Abstract
Hedyosmum purpurascens is an endemic species found in the Andes of Ecuador and it is characterized by its pleasant smell. In this study, essential oil (EO) from H. purpurascens was obtained by the hydro-distillation method with a Clevenger-type apparatus. The identification of the chemical composition was carried out by GC–MS and GC–FID in two capillary columns, DB-5ms and HP-INNOWax. A total of 90 compounds were identified, representing more than 98% of the total chemical composition. Germacrene-D, ϒ-terpinene, α-phellandrene, sabinene, O-cymene, 1,8-cineole and α-pinene accounted for more than 59% of the EO composition. The enantioselective analysis of the EO revealed the occurrence of (+)-α-pinene as a pure enantiomer; in addition, four pairs of enantiomers were found (α-phellandrene, o-cymene, limonene and myrcene). The biological activity against microbiological strains and antioxidants and the anticholinesterase properties were also evaluated and the EO showed a moderate anticholinesterase and antioxidant effect, with an IC50 value of 95.62 ± 1.03 µg/mL and a SC50 value of 56.38 ± 1.96. A poor antimicrobial effect was observed for all the strains, with MIC values over 1000 µg/mL. Based on our results, the H. purpurasens EO presented remarkable antioxidant and AChE activities. Despite these promising results, further research seems essential to validate the safety of this medicinal species as a function of dose and time. Experimental studies on the mechanisms of action are essential to validate its pharmacological properties.
1. Introduction
Chloranthaceae is a taxonomic family consisting of herbs, shrubs and aromatic trees of four genera, Ascarina, Chloranthus, Hedyosmum and Sarcandra [1]. The species are distributed in the tropical ecosystems of Latin America, Asia and the Pacific [2]. In Ecuador, 16 species of the Chloranthaceae family are recognized [3] and the majority are endemic. The importance of this taxonomic category resides in its primitive origins, applications and distribution patterns, as well as the secretory cells in its leaves and stems, which are common in the Angioespermae family [4]. Most of the chemical constituents of the Chloranthaceae family include sesquiterpenes, terpenoids, coumarins, flavonoids and lignans [5]. In particular, Hedyosmum is the most abundant genus of this family, comprising about 40 species located in the mountainous areas of Intertropical America and Southeast Asia [6]. The Hedyosmum genus occurs within the geographic limits of cloud forests and in the central Andes, where 50% of its total species are located [7]. Despite their scarce local abundance, these plants have been used in a vast number of applications, such as in ornaments and in the pharmaceutical industry, as fortifiers and antifungal agents, due to the presence of important secondary metabolites, which are the main sources of active ingredients in drugs used to treat respiratory infections and diarrheal diseases [8], In addition, they are used for food purposes, as additives, to improve the organoleptic character of fortifying beverages and liquors [4]. The species of the genus Hedyosmum are known to provide biological/pharmacological alternatives, such as antibacterial and antifungal properties, to combat common fungi or infections. In fact, there are studies that demonstrate the veracity of the properties possessed by the essential oils of some species from this genus, such as H. bonpladianum, in which the analgesic activity was attributed to isolated glycosidic flavonoids [9].
Hedyosmum purpurascens is an herbaceous dioecious plant approximately of 2 to 4 m in height. It possesses brittle branches, purple leaves, a small oval shape with petioles connate around the stem, male inflorescences visible on its last leaves, female inflorescences formed by spikelets located individually in the cavities of the crown, forming small clusters of multiple flowers arranged in the form of cymules and fruits with endocarps and mesocarps of considerable thickness. It grows naturally along the roads of the province of Loja towards the Zamora, Catamayo, Valladolid and Saraguro cantons [2]. There are approximately four endemic species within the Podocarpus National Park, in a small extension of the southern montane evergreen forest of the eastern Cordillera of the Andes [10,11,12].
Despite the age of the species of the Hedyosmum genus, only 20 species have been analyzed: H. traslucidum [13], H. brasiliense [4], H. angustifolium, H. scabrum [1], H. arborescens [14], H. mexicanum, H. bonplandianum and H. costaricensis [15], H. colombianun [16], H. sprucei [17], H. glabratum [18], H. anisodorum, H.uniflorum [2], H. luttynii [2], H. crenatum, H. nutans, H. scaberrimum, H. maxima, H. goudotianum [19] and H. racemosun [20]. Therefore, the aim of this research was to determine the chemical characterization, the enantiomeric distribution and the biological activity of the essential oil of Hedyosmum purpurascens. The main purpose was to enrich and promote the knowledge of the vernacular vegetation of our country, including the possible use of this essential oil in the isolation of new compounds and the synthesis of products for food, as well as in medicinal or pharmaceutical applications.
2. Results
2.1. Physical Properties
The aerial parts of the Hedyosmun purpurasens were hydro-distilled to successfully obtain 9.18 mL of a pure essential oil, with a yield of 0.16% (v/w). In terms of its sensory characteristics, the EO displayed a yellowish-green color and a slightly sour fresh aroma. The EO revealed a density of 0.87 ± 0.02 g/mL, a refractive index [n20] = 1.4831 ± 0.01 and an average specific optical rotation = −4.78 ± 0.01.
2.2. Chemical Constituents of Essential Oil
After the integration of the chromatograms, ninety chemical compounds were identified, representing 97.78%, in the DB-5ms column and 97.18%, in the HP-INNOWax column, of the total constituents (Table 1). The 10 most abundant compounds (representing 64.68 and 59.46% in the DB-5ms and HP-INNOwax, respectively), were Germacrene-D (17.80, 15. 73%), ϒ-terpinene (4.13, 4.53%), α-phellandrene (8.11, 10.84%), sabinene (7.05, 12.33%), ο-cymene (6.62, 3.77%), 1,8-cineole (6.62, 6.62%), α-pinene (5.84, 0.10%), β-pinene (3.29, 0.27%), bisabolene-E-ϒ (2.87, 1.51%) and limonene (2.35, 3.76%).

Table 1.
Chemical composition of H. purpurascens EO in DB-5ms and HP-INNOWAx columns.
The EO obtained through the DB-5ms and HP-INNOWAx was characterized by the presence of thirty-two sesquiterpene hydrocarbons (SH), twenty-one monoterpene hydrocarbons (MH), eight oxygenated monoterpenes (OM), seven oxygenated sesquiterpenes (OS) and fifteen other compounds. The SH represented 30.13% and 34.22%, the MH 51.29% and 43.43%, the OM 9.53% and 8.77%, the OS 1.50% and 2.39% and the remaining compounds 5.56% and 10.54%, respectively.
2.3. Enantiomeric Composition
When the first enantioselective analysis of the H. purpurascens EO was conducted (see Table 2), the (+)-α-Pinene completely occurred as a pure enantiomer and, additionally, four other pairs of optical isomers were found. Among these, the (+)-α Phellandrene presented one of the highest rates of enantiomeric excess, followed by the (+)-o-Cymene, with values of 83.49 ± 0.01 and 79.40 ± 3.41, respectively. Similarly, the lowest enantiomeric excess was presented by the (−)-Myrcene, with a value of 22.67 ± 0.31%.

