Abstract
1-(3-aryl)-3-(dimethylamino)prop-2-en-1-one (enaminones) derivatives and the diazonium salt of para-chloroaniline were used to synthesize several novel disperse azo dyes with high yield and the use of an environmentally friendly approach. At 100 and 130 °C, we dyed polyester fabrics using the new synthesized disperse dyes. At various temperatures, the dyed fabrics’ color intensity was assessed. The results we obtained showed that dyeing utilizing a high temperature method at 130 °C was enhanced than dyeing utilizing a low temperature method at 100 °C. Reusing dye baths once or twice was a way to achieve two goals at the same time. The first was obtaining a dyed product at no cost, and the second was a way to treat the wastewater of dyeing bath effluents and reuse it again. Good results were obtained for the fastness characteristics of polyester dyed with disperse dyes. When the disperse dyes were tested against certain types of microbes and cancer cells, they demonstrated good and encouraging findings for the potential to be used as antioxidants and antimicrobial agents.
1. Introduction
Since ancient times, natural dyes have played an important role in the lifestyle and beliefs of people and have gained their appreciation and respect. The dye industry has grown rapidly in general but especially in the last twenty years after the great demand for it has increased [1,2,3]. Organic substances called azo dyes account for more than half of the dyes now used. Since azo dyes have a variety of useful qualities, they are frequently used in industrial dyes [4,5,6]. Azo dyes are essential. They have many uses and are widely used in a variety of industries, including the food, medical, pharmaceutical, leather, and textile industries [7]. These dyes are also utilized in various processes, such as photosensitive dyes and thermal and laser printing. Polyester is a hydrophobic fiber. This leads to the use of disperse dyes in its aqueous dyeing, which can be carried out at high or moderate temperatures [8,9,10,11]. Where does the newness of life come from? The answer to this question is manufacturing processes because this is the main tool for the economic growth of nations because it plays a major role in the economic development of any country. The textile industry sector is of utmost importance, not only because it has many diverse job opportunities but because this industry is also able to create integration with other economic sectors. Therefore, the textile industry sector is considered the most expanded and widespread sector. Among the main raw materials on which the textile industry sector depends are synthetic dyes, especially azo dyes, synthetic fibers, especially polyester fibers, and treated water. Therefore, the synthesis of new azo dyes and the possibility of treating effluents is the solid foundation of this industry [12,13,14,15]. If we shed light on the liquid waste coming out of the textile industry, it comprises a large amount of color left over from dyeing and printing processes, suspended solids, dissolved solids, and salt. The effluent is more polluted due to the presence of dyes and chemicals in the printing, dyeing, and printing processes, which are not easily degraded using conventional processing methods. To remove pollutants from liquid waste in textiles, work has been carried out to develop different treatment techniques, such as chemical, biological, and physical treatment, or combining them; this requires a lot of money, effort, and time [16,17,18,19]. When auxiliary chemicals are used for more than one dyeing cycle or two dyeing cycles, in this case, the dye bath can be used again for dyeing once or more [8,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. This lowers the cost of dyeing, which in turn reduces the amount of wastewater and pollutants discharged. It is also a simple approach to the treatment of water used after the dyeing process. To our knowledge, no research has been conducted on the use of enaminone derivatives in the creation of disperse dyes, as can be seen from the literature survey [1,25,30,31], except for what we have published since 2015, but in 2017, Alassar et al. [39] created a variety of disperse dyes for dyeing polyester using enaminone derivatives. Since 2015, we have focused on creating an environmentally friendly and innovative program for synthesizing various disperse dyes based on enaminones, known for their exceptional brightness in deeper and darker shades. They also have a greater brightness than their aromatic or benzene counterparts. In this study, novel disperse dyes based on enaminone derivatives were synthesized via coupling the diazonium salt of para-chloroaniline with synthesized enaminones (Figure 1). These disperse dyes were utilized to dye substrate (polyester fabrics) via low temperature method at 100 °C or via high temperature method at 130 °C. We accomplished this by assessing dyeing efficiency using K/S values to determine the color intensity of the dyed polyester materials. These dyes were used to assess the polyester fabrics’ fastness characteristics.

