The Role of Natural and Semi-Synthetic Compounds in Ovarian Cancer: Updates on Mechanisms of Action, Current Trends and Perspectives
Abstract
1. Introduction
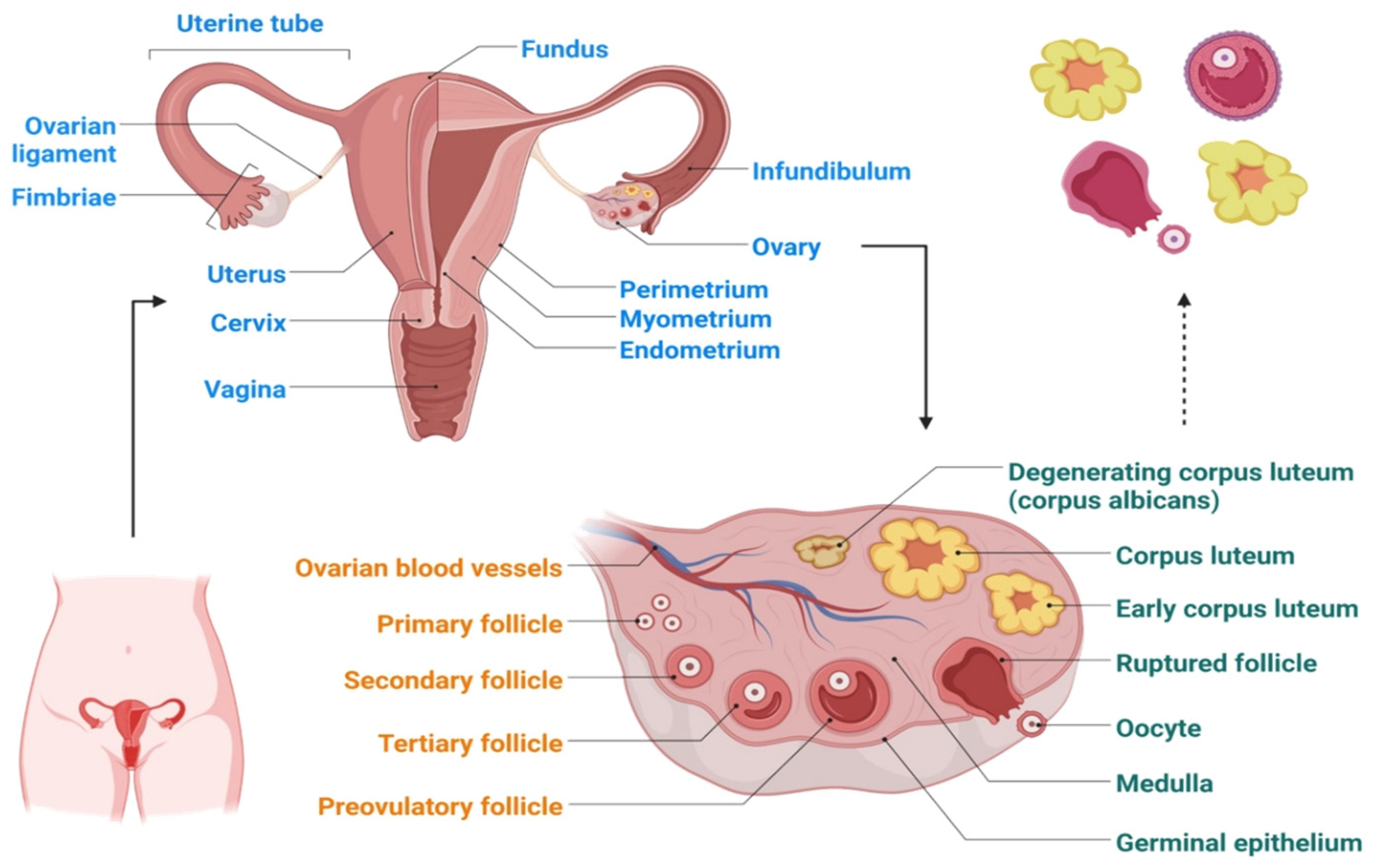
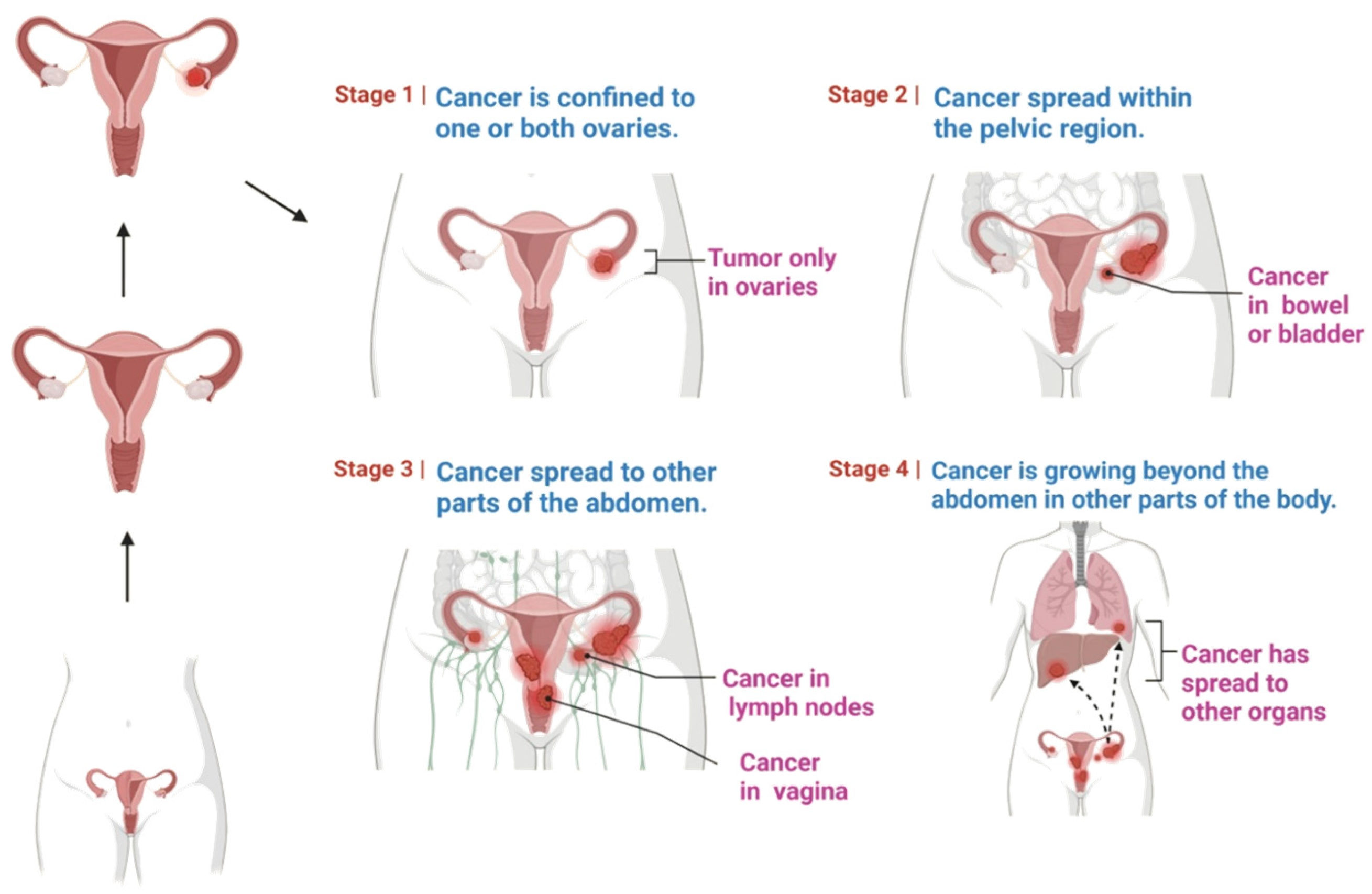
2. Etiology of Ovarian Cancer
3. Risk Factors for Ovarian Cancer
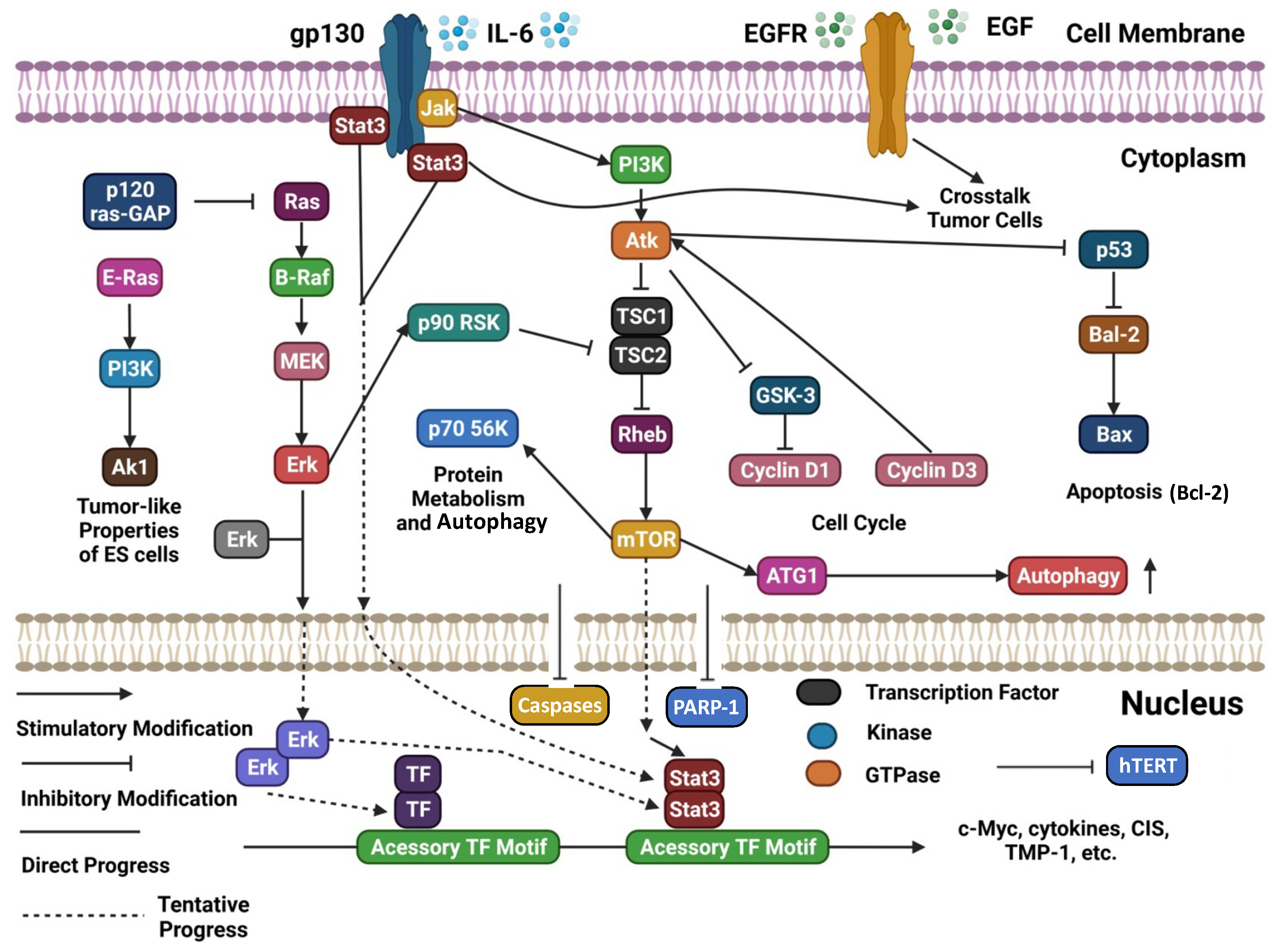
4. Ovarian Cancer Carcinogenesis and Progression: Molecular Mechanisms
5. Molecular Mechanisms Underlying Bioactivity of Natural Products
5.1. Compounds Inducing Apoptosis and Cytotoxicity and Inhibiting Proliferation
5.2. Interference with Reactive Oxygen Species (ROS) Damage and with Nucleic Acid Repair
5.3. Modulation of Inflammation
5.4. Suppression of Events Related to Disease Progression: Cell Migration and Angiogenesis
5.5. Regulation of Tumor Micro Environment
5.6. Other Mechanisms Related to Dysregulation of Cell Cycle
5.7. Natural Constituents Modulating Resistance to Chemotherapeutic Agents
6. A Focus on Selected Natural Compounds with Promising Activity against Ovarian Cancer
6.1. Curcumin
6.1.1. Antiproliferative and Proapoptotic Activity
6.1.2. Anti-Metastatic Activity
6.2. Resveratrol
