Abstract
A series of new fluorinated quinoline analogs were synthesized using Tebufloquin as the lead compound, 2-fluoroaniline, ethyl 2-methylacetoacetate, and substituted benzoic acid as raw materials. Their structures were confirmed by 1H NMR, 13C NMR, and HRMS. The compound 8-fluoro-2,3-dimethylquinolin-4-yl 4-(tert-butyl)benzoate (2b) was further determined by X-ray single-crystal diffraction. The antifungal activity was tested at 50 μg/mL, and the bioassay results showed that these quinoline derivatives had good antifungal activity. Among them, compounds 2b, 2e, 2f, 2k, and 2n exhibited good activity (>80%) against S. sclerotiorum, and compound 2g displayed good activity (80.8%) against R. solani.
1. Introduction
Nitrogen-containing heterocycles are important skeletons in synthetic chemistry [1,2,3,4,5]. In the development of heterocyclic pesticides, quinoline compounds, as a class of heterocyclic compounds with a quinoline skeleton [6], can be easily modified in structure, and play an important role in the development of new pesticides. Quinoline compounds not only have good antifungal [7,8,9,10], herbicidal [11], and anti-insecticidal [12] activities, but also have anticancer [13,14], antimalarial [15,16,17], and antituberculosis [18,19] activities. Since Runge [20] first extracted quinoline from coal tar, many natural quinoline compounds were discovered.
Among the more than 1200 commercialized pesticides, 424 pesticides contain at least one fluorine atom [21]. After organic compounds are fluorinated, their physical and chemical properties such as lipophilicity, water solubility, and metabolic stability will undergo significant changes [22], due to that they [23,24] have the characteristics of small radius and large electronegativity. Therefore, many commercial medicines or pesticides heterocycles containing fluorine atoms were commercialized. For example (Figure 1), the fluoroquinoline fungicide Ipflufenoquin [25] was recently developed by Japan’s Soda Company. The Meiji Fruit Industry in Japan has developed the fungicide Tebufloquin [26], which has excellent control effects on rice blast, and the commercial antifungal Ciprofloxacin.
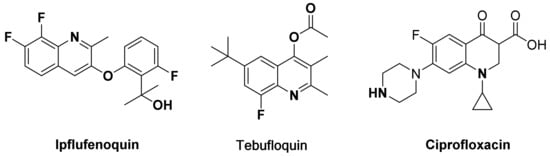
Figure 1.
Some commercialized pesticides or drugs with fluorinated quinoline derivatives.
In our previous work [27,28,29,30,31], many compounds based on the natural quinoline structure were synthesized and showed good activity. In recent work [32], we found that in the 8-position quinoline ring added p-chlorophenoxy group, some compounds showed certain activity. As we continued our effort to develop high-efficiency and low-toxicity new quinoline-based fungicides, the natural quinoline structure was selected as key core, 8-position of quinoline ring was replaced by fluorine atom, and the 4-position of hydroxyl on the quinoline ring was esterified (Figure 2 [32]). All new quinoline compounds were characterized by 1H NMR, 13C NMR, and HRMS, and their antifungal activity was tested.
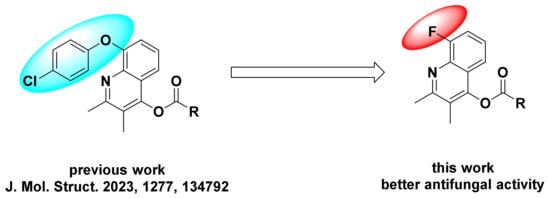
Figure 2.
Design strategy of the title compounds 2a–2p [32].
2. Results and Discussion
2.1. Synthesis and Spectra Analysis
The synthetic route of title fluorinated quinoline analogs is shown in Scheme 1. First, 2-fluorophenol was directly reacted with ethyl 2-methylacetoacetate under polyphosphoric acid (PPA) to give 2,3-dimethyl-4-hydroxyquinoline intermediate 1. The PPA was used as both a solvent and an acidic catalyst, and the reactants are directly one-step synthesis of quinoline rings. Then, intermediate 1 undergoes esterification with various substituted benzoic acids to generate the target compound. When EDC•HCl and DMAP are used as condensation agent, the solvent has a great influence on the yield of this reaction. It was found that using DMF as solvent can give higher yield than using dichloromethane (DCM) as solvent (Table 1).
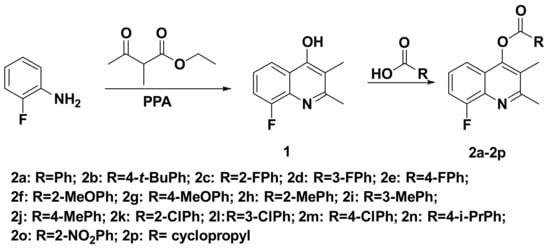
Scheme 1.
Synthesis route of title compounds 2a–2p.

Table 1.
Solvent effect of yield compound 2b.
The two CH3 protons are at 2.3 and 2.8 ppm, respectively. The protons of the benzene ring are assigned at 7~9 ppm. Taking compound 2b as an example, the protons of two methyl groups can be found at δ 2.32 ppm and δ 2.80 ppm as singlets in the 1H NMR, while in the 13C NMR data, they are found at 12.87 ppm and 24.37 ppm, respectively. The C=O was found at 163.73 ppm in the 13C NMR.
