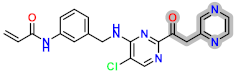
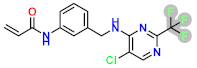
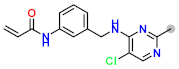
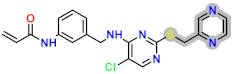
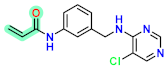
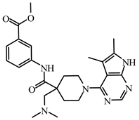
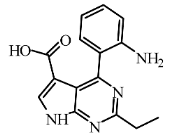
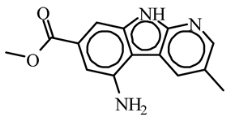
Abstract
This work aimed to find new inhibitors of the CYP3A4 and JAK3 enzymes, which are significant players in autoimmune diseases such as rheumatoid arthritis. Advanced computer-aided drug design techniques, such as pharmacophore and 3D-QSAR modeling, were used. Two strong 3D-QSAR models were created, and their predictive power was validated by the strong correlation (R2 values > 80%) between the predicted and experimental activity. With an ROC value of 0.9, a pharmacophore model grounded in the DHRRR hypothesis likewise demonstrated strong predictive ability. Eight possible inhibitors were found, and six new inhibitors were designed in silico using these computational models. The pharmacokinetic and safety characteristics of these candidates were thoroughly assessed. The possible interactions between the inhibitors and the target enzymes were made clear via molecular docking. Furthermore, MM/GBSA computations and molecular dynamics simulations offered insightful information about the stability of the binding between inhibitors and CYP3A4 or JAK3. Through the integration of various computational approaches, this study successfully identified potential inhibitor candidates for additional investigation and efficiently screened compounds. The findings contribute to our knowledge of enzyme–inhibitor interactions and may help us create more effective treatments for autoimmune conditions like rheumatoid arthritis.
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that significantly impacts the immune system [1]. It is characterized by persistent inflammation of the synovial joint lining, leading to substantial financial burdens, premature mortality, and a progressive decline in quality of life [2,3] This condition predominantly affects women and tends to manifest in older individuals [4]. Early identification of RA within the onset of symptoms is crucial for achieving optimal therapeutic outcomes, including limiting joint damage, preserving joint function, and minimizing radiological progression [5]. RA is the result of a combination of genetic and environmental factors, including variants of the major histocompatibility complex class II, tumor necrosis factor associated with factor 1 receptor, exposure to silica dust, and smoking, among others [6]. The underlying mechanisms of this disease remain partially understood, with autoimmune responses known to develop several years before the clinical symptoms become apparent [7].
Among the relevant molecular targets, Janus kinases (JAKs) are promising targets due to their essential role in regulating the JAK signal transducer and activator of the transcription (STAT) signaling pathway, which is associated with inflammation and immunity [8]. JAK inhibitors, including JAK1, JAK2, JAK3, and TYK2, have emerged as potential treatments for RA, with some of them receiving approval for use in this disease [9]. These medications modulate JAK signaling, reducing inflammation and alleviating symptoms while preserving joint integrity in RA patients [10]. Understanding the interactions between JAK inhibitors, with JAK3, and CYP3A4 is essential for developing safer and more effective treatments for RA [11]. Docking molecular studies have provided crucial preliminary information, but experimental validation remains imperative to ensure the efficacy and safety of JAK inhibitor-based treatments [12,13]. JAK enzymes are integral to transmitting signals from various cytokines and growth factors [14]. Recently, attention has focused on JAK3 (Janus kinase 3) as an important target in the treatment of RA, given its specific role in the cytokine signaling pathway [15]. In the context of RA, cytokine signaling plays a central role in chronic inflammation and joint damage, making JAK3 a preferred target [16]. It is noteworthy that some JAK inhibitors target both JAK3 and JAK1, but selectively targeting JAK3 offers potential advantages in terms of specificity and the profile of adverse effects [17]. It is essential to emphasize that decisions regarding the use of JAK-targeting medications for RA treatment should be made by qualified healthcare professionals, considering each patient’s individual needs and the associated risks and benefits of these drugs [10,18].
The metabolism of JAK inhibitors can also be influenced by other enzymes, including cytochrome P450 3A4 (CYP3A4), which is a cytochrome P450 enzyme found in the liver [19]. This enzyme plays a crucial role in the metabolism of many drugs, including some JAK inhibitors [20,21]. Inhibition of CYP3A4 can impact the efficacy and safety of treatment by altering the plasma concentrations of drugs metabolized by this enzyme [22]. Tofacitinib is a selective inhibitor of JAK3 that is used to treat certain autoimmune diseases, including rheumatoid arthritis, which helps reduce the activity of the immune system [15]. Tofacitinib is primarily metabolized by the enzyme CYP3A4 [23], and the inhibition of CYP3A4 can impact the tofacitinib metabolism, potentially leading to drug interactions [12,20,21]. Inhibition of CYP3A4 can potentially affect the metabolism and efficacy of tofacitinib, highlighting the importance of considering drug interactions when using it [24,25,26].
Herein, in this study, a series characterized by the presence of 1,4 Michael groups was investigated. The QSAR model allows for the design of molecules with groups that favor the Michael 1,4 reaction, while the DHRRR pharmacophore model is used to identify groups associated with α,β-unsaturated ketones through screening a database from PubChem, including JAK3 inhibitors. The α,β-unsaturated ketone is a functional group found in organic molecules, characterized by the presence of a carbonyl group (C=O) next to a double bond. The α,β-unsaturated ketones can undergo various chemical reactions, including the Michael 1,4 addition. This reaction involves the nucleophilic addition of a nucleophile to an acceptor enone, forming a bond between the β-carbon of the enone and the nucleophile. Table 1 provides a summary of the advantages and limitations of each reaction. It highlights the positive aspects, such as wide applicability and structural diversity in the case of covalent bond formation with a α,β-unsaturated ketone, as well as the selective formation of C-C bonds and simple reaction steps in the case of the Michael 1,4 reaction [27,28]. However, it also emphasizes limitations such as the limited reactivity of certain substrates and competitive reactions in the case of the Michael 1,4 reaction, as well as the formation of undesired by-products in the case of covalent bond formation with an α,β-unsaturated ketone [29].

Table 1.
Advantages and disadvantages of the α,β-Unsaturated ketones and Michael 1,4 reaction.
Today, the significance of computer-aided drug design (CADD) in chemistry for the discovery of new medications is undeniable [30,31,32,33]. This approach plays a pivotal role in identifying promising pharmaceutical compounds for the treatment of various diseases, including rheumatoid arthritis (RA). In this study, diverse CADD methodologies have facilitated the design and identification of a series of molecules exhibiting potent experimental inhibitory activity against JAK3, positioning them as potential candidates for potent RA treatments [34,35]. Three-dimensional models have guided the design and identification of these novel JAK3 and CYP3A4 inhibitory molecules, while both field-based and atom-based quantitative structure–activity relationship (QSAR) approaches, as well as pharmacophore modeling, have yielded significant results, with favorable validation. Furthermore, molecular dynamics (MD) simulations and MM/GBSA solvation energy have corroborated the findings from molecular docking, demonstrating that these newly designed molecules can form irreversible covalent bonds with Cys909 and reversible bonds with CYP3A4. These new molecules can thus be regarded as promising targets for the development of novel medications aimed at treating rheumatoid arthritis.
2. Results and Discussion
2.1. Static Results of QSAR Models
Static Analysis of Field-Based and Atom-Based Models
The field-based QSAR model was generated based on a training set of 59 molecules used to build the model (Table 2). As shown in Table 2, the best model (PLS factor = 4) offered good predictive power. The Leave-One-Out (LOO) method of cross-validation was adopted for the assessment of the predictive abilities of the models. The cross-validated value R2CV was 0.53. The R2 for the regression was 0.80, and the stability value was 0.80. The P value (6.13 × 10−15) also suggested a greater degree of confidence. The reliability of the model was tested via an external test set of 16 compounds. The RMSE (0.44), Q2 (0.763), and Pearson-r (0.88) models all further confirmed their robustness (Table 2). Activity residual values for the training set and the test set are shown in Table S1.

Table 2.
The statistical data for the field-based QSAR model.
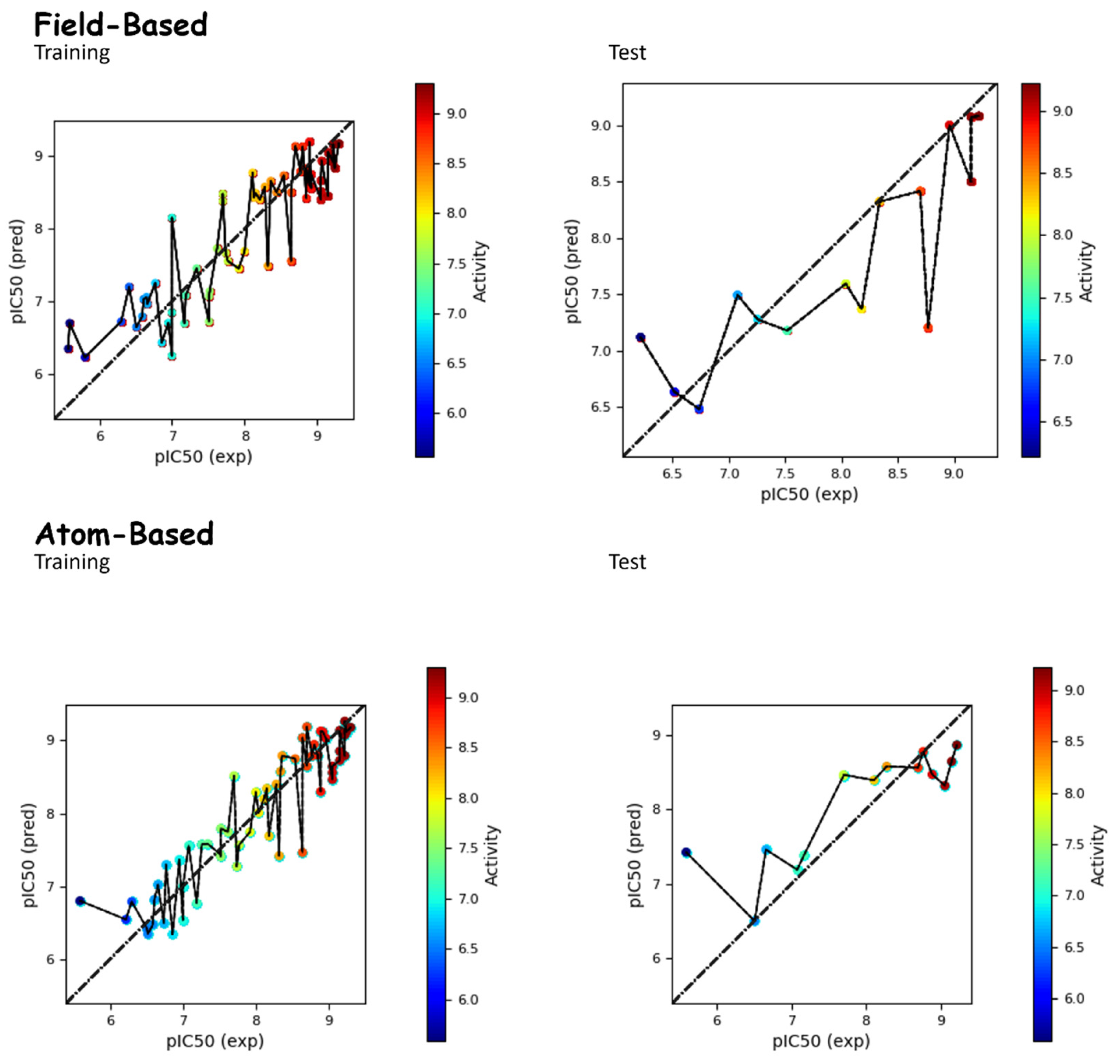
The atom-based QSAR model was generated based on a training set of 59 molecules that was used to build the model (Table 3). As shown in Table 3, the best model (PLS factor = 4) offered good predictive power. The Leave-One-Out (LOO) method of cross-validation was adopted for the assessment of the predictive abilities of the models. The cross-validated value R2CV was 0.56. The R2 for the regression was 0.87, and the stability value was 0.60. The p value (2.91 × 10−20) also suggested a greater degree of confidence. The reliability of the model was tested via an external test set of 13 compounds. The RMSE (0.66), Q2 (0.66), and Pearson-r (0.82) of the model all further confirmed its robustness (Table 3). The correlation between the experimental and predicted activities for the dataset is displayed in Figure 1.

Table 3.
The statistical data for the atom-based QSAR model.
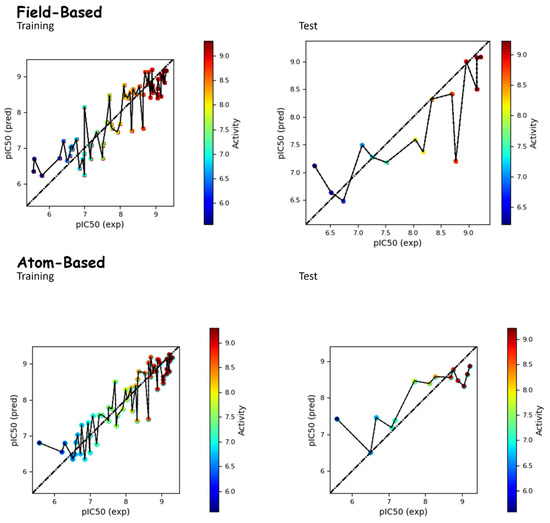
Figure 1.
Comparison between the actual and predicted pIC50 values of molecules in both the test and training sets for both field-based and atom-based approaches.
2.2. Contour Maps
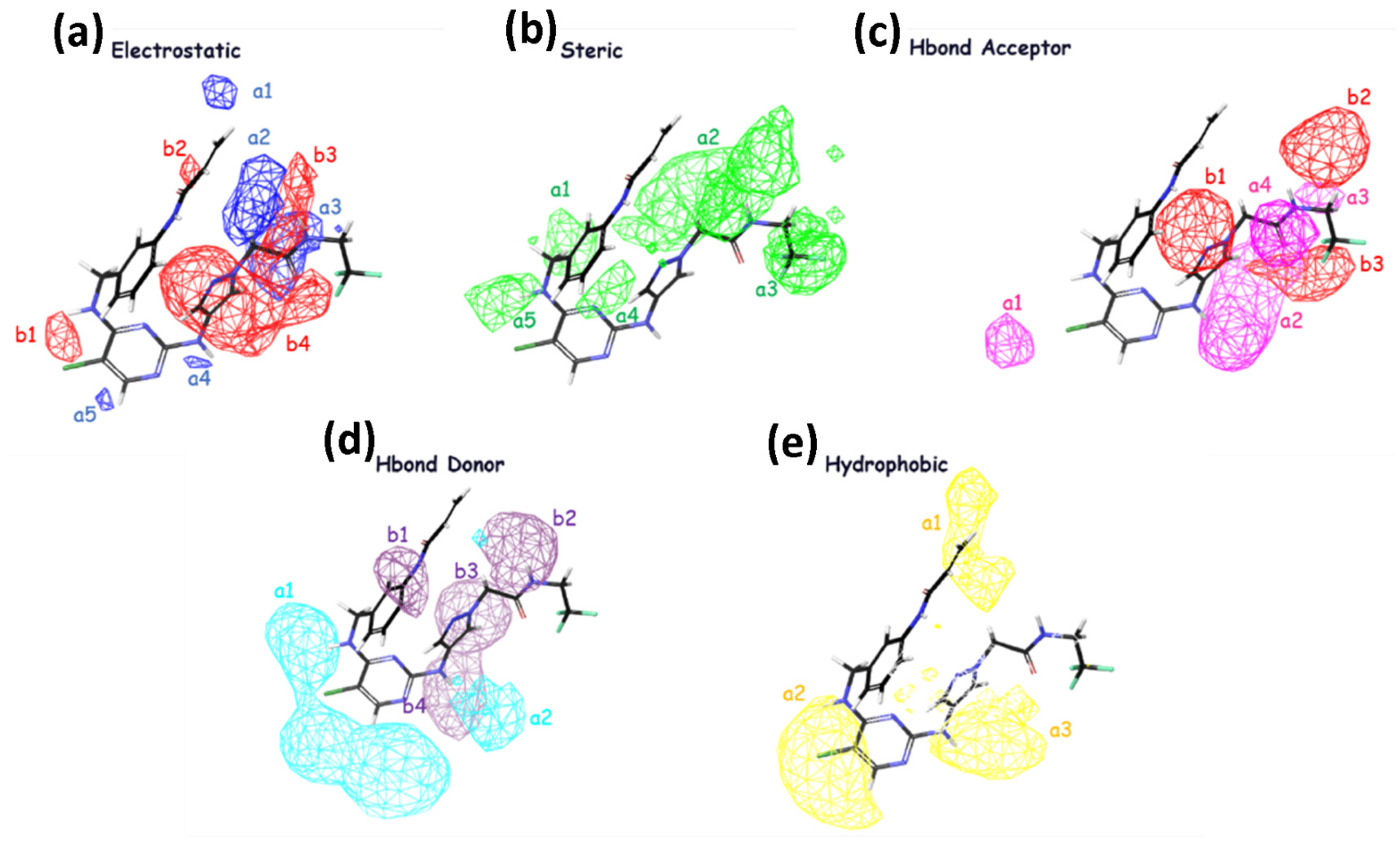
The significance of steric, hydrophobic, electrostatic, and hydrogen bond interactions (both as acceptor and donor) in explaining the studied properties is emphasized in field-based Model 4. This finding is consistent with the order of importance for increasing inhibitory activity, indicated by the coefficients reported in Table 4. The coefficients reveal that the steric group has the highest value, followed by hydrophobic, hydrogen bond acceptor, hydrogen bond donor, and electrostatic interactions, respectively, as presented in Table 4. In Figure 2, it is observed that the inhibitory activity is enhanced with groups that can generate electrostatic effects, favoring regions b3 and b4 with larger contours, suggesting their importance in these regions. The blue cubes represent regions where activity is favored, while the red regions correspond to disfavored regions for activity enhancement in regions a2 and a3. Regarding steric effects, it is noted that in regions a1–a5, increased biological activity is favored in the presence of more substituted groups. H-bond acceptor groups, depicted by larger contours in b1–b3, suggest the importance of H-bond donor groups in these regions, while the pink groups in a1–a4 disfavor the enhancement of inhibitory activity in these regions. For H-bond donor groups, all b1 groups in purple favor an increase in inhibitory activity, whereas the groups in cyan in regions a1–a2 disfavor the enhancement of inhibitory activity in the presence of H-bonds. In terms of hydrophobicity, the yellow contours suggest that in regions a1–a3, substituted hydrophobic groups favor an increase in inhibitory activity.

