Recent Advances in IRAK1: Pharmacological and Therapeutic Aspects
Abstract
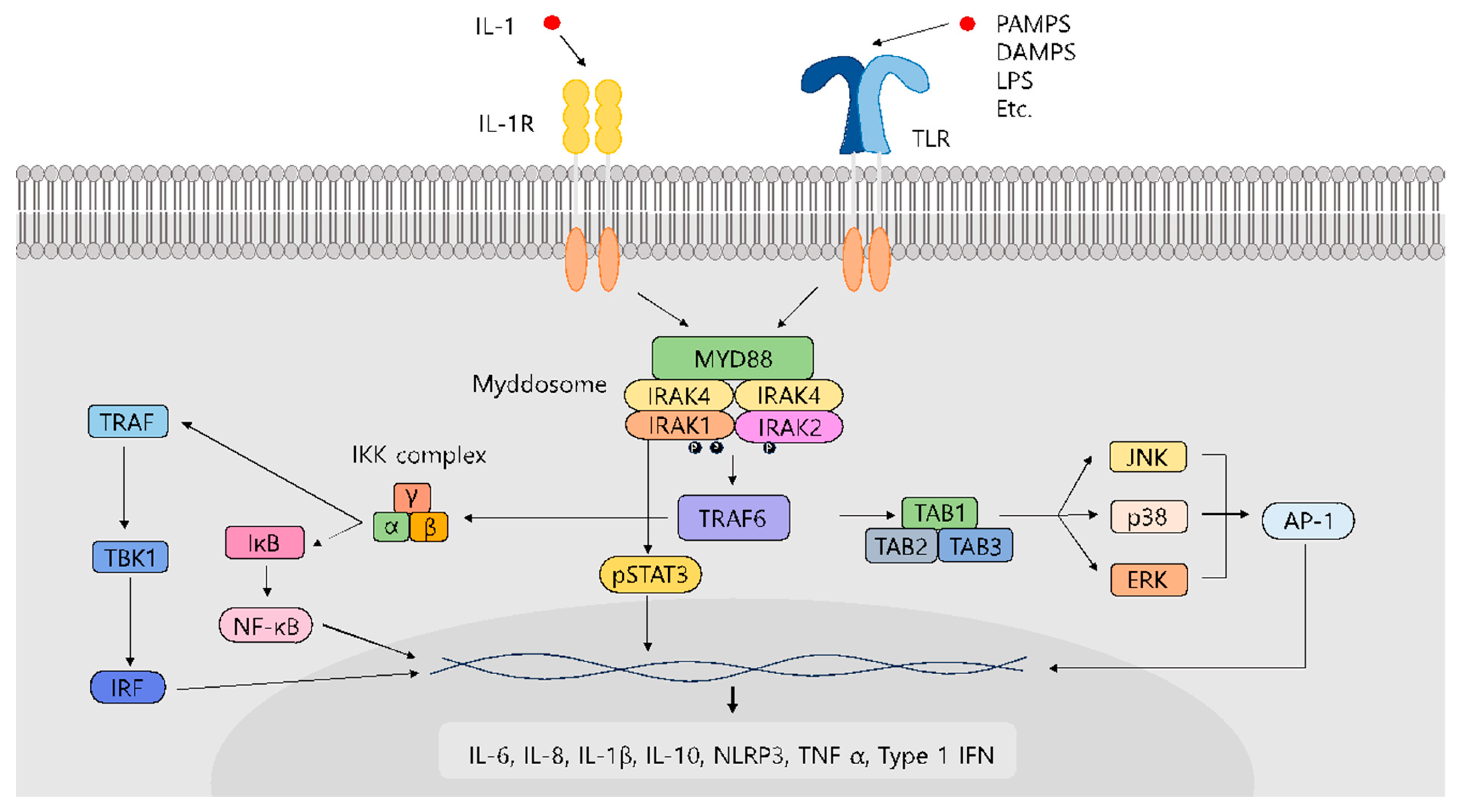
1. Introduction
2. IRAK1-Related Disease
2.1. Immune Disease
2.1.1. Inflammatory Disease
Sepsis
Fibrotic Disease—Myelofibrosis (MF)
GVHD
Dry Eye
2.1.2. Autoimmune Disease
SLE
Autoimmune Hypophysitis
2.1.3. Cardiovascular Disease
Atherosclerosis
2.1.4. Neurodegenerative Diseases
Alzheimer’s Disease (AD)
2.2. Oncology
2.2.1. Solid Tumor Malignancies
Glioma
Breast Cancer
Endometrial Cancer
Hepatocellular Carcinoma
Prostate Cancer
2.2.2. Hematologic Malignancy
Mutated B-Cell Lymphoma—ABC-DLBCL
AML
2.3. Viral Infection
HIV/SARS-CoV-2
3. IRAK1 Inhibitors and Degraders
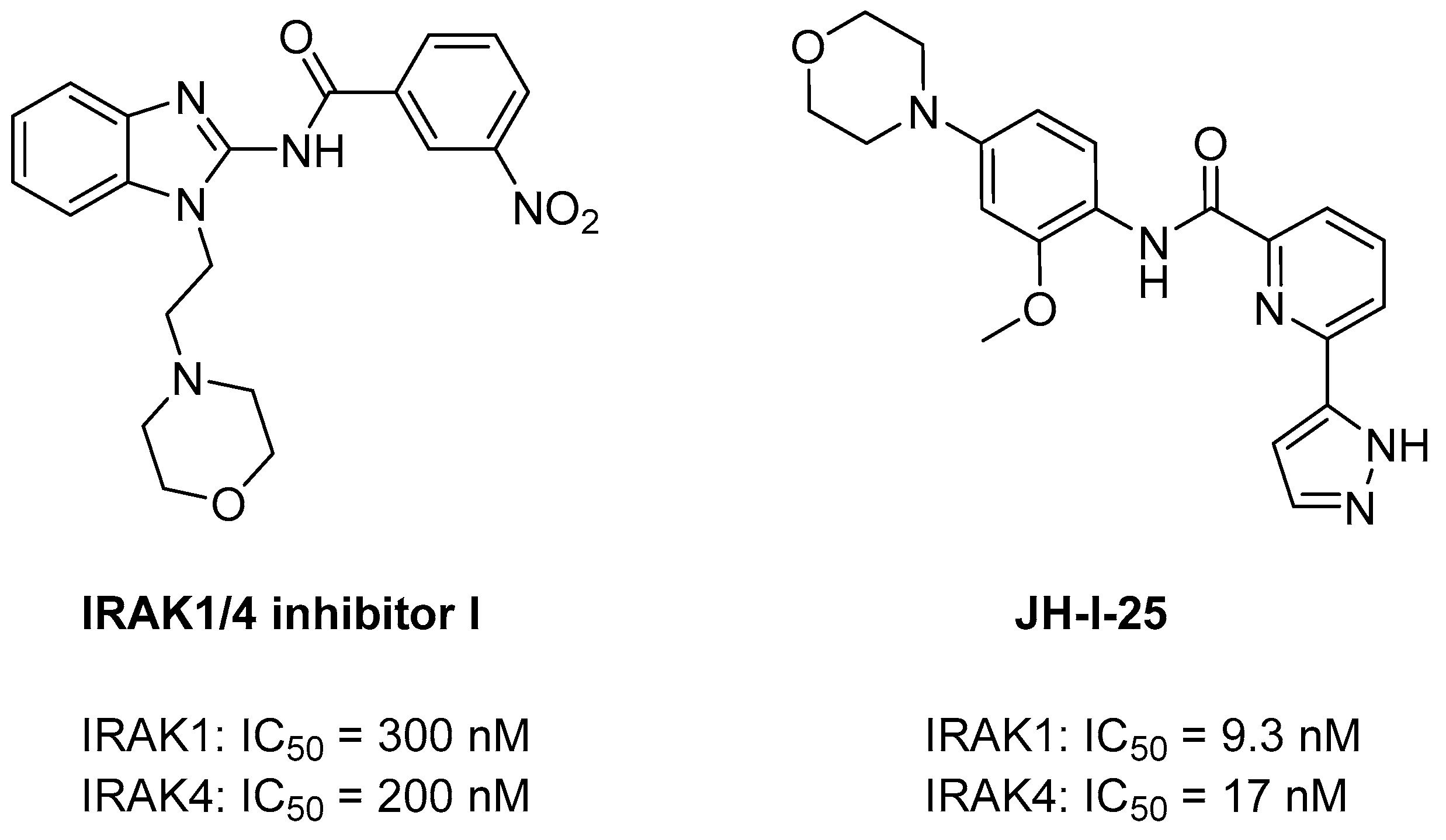
3.1. Dual IRAK1/4 Inhibitors
3.2. Selective IRAK1 Inhibitors
3.2.1. Pacritinib
3.2.2. Rosoxacin
3.2.3. 1,4-Naphthoquinone
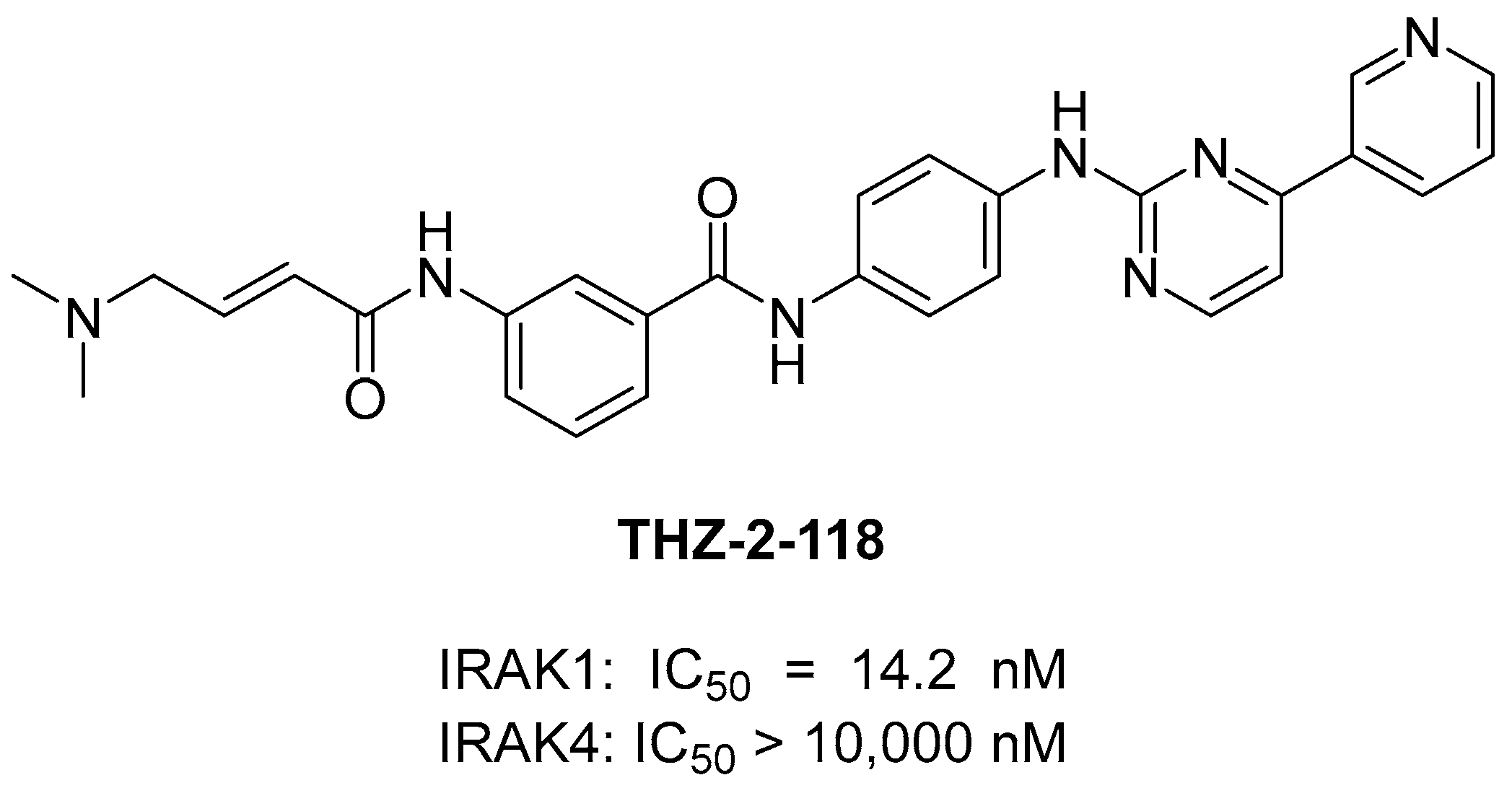
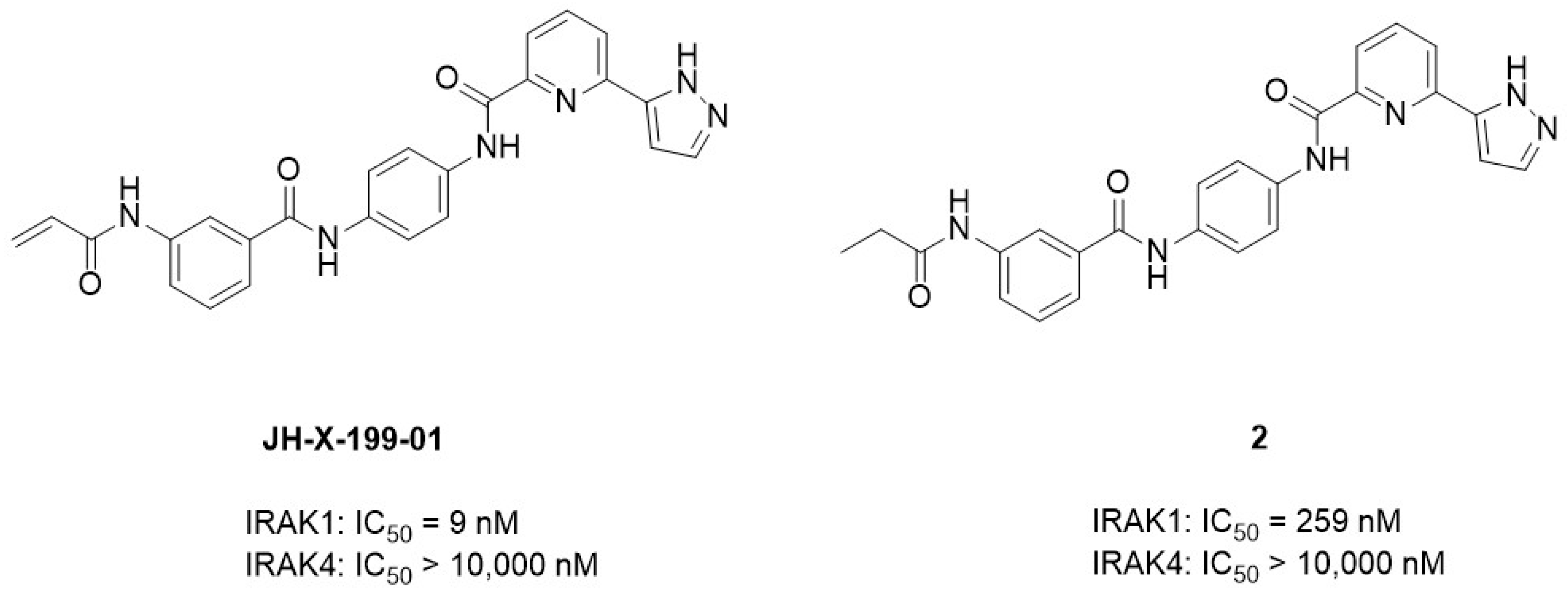
3.3. Covalent IRAK1 Inhibitor
3.4. Selective IRAK1 Degrader
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flannery, S.; Bowie, A.G. The interleukin-1 receptor-associated kinases: Critical regulators of innate immune signalling. Biochem. Pharmacol. 2010, 80, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Wesche, H.; Henzel, W.J.; Shillinglaw, W.; Li, S.Y.; Cao, Z.D. MyD88: An Adapter That Recruits IRAK to the IL-1 Receptor Complex. J. Immunol. 2013, 190, 5–15, reprinted in Immunity 1997, 7, 837–847. [Google Scholar] [CrossRef]
- Piccinini, A.M.; Midwood, K.S. DAMPening Inflammation by Modulating TLR Signalling. Mediat. Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef] [PubMed]
- Balka, K.R.; De Nardo, D. Understanding early TLR signaling through the Myddosome. J. Leukoc. Biol. 2019, 105, 339–351. [Google Scholar] [CrossRef]
- De Nardo, D.; Balka, K.R.; Gloria, Y.C.; Rao, V.R.; Latz, E.; Masters, S.L. Interleukin-1 receptor-associated kinase 4 (IRAK4) plays a dual role in myddosome formation and Toll-like receptor signaling. J. Biol. Chem. 2018, 293, 15195–15207. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X. IRAK4 in TLR/IL-1R signaling: Possible clinical applications. Eur. J. Immunol. 2008, 38, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef]
- Suzuki, N.; Suzuki, S.; Yeh, W.C. IRAK-4 as the central TIR signaling mediator in innate immunity. Trends Immunol. 2002, 23, 503–506. [Google Scholar] [CrossRef]
- Xu, M.; Liu, P.P.; Li, H.L. Innate Immune Signaling and Its Role in Metabolic and Cardiovascular Diseases. Physiol. Rev. 2019, 99, 893–948. [Google Scholar] [CrossRef]
- Su, L.C.; Xu, W.D.; Huang, A.F. IRAK family in inflammatory autoimmune diseases. Autoimmun. Rev. 2020, 19, 102461. [Google Scholar] [CrossRef]
