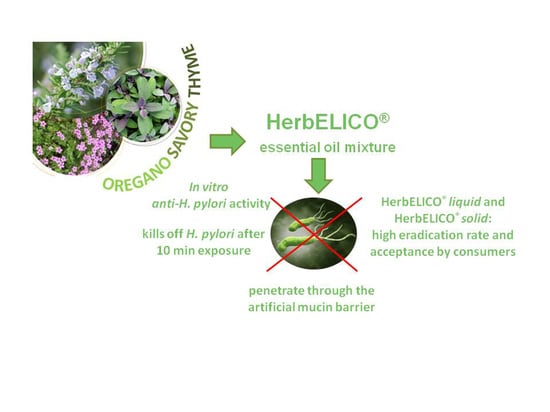
Savory, Oregano and Thyme Essential Oil Mixture (HerbELICO®) Counteracts Helicobacter pylori
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of HerbELICO Essential Oil Mixture
2.2. Determination of the MIC of HerbELICO® Essential Oil Mixture
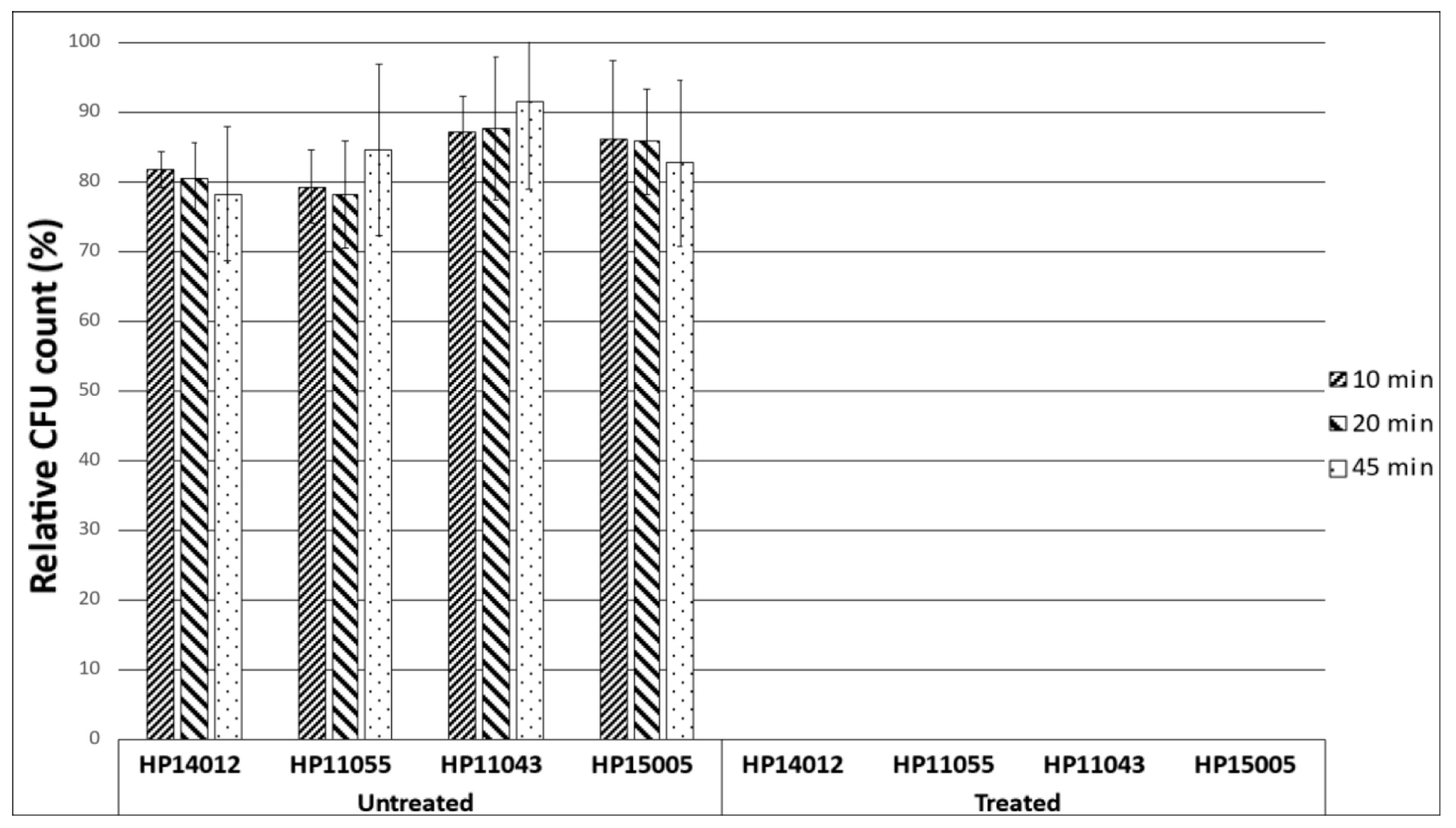
2.3. Time-Kill Assay
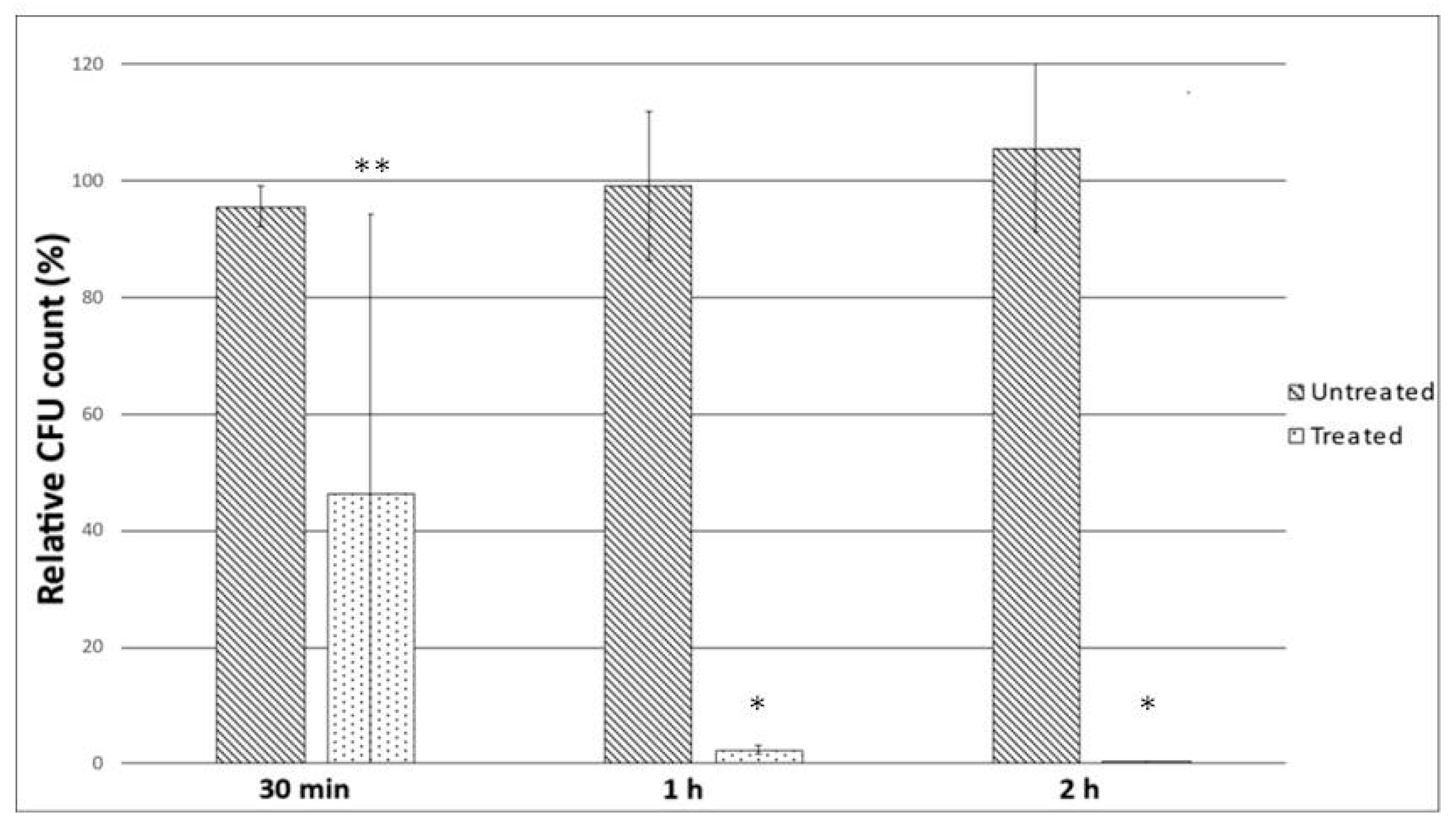
2.4. Mucin Penetration Assay
2.5. Customer Case Study
3. Discussion
4. Materials and Methods
4.1. HerbELICO® Essential Oil Mixture and Pharmaceutical Dosage Forms
4.2. GC-MS Analyses
4.3. Bacterial Strains and Culturing
4.4. Antimicrobial Medicinal Products Susceptibility Testing
4.5. Determination of the MIC of HerbELICO® Essential Oil Mixture
4.6. Time-Kill Assay
4.7. Mucin Penetration Assay
4.8. Consumer Case Study
4.8.1. Participants
4.8.2. Procedure
4.9. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Salih, B.A. Helicobacter pylori infection in developing countries: The burden for how long? Saudi J. Gastroenterol. 2009, 15, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Ghotaslou, R.; Leylabadlo, H.E.; Asl, Y.M. Prevalence of antibiotic resistance in Helicobacter pylori: A recent literature review. World J. Methodol. 2015, 5, 164–174. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). The Selection and Use of Essential Medicines: Report of the WHO Expert Committee, March 2011 (Including the 17th WHO Model List of Essential Medicines and the 3rd WHO Model List of Essential Medicines for Children); WHO Technical Report Series no. 965; WHO Press; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Ayala, G.; Escobedo-Hinojosa, W.I.; de la Cruz-Herrera, C.F.; Romero, I. Exploring alternative treatment for Helicobacter pylori infection. Word J. Gastroenterol. 