Table 4.
Statistical analysis of atom-based model in 3D-QSAR.
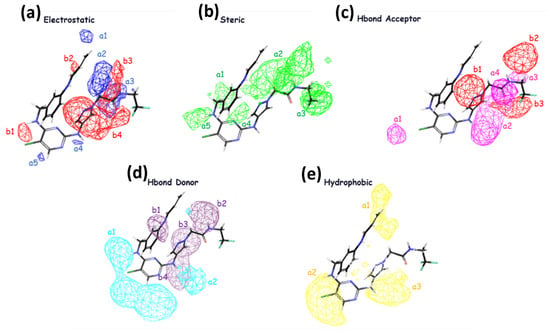
Figure 2.
The contour maps generated for the test set compounds depict different fields. Specifically, (a) Gaussian electrostatic fields are represented by blue color for favored electropositive regions and red color for disfavored electronegative regions. (b) Gaussian hydrogen bond acceptor fields are depicted using red color for favored regions and magenta color for disfavored regions. (c) Gaussian hydrogen bond donor fields are shown in purple for favored regions and cyan for disfavored regions. (d) Gaussian steric fields are displayed in green for favored regions and yellow for unfavorable regions. (e) Gaussian hydrophobic fields are represented by yellow color for favored regions and white color for disfavored regions.
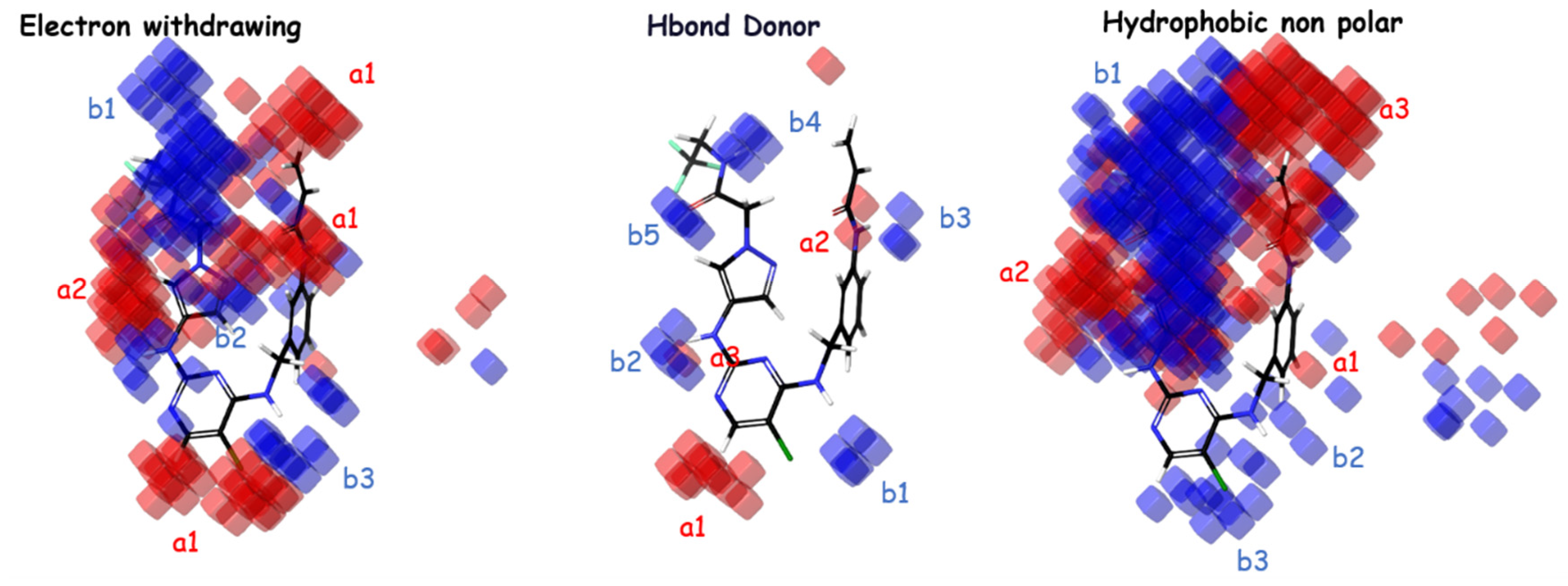
The importance of hydrophobic/nonpolar and electron-withdrawing interactions in the atom-based Model 4 is highlighted by these factors. According to the important coefficients presented in Table 4, the groups that most favor an increase in inhibitory activity, in descending order, are hydrophobic/nonpolar, electron-withdrawing, and hydrogen bond donor. The analysis of Figure 3 for electron-withdrawing in the indicated regions shows a distribution with similarities of blue/red cubes, suggesting that the corresponding electron-withdrawing groups correspond to favorable/unfavorable inhibition activity. Regarding H-bond donors, it can be observed from the distribution of blue/red cubes that in regions a1, b1, b2, and b4, the presence of groups with H-bonds favor/disfavor an increase in inhibitory activity. For hydrophobic nonpolar groups, it is evident that regions b2 and b3 favor an increase in inhibitory activity in the presence of hydrophobic groups compared to other regions, which exhibit a similar distribution of blue/red cubes.
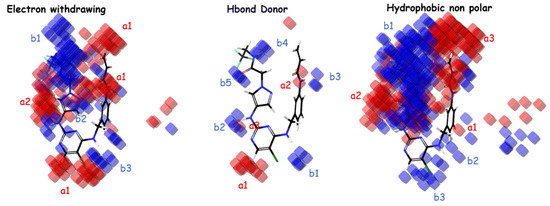
Figure 3.
The atom-based PHASE 3D-QSAR model utilizes visual representations to depict different molecular characteristics, such as electron-withdrawing, hydrogen bond donor, and hydrophobic properties. In these representations, blue-colored cubes indicate positive coefficients or an increase in activity, while red-colored cubes indicate negative coefficients or a decrease in activity.
2.3. Design of New Molecules Based on QSAR Models
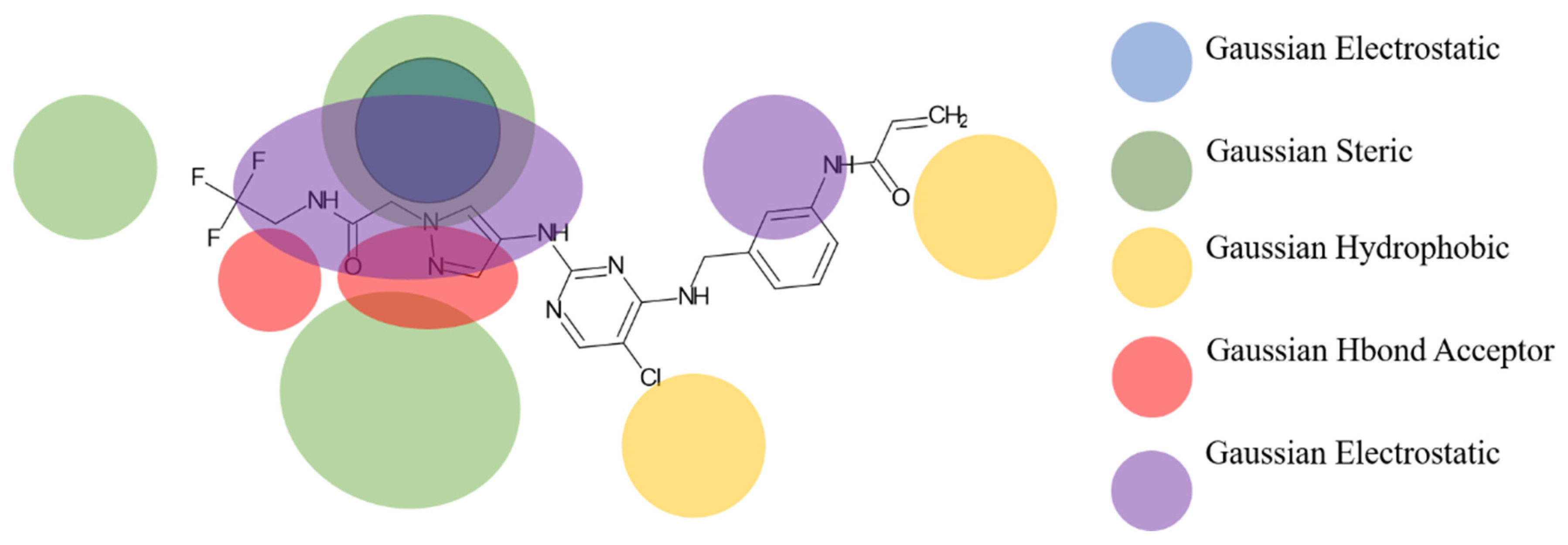
Based on the best models obtained from field-based and atom-based, the scheme in Figure 4 facilitates the analysis for designing new molecules presented in Table 5.
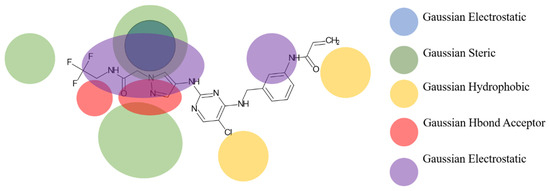
Figure 4.
Regions favoring the development of inhibitory activity pIC50.

Table 5.
Newly designed compounds with their predicted pIC50 for QSAR models.
2.4. Pharmacophore Model Analysis
The results of the pharmacophore hypothesis analysis include different scores for several models. These scores are utilized to assess the quality of pharmacophore models and their relevance for the identification of active chemical compounds. In this section, the results for the DDHRR_1 model will be discussed, along with global scores for other models for comparison (Figure 5).
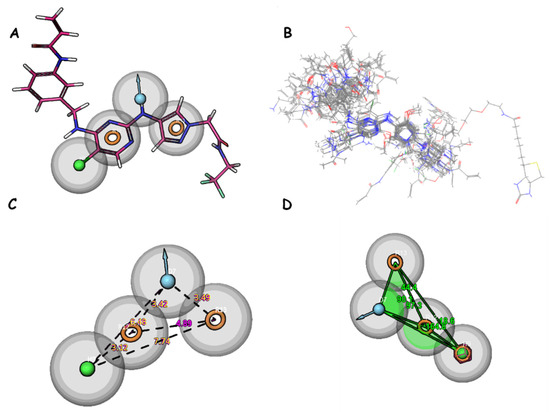
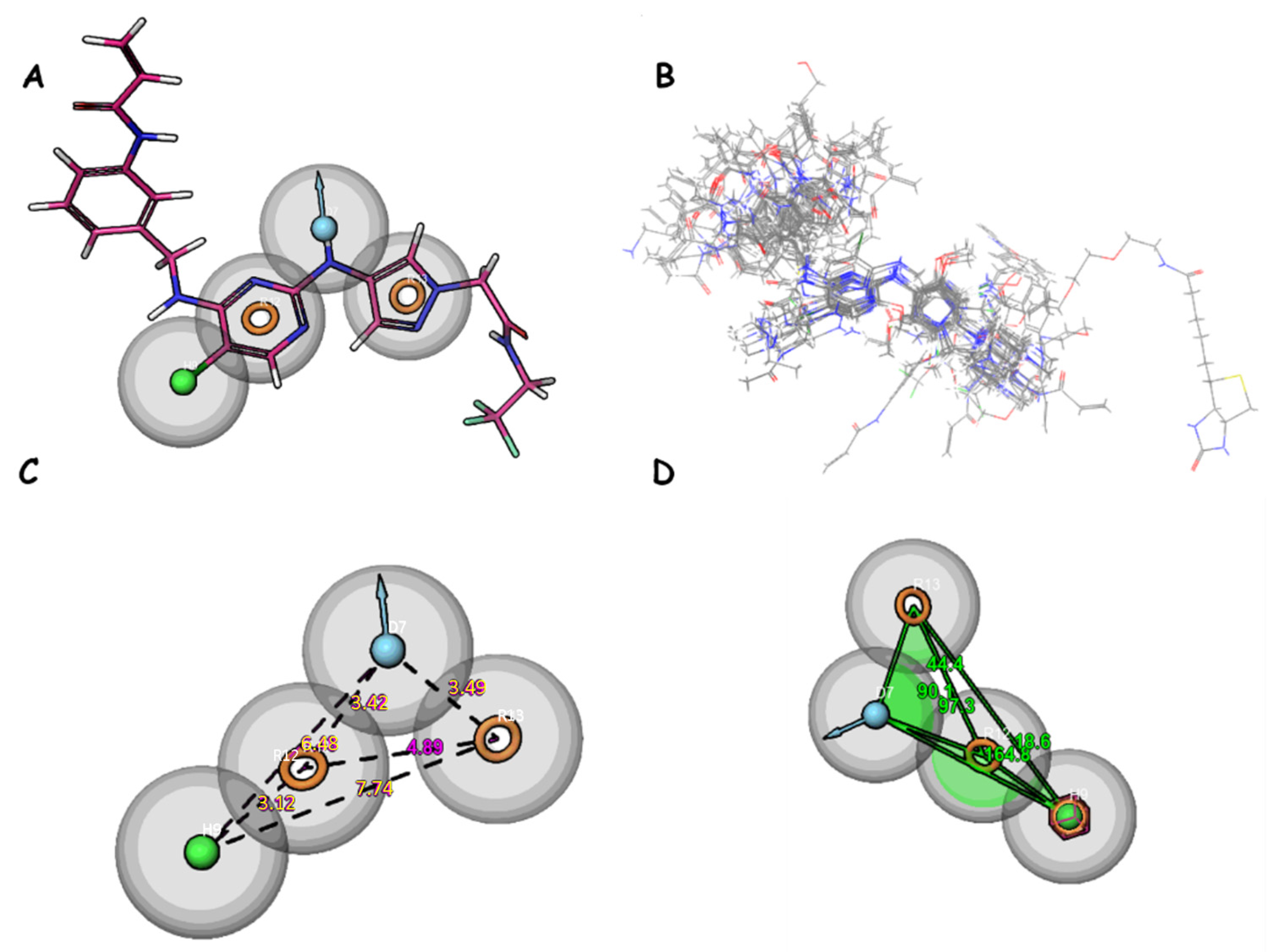
Figure 5.