- Rhyasen, G.W.; Starczynowski, D.T. IRAK signalling in cancer. Br. J. Cancer 2015, 112, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.M.; Chen, W.G.; Xiong, J.; Sherrod, C.J.; Henry, D.H.; Dittmer, D.P. Interleukin 1 receptor-associated kinase 1 (IRAK1) mutation is a common, essential driver for Kaposi sarcoma herpesvirus lymphoma. Proc. Natl. Acad. Sci. USA 2014, 111, E4762–E4768. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.; Beyaert, R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol. Cell 2003, 11, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Muzio, M.; Ni, J.; Feng, P.; Dixit, V.M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 1997, 278, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- Freihat, L.A.; Wheeler, J.I.; Wong, A.; Turek, I.; Manallack, D.T.; Irving, H.R. IRAK3 modulates downstream innate immune signalling through its guanylate cyclase activity. Sci. Rep. 2019, 9, 15468. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Strelow, A.; Fontana, E.J.; Wesche, H. IRAK-4: A novel member of the IRAK family with the properties of an IRAK-kinase. Proc. Natl. Acad. Sci. USA 2002, 99, 5567–5572. [Google Scholar] [CrossRef] [PubMed]
- Gottipati, S.; Rao, N.L.; Fung-Leung, W.P. IRAK1: A critical signaling mediator of innate immunity. Cell. Signal. 2008, 20, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Alnemri, T.; Kang, S.; Anderson, C.; Sagara, J.; Fitzgerald, K.A.; Alnemri, E.S. Cutting Edge: TLR Signaling Licenses IRAK1 for Rapid Activation of the NLRP3 Inflammasome. J. Immunol. 2013, 191, 3995–3999. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Gazzinelli, R.T. Regulation of innate immune signaling by IRAK proteins. Front. Immunol. 2023, 14, 1133354. [Google Scholar] [CrossRef]
- Sun, J.; Li, N.; Oh, K.S.; Dutta, B.; Vayttaden, S.J.; Lin, B.; Ebert, T.S.; De Nardo, D.; Davis, J.; Bagirzadeh, R.; et al. Comprehensive RNAi-based screening of human and mouse TLR pathways identifies species-specific preferences in signaling protein use. Sci. Signal. 2016, 9, ra3. [Google Scholar] [CrossRef]
- Zheng, Y.J.; He, J.Q. Interleukin Receptor Associated Kinase 1 Signaling and Its Association with Cardiovascular Diseases. Rev. Cardiovasc. Med. 2022, 23, 97. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.H.; Sidi, S. Targeting the Innate Immune Kinase IRAK1 in Radioresistant Cancer: Double-Edged Sword or One-Two Punch? Front. Oncol. 2019, 9, 1174. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Gao, J.; Chen, W.; Liu, C.; Shang, L.M.; Xu, M.D.; Fu, C.L.; Zhu, S.Y.; Niu, M.S.; Xu, K.L. Selective inhibition of interleukin-1 receptor-associated kinase 1 ameliorates lipopolysaccharide-induced sepsis in mice. Int. Immunopharmacol. 2020, 85, 106597. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, J.M.; Yang, G.; Wang, L.; Ficarro, S.B.; Buhrlage, S.; Wu, H.; Marto, J.A.; Treon, S.P.; Gray, N.S. Discovery of a Selective, Covalent IRAK1 Inhibitor with Antiproliferative Activity in MYD88 Mutated B-Cell Lymphoma. ACS Med. Chem. Lett. 2020, 11, 2238–2243. [Google Scholar] [CrossRef] [PubMed]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Federici, S.; Bishwas, T.; Németh, Z.H.; Deitch, E.A.; Thomas, J.A.; Spolarics, Z. IRAK1-dependent signaling mediates mortality in polymicrobial sepsis. Inflammation 2013, 36, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, X.