6.2.1. Antiproliferative and Proapoptotic Activity
6.2.2. Anti-Metastatic Activity
6.3. Ginsenosides
6.3.1. Antiproliferative and Proapoptotic Activity
6.3.2. Anti-Metastatic Activity
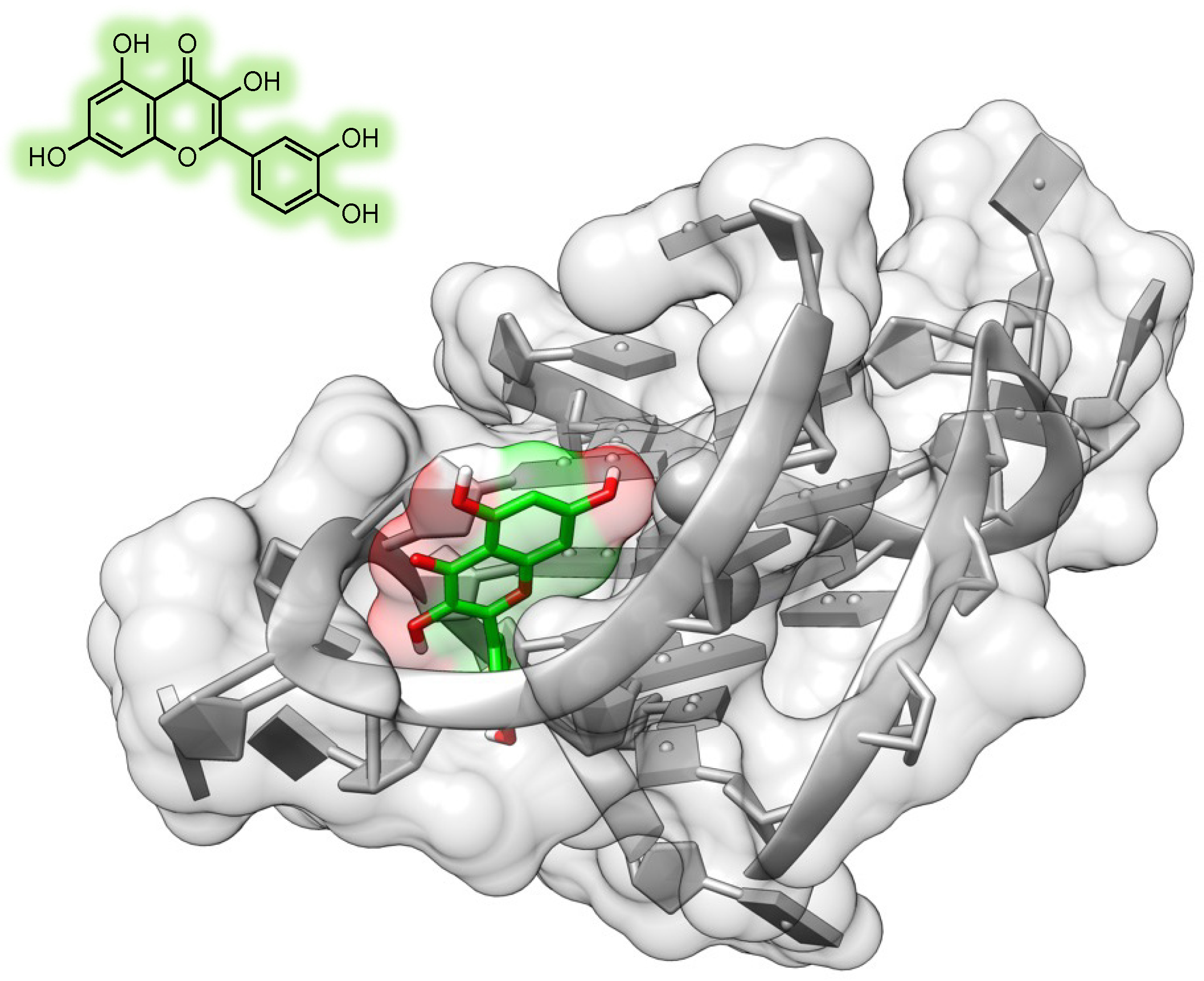
6.4. Quercetin
6.5. Semi-Synthetic Compounds
7. The Point of View of the Medicinal Chemist
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Duska, L.R.; Kohn, E.C. The New Classifications of Ovarian, Fallopian Tube, and Primary Peritoneal Cancer and Their Clinical Implications. Ann. Oncol. 2017, 28, viii8–viii12. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Dehghani, S.; Nosrati, R.; Ghanei, M.; Salmaninejad, A.; Rajaie, S.; Hasanzadeh, M.; Pasdar, A. Current Insights into the Metastasis of Epithelial Ovarian Cancer—Hopes and Hurdles. Cell. Oncol. 2020, 43, 515–538. [Google Scholar] [CrossRef] [PubMed]
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Rueger, R. Mechanisms and Targets Involved in Dissemination of Ovarian Cancer. Cancer Genom. Proteom. 2016, 13, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Scandurra, G.; Lombardo, V.; Gattuso, G.; Lavoro, A.; Distefano, A.B.; Scibilia, G.; Scollo, P. A Multidisciplinary Approach Remains the Best Strategy to Improve and Strengthen the Management of Ovarian Cancer (Review). Int. J. Oncol. 