(A) Pharmacophore features of DHRRR_1; (B) alignment of studied compounds; (C) distance between features; and (D) angles between features. Yellow circles represent aromatic rings. The blue balls indicate donor groups, while the green balls indicate hydrophobic groups.
For the DDHRR_1 model, a survival score of 6.265 is achieved, a site score of 0.837 is attained, a vector score of 0.951 is recorded, a volume score of 0.765 is registered, a BEDROC score of 0.932 is obtained, and a PhaseHypoScore of 1.308 is measured (Table 6 and Table 7). The survival score results from a combination of these scores with adjustable weights determined by the user. Scoring is executed on ligands within the active set, constituting ligands utilized in hypothesis development, for all selected variants listed in the variants table within the find common pharmacophores step. The vector score represents the average of the cosines of the angles between the vectors of each aligned ligand and those of the reference ligand. A good survival score is achieved by this model, indicating that active compounds can be effectively identified by it. A notably high site score is also achieved, suggesting precision in locating active sites. Furthermore, a high BEDROC score is recorded, indicating that active compounds can be rapidly retrieved by this model. Among models with comparable survival scores, DDHRR_1 stands out due to its high site score, implying that active site prediction is conducted exceptionally well by it. Additionally, when compared to similar models, DDHRR_1 boasts a high vector score, indicating that better discrimination between different spatial orientations of molecules is achieved. In terms of volume and BEDROC, DDHRR_1 is also competitive when compared to other models.

Table 6.
Various pharmacophore hypotheses were generated by utilizing the compounds and their corresponding activities.

Table 7.
Evaluation metrics for hypothesis DHRR_1.
Validation of Pharmacophore Models
It is essential to consider several metrics to evaluate the performance of a binary classification model. ROC (alpha) and RI (alpha) provide an overall assessment of the model’s discriminatory ability, but it is also important to consider other metrics such as precision, recall, and F-measure. These metrics allow us to assess the model’s performance at different classification thresholds and account for the imbalance between positive and negative classes. The results indicate that the pharmacophore model DHRR_1 achieved good performance scores, with an EF1% of 1.31. BEDROCK (alpha) and RI (alpha) are performance evaluation metrics for a binary classification model. ROC (alpha) is the area under the ROC curve using the alpha threshold for classification, and RI (alpha) is the true positive rate at a false positive rate corresponding to the alpha threshold (Table 7). These metrics are often used to evaluate a model’s ability to discriminate between positive and negative classes. It suggests that the ranking and scoring method used in the DHRR_1 analysis successfully captured all the known activities in the ranked list.
Among the 75 evaluated ligands, including both actives and decoys, a total of 58 molecules are identified as actives. This means that all known active molecules are included in the ranked list of ligands. These results suggest that the ranking method used for the DHRR_1 analysis effectively identified all known active molecules in the ranked list. The provided results suggest that the hypothesis phase achieved a high hypothesis PhaseHypoScore, strong enrichment (EF1%) in the top 1% of the results, and perfect matches for all expected targets. The high BEDROC160.9 score further indicates good early enrichment performance. The percentage of actives increases as we consider a larger percentage of decoys. In the top 10% of decoys, there are five actives, representing 10.4% of the total actives. The top 20% of decoys have 19 actives, representing 39.6% of the total actives. As we consider a larger percentage of results, the count and percentage of actives increase. In the top 2% of results, one active represents 2.1% of the total actives. In the top 5% of results, three actives represent 6.2% of the total actives. In the top 10% of results, five actives represent 10.4% of the total actives. In the top 20% of results, 11 actives represent 22.9% of the total actives. The enrichment factors (EFs) indicate a modest enrichment of actives in the sampled data, with EF values ranging from 1.1 to 1.3 depending on the sample size or the percentage of actives recovered.
The results demonstrate that the DHRR_1 model achieved strong performance in capturing known active molecules. The hypothesis phase showed high scores and strong enrichment, indicating its effectiveness in identifying active compounds. Additionally, the analysis revealed an increasing percentage of actives as larger result percentages were considered, suggesting a promising enrichment trend. These findings highlight the model’s ability to discriminate between positive and negative classes and its potential for identifying active compounds.
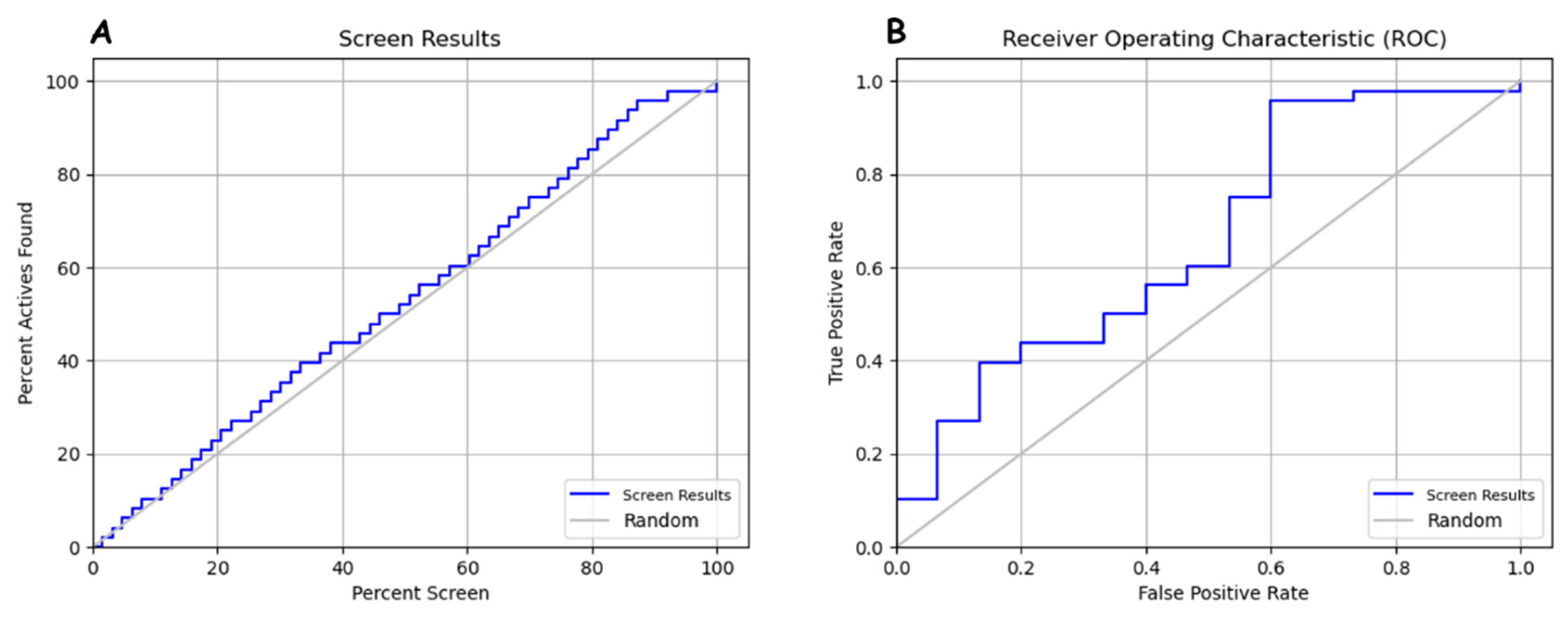
In Figure 6, it appears that the screen results curve behaves in a stair-step fashion in the true positive rate (TPR) zone, remaining above the random curve on an ROC graph. The fact that the screen results curve stays above the random curve in the TPR zone indicates that model DHRRR_1 performs better than mere random chance for detecting true positives. In other words, it can reliably detect positive examples. This configuration suggests that the pharmacophore or screening model strongly detects true positives while maintaining a relatively low false positive rate. This can be a good indicator of the ability to discriminate between positive and negative. Interpreting the relationship between the percentage of active compounds found (percent active found) and the percentage of screening (percent screen) about the screen results and random curves will help evaluate the pharmacophore model’s ability to identify actives in each screening set. Better performance is indicated by the screen results curve being above random and a rapid increase in the percentage of active compounds found as the percentage of screening increases.
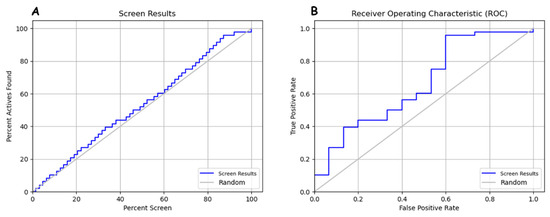
Figure 6.
(A) Relationship between percent active found and percent screen in model evaluation. (B) ROC curve analysis for pharmacophore model.
2.5. Identification of Compounds Using Pharmacophore Model DHRRR_1
The newly identified molecules obtained through the utilization of the DHRRR_1 pharmacophore model by screening a database of JAK3 inhibitor structures obtained from the literature and PubChem comprise a total of 568 compounds. The new compounds are identified based on the pharmacophore model in Figure 5, provided that the molecules exhibit a low RMSD of less than 0.5 Å and the structures of the compounds show a comparable similarity with the identified characteristic groups in the DHRR pharmacophore module (Table 8).

Table 8.
Newly identified compounds with their predicted pIC50 for QSAR models.
2.6. ADMET Analysis
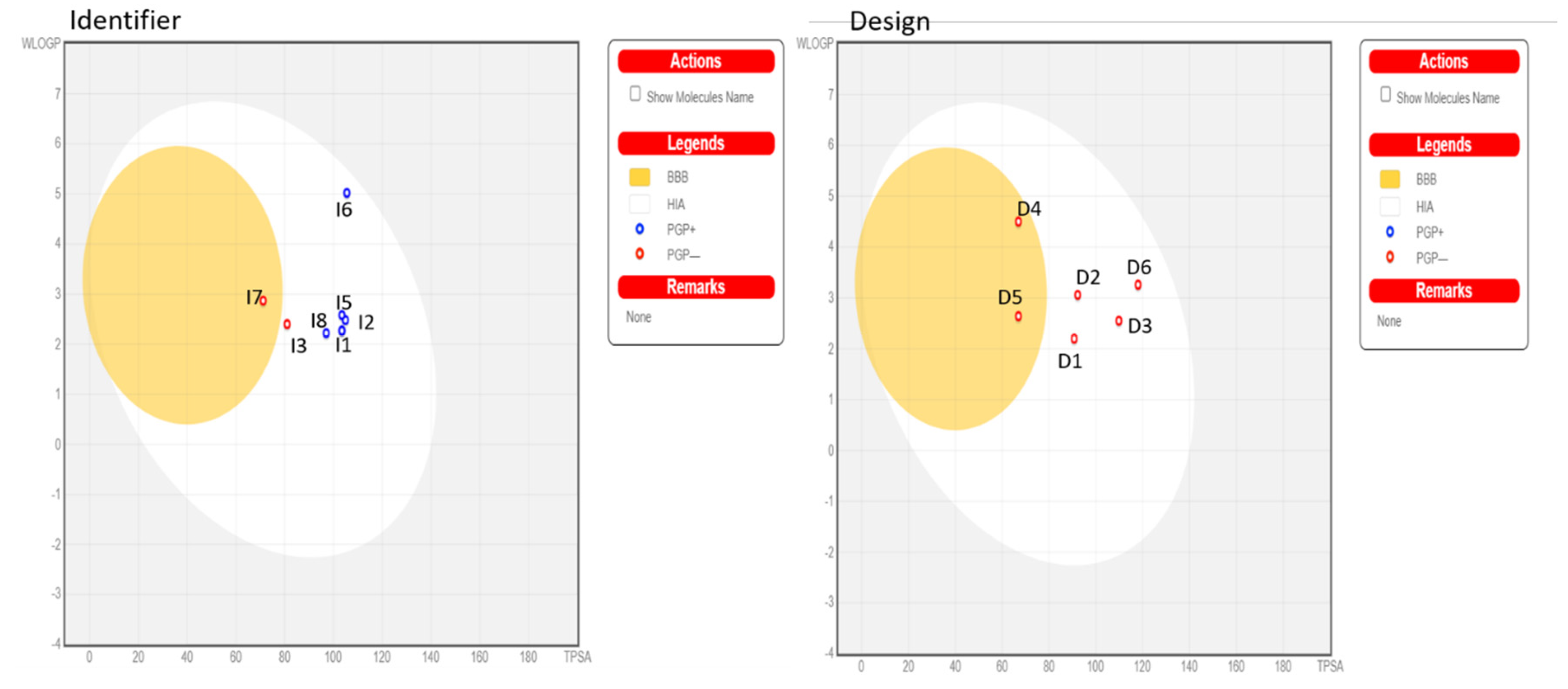
According to ADMET rules [36,37], for designing new compounds (Table 9 and Table 10), the value of logS reflects the drug’s solubility. The smaller the value, the less soluble the compound is in water. When logS are less than −6.0, the compounds are considered poorly soluble and insoluble. A molecule with less than 30% absorption is considered weakly absorbed, while molecules with an absorption greater than 30% are considered to have high absorption. The unit of BBB penetration is cm/s. Molecules with logBB greater than −1 are classified as BBB+ (Category 1), while molecules with logBB less than or equal to −1 are classified as BBB− (Category 0). BBB− indicates that the molecule has a low capacity to penetrate the blood–brain barrier (BBB) or does not penetrate at all. This may be desirable for certain drugs targeting the central nervous system (CNS) to minimize side effects or undesirable interactions with the brain. BBB+ indicates that the molecule has a high capacity to penetrate the BBB. This may be desirable for certain drugs that require direct access to the brain to be effective in treating CNS diseases. The output value represents the probability of being BBB+, ranging from 0 to 1. Molecules D1, D2, D3, and D6 did not show BBB penetration, whereas D4 and D5 demonstrated BBB penetration, suggesting a risk to the CNS. Since the inhibition of CYP3A4 remains a therapeutic target for diseases, especially rheumatoid arthritis, the results suggest that compounds D1, D2, and D5 could inhibit CYP3A4. The total clearance constant (TC), which indicates the drug’s clearance, is used to evaluate the drug’s half-life time. A low TC value indicates a long half-life time of the drug. It encompasses both hepatic and renal clearance and is important for bioavailability and determining dosage rates to achieve steady-state concentrations. All compounds exhibit low total clearance, ranging from −0.059 to 0.19 mL/min/kg, indicating a long half-life. For toxicity, the molecules did not show a positive AME test result.

Table 9.
ADMET evaluation of newly designed compounds.

Table 10.
ADMET assessment of newly identified molecules.
Similarly, in the ADMET analysis of the identified compounds, all molecules with logS greater than −6 suggest solubility. For absorption, all molecules exhibit high absorption rates above 30%. Regarding penetration, all molecules, except I7, penetrate the BBB and are classified as BBB−. In contrast, the other molecules are not classified as BBB+ and do not penetrate the BBB (Figure 7). For the inhibition of CYP3A4, molecules I1, I4, I5, and I6 demonstrate CYP3A4 inhibition. Regarding total clearance, the molecules show low clearance values ranging from 0.48 to 0.70 mL/min/kg. As for toxicity, molecules I1, I2, I4, I5, I7, and I8 do not yield a positive AMES test result compared to the other molecules. D1, D2, and I1 have been identified as the top compounds for the MD based on the ADMET rules, as well as their strong affinity.
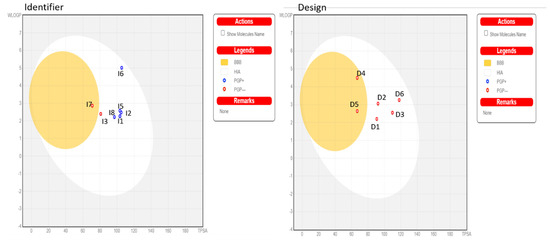
Figure 7.
SwissADME analysis of boiled egg.
