Characterization of Ginsenosides from the Root of Panax ginseng by Integrating Untargeted Metabolites Using UPLC-Triple TOF-MS
Abstract
1. Introduction
2. Results and Discussion
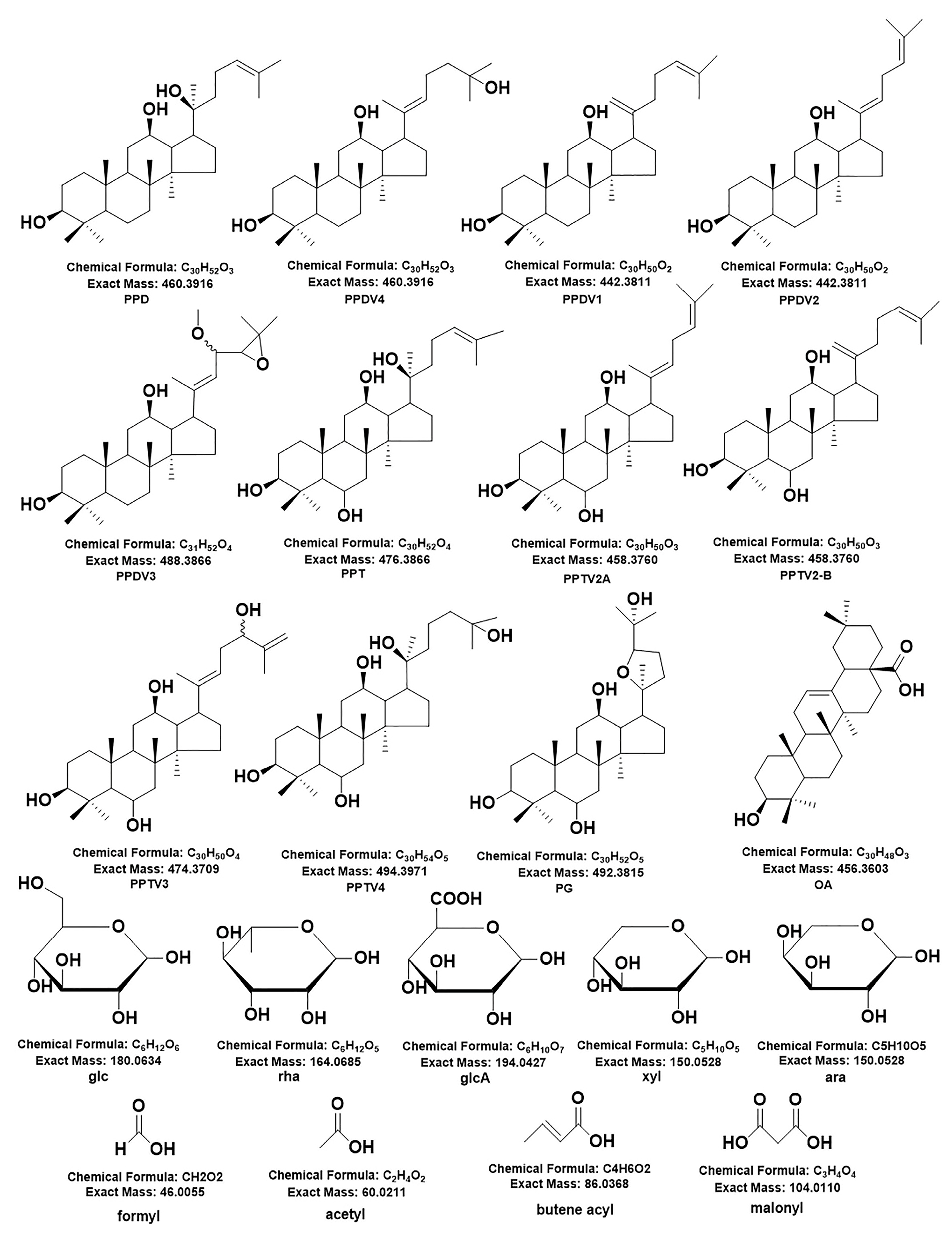
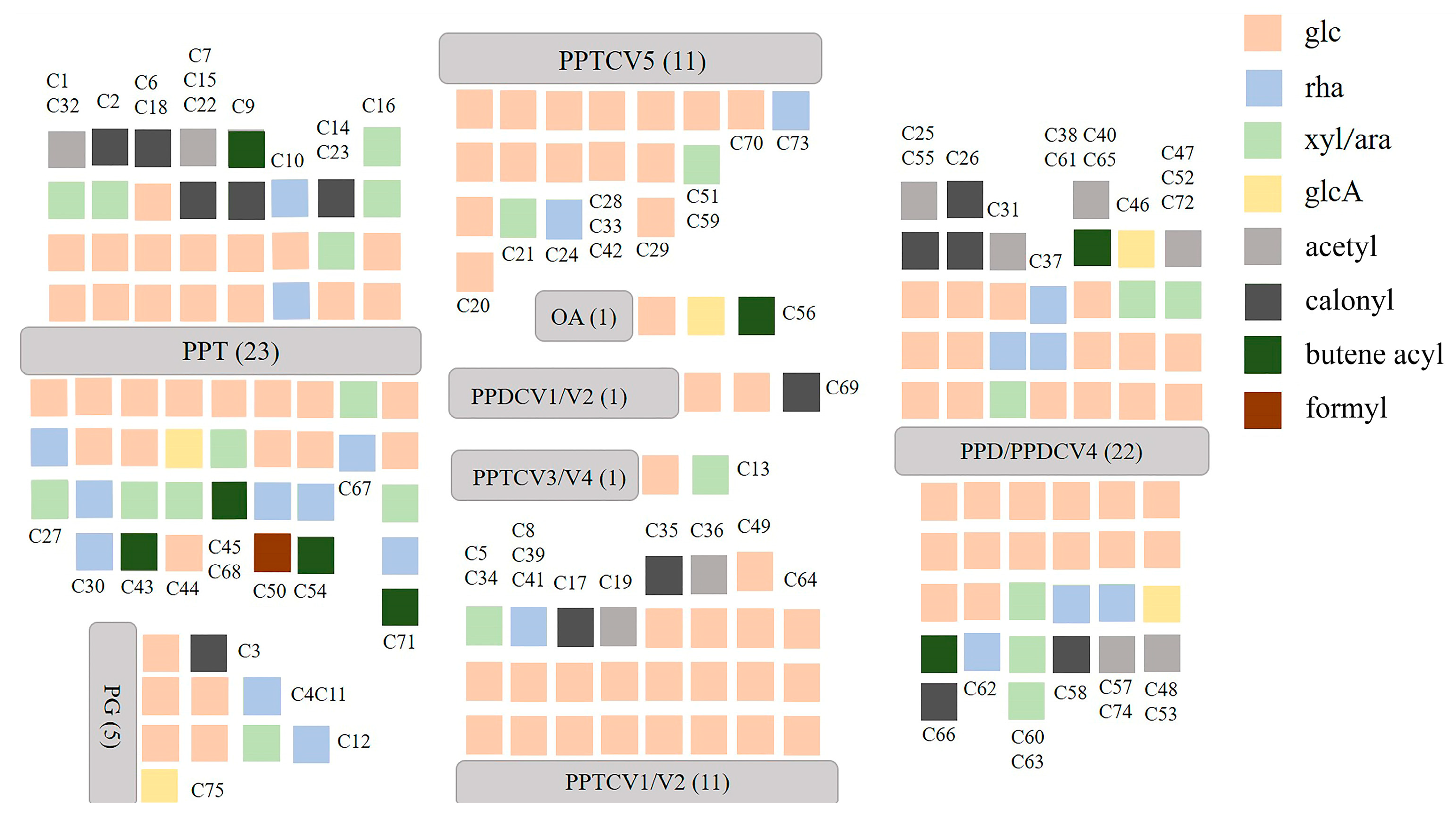
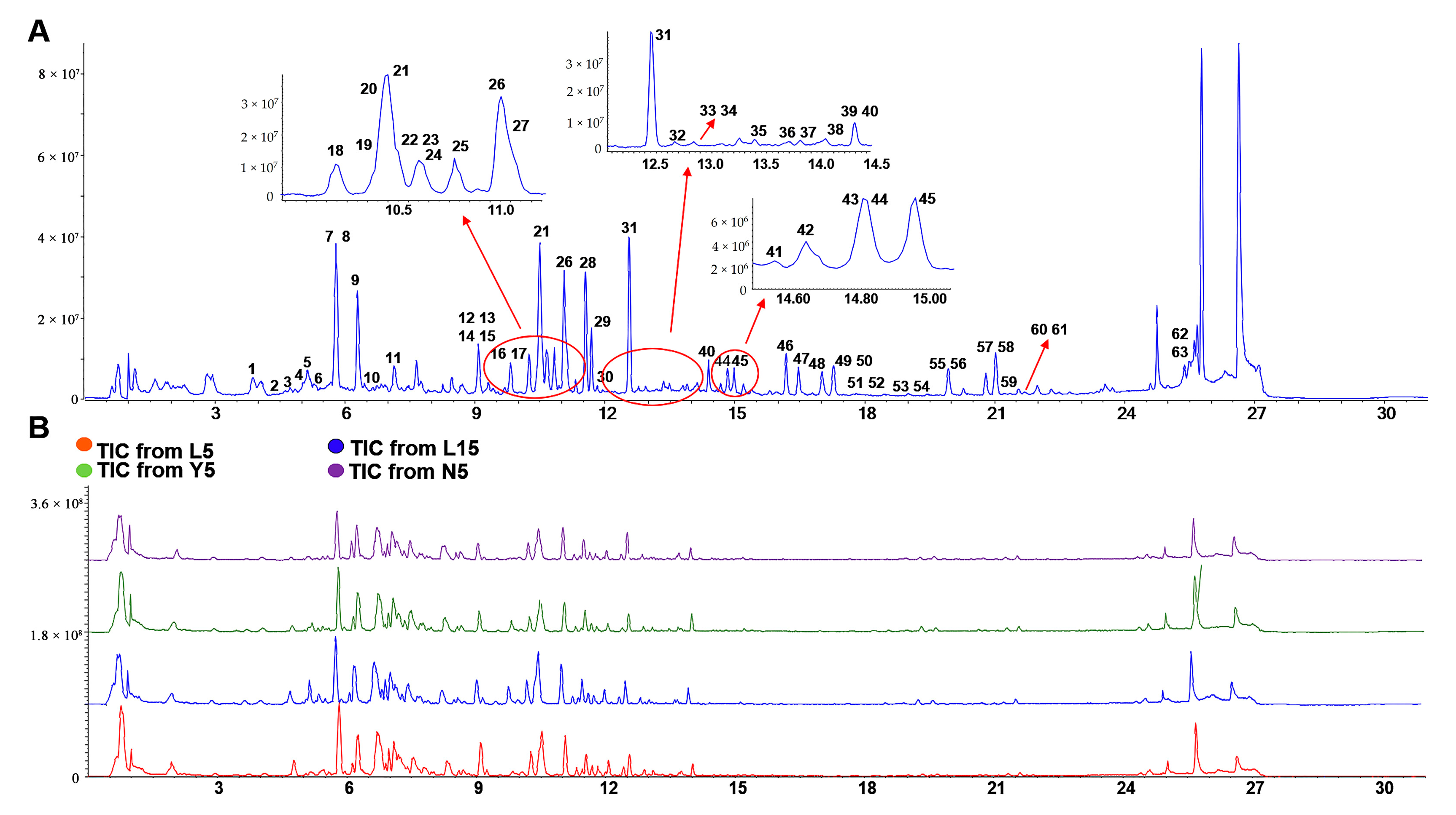
2.1. Analysis of the Structure of Ginsenosides
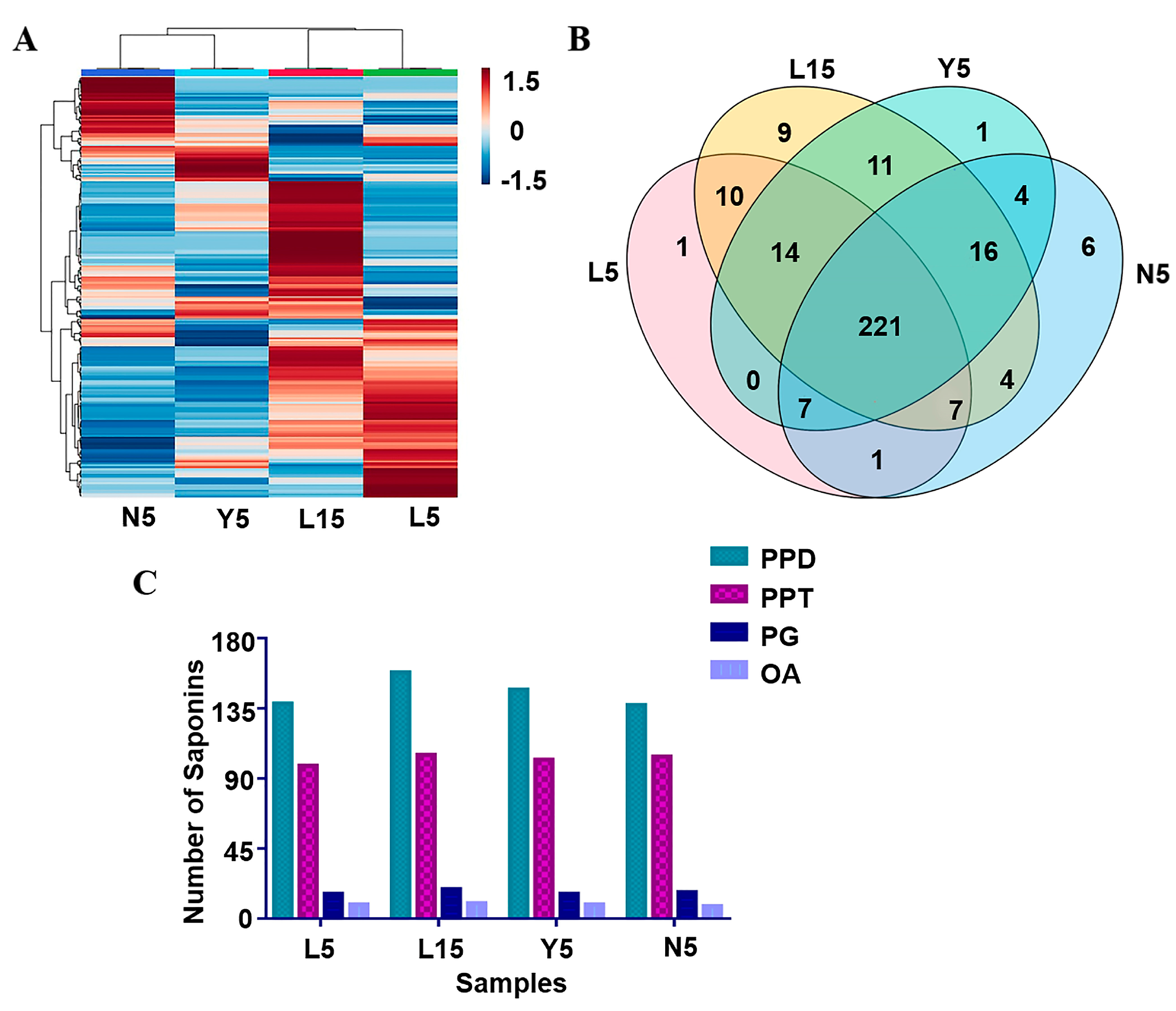
2.2. Multivariate Statistical Analysis of Samples
2.3. Discussion
3. Experimental
3.1. Materials
3.2. Standard Samples, Chemicals, and Reagents
3.3. Subsubsection Instruments Chromatographic Conditions and Parameters
3.4. Preparation of Samples and References Standards
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Isolation and analysis of ginseng: Advances and challenges. Nat. Prod. Rep. 2011, 28, 467–495. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Balan, P.; Popovich, D.G. Ginsenosides analysis of New Zealand-grown Forest Panax ginseng by LC-QTOF-MS/MS. J. Ginseng Res. 2020, 44, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.L.; Zhu, D.N.; Yang, X.W.; Xu, W.; Wang, Y.P. Development and validation of a UFLC-MS/MS method for simultaneous quantification of sixty-six saponins and their six aglycones: Application to comparative analysis of red ginseng and white ginseng. J. Pharm. Biomed. Anal. 2018, 159, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, D.; Pantuso, T. Panax ginseng. Am. Fam. Physician 2003, 68, 1539–1542. [Google Scholar] [PubMed]
