Abstract
Thermal-responsive block copolymers are a special type of macromolecule that exhibit a wide range of applications in various fields. In this contribution, we report a new type of polyacrylamide-based block copolymer bearing pyridine groups of polyethylene glycol-block-poly(N-(2-methylpyridine)-acrylamide; Px) that display distinct salt-induced lower critical solution temperature (LCST) behavior. Unexpectedly, the phase-transition mechanism of the salt-induced LCST behavior of Px block copolymers is different from that of the reported LCST-featured analogues. Moreover, their thermo-responsive behavior can be significantly regulated by several parameters such as salt species and concentration, urea, polymerization degree, polymer concentration and pH values. This unique thermal behavior of pyridine-containing block copolymers provides a new avenue for the fabrication of smart polymer materials with potential applications in biomedicine.
1. Introduction
Stimuli-responsive polymers that are able to change their own physical or chemical structures when triggered by external stimuli (temperature, pH, light, chemicals, etc.) have received a remarkable level of attention due to their potential applications in various fields such as drug delivery, biosensors and intelligent device manufacturing [1,2,3,4,5]. Over the past few decades, some stimuli-responsive polymers have been exploited for the construction of smart nanomaterials with different application potentials [6]. Among these, temperature-responsive polymers represent a special class that undergoes a phase transition in solvents at a critical solution temperature (lower critical solution temperature (LCST) or upper critical solution temperature (UCST)) [7]. Generally, LCST-type block copolymers (BCPs) in water are more attractive due to their potential bio-related applications [8,9,10,11,12]. For example, poly(N-isopropylacrylamide; PNIPAM)-based BCP is the most popular, with an LCST of around 32 °C—which approaches physiological temperatures [13]. PNIPAM bearing both hydrophilic (amide) and hydrophobic (isopropyl) groups has an LCTS due to the dehydration of its amide group upon heating, leading to a cloudy aqueous solution. Another class of polymers are the poly (oligo(ethylene glycol) (meth)-acrylate))-based BCPs that have an oligo ethylene glycol (OEG) as their temperature-responsive group, which exhibit a tunable LCST with a large range reaching up to 70 °C, depending upon the terminal groups (-Me or -Et) or their repeat units of oligo ethylene glycol [14,15,16].
It has been widely accepted that salts can enable the precipitation of certain proteins from aqueous solutions, which follows a recurring trend known as the Hofmeister series [17,18,19]. There is a general phenomenon that anions can cause this behavior to be more pronounced than cations can. The represented order of the anion species is: CO32− > SO42− > S2O32− > H2PO4− > F− > Cl− > Br− ~ NO3− > I− > ClO4− > SCN−. The left species of Cl− are called kosmotropes, while the ones on the right are referred to as chaotropes. In fact, changes in protein hydration induced by salts are one of the driving forces of liquid–liquid phase separation [20], which plays a critical role in the health and disease states of living organisms. Later, the Hofmeister series were used to explain the thermal-responsive behaviors of polymers in aqueous solutions based on the direct interactions of anions with macromolecules [21,22,23,24,25]. For example, Cremer and coworkers systematically studied specific ion effects on the basis of the Hofmeister Series on the thermal-responsive behavior of PNIPAM [26]. This has inspired tremendous efforts for the fabrication of smart polymeric materials with modulated thermal-responsive properties [27,28,29,30,31,32,33,34].
Herein, a new type of thermal-responsive polyacrylamide-based block-copolymer (Px) bearing pyridine groups was designed and synthesized through reversible addition-fragment chain transfer (RAFT) polymerization, showing unique salt-induced LCST behavior in aqueous solution. This thermo-responsive polymer bearing pyridine-containing acrylamide groups totally differs from traditional poly(acrylamide) analogs such as PNIPAM in terms of its chemical structure and responsive mechanisms. The significant influence on the thermal behaviors of the block copolymers exerted by molecular weight, salt concentration and species and pH values was systematically demonstrated. In addition, the obtained block copolymers were able to self-assemble into polymeric nano-objects in an aqueous solution—driven by the complementary hydrogen bonds in the N-(2-methylpyridine)-acrylamide (MPA)—that possess pH-responsive properties due to the protonation and deprotonation of their pyridine group. It can be anticipated that there will be a new class of thermo-responsive block polymers with pH responsive properties that can self-assemble into polymeric nanostructures driven by hydrogen bonding interactions.