2021, 59, 53. [Google Scholar] [CrossRef]
- Zhang, S.F.; Wang, X.Y.; Fu, Z.Q.; Peng, Q.H.; Zhang, J.Y.; Ye, F.; Fu, Y.F.; Zhou, C.Y.; Lu, W.G.; Cheng, X.D.; et al. TXNDC17 Promotes Paclitaxel Resistance via Inducing Autophagy in Ovarian Cancer. Autophagy 2015, 11, 225–238. [Google Scholar] [CrossRef]
- Yang, M.-F.; Lou, Y.-L.; Liu, S.-S.; Wang, S.-S.; Yin, C.-H.; Cheng, X.-H.; Huang, O.-P. Capn4 Overexpression Indicates Poor Prognosis of Ovarian Cancer Patients. J. Cancer 2018, 9, 304–309. [Google Scholar] [CrossRef] [PubMed]
- De, A.; De, A.; Papasian, C.; Hentges, S.; Banerjee, S.; Haque, I.; Banerjee, S.K. Emblica Officinalis Extract Induces Autophagy and Inhibits Human Ovarian Cancer Cell Proliferation, Angiogenesis, Growth of Mouse Xenograft Tumors. PLoS ONE 2013, 8, e72748. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.-M. The Origin and Pathogenesis of Epithelial Ovarian Cancer-a Proposed Unifying Theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Ottevanger, P.B. Ovarian Cancer Stem Cells More Questions than Answers. Semin. Cancer Biol. 2017, 44, 67–71. [Google Scholar] [CrossRef]