2014, 20, 1450–1469. [Google Scholar] [CrossRef] [PubMed]
- Kanamaru, T.; Nakano, Y.; Toyoda, Y.; Miyagawa, K.I.; Tada, M.; Kaisho, T.; Nakao, M. in vitro and in vivo antibacterial activities of TAK-083, an agent for treatment of Helicobacter pylori infection. Antimicrob. Agents Chemother. 2001, 45, 2455–2459. [Google Scholar] [CrossRef] [PubMed]
- Iwahori, A.; Hirota, Y.; Sampe, R.; Miyano, S.; Takahashi, N.; Sasatsu, M.; Kondo, I.; Numao, N. On the antibacterial activity of normal and reversed magainin 2 analogs against Helicobacter pylori. Biol. Pharm. Bull. 1997, 20, 805–808. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, J.; Xu, X.; Lai, R.; Zou, Q. An antimicrobial peptide with antimicrobial activity against Helicobacter pylori. Peptides 2007, 28, 1527–1531. [Google Scholar] [CrossRef]
- Loke, M.F.; Lui, S.Y.; Ng, B.L.; Gong, M.; Ho, B. Antiadhesive property of microalgal polysaccharide extract on the binding of Helicobacter pylori to gastric mucin. FEMS Immunol. Med. Microbiol. 2007, 50, 231–238. [Google Scholar] [CrossRef]
- Lembo, A.J.; Ganz, R.A.; Sheth, S.; Cave, D.; Kelly, C.; Levin, P.; Kazlas, P.T.; Baldwin, P.C.; Lindmark, W.R.; McGrath, J.R.; et al. Treatment of Helicobacter pylori infection with intra-gastric violet light phototherapy: A pilot clinical trial. Lasers Surg. Med. 2009, 41, 337–344. [Google Scholar] [CrossRef]