Figure 1.
Chemical structure of the synthesized dyes.
The innovation of this study lies in the production of a number of new disperse dyes based on enaminones, which contain substituted groups that give or withdraw electrons, which give them different bright colors; then, we used these new dyes in dyeing polyester fabrics and finally investigated the possibility of these new synthetic dyes having activity against some bacteria, fungi, and cancers; this gives them added value.
2. Results and Discussions
2.1. Chemistry
We can define any substance that contains an enamine group in addition to a carbonyl group by the term 1-(3-aryl)-3-(dimethylamino)prop-2-en-1-ones (enaminones). It is worth noting that enaminones serve as excellent building blocks for larger organic molecules because they contain many functional groups. Enaminones can be used to create a wide range of organic molecules for industrial purposes such as the production of many different dyes. Despite the very large number of structures made from enaminones in many different chemical industries, enaminones themselves are not easily synthesized; a catalyst is often required. In order to overcome the activation energy, many chemical reactions require an organic solvent, which is often harmful, but the synthesis of the enaminones that we successfully prepared did not require solvents. The multiple functional groups in enaminones can interact through many different routes. Many compounds can pass through amine chemistry. Weak nucleophiles can undergo 1, 4-addition, the carbon–carbon double bond can be reduced by a wide range of different compounds, and strong nucleophiles can be added to a ketone. Complementing our strategy toward synthesizing new dyes, we synthesized new, biologically active disperse dyes without using any solvents as an environmentally safe approach. In this study, we present the synthesis of these new dyes based on enaminones 3a–3e.Compounds 3a–3e were successfully synthesized by condensing acetophenone, m-methoxyacetophenone, m-chloroacetophenone, m-bromoacetophenone, and m-nitroacetophenone 1a–e with Dimethylformamide dimethylacetal (DMFDMA) 2. Compounds 3a–3e were coupled with the diazonium salt of p-chloroaniline 4 to synthesize novel disperse dyes 5a–5e (Scheme 1, Table 1 and Figure 2).
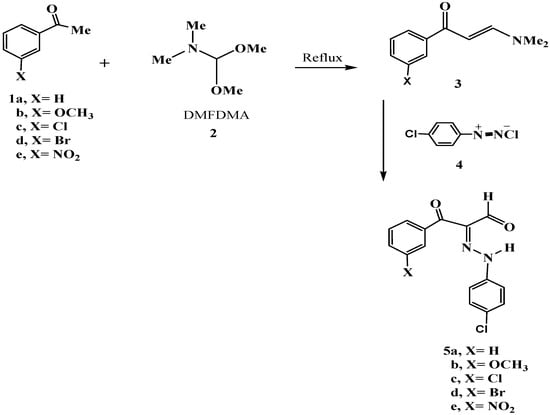
Scheme 1.
Synthesis of dyes 5a–e.

Table 1.
Characterization of the new dyes.
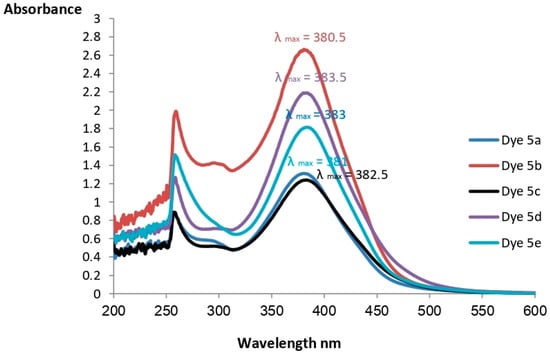
Figure 2.
Absorption spectrum in DMSO for the dyes.
Based on proton 1H NMR spectral data, the NH groups for dyes 5a–5e appear at δ 14.55, 14.67, 14.71, 14.70, and 14.83, respectively. CHO groups for dyes 5a–5e appear at δ 10.18, 10.16, 10.18, 10.17, and 10.23, respectively. Finally, aromatic protons for dyes 5a–5e appear at ~δ 7.12 to ~δ 8.96.
2.2. Dye Uptake
The major type of dye used in coloring polyester fabrics is disperse dyes, which are known to both the dye sciences and textile industries. By using a high temperature of about 130 °C or dyeing at 100 °C with a carrier present, the dyeing rate can be increased to a commercially excellent and consequently extremely acceptable standard. In order to increase dye absorption, speed up dyeing, and reduce dyeing temperature, dyeing polyester using a carrier has been extensively studied (Figure 3).

Figure 3.
Dyeing graph at 100 °C.
By making the polyester structure more swellable and less compressible, which makes it simpler for the chains to migrate partially and show improved dye absorption, dyeing polyester fabrics at a temperature of 130 °C causes a large rise in K/S values (Figure 4 and Table 2).
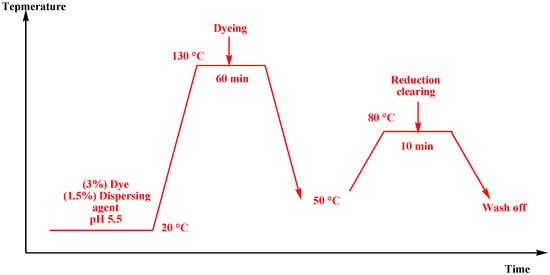
Figure 4.
Dyeing graph at 130 °C.

Table 2.
Color strengths at 130 °C.
The dissolution of the dye molecules and their solubility are further aided by the high temperature, which accelerates and increases the dye’s diffusion and penetrating ability.
According to Table 2’s color strength (K/S) results for the high-temperature dyeing polyesters, disperse dye 5a has a strong affinity for polyester fabrics and a K/S of 15.51. When the phenyl moiety of compound 5a was swapped out for an electron-withdrawing group like (C6H4Cl-m, C6H4Br-m, or C6H4NO2-m), the resulting compounds 5c, 5d, and 5e had lesser color strengths (K/S = 11.97, 14.31, and 12.14, respectively).
Similar results for the low-temperature dyeing polyesters are shown in Table 3, where the (K/S) values demonstrate that disperse dye 5a has an excellent affinity for polyester fabrics and a K/S of 14.62. When the phenyl moiety of compound 5a was swapped out for an electron-withdrawing group like (C6H4Cl-m, C6H4Br-m, or C6H4NO2-m), the resulting compounds 5c, 5d, and 5e had lesser color strengths (K/S = 12.75, 11.98, and 11.38, respectively).

