Abstract
Plant cell cultures of various yew species are a profitable source of taxoids (taxane diterpenoids) with antitumor activity. So far, despite intensive studies, the principles of the formation of different groups of taxoids in cultured in vitro plant cells have not been fully revealed. In this study, the qualitative composition of taxoids of different structural groups was assessed in callus and suspension cell cultures of three yew species (Taxus baccata, T. canadensis, and T. wallichiana) and two T. × media hybrids. For the first time, 14-hydroxylated taxoids were isolated from the biomass of the suspension culture of T. baccata cells, and their structures were identified by high-resolution mass spectrometry and NMR spectroscopy as 7β-hydroxy-taxuyunnanin C, sinenxane C, taxuyunnanine C, 2α,5α,9α,10β,14β-pentaacetoxy-4(20), 11-taxadiene, and yunnanxane. UPLC–ESI-MS screening of taxoids was performed in more than 20 callus and suspension cell lines originating from different explants and grown in over 20 formulations of nutrient media. Regardless of the species, cell line origin, and conditions, most of the investigated cell cultures retained the ability to form taxane diterpenoids. Nonpolar 14-hydroxylated taxoids (in the form of polyesters) were predominant under in vitro culture conditions in all cell lines. These results, together with the literature data, suggest that dedifferentiated cell cultures of various yew species retain the ability to synthesize taxoids, but predominantly of the 14-OH taxoid group compared to the 13-OH taxoids found in plants.
1. Introduction
Diterpenoids of the taxane group (taxoids) are specific for plants of the genus Taxus (yew, Taxaceae). More than 300 individual taxoid compounds have been isolated from different yew species [1,2]. These compounds can be divided into several classes based on the structure of the taxane skeleton and the nature and/or arrangement of the functional groups [1,2]. Three to six structural classes of taxoids are usually distinguished; however, the representatives of three groups are the most common among Taxus spp.: 13-hydroxylated taxoids of the baccatin III-type (baccatin III, paclitaxel, etc.), 14-hydroxylated taxoids of the taiwanxan-type (taxuyunnanin C, yunnanxane, etc.), and 11(15→1)-abeo-taxoids [1,2,3,4]. Paclitaxel (commercial synonym Taxol®) is a pharmaceutically important chemical that is widely used in cancer therapy [1].
The mechanism of taxol action is unique as it inhibits microtubule depolymerization. Taxol penetrates into cells and disrupts cytoskeleton functions, which causes the suppression or malfunctioning of various processes in eukaryotic cells. These include inhibition of cell proliferation and intracellular mobility, alterations of membrane structure and function, disruption of intracellular transport, compartmentation, signaling, etc. [5]. Taxol is highly effective in treating breast, ovarian, and lung cancer, which are the world’s most common cancer types [6]. Recently, however, the use of taxol has been hampered by a major concern—the emergence and development of tumor cells’ resistance to first-generation chemotherapeutic taxane drugs (13-hydroxylated taxoids (taxol) and its semisynthetic derivatives docetaxel, cabazitaxel, etc.) [7]. A pharmacological study of taxoids of other structural types showed that some taxoids, despite being structurally different from taxol, exhibited cytotoxic activity comparable to that of taxol derivatives in relation to certain tumor cell lines [8]. However, it was found that these “unusual” from the perspective of classical taxane diterpenoids chemotherapy agents are effective against tumor cell lines with a multidrug resistance phenotype [8,9]. Several taxoids, including 14-hydroxylated ones, can suppress the resistance of tumor cells to cytotoxic compounds by changing the work of transporters and the plasma membrane (in particular, ABC-transporters) [10]. Taxoids can inhibit epidermal growth factor receptor tyrosine kinase [11] and act as immunomodulators to activate the antitumor properties of effector cells [12]. Consequently, not only taxol but other taxoids of different structural types can be used in cancer chemotherapy as independent drugs or components of complex treatments. In addition to antitumor action, different types of taxoids demonstrate other biological activities, including antidiabetic (by inhibiting alpha-glucosidases and insulin resistance caused by inflammation, and disrupting the lipoxygenase cascade), anti-inflammatory (by impairing migration of leukocytes and development of a granuloma in the site of inflammation), analgesic, antipyretic, anticonvulsant (probably by modulating GABAA receptors), inhibition the formation of superoxide anion radical in neutrophils (by impairing phosphorylation and intracellular transport of proteins-subunits of NADPH oxidase of the plasma membrane), antimicrobial, fungicidal, antileishmanial and several others [13,14,15,16,17,18,19,20]. Based on the information presented, it is important to perform a detailed phytochemical study of not only the baccatin III-group taxoids (paclitaxel and its derivatives), but also other taxane diterpenoids.
The specific biology of yew species, including endemism, slow growth, difficulties in reproduction, and the slow and unstable accumulation of paclitaxel and other taxoids (0.001–0.03% of the dry weight) in wild plants, significantly limit the industrial production of taxoids from natural plant sources [1,2,3,4].
Plant cell culture can serve as an alternative source of taxoids. There is a pool of publications describing cell cultures of various yew species and their ability to produce taxoids [21]. However, many authors noted either the absence or only trace amounts of taxoids in the cell cultures of Taxus spp. [22]. These conclusions were driven primarily by the analysis of 13-hydroxylated taxoids (mostly paclitaxel, baccatin III, and some others) in cell cultures [21,22]. Many 13-OH taxoids are polar compounds, which determines the strategy of their chemical analysis using modern HPLC/UPLC systems with reversed-phase adsorbents [23,24]. However, taxane diterpenoids are very diverse in both structure and physicochemical characteristics. Representatives of several taxoid classes, for example, 14-hydroxylated taxoids, are hydrophobic compounds with a longer elution time in reversed-phase HPLC compared to 13-OH taxoids [23,24]. As a result, these compounds may remain unrevealed during the chemical analysis of cell cultures of Taxus spp. which is usually focused primarily on 13-OH taxoids. The abovementioned considerations suggest that the formation of taxoids of various structural classes in cultured yew cells is relatively underexplored and requires further investigation.
Furthermore, from a scientific viewpoint, isolated plant cells cultured in vitro are not analogous to the cells of whole plants [21]. As a result, many fundamentally important processes, including secondary metabolism, in cell cultures are different from those in plants [21,25]. Several studies demonstrated that the profile of secondary metabolites in plant cell cultures could be altered compared to their donor plants, which is often reflected in the promotion or suppression of the production of certain metabolite groups [21,25]. Meanwhile, there are very few publications related to the composition of structurally different taxoid groups in the in vitro cell cultures of different Taxus species [26,27,28,29].
In this work, we present for the first time a detailed UPLC–ESI-MS analysis of the structural diversity of taxoids in different lines (by explant origin) of callus and suspension cell cultures of three yew species (Taxus baccata L., T. canadensis Marshall, and T. wallichiana Zucc.) and two T. × media Rehder hybrids (T. × media cv. Aureovariegata and T. × media cv. Dovastaniana), grown in different nutrient media. The cell lines have been cultured in vitro for over 10 years, except for the cell culture of T. wallichiana, which is 5-years-old.