-P.; Li, Y.-J. MicroRNA-146a and Human Disease, Scand. J. Immunol. 2010, 71, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, X.; Zhang, X.; Ha, T.; Ma, H.; Liu, L.; Kalbfleisch, J.H.; Gao, X.; Kao, R.L.; Williams, D.L.; et al. Attenuation of Cardiac Dysfunction in Polymicrobial Sepsis by MicroRNA-146a Is Mediated via Targeting of IRAK1 and TRAF6 Expression. J. Immunol. 2015, 195, 672–682. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Gleitz, H.F.; Chifotides, H.T.; Harrison, C.N.; Verstovsek, S.; Vannucchi, A.M.; Rampal, R.K.; Kiladjian, J.-J.; Vainchenker, W.; Hoffman, R. Biological drivers of clinical phenotype in myelofibrosis. Leukemia 2023, 37, 255–264. [Google Scholar] [CrossRef]
- Kleppe, M.; Koche, R.; Zou, L.; van Galen, P.; Hill, C.E.; Dong, L.; De Groote, S.; Papalexi, E.; Somasundara, A.V.H.; Cordner, K. Dual targeting of oncogenic activation and inflammatory signaling increases therapeutic efficacy in myeloproliferative neoplasms. Cancer Cell 2018, 33, 29–43.e27. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, R.; Gangat, N.; Jimma, T.; Finke, C.M.; Lasho, T.L.; Pardanani, A.; Tefferi, A. Plasma cytokines in polycythemia vera: Phenotypic correlates, prognostic relevance, and comparison with myelofibrosis. Am. J. Hematol. 2012, 87, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, Y.; Ma, S.; Liang, Y.; Liu, C.; Shen, J.; Sun, Z.; Niu, M.; Xu, K.; Pan, B. Inhibition of IL-1 Receptor-Associated Kinase 1 Decreases Murine Acute Graft-versus-Host Disease While Preserving the Graft-versus-Lymphoma Effect. Transplant. Cell. Ther. 2022, 28, 134.e1–134.e10. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.; Agrawal, A.; Van Dyke, T.; Landreth, G.; McCauley, L.; Koh, A.; Maliszewski, C.; Akira, S.; Pulendran, B. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J. Immunol. 2004, 172, 4733–4743. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Hatcher, J.; Wang, J.; Liu, X.; Munshi, M.; Chen, J.; Xu, L.; Tsakmaklis, N.; Demos, M.; Kofides, A. A novel, highly selective IRAK1 inhibitor Jh-X-119-01 shows synergistic tumor cell killing with ibrutinib in MYD88 mutated B-cell lymphoma cells. Blood 2017, 130, 719. [Google Scholar] [CrossRef]
- Han, R.; Gao, J.; Wang, L.; Hao, P.; Chen, X.; Wang, Y.; Jiang, Z.; Jiang, L.; Wang, T.; Zhu, L. MicroRNA-146a negatively regulates inflammation via the IRAK1/TRAF6/NF-κB signaling pathway in dry eye. Sci. Rep. 2023, 13, 11192. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhang, M.; He, T.; Chen, S. The expression of miRNA-146a-5p and its mechanism of treating dry eye syndrome. J. Clin. Lab. Anal. 2021, 35, e23571. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.O.; Zhu, J.; Armstrong, D.L.; Yan, M.; Han, J.; Zhou, X.J.; Thomas, J.A.; Reiff, A.; Myones, B.L.; Ojwang, J.O. Identification of IRAK1 as a risk gene with critical role in the pathogenesis of systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA 2009, 106, 6256–6261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tian, Z.; Zhang, M.; Zhang, Y.; Ni, B.; Hao, F. Upregulated IL-1 receptor-associated kinase 1 (IRAK1) in systemic lupus erythematosus: IRAK1 inhibition represses Th17 differentiation with therapeutic potential. Immunol. Investig. 2018, 47, 468–483. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chen, Y.-T.; Lin, H.-H.; Li, Z.-Q.; Yang, J.-M.; Tzou, S.-C. Inhibition of IRAK1 Is an Effective Therapy for Autoimmune Hypophysitis in Mice. Int. J. Mol. Sci. 2022, 23, 14958. [Google Scholar] [CrossRef]
- Avlas, O.; Fallach, R.; Shainberg, A.; Porat, E.; Hochhauser, E. Toll-like receptor 4 stimulation initiates an inflammatory response that decreases cardiomyocyte contractility. Antioxid. Redox Signal. 2011, 15, 1895–1909. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Alvi, S.S.; Iqbal, D.; Khan, M.S. Insights into pharmacological mechanisms of polydatin in targeting risk factors-mediated atherosclerosis. Life Sci. 2020, 254, 117756. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, C.; Zhang, L.; Cao, X.; Ma, Q.; Deng, P.; Zhu, G.; Gao, C.; Li, B.; Pi, Y. IRAK1 mediates TLR4-induced ABCA1 downregulation and lipid accumulation in VSMCs. Cell Death Dis. 2015, 6, e1949. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Ardura-Fabregat, A.; Boddeke, E.; Boza-Serrano, A.; Brioschi, S.; Castro-Gomez, S.; Ceyzériat, K.; Dansokho, C.; Dierkes, T.; Gelders, G.; Heneka, M.T. Targeting neuroinflammation to treat Alzheimer’s disease. CNS Drugs 2017, 31, 1057–1082. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Microglia and astrocytes in Alzheimer’s disease: Implications for therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.C.; Croft, C.L.; Kurbatskaya, K.; O’Neill, M.J.; Hutton, M.L.; Hanger, D.P.; Garwood, C.J.; Noble, W. Astrocytes and neuroinflammation in Alzheimer’s disease. Biochem. Soc. Trans. 2014, 42, 1321. [Google Scholar] [CrossRef]
- Mandrekar-Colucci, S.; Landreth, G.E. Microglia and inflammation in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2010, 9, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Hoozemans, J.J.M.; Van Haastert, E.S.; Mulder, S.; Nielsen, H.; Veerhuis, R.; Ruijtenbeek, R.; Rozemuller, A.; Hilhorst, R.; Van Der Vies, S. Increased irak-4 kinase activity in Alzheimer’s disease; Inhibitory effect of irak-1/4 inhibitor i on pro-inflammatory cytokine secretion but not on uptake of amyloid beta by human glial cells. J. Clin. Cell Immunol. 2014, 5, 243. [Google Scholar]