- Shergis, J.L.; Zhang, A.L.; Zhou, W.; Xue, C.C. Panax ginseng in randomised controlled trials: A systematic review. Phytother. Res. 2013, 27, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, Y.; Yin, G.; Wang, J.; Wang, P.; Chen, Z.Y.; Wang, T.; Ren, G. Antimicrobial activities of Asian ginseng, American ginseng, and notoginseng. Phytother. Res. 2020, 34, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Arring, N.M.; Millstine, D.; Marks, L.A.; Nail, L.M. Ginseng as a Treatment for Fatigue: A Systematic Review. J. Altern. Complement Med. 2018, 24, 624–633. [Google Scholar] [CrossRef]
- Lorz, L.R.; Kim, M.Y.; Cho, J.Y. Medicinal potential of Panax ginseng and its ginsenosides in atopic dermatitis treatment. J. Ginseng Res. 2020, 44, 8–13. [Google Scholar] [CrossRef]
- Yun, M.; Yi, Y.S. Regulatory roles of ginseng on inflammatory caspases, executioners of inflammasome activation. J. Ginseng Res. 2020, 44, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.T. Advances in Ginsenosides. Biomolecules 2020, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.L.; He, Y.F.; Li, L.L.; Liu, S.Y. Fast quantitative analysis of ginsenosides in Asian ginseng (Panax ginseng C. A. Mayer) by using solid-phase methylation coupled to direct analysis in real time. Rapid Commun. Mass Spectrom 2016, 1, 111–115. [Google Scholar] [CrossRef]
- Wong, Y.L.; Chen, X.; Li, W.; Wang, Z.; Hung, Y.L.; Wu, R.; Chan, T.W. Differentiation of Isomeric Ginsenosides by Using Electron-Induced Dissociation Mass Spectrometry. Anal. Chem. 2016, 88, 5590–5594. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Han, H.; Yuan, X.; Park, E.; Lee, J.; Kim, J.H. A rapid, simultaneous and quantitative analysis of 26 ginsenosides in white and red Panax ginseng using LC–MS/MS. Appl. Biol. Chem. 2021, 6, 13. [Google Scholar] [CrossRef]