2. Results and Discussion
2.1. Synthesis and Characterization of Diblock Copolymers (Px) via RAFT Polymerization
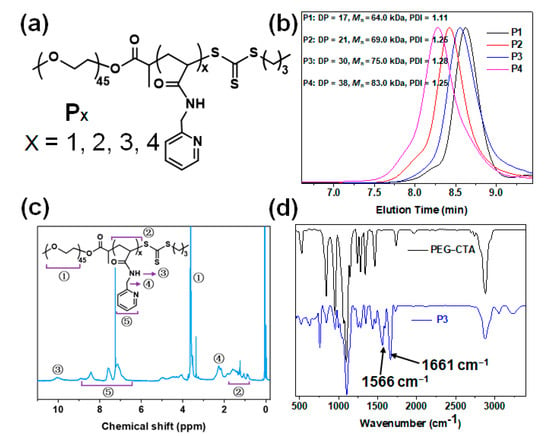
Px was synthesized via heat-initiated RAFT polymerization by employing macro-CTA (PEG-CTA) in the presence of MPA in ethanol at 70 °C for 24 h (Figure 1a). By controlling the molar ratio of the MPA and PEG-CTA, a series of block copolymers with different levels of PMPA were obtained. The GPC analysis showed that after polymerization, the molecular weight of the Px with a narrow index of polymer distribution (PDI < 1.3) increased with increases in the molar ratio of the MPA and PEG-CTA (Figure 1b). This suggests that the polymerization was controllable. Subsequently, the molecular structure of the Px was determined by proton nuclear magnetic resonance (1H NMR) and Fourier transform infrared spectrometer (FT-IR) after being purified by dialyzing in water, using a molecular cut-off 3.5 kDa. The 1H NMR spectrum exhibited typical pyridine peaks for PMPA at 8.4–7.1 ppm, and for PEG located at ca. 3.5 ppm (Figure 1c and Figure S1, Supplementary Materials). The FT-IR spectrum of the PEG-PMPA displayed representative signals at the 1661 and 1566 cm−1 wavelengths (Figure 1d), which illustrated the stretching vibration absorbance of the amido bonds and pyridine rings, respectively, and at 1103 cm−1—demonstrating the ether bond stretching vibration absorbance of the PEG backbone segments. These results confirmed the obtaining of PEG-PMPA via controllable RAFT polymerization.
2.2. Salt-Induced Thermal Response of Px
After obtaining the polymers, we first studied the different effects of salts, chemical additives and their concentrations on the aqueous thermal behaviors of the Px block copolymers. It has been shown that ionic strength is capable of influencing the solubility of macromolecules with amide groups by influencing hydrogen bonding between amide groups and water [26]. Thus, salt’s effects on the solubility of the obtained Px block copolymers were investigated. As homopolymers of PMPA are hardly homogeneously dispersed in water—even with a low DP (degree of polymerization) of only 20 (Figure S2)—purified block copolymers of Px with different DPs (17: P1, 21: P2, 30: P3, 38: P4) that are able to disperse in water after ultrasound for ca. 10 min at room temperature were selected. When continuously adding NaCl from 100 mM to 1.5 M into the solution, the 1H NMR spectra illustrated that the signals of the pyridine peaks increasingly weakened and became broad (Figure S3), which suggested that a high ionic strength could reduce the solubility of P2.