2. Results
2.1. Growth Characteristics of Taxus spp. Cell Cultures
2.1.1. Callus Cell Cultures
In this study, we used callus cell cultures of three yew species (Taxus baccata, T. canadensis, and T. wallichiana) and two T. × media hybrids: T. × media cv. Aureovariegata and T. × media cv. Dovastaniana. All callus and suspension cell cultures, except for T. wallichiana, were maintained by periodic subcultures for over 10 years and are represented by several (in some species, over 20) cell lines that originated from different explants. The T. wallichiana cell culture was maintained in an actively growing state for 5 years and is represented by a single line. The cultures were grown in more than 20 different media; the main differences in the media composition were growth regulators and the presence of antioxidants (polyvinylpyrrolidone) or adsorbents (activated charcoal). A complete list of the cell lines used and cultivation conditions is given in Table 1.

Table 1.
Callus cell cultures of Taxus spp. with nutrient media used for their initiation and maintenance, and increase in fresh weight.
Table 1 presents an increase in the fresh weight of callus cell lines measured on the 56th day of cultivation. For the majority of cultures, the increase in cell biomass ranged from 1.5 to 5.0, which is consistent with the literature [30]. For three cell lines of T. baccata and two cell lines of T. × media, relatively high values of the growth index (7.5–9.1) were recorded, which significantly exceeded those reported in the literature [30]. No correlation was observed between fresh weight accumulation and species, explant, or medium composition (Table 1). However, cell cultures originating from the 40-year-old tree in the botanical garden of the Moscow State University (lines Tb-msu) demonstrated very slow growth. Normally, growing cell cultures of this origin could be developed when the original callus line was cultured in liquid medium to form a cell suspension, followed by placing the cells back on solid medium (the “callus-suspension-callus” cycle). Callus cultures produced through this scheme demonstrated better growth than the original callus lines. In general, cell cultures growing in the presence of activated charcoal showed relatively high growth indices (from 3 to over 7.5).
2.1.2. Suspension Cell Cultures
Suspension cell cultures developed in this study had different but generally high growth characteristics. The maximum accumulation of dry biomass Mmax for all studied cell lines was within 7–16 g/L; growth index I ranged from 4 to 10; specific growth rate μ was 0.10–0.22 day–1; economic coefficient Y was 0.1–0.3; and biomass productivity P was 0.2–0.8 g/L per day. The highest growth parameters were recorded for suspension cell lines TmA-msu/B5-NB, Tb-msu/B5-NB, Tb-msu/B5-PB, and Tb-msu/B5-DK. Representative growth curves of cell lines Tb-msu/B5-PB-pvp and Tb-msu/B5-NB are shown in Figure 1.
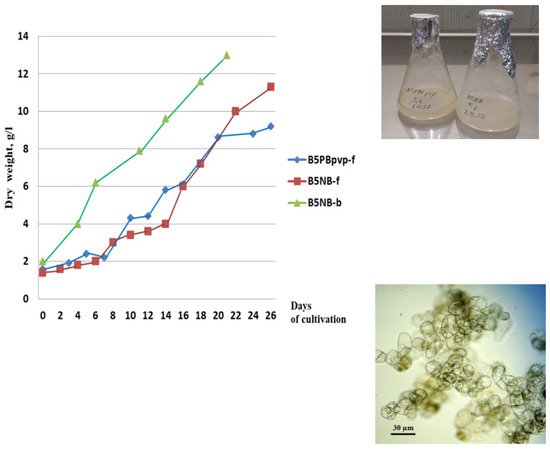
Figure 1.
Left—Growth curves of the suspension cell culture of Taxus baccata lines Tb-msu/B5-PB-pvp (B5PBpvp-f in the legend) and Tb-msu/B5-NB (B5NB-f) during cultivation in flasks and line Tb-msu/B5-NB during cultivation in 20-L bioreactor (B5NB-b) under semi-continuous regime (the third cycle of cultivation). Right—photograph of cell suspension cultures in 250-mL flasks and microphotograph of cells of Tb-msu/B5-NB line.
In order to accumulate a sufficient amount of biomass for the isolation of taxoids, suspension cell culture Tb-msu/B5-NB-ac (Taxus baccata) was cultured in a 20-L bubble-type bioreactor operated in a semi-continuous mode for four sequential growth cycles. The growth parameters of the cell culture improved gradually upon cell adaptation to bioreactor conditions (maximum biomass accumulation increased from 6.5 to 15 g/L, specific growth rate—from 0.12 to 0.20 day−1). Cell viability in all growth cycles was above 90%. A representative growth curve of the Tb-msu/B5-NB cell line during the third subculture cycle in the bioreactor is given in Figure 1.
The main growth parameters of the suspension cell culture in the subculture cycle shown in Figure 1 are presented in Table 2.

Table 2.
Growth characteristics of the suspension cell cultures of Taxus baccata grown in 250-mL flasks and 20-L bioreactors.
2.2. Phytochemical Screening of the Cell Cultures of Taxus spp.
2.2.1. Structural Identification of Taxoids in Cell Cultures
The first step in the phytochemical analysis was a detailed structural identification of toxoids present in the cell cultures. A UPLC–ESI-MS analysis of the taxoid composition was performed using biomass from callus cell cultures of T. baccata line Tb-nbg/R-NB-ac, grown on B5-PB-ac medium. This cell line was maintained by periodic subcultures for more than 10 years and showed the highest increase in fresh weight. Callus was taken to the lab for analysis on day 74 of the 42nd subcultivation. The positive ion detection mode (electrospray ionization) was selected to record chromatograms and mass spectra since it allows gathering the most information on the structure of taxane diterpenoids, as well as other natural compounds, during a single run due to molecule fragmentation in the ionization source [25,31].
The UPLC–ESI-MS chromatogram of the alcohol extract from this cell culture recorded in the total ion current mode (positive ions) is presented in Figure 2. Six peaks of compounds were found and eluted from the column within 3.5–11 min. The comparison of their MS spectra with the literature data suggested that they belong to the diterpenoids of the taxane group. These compounds were numbered 1 through 6 in order of increasing hydrophobicity, that is, increasing retention time on a reversed-phase chromatographic column.
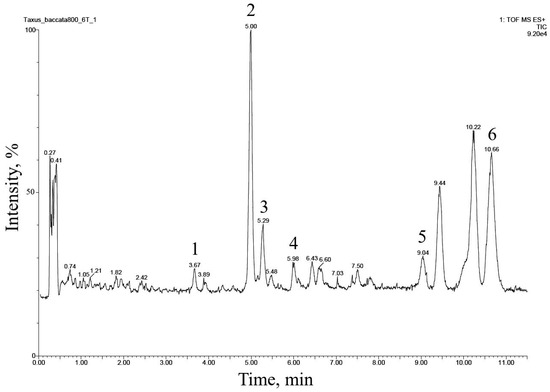
Figure 2.
UPLC–ESI-MS chromatogram (total ion current, positive ion mode) of an alcohol extract from the biomass of T. baccata callus cell culture (Tb-nbg /R-NB-ac line grown in a B5-PB-ac medium, 74 days of 42nd subcultivation). The description of peaks 1–6 is given in Table 3.