- Wang, H.P.; Zhang, Y.B.; Yang, X.W.; Zhao, D.Q.; Wang, Y.P. Rapid characterization of ginsenosides in the roots and rhizomes of Panax ginseng by UPLC-DAD-QTOF-MS/MS and simultaneous determination of 19 ginsenosides by HPLC-ESI-MS. J. Ginseng Res. 2016, 40, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, Y.; Zhang, H.; Wang, X.; Cong, P.; Xu, J.; Xue, C. Comparative lipid profile of four edible shellfishes by UPLC-Triple TOF-MS/MS. Food Chem. 2020, 310, 125947. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luan, G.; Zhou, W.; Meng, J.; Wang, H.; Hu, N.; Suo, Y. Subcritical water extraction, UPLC-Triple-TOF/MS analysis and antioxidant activity of anthocyanins from Lycium ruthenicum Murr. Food Chem. 2018, 249, 119–126. [Google Scholar] [CrossRef]
- Peng, X.; Luo, Y.; Wang, J.; Ji, T.; Yuan, L.; Kai, G. Integrated analysis of the transcriptome, metabolome and analgesic effect provide insight into potential applications of different parts of Lindera aggregata. Food Res. Int. 2020, 138, 109799. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Shi, X.; Zhao, L.; Lv, T.; Yuan, Q.; Hao, W.; Zhu, J. Anti-proliferative and an-ti-migratory effects of Scutellaria strigillosa Hemsley extracts against vascular smooth muscle cells. J. Ethnopharmacol 2019, 235, 155–163. [Google Scholar] [CrossRef]
- Li, K.K.; Yao, C.M.; Yang, X.W. Four new dammarane-type triterpene saponins from the stems and leaves of Panax ginseng and their cytotoxicity on HL-60 cells. Planta Med. 2012, 78, 189–192. [Google Scholar] [CrossRef]
- Tava, A.; Mella, M.; Avato, P.; Biazzi, E.; Pecetti, L.; Bialy, Z.; Jurzysta, M. New triterpenic saponins from the aerial parts of Medicago arabica (L.) huds. J. Agric. Food Chem. 2009, 57, 2826–2835. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yang, M.; Lu, Z.; Zhang, J.; Huang, H.; Liang, Y.; Guan, S.; Song, Y.; Wu, L.; Guo, D.A. Microbial transformation of 20(S)-protopanaxatriol-type saponins by Absidia coerulea. J. Nat. Prod. 2007, 70, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Lee, A.Y.; Kim, K.T.; Cho, E.J.; Lee, S. Novel Dammarane-Type Triterpene Saponins from Panax ginseng Root. Chem. Pharm. Bull. 2015, 63, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Qin, Q.; Guo, Y.; Sun, J.; Liu, S. Studies on the chemical transformation of 20(S)-protopanaxatriol (PPT)-type ginsenosides R(e), R(g2), and R(f) using rapid resolution liquid chromatography coupled with quadruple-time-of-flight mass spectrometry (RRLC-Q-TOF-MS). J. Agric. Food Chem. 2012, 60, 10007–10014. [Google Scholar] [CrossRef]
- Vinh, L.B.; Lee, Y.; Han, Y.K.; Kang, J.S.; Park, J.U.; Kim, Y.R.; Yang, S.Y.; Kim, Y.H. Two new dammarane-type triterpene saponins from Korean red ginseng and their anti-inflammatory effects. Bioorg. Med. Chem. Lett. 2017, 27, 5149–5153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, L.F.; Sakah, K.J.; Wu, Z.Z.; Liu, L.L.; Agyemang, K.; Gao, X.M.; Wang, T. Bio-active protopanaxatriol type saponins isolated from the roots of Panax notoginseng (Burk.) F. H. Chen. Molecules 2013, 18, 10352–10366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.L.; Yang, X.W. Four new ginsenosides from red ginseng with inhibitory activity on melanogenesis in melanoma cells. Bioorg. Med. Chem. Lett. 2015, 25, 3112–3116. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Ye, M.; Qiao, X.; Liu, C.F.; Miao, W.J.; Bo, T.; Tao, H.Y.; Guo, D.A. A strategy for efficient discovery of new natural compounds by integrating orthogonal column chromatography and liquid chromatography/mass spectrometry analysis: Its application in Panax ginseng, Panax quinquefolium and Panax notoginseng to characterize 437 potential new ginsenosides. Anal. Chim. Acta 2012, 739, 56–66. [Google Scholar] [PubMed]