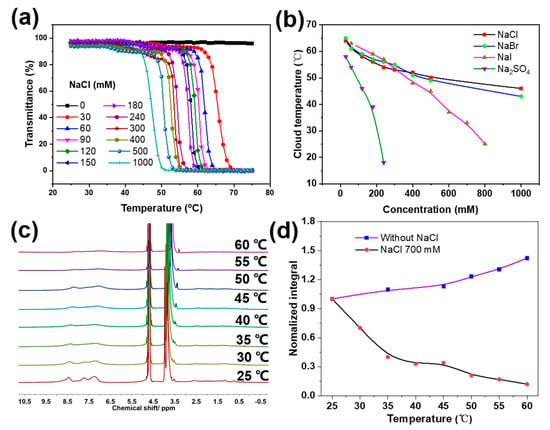
Next, temperature’s effect on the solubility of Px was systematically studied. As shown in Figure 2a (black line), turbidity experiments revealed that little transmittance (at 500 nm) change was observed with temperature increases from 20–75 °C in deionized water, which illustrated that no obvious aggregation occurred during heating in deionized water. Furthermore, the temperature-variable 1H NMR (TV-NMR) spectra displayed that, when enhancing the temperature from 25 to 60 °C, the proton signal peaks of the pyridine ring gradually became slightly sharpened and narrowed, following a gradual increase in the relative integrated value of the protons in the pyridine (Figure 2d, purple line). This can be ascribed to the fact that high temperatures, to a certain degree, weaken the inter-molecular hydrogen bonds between pyridine and amide bonds—increasing the water solubility of the polymer. It is well-known that the thermal properties of acetamide-based polymers such as PNIPAM can be influenced by anions based on the Hofmeister series in aqueous solutions [35]. Thus, we studied salt’s effects on the possible thermal behaviors of Px in water. Intriguingly, when we added even a small amount of NaCl (30 mM) into the P2 system (1 mg/mL), a cloud-point temperature (CPT) of 66 °C appeared, and when the concentration of NaCl was increased from 30 mM to 1 M, the CPT decreased from 66 °C to 47 °C (Figure 2a). Meanwhile, the TV-NMR analysis revealed that when increasing the temperature from 25 to 60 °C, with a concentration of NaCl of 700 mM in D2O, the proton peaks of the pyridine rings gradually weakened and became broad (Figure 2c), following an evident decrease in the relative integrated value of the protons in the pyridine (Figure 2d, black line)—which agrees with the turbidity results. Besides this, the effects of other anions including kosmotropes and chaotropes on the thermal behaviors of Px were also investigated. The results in Figure 2b and Figure S4 exhibit that all the selected anions, regardless of the presence of kosmotropes (Br− and I−) or chaotropes (SO42−), were able to induce the appearance of CPT. Furthermore, all the cloud-point temperatures of Px decreased with increases in the concentrations of NaCl, NaBr, NaI and Na2SO4. Additionally, the order-of-change range induced by the addition of salts was Na2SO4 >> NaI > NaBr ~ NaCl—indicating that the thermal response of Px influenced by anions did not follow the classical Hofmeister serial order, which differs from that of traditional synthesized temperature-responsive polymers such as PNIPAM or the family of poly (oligo(ethylene glycol)). This unique phenomenon may be mainly attributed to the electron-rich nitrogen present in pyridine structures that forms complementary hydrogen bonds with amide groups. The control experiment with the PEG-CTA and MPA monomer showed no turbidity variation with temperature increases up to 1 M NaCl concentrations (Figure S5), excluding the PEG block and a possible monomer residue effect on the salt-induced LCST. From all these results, it may be deduced that salts can significantly influence the solubility and unique thermal behaviors of Px block polymers—possibly via the polarization of an adjacent water molecule, weakening the hydrogen bonding interactions between water molecules and amides and, in contrast, enhancing the complementary hydrogen bonding between pyridines and amides.
Figure 2.
(a) Effect of NaCl concentration on the thermal response of P2. (b) Effect of various salts on the temperature–responsive behaviors of P2. (c) 1H NMR spectra of P2 at various temperatures with NaCl concentrations at 700 mM. (d) Plots of the integration area of the protons on the pyridine ring versus temperature with (black line) and without (purple line) NaCl.