2.7. Analysis Docking of Selected Molecules (Covalent Docking between Identified and Designed Molecules)
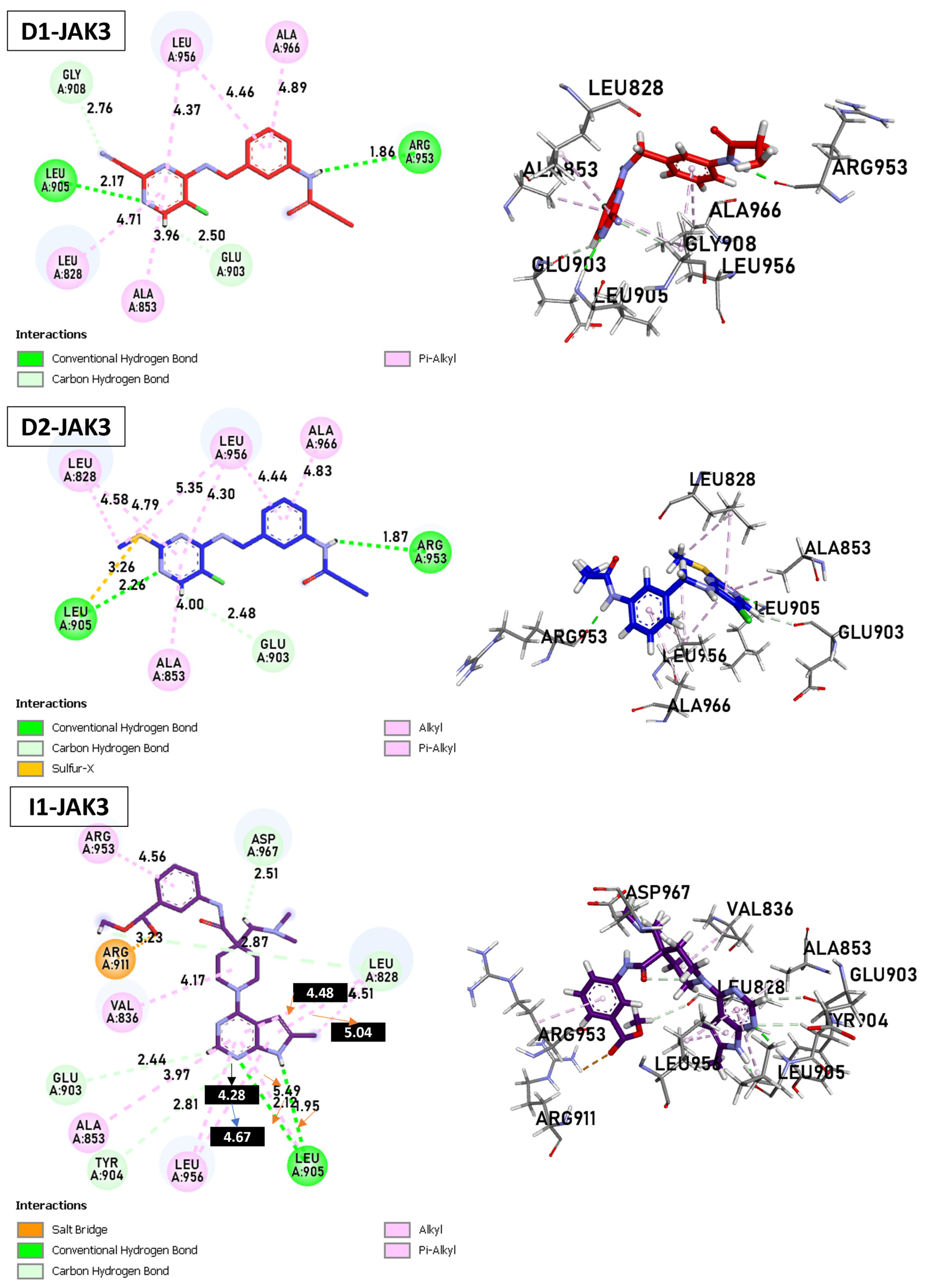
Today, covalent docking has emerged as an irreversible method with great potential for targeting autoimmune diseases, specifically focusing on the protein JAK3 (ID: 4Z16). JAK3 is one of the pioneering proteins that exhibit remarkable resolution and demonstrates a superior fit to experimental data through the better ligand structure. A co-crystallized ligand (4LH) has been identified, which forms a strong covalent bond with Cys909 by employing an acrylaldehyde moiety through a 1,4 Michael addition reaction. In this context, ligands D1 and D2 have been carefully selected and designed, incorporating a similar acrylaldehyde moiety (Figure 8). On the other hand, ligand I1 utilizes an ester group and undergoes covalent docking to establish its binding (Figure 8).
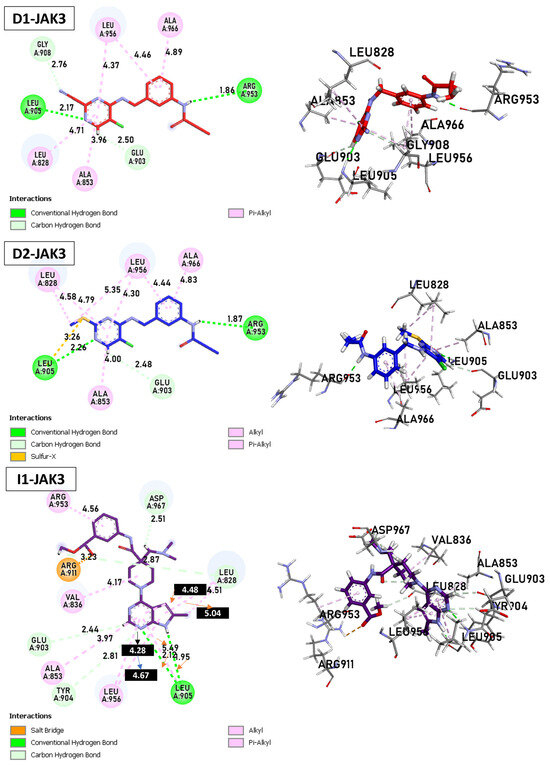
Figure 8.
Binding interactions of new compounds D1, D2, and I1 with JAK3 (PDB ID: 4Z16), illustrated in 3D and 2D diagrams.
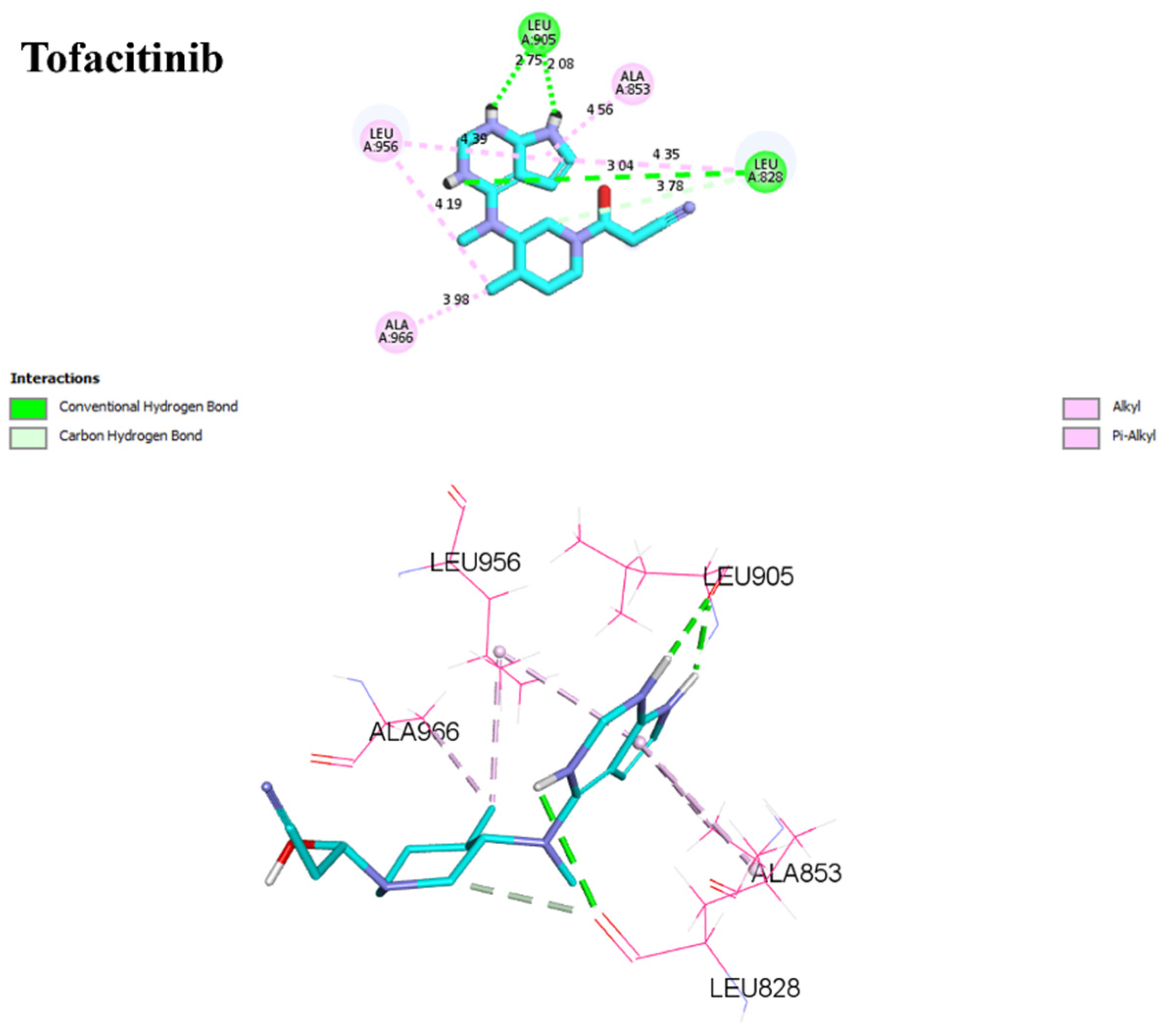
For D1, residues Arg953 and Leu905 form hydrogen bonds with distances of 1.86 and 2.17 Å. Leu828, Ala853, Leu956, and Ala966 form π–alkyl interactions with distances ranging from 3.96 to 4.89 Å. In the case of D2, Arg953, and Leu905 once again form hydrogen bonds, with distances of 1.87 and 2.26 Å, respectively. A sulfur–X interaction is observed towards Leu905, Leu828, and Leu956 at distances of 3.26, 4.58, and 4.79, respectively. The same π–alkyl-type interactions are observed toward residues Leu828, Ala853, Leu956, and Ala966, with similar distances to D1. Finally, I1 formed a salt bridge interaction with Arg911, with 3.23 Å. I1 forms two hydrogen bonds with Leu905, with respective distances of 2.12 and 1.95 Å. The π–alkyl and VdW interactions are observed between aromatic residues up to 3 Å. In Figure 9, tofacitinib formed multiple hydrogen bonds with Leu828 and Leu906 at distances of 3.04, 2.75, 2.08, and 3.78 Å. Additionally, hydrophobic interactions were observed between tofacitinib and residues Leu956, Ala966, Leu828 (4.35 Å), Ala853 (4.56 Å), and Leu956 (4.39 Å).
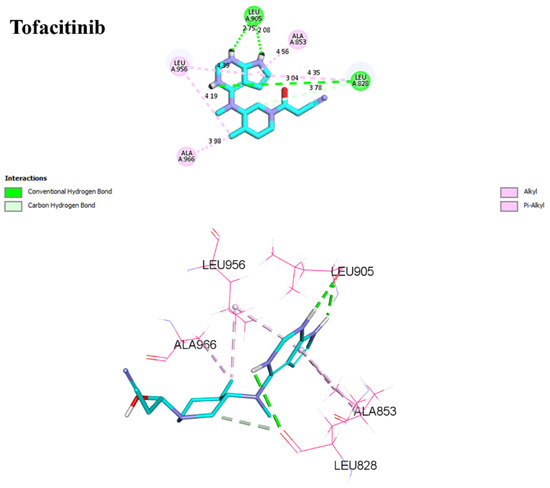
Figure 9.
Binding interactions of tofacitinib with JAK3 (PDB ID: 4Z16), illustrated in 3D and 2D diagrams.
Compared to the tofacitinib drug, compounds D1, D2, and I1 all form key hydrogen bonding and electrostatic interactions with the target protein, explaining their affinity. In the presence of covalent bonds with Cys909 and hydrogen bonds, non-covalent interactions increase, thereby enhancing affinity and promoting ligand binding.
2.8. Molecular Docking with CYP3A4
The metabolism of the enzyme CYP3A4, involving compounds D1, D2, and I2, was investigated (Figure 10). The enzyme (ID: 5VCC) selected for this study also exhibits a high resolution and good fit to experimental data through the better ligand structure. This protein choice provides valuable insights into the metabolic processes within the enzyme. Notably, a co-ligand, HEM, remains a target of interest due to its ability to activate CYP3A4. Consequently, there is a focus on inhibiting this enzyme as a potential strategy to modulate its activity.
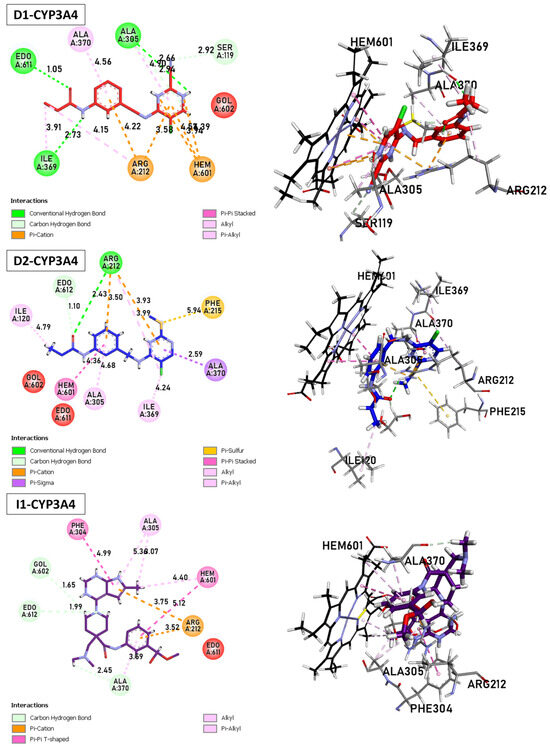
Figure 10.
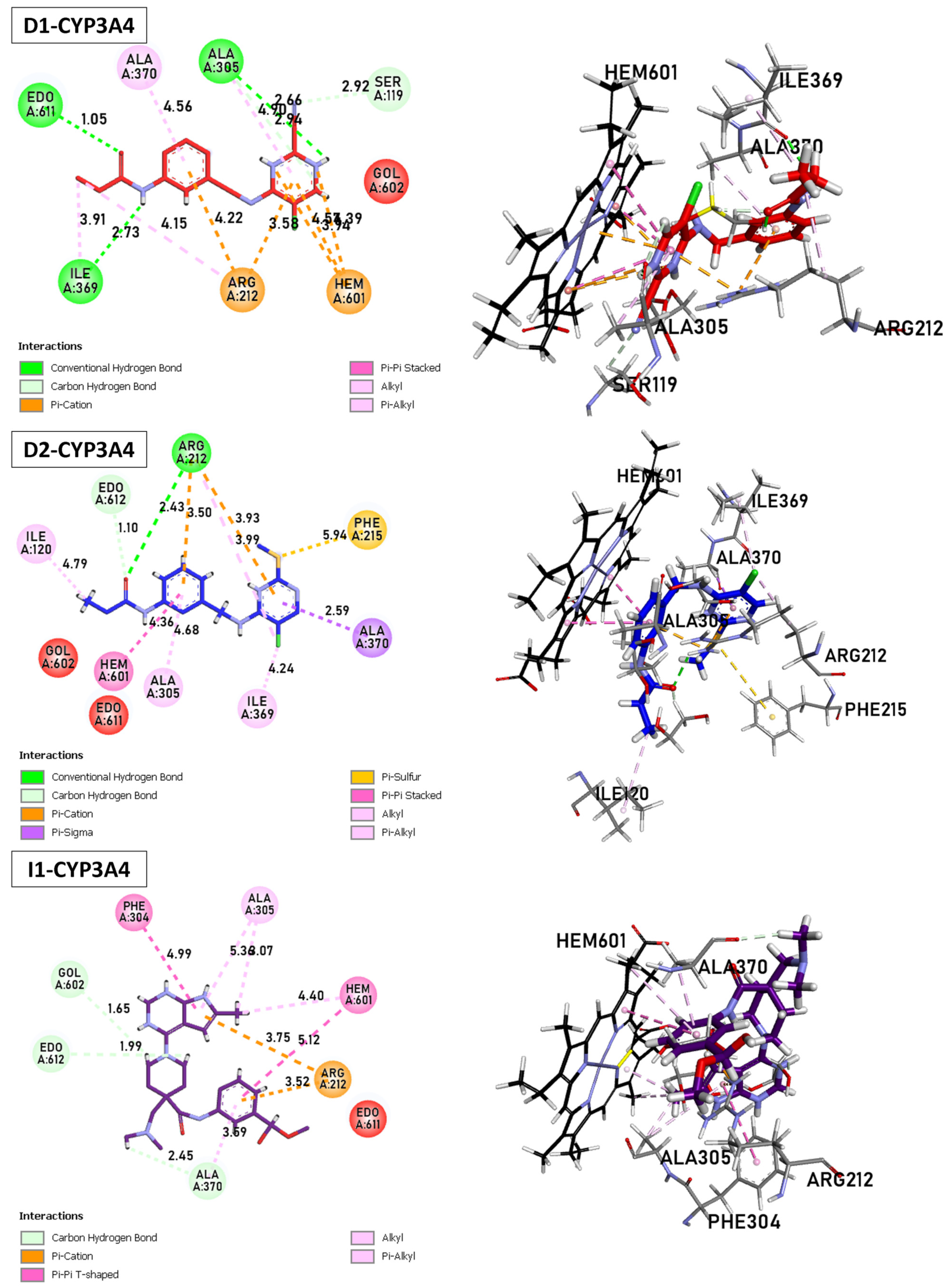
Binding Interactions of new compounds D1, D2, and I1 with CYP3A4 (PDB ID: 5VCC), illustrated in 3D and 2D diagrams.