- Yoshizaki, K.; Devkota, H.P.; Yahara, S. Four new triterpenoid saponins from the leaves of Panax japonicus grown in southern Miyazaki Prefecture (4). Chem. Pharm. Bull. 2013, 61, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, Y.; Xu, L.; Qin, M.; Yi, T.; Chen, H.; Zhao, Z. Localization of ginsenosides in the rhizome and root of Panax ginseng by laser microdissection and liquid chromatography-quadrupole/time of flight-mass spectrometry. J. Pharm. Biomed. Anal. 2015, 105, 121–133. [Google Scholar] [CrossRef]
- Shi, J.; Cai, Z.; Chen, S.; Zou, L.; Liu, X.; Tang, R.; Ma, J.; Wang, C.; Chen, J.; Tan, M. Qualitative and quantitative analysis of saponins in the flower bud of Panax ginseng (Ginseng Flos) by UFLC-Triple TOF-MS/MS and UFLC-QTRAP-MS/MS. Phytochem. Anal. 2020, 31, 287–296. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Yahara, S. New triterpenoid saponins from fruits specimens of Panax japonicus collected in Kumamoto and Miyazaki prefectures (1). Chem. Pharm. Bull. 2012, 60, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Zhou, R.X.; Sun, C.K.; Jin, Y.H.; Yu, H.S.; Zhang, T.Y.; Xu, L.Q.; Jin, F.X. Preparation of minor ginsenosides C-Mc, C-Y, F2, and C-K from American ginseng PPD-ginsenoside using special ginsenosidase type-I from Aspergillus niger g.848. J. Ginseng Res. 2015, 39, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Xu, S.Y.; Zhang, Y.; Zhang, H.; Liu, M.N.; Liu, H.; Gao, Y.; Xue, X.; Xiong, H.; Lin, R.C.; et al. Chemical Comparison of Two Drying Methods of Mountain Cultivated Gin-seng by UPLC-QTOF-MS/MS and Multivariate Statistical Analysis. Molecules 2017, 22, 717. [Google Scholar] [CrossRef] [PubMed]