- Liu, X.; Jiao, K.; Jia, C.-c.; Li, G.-x.; Yuan, Q.; Xu, J.-k.; Hou, Y.; Wang, B. BAP31 regulates IRAK1-dependent neuroinflammation in microglia. J. Neuroinflamm. 2019, 16, 281. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Ma, Y.; Zhao, X.; Sun, X.; Wang, Y.; Zhang, X. Comprehensive pan-cancer analysis of IRAK family genes identifies IRAK1 as a novel oncogene in low-grade glioma. J. Oncol. 2022, 2022, 6497241. [Google Scholar] [CrossRef]
- Pilarsky, C.; Wenzig, M.; Specht, T.; Saeger, H.D.; Grützmann, R. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia 2004, 6, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Nam, D.-H.; Ram, Z.; Poon, W.-s.; Wang, J.; Boldbaatar, D.; Mao, Y.; Ma, W.; Mao, Q.; You, Y. Clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett. 2021, 499, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, Y.; Zhao, X.; Ma, Y.; Xie, Y.; Liu, S.; Hui, B.; Shi, X.; Sun, X.; Zhang, X. Radiation induces IRAK1 expression to promote radioresistance by suppressing autophagic cell death via decreasing the ubiquitination of PRDX1 in glioma cells. Cell Death Dis. 2023, 14, 259. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Bakhoum, S.F. The cytosolic DNA-sensing cGAS–STING pathway in cancer. Cancer Discov. 2020, 10, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Song, Z.; Shen, A.; Chen, T.; Zhang, A. Small molecules targeting the innate immune cGAS–STING–TBK1 signaling pathway. Acta Pharm. Sin. B 2020, 10, 2272–2298. [Google Scholar] [CrossRef] [PubMed]
- Wee, Z.N.; Yatim, S.M.J.; Kohlbauer, V.K.; Feng, M.; Goh, J.Y.; Bao, Y.; Lee, P.L.; Zhang, S.; Wang, P.P.; Lim, E. IRAK1 is a therapeutic target that drives breast cancer metastasis and resistance to paclitaxel. Nat. Commun. 2015, 6, 8746. [Google Scholar] [CrossRef] [PubMed]
- Long, J.P.; Dong, L.F.; Chen, F.F.; Fan, Y.F. miR-146a-5p targets interleukin-1 receptor-associated kinase 1 to inhibit the growth, migration, and invasion of breast cancer cells. Oncol. Lett. 2019, 17, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.; Lin, J.; Lv, C.; Qiao, G. miR-146a enhances the sensitivity of breast cancer cells to paclitaxel by downregulating IRAK1. Cancer Biother. Radiopharm. 2022, 37, 624–635. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.; Li, Y.; Su, R. MiR-192-5p-Modified tumor-associated macrophages-derived exosome suppressed endometrial cancer progression through targeting IRAK1/NF-κB signaling. Reprod. Sci. 2022, 29, 436–447. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shen, Q.; Son, K.; Kim, H.S.; Yang, H.D.; Na, M.J.; Shin, E.; Yu, S.; Kang, K.; You, J.S. SMARCA4 oncogenic potential via IRAK1 enhancer to activate Gankyrin and AKR1B10 in liver cancer. Oncogene 2021, 40, 4652–4662. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wei, T.; Chen, Y.; Yang, L.; Wu, X. Downregulation of irak1 prevents the malignant behavior of hepatocellular carcinoma cells by blocking activation of the mapks/nlrp3/il-1β pathway. Onco Targets Ther. 2020, 13, 12787–12796. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Oseni, S.O.; Nguyen, C.; Pavlovic, M.; Kumi-Diaka, J. Dysregulation of Interleukin-1 receptor-associated kinase 1 promotes prostate cancer-associated chronic inflammation and aggressiveness. Cancer Res. 2019, 79, 1487. [Google Scholar] [CrossRef]