D1: The shortest hydrogen bond is formed between Arg212 and Hem601, with 3.24 Å. Other hydrogen bonds are observed with Ala305 (2.66 Å), Ile369 (2.73 Å), and Ser119 (3.49 Å). Carbon–hydrogen bond interactions are also present with Ala305, Ser119, and Ala370. The π–cation interactions between Arg212 and Hem601/Hem601 are observed, with distances ranging from 3.58 to 4.22 Å. Several stacked π–π and T-shaped interactions between Hem601/Hem601 and aromatic residues of CYP3A4, such as Phe215 and Phe304. Finally, alkyl interactions are present between Hem601 and Ile369, Ile120 and Ala305, as well as between Arg212 and Hem601. D2: Similar to D1, the shortest hydrogen bond involves Arg212 and Hem601, with 3.14 Å. Another bond of this type is formed with Ile369 (4.24 Å). The π–cation and alkyl interactions are observed between Arg212 and Hem601. Numerous stacked π–π and π–alkyl interactions are found between Hem601 and aromatic residues Phe and Ala. D3 The shortest carbon–hydrogen bonds are established between Edo612/Gol602 and Hem601, with distances of 1.99 and 1.65 Å, respectively. The π–cation interactions (3.52–3.75 Å), π–H-bond donor (3.42 Å), and T-shaped π–π interactions are present between Arg212, Ser119, Ala370, and Hem601. Finally, alkyl interactions involve the residues Hem601, Ala305, and Ala370.
D1 and D2 inhibitors, as well as I1, target the Hem molecule to decrease its activity, which in turn affects the activity of CYP3A4. The shortest hydrogen bond in both D1 and D2 inhibitors involves Arg212 and Hem601. This interaction suggests a strong binding between the inhibitor and the target molecule. Additionally, other hydrogen bonds are observed with different residues, such as Ala305, Ile369, and Ser119, indicating multiple binding sites and potential stability of the inhibitor Hem complex. Both D1 and D2 inhibitors exhibit π–cation interactions between Arg212 and Hem601, indicating the presence of positive charges in the inhibitor that interact with the π–electron system of Hem601. These interactions contribute to the binding affinity between the inhibitor and Hem.
Furthermore, alkyl interactions are observed between the inhibitor and various residues, suggesting hydrophobic interactions that can enhance the stability of the inhibitor–Hem complex. D1 specifically shows stacked π–π and T-shaped interactions between Hem601 and aromatic residues of CYP3A4, such as Phe215 and Phe304. These interactions indicate potential binding between the inhibitor and the aromatic residues of the target enzyme, which can affect the enzyme’s activity by interfering with its active site or substrate binding. In D2, numerous stacked π–π and π–alkyl interactions are found between Hem601 and aromatic residues Phe and Ala. These interactions suggest a strong binding between the inhibitor and the target molecule, potentially disrupting the activity of CYP3A4. Overall, these inhibitors (D1, D2, and I1) exhibit various interactions with the Hem molecule, including hydrogen bonds, π–cation interactions, and alkyl interactions. These interactions contribute to the inhibition of Hem activity and subsequently impact the activity of CYP3A4, a crucial enzyme in drug metabolism.
2.9. Molecular Dynamics Simulation Analysis
2.9.1. RMSD, RMSF, RoG, and SASA Analyses
The analyses of RMSD, RMSF, RoG, SASA, flexibility, and PCA provide crucial insights into different aspects of the studied complexes. RMSD measures the average deviations of atomic positions between initial and final structures, enabling the assessment of stability and conformational variations in the complexes. RMSF quantifies the average fluctuations of atomic positions during the simulation, revealing the flexibility of residues and their relative stability. RoG measures the three-dimensional compactness of a molecule, providing information about the size and shape of the studied complex. SASA evaluates the solvent-accessible surface of a molecule, giving insights into the accessibility of residues and their exposure to the environment. FEL measures a molecule’s ability to deform or change conformation, thus revealing its structural plasticity. PCA analysis is a statistical technique that reduces data complexity by identifying the main modes of variation in the studied structures. These parameters yield results that enable the assessment of stability, flexibility, compactness, solvent accessibility, interactions, and variation modes of the studied complexes, contributing to a better understanding of their structural behavior and properties.
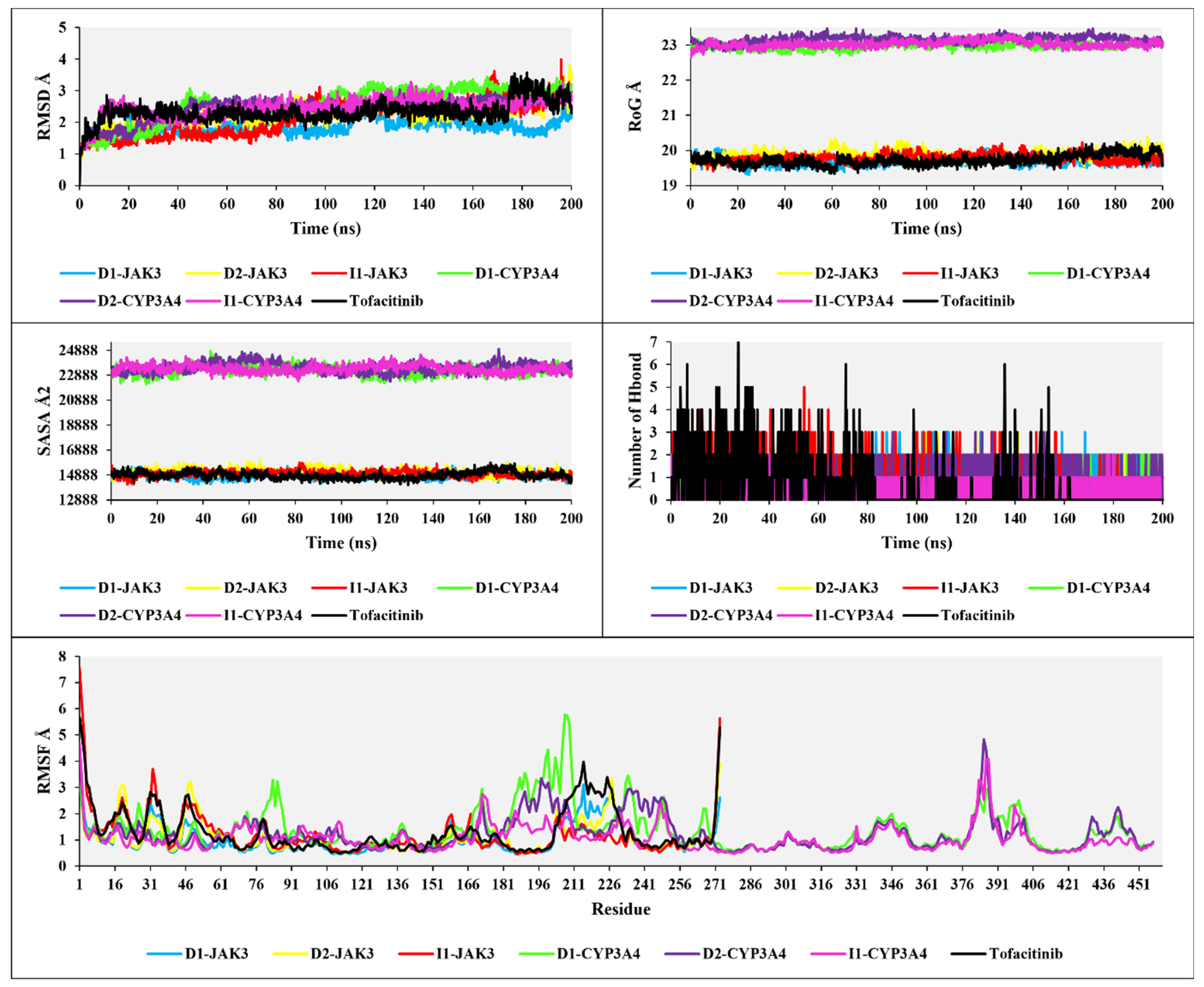
The analyses of RMSD, RMSF, RoG, and SASA in Figure 11 provide the following results: The RMSD analysis for the three compounds, D1, D2, and I1, with JAK3 and CYP3A4, shows RMSD values of 1.5 Å, 2 Å, 2.2 Å, 2.6 Å, 2.5 Å, 2.4 Å, and 2 Å, respectively. The RMSD analysis, compared to tofacitinib, demonstrates favorable stability, with some exceptions in the RMSD changes. For D1-JAK3, there is a fluctuation ranging from 1.5 Å to 2 Å between 115 ns and 125 ns. D2-JAK3 exhibits a variation between 2 Å and 3.5 Å between 190 ns and 200 ns. I3-JAK3 experiences an increase in RMSD between 80 ns and 100 ns, with a variation ranging from 1.5 Å to 2.5 Å, indicating stability. D1-CYP3A4 shows an increase between 30 ns and 40 ns, with an RMSD variation reaching up to 2.5 Å, followed by stability until 200 ns. D2-CYP3A4 undergoes an increase between 20 ns and 40 ns, with an RMSD variation up to 2.5 Å, followed by stability. I1-CYP3A4, after 5 ns, exhibits favorable stability up to 200 ns. The RMSD analyses of the compounds D1, D2, and I1 with JAK3 and CYP3A4 reveal overall stability, indicating promising stabilities.
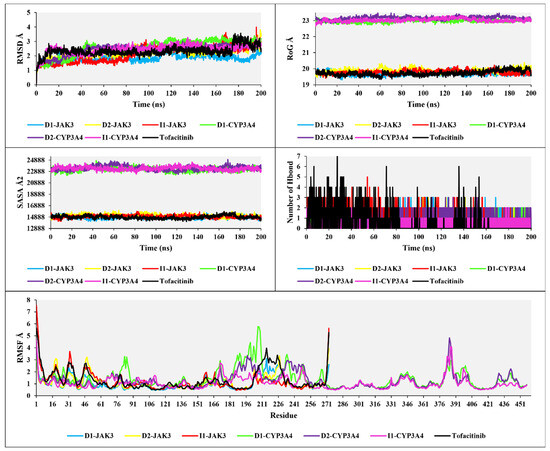
Figure 11.
The plots of RMSD, RoG, SASA, H-bond, and RMSF analyses of new compounds with tofacitinib drug.
The analyses of RoG and SASA for the compounds with JAK3 and CYP3A4 in interaction show a stable RoG ranging from 19 to 20 Å for the compounds with JAK3 and a similarly stable RoG ranging from 23 to 23.3 Å during the 200 ns simulation. Likewise, for SASA, with JAK3 present, a stable SASA ranging from 14,985 to 15,060 Å2 is observed, while with CYP3A4, it is situated between 22,990 and 23,225 Å2. These results indicate good structural complementarity and a robust binding between the protein and the ligand. The stability of the solvent-accessible surface also suggests that the ligand is well-positioned and protected from the aqueous environment, which is generally beneficial for efficient and specific interaction with the target protein.
The H-bonds for the three compounds interacting with JAK3 and CYP3A4 reveal H-bonds ranging from a maximum of seven to a minimum of one. Compared to tofacitinib, the H-bond results for D1, D2, and I1 in interaction with JAK3 and CYP3A4 show consistent H-bond interactions without any disruptions, with a minimum of one H-bond observed. In contrast, tofacitinib experiences H-bond disruptions between 80 ns and 200 ns. The H-bond analysis indicates stable and favorable interactions between the compounds and JAK3/CYP3A4, consistent with H-bonds throughout the simulation. This suggests that the studied compounds exhibit strong binding affinity and potential for effective interaction with the target proteins. The comparison to tofacitinib highlights the stability of the observed H-bond interactions, further supporting the potential of the studied compounds as promising candidates for further drug design and development exploration.
The analysis of RMSF for the compounds D1, D2, and I1 in interaction with JAK3, considering 275 residues, reveals rigid flexibility and overall stability of the residues. Compared to tofacitinib, there are exceptions for certain residues where the RMSF values exceed three, indicating relatively higher fluctuation and lower stability. However, the superposition of the RMSF values suggests a generally stable RMSF pattern for the studied complexes. Furthermore, when analyzing the RMSF with 450 residues for D1, D2, and I1 in interaction with CYP3A4, a similar stable RMSF pattern is observed compared to tofacitinib. The RMSF analysis indicates that the compounds D1, D2, and I1, in interaction with JAK3, exhibit rigid flexibility and overall stability of the residues. Although there are exceptions with higher RMSF values for specific residues, the superposition analysis suggests a stable RMSF pattern for the studied complexes. Additionally, when considering the interaction with CYP3A4, the compounds D1, D2, and I1 also show a stable RMSF comparable to tofacitinib.
The comprehensive analyses of RMSD, RoG, SASA, H-bonds, and RMSF provide valuable insights into the stability and structural characteristics of D1, D2, and I1 in interaction with JAK3 and CYP3A4. The RMSD analysis reveals overall stability with some exceptions, indicating promising stability for the studied complexes. The RoG and SASA analyses demonstrate good structural complementarity and robust binding between the proteins and ligands. The H-bond analysis indicates stable and consistent interactions, suggesting strong binding affinity. Moreover, the RMSF analysis highlights the residues’ generally stable and rigid flexibility with some exceptions.