- Vu-Huynh, K.L.; Nguyen, H.T.; Van Le, T.H.; Ma, C.T.; Lee, G.J.; Kwon, S.W.; Park, J.H.; Nguyen, M.D. Accumulation of Saponins in Underground Parts of Panax vietnamensis at Different Ages Analyzed by HPLC-UV/ELSD. Molecules 2020, 25, 3086. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, S.; Li, C.J.; Ma, J.; Chen, F.Y.; Peng, Y.; Wang, X.L.; Zhang, D.M. Dammarane-type saponins from the leaves of Panax notoginseng and their neuroprotective effects on damaged SH-SY5Y cells. Phytochemistry 2018, 145, 10–17. [Google Scholar] [CrossRef]
- Wan, J.B.; Zhang, Q.W.; Hong, S.J.; Li, P.; Li, S.P.; Wang, Y.T. Chemical investigation of saponins in different parts of Panax notoginseng by pressurized liquid extraction and liquid chromatography-electrospray ionization-tandem mass spectrometry. Molecules 2012, 17, 5836–5853. [Google Scholar] [CrossRef]
- Zhang, C.X.; Wang, X.Y.; Lin, Z.Z.; Wang, H.D.; Qian, Y.X.; Li, W.W.; Yang, W.Z.; Guo, D.A. Highly selective monitoring of in-source fragmentation sapogenin product ions in positive mode enabling group-target ginsenosides profiling and simultaneous identification of seven Panax herbal medicines. J. Chromatogr. A 2020, 1618, 460850. [Google Scholar] [CrossRef]
- Liao, P.Y.; Wang, D.; Zhang, Y.J.; Yang, C.R. Dammarane-type glycosides from steamed notoginseng. J. Agric. Food Chem. 2008, 56, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Murakami, T.; Ueno, T.; Yashiro, K.; Hirokawa, N.; Murakami, N.; Yamahara, J.; Matsuda, H.; Saijoh, R.; Tanaka, O. Bioactive