2.2. Structure Determination
A colorless crystal of 8-fluoro-2,3-dimethylquinolin-4-yl 4-(tert-butyl)benzoate, 2b, suitable for X-ray diffraction study, was cultivated in the test tube from EtOH by self-volatilization. A crystal with dimensions of 0.36 mm × 0.28 mm × 0.18 mm was mounted on a Bruker APEX-II CCD diffractometer equipped with graphite-monochromatic MoKα radiation (λ = 0.71073 Å). The crystal structure was solved by direct methods with SHELXS-97 [33] and refined by full-matrix least-squares refinements based on F2 with SHELXL-97. All nonhydrogen atoms were refined anisotropically, and all hydrogen atoms were located in the calculated positions and refined with a riding model. The detailed crystal data are listed in Table 2.

Table 2.
Crystal data and structure refinement of compound 2b.
2.3. Fungicidal Activity and SAR
The antifungal activities of compounds 2a–2p against ten phytopathogenic fungi are listed in Table 3. Generally, these compounds showed good antifungal activity. For example, compounds 2b(4-Bu), 2e(4-F), 2g(4-OMe) and 2p displayed moderate activity (40~60%) against A. solani. For the P. oryae, compounds 2b, 2d, 2f, and 2p also exhibited moderate activity (40~60%), which is the same as the A. solani. The inhibition rate of compound 2f against P. capsicum reached 58.1%, which was the same as that of the positive control Tebufloquin (58.1%). Compounds 2e and 2j were both 45% inhibitory to F. oxysporum, which is a little better than the positive control Tebufloquin (42.9%). Among these fungi, compounds 2b, 2e, 2f, 2k, and 2n possessed good activity (>80%) against S. sclerotiorum, which were higher than the positive control Tebufloquin (75.0%). Compounds 2b and 2d also had good activity (53.8%) against B. cinerea, which was a little lower than that of the positive control Tebufloquin (56.7%), but the activity of compound 2n (57.7%) was a little higher than that of the positive control Tebufloquin (56.7%). For the R. solani, compounds 2g (80.8%) and 2p (76.9%) exhibited good activity, which were higher than the positive control Tebufloquin (69.7%). The inhibition rates of compounds 2b, 2f, and 2n against C. arachidicola were 46.7%, 46.7%, and 60%, respectively, which were higher than the positive control Tebufloquin (37.5%). The inhibitory rates of compounds 2e, 2f, 2k, and 2n, against P. piricola were 72.0%, 76.0%, 76.0%, and 76.0%, respectively, which were also higher than the positive control Tebufloquin (65.4%). Notably, all the title compounds had lower inhibition rates against G. zeae; only compounds 2b and 2n exceeded 30%. From Table 3, it can be concluded that substitution on the benzene ring can influence the activity. The 4-position of the benzene ring had an electron-given group with better activity, such as compounds 2b(4-Bu), 2g(4-MeO), 2j(4-Me), and 2n(4-i-Pr), while the 4-position of the benzene ring had an electron-withdraw group, which had low activity, except for compound 2e(4-F), which may be the influence of the fluorine atom. When the R was an alkyl group, such as compound 2p (cyclopropyl group), it also exhibited good activity.

Table 3.
The fungicidal activity (% inhibition) of compounds 2a–2p and Tebufloquin (FP) at 50 μg/mL on A. solani (AS), G. zeae (GZ), P. oryae (PO), P. capsici (PC), S. sclerotiorum (SS), B. cinerea (BC), R. solani (RS), F. oxysporum (FO), C. arachidicola (CA), and P. piricola (PP). CK = control.
3. Materials and Methods
3.1. Instruments
Melting points were determined using an X-4 apparatus and uncorrected. 1H NMR and 13C NMR spectra were measured on a Bruker AC-P500 and AC-P400 instrument using TMS as an internal standard and deuterated chloroform, CDCl3, as the solvent. HR-ESI-MS was tested using an Agilent 1100 HPLC-JEOL AccuTOF instrument. All reagents were of analytical grade or were freshly prepared before use.
3.2. Synthesis
3.2.1. Synthesis of Intermediate 1
The synthetic route is shown in Scheme 1. In a 250 mL three-necked flask, 2-fluoroaniline (11.11 g, 100.00 mmol), ethyl 2-methylacetoacetate (14.42 g, 100.00 mmol), and polyphosphoric acid (50.69 g, 150.00 mmol) were added, and the mixture was heated at 150 °C. After the reaction was completed, the mixture was cooled to room temperature. The three-necked flask was placed in an ice bath, and the pH was adjusted to 7–8 by 10% aqueous sodium hydroxide solution. Then, it was filtered and dried to give 8-fluoro-2,3-dimethylquinolin-4-ol (intermediate 1), white solid with the yield of 89.2%, mp 230–231 °C. 1H NMR (500 MHz, DMSO-d6) δ: 11.36 (s, 1H, OH), 7.88 (d, J = 8.1 Hz, 1H, Ph), 7.52–7.46 (m, 1H, Ph), 7.26–7.19 (m, 1H, Ph), 2.43 (s, 3H, CH3), 1.98 (s, 3H, CH3).
3.2.2. Synthesis of Target Compounds 2
The synthetic route is shown in Scheme 1. In a 50 mL round-bottomed flask, intermediate 1 (0.20 g, 1.05 mmol), substituted benzoic acid (1.16 mmol), EDC•HCl (0.24 g, 1.26 mmol), 4-dimethylaminopyridine (DMAP) (0.13 g, 1.05 mmol), and DMF (10 mL) were stirred at room temperature. After the reaction was completed, water (30 mL) was added, it was extracted with ethyl acetate (10 mL × 3), and the organic phases were combined, washed with saturated brine (10 mL × 3), dried over anhydrous sodium sulfate, filtered, and purified by column chromatography to obtain the target compounds 2a–2p.