The preliminary analysis of the MS spectra of the detected compounds indicated (Table 3) that all of them belong to neutral taxoids with molecules without nitrogen-containing functional groups. This conclusion was supported by the fact that ions with odd m/z values predominated in the MS spectra of all compounds. In addition, intense signals of adduct ions [M + NH4]+ and [M + Na]+ were observed, and there were almost no signals of protonated ions [M + H]+ (Table 3) [23,24,28].

Table 3.
Results of UPLC–ESI-MS analysis (positive ion mode) of an alcohol extract from the biomass of T. baccata callus culture (Tb-nbg R-NB-ac line grown in a B5-PB-ac medium, 74 days of 42nd subculturing cycle). Peak numbers correspond to those in Figure 2.
The fragmentation of compounds 1–6 in the ionization source suggested that they all belong to the so-called “regular” taxoids containing a taxa-4(20),11-diene skeleton [1,23,24,28]. Based on the number of substituents in taxa-4(20),11-diene core fragment, compounds 1–6 could be divided into two structural subclasses: (1) compounds 1, 2, and 5, derivatives containing five substituents (the presence of a pair of characteristic ions with m/z 281 and 263), and (2) compounds 3, 4, and 6 containing four substituents (the presence of a pair of characteristic ions with m/z 283 and 265) [1,23,24,28].
By their chemical nature, the substituents in the taxadiene skeleton of the identified compounds are hydroxyl groups esterified (in various combinations) with aliphatic acid residues [1,23,24,28]. The following acyl substituents were identified: acetic acid (identified in all compounds based on the presence of neutral losses of 77 (C2H4O2 + NH3), 60 (C2H4O2), and/or 42 Da (C2H2O) upon fragmentation of the [M+NH4] adduct ion in the ionization source), hydroxymethylbutanoic acid (for compound 3, neutral loss of 118 Da (C5H10O3)), methylbutanoic acid (for compounds 5 and 6, neutral loss of 119 Da (C5H10O2 + NH3)).
The described patterns of MS fragmentation (positive ion mode) suggested that taxoids 1–6 belonged to the structural group of taiwanxan (14-hydroxylated taxoids).
The 14-hydroxylated taxoids were also predominant in the extract of the cell biomass of a suspension culture of T. baccata, line Tb-msu/B5-NB, grown in a 20-L bioreactor, as confirmed by UPLC–ESI-MS (Figure 3 and Table 4). Many of these compounds were identical, in terms of relative chromatographic behavior and mass spectrometry data, to taxoids identified in T. baccata callus culture.
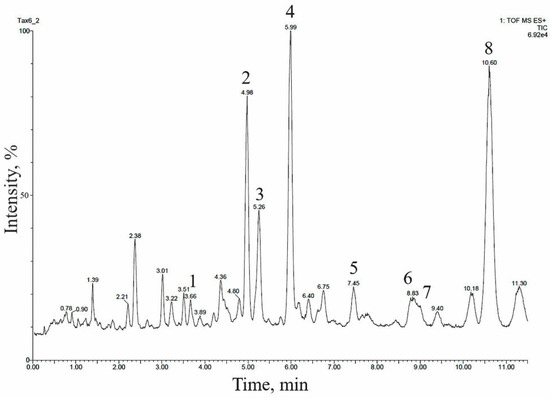
Figure 3.
UPLC–ESI-MS chromatogram (total ion current, positive ion mode) of an alcohol extract from the biomass of T. baccata cell suspension culture (Tb-msu/B5-NB-Car line grown in a 20-L bioreactor). The description of peaks 1–8 is given in Table 4.

Table 4.
Results of UPLC–ESI-MS analysis (positive ion mode) of an alcohol extract from the biomass of T. baccata cell suspension culture (Tb-msu/B5-NB-ac line grown in a 20-L bioreactor). Peak numbers correspond to those in Figure 3.
In order to verify the structural identification of the detected taxoids, we isolated major diterpenoids from 93 g of air-dried biomass from a suspension culture of T. baccata (Tb-msu/B5-NB) grown in a 20-L bioreactor.
Five taxoids were present in the cell biomass in amounts sufficient for preparative isolation (analytical TLC results); they were identified by specific staining (pink-lilac color) upon the development of the TLC plate with an anisaldehyde–sulfuric acid reagent [1]. The detected compounds were designed in decreasing order of polarity (relative mobility (Rf) in the ethyl acetate–hexane system (1:1, v/v)) as follows: I (Rf 0.3), II (Rf 0.5), III (Rf 0.7), IV (Rf 0.8), and V (Rf 0.9). Using conventional column chromatography and semipreparative TLC, these compounds were isolated in pure form with the following yields (% of dry cell mass): I 0.0006%, II 0.0030%, III 0.0050%, IV 0.0080%, and V 0.014%. The structures of the isolated glycosides were determined using high-resolution mass spectrometry and NMR spectroscopy.
The results of the interpretation of the 13C NMR spectrum of taxoid I suggest that the isolated compound has the skeleton of taxa-4(20),11-diene substituted with hydroxyl groups at positions C2, C5, C7, C10, and C14 (Supplementary Table S1). The hydroxyl groups at C2, C5, C10, and C14 are esterified with four acetic acid residues. The order of attachment of acyl fragments to the diterpene backbone was determined by interpreting the results of the 1H–13C HMBC experiment (Figure 4). The stereochemistry of substituents in the molecule of compound I was determined based on the analysis of the spin-spin coupling constants of the corresponding protons and their comparison with the published data (Supplementary Table S2) [27,28,29,32].
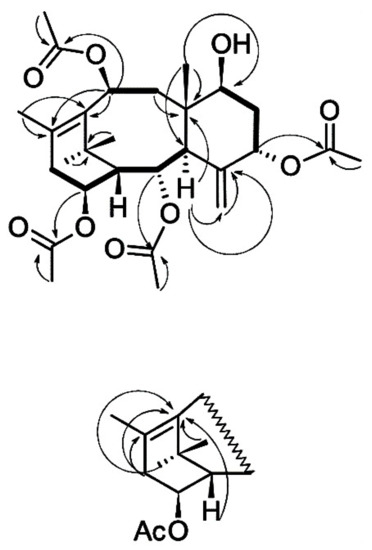
Figure 4.
Structure and key 1H–1H COSY (bold lines) and 1H–13C HMBC (arrows) correlations of taxoid I isolated from T. baccata cell suspension culture.
Thus, the interpretation of the 1H and 13C NMR spectra of the compound I and analysis of the literature data [27,28,29,32] suggest that this taxoid has the structure of 7β-hydroxy-2α,5α,10β,14β-tetraacetoxy-4(20),11-taxadiene (Figure 4) and corresponds to 7β-hydroxy-taxuyunnanine C, which was first isolated from T. cuspidata cell culture [32]. The described structure is also consistent with the results of high-resolution mass spectrometry of the isolated taxoid: the formula C28H40O9 is confirmed by the presence of a signal of the [M + Na]+ adduct ion at m/z 543.2572 in the spectra of positive ions of this compound (calculated value m/z 543.2565). 7β-Hydroxy-taxuyunnanine C can be classified as a rare and/or unusual taxoid: it is one of a few 14-hydroxylated taxoids having a hydroxyl group at the C7 position of 4(20),11-taxadiene; only two taxoids with a similar structure are currently known [1,2,3,4]. There is only one report on discovering this taxoid first isolated from T. cuspidata callus cell culture [32]. Thus, 7β-hydroxy-taxuyunnanine C was detected for the first time in T. baccata cell culture.