saponins and glycosides. VIII. Noto-ginseng (1): New dammarane-type triterpene oligoglycosides, notoginsenosides-A, -B, -C, and -D, from the dried root of Panax notoginseng (Burk.) F.H. Chen. Chem. Pharm. Bull. 1997, 45, 1039–1045. [Google Scholar] [CrossRef]
- Li, M.; Liu, F.; Jin, Y.R.; Wang, X.Z.; Wu, Q.; Liu, Y.; Li, X.W. Five New Triterpenoid Saponins from the Rhizomes of Panacis majoris and Their Antiplatelet Aggregation Activity. Planta Med. 2017, 83, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhu, H.; Tan, J.; Wang, H.; Dong, Q.; Wu, F.; Liu, Y.; Li, P.; Liu, J. Non-Targeted Metabolomic Analysis of Methanolic Extracts of Wild-Simulated and Field-Grown American Ginseng. Molecules 2019, 24, 1053. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Zhang, W.D.; Chen, H.S.; Gu, Z.B.; Li, T.Z.; Chen, W.S. New triterpenoid saponins from bulbs of Bolbostemma paniculatum. Planta Med. 2004, 70, 458–464. [Google Scholar] [PubMed]
- Le, T.H.; Lee, G.J.; Vu, H.K.; Kwon, S.W.; Nguyen, N.K.; Park, J.H.; Nguyen, M.D. Ginseng Saponins in Different Parts of Panax vietnamensis. Chem. Pharm. Bull. 2015, 63, 950–954. [Google Scholar] [CrossRef]
- Chen, L.H.; Zhang, Y.B.; Yang, X.W.; Xu, W.; Wang, Y.P. Characterization and quantification of ginsenosides from the root of Panax quinquefolius L. by integrating untargeted metabolites and targeted analysis using UPLC-Triple TOF-MS coupled with UFLC-ESI-MS/MS. Food Chem. 2022, 384, 132466. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Zhang, D.; Yang, D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar] [CrossRef]