8-fluoro-2,3-dimethylquinolin-4-yl benzoate (2a). White solid, yield 56.1%, mp 176~178 °C; 1H NMR (500 MHz, CDCl3) δ: 8.36–8.30 (m, 2H, Ph), 7.80–7.69 (m, 1H, Ph), 7.60 (t, J = 7.8 Hz, 2H, Ph), 7.56–7.49 (m, 1H, Ph), 7.42–7.29 (m, 2H, Ph), 2.81 (s, 3H, CH3), 2.33 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 163.75, 160.83, 157.74 (d, J = 255.8 Hz), 151.72 (d, J = 4.5 Hz), 137.55 (d, J = 12.2 Hz), 134.38, 130.49 (2C), 128.97 (2C), 128.21, 125.99 (d, J = 8.2 Hz), 123.58 (d, J = 2.4 Hz), 123.23, 116.72 (d, J = 4.7 Hz), 113.24 (d, J = 19.2 Hz), 24.39, 12.89; HRMS (ESI) for C18H14FNO2 m/z: Calculated, 296.1081, found, 296.1087 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 4-(tert-butyl)benzoate (2b). White solid, yield 59.3%, mp 171~173 °C; 1H NMR (500 MHz, CDCl3) δ: 8.29–8.19 (m, 2H, Ph), 7.61 (d, J = 8.6 Hz, 2H, Ph), 7.56–7.50 (m, 1H, Ph), 7.40–7.30 (m, 2H, Ph), 2.80 (s, 3H, CH3), 2.32 (s, 3H, CH3), 1.41 (s, 9H, C(CH3)3); 13C NMR (151 MHz, CDCl3) δ: 163.73, 160.82, 158.37, 157. 73 (d, J = 256.7 Hz), 151.82 (d, J = 4.5 Hz), 137.52 (d, J = 12.1 Hz), 130.44 (2C), 125.97 (2C), 125.91 (d, J = 8.2 Hz), 125.38, 123.68 (d, J = 2.3 Hz), 123.28, 116.79 (d, J = 4.7 Hz), 113.18 (d, J = 19.2 Hz), 35.35, 31.11 (3C), 24.37, 12.87; HRMS (ESI) for C22H22FNO2 m/z: Calculated, 352.1707, found, 352.1713 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 2-fluorobenzoate (2c). White solid, yield 67.2%, mp 144~146 °C; 1H NMR (500 MHz, CDCl3) δ: 8.24–8.17 (m, 1H, Ph), 7.75–7.67 (m, 1H, Ph), 7.58 (d, J = 8.1 Hz, 1H, Ph), 7.44–7.28 (m, 4H, Ph), 2.81 (s, 3H, CH3), 2.35 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 162.54 (d, J = 262.0 Hz), 161.43 (d, J = 4.0 Hz), 160.87, 157.70 (d, J = 255.9 Hz), 151.46 (d, J = 4.6 Hz), 137.50 (d, J = 12.2 Hz), 136.04 (d, J = 9.1 Hz), 132.80, 126.11 (d, J = 8.2 Hz), 124.55 (d, J = 3.9 Hz), 123.39 (d, J = 2.4 Hz), 123.18, 117.53 (d, J = 22.1 Hz), 116.89 (d, J = 9.7 Hz), 116.75 (d, J = 4.7 Hz), 113.32 (d, J = 19.2 Hz), 24.32, 12.96; HRMS (ESI) for C18H13F2NO2 m/z: Calculated, 314.0987, found, 314.0993 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 3-fluorobenzoate (2d). White solid, yield 66.2%, mp 146~148 °C; 1H NMR (500 MHz, CDCl3) δ: 8.17–8.09 (m, 1H, Ph), 8.03–7.97 (m, 1H, Ph), 7.63–7.56 (m, 1H, Ph), 7.54–7.49 (m, 1H, Ph), 7.48–7.42 (m, 1H, Ph), 7.42–7.33 (m, 2H, Ph), 2.81 (s, 3H, CH3), 2.32 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 162.80 (d, J = 249.2 Hz), 162.65 (d, J = 3.1 Hz), 160.88, 157.75 (d, J = 256.1 Hz), 151.48 (d, J = 4.4 Hz), 137.53 (d, J = 12.1 Hz), 130.73 (d, J = 7.8 Hz), 130.29 (d, J = 7.5 Hz), 126.25 (d, J = 3.1 Hz), 126.15 (d, J = 8.2 Hz), 123.36 (d, J = 2.3 Hz), 123.16, 121.56 (d, J = 21.3 Hz), 117.35 (d, J = 23.3 Hz), 116.54 (d, J = 4.7 Hz), 113.37 (d, J = 19.2 Hz), 24.34, 12.90; HRMS (ESI) for C18H13F2NO2 m/z: Calculated, 314.0987, found, 314.0993 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 4-fluorobenzoate (2e). White solid, yield 48.5%, mp 152~154 °C; 1H NMR (500 MHz, CDCl3) δ: 8.48–8.22 (m, 2H, Ph), 7.55–7.46 (m, 1H, Ph), 7.41–7.32 (m, 2H, Ph), 7.31–7.25 (m, 2H, Ph), 2.81 (s, 3H, CH3), 2.32 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 166.65 (d, J = 256.6 Hz), 162.78, 160.88, 157.73 (d, J = 256.0 Hz), 151.60 (d, J = 4.5 Hz), 137.50 (d, J = 12.2 Hz), 133.19 (d, J = 9.6 Hz, 2C), 126.09 (d, J = 8.2 Hz), 124.43 (d, J = 3.0 Hz), 123.50 (d, J = 2.5 Hz), 123.24, 116.60 (d, J = 4.7 Hz), 116.29 (d, J = 22.2 Hz, 2C), 113.33 (d, J = 19.2 Hz), 24.31, 12.89; HRMS (ESI) for C18H13F2NO2 m/z: Calculated, 314.0987, found, 314.0993 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 2-methoxybenzoate (2f). White solid; yield 51.3%; mp 129~130 °C; 1H NMR (500 MHz, CDCl3) δ: 8.15 (dd, J = 7.9, 1.6 Hz, 1H, Ph), 7.69–7.61 (m, 2H, Ph), 7.44–7.30 (m, 2H, Ph), 7.17–7.09 (m, 2H, Ph), 3.99 (s, 3H, OCH3), 2.80 (s, 3H, CH3), 2.36 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 163.20, 160.81, 160.16, 157.70 (d, J = 255.5 Hz), 151.88 (d, J = 4.5 Hz), 137.54 (d, J = 12.1 Hz), 135.08, 132.52, 125.85 (d, J = 8.2 Hz), 123.76 (d, J = 2.5 Hz), 123.29, 120.50, 117.93, 117.10 (d, J = 4.6 Hz), 113.10 (d, J = 19.2 Hz), 112.36, 56.04, 24.40, 12.94; HRMS (ESI) for C19H16FNO3 m/z: Calculated, 326.1187, found, 326.1192 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 4-methoxybenzoate (2g). White solid, yield 57.3%, mp 86~88 °C; 1H NMR (500 MHz, CDCl3) δ: 8.35–8.20 (m, 2H, Ph), 7.55–7.50 (m, 1H, Ph), 7.40–7.30 (m, 2H, Ph), 7.09–7.03 (m, 2H, Ph), 3.94 (s, 3H, OCH3), 2.80 (s, 3H, CH3), 2.32 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 163.48, 162.31, 160.83, 157.73 (d, J = 255.7 Hz), 151.87 (d, J = 4.6 Hz), 137.52 (d, J = 12.0 Hz), 132.84 (2C), 125.89 (d, J = 8.2 Hz), 123.78 (d, J = 2.4 Hz), 123.32, 120.40, 116.82 (d, J = 4.7 Hz), 114.15 (2C), 113.17 (d, J = 19.2 Hz), 55.60, 24.38, 12.89; HRMS (ESI) for C19H16FNO3 m/z: Calculated, 326.1187, found, 326.1192 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 2-methylbenzoate (2h). White solid, yield 60.5%, mp 150~152 °C; 1H NMR (500 MHz, CDCl3) δ: 8.39 (d, J = 7.8 Hz, 1H, Ph), 7.64–7.50 (m, 2H, Ph), 7.48–7.31 (m, 4H, Ph), 2.81 (s, 3H, CH3), 2.70 (s, 3H, CH3), 2.34 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 164.03, 160.83, 157.76 (d, J = 255.8 Hz), 151.80 (d, J = 4.5 Hz), 142.34, 137.55 (d, J = 12.1 Hz), 133.58, 132.41, 131.47, 126.99, 126.30, 125.98 (d, J = 8.2 Hz), 123.72 (d, J = 2.4 Hz), 123.25, 116.76 (d, J = 4.7 Hz), 113.21 (d, J = 19.2 Hz), 24.37, 22.10, 12.95; HRMS (ESI) for C19H16FNO2 m/z: Calculated, 310.1238, found, 310.1243 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 3-methylbenzoate (2i). White solid, yield 63.1%, mp 126~128 °C; 1H NMR (500 MHz, CDCl3) δ: 8.17–8.07 (m, 2H, Ph), 7.58–7.51 (m, 2H, Ph), 7.48 (t, J = 7.9 Hz, 1H, Ph), 7.41–7.32 (m, 2H, Ph), 2.81 (s, 3H, CH3), 2.50 (s, 3H, CH3), 2.32 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 163.92, 160.85, 157.72 (d, J = 255.8 Hz), 151.80 (d, J = 4.5 Hz), 138.93, 137.51 (d, J = 12.2 Hz), 135.16, 130.97, 128.86, 128.12, 127.65, 125.97 (d, J = 8.2 Hz), 123.62 (d, J = 2.4 Hz), 123.26, 116.76 (d, J = 4.6 Hz), 113.24 (d, J = 19.2 Hz), 24.34, 21.34, 12.89; HRMS (ESI) for C19H16FNO2 m/z: Calculated, 310.1238, found, 310.1243 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 4-methylbenzoate (2j). White solid, yield 66.5%, mp 122~124 °C; 1H NMR (500 MHz, CDCl3) δ: 8.21 (d, J = 8.2 Hz, 2H, Ph), 7.56–7.50 (m, 1H, Ph), 7.43–7.30 (m, 4H, Ph), 2.80 (s, 3H, CH3), 2.51 (s, 3H, CH3), 2.32 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 163.81, 160.84, 157.73 (d, J = 255.7 Hz), 151.82 (d, J = 4.5 Hz), 145.43, 137.51 (d, J = 12.2 Hz), 130.55 (2C), 129.68 (2C), 125.94 (d, J = 8.2 Hz), 125.43, 123.68 (d, J = 2.4 Hz), 123.29, 116.78 (d, J = 4.7 Hz), 113.21 (d, J = 19.2 Hz), 24.35, 21.87, 12.88; HRMS (ESI) for C19H16FNO2 m/z: Calculated, 310.1238, found, 