2.9.2. PCA and FEL Analyses
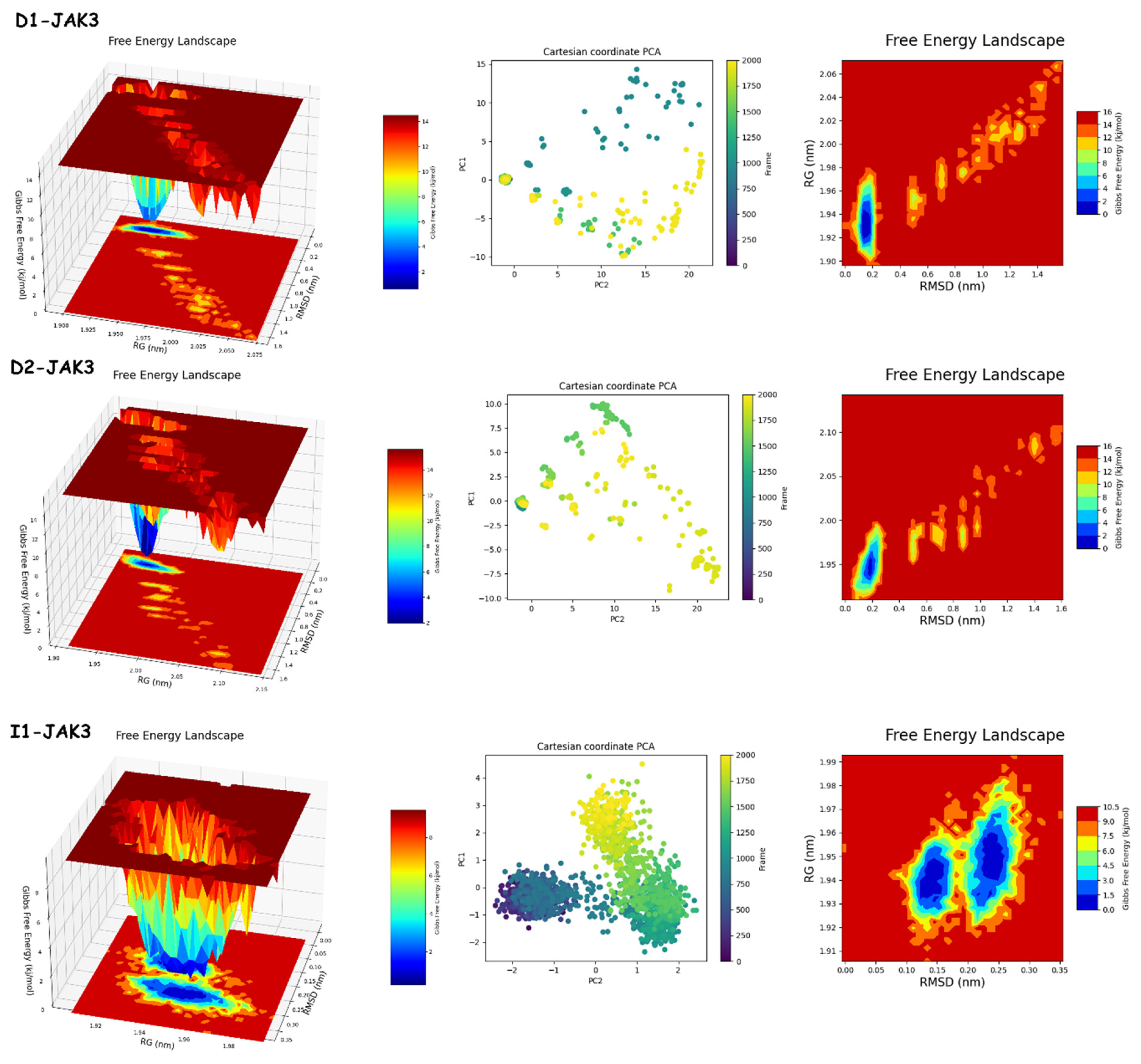
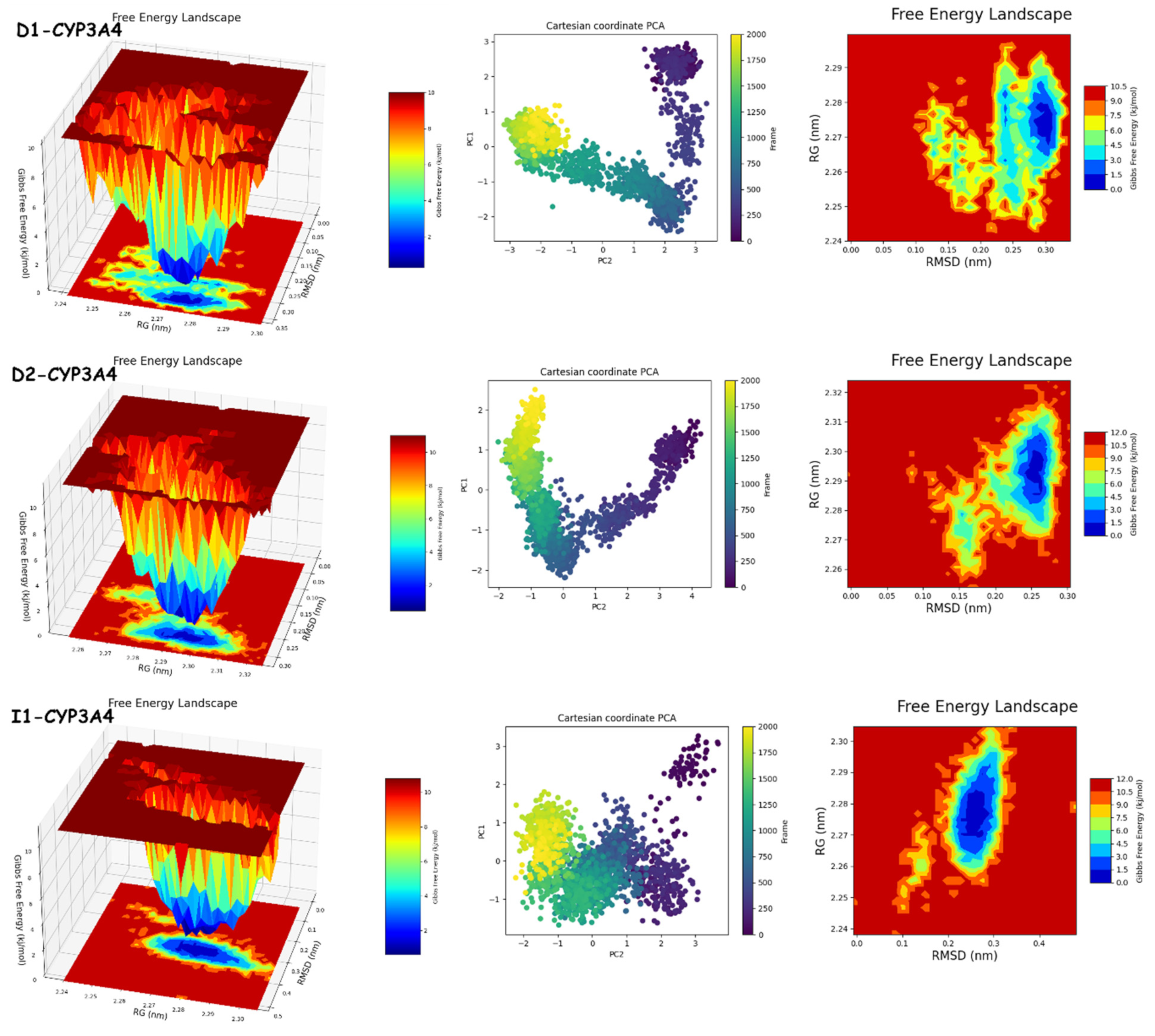
The results of PCA and FEL in Figure 12 and Figure 13 provide the following. First, the PCA results are as follows: D1-JAK3 experienced a PCA for PC1 and PC2 ranging from −10–15 to 0–20, respectively. D2-JAK3 experienced a PCA for PC1 and PC2 ranging from −10–15 to 0–20, respectively. I1-JAK3 experienced a PCA for PC1 and PC2 ranging from −2–10 to −2–2, respectively. D1-CYP3A4 experienced a PCA for PC1 and PC2 ranging from −2–3 to −3–3, respectively. D2-CYP3A4 experienced a PCA for PC1 and PC2 ranging from −2–2 to −2–4, respectively. I1-CYP3A4 experienced a PCA for PC1 and PC2 ranging from −1–3 to −2–3, respectively. Secondly, FEL: D1-JAK3 had a stable conformation energy minimum between an RMSD of 0.19 nm and an RoG of 1.94 nm. D2-JAK3 had a stable conformation energy minimum between an RMSD of 0.19 nm and an RoG of 1.95 nm. I1-JAK3 had two stable confirmation energy minima located between an RMSD of 0.15 nm and an RoG of 1.94 nm and between an RMSD of 0.24 nm and an RoG of 1.95 nm. D1-CYP3A4 had two stable confirmation energy minima located between an RMSD of 0.3 nm and an RoG of 2.27 nm. D2-CYP3A4 had two stable confirmation energy minima located between an RMSD of 0.2209 nm and an RoG of 2.29. I1-CYP3A4 had two stable confirmation energy minima located between an RMSD of 0.15 nm and an RoG of 2.27 nm.
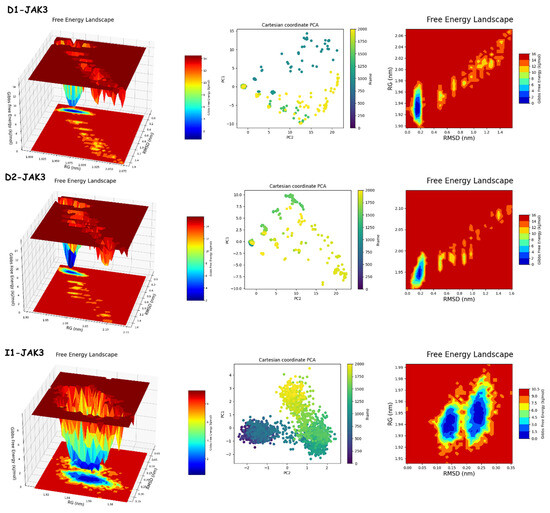
Figure 12.
PCA and FEL analyses of new compounds.
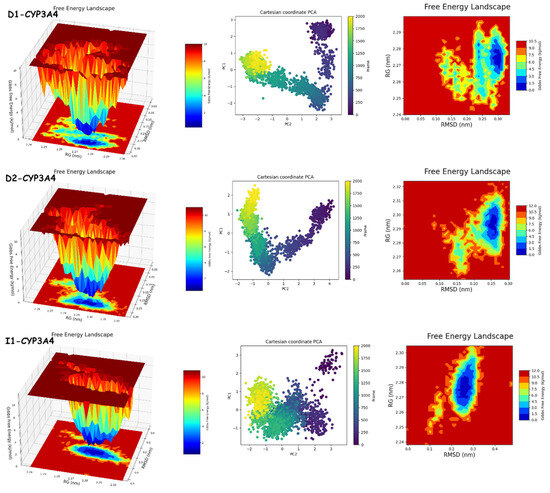
Figure 13.
Comparative analysis of FEL and PCA graphs in 2D and 3D for compounds D1, D2, and I1.
The results of the PCA analysis indicate the principal components (PC1 and PC2) for each protein (D1-JAK3, D2-JAK3, I1-JAK3, D1-CYP3A4, D2-CYP3A4, and I1-CYP3A4) and their corresponding ranges. On the other hand, the FEL analysis provides information about the stable conformation energy minima for each protein, as indicated by their respective RMSD and RoG values. These findings contribute to a better understanding of the structural characteristics and dynamics of the proteins under investigation.
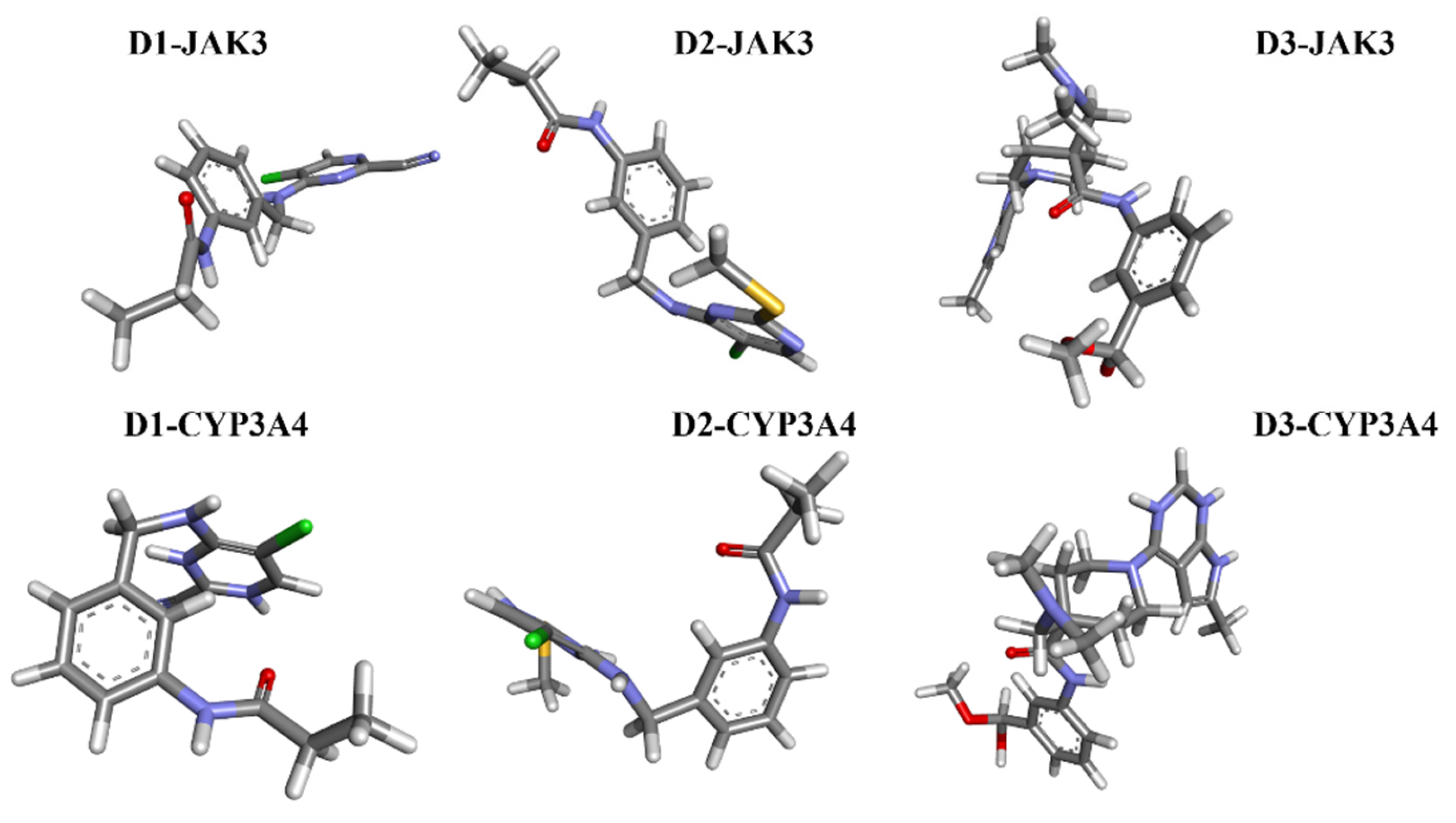
The analyses of FEL and PCA allow for the extraction of stable conformations corresponding to each divergent minimum during the 200 ns simulation on the target. The results in Figure 14 suggest that the new compounds can adopt multiple stable conformations in the active site to achieve inhibition of the studied proteins, JAK3 and CYP3A4.
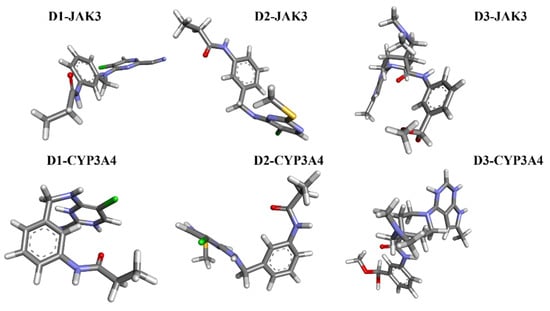
Figure 14.
The stable conformational structure for each new compound that favors the energy minima of FEL and PCA.
2.10. MM/GBSA Analysis
The results of MM/GBSA analysis can be used to prioritize compounds for further optimization or guide the design of new ligands with improved binding affinity. The MM/GBSA analysis is a valuable tool for understanding and predicting the binding energetics of molecular complexes, aiding in structure-based drug design and optimization.
The results of the MM/GBSA analysis show the energy contributions of different components and complexes (Table 11). ΔTOTAL represents negative values that indicate favorable binding, while positive values suggest unfavorable binding. In this case, D1-JAK3, D2-JAK3, and I1-JAK3 show negative ΔTOTAL values, indicating favorable binding to JAK3. On the other hand, D1-CYP3A4, D2-CYP3A4, and I1-CYP3A4 exhibit positive ΔTOTAL values, suggesting less favorable binding to CYP3A4. ΔGSOLV represents the change in solvation energy upon complex formation. Positive values indicate an increase in solvation energy, while negative values suggest a decrease. Notably, I1-JAK3 shows a significantly positive ΔGSOLV value, indicating a substantial increase in solvation energy upon complex formation.

Table 11.
Energy contributions and binding characteristics of complexes.