Table 2.
Enantiomeric GC analysis of Hedyosmum purpurascens EO.
2.4. Antimicrobial Activity
Table 3 shows the minimum inhibitory concentration (MIC) values of the essential oil against the tested microorganisms. The EO of the H. purpurascens was found to be inactive at the highest dose tested for the Gram-negative microorganisms and the Candida albicans strain. However, for the rest of the strains, the antibacterial capacity proved to be more efficient, particularly for Staphylococcus aureus and Aspergillus niger, each with a MIC value of 1000 µg/mL. Nevertheless, insufficient relevant outcomes were recorded to propose this EO as an antimicrobial efficient agent for future research.

Table 3.
Antimicrobial activities of Hedyosmun purpurascens essential oil.
2.5. Antioxidant Activity
The antioxidant potential of the H. purpurascens species was evaluated for the DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radicals. The analysis was based on the scavenging effect exerted by the EO sample on the 50% radical reduction (SC50). Trolox was used as an antioxidant positive control. The essential oil of the H. purpurasens essential was not active at the highest dose tested for the DPPH. Instead, it showed a high ABTS uptake capacity, of 56.38 ± 1.96 µg/mL. The results are shown in Table 4.

Table 4.
Half scavenging capacity of H. purpurascens EO.
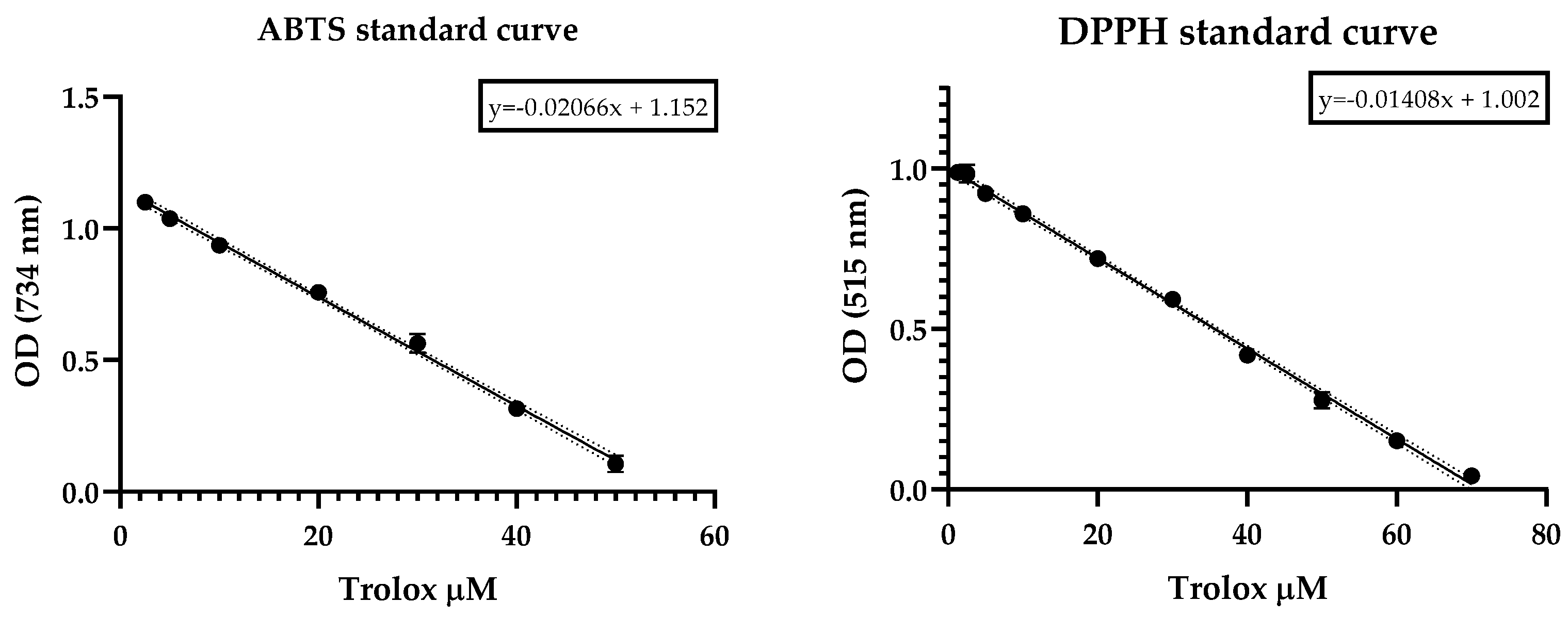
The Trolox antioxidant capacity was measured according to the same method as that used for the DPPH and ABTS radicals and the SC50 was calculated from the dose-response-curve fitting (Figure 1).
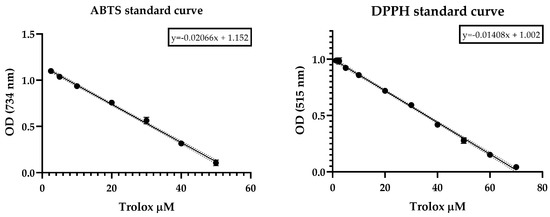
Figure 1.
Scavenging effect of Trolox on ABTS and DPPH radicals revealed as a rapid decrease in optical density as a function of the dose.
