neotrop. Entomol. 2013, 42, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Demirak, M.Ş.Ş.; Canpolat, E. Plant-based bioinsecticides for mosquito control: Impact on insecticide resistance and disease transmission. Insects 2022, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Silvério, M.R.S.; Espindola, L.S.; Lopes, N.P.; Vieira, P.C. Plant natural products for the control of Aedes aegypti: The main vector of important arboviruses. Molecules 2020, 25, 3484. [Google Scholar] [CrossRef]
- Roiz, D.; Wilson, A.L.; Scott, T.W.; Fonseca, D.M.; Jourdain, F.; Müller, P.; Velayudhan, R.; Corbel, V. Integrated Aedes management for the control of Aedes-borne diseases. PLoS Negl. Trop Dis. 2018, 12, e0006845. [Google Scholar] [CrossRef]
- Okoli, B.J.; Ladan, Z.; Mtunzi, F.; Hosea, Y.C.; Vitex Negundo, L. Essential oil: Odorant binding protein efficiency using molecular docking approach and studies of the mosquito repellent. Insects 2021, 12, 1061. [Google Scholar] [CrossRef]
- Thanigaivel, A.; Senthil-Nathan, S.; Vasantha-Srinivasan, P.; Edwin, E.S.; Ponsankar, A.; Selin-Rani, S.; Pradeepa, V.; Chellappandian, M.; Kalaivani, K.; Abdel-Megeed, A.; et al. Chemicals isolated from Justicia adhatoda Linn reduce fitness of the mosquito, Aedes aegypti L. Arch. Insect Biochem. Physiol. 2017, 94, e21384. [Google Scholar] [CrossRef]
- WHO. Guidelines for Laboratory and Field Testing of Mosquito Larvicides. Available online: https://www.who.int/publications/i/item/WHO-CDS-WHOPES-GCDPP-2005.13 (accessed on 16 February 2024).
- Abbott, W.S. A Method of computing the effectiveness of an insecticide. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [CrossRef]
- Brogdon, W.; Chan, A. Guideline for Evaluating Insecticide Resistance in Vectors Using the CDC Bottle Bioassay (with Inserts 1 (2012) and 2 (2014); CDC Technical Report; CDC: Atlanta, Georgia, 2010. [Google Scholar]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 10-193263321. [Google Scholar]
- Yang, X.; Liu, Y.; Gan, J.; Xiao, Z.X.; Cao, Y. FitDock: Protein–Ligand Docking by Template Fitting. Brief Bioinform. 2022, 23, bbac087. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.X.; Cao, Y. CB-Dock2: Improved Protein-Ligand Blind Docking by Integrating Cavity Detection, Docking and Homologous Template Fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.L.; Jones, M.M.; Olguin, E.; Alberts, B. Bioassays with Arthropods, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781315373775. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: New York, NY, USA, 1971. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).