ΔGGAS represents the change in the gas–phase interaction energy upon complex formation. Negative values indicate favorable interactions, while positive values suggest unfavorable interactions. In this case, D1-JAK3, D2-JAK3, and I1-JAK3 exhibit negative ΔGGAS values, indicating favorable gas–phase interactions with JAK3. ΔESURF represents the change in the surface energy upon complex formation. Negative values suggest a decrease in surface energy, indicating favorable binding. All complexes show negative ΔESURF values, indicating favorable changes in surface energy. ΔEGB represents the change in the generalized Born energy upon complex formation. Positive values indicate an increase in the generalized Born energy, while negative values suggest a decrease. Notably, I1-JAK3 shows a significantly positive ΔEGB value, indicating a substantial increase in the generalized Born energy upon complex formation. ΔEEL represents the change in the electrostatic energy upon complex formation. Negative values indicate favorable electrostatic interactions, while positive values suggest unfavorable interactions. In this case, D1-JAK3 and D2-JAK3 exhibit negative ΔEEL values, indicating favorable electrostatic interactions with JAK3. ΔVDWAALS represents the change in the van der Waals energy upon complex formation. Negative values indicate favorable van der Waals interactions, while positive values suggest unfavorable interactions. Notably, D2-CYP3A4 and I1-CYP3A4 show negative ΔVDWAALS values, indicating favorable van der Waals interactions with CYP3A4.
3. Methods and Materials
3.1. Dataset
The dataset used in this study was collected based on previous work and included 75 inhibitors of JAK3 (Table S1, Supplementary Materials), along with their experimentally determined inhibitory biological activity values [38,39]. These biological activity values were converted into pIC50 using the formula −log (IC50 × 10−9). The dataset was randomly divided without any specific rule for this type of study. Fifty-nine molecules were included in the training set, with consideration given to the most active molecule in the training set. Sixteen molecules were reserved for the test set. The table below presents the predicted pIC50 values by both field-based and atom-based QSAR models, along with the residual errors compared to the experimental pIC50 values.
3.2. Building Robust Models: Exploring Three-Dimensional QSAR in Development
3.2.1. Preparation of Ligands
The accurate alignment of compounds is crucial for ensuring the quality and predicted activity of both the 3D-QSAR and pharmacophore models [40]. Initially, the molecular structures were in the 2D-SDF format and subsequently converted into 3D structures (Figure 5).
The LigPrep module of Schrödinger version 2021-3 was used to prepare the ligands, ensuring the generation of high-quality structures with appropriate ionization states, tautomeric forms, ring conformations, and stereochemistry. All the molecules underwent energy minimization using the OPLS_2005 force field to optimize their structures. The alignment of the molecules was achieved using the alignment tool in Schrödinger version 2021-3 into Maestro using the Flexible Ligand Alignment Panel, which provided the capability to execute a versatile alignment for the selected entries in the Project Table. The initial selected entry functioned as a template and remained unchanged. Subsequent ligands underwent a ligand torsional search utilizing ConfGen [41]. The conformers generated by ConfGen were then sequentially aligned with the reference ligand, and the conformer exhibiting the optimal overlap with the reference ligand was selected. This chosen conformer replaced the existing entry, so it was essential to duplicate the original structures if you wished to retain them. It is recommended to employ well-minimized structures as input for flexible ligand alignment. The structures must contain hydrogens, and implicit hydrogens are not allowed [42], taking into consideration the template molecule with the highest pIC50 value.
3.2.2. Field-Based and Atom-Based 3D-QSAR
Researchers have favored quantitative structure–activity relationships (QSARs) for optimizing lead compounds over the years. Nevertheless, conventional QSAR models usually involve only rough approximations of 3D structures. There are two methods available in Maestro for QSAR modeling: atom-based QSAR utilizes atom types and their occupancy within a grid of cubes as independent variables for fitting and predicting properties, and field-based QSAR implements the ComFA/ComSIA approach, using potential values on a grid for fitting and predicting properties. In Maestro, make your selection accordingly.
The PHASE module from Maestro, an interface of Schrödinger’s version 2021-3 utility, was utilized to develop 3D-QSAR models. To better understand the correlation between structural features and biological activity, we aimed to develop both atom-based and field-based 3D-QSAR models. The models were developed by randomly selecting a training set and a test set, following the 80:20 split recommended in the literature, and widely adopted [43,44]. However, we took steps to ensure that the developed models were not the result of random chance, and they were further evaluated for internal and external validation to determine their statistical significance. To ensure the reliability of the developed models, both active and inactive molecules were included in both the training and test sets. The same strategy was used for MLR-based QSAR models, and in all cases, we thoroughly assessed the robustness of our models. The dataset was randomly divided into an 80% training set and a 20% test set, with a PLS factor of 4 applied to both 3D-QSAR models. The random selection made by software was visually verified to ensure diversity among the molecules in the training and test sets. We maintained a 1 Å grid spacing for the selected hypothesis. We developed four atom-based 3D-QSAR models and four field-based models (Table 2, Table 3, Table 4, Table 5 and Table 12). For both the field-based and atom-based models, the training set consisted of 75 molecules, while the test set consisted of 13 molecules (Table S1). The Gaussian field-based 3D-QSAR models incorporated descriptors such as Gaussian steric, electrostatic, hydrophobic, hydrogen bond donor, and hydrogen bond acceptor. In the field-based models, Gaussian intensities were considered as independent variables. Finally, the best-selected 3D-QSAR models were developed to visualize the 3D contour maps associated with structural features (Figure 1). The visualization of QSAR models is important for optimizing the scaffolds.

Table 12.
Statistical analysis of field-based model in 3D-QSAR.
3.2.3. Evaluating the Predictive Power of 3D-QSAR Models: A Comparative Analysis
The essential metrics used for evaluating three-dimensional quantitative structure–activity relationship (3D-QSAR) models will be explored. These metrics provide a crucial insight into the quality and reliability of models, guiding the compound optimization process. R2 (coefficient of determination): The proportion of the variance in the dependent variable explained by the independent variables is measured. An optimal fit is indicated by an R2 close to R2CV (cross-validated coefficient of determination): As with R2, this is calculated using cross-validation methods, assessing the model’s ability to generalize to independent data. RMSE (root mean square error): The average of errors between predicted and observed values is indicated, providing an overall measure of model accuracy. Q2 (cross-validated coefficient of determination): Similar to R2CV, it evaluates how well the model predicts new, unseen data using cross-validation methods. These metrics, when employed, offer a comprehensive understanding of the predictive power and reliability of 3D-QSAR models, contributing to informed decision making in compound design and optimization.
The evaluation of the 3D-QSAR model involved the assessment of key statistical parameters, including the squared cross-validation coefficient (Q2), squared non-cross-validation coefficient (R2), predictive R2, and standard error of estimate (SEE). To determine the internal quality of the developed model, the Q2 value was considered, with a criterion of >0.5 deemed statistically significant. The R2 value was utilized as a relative measure of the regression fit, with a value close to 1.0 indicating a solid fit. Additionally, the standard error of estimate provided insights into the variation in residuals or the regression line [45,46].
3.3. Pharmacophore Hypothesis Generation
The pharmacophore hypothesis is a widely used approach in pharmaceutical chemistry that aims to identify and model the essential interactions between a drug molecule and its biological target. This method is based on the understanding that specific structural or chemical characteristics of the molecule are crucial for its biological activity [12,47,48]. Advanced Schrödinger software version 2021 provides sophisticated tools for generating and validating pharmacophore hypotheses by utilizing information on molecular interactions, such as hydrogen bonds, electrostatic interactions, and hydrophobic interactions [42].
To prepare the structure data file for our test compounds, we utilized the LigPrep panel integrated within Schrödinger software version 2021-3. The ligand chemistry was appropriately normalized and extrapolated for pharmacophore modeling using PHASE, an automated process that aligns the ligands based on their optimal arrangement and shared properties (Figure 5B). The geometrical optimization through condensed Newton conjugate gradient (TNCG) minimization employed the OPLS_2005 force field. Ligands were prepared using LigPrep (Schrödinger version 2021-3) with Epik, adjusting the pH to 7 ± 2.0 units to account for protonation and tautomeric states and utilizing the OPLS_2005 force field. Subsequently, the prepared ligands were imported into the Maestro workspace, and their experimental binding affinities (pIC50) were used to categorize them as active or inactive. The pIC50 values were calculated using the equation pIC50 = −log (IC50), where an IC50 affinity of ≤50 nM corresponded to a pIC50 value greater than 7.5. Inactive molecules were identified using a threshold of 10 µM or a pIC50 value below 7.5. The assumption requirement was set to match at least 50% of the active compounds, and a minimum of five features were preferred for a successful match. The assumption difference criteria remained at their default settings, except for donor and negative molecules, where ionic features were assigned a value of 1 to ensure compatibility between the acceptor and negative features.
3.4. Molecular Docking
Irreversible (covalent) docking and reversible (non-covalent) docking are two distinct approaches used in the field of molecular docking, a computational technique employed to predict the binding affinity and orientation of a small molecule (ligand) within a receptor or target protein [49].
Irreversible (Covalent Docking)
Irreversible (covalent) docking involves the formation of a covalent bond between the ligand and the target protein. In this approach, the ligand is designed to contain a reactive functional group that can form a stable covalent bond with specific amino acid residue in the target protein, typically a nucleophilic residue such as a cysteine or a serine. Once the covalent bond is formed, it is generally irreversible, meaning that the ligand cannot easily dissociate from the target protein. Irreversible docking is often used when designing drugs that require long-lasting or irreversible inhibition of their target proteins [50,51].
3.5. Protein Structure Preparation
The protein structures of JAK3 (PDB ID: 4Z16) were obtained from the RCSB Protein Data Bank. They were imported into the Maestro program for further processing using the Protein Preparation Wizard in Schrödinger software. The aim was to optimize the protein structure, maximize H-bond interactions of side chains, and perform energy minimization using the OPLS_2005 force field [52]. In the case of the model protein 4Z16, the ligands were covalently bound to Cys909. Two approaches were taken for treatment: one involved disconnecting the co-crystallized ligand from Cys909, minimizing the protein, and saving it as a non-covalent model; the other involved maintaining the covalent bond between the ligand and Cys909 during minimization for covalent docking.
The selection of proteins for this study is driven by several factors: superior resolution compared to other proteins, absence of mutations, and a more favorable alignment of the ligand structure with experimental data. Specifically, JAK3 (4Z16) was employed in the research due to its distinction as the first protein featuring a co-crystallized ligand containing an acrylaldehyde group. This structural attribute facilitates the formation of a covalent bond with Cys909, effectively inhibiting JAK3. The decision to use JAK3 (ID: 4Z16) is rooted in its recognition as one of the primary proteins capable of elucidating the presence of the covalent bond between JAK3 and the Cys909 residue. Moreover, the co-crystallized ligand in the study is identified as an EGFR inhibitor, underscoring its efficacy in treating autoimmune diseases. Furthermore, JAK3 (ID: 4Z16) is acknowledged as one of the target proteins with superior resolution compared to other types found on UniProt and PDB. The requisite metrics are also available on PDB, and JAK3 (ID: 4Z16) does not manifest any mutations. Similarly, for CYP3A4 (ID: 5VCC), an in-depth search was conducted among proteins that could yield desired results with high resolution and the metrics available on PDB, taking into consideration the presence of mutation(s).
3.6. Schrödinger Covalent Docking
To study the effect of covalent docking, ligand JAK3 inhibitors were docked to the model protein 4Z16 using the covalent dock program within Schrödinger software suite. The setup process involved specifying Michael addition or Ketone-Cysteine as the reaction type for the new compounds designed, and the scoring function was set to Extra Precision. Default parameters were used for all other settings.
Reversible (Non-Covalent)
Reversible (non-covalent) docking involves the prediction of the non-covalent interactions between the ligand and the target protein. These interactions can include hydrogen bonding, van der Waals forces, hydrophobic interactions, and electrostatic interactions. Reversible docking predicts the most favorable binding pose and affinity of the ligand within the target protein without the formation of a covalent bond. Reversible docking is widely used in drug discovery and virtual screening to identify potential lead compounds that can bind to the target protein with high affinity and specificity. One of the advantages of reversible docking is the possibility of ligand dissociation, which allows for the development of drugs with desirable pharmacokinetic properties and reduced toxicity risks.
Before conducting molecular docking, the ligands intended for docking were optimized using Avogadro software 2.0. Subsequently, we obtained the structures of JAK3 and CYP3A4 from the RCSB database (PDB ID:4Z16 and 5VCC). The crystal complex of 4Z16 comprises water molecules and the co-crystallized ligand 4LH bound to the protein 4Z16. To prepare the protein, we eliminated all water molecules and 4LH and added polar hydrogens to the JAK3 protein structure using Discovery Studio software 2021. Similar steps were followed for 5VCC, except that the included ligands were not deleted because they are an important part of the metabolism of CYP3A4. After preparing the ligands and protein, molecular docking was carried out using AD4 and AutoVina to explore the active site of 4Z16 and 5VCC, which is determined by the region encompassing the co-crystallized ligands (4LH and HEM) [53]. The three-dimensional grid was established using the AUTOGRID algorithm, which calculates the binding energy between ligands and their receptor. The default grid size for JAK3 with tofacitinib and CYP3A4 with new compounds was set to x = 60, y = 60, and z = 60, with a spacing of 0.375 Å between grid points. The center of the grid corresponds to the active site of the receptors JAK3-4LH and CYP3A4 HEM, with coordinates (x = −6.68875 Å, y = −14.7757 Å, and z = 1.89597 Å) and (x = −19.3381 Å, y = −30.475 Å, and z = 17.7174 Å), respectively. The docking results obtained from AD4 and Vina were visualized using Discovery Studio software 2021.
3.7. Predictive Toxicity Analysis and Bioactivity Assessment
ADMET analysis, medicinal chemistry, and the assessment of lead-like and drug-like properties were conducted using readily accessible online tools. These tools, such as SwissADME (http://www.swissadme.ch/ (accessed on 22 April 2022)) [54] and pkCSM (http://structure.bioc.cam.ac.uk/pkcsm (accessed on 23 April 2022)) [36], evaluate drug candidates and compounds to determine their potential toxicity for human use.
3.8. Molecular Dynamics Simulation
The newly created compounds, which exhibited enhanced binding affinity with JAK3 and CYP3A4, underwent all-atom molecular dynamics simulations using GROMACS 2021 (Groningen Machine for Chemical Simulation) software [55,56]. Before initiating the MD simulations, the CHARMM-GUI web server [57] was employed to generate the initial input parameters, implementing the CHARMM36 force field [58,59,60,61]. The simulation was conducted at a pH of 7. Before entering the production phase, each complex was solved within a rectangular grid box, surrounded by TIP3P water molecules, and supplemented with the requisite counter-ions (Na+, Cl−) to maintain a salt concentration of 0.15 M, achieved through Monte Carlo ion displacement. Energy minimization was executed for each system using the steepest descent algorithm, encompassing a maximum of 50,000 steps and a maximum force of 10.0 kJ/mol. The temperature and atmospheric pressure were set to 310 K and 1.01325 bar, respectively. For NVT equilibration, two stages were carried out, each lasting 10 ns. Canonical (NVT) and isothermal-isobaric (NPT) ensembles were utilized for equilibrating each system. Subsequently, MD simulations were conducted for a duration of 200 nanoseconds. To assess the structural stability of the designed molecules, various parameters, including root mean square deviation (RMSD), the radius of gyration (RoG), solvent accessible surface area (SASA), and root mean square flexibility (RMSF), were analyzed based on the dynamics trajectory results.
3.9. Evaluating Binding Free Energy with Molecular Mechanics/Generalized Born Surface Area (MM/GBSA)
The Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) method is an efficient force field technique employed to evaluate the binding free energy of a system, specifically in kcal/mol [62]. For more information, free binding energy ([12]) can be visited. These calculations were conducted by utilizing the last 200 frames and were determined through the application of the following equations.
ΔGbind = Gcomplex − Gprotein − Gligand
ΔGbind = ΔGgas + ΔGsol − TΔS
ΔGgas = Bond + Angle + Dihed + EEL + VdW
ΔGsol = ΔEGB + ΔESURF
ΔGbind represents the total binding free energy of the system, as elucidated in Equation (1), and is determined through the utilization of Equation (2). TΔS denotes the change in conformational entropy that arises from the binding of the ligand at a specific temperature. ΔGgas encompasses the combined contributions stemming from bond, angle, dihedral, EEL (the electrostatic element of internal energy), and van der Waals energies, as expounded in Equation (3). The internal energy relates to the oscillations and rotations of individual bond torsional angles. Solvation-free energy (ΔGsol) is composed of both ΔEGB (the polar component of solvation energy) and ΔESURF (the nonpolar component of solvation energy), as detailed in Equation (4).