2.6. Anticholinesterase Activity
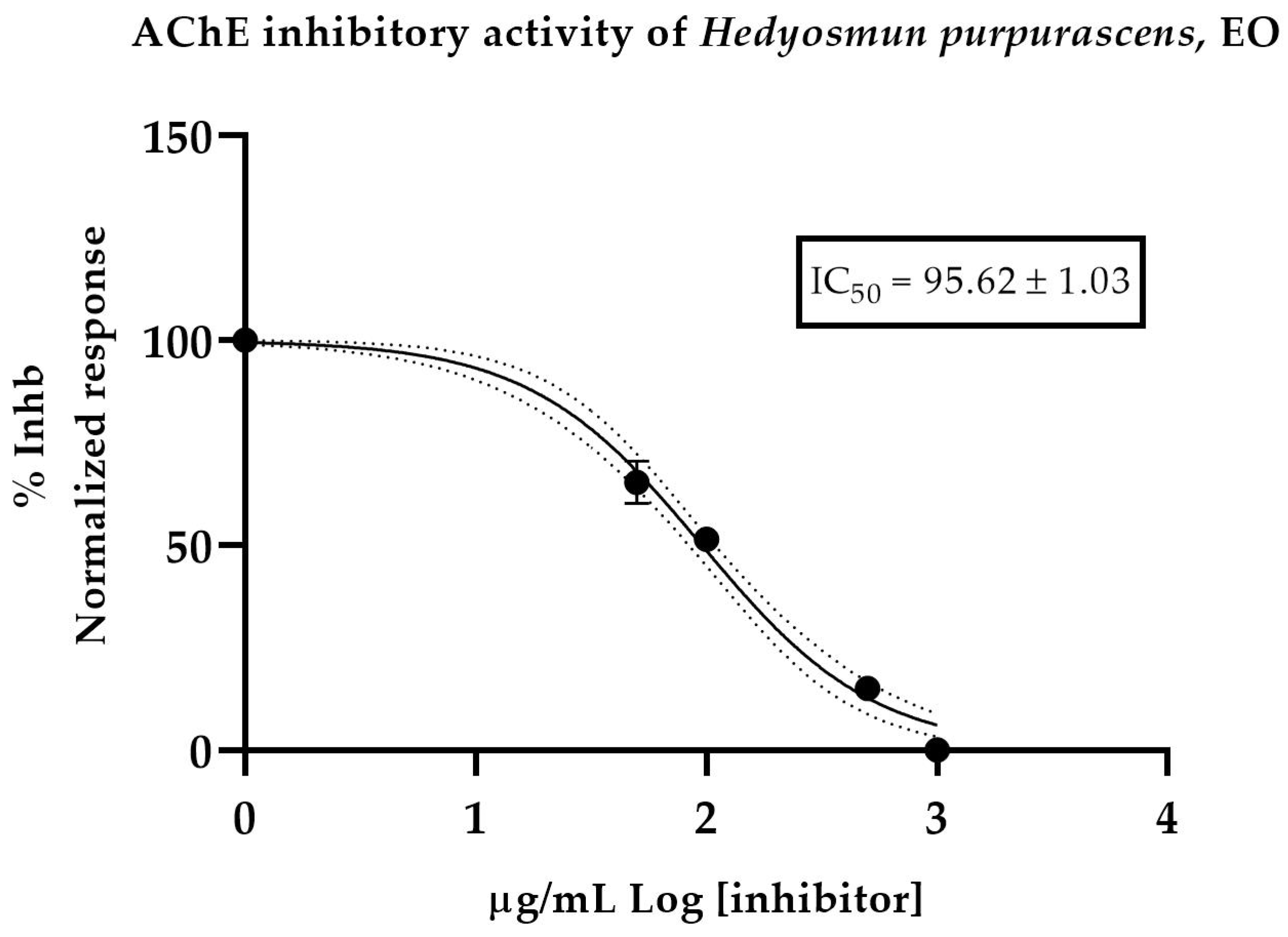
Five samples of H. purpurascens EO dissolved with MeOH were tested. Donepezil-hydrochloride was used as a positive control, with IC50 of 13.6 ± 1.02 µM. The results showed a moderate effect, with an IC50 value of 95.62 ± 1.03 µg/mL (Figure 2).
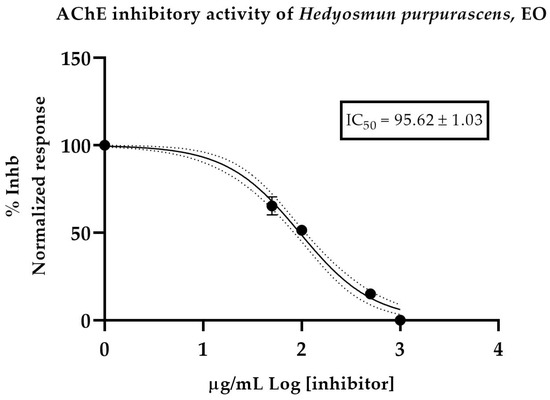
Figure 2.
Half-maximum inhibitory concentration of H. purpurascens EO against acetylcholinesterase.
3. Discussion
The yield of the H. purpurascens EO was estimated based on the mass quantity of the oil obtained and the fresh plant material. The yield obtained is based on the operating conditions under which the whole process was developed, such as the meteorology of plant growth, harvest time, storage of the species, moisture content of the plant, type of hydro-distiller used and steam thermal conditions [69]. On the other hand, the results for the physical properties were in accordance with those of other studies carried out on species of the same genus, such as H. racemosum, including the density, with a value of 0.897 g/mL and the refractive index, of 1.4911 [20].
Regarding the qualitative analysis of the EO, more than 95% of the total constituents of the volatile fraction of the sample were identified in the chromatographic columns, DB-5ms and HP-INNOWax. Considering the main compounds found in the oil, it can be inferred that the essential oil of H. purpurasens is more closely related to certain studies of species of the same genus; the only difference is in the quantity in the total oil. It was reported that in H. scabrum, the major compounds were germacrene-D (13.0%), δ-3-carene (12.1%), α-gurjunene (6.6%), 3′,4′-dimethoxypropiophenone (6.6%), 1,8-cineole (5.7%) and α-phellandrene (2.8%), while in H. angustifolium species, the main compounds were α-Pinene (24.0%), β-pinene (23.5%), sabinene (6.4%), linalool (6.1%), 1,8-cineole (3.7%) and germacrene D (3.1%) [70]. In contrast, in a study by Nurliyana et al. [71], the Chloranthus erectus EO, a species in the same family as H. purpurascens, was analyzed. It was evident that the major compounds in this species are the total opposite of those found in this study, since germacrene-D represented only 0.05%.
The EO composition included SH (30.13%) and MH (51.29%), among which α-pinene, germacrene-D, α-cadinene and α-phellandrene were the most representative. According to Cao et al. [72], these types of compound were considered the main constituents in the essential oils of the Chloranthaceae family, with germacrene-D and cadinene considered some of the main chemotypes. Similarly, the fact that hydrocarbons were found in a greater proportion than the oxygenated hydrocarbons was due to the fact that they are organic compounds with high levels of volatility and, therefore, as they act as regulators of water potential, their extraction occurs more easily. In contrast, in other species, as reported by Stashenko et al. [73], in the EO of L. alba, MO predominated (55%); the same was observed for A. triphylla, where the MO >70%, since the MWHD (microwave-assisted hydrodistillation) extraction method was used, in which obtaining the EO presents a higher yield [74]. Finally, the presence of other types of compound (5.56%), such as vetivenic acid, E-nuciferol, citronellyl pentanoate, myrtenyl acetate, citronellyl acetate and E-isocroweacin, which have not been reported in other studies, indicated the variation in the chemical compositions of the EO due to the presence of specific chemotypes.
In order to demonstrate the therapeutic properties of H. purpurascens, it was found that germacrene-D, the main component of H. scabrum and H. purpurascens, is a strong antioxidant, given its extracyclic methylene content and its ability to eliminate superoxide anions [75]. Similarly, ϒ-Terpinene possesses biological anti-inflammatory, antimicrobiological and analgesic activities, due to which species containing this compound are used as expectorants and diuretics, as well as in treatment for the relief of muscle pain, fever and asthma [76].
In this study, the antimicrobial activity and Anti-AChE activity of H. purpurasens is reported for the first time. Compared with other EOs and with the positive control, our prepared EO had weak antimicrobial activity. Although there are no criteria that are widely used to assess the potency of MIC values, some authors suggested a classification for extracts and essential oils and found that a MIC value between 101 and 500 µg/mL can usually be considered to represent a strong activity [77].