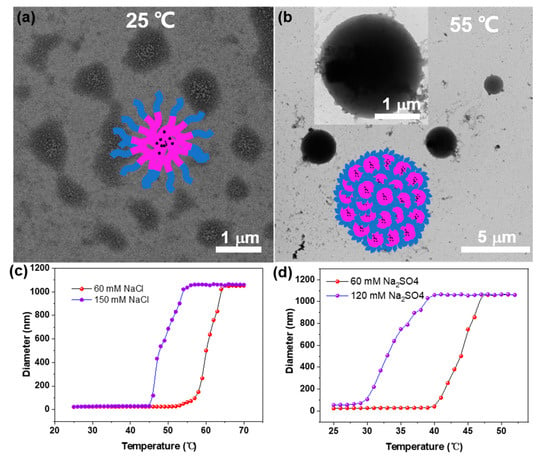
Subsequently, aggregation behavior was observed upon heating. As shown in Figure 3a, it was found that the P2 formed irregular, loose aggregates at 25 °C with NaCl concentrations of 500 mM, driven by complementary hydrogen bonding between the pyridine and amides—subsequently assembled into large micelles with a size of several micrometers (Figure 3b). The temperature-variable DLS (TV-DLS) results showed a significant size increase with temperature enhancement at different NaCl and Na2SO4 concentrations (Figure 3c,d). Intriguingly, this thermal response was totally responsive (Figure S6).
Figure 3.
TEM images of the P2 at (a) 25 °C and (b) 55 °C with 500 mM NaCl. The diameter variation of P2 upon the temperature increases with different concentrations of (c) NaCl and (d) Na2SO4.
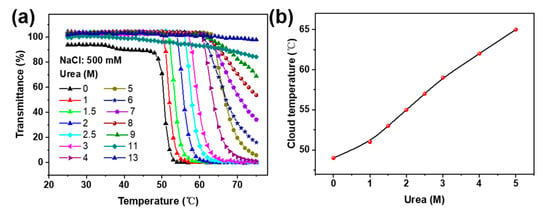
In order to demonstrate the key role of hydrogen-bonding interactions between the pyridine and amides in the temperature response of the Px, varying amounts of urea—which is commonly utilized to disturb hydrogen bonds—were added into the solution [36]. It was observed that with increases in urea concentrations from 0 to 7 M, the CPT of the P2 (1 mg/mL, 500 mM NaCl) significantly increased from ca. 45 to 70 °C, and when adding urea at over 8 M, no obvious temperature response was observed (Figure 4)—suggesting that urea could dramatically enhance the aqueous solubility of Px.
Figure 4.
(a) Effect of urea concentration on the thermal response of P2 in NaCl aqueous solution. (b) Plots of the cloud-point temperature versus urea concentration in NaCl solution.
After verifying the salt effect on the temperature properties of the Px, we next studied the effect of DP and its concentration on the thermal response of Px. As illustrated in Figure S7, four kinds of Px with a PMPA DP of 17, 21, 30 and 38, respectively, were evaluated at various concentrations (0.5 mg/mL, 1 mg/mL and 2 mg/mL) and 500 mM NaCl. Generally, with increases in both the DP and its concentration, the cloud-point temperature shifted to a lower temperature. Temperature-dependent turbidity curves exhibited that the cloud-point temperature shifted from 61 to 41 °C (0.5 mg/mL), 59 to 37 °C (1 mg/mL) and 57 to 31 °C (2 mg/mL) with increases in the DP of the PMPA (Figure S7a)—possibly due to the increased efficiency of intermolecular aggregation with molecular weight increases. Notably, when the DP of the PMPA part was 38, it was found that the transmittance was lowered to 27 % (2 mg/mL) at 20 °C, which implies the formation of large aggregates from P4 at a DP of 38. Subsequently, the temperature-responsive behavior of Px was investigated at different concentrations. It was observed that when increasing the concentration from 0.5 to 2 mg/mL at NaCl 500 mM, the cloud-point temperature decreased from 61 to 57 for DP 17, 54 to 50 for DP 21, 44 to 39 for DP 30 and 41 to 31 for DP 38 (Figure S7b), which could be attributed to the increased efficiency of the aggregation of intermolecular chains with increasing concentrations.