- Cogo, L.L.; Monteiro, C.L.B.; Miguel, M.D.; Miguel, O.G.; Cunico, M.M.; Ribeiro, M.L.; Camargo, E.R.; Kussen, G.M.B.; Nogueira, K.D.S.; Costa, L.M.D. Anti-Helicobacter pylori activity of plant extracts traditionally used for the treatment of gastrointestinal disorders. Braz. J. Microbiol. 2010, 41, 304–309. [Google Scholar] [CrossRef]
- Baker, D.A. Plants against Helicobacter pylori to combat resistance: An ethnopharmacological review. Biotechnol. Rep. 2020, 26, e00470. [Google Scholar] [CrossRef]
- Ohno, T.; Kita, M.; Yamaoka, Y.; Imamura, S.; Yamamoto, T.; Mitsufuji, S.; Kodama, T.; Kashima, K.; Imanishi, J. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter 2003, 8, 207–215. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Takeuchi, H.; Trang, V.T.; Morimoto, N.; Nishida, Y.; Matsumura, Y.; Sugiura, T. Natural products and food components with anti-Helicobacter pylori activities. World J. Gastroenterol. 2014, 20, 8971–8978. [Google Scholar]
- Harmati, M.; Gyukity-Sebestyen, E.; Dobra, G.; Terhes, G.; Urban, E.; Decsi, G.; Mimica-Dukić, N.; Lesjak, M.; Simin, N.; Pap, B.; et al. Binary mixture of Satureja hortensis and Origanum vulgare subsp. hirtum essential oils: In vivo therapeutic efficiency against Helicobacter pylori infection. Helicobacter 2017, 22, e12350. [Google Scholar]