Regarding the cholinesterase inhibitory activity, the H. purpurasens EO exerted moderate inhibitory potency, with an IC50 value of 56.38 µg/mL; similarly, more recent studies indicated a moderate inhibition of AChE obtained from EOs compared to positive controls, which is in accordance with the reported activity of the EO of H. brasiliense of 69.82% at a dose of 1 mg/mL [78]. A study of H. strigosum reported a value of 137.6 µg/mL [69]. Many species of the genus Hedyosmum have been studied for their antibacterial or antioxidant potential and chemical profile, but little or no inhibitory potential against acetylcholinesterase has been isolated from the EO in this genus. Information on the chemicals used and their inhibitory potential may exist and can be found in the literature.
Although ABTS and DPPH antiradical assays are well recognized and used around the scientific community, the ABTS assay has been confirmed to show better results in detecting the antioxidant properties in foods or in natural matrices, such as extracts or EOs [79]. Therefore, it is noteworthy that our EO displayed better results in ABTS assay; however, further analyses should be carried out to confirm this activity, including the use of another antioxidant assay, such as ORAC or FRAP. The aim of another report will be to analyze the antioxidant properties of the main constituents in essential oils.
4. Materials and Methods
4.1. Reagents
Dichloromethane, anhydrous sodium sulphate, 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), acetylcholinesterase from Electrophorus electricus, phosphate buffered saline, Ellman’s reagent (5,5′-dithiobis(2-nitrobenzoic acid), acetylthiocholine iodide and donepezil hydrochloride. The standard aliphatic hydrocarbons were purchased from Chem Service (Sigma-Aldrich, St. Louis, MO, USA), dimethyl sulfoxide was purchased from Merck and helium ultra-pure gas was purchased from INDURA (Guayaquil, Ecuador). All chemicals were of analytical grade and used without further purifications.
4.2. Plant Material
Aerial parts of H. purpurascens were collected in October 2020 in El Tiro sector, at the border between Loja and Zamora Chinchipe, Ecuador (latitude 3°58′59″ S, longitude 79°08′05″ W). The plant material was harvested under permit MAE-DBN-2016-048 of the Ministry of Environment of Ecuador (MAE) and was authenticated by Dr. Nixon Cumbicus, botanist at Herbarium UTPL. A specimen sample was deposited at the Herbarium of the Universidad Técnica Particular de Loja (HUTPL) with voucher code PPN-as-057.
4.3. Isolation of the Essential Oil
Aerial plant material (c.a. 5000 g) was subjected to hydro-distillation immediately after harvesting in a Clevenger-type apparatus for 3 h. The essential oil was dried over anhydrous sodium sulfate to remove the moisture and then stored at −4 °C until use for the biological and chemical assays. The procedure was performed three times.
4.4. Physical Properties of Essential Oil
The relative density, refractive index and optical rotation of EO were determined by triplicate at 20 °C. Relative density was determined according to the AFNOR NF T 75-11 method (corresponds to ISO 279:1998), refractive index according to the AFNOR method NF 75-112 (ISO 280: 1998) using the refractometer model ABBE (BOECO, Hamburg, Germany) and, finally, the specific optical determination of rotation using the standard method ISO 592-1998 with an Hannon P-810 automatic polarimeter (Jinan Hanon Instruments Co., Ltd., Jinan, China).
4.5. Chemical Characterization of Essential Oil
4.5.1. Sample Preparation
Quantitative and qualitative characterization of EO from H. purpurascens required sample preparation of the volatile fractions. The EO was diluted with dichloromethane to reach a concentration of 1%. The samples were used in the chemical analyses described below.
4.5.2. Qualitative and Quantitative Analysis
Qualitative identification was performed using gas chromatography coupled with mass spectrometry (GC–MS): Thermo Fisher Scientific model Trace 1310 gas chromatograph (GC) equipped with Thermo Scientific AI/AS 1300 liquid-sampling automation and model ISQ7000 Mass Spectrometer Single Quadrupole (Waltham, MA, USA). Referring to the experimental conditions of our study, the mass-spectra electronic impact was measured at 70 eV and scanned mass range was set at 40– 400 m/z. Helium was the carrier gas, with a constant flow of 1.00 mL/min. Oven-temperature program was set to an initial temperature of 60 °C for 5 min and then increased to 250 °C at 3 °C/min gradient operation. The ion source temperature was set to 230 °C and the quadrupole temperature to 150 °C. Capillary columns were DB-5 ms (5% phenylmethylpolysiloxane, 30 m × 0.25 mm id, 0.25 μm of film thickness) and HP-INNOWax (polyethylene glycol, film 30 m × 0.25 mm id; thickness 0.2 mm; J & W Scientific, Folsom, CA, USA). The procedure was performed in triplicate.
The aromatic compounds were identified by comparing mass spectra and linear retention index (LRI) with those reported in the literature. The LRI was determined experimentally by Van Den Dool and Krats [80] by injecting a series of homologous C9 to C24 alkanes.
Quantitative analysis of EO was performed using gas chromatography coupled with a flame-ionization detector (GC–FID). The prepared samples were injected under the same analytical conditions as the qualitative GC–MS method with the same column. The percentage of aromatics was determined by comparing the total area of the GC peaks with the identified peaks [39].
4.5.3. Enantioselective Analysis
Enantioselective analysis was performed on the GC–MS instrument mentioned above using a chiral capillary column MEGA-DEX-DET-Beta (diethyl-tert-butylsilyl-β-cyclodextrin; 25 m × 0.25 mm × 0.25 μm) selective analysis. Analytical conditions were the same as for the qualitative oil analysis, except that the oven program was held at 60 °C (5 min) and then increased at a rate of 2 °C to 220 °C for 5 min. The enantiomeric distribution and enantiomeric excess of each enantiomeric pair were determined by comparison with authentic reference compounds.
4.6. Anticholinesterase Activiy
The AChE inhibition was measured using the method developed by Ellman et al. [81], with minor modifications, as in Rhee et al. [82]. The AChE inhibition was demonstrated after addition of acetylthiocholine as an enzyme substrate and addition of different concentrations of EO dissolved in methanol. The enzymatic reaction was monitored at 405 nm for 60 min in a microplate reader (EPOCH 2, BioTek, Winooski, VT, USA). Final concentrations of 1000, 500, 100, 50 and 10 µg/mL EO in MeOH were prepared to assess enzyme inhibition. The assay was performed in triplicate in 96-well microplates. Donepezil was used as a positive control. The IC50 values were calculated from the progression curves using Graph Pad Prism software.