Table 3.
K/S values of the dyed substrate at 100 °C.
The results in Table 2 and Table 3 clearly indicate that the color intensity K/S values of polyester fabrics dyed with the new disperse dyes at a temperature of 130 °C are better than the color intensity K/S values of the polyester fabrics dyed with the new disperse dyes at a temperature of 100 °C. One of the major water-intensive industries is the textile sector, particularly when it comes to wet processes.
2.3. Fastness Properties
Polyester fabrics were colored using the synthesized disperse dyes, 5a–e. At 130 °C (Table 4) or 100 °C (Table 5), the dyed polyester textiles performed well for rubbing, reasonably well for light fastness, and extremely well for washing and perspiration fastness. We found that the values of light fastness for polyester fabrics dyed with dyes 5a to 5e at 130 °C range from 3–4 to 4, and that the values of light fastness for polyester fabrics dyed with dyes 5a to 5e at 100 °C range from 4 to 4–5, which shows that the stability against the light of polyester fabric samples dyed at 100 °C is better than those dyed at 130 °C.

Table 4.
Fastness properties of the dyed substrate at 130 °C.

Table 5.
Fastness properties of the polyester fabrics dyed at 100 °C.
We found that the stability values of rubbing fastness for polyester fabrics dyed at 100 °C were lower than the stability values of rubbing fastness for polyester fabrics dyed at 130 °C, especially for dyes 5b to 5d.
We found that the values of washing fastness and perspiration fastness for polyester fabrics dyed with dyes 5a to 5e at a temperature of 130 °C were the same as the values of washing fastness and perspiration fastness for polyester fabrics dyed with dyes 5a to 5e at a temperature of 100 °C.
2.4. Dyebath Reuse
Numerous environmental contaminants are also produced by this sector. We employed a straightforward technique to purify the water used after the dyeing process by recycling the dyeing bath once or twice more for dyeing, which was an inventive step to eliminate contaminants from liquid waste in textiles. As a result, dyeing was much less expensive, which lowered the quantity of wastewater and other pollutants generated that are released into the environment and endanger the ecosystem.
When polyester fabrics were dyed with the prepared disperse dyes at 130 or 100 °C, it was found that the dye effluents contained a significant amount of dye that was harmful to the environment. Therefore, in order to make the most of the dye that was used while preventing the disposal of any colored waste that had a detrimental influence on the environment, our strategy was to employ the dye effluent waste in dyeing new polyester fabrics. Based on the information in Table 6, we can see that for all disperse dyes, the color strength K/S value of the dye bath reuse procedure varied by ~6% and ~1.5% during the first and second re-dyeing processes at 130 °C from its initial value.

Table 6.
K/S values of the dyed substrate at 130 °C.
Based on the information in Table 7, we can see that for all disperse dyes, the color strength K/S value of the dye bath reuse procedure varied by~30% and ~5% during the first and second re-dyeing processes at 100 °C from its initial value.

Table 7.
K/S values of the dyed substrate at 100 °C.
Polyester fabrics were colored using the synthesized disperse dyes, 5a–e. At 130 °C (Table 8) or 100 °C (Table 9), we found that the stability values of rubbing fastness for polyester fabrics dyed at 130 °C were lower than the stability values of rubbing fastness for polyester fabrics dyed at 100 °C, especially for dyes 5b and 5c.

Table 8.
Fastness properties of the dyed substrate at 130 °C.

Table 9.
Fastness properties of the dyed substrate at 100 °C.
We found that the values of perspiration fastness and washing fastness for polyester fabrics dyed with dyes 5a to 5e at a temperature of 130 °C were the same as the values of perspiration fastness and washing fastness for polyester fabrics dyed with dyes 5a to 5e at a temperature of 100 °C.
2.5. Antimicrobial Activity
At least three microorganisms are physiologically active against each of the research dyes, as shown in Table 10 and Figure 5. Dyes 5a and 5d showed excellent microbial activity against Candida albicans, Escherichia coli, and Staphylococcus aureus, whereas dyes 5b and 5e showed very good microbial activity against Candida albicans, Escherichia coli, and Staphylococcus aureus, while dye 5c showed good microbial activity against Candida albicans, Escherichia coli, and Staphylococcus aureus. On the contrary, dyes 5a to 5e did not show any activity against Aspergillus niger.

Table 10.
The antimicrobial activity of dyes 5a–e against the different test microbes.
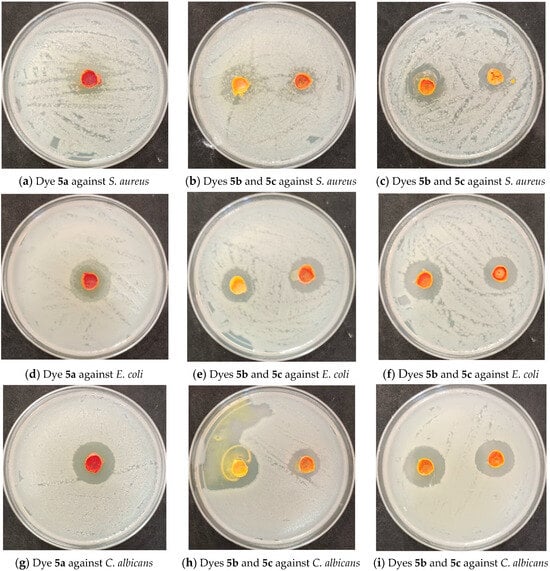
Figure 5.
The images display the disperse dyes’ zone of inhibition.
2.6. In Vitro Cytotoxicity Evaluation
The newly created disperse dyes 5a–e were tested for their ability to inhibit the growth of four human cell lines: HepG-2 (for hepatocellular carcinoma), HCT-116 (for colon carcinoma), MCF-7 (for breast cancer), and A-549 (for lung cancer). The IC50 (g/mL) values—the concentrations needed to prevent 50% of the growth of the culture when the cells are exposed to the tested disperse dyes for 48 h—were calculated using various concentrations of the four disperse dyes. The mean IC50 was used to calculate the cytotoxic activity in three different experiments. The data are provided in Table 11 and Figure 6, Figure 7, Figure 8 and Figure 9 which reveal that compounds 5a and 5c had the highest cytotoxic activity against the HePG-2, HCT-116, MCF-7, and A-549 cells when compared to cisplatin as a reference drug.