- Bergonzelli, G.E.; Donnicola, D.; Porta, N.; Corthésy-Theulaz, I.E. Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob. Agents Chemother. 2003, 47, 3240–3246. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, D.; Mobarez, A.M.; Tohidpour, A. Anti-Helicobacter pylori activities of shoya powder and essential oils of Thymus vulgaris and Eucalyptus globulus. Open Microbiol. J. 2012, 6, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, L.; Di Stefano, A.; Cacciatore, I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018, 17, 903–921. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Moreno, Y.; Barat, J.M. In vitro antimicrobial activity of immobilised essential oil components against Helicobacter pylori. World J. Microbiol. Biotechnol. 2020, 36, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Korona-Glowniak, I.; Glowniak-Lipa, A.; Ludwiczuk, A.; Baj, T.; Malm, A. The in vitro activity of essential oils against Helicobacter pylori growth and urease activity. Molecules 2020, 25, 586. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Simin, N.; Orcic, D.; Francisković, M.; Knezević, P.; Beara, I.; Aleksić, V.; Svirčev, E.; Buzas, K.; Mimica-Dukić, N. Binary and tertiary mixtures of Satureja hortensis and Origanum vulgare essential oils as potent antimicrobial agents against Helicobacter pylori. Phytother Res. 2016, 30, 476–484. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration Web Site. Generally Recognized as Safe. Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 30 March 2022).
- U.S. Food and Drug Administration Web Site. Substances Generally Recognised as Safe. Code of Federal Regulations 21 CFR 182.20. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=182.20 (accessed on 30 March 2022).