- Jang, W.; Jang, Y.; Kim, N.H.; Waminal, N.E.; Kim, Y.C.; Lee, J.W.; Yang, T.J. Genetic diversity among cultivated and wild Panax ginseng populations revealed by high-resolution microsatellite markers. J. Ginseng Res. 2020, 44, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, C.Z.; Zhou, C.J.; Wang, B.; Zhang, C.F.; Wu, X.H.; Yuan, C.S. Adulteration and cultivation region identification of American ginseng using HPLC coupled with multivariate analysis. J. Pharm. Biomed. Anal. 2014, 99, 8–15. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Fu, X.; Qu, Y.; Chen, L.; Liu, X.; He, Z.; Xu, J.; Yang, J.; Ma, W.; Li, J.; et al. Characterization of Ginsenosides from the Root of Panax ginseng by Integrating Untargeted Metabolites Using UPLC-Triple TOF-MS. Molecules 2023, 28, 2068. https://doi.org/10.3390/molecules28052068
Sun Y, Fu X, Qu Y, Chen L, Liu X, He Z, Xu J, Yang J, Ma W, Li J, et al. Characterization of Ginsenosides from the Root of Panax ginseng by Integrating Untargeted Metabolites Using UPLC-Triple TOF-MS. Molecules. 2023; 28(5):2068. https://doi.org/10.3390/molecules28052068
Chicago/Turabian StyleSun, Yizheng, Xiaojie Fu, Ying Qu, Lihua Chen, Xiaoyan Liu, Zichao He, Jing Xu, Jiao Yang, Wen Ma, Jun Li, and et al. 2023. "Characterization of Ginsenosides from the Root of Panax ginseng by Integrating Untargeted Metabolites Using UPLC-Triple TOF-MS" Molecules 28, no. 5: 2068. https://doi.org/10.3390/molecules28052068
APA StyleSun, Y., Fu, X., Qu, Y., Chen, L., Liu, X., He, Z., Xu, J., Yang, J., Ma, W., Li, J., Guo, Q., & Zhang, Y. (2023). Characterization of Ginsenosides from the Root of Panax ginseng by Integrating Untargeted Metabolites Using UPLC-Triple TOF-MS. Molecules, 28(5), 2068. https://doi.org/10.3390/molecules28052068