2.3. pH-Responsive Properties of Px
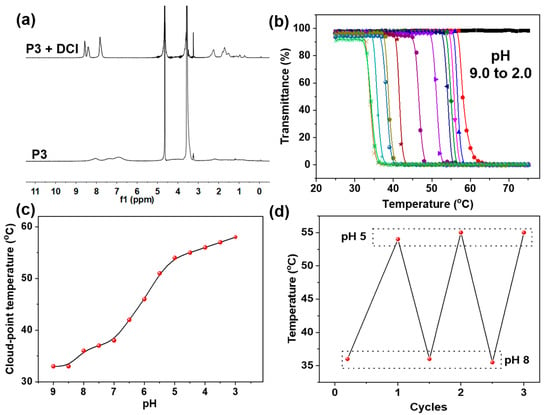
Considering that pyridine is structurally sensitive to acid, the pH effect on the obtained Px polymers was investigated. It can be anticipated that the pH value can be used to not only tune the solubility of Px, but also to produce an effect on their thermal behaviors in aqueous solution. The 1H NMR spectra (Figure 5a) showed that after adding 0.2 M DCl (deuterium chloride) into the purified P3 (10 mg/mL) D2O solution, all the signals of the PMPA block became dramatically sharp and strong, manifesting that an acid can significantly enhance the solubility of the block copolymers (Px) due to the protonation of their pyridine groups. Furthermore, we systematically investigated pH effects on the temperature-responsive behaviors of P3 in PBS buffer solution containing a certain amount of salt (ionic strength ≈ 0.5 mol/L). It was found that when varying the pH from 9.0 to 3.0, the CPT of the P3 shifted from 33 to 57 °C (Figure 5b,c), which was ascribed to the constant protonation that occurred with pH decreases. However, when lowering the pH to 2, the CPT disappeared (Figure 5b)—possibly due to the fact that the dense protonation of pyridine moieties completely prevents the aggregation of PMPA blocks. In addition, their CPT values can be reversibly regulated by alternatively changing the pH values (Figure 5d). All these results indicate that the resultant Px block copolymers possessed a remarkable pH-responsive feature due to the protonation and deprotonation of their pyridine groups. Furthermore, at the same time, the CPT of the polymer could be precisely tuned within a large range by altering the pH in buffer solution.
Figure 5.
(a) 1H NMR spectra of P3 before and after the addition of DCl in D2O. (b) Effect of pH values on the thermal-responsive behaviors of P3 in PBS buffer. (c) Plots of the cloud-point temperature depending on the pH values in PBS buffer. (d) Reversible cycles of cloud-point temperature by alternatively changing the pH between 8 and 5.
3. Materials and Methods
3.1. Materials
Acryloyl chloride (Aladdin, 98%), 4-Dimethylaminopyridine (Aladdin, 98%), Methoxypolyethylene glycols (2000; Aladdin, 98%), 2-Picolylamine (Aladdin, 99%), 2-Butylsulfanyl-thiocarbonylsulfanyl-propionic acid (Aladdin, 98%), Triethylamine (Aladdin, 98%), Azobisisobutyronitrile (Aladdin, 98%) and 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (Aladdin, 98%) were purchased from Aladdin Reagents Co. Ltd. (Shanghai, China).
3.2. Characterizations
NMR spectra were obtained from Agilent DD2 600 MHz of Bruker BioSpin International (Billerica, MA, USA). CDCl3 and D2O were used as solvents. The structures of the nanomaterials were observed by transmission electron microscopy (TEM, HT-7700 microscope, Hitachi, Japan). Dynamic light scattering (DLS) was performed using a Delsa Max Pro from Beckman Coulter (Brea, CA, USA). UV-Vis Absorption Spectra: UV-vis spectra were recorded on an Analytikjena Specord S 600 (Jena, Germany) via using a cuvette to scan at 200–800 nm. FT-IR spectra were collected using a Nicolei 6670 FT-IR spectrometer (Madison, WI, USA).