NMR spectroscopy and high-resolution mass spectrometry of compounds II–V were performed in a similar way and revealed that they have the structures of sinenxane C, taxuyunnanine C, 2α,5α,9α,10β,14β-pentaacetoxy-4(20),11-taxadiene, and yunnanxane, respectively (Supplementary Tables S1 and S2). These taxoids were also previously isolated from cell cultures of different yew species (T. chinensis, T. chinensis var. mairei, T. × media, T. wallichiana, and T. cuspidata) [23,24,25,26,27,28,29,33,34]. However, these 14-hydroxylated taxoids were found for the first time in T. baccata cells cultured in vitro.
Thus, the preparative isolation and structural study of individual taxoids confirm the results of their identification performed using UPLC–ESI-MS. 14-hydroxylated taxoids predominated in callus and suspension cultures of T. baccata cells.
2.2.2. Screening of Taxoids in the Cell Cultures of Various Yew Species, Provenance and Cultivation Conditions
In order to reveal any general pattern in the accumulation of taxoids of different structural groups among the cell cultures of Taxus spp., we performed the UPLC–ESI-MS phytochemical screening of taxoids from all available cell cultures (over 20 cell lines in total). These cell lines belonged to different yew species and were induced from different donor plants using different explants in different media. Identification of 14-OH taxoids was accomplished by comparing their retention times and mass spectra with standard samples of 14-OH taxoids isolated at the first step of this study. Sinenxane B and taxuyunnanine B were identified by comparing the results of mass spectrometry with the literature [23,24]. Commercial standard samples of Baccatin III, 10-deacetyl-7-xylosyl taxol, cephalomannine, paclitaxel, and taxusin were used to identify 13-hydroxylated taxoids.
The results of screening the biomass of callus cell cultures are presented in Table 5.

Table 5.
Taxoids identified in callus cell cultures of Taxus spp.
As a result, 13-hydroxylated taxoids (paclitaxel, baccatin III, etc.) were not detected in the cell samples of any callus cell cultures of Taxus spp., while 14-hydroxylated taxoids were found in the biomass of almost all studied callus lines. Only four out of the 45 samples tested did not have taxoids.
The screening results emphasized the role of the genotype (the type of plant used for culture induction) in the formation of taxoids in cell cultures. Among the studied callus cultures, T. × media cv. Dovastaniana showed the lowest number of toxoids, and their accumulation in the cell biomass was unstable: only 2 out of 4 biomass samples from this cell culture line contain 14-OH taxoids.
Similar results were obtained for suspension cultures of Taxus spp. grown in flasks (Table 6): 13-OH taxoids were absent in all samples, while 14-OH taxoids were found in most samples.

Table 6.
Taxoids identified in suspension cell cultures of Taxus spp.
At the same time, taxoids were not detected in any of the samples of suspension cultures of T. × media cells but were present, with one exception, in all analyzed samples of cell cultures of other species of Taxus spp. This confirmed the earlier conclusion about the low capability of T. × media cell cultures to form taxoids.
The qualitative composition of 14-OH taxoids in the biomass of Taxus spp. may somewhat vary depending on growing conditions. For example, out of the 8 taxoids found in the biomass of the suspension culture of T. baccata cells (Tb-msu/B5-NB) grown in a bioreactor, only sinenxane C is stably present in the cell culture grown in flasks.
2.2.3. Screening of Taxoids Released to Cultivation Medium
Paclitaxel and some other 13-OH taxoids can be secreted into the apoplast and the growth medium during the in vitro growth of yew cells [35]. Therefore, we performed the UPLC–ESI-MS screening of culture media of callus and suspension cell cultures studied in this work.
As a result, 13-hydroxylated taxoids were not detected in any culture media samples (Supplementary Tables S4 and S5), while 14-OH were present in most media of the studied callus and suspension cell cultures. 14-OH taxoids were detected in 21 of 28 test samples of callus cultures and in 6 out of 8 samples of suspension cultures (Supplementary Tables S3 and S4). Taxoids were often absent in the culture media of T. × media cells: they were not detected in 4 out of 8 test samples of callus cultures and in none of the suspension cultures.
3. Discussion
There are a number of publications on plant cell cultures of different Taxus species. The main goal of the majority of those studies is practical: to obtain well-growing cell cultures and develop strategies for enhanced production of taxoids, primarily of commercially valuable paclitaxel [21].
Some authors reported the development of plant cell cultures accumulating taxol in amounts comparable to its content in plants; however, in most cases, taxol was absent in the cell cultures or present in trace amounts [22]. In suspension cell culture, paclitaxel was first discovered in 1989 in the cell culture of T. brevifolia [36].
Several trends could be found while analyzing the available literature sources on yew cell cultures [22,30,35]:
- In most publications, studies were performed on relatively "young” cell cultures, 1-2 years after induction, while the content of secondary compounds may change in cell cultures during long-term cultivation.
- The majority of the studies, with rare exceptions, only focused on the analysis of a few industrially valuable 13-OH-hydroxylated compounds (paclitaxel, baccatin III); other groups of taxoids were not screened.
- Most studies were performed using cell cultures of one yew species only, which did not allow generalizing on potential trends of taxoid formation in the cell cultures of different Taxus species.
In the present study, taxoids of various groups were screened in the plant cell cultures of different Taxus species, and long-term (at least 10 years) grown cell cultures were mostly used for analysis.
The results indicate that, regardless of the species, cell line, or cultivation conditions, most of the investigated cell cultures of Taxus spp. retain the ability to form taxane diterpenoids. However, in the in vitro cultured yew cells, the metabolism of taxoids was shifted towards the predominant formation of non-polar 14-hydroxylated derivatives (in the form of polyesters), while more hydrophilic and toxic 13-oxygenated (in particular, 13-hydroxylated) taxoids were predominant in the aerial parts of intact plants that were used as explants for culture induction [1,2,3,4,21,22,23,24]. A comparison of the obtained results with literature data [26,27,28,29,33,34] confirms that this is a general pattern and is observed in plant cell cultures of almost all yew species, in which 14-OH taxoids were analyzed.
The reasons for such a metabolic shift may lie in the unique physiology of a plant cell culture as a population of dedifferentiated proliferating cells [21]. Many processes in cells grown in vitro, including secondary metabolism, differ significantly from those in whole plants due to the absence of organismic control, a different signaling system, and altered compartmentation [21]. The formation of 14-OH taxoids in plant cell cultures may be due to their lower toxicity for proliferating cells compared to 13-OH derivatives. For example, paclitaxel and some of its homologues disrupt the functioning of the cytoskeleton, which is lethal for most eukaryotic cells [37].