310.1243 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 2-chlorobenzoate (2k). White solid, yield 46.3%, mp 116~118 °C; 1H NMR (500 MHz, CDCl3) δ: 8.25 (dd, J = 7.8, 1.2 Hz, 1H, Ph), 7.66–7.56 (m, 3H, Ph), 7.51–7.47 (m, 1H, Ph), 7.44–7.39 (m, 1H, Ph), 7.39–7.34 (m, 1H, Ph), 2.82 (s, 3H, CH3), 2.37 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 162.43, 160.84, 157.71 (d, J = 255.7 Hz), 151.44 (d, J = 3.5 Hz), 137.53 (d, J = 11.9 Hz), 134.94, 134.03, 132.34, 131.81, 127.87, 127.07, 126.14 (d, J = 8.0 Hz), 123.37 (d, J = 2.6 Hz), 123.17, 116.74 (d, J = 4.0 Hz), 113.31 (d, J = 19.1 Hz), 24.35, 13.05; HRMS (ESI) for C18H13ClFNO2 m/z: Calculated, 330.0692, found, 330.0697 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 3-chlorobenzoate (2l). White solid, yield 68.5%, mp 148~150 °C; 1H NMR (500 MHz, CDCl3) δ: 8.30 (t, J = 1.7 Hz, 1H, Ph), 8.24–8.16 (m, 1H, Ph), 7.74–7.68 (m, 1H, Ph), 7.57–7.48 (m, 2H, Ph), 7.42–7.34 (m, 2H, Ph), 2.81 (s, 3H, CH3), 2.32 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 162.59, 160.88, 157.74 (d, J = 256.1 Hz), 151.47 (d, J = 4.6 Hz), 137.51 (d, J = 12.2 Hz), 135.23, 134.43, 130.44, 130.32, 129.92, 128.59, 126.17 (d, J = 8.2 Hz), 123.34 (d, J = 2.4 Hz), 123.16, 116.53 (d, J = 4.7 Hz), 113.39 (d, J = 19.2 Hz), 24.32, 12.91; HRMS (ESI) for C18H13ClFNO2 m/z: Calculated, 330.0692, found, 330.0697 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 4-chlorobenzoate (2m). White solid, yield 70.2%, mp 183~185 °C; 1H NMR (500 MHz, CDCl3) δ: 8.32–8.21 (m, 2H, Ph), 7.62–7.56 (m, 2H, Ph), 7.53–7.46 (m, 1H, Ph), 7.42–7.33 (m, 2H, Ph), 2.81 (s, 3H, CH3), 2.31 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 162.94, 160.85, 157.76 (d, J = 256.0 Hz), 151.50 (d, J = 4.6 Hz), 141.09, 137.55 (d, J = 12.2 Hz), 131.83 (2C), 129.40 (2C), 126.61, 126.10 (d, J = 8.2 Hz), 123.41 (d, J = 2.5 Hz), 123.17, 116.55 (d, J = 4.8 Hz), 113.33 (d, J = 19.2 Hz), 24.39, 12.90; HRMS (ESI) for C18H13ClFNO2 m/z: Calculated, 330.0692, found, 330.0697 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 4-isopropylbenzoate (2n). White solid, yield 46.5%, mp 144~146 °C; 1H NMR (500 MHz, CDCl3) δ: 8.29–8.21 (m, 2H, Ph), 7.57–7.50 (m, 1H, Ph), 7.45 (d, J = 8.2 Hz, 2H, Ph), 7.40–7.30 (m, 2H, Ph), 3.13–2.99 (m, 1H, CH), 2.80 (s, 3H, CH3), 2.32 (s, 3H, CH3), 1.34 (s, 3H, CH3), 1.33 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 163.76, 160.82, 157.73 (d, J = 255.7 Hz), 156.14, 151.82 (d, J = 4.5 Hz), 137.53 (d, J = 12.2 Hz), 130.73 (2C), 127.11 (2C), 125.91 (d, J = 8.2 Hz), 125.76, 123.68 (d, J = 2.3 Hz), 123.28 (s), 116.80 (d, J = 4.7 Hz), 113.18 (d, J = 19.2 Hz), 34.47, 24.38, 23.69 (2C), 12.87; HRMS (ESI) for C21H20FNO2 m/z: Calculated, 338.1551, found, 338.1556 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl 2-nitrobenzoate (2o). White solid, yield 46.3%, mp 204~206 °C, 1H NMR (500 MHz, CDCl3) δ: 9.17 (t, J = 1.8 Hz, 1H, Ph), 8.64 (d, J = 7.7 Hz, 1H, Ph), 8.62–8.57 (m, 1H, Ph), 7.84 (t, J = 8.0 Hz, 1H, Ph), 7.51–7.46 (m, 1H, Ph), 7.43–7.35 (m, 2H, Ph), 2.83 (s, 3H, CH3), 2.34 (s, 3H, CH3); 13C NMR (151 MHz, CDCl3) δ: 161.77, 160.90, 157.78 (d, J = 256.3 Hz), 151.16 (d, J = 4.6 Hz), 148.64, 137.60 (d, J = 12.2 Hz), 135.99, 130.38, 129.98, 128.74, 126.32 (d, J = 8.3 Hz), 125.34, 123.08 (d, J = 2.4 Hz), 123.06, 116.32 (d, J = 4.7 Hz), 113.50 (d, J = 19.2 Hz), 24.41, 12.97; HRMS (ESI) for C18H13FN2O4 m/z: Calculated, 341.0932, found, 341.0938 [M + H]+.