- Schagdarsurengin, U.; Breiding, V.; Loose, M.; Wagenlehner, F.; Dansranjav, T. Interleukin-1 receptor associated kinase 1 (IRAK1) is epigenetically activated in luminal epithelial cells in prostate cancer. Front. Oncol. 2022, 12, 991368. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Commane, M.; Burns, C.; Vithalani, K.; Cao, Z.; Stark, G.R. Mutant cells that do not respond to interleukin-1 (IL-1) reveal a novel role for IL-1 receptor-associated kinase. Mol. Cell. Biol. 1999, 19, 4643–4652. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.E.; Whitehead, A.S. IRAK1b, a novel alternative splice variant of interleukin-1 receptor-associated kinase (IRAK), mediates interleukin-1 signaling and has prolonged stability. J. Biol. Chem. 2001, 276, 29037–29044. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, J.; Shen, B.; Kong, L.; Liu, Y.; Tu, W.; Wang, W.; Cai, X.; Wang, X.; Cheng, N. Discovery of highly potent and selective IRAK1 degraders to probe scaffolding functions of IRAK1 in ABC DLBCL. J. Med. Chem. 2021, 64, 10878–10889. [Google Scholar] [CrossRef] [PubMed]
- Pasqualucci, L. Molecular pathogenesis of germinal center-derived B cell lymphomas. Immunol. Rev. 2019, 288, 240–261. [Google Scholar] [CrossRef]
- Hosseini, M.M.; Kurtz, S.E.; Abdelhamed, S.; Mahmood, S.; Davare, M.A.; Kaempf, A.; Elferich, J.; McDermott, J.E.; Liu, T.; Payne, S.H. Inhibition of interleukin-1 receptor-associated kinase-1 is a therapeutic strategy for acute myeloid leukemia subtypes. Leukemia 2018, 32, 2374–2387. [Google Scholar] [CrossRef]
- Campbell, G.R.; Rawat, P.; Spector, S.A. Pacritinib Inhibition of IRAK1 Blocks Aberrant TLR8 Signalling by SARS-CoV-2 and HIV-1-Derived RNA. J. Innate Immun. 2023, 15, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, A.T.C. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet 2008, 372, 293–299. [Google Scholar]
- Utay, N.S.; Hunt, P.W. Role of immune activation in progression to AIDS. Curr. Opin. HIV AIDS 2016, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, A.R.; Zheng, Y.; Bosch, R.J.; Krishnan, S.; Rodriguez, B.; Hunt, P.W.; Plants, J.; Seth, A.; Wilson, C.C.; Deeks, S.G. Soluble markers of inflammation and coagulation but not T-cell activation predict non–AIDS-defining morbid events during suppressive antiretroviral treatment. J. Infect. Dis. 2014, 210, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Nanda, A.; Vura, N.V.R.K.; Gravenstein, S. COVID-19 in older adults. Aging Clin. Exp. Res. 2020, 32, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Bordallo, B.; Bellas, M.; Cortez, A.F.; Vieira, M.; Pinheiro, M. Severe COVID-19: What have we learned with the immunopathogenesis? Adv. Rheumatol. 2020, 60, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ji, P.; Pang, J.; Zhong, Z.; Li, H.; He, C.; Zhang, J.; Zhao, C. Clinical characteristics of 3062 COVID-19 patients: A meta-analysis. J. Med. Virol. 2020, 92, 1902–1914. [Google Scholar] [CrossRef]