- Akilli, H.; Rahatli, S.; Tohma, Y.A.; Karakas, L.A.; Altundag, O.; Ayhan, A. Effect of Increased Number of Neoadjuvant Chemotherapy Cycles on Tumor Resectability and Pathologic Response in Advanced Stage Epithelial Ovarian Cancer. J. BUON 2018, 23, 111–115. [Google Scholar]
- Ribaudo, G. Synthesis of Flavonoids or Other Nature-Inspired Small Molecules. Molbank 2022, 2022, M1313. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rajabi, S.; Martorell, M.; López, M.D.; Toro, M.T.; Barollo, S.; Armanini, D.; Fokou, P.V.T.; Zagotto, G.; Ribaudo, G.; et al. Plant Natural Products with Anti-Thyroid Cancer Activity. Fitoterapia 2020, 146, 104640. [Google Scholar] [CrossRef] [PubMed]
- Zorzan, M.; Collazuol, D.; Ribaudo, G.; Ongaro, A.; Scaroni, C.; Zagotto, G.; Armanini, D.; Barollo, S.; Galeotti, F.; Volpi, N.; et al. Biological Effects and Potential Mechanisms of Action of Pistacia Lentiscus Chios Mastic Extract in Caco-2 Cell Model. J. Funct. Foods 2019, 54, 92–97. [Google Scholar] [CrossRef]