- Türkmenoğlu, A.; Özmen, D. Allergenic components, biocides, and analysis techniques of some essential oils used in food products. J. Food Sci. 2021, 86, 2225–2241. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Business Media: Carol Stream, IL, USA, 2012. [Google Scholar]
- Preuss, H.G.; Echard, B.; Enig, M.; Brook, I.; Elliott, T.B. Minimum inhibitory concentrations of herbal essential oils and monolaurin for gram-positive and gram-negative bacteria. Mol. Cell. Biochem. 2005, 272, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Alternat. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; van der Zee, R.; Koets, A.P.; de Graaff, A.M.; van Knapen, F.; Gaastra, W.; Haagsman, H.P.; Veldhuizen, E.J. Carvacrol induces heat shock protein 60 and inhibits synthesis of flagellin in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007, 73, 4484–4490. [Google Scholar] [CrossRef] [PubMed]
- Coudron, P.E.; Stratton, C.W. Use of time-kill methodology to assess antimicrobial combinations against metronidazole-susceptible and metronidazole-resistant strains of Helicobacter pylori. Antimicrob. Agents Chemother. 1995, 39, 2641–2644. [Google Scholar] [CrossRef] [PubMed]
- Sivam, G.P.; Lampe, J.W.; Ulness, B.; Swanzy, S.R.; Potter, J.D. Helicobacter pylori—in vitro susceptibility to garlic (Allium sativum) extract. Nutr. Cancer 1997, 27, 118–121. [Google Scholar] [CrossRef]
- Al Somal, N.; Coley, K.E.; Molan, P.C.; Hancock, B.M. Susceptibility of Helicobacter pylori to the antibacterial activity of manuka honey. J. R. Soc. Med. 1994, 87, 9–12. [Google Scholar] [CrossRef]
- Byrd, J.C.; Bresalier, R.S. Alterations in gastric mucin synthesis by Helicobacter pylori. World J. Gastroenterol. 2000, 6, 475–482. [Google Scholar]
- Celli, J.P.; Turner, B.S.; Afdhal, N.H.; Keates, S.; Ghiran, I.; Kelly, C.P.; Ewoldt, R.H.; McKinley, G.H.; So, P.; Erramilli, S.; et al. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc. Natl. Acad. Sci. USA 2009, 106, 14321–14326. [Google Scholar] [CrossRef] [PubMed]