The pattern of taxoid accumulation described in the present work is unique compared to cell cultures of most other plant taxa, where the secondary metabolism upon cell dedifferentiation is usually shifted towards increased formation of more polar/hydrophilic compounds [21,25]. This might be explained by comparing the results of this study with phytochemical investigations of yew plants. Taxus species tend to accumulate hydrophobic secondary metabolites [4]. For example, phenolic compounds in yews are mainly represented by biflavones, lignans, esters of catechins, etc. In Taxus spp., glycosylated secondary metabolites (except for cyanogenic glycosides, some phenolic minor derivatives, xylosides, and very rarely taxoid glucosides) are less common [4,38] than in angiosperms [39].
The results reported here have both fundamental and applied significance, since they demonstrate the importance of Taxus spp. cell cultures as renewable and environmentally friendly sources of 14-hydroxylated taxoids. Taxoids with 14-hydroxylated structures have a wide range of practical uses. For example, 14-OH taxoids synthesized by in vitro cultured yew cells decrease tumor cell resistance to cytotoxic compounds through disruption of plasma membrane ABC transporters by direct non-covalent binding to these proteins and/or modulation of MAP signaling. Therefore, 14-OH taxoids can be used as components of complex cancer chemotherapy programs [10,40]. Furthermore, 14-OH taxoids isolated from yew cell cultures can be used as intermediate compounds for chemical modifications and biotransformation to generate new taxane drugs [41,42]. 14-OH taxoids can also be used to treat other diseases besides cancer. It has been demonstrated that many natural 14-OH taxoids act as effective alpha-glucosidase inhibitors and are useful in the treatment of diabetes [17]. The 14-OH taxoids can act as nerve growth factor (NGF) mimetics, making them useful in the prevention of side effects associated with classical cytostatics (taxol, cisplatin, and vincristine) and Alzheimer’s disease [4,42]. Accordingly, yew cell cultures can be used as sources of natural compounds important to human physiology and medicine. This study adds to the literature’s already established fact that yew cell cultures in vitro are an excellent source of raw material for isolating unusual and rare (not typical for intact yew plants) taxoids with unique biological properties [8].
4. Materials and Methods
4.1. Plant Material
4.1.1. Callus Cell Cultures
Callus cell cultures of three yew species (Taxus baccata, T. canadensis, and T. wallichiana) and two T. × media hybrids (T. × media cv. Aureovariegata and T. × media cv. Dovastaniana) were used in the study. Callus cultures of T. baccata cells were obtained in 2007–2009 from two plants: a 40-year-old tree from the Botanical Garden of the Moscow State University, Moscow (MSU Botanical Garden; Tb-msu line) and an 800-year-old tree from the Nikitsky Botanical Garden, Crimea (Tb-800 line). Callus cultures of T. canadensis were obtained in 2008 from a 40-year-old plant from the Botanical Garden of Moscow State University (lines Tc-msu); callus cultures of T. × media were developed in 2007–2008 from 30-year-old plants from the Botanical Garden of Moscow State University (lines TmD-msu (cv. Dovastaniana) and TmA-msu (cv. Aureovariegata)). A callus culture of T. wallichiana was induced in 2016 from a 50-year-old tree from the Central Botanical Garden of the National Academy of Sciences of Belarus, Minsk (NASB Botanical Garden; line TW-Bel). Stem segments (a small section of the stem with 1–3 leaves) were used as explants. Each line was obtained from a single explant on a specific medium. For each line, the induction medium is designated as “Im” (initial medium). The conditions for culture induction were described earlier [43].
The developed cell lines were grown on media of different compositions, as described below.
Three mineral salt formulations were used: Gamborg’s medium (B5), Reinart’s medium (R), and White’s medium (W). All media contained vitamins as described by Gamborg (nicotinic acid 0.5 mg/L, pyridoxine 0.1 mg/L, thiamine chloride 0.1 mg/L, Serva, St. Louis, Missouri, USA), 3% sucrose (Merck, Germany), and 0.55% agar (Merck, Germany).
The following combinations of growth regulators were used: NB—1-NAA (2 mg/L) and BAP (0.3 mg/L); PB—picloram (1 mg/L) and BAP (0.3 mg/L); DK—2,4-D (1 mg/L) and kinetin (0.3 mg/L); all growth regulators were purchased from Serva. The media also differed by the presence of polyvinylpyrrolidone (PanReac AppliChem, molecular weight 4000, 1.0 g/L) or activated charcoal (Fluka, 500 mg/L). These media are marked “PVP” and “ac,” respectively. Some callus cultures were obtained from the corresponding suspension cell cultures according to the scheme “callus → suspension → callus.” A complete list of cell lines used and their media are given in Table 1.
Calli was grown in the dark at 26 °C. The fresh biomass gain (the callus-to-transplant weight ratio) of callus cell cultures was determined on the 56th day of growth, as described earlier [43].
4.1.2. Suspension Cell Cultures
Suspension cell cultures were obtained from the corresponding callus cultures using a standard procedure [43]. Suspension cultures of T. × media, T. canadensis, and T. baccata cells were obtained in 2009–2010, suspension cell culture of T. wallichiana was developed in 2016 [44].
Suspension cell cultures were grown in media containing Gamborg (B5) mineral salts. The medium composition for each suspension cell line was similar to the corresponding callus line except for the absence of agar. The suspension cell cultures were grown in the dark using an orbital shaker (100 ± 10 rpm) at 26 ± 1 ° C and relative humidity of 70 ± 5%. Flasks of 250, 500, and 1000 mL volume filled with, respectively, 40, 80, and 160 mL of culture medium were used for the cultivation of cell suspensions. Cultures were transferred to the fresh medium (subcultured) on the 28th day of cultivation; the inoculum-to-fresh medium ratio was 1:4. To characterize the growth and physiological state of the cell cultures, the dry and fresh weights of the cells and their viability were determined as described earlier [43,44].
The growth index (I), specific growth rate (μ), biomass doubling time (τ), economic coefficient (Y), and biomass productivity (P) were calculated using the following equations [45,46]:
where Xmax and X0 are the maximum and initial values of the growth criterion (dry or fresh weight of cells), respectively;
where X2 and X1 are the values of the growth criterion (dry or fresh weight of cells) at time points t2 and t1, respectively (calculated for the exponential growth phase);
where Xmax and X0 are the maximum and initial concentrations of dry cell biomass (g/L), respectively, and S0 is the initial concentration of the substrate (sucrose) in the medium (g/L of the medium);
where X0 and Xi are the amounts of dry biomass at the beginning of cultivation and at time ti, respectively.
I= Xmax/X0,
µ (day–1) = (ln X2 − ln X1)/(t2 − t1),
τ (day) = ln 2/µ;
Y= (Xmax − X0)/S0,
P(g/L day) = (Xi − X0)/(ti − t0),
In addition, a suspension culture of T. baccata cells (Tb-msu/B5-NB) was grown in a 20-L bubble-type conical bioreactor designed at the Department of Cell Biology and Biotechnology, Timiryazev Institute of Plant Physiology, Russian Academy of Sciences, with a total volume of 20 L and a working volume of 15 L [47]. A cell culture grown in flasks was used as an inoculum; the density of the inoculum was 2 g/L by dry weight. Depending on the growth cycle phase, the air was supplied at a rate of 0.1–1.0 L/L/min. The concentration of dissolved oxygen pO2 was maintained at 10–40% of saturation in the absence of intense foaming.