4.7. Antimicrobial Activity
Minimal inhibitory concentration was determined by means of the broth-microdilution method assessing the effect of the EO against seven ATCC reference strains. Three gram-positive bacteria, Enterococcus faecalis ATCC 19433, Enterococcus faecium ATCC 27270 and Staphylococcus aureus ATCC 25923, two gram-negative bacteria, Escherichia coli (O157:H7) ATCC 43888 and Pseudomonas aeruginosa ATCC 10145 and two fungi, Candida albicans ATCC 10231 and Aspergillus niger ATCC 6275, were employed. The EO was diluted at concentration of 80 mg/mL and two-fold serial-dilution method was used to achieve concentrations ranging from 4000 to 31.25 μg/mL, with a final cell concentration of 5 × 105 cfu/mL for bacteria, 2.5 × 105 cfu/mL for yeast and 5 × 104 spores/mL for sporulated fungi. The method was developed in a 96-microwell plate and Mueller Hinton II (MH II), for bacteria and Sabouraud broth, for fungi, were used as assay media. The entire method was described in a previous report by our research group [69]. Antimicrobial commercial agents, such as ampicilin 1 mg/mL solution for S. aureus, E. faecalis and E. faecium, ciprofloxacin 1 mg/mL solution for P. aeruginosa and E. coli and, finally, amphotericin B 250 µg/mL for the two tested fungi, were used as positive controls. The DMSO was used as negative control at a maximum concentration of 5%.
4.8. Antioxidant Spectrophotometric Analysis
4.8.1. DPPH Assay
The DPPH free-radical-scavenging method was developed by Taipong et al. [83] according to the proposed methodology [84], which was slightly modified to use 2,2-diphenyl-1-picrylhydryl (DPPH-). A working solution in methanol with an adjusted absorbance of 1.1 ± 0.01 (EPOCH 2 microplate reader (BIOTEK, Winooski, VT, USA)) was prepared from 625-micrometer DPPH, methanol-stabilized. The antiradical reactions between different concentrations of EO (two-fold serial dilutions ranging from 1000 to 7.81 µg/mL) and DPPH were carried out as follows: in total, 270 µL of DPPH-adjusted working solution were added in a 96 microplate with 30 µL of EO sample. The reaction was monitored at 515 nm in darkness for 60 min at room temperature. Trolox and methanol were used as positive and blank controls, respectively. Results were expressed as SC50 (50% radical scavenging concentration) and calculated from a curve fit of GraphPad Prism v.8.0.1 data. Measurements were performed in triplicate. A calibration standard curve of Trolox was built with concentrations ranging from 1.25 to 70 µM, employing the same method as described above. Linear regression model was obtained by plotting the Trolox concentration vs. absorbance decrease inDPPH radical .
4.8.2. ABTS Assay
Antioxidant capacity against the ABTS• cation (2,2’-azinobis-3-ethylbenzothiazoline-6-sulfonic acid) was determined as reported by Arnao et al. [85] and Thaipong et al. [83], with slightly modifications, as follows. Briefly, the assay begins with the preparation of a stock solution of free radicals by reacting equal volumes of water solutions of ABTS (7.4 µM) and potassium persulfate (2.6 µM) for 14 h at constant stirring. A working solution was prepared by dissolving an aliquot of the stock solution in methanol until the absorbance reached 1.1 ± 0.02 at 734 nm (EPOCH 2 microplate reader (BIOTEK, Winooski, VT, USA)). The antiradical response was measured by reacting 270 µL of ABTS working solution and 30 µL of EO at the same concentrations employed in DPPH antiradical assay and monitored in darkness at room temperature for 60 min at 734 nm. Trolox and methanol were used as positive and blank controls, respectively. Results are expressed as SC50 (50% radical scavenging concentration) and calculated from a curve fit of GraphPad Prism v.8.0.1 data. Measurements were performed in triplicate. A calibration standard curve of Trolox was built with concentrations ranging from 1.25 to 50 µM, employing the same method as described above. Linear regression model was obtained by plotting the Trolox concentration vs. absorbance decreasing of ABTS cation radical .
5. Conclusions
The EO of Hedyosmum purpurascens was obtained from the aerial parts of the plant, with a very low extraction yield of 0.16%. The chemical composition of the EO included ca. 90 compounds, of which germacrene-D-, α-phellandrene and sabinene were the most heavily represented. The enantioselective analysis revealed the presence of one optically pure enantiomer and four other pairs of optical isomers, which were (+)-α-pinene, (−) myrcene, (+) α-phellandrene, (+)-limonene and (+) -o-, cymene. The biological activity of the H. purpurascens revealed that the EO was inactive for Gram-negative microorganisms. A moderate effect of 95.62 and 56.38 µg/mL was obtained from the AChE enzyme and ABTS assays, respectively. The anticholinesterase and antioxidant effects observed for this species allow us to consider it as a novel candidate for the pharmaceutical industry. This study is a contribution of new knowledge about the native aromatic species of Ecuador.
Author Contributions
Conceptualization, V.M., J.C. and L.C.; methodology, J.C., L.C. and L.N.C.; formal analysis, L.C., J.C. and V.M.; investigation, J.C., L.N.C., L.C. and V.M; data curation, J.C. and V.M.; writing—original draft preparation, J.C. and V.M.; writing—review and editing, J.C. and V.M.; supervision, V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the Universidad Técnica Particular de Loja (UTPL) for supporting this investigation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lorenzo, D.; Loayza, I.; Dellacassa, E. Composition of the essential oils form leaves of two Hedyosmum spp from Bolivia. Flavor Fragr. J. 2003, 18, 32–35. [Google Scholar] [CrossRef]
- Forero, E.; Mori, S. The Organization for Flora Neotropica. Brittonia 1995, 47, 379–393. [Google Scholar] [CrossRef]
- Eras, M.T.; Mendoza, Z.A.; Tamayo, J.P. Diversidad florística, endemismo y estado de conservación de los componentes arbustivo y herbáceo de un bosque andino en el sur del Ecuador. Bosques Latid. Cero 2021, 11, 83–96. [Google Scholar]
- Kirchner, K.; Wisniewski, A., Jr.; Bella-Cruz, A.; Biavatti, M.W.; Netz, A.D. Chemical composition and antimicrobial activity of Hedyosmum brasiliense Miq., Chloranthaceae, essential oil. Braz. J. Pharmacogn. 2010, 20, 692–699. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, D.; Fan, G.; Wang, R.; Lu, X.; Gu, Y.; Shi, Q.W. Constituents from Chloranthaceae plants and their biological activities. Heterocycl. Commun. 2016, 22, 175–220. [Google Scholar] [CrossRef]
- Ulloa, U.; Moler, J. Floras. Available online: http://www.efloras.org/florataxon.aspx?flora_id=201&taxon_id=114826 (accessed on 15 September 2022).
- Leon-Yanez, S.; Valencia, R.; Pitman, N.; Endara, L.; Ulloa, C.; Navarrete, H. Libro Rojo de las Plantas Endémicas del Ecuador; Publicaciones del Herbario QCA, Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2019; Available online: https://bioweb.bio/floraweb/librorojo/hom (accessed on 29 September 2022).