- Carsetti, R.; Zaffina, S.; Piano Mortari, E.; Terreri, S.; Corrente, F.; Capponi, C.; Palomba, P.; Mirabella, M.; Cascioli, S.; Palange, P. Different innate and adaptive immune responses to SARS-CoV-2 infection of asymptomatic, mild, and severe cases. Front. Immunol. 2020, 11, 610300. [Google Scholar] [CrossRef]
- Wang, L.; Qiao, Q.; Ferrao, R.; Shen, C.; Hatcher, J.M.; Buhrlage, S.J.; Gray, N.S.; Wu, H. Crystal structure of human IRAK1. Proc. Natl. Acad. Sci. USA 2017, 114, 13507–13512. [Google Scholar] [CrossRef]
- Powers, J.P.; Li, S.Y.; Jaen, J.C.; Liu, J.Q.; Walker, N.P.C.; Wang, Z.L.; Wesche, H. Discovery and initial SAR of inhibitors of interleukin-1 receptor-associated kinase-4. Bioorg. Med. Chem. Lett. 2006, 16, 2842–2845. [Google Scholar] [CrossRef] [PubMed]
- Somani, V.K.; Zhang, D.X.; Dodhiawala, P.B.; Lander, V.E.; Liu, X.T.; Kang, L.I.; Chen, H.P.; Knolhoff, B.L.; Li, L.; Grierson, P.M.; et al. IRAK4 Signaling Drives Resistance to Checkpoint in Pancreatic Ductal Adenocarcinoma. Gastroenterology 2022, 162, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.K.; Bolanos, L.C.; Dexheimer, P.J.; Karns, R.A.; Aronow, B.J.; Komurov, K.; Jegga, A.G.; Casper, K.A.; Patil, Y.J.; Wilson, K.M.; et al. IRAK1 is a novel DEK transcriptional target and is essential for head and neck cancer cell survival. Oncotarget 2015, 6, 43395–43407. [Google Scholar] [CrossRef] [PubMed]
- Buckley, G.M.; Gowers, L.; Higueruelo, A.P.; Jenkins, K.; Mack, S.R.; Morgan, T.; Parry, D.M.; Pitt, W.R.; Rausch, O.; Richard, M.D.; et al. IRAK-4 inhibitors. Part 1: A series of amides. Bioorg. Med. Chem. Lett. 2008, 18, 3211–3214. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Pacritinib: First Approval. Drugs 2022, 82, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J. Pacritinib for the treatment of patients with myelofibrosis and thrombocytopenia. Expert Rev. Hematol. 2022, 15, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.W.; Fleischman, A.; Al-Fayoumi, S.; Mascarenhas, J.O.; Yu, Q.; Agarwal, A. Inhibition of interleukin-1 receptor-associated kinase 1 (IRAK1) as a therapeutic strategy. Oncotarget 2018, 9, 33416–33439. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.; Goh, K.C.; Novotny-Diermayr, V.; Tan, Y.C.; Madan, B.; Amalini, C.; Ong, L.C.; Kheng, B.; Cheong, A.; Zhou, J.; et al. Pacritinib (SB1518), a JAK2/FLT3 inhibitor for the treatment of acute myeloid leukemia. Blood Cancer J. 2011, 1, e44. [Google Scholar] [CrossRef]
- Goh, J.Y.; Feng, M.; Wang, W.Y.; Oguz, G.; Yatim, S.; Lee, P.L.; Bao, Y.; Lim, T.H.; Wang, P.P.; Tam, W.L.; et al. Chromosome 1q21.3 amplification is a trackable biomarker and actionable target for breast cancer recurrence. Nat. Med. 2017, 23, 1319–1330. [Google Scholar] [CrossRef]