8-fluoro-2,3-dimethylquinolin-4-yl cyclopropanecarboxylate (2p). White solid, yield 32.8%, mp 115~117 °C; 1H NMR (500 MHz, CDCl3) δ: 7.51 (d, J = 8.3 Hz, 1H, Ph), 7.44–7.38 (m, 1H, Ph), 7.37–7.30 (m, 1H, Ph), 2.77 (s, 3H, CH3), 2.27 (s, 3H, CH3), 2.09–2.02 (m, 1H, CH), 1.32–1.28 (m, 2H, CH2), 1.21–1.12 (m, 2H, CH2); 13C NMR (151 MHz, CDCl3) δ: 171.99, 160.75, 157.71 (d, J = 255.6 Hz), 151.42 (d, J = 4.5 Hz), 137.47 (d, J = 12.1 Hz), 125.88 (d, J = 8.2 Hz), 123.57 (d, J = 2.5 Hz), 123.06, 116.61 (d, J = 4.6 Hz), 113.13 (d, J = 19.2 Hz), 24.35, 12.71, 12.66, 9.57 (2C); HRMS (ESI) for C15H14FNO2 m/z: Calculated, 260.1081, found, 260.1087 [M + H]+.
3.3. Fungicide Bioassays
The fungicidal activities of title chiral niacinamide derivatives 2a–2p were tested in vitro against A. solani (AS), G. zeae (GZ), P. oryae (PO), P. capsici (PC), S. sclerotiorum (SS), B. cinerea (BC), R. solani (RS), F. oxysporum (FO), C. arachidicola (CA), and P. piricola (PP), and their relative percent inhibition (%) was determined using the mycelium growth rate method according to the previous work [34,35]. Tebufloquin was used as the positive control. Each compound was dissolved in DMSO with 1% Tween to prepare the 500 μg/mL stock solution. The ten fungi were inoculated into a Petri dish containing 50 μg/mL stock solution and incubated in a 27 °C incubator. A DMSO solvent containing 1% Tween was used as a blank assay. The fungicidal effect was determined 48–72 h later. The inhibition rate of the compound compared to the blank assay was calculated by the following equation:
where CK is the average diameter of the mycelium in the blank test, and AI is the average value of the mycelium in the presence of these compounds.
inhibition (%) = (CK − AI)/CK × 100%
3.4. Crystal Structure
The structure of compound 8-fluoro-2,3-dimethylquinolin-4-yl 4-(tert-butyl)benzoate 2b was further characterized by single-crystal X-ray diffraction, and is illustrated in Figure 3. As shown in Figure 3, the phenyl ring (C(13)~C(18) is nearly vertical with the quinoline ring, which has a dihedral angle (θ) of 76.2° with plane equation 2.499x + 3.956y + 7.124z = 5.9084 and 5.915x + 3.757y + −6.977z = 3.1104, respectively. The torsion angles of C(9)-O(1)-C(12)-C(13) and O(2)-C(12)-C(13)-C(14) were −171.3(3)° and −175.7(3)°, respectively, which showed that the ester group is nearly in the same plane as the phenyl ring. Compound 2b had weak interaction, which formed an infinite one-dimensional chain structure along the a-axis (Figure 4).
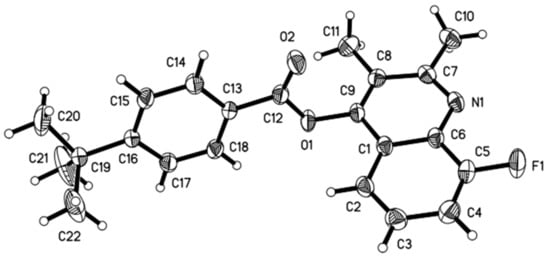
Figure 3.
Molecular structure of the title compound 2b.
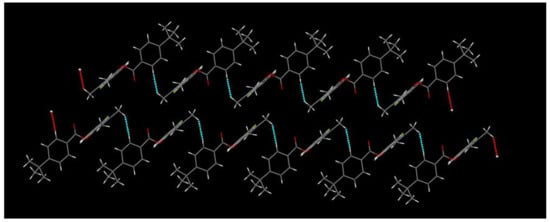
Figure 4.
Chain structure of compound 2b.
4. Conclusions
In summary, sixteen new fluorinated quinoline compounds were synthesized, and their structures were characterized by 1H NMR, 13C NMR, X-ray diffraction, and HRMS. The antifungal activity results showed that some of the compounds exhibited good antifungal activity at 50 μg/mL. Among them, compounds 2b, 2e, 2f, 2k, and 2n exhibited good antifungal activity (>80%) against S. sclerotiorum. The antifungal activity of compound 2g against R. solani was 80.8%. This can give new clues for designing new highly active quinoline compounds in the next study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28083373/s1, Figures for 1H NMR, 13C NMR, and HRMS.