4.2. Biochemical Analysis of the Cell Cultures
4.2.1. Sample Preparation for Taxoids Screening
A sample (25 mg) of powdered air-dry biomass or dried culture medium was extracted three times with 1 mL of 96% ethanol for 30 min in an ultrasonic bath (Sapfir, Moscow, Russia), then centrifuged at 130,000 rpm for 10 min; the supernatant was collected into a pear-shaped flask. The combined alcohol extracts were evaporated under vacuum by heating to 50 °C in a water bath. The resulting dry extract was dissolved in 1 mL of distilled water and applied to a Supelclean ENVI-18 solid-phase extraction cartridge (Supelco, St. Louis, Missouri, USA). The cartridge was washed with 3 mL of water, and the analytes were desorbed with 3 mL of ethanol. The resulting solution was evaporated in a vacuum at 50 °C. Before analysis, the extracts were dissolved in an acetonitrile–water mixture (1:1, by volume). When analyzing the culture media of suspension cell cultures, 10 mL of the medium was applied to a cartridge for solid-phase extraction. Further preparation of samples was carried out according to the procedure described above. For most cell cultures, the analysis was carried out for two to four different subcultures (in the tables, the results for different subcultures are presented separately).
4.2.2. UPLC–ESI-MS Analysis of Taxoids
The analysis was performed using a Waters Acquity UPLC system (Waters, Milford, MA, USA) equipped with a Xevo QTof hybrid quadrupole time-of-flight mass spectrometer (Waters, Milford, MA, USA). A sample (1 μL) was injected in an ACQUITY UPLC BEH Phenyl column (50 × 2.1 mm, 1.7 μm; Waters, Drinagh, County Wexford, Ireland). The column temperature was 40 °C, and the flow rate of the mobile phase was 0.4 mL/min. A 0.1% (by volume) solution of formic acid in water (solvent A) and a 0.1% (by volume) solution of formic acid in acetonitrile (solvent B) were used as the mobile phase.
Chromatographic separation was performed in the gradient elution mode. The gradient elution was performed by the following program (B, % by volume): 0–1 min, 35%; 1–7 min, 35 → 45%; 7–17 min, 45%; 17–17.5 min, 45 → 95%; 17.5–19 min, 95%; 19–19.5 min, 95 → 35%.
The analysis was carried out in the positive-ion mode in the m/z range of 100–1200. Ionization source parameters were as follows: ionization source temperature 120 °C; desolvation temperature 250 °C; capillary voltage 3.0 kV; sample injection cone voltage 30 V; nitrogen (desolvation gas) flow rate 600 L/h.
Commercial standard samples of baccatin III, cephalomannine, paclitaxel (Sigma-Aldrich, St. Louis, MO, USA), 10-deacetyl-7-xylosyl taxol, and taxusin (ChromaDex, Irvine, CA, USA) were used to identify 13-OH taxoids.
4.2.3. Preparative Isolation of Taxoids from T. baccata Cell Culture
Preparative isolation of diterpenoids was performed using 93 g of air-dry biomass from a suspension culture of T. baccata cells (line Tb-msu/B5-NB) grown in a 20-L bubble-type bioreactor. Diterpenoids were separated using a combination of classical column chromatography with silica gel (Silicagel 60, grade 7734, 70-230 mesh, Sigma-Aldrich, St. Louis, MO, USA) and semi-preparative TLC (Uniplate Silica gel GF, Analtech, Newark, DE, USA) according to published procedures [28,29].
4.2.4. High-Resolution Mass Spectrometry
High-resolution mass spectra with electrospray ionization were recorded using a Bruker micrOTOF II instrument as described earlier [48].
4.2.5. NMR Spectroscopy
The 1H and 13C NMR spectra of the isolated compounds were measured in chloroform-d using a Bruker Avance AV600 instrument (Germany); tetramethylsilane was used as the internal standard. Signals in the 1H and 13C NMR spectra were assigned using two-dimensional NMR experiments (1H–1H COSY, TOCSY, 1H–13C HSQC, and HMBC).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28052178/s1, Table S1: 13C NMR spectra (125 MHz, CDCl3) of taxoids I–V isolated from T. baccata cell suspension culture; Table S2: 1H NMR spectra (600 MHz, CDCl3) of taxoids I–V isolated from T. baccata cell suspension culture; Table S3: Taxoids detected in culture medium of the callus cell lines of Taxus spp.; Table S4: Taxoids detected in the cultivation medium of suspension cell cultures of Taxus spp.
Author Contributions
D.V.K.: Conceptualization, liquid chromatography-mass spectrometry, NMR spectra, writing—original draft preparation, review, and editing; E.V.D.: Taxus spp. plant cell cultures, writing—review and editing; E.B.G.: plant cell cultures, writing—review and editing; A.M.N.: supervision, writing—original draft preparation, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Cultivation of plant cell suspensions was performed using the equipment of the large-scale research facilities “Experimental biotechnological facility” and “All-Russian Collection of cell cultures of higher plants” of the IPPRAS (EBF IPPRAS and ARCCC HP IPPRAS). Cell strain cultivation and bioreactor production of biomass were financially supported within the state assignments of the Ministry of Science and Higher Education of the Russian Federation, themes No. 122042700045-3 (cell culture obtainment and maintenance) and No. 122042600086-7 (bioreactor production of biomass), respectively. Evaluation of taxoids in cell cultures was financially supported by Russian Science Foundation project no. 19-14-00387 and Russian Foundation for Basic Research project no. 20-54-00014 Bel_a.