3.3. Synthesis of Px
MPA, PEG-CTA and AIBN with specific molar ratios were added into 1 mL ethanol. After 3 cycles of freezing and thawing, the reaction was kept at 70 °C for 24 h to ensure that conversion approached 100%. MPA and PEG-CTA were synthesized according to our previous work [36].
4. Conclusions
In summary, we synthesized a new type of polyacrylamide-based block copolymer bearing pyridine groups that showed salt-induced LCST behavior. The LCST can be tuned by several external factors such as salt species and concentration, pH value, urea concentration, molecular concentration and the DP of the PMPA block. It is notable that the mechanisms of the thermo-responsive behaviors of the PMPA block did not strictly follow the order of the Hofmeister series, showing different mechanisms to those of traditional LCST-possessed polymers. We believe that this novel kind of thermal-responsive block-copolymer and its assemblies possess great potential in a wide range of applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28072921/s1, Figure S1: 1H NMR spectra of P1, P2, P3 and P4 in CDCl3; Figure S2: 1H NMR spectrum of PMPA20. The inset showed that PMPA20 was insoluble in deionized water at room temperature; Figure S3: (a) 1H NMR spectra of P2 depending on the concentration of NaCl. (b) Plots of the integration area of the protons on pyridine ring versus concentration of NaCl; Figure S4: Effect of NaBr (a), NaI (b) and Na2SO4 (c) concentration on thermal response of P2; Figure S5: Turbidity change of PEG-CTA and MPA in 1 M NaCl aqueous solution. Figure S6: DLS results of P2 at 25 °C and 55 °C, respectively. Figure S7: Effect of (a) DP of PMPA and (b) concentration on the thermal responsive behaviors of Px.
Author Contributions
Conceptualization, G.Y. and X.L.; funding acquisition, G.Y. and L.Y.; investigation, G.Y., P.S. and L.Y.; methodology, Y.T. and D.F.; resources, G.Y., P.S. and L.Y.; software, W.Z. and Z.W.; visualization, Y.T. and D.F.; validation, Y.T. and D.F.; formal analysis, W.Z. and Z.W., data curation, Y.T., W.Z. and Z.W.; writing—original draft preparation, Y.T. and G.Y.; writing—review and editing, Y.T. and D.F.; supervision, G.Y.; project administration, G.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge open funding from Key Laboratory of Jiangxi Province for Persistent Pollutants Control and Resources Recycle (No. ES202080079), as well as the support of undergraduate student research project 202210364021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this work are available in the article and Supplementary Materials.
Acknowledgments
The authors acknowledge the financial support of the Anhui Province Natural Science Funds (2008085QE209) and K2020-03 from the State Key Laboratory of Molecular Engineering of Polymers (Fudan University) and open funding from Key Laboratory of Jiangxi Province for Persistent Pollutants Control and Resources Recycle (No. ES202080079), as well as the support of undergraduate student research project 202210364021.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Reineke, T.M. Stimuli-responsive polymers for biological detection and delivery. ACS Macro Lett. 2016, 5, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.M.; Liu, R.J.; Yan, Q. Biological stimuli-responsive polymer systems: Design, construction and controlled self-assembly. Chin. J. Polym. Sci. 2018, 36, 347–365. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Q.; Li, X.; Serpe, M.J. Stimuli-responsive polymers for sensing and actuation. Mater. Horizons 2019, 6, 1774–1793. [Google Scholar] [CrossRef]