- Povolo, C.; Foschini, A.; Ribaudo, G. Optimization of the Extraction of Bioactive Molecules from Lycium Barbarum Fruits and Evaluation of the Antioxidant Activity: A Combined Study. Nat. Prod. Res. 2018, 33, 2694–2698. [Google Scholar] [CrossRef]
- Dutta, R.; Khalil, R.; Green, R.; Mohapatra, S.S.; Mohapatra, S. Withania Somnifera (Ashwagandha) and Withaferin A: Potential in Integrative Oncology. Int. J. Mol. Sci. 2019, 20, 5310. [Google Scholar] [CrossRef]
- Mominur Rahman, M.; Islam, F.; Saidur Rahaman, M.; Sultana, N.A.; Fahim, N.F.; Ahmed, M. Studies on the Prevalence of HIV/AIDS in Bangladesh Including Other Developing Countries. Adv. Tradit. Med. 2021, 1–12. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Akash, S.; Shohag, S.; Ahmed, L.; Supti, F.A.; Rauf, A.; Aljohani, A.S.M.; Al Abdulmonem, W.; Khalil, A.A.; et al. Naphthoquinones and Derivatives as Potential Anticancer Agents: An Updated Review. Chem. Biol. Interact. 2022, 368, 110198. [Google Scholar] [CrossRef]
- Islam, M.R.; Akash, S.; Rahman, M.M.; Nowrin, F.T.; Akter, T.; Shohag, S.; Rauf, A.; Aljohani, A.S.M.; Simal-Gandara, J. Colon Cancer and Colorectal Cancer: Prevention and Treatment by Potential Natural Products. Chem. Biol. Interact. 2022, 368, 110170. [Google Scholar] [CrossRef]