- Mera, R.; Fontham, E.T.; Bravo, L.E.; Bravo, J.C.; Piazuelo, M.B.; Camargo, M.C.; Correa, P. Long term follow up of patients treated for Helicobacter pylori infection. Gut 2005, 54, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
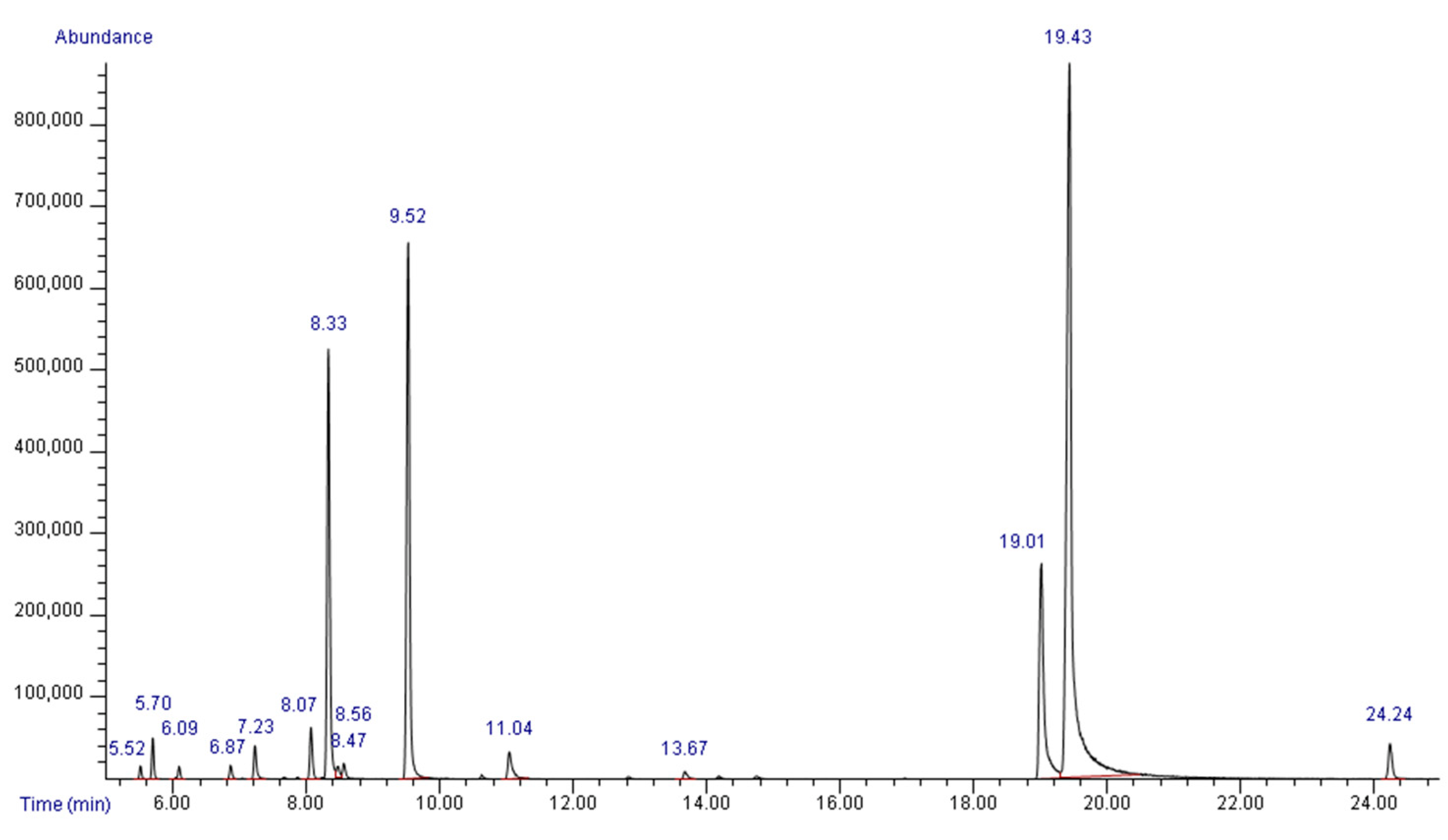
| AIexp | AIlit | Compound | % of Total Peak Area |
|---|---|---|---|
| 925 | 924 | α-Thujene | 0.32 |
| 932 | 932 | α-Pinene | 0.99 |
| 947 | 946 | Camphene | 0.37 |
| 976 | 974 | β-Pinene | 0.36 |
| 990 | 988 | β-Myrcene | 0.96 |
| 1005 | 1002 | α-Phellandrene | 0.05 |
| 1016 | 1014 | α-Terpinen | 1.51 |
| 1023 | 1020 | p-Cymene | 13.35 |
| 1027 | 1024 | Limonene | 0.42 |
| 1030 | 1026 | 1,8-Cineole | 0.52 |
| 1057 | 1054 | γ-terpinene | 18.20 |
| 1088 | 1086 | α-Terpinolene | 0.13 |
| 1100 | 1095 | Linalool | 1.45 |
| 1141 | 1141 | Camphor | 0.10 |
| 1164 | 1165 | Borneol | 0.35 |
| 1174 | 1174 | Terpinen-4-ol | 0.12 |
| 1186 | 1186 | α-Terpineol | 0.19 |
| 1292 | 1289 | Thymol | 11.62 |
| 1302 | 1298 | Carvacrol | 47.44 |
| 1418 | 1417 | β-Caryophyllene | 1.56 |
| Strain | Patient Origin | MIC (mg/L) | |||||
|---|---|---|---|---|---|---|---|
| MTZ | AMX | CIP | TET | CLA | RIF | ||
| HP08058 | Sudan | 256 † | 0.016 | 32 † | 0.023 | 8 † | 1.5 † |
| HP11043 | Afghanistan | 256 † | 0.016 | 0.064 | 0.125 | 8 † | 32 † |
| HP11049 | Sudan | 32 † | 0.016 | 0.047 | 0.016 | 12 † | 0.38 |
| HP11055 | Vietnam | 256 † | 0.016 | 0.125 | 0.023 | 256 † | 0.25 |
| HP13050 | Sudan | 0.25 | 0.016 | 0.094 | 0.016 | 0.016 | 0.38 |
| HP13064 | Iran | 256 † | 0.016 | 0.5 | 0.016 | 256 † | 2 † |
| HP14012 | Vietnam | 0.064 | 0.016 | 32 † | 0.125 | 256 † | 2 † |
| HP15005 | Eritrea | 256 † | 0.016 | 0.047 | 0.016 | 0.016 | 0.38 |
| HP15026 | Afghanistan | 256 † | 0.016 | 32 † | 0.016 | 8 † | 0.38 |
| HP15035 | Iran | 256 † | 0.19 † | 0.032 | 0.5 | 1 † | 0.125 |
| HP15065 | India | 256 † | 0.016 | 32 † | 0.016 | 256 † | 0.25 |
| HP16024 | Vietnam | 0.75 | 0.125 | 32 † | 0.047 | 256 † | 1.5 † |
| HP16033 | Bangladesh | 256 † | 0.125 | 0.016 | 0.016 | 32 † | 0.094 |
| HP16035 | Vietnam | 256 † | 0.016 | 0.008 | 0.032 | 2 † | 0.38 |