- Grzelczak, M.; Liz-Marzán, L.M.; Klajn, R. Stimuli-responsive self-assembly of nanoparticles. Chem. Soc. Rev. 2019, 48, 1342–1361. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, L.; Du, Q.; Gou, L.; Zhang, L.; Chai, Y.; Zhang, R.; Shi, T.; Chen, G. Polymorphism of Kdo-Based Glycolipids: The Elaborately Determined Stable and Dynamic Bicelles. CCS Chem. 2022, 4, 2228–2238. [Google Scholar] [CrossRef]
- Montero de Espinosa, L.; Meesorn, W.; Moatsou, D.; Weder, C. Bioinspired polymer systems with stimuli-responsive mechanical properties. Chem. Rev. 2017, 117, 12851–12892. [Google Scholar] [CrossRef]
- Sun, W.; An, Z.; Wu, P. UCST or LCST? Composition-Dependent Thermoresponsive Behavior of Poly (N-acryloylglycinamide-co-diacetone acrylamide). Macromolecules 2017, 50, 2175–2182. [Google Scholar] [CrossRef]
- Wei, P.; Cornel, E.J.; Du, J. Breaking the corona symmetry of vesicles. Macromolecules 2021, 54, 7603–7611. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly (N-isopropylacrylamide) phase diagrams: Fifty years of research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, X.X.; Luo, J.; Liu, H. Effects of substitution groups on the RAFT polymerization of N-alkylacrylamides in the preparation of thermosensitive block copolymers. Macromolecules 2007, 40, 6481–6488. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, Z.; Zhu, X.X. Rational design of thermoresponsive polymers in aqueous solutions: A thermodynamics map. Prog. Polym. Sci. 2019, 90, 269–291. [Google Scholar] [CrossRef]
- Panja, S.; Dey, G.; Bharti, R.; Kumari, K.; Maiti, T.K.; Mandal, M.; Chattopadhyay, S. Tailor-made temperature-sensitive micelle for targeted and on-demand release of anticancer drugs. ACS Appl. Mater. Interfaces 2016, 8, 12063–12074. [Google Scholar] [CrossRef] [PubMed]
- Sahn, M.; Yildirim, T.; Dirauf, M.; Weber, C.; Sungur, P.; Hoeppener, S.; Schubert, U.S. LCST behavior of symmetrical PNiPAm-b-PEtOx-b-PNiPAm triblock copolymers. Macromolecules 2016, 49, 7257–7267. [Google Scholar] [CrossRef]
- Wang, K.; Chen, S.; Zhang, W. A New Family of Thermo-, pH-, and CO2-Responsive Homopolymers of Poly [Oligo (ethylene glycol) (N-dialkylamino) methacrylate]. Macromolecules 2017, 50, 4686–4698. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Q.; Lu, G.; Zhang, Y.; Zhou, Y.; Chen, S.; Ma, Q.; Liu, G.; Zeng, Y. Acid-Labile Temperature-Responsive Homopolymers and a Diblock Copolymer Bearing the Pendent Acetal Group. Macromolecules 2021, 54, 3725–3734. [Google Scholar] [CrossRef]
- Jochum, F.D.; Theato, P. Temperature-and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef] [PubMed]
- Kunz, W.; Nostro, P.L.; Ninham, B.W. The present state of affairs with Hofmeister effects. Curr. Opin. Colloid Interface Sci. 2004, 9, 1–18. [Google Scholar] [CrossRef]
- Lo Nostro, P.; Fratoni, L.; Ninham, B.W.; Baglioni, P. Water absorbency by wool fibers: Hofmeister effect. Biomacromolecules 2002, 3, 1217–1224. [Google Scholar] [CrossRef]
- Boström, M.; Williams, D.R.M.; Ninham, B.W. Specific ion effects: Why DLVO theory fails for biology and colloid systems. Phys. Rev. Lett. 2001, 87, 168103. [Google Scholar] [CrossRef]
- Hyman, A.A.; Weber, C.A.; Jülicher, F. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 2014, 30, 39–58. [Google Scholar] [CrossRef]