Table 11.
Antitumor activities of the dyes 5a–e.
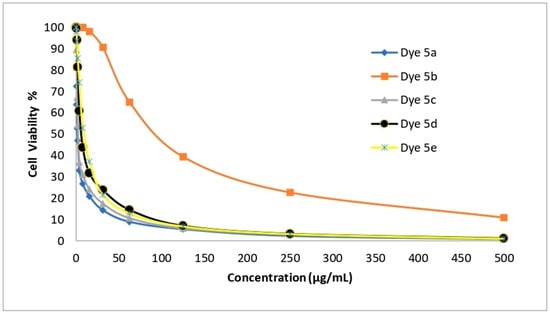
Figure 6.
The ability of disperse dyes 5a–e to inhibit the growth of HepG-2 cells.
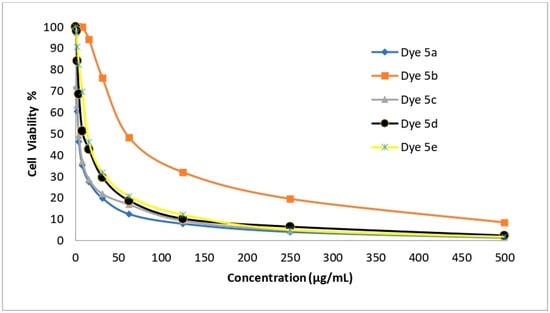
Figure 7.
The ability of disperse dyes 5a–e to inhibit the growth of HCT-116 cells.
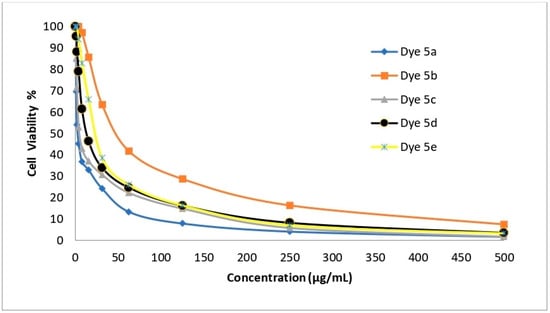
Figure 8.
The ability of disperse dyes 5a–e to inhibit the growth of A-549 cells.
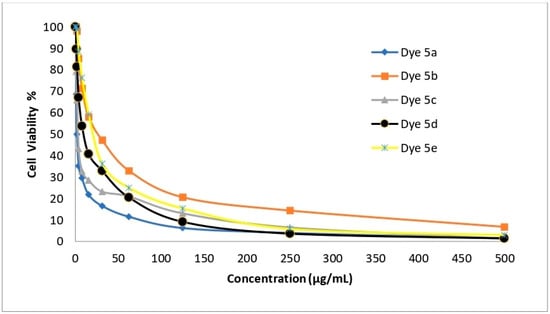
Figure 9.
The ability of disperse dyes 5a–e to inhibit the growth of MCF-7 cells.
Table 11 and Figure 6, Figure 7, Figure 8 and Figure 9 demonstrate the highly substantial activity of disperse dye 5a, with IC50 values in HePG-2, A-549, HCT-116, and MCF-7 cells of 1.48, 2.91, 3.48, and 1.99 g/mL, respectively. Compounds 5c and 5d were created by substituting the phenyl moiety in compound 5a with electron-withdrawing groups like C6H4Cl-m and C6H4Br-m, and their respective IC50 values were 2.48, 5.03, 3.89, and 3.39 and 6.38, 13.67, 8.92, and 10.03 g/mL.
With an IC50 similar to cisplatin, this modification successfully generated potent anticancer activity against the four cell lines. Interestingly, compound 5a had an IC50 value of 1.48, 2.91, and 1.99 g/mL in each case vs. 3.69, 7.49, and 5.68 g/mL for cisplatin and was more effective at killing HePG-2, A-549 and MCF-7 cells. Also, compound 5c had an IC50 value of 2.48, 5.03, and 3.39 g/mL in each case vs. 3.69, 7.49, and 5.68 g/mL for cisplatin and was more effective at killing HePG-2, A-549, and MCF-7 cells.
Obstinately, when the phenyl moiety of compound 5a was substituted by an electron-withdrawing group such as (C6H4NO2-m) (IC50 = 9.23, 24.60, 14.28, and 21.76) in HePG-2, A-549, HCT-116, and MCF-7 cells, the anticancer activity of dye 5e was reduced.
2.7. In Vitro Cytotoxic Activity toward Normal Human WI-38
Disperse dyes 5a–5e were tested for their cytotoxic effect against the normal human lung cell line (WI-38) in order to determine whether the synthesized dyes may be safe for use on normal cells. IC50 values were used to express the results (see Table 12 and Figure 10). The cytotoxic impact of disperse dyes 5a, 5b, 5c, 5d, and 5e on WI-38 cells was found to have a non-significant cytotoxic impact on (WI-38), with CC50 values of 21.23, 241.94, 55.60, 59.82, and 56.91, respectively. Badisa et al. [35] reported that a chemical exhibits specific toxicity to cancer cells when its selective index value is more than two. Conversely, a substance with a selective index value less than two is thought to be generally hazardous, meaning it can also occasion cytotoxicity on normal cells. As anticancer agents, disperse dyes 5a, 5b, 5c, 5d, and 5e demonstrated an excellent selectivity index (WI-38/HepG-2, WI-38/A-549, WI-38/HCT-116, and WI-38/MCF-7) range of 2.3 to 22.4, which contributed to their good safety profile.