| HP16037 | India | 0.38 | 0.016 | 3 † | 0.064 | 0.032 | 1 |
| HP16046 | Vietnam | 0.25 | 0.125 | 0.032 | 0.38 | 256 † | 0.25 |
| HP16051 | Vietnam | 256 † | 0.016 | 0.032 | 0.032 | 32 † | 3 † |
| HP17023 | Lebanon | 0.25 | 0.047 | 0.047 | 0.047 | 0.094 | 2 |
| HP17026 | Eritrea | 256 † | 0.016 | 0.032 | 0.016 | 256 † | 0.25 |
| HP17029 | Mauritius | 0.5 | 0.016 | 0.032 | 0.016 | 0.016 | 0.5 |
| Strain | HerbELICO® Essential Oil Mixture | |
|---|---|---|
| MIC (v/v %) | MIC (g/L) | |
| HP08058 | 4 | 36.6 |
| HP11043 | 5 | 45.7 |
| HP11049 | 4 | 36.6 |
| HP11055 | 5 | 45.7 |
| HP13050 | 4 | 36.6 |
| HP13064 | 5 | 45.7 |
| HP14012 | 5 | 45.7 |
| HP15005 | 4 | 36.6 |
| HP15026 | 4 | 36.6 |
| HP15035 | 5 | 45.7 |
| HP15065 | 5 | 45.7 |
| HP16024 | 5 | 45.7 |
| HP16033 | 5 | 45.7 |
| HP16035 | 5 | 45.7 |
| HP16037 | 5 | 45.7 |
| HP16046 | 5 | 45.7 |
| HP16051 | 4 | 36.6 |
| HP17023 | 4 | 36.6 |
| HP17026 | 5 | 45.7 |
| HP17029 | 5 | 45.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, I.; Chua, E.G.; Tay, A.C.Y.; Kostrešević, A.; Pavlović, B.; Jončić Savić, K. Savory, Oregano and Thyme Essential Oil Mixture (HerbELICO®) Counteracts Helicobacter pylori. Molecules 2023, 28, 2138. https://doi.org/10.3390/molecules28052138
Nikolić I, Chua EG, Tay ACY, Kostrešević A, Pavlović B, Jončić Savić K. Savory, Oregano and Thyme Essential Oil Mixture (HerbELICO®) Counteracts Helicobacter pylori. Molecules. 2023; 28(5):2138. https://doi.org/10.3390/molecules28052138
Chicago/Turabian StyleNikolić, Ivan, Eng Guan Chua, Alfred Chin Yen Tay, Aleksandra Kostrešević, Bojan Pavlović, and Katarina Jončić Savić. 2023. "Savory, Oregano and Thyme Essential Oil Mixture (HerbELICO®) Counteracts Helicobacter pylori" Molecules 28, no. 5: 2138. https://doi.org/10.3390/molecules28052138
APA StyleNikolić, I., Chua, E. G., Tay, A. C. Y., Kostrešević, A., Pavlović, B., & Jončić Savić, K. (2023). Savory, Oregano and Thyme Essential Oil Mixture (HerbELICO®) Counteracts Helicobacter pylori. Molecules, 28(5), 2138. https://doi.org/10.3390/molecules28052138