Institutional Review Board Statement
The Institutional Review Board Statement was waived for this work.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used in this study was gathered from open literature sources or scientific journals under institutional subscription.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.-F.; Shi, Q.-W.; Dong, M.; Kiyota, H.; Gu, Y.-C.; Cong, B. Natural Taxanes: Developments Since 1828. Chem. Rev. 2011, 111, 7652–7709. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.M.; Conner, C.F. Taxanes and taxoids of the genus Taxus—A comprehensive inventory of chemical diversity. Phytochemistry 2021, 190, 112829. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E.; Kingston, D.G.I. The Taxane Diterpenoids. J. Nat. Prod. 1999, 62, 1448–1472. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G. The phytochemistry of the yew tree. Nat. Prod. Rep. 1995, 12, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Mikuła-Pietrasik, J.; Witucka, A.; Pakuła, M.; Uruski, P.; Begier-Krasińska, B.; Niklas, A.; Tykarski, A.; Książek, K. Comprehensive review on how platinum- and taxane-based chemotherapy of ovarian cancer affects biology of normal cells Cell. Mol. Life Sci. 2019, 76, 681–697. [Google Scholar] [CrossRef]
- Amaya, C.; Luo, S.; Baigorri, J.; Baucells, R.; Smith, E.R.; Xu, X.-X. Exposure to low intensity ultrasound removes paclitaxel cytotoxicity in breast and ovarian cancer cells. BMC Cancer 2021, 21, 981. [Google Scholar] [CrossRef]
- Maloney, S.M.; Hoover, C.A.; Morejon-Lasso, L.V.; Prosperi, J.R. Mechanisms of Taxane Resistance. Cancers 2020, 12, 3323. [Google Scholar] [CrossRef]
- Galletti, E.; Magnani, M.; Renzulli, M.L.; Botta, M. Paclitaxel and docetaxel resistance: Molecular mechanisms and development of new generation taxanes. ChemMedChem 2007, 2, 920–942. [Google Scholar] [CrossRef]
- Kobayashi, J.; Shigemori, H.; Hosoyama, H.; Chen, Z.; Akiyama, S.; Naito, M.; Tsuruo, T. Multidrug resistance reversal activity of taxoids from Taxus cuspidata in KB-C2 and 2780AD cells. Jpn. J. Cancer Res. 2000, 91, 638–642. [Google Scholar] [CrossRef]
- Hasegawa, T.; Bai, J.; Dai, J.; Bai, L.; Sakai, J.; Nishizawa, S.; Bai, Y.; Kikuchi, M.; Abe, M.; Yamori, T.; et al. Synthesis and structure-activity relationships of taxuyunnanine C derivatives as multidrug resistance modulator in MDR cancer cells. Bioorg. Med. Chem. Lett. 2007, 17, 3722–3728. [Google Scholar] [CrossRef]
- Qayum, M.; Nisar, M.; Rauf, A.; Khan, I.; Kaleem, W.A.; Raza, M.; Karim, N.; Saleem, M.A.; Bawazeer, S.; Uysal, S.; et al. In-vitro and in-silico anticancer potential of taxoids from Taxus wallichiana Zucc. Biol. Futur. 2019, 70, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.K.; Pal, A.; Maulik, P.R.; Kaur, T.; Garg, A.; Khanuja, S.P. Taxoid from the needles of the Himalayan yew Taxus wallichiana with cytotoxic and immunomodulatory activities. Bioorg. Med. Chem. Lett. 2006, 16, 2446–2449. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.; Khan, I.; Simjee, S.U.; Gilani, A.H.; Obaidullah; Perveen, H. Anticonvulsant, analgesic and antipyretic activities of Taxus wallichiana Zucc. J. Ethnopharmacol. 2008, 116, 490–494. [Google Scholar] [CrossRef]
- Tong, J.; Lu, J.; Zhang, N.; Chi, H.; Yamashita, K.; Manabe, M.; Kodama, H. Effect of seven tricyclic diterpenoids from needles of Taxus media var. Hicksii on stimulus-induced superoxide generation, tyrosyl or serine/threonine phosphorylation and translocation of cytosolic compounds to the cell membrane in human neutrophils. Planta Med. 2009, 75, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Polonio, T.; Efferth, T. Leishmaniasis: Drug resistance and natural products (review), Int. J. Mol. Med. 2008, 22, 277–286. [Google Scholar] [CrossRef]
- Binwal, M.; Babu, V.; Israr, K.M.M.; Kashyap, P.K.; Maurya, A.K.; Padalia, R.C.; Tandon, S.; Bawankule, D.U. Taxoids-rich extract from Taxus wallichiana alleviates high-fat diet-induced insulin resistance in C57BL/6 mice through inhibition of low-grade inflammation. Inflammopharmacology 2023, 3, 451–464. [Google Scholar] [CrossRef]
- Dang, P.H.; Nguyen, H.X.; Duong, T.T.T.; Tran, T.K.T.; Nguyen, P.T.; Vu, T.K.T.; Vuong, H.C.; Phan, N.H.T.; Nguyen, M.T.T.; Nguyen, N.T.; et al. α-Glucosidase Inhibitory and Cytotoxic Taxane Diterpenoids from the Stem Bark of Taxus wallichiana. J. Nat. Prod. 2017, 80, 1087–1095. [Google Scholar] [CrossRef]
- Qayum, M.; Nisar, M.; Shah, M.R.; Adhikari, A.; Kaleem, W.A.; Khan, I.; Khan, N.; Gul, F.; Khan, I.A.; Zia-Ul-Haq, M.; et al. Analgesic and antiinflammatory activities of taxoids from Taxus wallichiana Zucc. Phytother. Res. 2012, 26, 552–556. [Google Scholar] [CrossRef]
- Sharma, H.; Garg, M. A review of traditional use, phytoconstituents and biological activities of Himalayan yew, Taxus wallichiana. J. Integr. Med. 2015, 13, 80–90. [Google Scholar] [CrossRef]
- Küpeli, E.; Erdemoğlu, N.; Yeşilada, E.; Sener, B. Anti-inflammatory and antinociceptive activity of taxoids and lignans from the heartwood of Taxus baccata L. J. Ethnopharmacol. 2003, 89, 265–270. [Google Scholar] [CrossRef]
- Nosov, A.M.; Popova, E.V.; Kochkin, D.V. Isoprenoid Production via Plant Cell Cultures: Biosynthesis, Accumulation and Scaling-Up to Bioreactors. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.-Y., Murthy, H.N., Zhong, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 563–623. [Google Scholar] [CrossRef]
- Fu, C.; Li, L.; Wu, W.; Li, M.; Yu, X.; Yu, L. Assessment of genetic and epigenetic variation during long-term Taxus cell culture. Plant Cell Rep. 2012, 3, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Madhusudanan, K.P.; Chattopadhyay, S.K.; Tripathi, V.K.; Sashidhara, K.V.; Kukreja, A.K.; Jain, S.P. LC-ESI-MS analysis of taxoids from the bark of Taxus wallichiana. Biomed. Chromatogr. 2002, 16, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Madhusudanan, K.P.; Chattopadhyay, S.K.; Tripathi, V.; Sashidhara, K.V.; Kumar, S. MS/MS profiling of taxoids from the needles of Taxus wallichiana. Phytochem. Anal. 2002, 13, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Kochkin, D.V.; Galishev, B.A.; Titova, M.V.; Popova, E.V.; Nosov, A.M. Chromato-Mass-Spectrometric Identification of Glycosides of Phenylethylamides of Hydroxycinnamic Acids in a Suspension Cell Culture of Mandragora turcomanica. Rus. J. Plant Physiol. 2021, 68, 973–980. [Google Scholar] [CrossRef]