Author Contributions
Methodology and Investigation, X.S. and W.Y.; editing, L.M. and J.S.; Resources, L.H.; Writing—review & editing, X.H.; Supervision, N.S. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Zhejiang Provincial Natural Science Foundation of China (No. LTGN23C140002, LY19C140002, LY19B020009), Natural Science Foundation of China (No. 32001929), the Talent Start Foundation of Huangshan University (No. 2018xkjq018), and the Chemical Company for Research (KYY-HX-20210140, KYY-HX-20190720).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Dhakshinamoorthy, A.; Garcia, H. Metal-organic frameworks as solid catalysts for the synthesis of nitrogen-containing heterocycles. Chem. Soc. Rev. 2014, 43, 5750–5765. [Google Scholar] [CrossRef] [PubMed]
- Lamberth, C. Heterocyclic chemistry in crop protection. Pest Manag. Sci. 2019, 69, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Prandi, C.; Occhiato, E.G. From synthetic control to natural products: A focus on N-heterocycles. Pest Manag. Sci. 2019, 75, 2385–2402. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Mohammadkhani, L. Synthesis of various N-heterocycles using the four-component Ugi reaction. Adv. Heterocycl. Chem. 2020, 131, 351–403. [Google Scholar]
- Wu, X.Q.; Li, W.F. The applications of beta-keto amides for heterocycle synthesis. J. Heterocycl. Chem. 2022, 59, 1445–1490. [Google Scholar] [CrossRef]
- Rajendran, S.; Sivalingam, K.; Jayarampillai, R.P.K.; Wang, W.L.; Salas, C.O. Friedländer’s synthesis of quinolines as a pivotal step in the development of bioactive heterocyclic derivatives in the current era of medicinal chemistry. Chem. Biol. Drug Des. 2022, 100, 1042–1085. [Google Scholar] [CrossRef]
- Prajapati, S.M.; Patel, K.D.; Vekariya, R.H.; Panchal, S.N.; Pate, H.D. Recent advances in the synthesis of quinolines: A review. RSC Adv. 2014, 4, 24463–24476. [Google Scholar] [CrossRef]
- Bouzian, Y.; Karrouchi, K.; Sert, Y.; Lai, C.H.; Mahi, L.; Ahabchane, N.H.; Talbaoui, A.; Mague, J.T.; Essassi, E. Synthesis, spectroscopic characterization, crystal structure, DFT, molecular docking and in vitro antibacterial potential of novel quinoline derivatives. J. Mol. Struct. 2020, 1209, 127940. [Google Scholar] [CrossRef]
- Dolan, N.; Gavin, D.P.; Eshwika, A.; Kavanagh, K.; McGinley, J.; Stephens, J.C. Synthesis, antibacterial and anti-MRSA activity, in vivo toxicity and a structure activity relationship study of a quinoline thiourea. Bioorg. Med. Chem. Lett. 2016, 26, 630–635. [Google Scholar] [CrossRef]
- Abdullah, M.I.; Mahmood, A.; Madni, M.; Masood, S.; Kashif, M. Synthesis, characterization, theoretical, anti-bacterial and molecular docking studies of quinoline based chalcones as a DNA gyrase inhibitor. Bioorg. Chem. 2014, 54, 31–37. [Google Scholar] [CrossRef]
- Wang, D.W.; Lin, H.Y.; Cao, R.J.; Chen, T.; Wu, F.X.; Hao, G.F.; Chen, Q.; Yang, W.C.; Yang, G.F. Synthesis and Herbicidal Activity of Triketone−Quinoline Hybrids as Novel 4 Hydroxyphenylpyruvate Dioxygenase Inhibitors. J. Agric. Food Chem. 2015, 63, 5587–5596. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wagerle, T.; Long, J.K.; Lahm, G.P.; Barry, J.D.; Smith, R.M. Insecticidal quinoline and isoquinoline isoxazolines. Bioorg. Med. Chem. Lett. 2014, 24, 4026–4030. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Chandra, V.; Jain, P.K.; Pathak, K.; Pathak, D.; Vaidya, A. Comprehensive Review on Current Developments of Quinoline-Based Anti-cancer Agents. Arab. J. Chem. 2020, 12, 4920–4946. [Google Scholar] [CrossRef]
- Serda, M.; Kalinowski, D.S.; Mrozek-Wilczkiewicz, A.; Musiol, R.; Szurko, A.; Ratuszna, A.; Pantarat, N.; Kovacevic, Z.; Merlot, A.M.; Richardson, D.R.; et al. Synthesis and characterization of quinoline-based thiosemicarbazones and correlation of cellular iron-binding effificacy to anti-tumor effificacy. Bioorg. Med. Chem. Lett. 2012, 22, 5527–5531. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Panda, S.S.; Hall, C.D. Quinine conjugates and quinine analogues as potential antimalarial agents. Eur. J. Med. Chem. 2015, 97, 335–355. [Google Scholar] [CrossRef]
- Shah, R.B.; Valand, N.N.; Sutariya, P.G.; Menon, S.K. Design, synthesis and characterization of quinoline–pyrimidine linked calix[4]arene scaffolds as anti-malarial agents. J. Incl. Phenom. Macrocycl. Chem. 2016, 84, 172–178. [Google Scholar] [CrossRef]