4. Conclusions
In conclusion, this 3D-QSAR study utilizing field-based and atom-based approaches and the DHRRR pharmacophore model has provided valuable insights into the design and identification of JAK3 and CYP3A4 inhibitor compounds. The favorable validation results have instilled confidence in using these models for in silico prediction of compound activity. The study has led to the discovery of novel compounds by applying 3D-QSAR on a collected series of molecules with known activity. Screening of a database of molecules, based on their inhibitory activity against JAK3, has resulted in identifying three hits exhibiting strong affinity and favorable non-toxicity. These hits were further evaluated using molecular docking and ADMET analysis to assess their pharmaceutical properties. To validate the potential of these compounds, confirmation was sought through MD simulation and MM/GBSA analysis. These simulations’ results support the identified compounds’ promising nature, indicating their potential as candidates for drug design and development. These findings highlight the importance of utilizing 3D-QSAR techniques in designing novel compounds, which can lead to the discovery of potent inhibitors. Further exploration and experimental validation are warranted to fully exploit the potential of these compounds and progress them toward becoming viable drug candidates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29010023/s1, Table S1. Activity Experimental, Prediction, and Error Analysis for Series of Cys909 Linkage Agents.
Author Contributions
A.F. conducted analysis and investigation, designed compounds, and wrote, and prepared the manuscript. R.A. conducted analysis, reviewed, and edited the manuscript. J.G. and A.A. conducted analysis, reviewed, and edited the manuscript. M.H.A.M. and M.A. conducted analysis, reviewed, and edited the manuscript. M.E. conducted analysis, edited the manuscript, supervised the entire research, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a small group research project under grant number RGP1/312/44.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors wish to express their gratitude to the UM6P University, with special recognition extended to Rachid EL FATIMY and Bouchra CHAOUNI for their invaluable technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, J.; Liu, Z.; Pang, Y.; Zhou, H. The interaction between nanoparticles and immune system: Application in the treatment of inflammatory diseases. J. Nanobiotechnol. 2022, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Kumar, U.; Dada, R. Yoga and its impact on chronic inflammatory autoimmune arthritis. Front. Biosci. Elite 2020, 13, 77–116. [Google Scholar] [CrossRef]
- Sangha, P.S.; Thakur, M.; Akhtar, Z.; Ramani, S.; Gyamfi, R.S. The Link between Rheumatoid Arthritis and Dementia: A Review. Cureus 2020, 12, e7855. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.A.; Semmelink, J.F.; Denis, S.W.; van de Sande, M.G.H.; Houtkooper, R.H.L.; van Baarsen, L.G.M. Altered lipid metabolism in synovial fibroblasts of individuals at risk of developing rheumatoid arthritis. J. Autoimmun. 2023, 134, 102974. [Google Scholar] [CrossRef] [PubMed]
- Haville, S.; Deane, K.D. Pre-RA: Can early diagnosis lead to prevention? Best Pract. Res. Clin. Rheumatol. 2022, 36, 101737. [Google Scholar] [CrossRef] [PubMed]
- Padyukov, L. Genetics of rheumatoid arthritis. Semin. Immunopathol. 2022, 44, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M.; Simons, M. CNS remyelination and inflammation: From basic mechanisms to therapeutic opportunities. Neuron 2022, 110, 3549–3565. [Google Scholar] [CrossRef]
- Godoi, M.A.; Camilli, A.C.; Gonzales, K.G.A.; Costa, V.B.; Papathanasiou, E.; Leite, F.R.M.; Guimarães-Stabili, M.R. JAK/STAT as a Potential Therapeutic Target for Osteolytic Diseases. Int. J. Mol. Sci. 2023, 24, 10290. [Google Scholar] [CrossRef]
- Winthrop, K.L. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 234–243. [Google Scholar] [CrossRef]
- Nezamololama, N.; Fieldhouse, K.; Metzger, K.; Gooderham, M. Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: A review of abrocitinib, baricitinib, and upadacitinib. Drugs Context 2020, 9, 2020-8-5. [Google Scholar] [CrossRef]
- Liu, C.; Kieltyka, J.; Fleischmann, R.; Gadina, M.; O’Shea, J.J. A Decade of JAK Inhibitors: What Have We Learned and What May Be the Future? Arthritis Rheumatol. 2021, 73, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Faris, A.; Ibrahim, I.M.; Al Kamaly, O.; Saleh, A.; Elhallaoui, M. Computer-Aided Drug Design of Novel Derivatives of 2-Amino-7,9-dihydro-8H-purin-8-one as Potent Pan-Janus JAK3 Inhibitors. Molecules 2023, 28, 5914. [Google Scholar] [CrossRef] [PubMed]
- Faris, A.; Ibrahim, I.M.; Hadni, H.; Elhallaoui, M. High-throughput virtual screening of phenylpyrimidine derivatives as selective JAK3 antagonists using computational methods. J. Biomol. Struct. Dyn. 2023, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Yang, L.; Addison, G. Current Status in the Discovery of Covalent Janus Kinase 3 (JAK3) Inhibitors. Mini Rev. Med. Chem. 2019, 19, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, Q.; Li, W.; Ge, G.; Peng, J.; Xu, Y.; Yang, H.; Bai, J.; Geng, D. Comprehensive overview of microRNA function in rheumatoid arthritis. Bone Res. 2023, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yin, Y.; Shi, G.; Zhou, Y.; Shao, S.; Wei, Y.; Wu, L.; Zhang, D.; Sun, L.; Zhang, T. A highly selective JAK3 inhibitor is developed for treating rheumatoid arthritis by suppressing γc cytokine–related JAK-STAT signal. Sci. Adv. 2022, 8, eabo4363. [Google Scholar] [CrossRef]
- Baselga, J. Targeting tyrosine kinases in cancer: The second wave. Science 2006, 312, 1175–1178. [Google Scholar] [CrossRef]
- Wang, X.; Rao, J.; Tan, Z.; Xun, T.; Zhao, J.; Yang, X. Inflammatory signaling on cytochrome P450-mediated drug metabolism in hepatocytes. Front. Pharmacol. 2022, 13, 1043836. [Google Scholar] [CrossRef]
- Veeravalli, V.; Dash, R.P.; Thomas, J.A.; Babu, R.J.; Madgula, L.M.V.; Srinivas, N.R. Critical Assessment of Pharmacokinetic Drug–Drug Interaction Potential of Tofacitinib, Baricitinib and Upadacitinib, the Three Approved Janus Kinase Inhibitors for Rheumatoid Arthritis Treatment. Drug Saf. 2020, 43, 711–725. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Zhang, P.; Li, Y.; Fu, M.; Lin, H.; Feng, S.; Shen, K.; Yu, G.; Li, X. Effect of CYP3A4 induction and inhibition on the pharmacokinetics of SHR0302 in healthy subjects. Br. J. Clin. Pharmacol. 2023, 89, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, C.; Liu, G.; Liu, R.; Chen, Y.; Li, W.; Cao, Z.; Zhao, B.; Lu, C.; Liu, Y. Drug-Metabolizing Cytochrome P450 Enzymes Have Multifarious Influences on Treatment Outcomes. Clin. Pharmacokinet. 2021, 60, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, W.; Li, Q.; Chen, Y.; Zhao, G.; Peng, Y.; Zheng, J. Tofacitinib Is a Mechanism-Based Inactivator of Cytochrome P450 3A4. Chem. Res. Toxicol. 2019, 32, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Radu, A.-F.; Bungau, S.G.; Negru, A.P.; Uivaraseanu, B.; Bogdan, M.A. Novel Potential Janus Kinase Inhibitors with Therapeutic Prospects in Rheumatoid Arthritis Addressed by In Silico Studies. Molecules 2023, 28, 4699. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Chen, M.; Hu, Y.; Lan, Y.; Gan, L.; You, G.; Yue, M.; Wang, H.; Xia, B.; Zhao, J.; et al. CYP3A4/5 mediates the metabolic detoxification of humantenmine, a highly toxic alkaloid from Gelsemium elegans Benth. J. Appl. Toxicol. 2019, 39, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Wall, M.; Corboy, G.P.; Taubenheim, N.; Gregory, G.P.; Opat, S.; Shortt, J. Failure of tofacitinib to achieve an objective response in a DDX3X-MLLT10 T-lymphoblastic leukemia with activating JAK3 mutations. Mol. Case Stud. 2020, 6, a004994. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yu, C.; Denman, R.J.; Zhang, W. Recent advances in dynamic covalent chemistry. Chem. Soc. Rev. 2013, 42, 6634–6654. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.S.; Jacobsen, E.N. Asymmetric Catalysis by Chiral Hydrogen-Bond Donors. Angew. Chem. Int. Ed. 2006, 45, 1520–1543. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Heterogeneous Catalysts for the One-Pot Synthesis of Chemicals and Fine Chemicals. Chem. Rev. 2011, 111, 1072–1133. [Google Scholar] [CrossRef]
- Kapetanovic, I.M. Computer-aided drug discovery and development (CADDD): In silico-chemico-biological approach. Chem. Biol. Interact. 2008, 171, 165–176. [Google Scholar] [CrossRef]
- Lu, F.; Wang, D.; Li, R.-L.; He, L.-Y.; Ai, L.; Wu, C.-J. Current strategies and technologies for finding drug targets of active components from traditional Chinese medicine. Front. Biosci. Landmark 2021, 26, 572–589. [Google Scholar] [CrossRef]
- Nascimento, I.J.d.S.; de Aquino, T.M.; da Silva-Júnior, E.F. The New Era of Drug Discovery: The Power of Computer-aided Drug Design (CADD). Lett. Drug Des. Discov. 2022, 19, 951–955. [Google Scholar] [CrossRef]
- da Silva-Júnior, E.F. “You’ve got the Body I’ve got the Brains”—Could the current AI-based tools replace the human ingenuity for designing new drug candidates? Bioorg. Med. Chem. 2023, 94, 117475. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.A.; Thathan, J.; Jupudi, S. Pharmacophore modeling, atom based 3D-QSAR, molecular docking and molecular dynamics studies on Escherichia coli ParE inhibitors. Comput. Biol. Chem. 2020, 84, 107197. [Google Scholar] [CrossRef] [PubMed]
- Rondla, R.; PadmaRao, L.S.; Ramatenki, V.; Haredi-Abdel-Monsef, A.; Potlapally, S.R.; Vuruputuri, U. Selective ATP competitive leads of CDK4: Discovery by 3D-QSAR pharmacophore mapping and molecular docking approach. Comput. Biol. Chem. 2017, 71, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Shu, L.; Chen, C.; Huan, X.; Huang, H.; Wang, M.; Zhang, J.; Yan, Y.; Liu, J.; Zhang, T.; Zhang, D. Design, synthesis, and pharmacological evaluation of 4- or 6-phenyl-pyrimidine derivatives as novel and selective Janus kinase 3 inhibitors. Eur. J. Med. Chem. 2020, 191, 112148. [Google Scholar] [CrossRef]
- Tan, L.; Akahane, K.; McNally, R.; Reyskens, K.M.; Ficarro, S.B.; Liu, S.; Herter-Sprie, G.S.; Koyama, S.; Pattison, M.J.; Labella, K. Development of selective covalent Janus kinase 3 inhibitors. J. Med. Chem. 2015, 58, 6589–6606. [Google Scholar] [CrossRef]
- Yadav, D.K.; Saloni; Sharma, P.; Misra, S.; Singh, H.; Mancera, R.L.; Kim, K.; Jang, C.; Kim, M.; Pérez-Sánchez, H.; et al. Studies of the benzopyran class of selective COX-2 inhibitors using 3D-QSAR and molecular docking. Arch. Pharm. Res. 2018, 41, 1178–1189. [Google Scholar] [CrossRef]
- Ozgencil, F.; Eren, G.; Ozkan, Y.; Guntekin-Ergun, S.; Cetin-Atalay, R. Identification of small-molecule urea derivatives as novel NAMPT inhibitors via pharmacophore-based virtual screening. Bioorg. Med. Chem. 2020, 28, 115217. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2021-1, Maestro—Schrödinger, LLC.: New York, NY, USA, 2021.
- Janet, J.P.; Kulik, H.J. Resolving Transition Metal Chemical Space: Feature Selection for Machine Learning and Structure–Property Relationships. J. Phys. Chem. A 2017, 121, 8939–8954. [Google Scholar] [CrossRef] [PubMed]
- Jawarkar, R.D.; Zaki, M.E.A.; Al-Hussain, S.A.; Abdullah Alzahrani, A.Y.; Ming, L.C.; Samad, A.; Rashid, S.; Mali, S.; Elossaily, G.M. Mechanistic QSAR analysis to predict the binding affinity of diverse heterocycles as selective cannabinoid 2 receptor inhibitor. J. Taibah Univ. Sci. 2023, 17, 2265104. [Google Scholar] [CrossRef]
- Clark, M.; Cramer, R.D.; Opdenbosch, N.V. Validation of the general purpose tripos 5.2 force field. J. Comput. Chem. 1989, 10, 982–1012. [Google Scholar] [CrossRef]
- Shinde, M.G.; Modi, S.J.; Kulkarni, V.M. QSAR and Molecular Docking of Phthalazine Derivatives as Epidermal Growth Factor Receptor (EGFR) Inhibitors. J. Appl. Pharm. Sci. 2017, 7, 181–191. [Google Scholar] [CrossRef][Green Version]
- Giordano, D.; Biancaniello, C.; Argenio, M.A.; Facchiano, A. Drug Design by Pharmacophore and Virtual Screening Approach. Pharmaceuticals 2022, 15, 646. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y. Pharmacophore modeling and applications in drug discovery: Challenges and recent advances. Drug Discov. Today 2010, 15, 444–450. [Google Scholar] [CrossRef]
- Aljoundi, A.; Bjij, I.; El Rashedy, A.; Soliman, M.E.S. Covalent Versus Non-covalent Enzyme Inhibition: Which Route Should We Take? A Justification of the Good and Bad from Molecular Modelling Perspective. Protein J. 2020, 39, 97–105. [Google Scholar] [CrossRef]
- Tivon, B.; Gabizon, R.; Somsen, B.A.; Cossar, P.J.; Ottmann, C.; London, N. Covalent flexible peptide docking in Rosetta. Chem. Sci. 2021, 12, 10836–10847. [Google Scholar] [CrossRef]
- Faris, A.; Ibrahim, I.M.; Alnajjar, R.; Hadni, H.; Bhat, M.A.; Yaseen, M.; Chakraborty, S.; Alsakhen, N.; Shamkh, I.M.; Mabood, F.; et al. QSAR-driven screening uncovers and designs novel pyrimidine-4,6-diamine derivatives as potent JAK3 inhibitors. J. Biomol. Struct. Dyn. 2023, 1–30. [Google Scholar] [CrossRef]
- Stortz, C.A.; Johnson, G.P.; French, A.D.; Csonka, G.I. Comparison of different force fields for the study of disaccharides. Carbohydr. Res. 2009, 344, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for Ligand-Receptor Docking. Curr. Protoc. Bioinform. 2008, 24, 8.14.1–8.14.40. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Faris, A.; Ibrahim, I.M.; Chakraborty, S.; Kamaly, O.A.; Alshawwa, S.Z.; Hallaoui, M.E. Identification of Selective JAK3/STAT1 and CYP34A from Pyrazolopyrimidine Derivatives: A Search for Potential Drug Targets for Rheumatoid Arthritis using In-silico Drug Discovery Techniques. Lett. Drug Des. Discov. 2023, 20, 1–26. [Google Scholar] [CrossRef]
- Faris, A.; Hadni, H.; Saleh, B.A.; Khelfaoui, H.; Harkati, D.; Ait Ahsaine, H.; Elhallaoui, M.; El-Hiti, G.A. In silico screening of a series of 1,6-disubstituted 1H-pyrazolo[3,4-d]pyrimidines as potential selective inhibitors of the Janus kinase 3. J. Biomol. Struct. Dyn. 2023, 1–19. [Google Scholar] [CrossRef]
- Huang, J.; MacKerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef]
- Ziada, S.; Diharce, J.; Raimbaud, E.; Aci-Sèche, S.; Ducrot, P.; Bonnet, P. Estimation of Drug-Target Residence Time by Targeted Molecular Dynamics Simulations. J. Chem. Inf. Model. 2022, 62, 5536–5549. [Google Scholar] [CrossRef]
- Zhang, X.; Perez-Sanchez, H.; Lightstone, F.C. A Comprehensive Docking and MM/GBSA Rescoring Study of Ligand Recognition upon Binding Antithrombin. Curr. Top. Med. Chem. 2017, 17, 1631–1639. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).