- Isobe, N.; Shimizu, S. Salt-induced LCST-type thermal gelation of methylcellulose: Quantifying non-specific interactions via fluctuation theory. Phys. Chem. Chem. Phys. 2020, 22, 15999–16006. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.P.; Elliott, G.R.; Robertson, H.; Kumar, A.; Wanless, E.J.; Webber, G.B.; Craig, V.S.J.; Andersson, G.G.; Page, A.J. Understanding specific ion effects and the Hofmeister series. Phys. Chem. Chem. Phys. 2022, 24, 12682–12718. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hua, M.; Alsaid, Y.; Du, Y.; Ma, Y.; Zhao, Y.; Lo, C.; Wang, C.; Wu, D.; Yao, B.; et al. Poly (Vinyl Alcohol) Hydrogels with Broad-Range Tunable Mechanical Properties via the Hofmeister Effect. Adv. Mater. 2021, 33, 2007829. [Google Scholar] [CrossRef]
- Salis, A.; Ninham, B.W. Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, G. Ionic effects on synthetic polymers: From solutions to brushes and gels. Soft Matter 2020, 16, 4087–4104. [Google Scholar] [CrossRef]
- Zhang, Y.; Furyk, S.; Bergbreiter, D.E.; Cremer, P.S. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J. Am. Chem. Soc. 2005, 127, 14505–14510. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, J.; Yan, Y.; Hai, Z.; Hua, Z.; Chen, G. Multi-Stimuli-Triggered Shape Transformation of Polymeric Filaments Derived from Dynamic Covalent Block Copolymers. Biomacromolecules 2020, 21, 4159–4168. [Google Scholar] [CrossRef]
- Lian, L.L.; Xu, S.Y.; Yuan, H.Y.; Liu, G.M. The Anion Binding Affinity Determines the Strength of Anion Specificities of Thermosensitive Polymers. Chin. J. Polym. Sci. 2021, 39, 1351–1356. [Google Scholar] [CrossRef]
- Wei, P.; Cook, T.R.; Yan, X.; Huang, F.; Stang, P.J. A discrete amphiphilic organoplatinum (II) metallacycle with tunable lower critical solution temperature behavior. J. Am. Chem. Soc. 2014, 136, 15497–15500. [Google Scholar] [CrossRef]
- Fan, X.; Liu, H.; Gao, Y.; Zou, Z.; Craig, V.S.J.; Zhang, G.; Liu, G. Forward-Osmosis Desalination with Poly (Ionic Liquid) Hydrogels as Smart Draw Agents. Adv. Mater. 2016, 28, 4156–4161. [Google Scholar] [CrossRef]
- Beyer, V.P.; Becer, C.R. Thermoresponsive polymers in non-aqueous solutions. Polym. Chem. 2022, 13, 6423–6474. [Google Scholar]
- Ding, Y.; Yan, Y.; Peng, Q.; Wang, B.; Xing, Y.; Hua, Z.; Wang, Z. Multiple stimuli-responsive cellulose hydrogels with tunable LCST and UCST as smart windows. ACS Appl. Polym. Mater. 2020, 2, 3259–3266. [Google Scholar] [CrossRef]
- Ercole, F.; Kim, C.J.; Dao, N.V.; Tse, W.K.; Whittaker, M.R.; Caruso, F.; Quinn, J.F. Synthesis of Thermoresponsive, Catechol-Rich Poly (ethylene glycol) Brush Polymers for Attenuating Cellular Oxidative Stress. Biomacromolecules 2022, 24, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Phunpee, S.; Ruktanonchai, U.R.; Chirachanchai, S. Tailoring a UCST-LCST-pH Multiresponsive Window through a Single Polymer Complex of Chitosan–Hyaluronic Acid. Biomacromolecules 2022, 23, 5361–5372. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.A.; Okur, H.I.; Yan, C.; Yang, T.; Heyda, J.; Cremer, P.S. Weakly hydrated anions bind to polymers but not monomers in aqueous solutions. Nat. Chem. 2022, 14, 40–45. [Google Scholar] [CrossRef]
- Ren, H.; Wei, Z.; Wei, H.; Yu, D.; Li, H.; Bi, F.; Xu, B.; Zhang, H.; Hua, Z.; Yang, G. Pyridine-containing block copolymeric nano-assemblies obtained through complementary hydrogen-bonding directed polymerization-induced self-assembly in water. Polym. Chem. 2022, 13, 3800–3805. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).