- Torres-Rodríguez, S.H.; Tovar-Torres, M.C.; Garcia, V.J.; Lucena, M.E.; Araujo-Baptista, L. Composición química del aceite esencial de de las hojas de Hedyosmum Luteynii Todzia (Chloranthaceae). Rev. Peru. De Biol. 2018, 25, 173–178. [Google Scholar] [CrossRef]
- Cardenas, L.; Rodriguez, J.; Villaverde, M.; Rivera, R.; Cadena, R.; Otero, J. The analgesic activity of Hediosmum bonplandianum. Planta Med. 1993, 59, 26–27. [Google Scholar] [CrossRef]
- Lozano, P.; Delgado, T.; Aguirre, Z. Estado Actual de la Flora Endémica Exclusiva y su Distribución en el Occidente del Parque Nacional Podocarpus; Publicaciones del Herbario y Jardín Botánico Reinaldo Espinosa: Loja, Ecuador, 2003. [Google Scholar]
- Lozano, P.; Delgado, T.; Aguirre, Z. Endemismo una Herramienta para la Conservación. Parque Nacional Podocarpus; Publicaciones del Herbario y Jardín Botánico Reinaldo Espinosa: Loja, Ecuador, 2004. [Google Scholar]
- Lozano, P.; Bussmann, R.; Küppers, M. Diversidad florística del bosque montano en el Occidente del Parque Nacional Podocarpus, Sur del Ecuador y su influencia en la flora pionera en deslizamientos naturales. Rev. UDO Agrícola 2007, 7, 142–159. [Google Scholar]
- Zamora-Birbano, A.M.; Arturo-Perdomo, D.E. Composición química del aceite esencial de hojas Hedyosmum traslucidum Cuatrec., Chloranthaceae (Granizo). Bol. Latinoam. Caribe Plantas Med. Aromáticas 2016, 15, 192–198. [Google Scholar]
- Sylvestre, M.; Pichette, A.; Longtin, A.; Martin, M.-A.C.D.K.; Bercion, S.R.; Legault, J. Chemical Composition of Leaf Essential Oil of Hedyosmum arborescens and Evaluation of its Anticancer Activity. Nat. Prod. Commun. 2007, 2, 1269–1272. [Google Scholar] [CrossRef]
- Mundina, M.; Roser, V.; Felix, T.; Cicciò, J.F.; Adezt, T.I.; Casanova, J.; Cañigueral, S. Composition of the essential oils from leaves and fruits of three Hedyosmum species from Costa Rica. Flavour Fragr. J. 2000, 15, 201–205. [Google Scholar] [CrossRef]
- Delgado, P.A.; Quijano, C.E.; Morales, G.; Pino, J.A. Composition of the Essential Oil from Leaves and Fruits of Hedyosmum colombianum Cuatrec. Grown in Colombia. J. Essent. Oil Res. 2010, 22, 234–236. [Google Scholar] [CrossRef]
- Guerrini, A.; Gianni, S.; Grandini, A.; Spagnoletti, A.; Asanza, M.; Scalvenzi, L. Cytotoxic Effect and TLC Bioautography-Guided Approach to Detect Health Properties of Amazonian Hedyosmum sprucei Essential Oil. Evid. Based Complement. Altern. Med. 2016, 2016, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Danis, M.; Ávila, D.; Ortega, J.; Peña, N.; Rojas, L.; Cepeda, Y. Composición química del aceite esencial de Hedyosmum glabratum. Ciencia 2012, 20, 68–72. [Google Scholar]
- Grandtner, M.M.; Cheverette, J. Dictionay of Trees, 1st ed.; Laval University: Quebec, QC, Canada, 2013; ISBN 9780123969545. [Google Scholar]
- Valarezo, E.; Morocho, V.; Cartuche, L.; Chamba-Granda, F.; Correa-Conza, M.; Jaramillo-Fierro, X.; Meneses, M.A. Variability of the Chemical Composition and Bioactivity between the Essential Oils Isolated from Male and Female Specimens of Hedyosmum racemosum (Ruiz y Pav) G. Don. Molecules 2021, 26, 4613. [Google Scholar] [CrossRef]
- Bisio, A.; Ciarallo, G.; Romussi, G.; Fontana, N.; Mascolo, N.; Capasso, R.; Biscardi, D. Chemical Composition of Essential Oils from some Salvia species. Phytother. Res. 1998, 12, s117–s120. [Google Scholar] [CrossRef]
- Grujic-Jovanovic, S.; Skaltsa, H.D.; Marin, P.; Sokovic, M. Composition and Antibacterial activity of the essential oil of six Stachys species from Serbia. Flavour Fragr. J. 2004, 19, 139–144. [Google Scholar] [CrossRef]
- Capetanos, C.; Saroglou, V.; Marin, P.D.; Simic, A.; Skaltsa, H.D. Essential oil analysis of two endemic Eringium species from Serbia. J. Serb. Chem. Soc. 2007, 72, 961–965. [Google Scholar] [CrossRef]
- Narain, N.; Galvao, M.S.; Madruga, M.S. Volatile compounds captured through purge and trap technique in caja-umbu (Spondias sp.) fruits during maturation. Food Chem. 2007, 102, 726–731. [Google Scholar] [CrossRef]
- Saroglou, V.; Marin, P.D.; Rancic, A.; Veljic, M.; Skaltsa, H. Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochem. Syst. Ecol. 2007, 35, 146–152. [Google Scholar] [CrossRef]
- Hua, C.X.; Wang, G.R.; Lei, Y. Evaluation of essential oil composition and DNA diversity of mint resources from China. Afr. J. Biotechnol. 2011, 10, 16740–16745. [Google Scholar]
- Skaltsa, H.D.; Demetzos, C.; Lazari, D.; Sokovic, M. Essential oil analysis and antimicrobial activity of eight Stachys species from Greece. Phytochemistry 2003, 64, 743–752. [Google Scholar] [CrossRef]
- Riela, S.; Bruno, M.; Rosselli, S.; Saladino, M.L.; Caponetti, E.; Formisano, C.; Senatore, F. A study on the essential oil of Ferulago campestris: How much does extraction method influence the oil composition? J. Sep. Sci. 2011, 34, 483–492. [Google Scholar] [CrossRef]
- Frizzo, C.D.; Atti-Serafini, L.; Laguna, S.E.; Cassel, E.; Lorenzo, D.; Dellacassa, E. Essential oil variability in Baccharis uncinella DC and Baccharis dracunculifolia DC growing wild in southern Brazil, Bolivia, and Uruguay. Flavour Fragr. J. 2008, 23, 99–106. [Google Scholar] [CrossRef]
- Hachicha, S.F.; Skanji, T.; Barrek, S.; Ghrabi, A.G.; Zarrouk, H. Composition of. the essential oil of Teucrium ramosissimum Desf. (Lamiaceae) from Tunisia. Flavour Fragr. J. 2007, 22, 101–104. [Google Scholar] [CrossRef]
- Bedoussac, L.; Journet, E.P.; Hauggaard-Nielsen, H.; Naudin, C.; Corre-Hellou, G.; Jensen, E.S.; Justes, E. Ecological principles underlying the increase of productivity achieved by cereal-grain legume intercrops in organic farming. A review. Agron. Sustain. Dev. 2015, 35, 911–935. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Demirci, B.; Özek, T.; Akalin, E.; Özhatay, N. Micro-distilled volatile compounds from Ferulago species growing in western Turkey. Pharm. Biol. 2002, 40, 466–471. [Google Scholar] [CrossRef]