- Dobson, R.A.; O’Connor, J.R.; Poulin, S.A.; Kundsin, R.B.; Smith, T.F.; Came, P.E. In vitro antimicrobial activity of rosoxacin against Neisseria gonorrhoeae, Chlamydia trachomatis, and Ureaplasma urealyticum. J. Antimicrob. Chemother. 1980, 18, 738–740. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Yang, J.M.; Chen, C.C. GEMDOCK: A generic evolutionary method for molecular docking. Proteins 2004, 55, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, I.S.; Hatmal, M.M.; Abuarqoub, D.; Esawi, E.; Zalloum, H.; Wehaibi, S.; Nsairat, H.; Alshaer, W. 1,4-Naphthoquinone Is a Potent Inhibitor of IRAK1 Kinases and the Production of Inflammatory Cytokines in THP-1 Differentiated Macrophages. ACS Omega 2021, 6, 25299–25310. [Google Scholar] [CrossRef]
- Bennett, J.; Starczynowski, D.T. IRAK1 and IRAK4 as emerging therapeutic targets in hematologic malignancies. Curr. Opin. Hematol. 2022, 29, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.N.; Young, R.M.; Schmitz, R.; Jhavar, S.; Xiao, W.; Lim, K.H.; Kohlhammer, H.; Xu, W.; Yang, Y.; Zhao, H.; et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011, 470, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Kubacz, M.; Kusowska, A.; Winiarska, M.; Bobrowicz, M. In Vitro Diffuse Large B-Cell Lymphoma Cell Line Models as Tools to Investigate Novel Immunotherapeutic Strategies. Cancers 2022, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Bosmann, M.; Ward, P.A. The inflammatory response in sepsis. Trends Immunol. 2013, 34, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Li, L. Reduced oxidative tissue damage during endotoxemia in IRAK-1 deficient mice. Mol. Immunol. 2012, 50, 244–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Z.; Wesche, H.; Stevens, T.; Walker, N.; Yeh, W.C. IRAK-4 inhibitors for inflammation. Curr. Top. Med. Chem. 2009, 9, 724–737. [Google Scholar] [CrossRef]
- Picard, C.; Puel, A.; Bonnet, M.; Ku, C.L.; Bustamante, J.; Yang, K.; Soudais, C.; Dupuis, S.; Feinberg, J.; Fieschi, C.; et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 2003, 299, 2076–2079. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.M.; Hwang, N.-H.; Hyun, J.-S.; Shin, D. Recent Advances in IRAK1: Pharmacological and Therapeutic Aspects. Molecules 2024, 29, 2226. https://doi.org/10.3390/molecules29102226
Kim KM, Hwang N-H, Hyun J-S, Shin D. Recent Advances in IRAK1: Pharmacological and Therapeutic Aspects. Molecules. 2024; 29(10):2226. https://doi.org/10.3390/molecules29102226
Chicago/Turabian StyleKim, Kyeong Min, Na-Hee Hwang, Ja-Shil Hyun, and Dongyun Shin. 2024. "Recent Advances in IRAK1: Pharmacological and Therapeutic Aspects" Molecules 29, no. 10: 2226. https://doi.org/10.3390/molecules29102226
APA StyleKim, K. M., Hwang, N.-H., Hyun, J.-S., & Shin, D. (2024). Recent Advances in IRAK1: Pharmacological and Therapeutic Aspects. Molecules, 29(10), 2226. https://doi.org/10.3390/molecules29102226