- Bai, J.; Kitabatake, M.; Toyoizumi, K.; Fu, L.; Zhang, S.; Dai, J.; Sakai, J.; Hirose, K.; Yamori, T.; Tomida, A.; et al. Production of Biologically Active Taxoids by a Callus Culture of Taxus cuspidata. J. Nat. Prod. 2004, 67, 58–63. [Google Scholar] [CrossRef]
- Bai, J.; Ito, N.; Sakai, J.; Kitabatake, M.; Fujisawa, H.; Bai, L.; Dai, J.; Zhang, S.; Hirose, K.; Tomida, A.; et al. Taxoids and Abietanes from Callus Cultures of Taxus cuspidata. J. Nat. Prod. 2005, 68, 497–501. [Google Scholar] [CrossRef]
- Zhao, C.F.; Yu, L.J.; Li, L.Q.; Xiang, F. Simultaneous identification and determination of major taxoids from extracts of Taxus chinensis cell cultures. Z. Naturforsch. C J. Biosci. 2007, 62, 1–10. [Google Scholar] [CrossRef]
- Ma, W.; Stahlhut, R.W.; Adams, T.L.; Park, G.L.; Evans, W.A.; Blumenthal, S.G.; Gomez, G.A.; Nieder, M.H.; Hylands, P.J. Yunnanxane and its homologous esters from cell cultures of Taxus chinensis var. Mairei. J. Nat. Prod. 1994, 57, 1320–1324. [Google Scholar] [CrossRef]
- Furmanowa, M.; Glowniak, K.; Syklowska-Baranek, K.; Zgórka, G.; Józefczyk, A. Effect of picloram and methyl jasmonate on growth and taxane accumulation in callus culture of Taxus × media var. Hatfieldii. Plant Cell Tiss. Organ Cult. 1997, 49, 75–79. [Google Scholar] [CrossRef]
- Kochkin, D.V.; Galishev, B.A.; Glagoleva, E.S.; Titova, M.V.; Nosov, A.M. Rare triterpene glycoside of ginseng (ginsenoside malonyl-Rg1) detected in plant cell suspension culture of Panax japonicus var. repens. Rus. J. Plant Physiol. 2017, 64, 649–656. [Google Scholar] [CrossRef]
- Fedoreyev, S.A.; Vasilevskaya, N.A.; Veselova, M.V.; Denisenko, V.A.; Dmitrenok, P.S.; Ozhigova, I.T.; Muzarok, T.I.; Zhuravlev, Y.N. A new C-14 oxygenated taxane from Taxus cuspidata cell culture. Fitoterapia 1998, 69, 430–432. [Google Scholar]
- Li, F.L.; Ma, X.J.; Hu, X.L.; Hoffman, A.; Dai, J.G.; Qiu, D. Antisense-induced suppression of taxoid 14b-hydroxylase gene expression in transgenic Taxus x media cells. Afr. J. Biotechnol. 2011, 10, 8720–8728. [Google Scholar] [CrossRef]
- Agrawal, S.; Banerjee, S.; Chattopadhyay, S.K.; Kulshrestha, M.; Madhusudanan, K.P.; Mehta, V.K.; Kumar, S. Isolation of taxoids from cell suspension cultures of Taxus wallichiana. Planta Med. 2000, 66, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Fornalè, S.; Esposti, D.D.; Navia-Osorio, A.; Cusidò, R.M.; Palazòn, J.; Teresa Piñol, M.; Bagni, N. Taxol transport in Taxus baccata cell suspension cultures. Plant Physiol. Biochem. 2002, 40, 81–88. [Google Scholar] [CrossRef]
- Christen, A.A.; Bland, J.; Gibson, D.M. Cell cultures as a means to produce taxol. Proc. Am. Assoc. Cancer Res. 1989, 30, 566. [Google Scholar]
- Kim, B.J.; Gibson, D.M.; Shuler, M.L. Relationship of viability and apoptosis to taxol production in Taxus sp. suspension cultures elicited with methyl jasmonate. Biotechnol. Prog. 2005, 21, 700–707. [Google Scholar] [CrossRef]
- Gai, Q.-Y.; Jiao, J.; Wang, X.; Liu, J.; Fu, Y.-J.; Lu, Y.; Wang, Z.-Y.; Xu, X.-J. Simultaneous determination of taxoids and flavonoids in twigs and leaves of three Taxus species by UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2020, 189, 113456. [Google Scholar] [CrossRef]
- Wilson, A.E.; Tian, L. Phylogenomic analysis of UDP-dependent glycosyltransferases provides insights into the evolutionary landscape of glycosylation in plant metabolism. Plant J. 2019, 100, 1273–1288. [Google Scholar] [CrossRef]
- Mei, M.; Xie, D.; Zhang, Y.; Jin, J.; You, F.; Li, Y.; Dai, J.; Chen, X. A New 2α,5α,10β,14β-tetraacetoxy-4(20),11-taxadiene (SIA) Derivative Overcomes Paclitaxel Resistance by Inhibiting MAPK Signaling and Increasing Paclitaxel Accumulation in Breast Cancer Cells. PLoS ONE 2014, 9, e104317. [Google Scholar] [CrossRef]
- Liu, X.; Chen, R.; Xie, D.; Mei, M.; Zou, J.; Chen, X.; Dai, J. Microbial transformations of taxadienes and the multi-drug resistant tumor reversal activities of the metabolites. Tetrahedron 2012, 68, 9539–9549. [Google Scholar] [CrossRef]
- Hayakawa, K.; Itoh, T.; Niwa, H.; Mutoh, T.; Sobue, G. NGF prevention of neurotoxicity induced by cisplatin, vincristine and taxol depends on toxicity of each drug and NGF treatment schedule: In vitro study of adult rat sympathetic ganglion explants. Brain Res. 1998, 794, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Globa, E.B.; Demidova, E.V.; Turkin, V.V.; Makarova, S.S.; Nosov, A.M. Callus and suspension cell culture induction of four yew species: Taxus canadensis, T. baccata, T. cuspidata and T. media. Biotechnology 2009, 3, 54–59. (In Russian) [Google Scholar]
- Globa, E.B.; Demidova, E.V.; Gaysinskiy, V.V.; Kochkin, D.V. Obtainment and characterization of callus and suspension cell culture of Taxus wallichiana Zucc. Vestn. Severo-Vostochn. Fed. Univ. M.K. Ammosova. 2018, 2, 18–25. (In Russian) [Google Scholar] [CrossRef]
- Nosov, A.M. Methods of evaluation and characterization of growth of cell cultures of higher plants. In Molecular-Genetic and Biochemical Methods in Modern Plant Biology; Kuznetsov, V.V., Ed.; BIONOM: Moscow, Russia, 2011; pp. 386–403. (In Russian) [Google Scholar]
- Demidova, E.V.; Reshetnyak, O.V.; Oreshnikov, A.V.; Nosov, A.M. Growth and biosynthetic characteristics of ginseng (Panax japonicus var. repens) deep-tank cell culture in bioreactors. Rus. J. Plant Physiol. 2006, 53, 134–140. [Google Scholar] [CrossRef]
- Povydysh, M.N.; Titova, M.V.; Ivanov, I.M.; Klushin, A.G.; Kochkin, D.V.; Galishev, B.A.; Popova, E.V.; Ivkin, D.Y.; Luzhanin, V.G.; Krasnova, M.V.; et al. Effect of Phytopreparations Based on Bioreactor-Grown Cell Biomass of Dioscorea deltoidea, Tribulus terrestris and Panax japonicus on Carbohydrate and Lipid Metabolism in Type 2 Diabetes Mellitus. Nutrients 2021, 13, 3811. [Google Scholar] [CrossRef]
- Belyakov, P.A.; Kadentsev, V.I.; Chizhov, A.O.; Kolotyrkina, N.y.G.; Shashkov, A.S.; Ananikov, V.P. Mechanistic insight into organic and catalytic reactions by joint studies using mass spectrometry and NMR spectroscopy. Mend. Commun. 2010, 20, 125–131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