Table 12.
In vitro cytotoxic activity against normal cell lines (WI-38) and selectivity index (the ratio between the IC50 value of normal cells and the IC50 value of cancer cells) for the newly synthesized dyes.
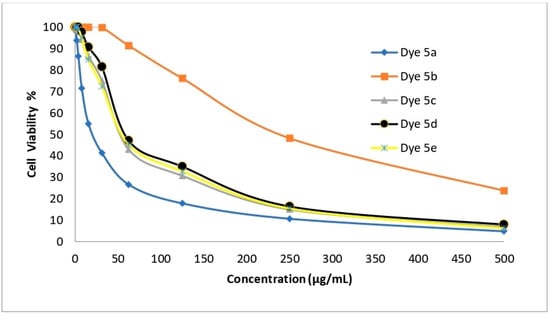
Figure 10.
WI-38 human lung fibroblast normal cells.
2.8. Antioxidant Activity
The ability of the four disperse dyes to scavenge free radicals as well as their antioxidant activity was evaluated in vitro (DPPH). The antioxidant activity of the dyes was measured using the IC50 value, which is the dose required to reduce the formation of DPPH radicals by 50%. According to the data in Table 13, disperse dye 5c has poor antioxidant activity (IC50 values of 195.75 when compared to ascorbic acid, which has antioxidant activity (IC50 value of 10.62; see Figure 11).