- Maggio, A.; Bruno, M.; Guarino, R.; Senatore, F.; Ilardi, V. Contribution to a Taxonomic Revision of the Sicilian Helichrysum Taxa by PCA Analysis of Their Essential-Oil Compositions. Chem. Biodivers. 2016, 13, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Charoensiddhi, S.; Anprung, P. Bioactive compounds and volatile compounds of Thai bael fruit (Aegle marmelos (L.) Correa) as a valuable source for functional food ingredients. Int. Food Res. J. 2008, 15, 287–295. [Google Scholar]
- Arango, A.M.P.; Loaiza, L.M.R.; Cabra, J.L.R. Identificación de componentes químicos del aceite esencial de romero (Rosmarinus officinalis L.) proveniente de cultivos orgánicos en la zona alta andina. Rev. Colomb. Investig. Agroind. 2018, 5, 6–19. [Google Scholar] [CrossRef]
- Cozzani, S.; Muselli, A.; Desjobert, J.M.; Bernardini, A.F.; Tomi, F.; Casanova, J. Chemical composition of essential oil of Teucrium polium subsp. capitatum (L.) from Corsica. Flavour Fragr. J. 2005, 20, 436–441. [Google Scholar] [CrossRef]
- Cheraif, I.; Jannet, H.B.; Hammami, M.; Khouja, M.L.; Mighri, Z. Chemical composition and antimicrobial activity of essential oils of Cupressus arizonica Greene. Biochem. Syst. Ecol. 2007, 35, 813–820. [Google Scholar] [CrossRef]
- Patiño-Bayona, W.R.; Plazas, E.; Bustos-Cortes, J.J.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Essential Oils of Three Hypericum Species from Colombia: Chemical Composition, Insecticidal and Repellent Activity Against Sitophilus zeamais Motsch.(Coleoptera: Curculionidae). Rec. Nat. Prod. 2021, 15, 111–121. [Google Scholar] [CrossRef]
- Amri, I.; Hamrouni, L.; Hanana, M.; Gargouri, S.; Fezzani, T.; Jamoussi, B. Chemical composition, physico-chemical properties, antifungal and herbicidal activities of Pinus halepensis Miller essential oils. Biol. Agric. Hortic. 2013, 29, 91–106. [Google Scholar] [CrossRef]
- Couladis, M.; Tzakou, O.; Stojanovic, D.; Mimica-Dukic, N.; Jancic, R. The essential oil composition of Salvia argentea L. Flavour Fragr. J. 2001, 16, 227–229. [Google Scholar] [CrossRef]
- Wannes, W.A.; Mhamdi, B.; Marzouk, B. GC comparative analysis of least essential oils from two Myrtle varieties at different phenological stages. Chromatographia 2009, 69, 145–150. [Google Scholar] [CrossRef]
- Özcan, M.; Akgül, A.; Başcr, K.H.C.; Özck, T.; Tabanca, N. Essential oil composition of sea fennel (Crithmum maritimum) form Turkey. Food Nahrung 2001, 45, 353–356. [Google Scholar] [CrossRef]
- Formisano, C.; Senatore, F.; Bancheva, S.; Bruno, M.; Maggio, A.; Rosselli, S. Volatile components of C. bracteata and C. pannonica subsp. pannonica growing wild in Croatia. Nat. Prod. Commun. 2010, 5, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Solis-Quispe, L.; Tomaylla-Cruz, C.; Callo-Choquelvica, Y.; Solís-Quispe, A.; Rodeiro, I.; Hernández, I.; Pino, J.A. Chemical composition, antioxidant and antiproliferative activities of essential oil from Schinus areira L. and Minthostachys spicata (Benth.) Epl. grown in Cuzco, Peru. J. Essent. Oil Res. 2016, 28, 234–240. [Google Scholar] [CrossRef]
- Taarit, M.B.; Msaada, K.; Hosni, K.; Chahed, T.; Marzouk, B. Essential oil composition of Salvia verbenaca L. growing wild in Tunisia. J. Food Biochem. 2010, 34, 142–151. [Google Scholar] [CrossRef]
- Salinas, M.; Bec, N.; Calva, J.; Ramírez, J.; Andrade, J.M.; Larroque, C.; Armijos, C. Chemical composition and anticholinesterase activity of the essential oil from the Ecuadorian plant Salvia pichinchensis Benth. Rec. Nat. Prod. 2020, 14, 276–285. [Google Scholar] [CrossRef]
- Mazzoni, V.; Tomi, F.; Casanova, J. A daucane-type sesquiterpene from Daucus carota seed oil. Flavour Fragr. J. 1999, 14, 268–272. [Google Scholar] [CrossRef]
- Choi, H.S. Characterization of Citrus unshiu (C. unshiu Marcov. forma Miyagawa-wase) blossom aroma by solid-phase microextraction in conjunction with an electronic nose. J. Agric. Food Chem. 2003, 51, 418–442. [Google Scholar] [CrossRef]
- Padalia, R.C.; Verma, R.S.; Amit, C.; Velusamy, S.; Chanotiya, C.S. Essential oil composition of sixteen elite cultivars of Mentha from western Himalayan region, India. Maejo Int. J. Sci. Technol. 2013, 7, 83–93. [Google Scholar]
- Ferretti, G.; Maggi, F.; Tirillini, B. Essential oil composition of Hypericum richeri Vill. from Italy. Flavour Fragr. J. 2005, 20, 295–298. [Google Scholar] [CrossRef]
- Setzer, W.N.; Stokes, S.L.; Penton, A.F.; Takaku, S.; Haber, W.A.; Hansell, E.; McKerrow, J.H. Cruzain inhibitory activity of leaf essential oils of Neotropical Lauraceae and essential oil components. Nat. Prod. Commun. 2007, 2, 1203–1212. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Neutrophil Immunomodulatory Activity of (−)-Borneol, a Major Component of Essential Oils Extracted from Grindelia squarrosa. Molecules 2022, 27, 4897. [Google Scholar] [CrossRef] [PubMed]
- Paolini, J.; Muselli, A.; Bernardini, A.F.; Bighelli, A.; Casanova, J.; Costa, J. Thymol derivatives from essential oil of Doronicum corsicum L. Flavour Fragr. J. 2007, 22, 479–487. [Google Scholar] [CrossRef]
- Ali, A.; Tabanca, N.; Demirci, B.; Blythe, E.K.; Başer, K.E.M.A.L.; Khan, I.A. Chemical composition and biological activity of essential oils from four Nepeta species and hybrids against Aedes aegypti (L.) (Diptera: Culicidae). Rec. Nat. Prod. 2016, 10, 137–147. [Google Scholar]
- Bouhlel, C.; Dolhem, G.A.; Fernandez, X.; Antoniotti, S. Model study of the enzymatic modification of natural extracts: Peroxidase-based removal of eugenol from rose essential oil. J. Agric. Food Chem. 2012, 60, 1052–1058. [Google Scholar] [CrossRef]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A 1999, 503, 1–24. [Google Scholar] [CrossRef]