- Mullié, C.; Taudon, N.; Degrouas, C.; Jonet, A.; Pascual, A.; Agnamey, P.; Sonnet, P. Enantiomerically pure amino-alcohol quinolines: In vitro anti-malarial activity in combination with dihydroartemisinin, cytotoxicity and in vivo efficacy in a Plasmodium berghei mouse model. Malar. J. 2014, 13, 407. [Google Scholar] [CrossRef]
- Patel, S.R.; Gangwal, R.; Sangamwar, A.T.; Jain, R. Synthesis, biological evaluation and 3D-QSAR study of hydrazide, semicarbazide and thiosemicarbazide derivatives of 4-(adamantan-1-yl)quinoline as anti-tuberculosis agents. Eur. J. Med. Chem. 2014, 85, 255–267. [Google Scholar] [CrossRef]
- Mandewale, M.C.; Thorat, B.; Nivid, Y.; Jadhav, R.; Nagarsekar, A.; Yamgar, R. Synthesis, structural studies and antituberculosis evaluation of new hydrazone derivatives of quinoline and their Zn(II) complexes. J. Saudi Chem. Soc. 2018, 22, 218–228. [Google Scholar] [CrossRef]
- Runge, F.F. Ueber einige produkte der steinkohlendestillation. Ann. Phys. 1834, 107, 65–78. [Google Scholar] [CrossRef]
- Ogawa, Y.; Tokunaga, E.; Kobayashi, O. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 2020, 23, 101467–101520. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P. Latest generation of halogen-containing pesticides. Pest Manag. Sci. 2017, 73, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Zhao, W.; Shen, Z.H.; Xing, J.H.; Yuan, J.; Yang, G. Synthesis, nematocidal activity and docking study of novel chiral 1-(3-chloropyridin-2-yl)-3-(trifluoromethyl)-1H-pyrazole-4-carboxamide derivatives. J. Heterocycl. Chem. 2017, 54, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Zhao, W.; Shen, Z.H.; Xing, J.H.; Xu, T.M.; Peng, W.L. Synthesis, nematocidal activity and SAR study of novel difluoromethylpyrazole carboxamide derivatives containing flexible alkyl chain moieties. Eur. J. Med. Chem. 2017, 125, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Atsushi, S.; Mitsuyuki, Y.; Shigeru, U.; Yoshinori, M. Agrochemical Composition in Form of Aqueous Suspension. AU Patent 2014213426, 29 January 2014. [Google Scholar]
- Takeshi, T.; Makoto, M. Fungicidal Compositions for the Control of Paddy Rice Disease. PubChem Patent WO2004039156, 29 October 2003. [Google Scholar]
- Cai, P.P.; Cheng, L.; Tan, C.X.; Weng, J.Q.; Xu, T.M. New Quinoline Carbonate Derivatives with Perfluoroisopropyl Hybrid: Design, Synthesis, and Fungicidal Activity. Indian J. Heterocycl. Chem. 2019, 29, 243–247. [Google Scholar]
- Cheng, L.; Cai, P.P.; Zhang, R.R.; Han, L.; Tan, C.X.; Weng, J.Q.; Xu, T.M.; Liu, X.H. Synthesis and Biological Activity of Some New 6-perfluoropropanyl Quinoline Derivatives. J. Heterocycl. Chem. 2018, 55, 2585–2589. [Google Scholar] [CrossRef]
- Fang, Y.M.; Zhang, R.R.; Shen, Z.H.; Tan, C.X.; Weng, J.Q.; Xu, T.M.; Liu, X.H.; Huang, H.Y.; Wu, H.K. Synthesis and Antifungal Activity of Some 6-tert-butyl-8-chloro-2,3-dimethylquinolin-4-ol Derivatives against Pyricularia oryae. Lett. Drug Des. Discov. 2018, 15, 1314–1318. [Google Scholar] [CrossRef]
- Fang, Y.M.; Zhang, R.R.; Shen, Z.H.; Wu, H.K.; Tan, C.X.; Weng, J.Q.; Xu, T.M.; Liu, X.H. Synthesis, Antifungal Activity, and SAR Study of Some New 6-Perfluoropropanyl Quinoline Derivatives. J. Heterocycl. Chem. 2018, 55, 240–245. [Google Scholar] [CrossRef]
- Liu, X.H.; Fang, Y.M.; Xie, F.; Zhang, R.R.; Shen, Z.H.; Tan, C.X.; Weng, J.Q.; Xu, T.M.; Huang, H.Y. Synthesis and in vivo fungicidal activity of some new quinoline derivatives against rice blast. Pest Manag. Sci. 2017, 73, 1900–1907. [Google Scholar] [CrossRef]
- Sun, X.P.; Yu, W.; Min, L.J.; Han, L.; Sun, N.B.; Liu, X.H. Synthesis, crystal structure and antifungal activities of new quinolone derivatives. J. Mol. Struct. 2023, 1277, 134792. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS97 and SHELXL97; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Min, L.J.; Wang, H.; Bajsa-Hirschel, J.; Yu, C.S.; Wang, B.; Yao, M.M.; Han, L.; Cantrell, C.L.; Duke, S.O.; Sun, N.B.; et al. Novel dioxolane ring compounds for the management of Phytopathogen diseases as ergosterol biosynthesis inhibitors: Synthesis, biological activities and molecular docking. J. Agric. Food Chem. 2022, 70, 11470–11484. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Wen, Y.H.; Cheng, L.; Xu, T.M.; Wu, N.J. Design, Synthesis, Pesticidal Activities of Pyrimidin-4-amine Derivatives Bearing a 5-(Trifluoromethyl)-1,2,4-oxadiazole Moiety. J. Agric. Food Chem. 2021, 69, 6968–6980. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).