Table 13.
Antioxidant activities of dyes 5a–e.
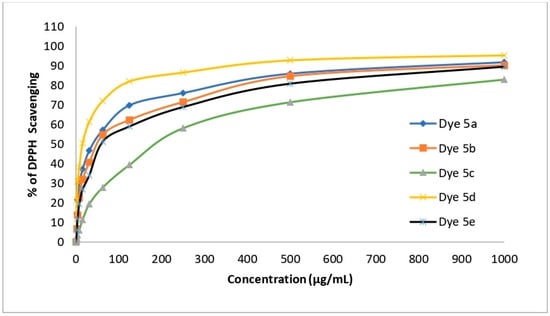
Figure 11.
Antioxidant activities of disperse dyes 5a–e.
Contrarily, dye 5d displays extremely potent antioxidant activity, with an IC50 of 15.33.
Dye 5a has very good antioxidant activity, with an IC50 of 40.99. Disperse dyes 5b and 5e have good antioxidant activity (IC50 values of 51.87 and 60.18, respectively).
3. Materials and Methods
The NMR spectra were analyzed at 300 MHz using a Mercury-300BB spectrometer (Palo Alto, CA, USA). By comparing material fluctuations to an internal reference of tetramethylsilane (TMS), the ppm findings were recorded at the Faculty of science, Cairo University. The Fourier-transforminfrared (FT-IR) spectra were controlled using a JASCO FT/IR4700 spectrophotometer (Tokyo, Japan). The elemental analyses (C, H, and N) were completed using a PerkinElmer 2400 analyzer (Palo Alto, CA, USA). The solvents used in this exploratory inquiry for both the synthesis methods and the spectroscopic estimations were given by Flukaand Aldrich (Cairo, Egypt).
3.1. Preparation of 1-(3-Aryl)-3-(dimethylamino)prop-2-en-1-ones 3a–e
Following the guidelines provided in our previous work [1], compounds 3a–e were produced. DMFDMA (1.19 g, 0.01 mol) was refluxed for ten hours with acetophenone, m-methoxyacetophenone, m-chloroacetophenone, m-bromoacetophenone, and m-nitroacetophenone. Thin-layer chromatography (TLC) was used to assess how successfully the reactions had taken place. Petroleum ether was employed to treat the reaction mixture once it had cooled to room temperature. The solid product that had been produced was filtered and re-crystallized.
3.2. Synthesis of Dyes
A cold solution of the diazonium salt 4 [10 mmol] was added to a cold solution of 1-(3-aryl)-3-(dimethylamino)prop-2-en-1-ones 3a–e in ethanol that contained sodium sulfate by adding a cold solution of sodium nitrite (0.69 g) in water to a cold solution of p-chloroaniline (10 mmol) in concentrated HCl acid at a temperature of 0 to 5 °C. For one hour, the mixture was stirred at a temperature of 5 °C. The generated solid precipitate was filtered out and crystallized into orange crystals using the proper solvents as listed in Table 1.
3.3. Dyeing Procedure
The suitable amount of dye(3% shades) was dissolved in a few drops of dimethylformamide (DMF) to create a dispersion of disperse dyes, which was then added dropwise via stirring to the dye bath (liquor ration: 1:30) containing (1.5%) of dispersing agent and (1%) of an anionic eco-friendly carrier in the case of dyeing at 100 °C or with (1.5%) of dispersing agent in case of dyeing at 130 °C using an infrared dyeing machine (Starlet 3). The polyester textiles were added when the pH of the dye bath was changed to 5.5. At a rate of 3 °C/min, the dye bath had to be heated to 100 °C and held there for 60 min. The dyed polyester materials were first reduced (1 g/L sodium hydroxide and 1 g/L sodium hydrosulfite, 10 min, 80 °C) and then cleaned after being chilled to 50 °C. After rinsing with cold water, the samples were air-dried.
3.4. Fastness Properties Tests
According to the American Association of Textile Chemists and Colorists, the blue wool scale (grades 1–8) and grey scale (grades 1–5) were used to evaluate the dyed samples’ fastness properties under various conditions, including rubbing, washing, perspiration, and light fastness [1,25].
3.4.1. Light Fastness
The test is run continuously for 35 h. It may be deduced that the type of fabric into which the dye is put ultimately causes the color of the colored fabric to intensify with increasing dye concentration. This is because different chemical groups present in diverse fabrics can significantly affect a dye’s light resistance index when used on a particular fabric. According to the wavelength dispersion of the light flowing in, not all absorptions start bleaching equally. The rate at which some colorants fade can be significantly influenced by the level of moisture as well as the nature of the environment. The color variations of the examined materials were noted using the blue scale (1–8).
3.4.2. Washing Fastness
To assess washing fastness, the ISO 105-C02 procedure from 1989 was employed [10]. The test pieces were sandwiched between a pair of samples of both wool and cotton fabric, which were then immersed in a solution made up of water with a non-ionic detergent concentration of 5 g/L (1:50) for half an hour at 60 °C. The samples were removed after a predetermined period of time, cleaned twice with sporadic hand pressure, and then dried, with assessments of washing fastness taking place.
3.5. Color Strength
The Kubelka–Munk equation was used to calculate the intensity of each color.
where K represents the absorption coefficient, S represents the scattering coefficient, R represents the decimal percentage of the dyed cloth’s reflectance, and R0 represents the decimal fraction of the non-dyed fabric’s reflectance.
The International Committee on Illumination (CIE) developed the CIELAB (colorspace) psychometric coordinates in 1976. (L*) stands for lightness, while the hue of the dye is expressed as (h*) values and (C*) for chroma. The colorhues of the dyed example were approximated using the CIELAB psychometric coordinates (a*) and (b*), where (a*) denotes the red–green axis and (b*) denotes the yellow–blue axis
3.6. Evaluation of the Biological Activities of the Disperse Dyes
The antimicrobial, anticancer, and antioxidant properties of the synthesized dyes were evaluated according to what we recently published [1,9,28].
4. Conclusions