- Cavalli, J.F.; Tomi, F.; Bernardini, A.F.; Casanova, J. Composition and chemical variability of the bark oil of Cedrelopsis grevei H. Baillon from Madagascar. Flavour Fragr. J. 2003, 18, 532–538. [Google Scholar] [CrossRef]
- Kundakovic, T.; Fokialakis, N.; Kovacevic, N.; Chinou, I. Essential oil composition of A. lingulata and A. umbellate. Flavour Fragr. J. 2007, 22, 184–187. [Google Scholar] [CrossRef]
- Ennigrou, A.; Casabianca, H.; Laarif, A.; Hanchi, B.; Hosni, K. Maturation-related changes in phytochemicals and biological activities of the Brazilian pepper tree (Schinus terebinthifolius Raddi) fruits. S. Afr. J. Bot. 2017, 108, 407–415. [Google Scholar] [CrossRef]
- Montalván, M.; Peñafiel, M.A.; Ramírez, J.; Cumbicus, N.; Bec, N.; Larroque, C.; Gilardoni, G. Chemical composition, enantiomeric distribution, and sensory evaluation of the essential oils distilled from the Ecuadorian species Myrcianthes myrsinoides (Kunth) Grifo and Myrcia mollis (Kunth) dc.(Myrtaceae). Plants 2019, 8, 511. [Google Scholar] [CrossRef]
- Viljoen, A.M.; Kamatou, G.P.; Coovadia, Z.H.; Özek, T.; Başer, K.H.C. Rare sesquiterpenes from South African Pteronia species. S. Afr. J. Bot. 2010, 76, 146–152. [Google Scholar] [CrossRef]
- Kacem, N.; Roumy, V.; Duhal, N.; Merouane, F.; Neut, C.; Christen, P.; Rhouati, S. Chemical composition of the essential oil from Algerian Genista quadriflora Munby and determination of its antibacterial and antifungal activities. Ind. Crops Prod. 2016, 90, 87–93. [Google Scholar] [CrossRef]
- Carroll, J.F.; Tabanca, N.; Kramer, M.; Elejalde, N.M.; Wedge, D.E.; Bernier, U.R.; Zhang, S. Essential oils of Cupressus funebris, Juniperus communis, and J. chinensis (Cupressaceae) as repellents against ticks (Acari: Ixodidae) and mosquitoes (Diptera: Culicidae) and as toxicants against mosquitoes. J. Vector Ecol. 2011, 36, 258–268. [Google Scholar] [CrossRef]
- Calvopiña, K.; Malagón, O.; Capetti, F.; Sgorbini, B.; Verdugo, V.; Gilardoni, G. A New Sesquiterpene Essential Oil from the Native Andean Species Jungia rugosa Less (Asteraceae): Chemical Analysis, Enantiomeric Evaluation, and Cholinergic Activity. Plants 2021, 10, 2102. [Google Scholar] [CrossRef]
- Gavyar, P.; Amiri, H. Chemical composition of essential oil and antioxidant activity of Postia puberula, an endemic species from Iran. Acta Sci. Pol. Hortorum Cultus 2019, 18, 119–128. [Google Scholar] [CrossRef]
- Song, H.S.; Sawamura, M.; Ito, T.; Kawashimo, K.; Ukeda, H. Quantitative determination and characteristic flavour of Citrus junos (yuzu) peel oil. Flavour Fragr. J. 2020, 15, 245–250. [Google Scholar] [CrossRef]
- Xu, G.L.; Geng, D.; Xie, M.; Teng, K.Y.; Tian, Y.X.; Liu, Z.Z.; She, G.M. Chemical composition, antioxidative and anticancer activities of the essential oil: Curcumae rhizoma–Sparganii rhizoma, a traditional herb pair. Molecules 2015, 20, 15781–15796. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas, Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 10-1932633219. [Google Scholar]
- Cartuche, L.; Calva, J.; Valarezo, E.; Chuchuca, N.; Morocho, V. Chemical and Biological Activiy Profiling of Hedyosmum strigosum Todzia. Essential Oil, and Aromatic Native Shrub from Southern Ecuador. Plants 2022, 11, 2832. [Google Scholar] [CrossRef] [PubMed]
- Huong, B.V.; Anh, L.D.N.; Vuong, T.B. Chemical Composition of Essential Oils from Hedyosmum orientale. Chem. Nat. Compd. 2021, 57, 378–381. [Google Scholar] [CrossRef]
- Nurliyana, A.L.; Noorsiha, A. Volatile composition and antifungal properties of Chloranthus erectus leaves oil. Unravelling Nat. Treasures Secrets: Curr. Species Interest 2018, 16, 97. [Google Scholar]
- Cao, C.M.; Peng, Y.; Shi, Q.W.; Xiao, P.G. Chemical constituents and bioactivities of plants of chloranthaceae. Chem. Biodivers. 2008, 5, 219–238. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Jaramillo, B.E.; Martínez, J.R. Comparación de la composición química y de la actividad antioxidante in vitro de los metabolitos secundarios volátiles de plantas de la familia Verbenaceae. Rev. Acad. Colomb. Cienc 2003, 27, 579–597. [Google Scholar]
- Guarín, O.D.; Barajas-Solano, A.F. Hidrodestilación asistida con microondas (MWHD) para la extracción de hidrolatos de plantas aromáticas. Rev. Politécnica 2015, 11, 51–55. [Google Scholar]
- Dickson, R.A.; Houghton, P.J.; Hylands, P.J. Antibacterial and antioxidant cassane diterpenoids from Caesalpinia benthamiana. Phytochemistry 2007, 68, 1436–1441. [Google Scholar] [CrossRef]
- Hani, I.B.; Mazura, M.P.; Juliza, M.; Fadzureena, J.; Vimala, S.; Farizan, N. In-vitro anti-inflammatory and antioxidant evaluation of leaf extract of Baeckea frutescens L. In Proceedings of the Seminar on Medicinal and Aromatic Plants: Harnessing the Tropical Herbal Heritage: Recent Advances in R&D and Commercialization; Forest Research Institute Malaysia: Kuala Lumpur, Malaysia, 2010. [Google Scholar]
- Van Vuuren, S.; Holl, D. Antimicrobial natural product research: A review from a South African perspective for the years 2009–2016. J. Ethnopharmacol 2017, 17, 236–252. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, J.P.; do Amaral, W.; Alberton, M.D.; Paim, M.; Da Silva, L.E. Chemical Composition, Antibacterial Potential and Enzymatic Inhibition of the Hedyosmum brasiliense Mart-Chloranthaceae. In Produtos Naturais E Suas Aplicacoes: Da Comunidade Para o Laboratorio. Ed. Científica Digit. 2021, 1, 301–315. [Google Scholar]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperatura programmed gas-Liquid partition chromatography. J. Chromatogr 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Rhee, I.K.; van de Meent, M.; Ingkaninan, K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).