This study successfully presents the effective synthesis of novel disperse dyes with high productivity through the reaction of acetophenone derivatives with DMFDMA and then coupling with the diazonium salt of p-chloroaniline. These new dyes were used for dyeing polyester fabrics at different temperatures, producing colors ranging from pale orange to orange. A simple, effective method as one of the sustainable treatment methods for water emerging from dyeing baths and the possibility of using it again was investigated. The results showed that dyeing polyester fabrics at high temperatures is environmentally friendly and superior to dyeing at low temperatures, based on the color intensity of the dyed fabrics. The fastness properties of dyed fabrics gave excellent results for their fastness to washing, perspiration, and rubbing and fair results for their light fastness. The outcomes also demonstrated that these new disperse dyes are active against a variety of bacteria and cancer cells. Additionally, the normal cell line (lung WI-38) was used to evaluate the cytotoxic effects of the synthesized dyes, and the dyes showed a satisfactory safety profile as anticancer agents. The outcomes demonstrate the potential of these disperse as antioxidants, antibacterial, and anticancer agents. The above clarification reveals that we have presented comprehensive work not only in preparing a series of new disperse dyes and the possibility of using them in dyeing polyester fabrics but also in demonstrating the added value of these dyes due to their activity against some bacteria and cancers. Also, we present a simple approach that is effective as one of the sustainable wastewater treatment approaches, whereby it can treat the wastewater emerging from the dyeing process and achieve two goals at the same time, the first of which is obtaining no-cost dyed polyester fabrics and the second is the success of the wastewater treatment process and the possibility of using the treated wastewater again in dyeing wet processes, which has a positive impact on the environment if it is thrown into waterways without further treatment processing. To obtain novel effective disperse dyes for future development, compounds 3a to 3e require further structural enhancement by reacting dimethylformamide dimethyl acetal with o-substituted acetophenone/or ketones. In addition, the synthesis of further derivatives without using solvents in an environmentally safe manner is required.
Author Contributions
Methodology, M.A.E.-A.; software, K.M.A.A.; validation, M.A.E.-A.; investigation, K.M.A.A.; resources, K.M.A.A.; data curation, M.A.E.-A.; writing—original draft preparation, K.M.A.A. and M.A.E.-A.; writing—review and editing K.M.A.A. and M.A.E.-A.; supervision, A.A.F.W. and M.A.E.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets used and analyzed supporting the conclusions of this article are available upon request; further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors sincerely appreciate Benha University and the National Research Centre for the facilities and materials they provided for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abdelmoteleb, K.M.A.; El-Apasery, M.A.; Wasfy, A.A.F.; Ahmed, S.M. Synthesis of New Monoazo Disperse Dyes for Dyeing Polyester Fabric Using Two Different Dyeing Methods: Demonstration of Their Antibacterial and Anticancer Activities. Polymers 2023, 15, 3052. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadi, N.A.; Ibrahim, M.R.; Abdelhamid, I.A.; Elnagdi, M.H. Arylhydrazonals as the aldehyde component in Baylis–Hillman reactions. Tetrahedron 2008, 64, 8202–8205. [Google Scholar] [CrossRef]
- A Al-Awadi, N.; Elnagdi, M.H.; A Ibrahim, Y.; Kaul, K.; Kumar, A. Efficient synthesis of 3-aroylcinnolines from aryl methyl ketones. Tetrahedron 2001, 57, 1609–1614. [Google Scholar] [CrossRef]
- Abdelrahman, M.A.; Salama, I.; Gomaa, M.S.; Elaasser, M.M.; Abdel-Aziz, M.M.; Soliman, D.H. Design, synthesis and 2D QSAR study of novel pyridine and quinolone hydrazone derivatives as potential antimicrobial and antitubercular agents. Eur. J. Med. Chem. 2017, 138, 698–714. [Google Scholar] [CrossRef]
- Kascheres, C.M. The chemistry of enaminones and small rings: Our contribution. J. Braz. Chem. Soc. 2003, 14, 945–969. [Google Scholar] [CrossRef]
- Pieczonka, A.M.; Strzelczyk, A.; Sadowska, B.; Mlostoń, G.; Stączek, P. Synthesis and evaluation of antimicrobial activity of hydrazones derived from 3-oxido-1H-imidazole-4-carbohydrazides. Eur. J. Med. Chem. 2013, 64, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Shawali, A.S. Synthesis, Reactions and Antitumour Screening of new Enaminones. J. Chem. Res. 2010, 34, 630–634. [Google Scholar] [CrossRef]
- Šimůnek, P.; Macháček, V. The structure and tautomerism of azo coupled β-Enaminones. Dye. Pigment. 2010, 86, 197–205. [Google Scholar] [CrossRef]
- Al-Etaibi, A.M.; El-Apasery, M.A. Nano TiO2 Imparting Multifunctional Performance on Dyed Polyester Fabrics with some Disperse Dyes Using High Temperature Dyeing as an Environmentally Benign Method. Int. J. Environ. Res. Public Health 2020, 17, 1377. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Narasimhan, B. Hydrazides/Hydrazones as Antimicrobial and Anticancer Agents in the New Millennium. Mini-Rev. Med. Chem. 2013, 13, 971–987. [Google Scholar] [CrossRef]
- De Moraes, T.A.P.; Filha, M.J.S.; Camara, C.A.; Silva, T.M.S.; Soares, B.M.; Bomfim, I.S.; Pessoa, C.; Ximenes, G.C.; Silva, V.A. Synthesis and Cytotoxic Evaluation of a Series of 2-Amino-Naphthoquinones against Human Cancer Cells. Molecules 2014, 19, 13188–13199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xue, M.; Huang, K.; Liu, Z. Textile dyeing wastewater treatment. Adv. Treat. Text. Effl. 2011, 5, 91–116. [Google Scholar]
- Bhatt, P.; Rani, A. Textile dyeing and printing industry: An environmental hazard. Asian Dye. 2013, 10, 51–54. [Google Scholar]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Rodrigues, C.S.; Madeira, L.M.; Boaventura, R.A. Synthetic textile dyeing wastewater treatment by integration of advanced oxidation and biological processes—Performance analysis with costs reduction. J. Environ. Chem. Eng. 2014, 2, 1027–1039. [Google Scholar] [CrossRef]
- Vergili, I.; Kaya, Y.; Sen, U.; Gönder, Z.B.; Aydiner, C. Techno-economic analysis of textile dye bath wastewater treatment by integrated membrane processes under the zero liquid discharge approach. Resour. Conserv. Recycl. 2012, 58, 25–35. [Google Scholar] [CrossRef]
- Starling, M.C.V.M.; Castro, L.A.S.; Marcelino, R.B.P.; Leão, M.M.D.; Amorim, C.C. Optimized treatment conditions for textile wastewater reuse using photocatalytic processes under UV and visible light sources. Environ. Sci. Pollut. Res. 2016, 24, 6222–6232. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Sangal, V.K.; Kushwaha, J.P. Parametric study of electro-fenton treatment for real textile wastewater, disposal study and its cost analysis. Int. J. Environ. Sci. Technol. 2019, 16, 801–810. [Google Scholar] [CrossRef]
- Kobya, M.; Gengec, E.; Demirbas, E. Operating parameters and costs assessments of a real dye house wastewater effluent treated by a continuous electrocoagulation process. Chem. Eng. Process. Process Intensif. 2016, 101, 87–100. [Google Scholar] [CrossRef]
- Metwally, K.A.; Abdel-Aziz, L.M.; Lashine, E.-S.M.; Husseiny, M.I.; Badawy, R.H. Hydrazones of 2-aryl-quinoline-4-carboxylic acid hydrazides: Synthesis and preliminary evaluation as antimicrobial agents. Bioorganic Med. Chem. 2006, 14, 8675–8682. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.U.; Adeel, S.; Saif, M.J.; Khosa, M.K.; Anjum, M.N.; Kamran, M.; Zuber, M.; Asif, M. Ultrasonic Assisted Improvement in Dyeing Behaviour of Polyester Fabric Using Disperse Red 343. Pol. J. Environ. Stud. 2019, 29, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Lozada, M.C.; Soria-Arteche, O.; Apan, M.T.R.; Nieto-Camacho, A.; Enríquez, R.G.; Izquierdo, T.; Jiménez-Corona, A. Synthesis, cytotoxic and antioxidant evaluations of amino derivatives from perezone. Bioorganic Med. Chem. 2012, 20, 5077–5084. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Madhu, K. Synthesis and in vitro and in vivo antimycobacterial activity of isonicotinoyl hydrazones. Bioorganic Med. Chem. Lett. 2005, 15, 4502–4505. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, G.D.; Muñoz, A.N.; Sturm, G.S.; Stankiewicz, A. A helicopter view of microwave application to chemical processes: Reactions, separations, and equipment concepts. Rev. Chem. Eng. 2014, 30, 233–259. [Google Scholar] [CrossRef]
- El-Apasery, M.A.; Abdellatif, M.E.A.; Yassin, F.A.; Ahmed, S.M. Synthesis of novel disperse dyes based on arylazophenols: Synthesis, Characterizations and applications. Bull. Chem. Soc. Ethiop. 2023, 37, 993–1002. [Google Scholar] [CrossRef]
- Iskender, M.A.; Becerir, B.; Koruyucu, A. Carrier Dyeing of Different Energy Level Disperse Dyes on Polyester Fabric. Text. Res. J. 2005, 75, 462–465. [Google Scholar] [CrossRef]
- Alnajjar, A.; Abdelkhalik, M.M.; Al-Enezi, A.; Elnagdi, M.H. Enaminones as building blocks in heterocyclic syntheses: Reinves-tigating the product structures of enaminones with malononitrile. A novel route to 6-substituted-3-oxo-2,3- dihydro-pyridazine-4-carboxylic acids. Molecules 2009, 14, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Al-Etaibi, A.M.; El-Apasery, M.A. Can Novel Synthetic Disperse Dyes for Polyester Fabric Dyeing Provide Added Value? Polymers 2023, 15, 1845. [Google Scholar] [CrossRef]
- Delgado, V.; Ibacache, A.; Theoduloz, C.; Valderrama, J.A. Synthesis and in Vitro Cytotoxic Evaluation of Aminoquinones Structurally Related to Marine Isoquinolinequinones. Molecules 2012, 17, 7042–7056. [Google Scholar] [CrossRef]
- El-Apasery, M.A.; Abdellatif, M.; Yassin, F.; Ahmed, S.M. Synthesis of Novel Disperse Dyes based on Arylazophenols: Part 2. Anticancer Activities. Egypt. J. Chem. 2023, 66, 49–53. [Google Scholar] [CrossRef]
- El-Apasery, M.A.; Abdellatif, M.E.A.; Ahmed, S.M. Synthesis of Novel Disperse Dyes based on Arylazophenols: Part 3.High Temperature Dyeing of Polyester Fabrics. Egypt. J. Chem. 2023, 66, 21–25. [Google Scholar] [CrossRef]
- Sehim, A.E.; Amin, B.H.; Yosri, M.; Salama, H.M.; Alkhalifah, D.H.; Alwaili, M.A.; Abd Elghaffar, R.Y. GC-MS Analysis, Antibacterial, and Anticancer Activities of Hibiscus sabdariffa L. Methanolic Extract: In Vitro and In Silico Studies. Microorganisms 2023, 11, 1601. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microliter plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Delgado, V.; Sepúlveda, S.; Benites, J.; Theoduloz, C.; Calderon, P.B.; Muccioli, G.G. Synthesis and Cytotoxic Activity on Human Cancer Cells of Novel Isoquinolinequinone–Amino Acid Derivatives. Molecules 2016, 21, 1199. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Abo-Ashour, M.F.; Eldehna, W.M.; Nocentini, A.; Bonardi, A.; Bua, S.; Ibrahim, H.S.; Elaasser, M.M.; Kryštof, V.; Jorda, R.; Gratteri, P.; et al. 3-Hydrazinoisatin-based benzenesulfonamides as novel carbonic anhydrase inhibitors endowed with anticancer activity: Synthesis, in vitro biological evaluation and in silico insights. Eur. J. Med. Chem. 2019, 184, 111768. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2015, 6, 71–79. [Google Scholar] [CrossRef]
- Elassar, A.-Z.A.; Al-Mousawi, S.M.; Helal, M.H.; Elgazzar, M.E. Synthesis of N-substituted arylhydrazones: Applying to polyester fabric as disperse dyes. Pigment. Resin Technol. 2017, 46, 449–457. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).