- Rahman, M.M.; Wang, X.; Islam, M.R.; Akash, S.; Supti, F.A.; Mitu, M.I.; Harun-Or-Rashid, M.; Aktar, M.N.; Khatun Kali, M.S.; Jahan, F.I.; et al. Multifunctional Role of Natural Products for the Treatment of Parkinson’s Disease: At a Glance. Front. Pharmacol. 2022, 13, 4207. [Google Scholar] [CrossRef]
- Mukerjee, N.; Al-Khafaji, K.; Maitra, S.; Suhail Wadi, J.; Sachdeva, P.; Ghosh, A.; Buchade, R.S.; Chaudhari, S.Y.; Jadhav, S.B.; Das, P.; et al. Recognizing Novel Drugs against Keap1 in Alzheimer’s Disease Using Machine Learning Grounded Computational Studies. Front. Mol. Neurosci. 2022, 15, 638. [Google Scholar] [CrossRef]
- Semwal, P.; Painuli, S.; Anand, J.; Martins, N.C.; Machado, M.; Sharma, R.; Batiha, G.E.S.; Yaro, C.A.; Lorenzo, J.M.; Rahman, M.M. The Neuroprotective Potential of Endophytic Fungi and Proposed Molecular Mechanism: A Current Update. Evid. Based Complement. Altern. Med. 2022, 2022, 6214264. [Google Scholar] [CrossRef]
- Garg, S.; Singla, R.K.; Rahman, M.M.; Sharma, R.; Mittal, V. Evaluation of Ulcer Protective Activity of Morus alba L. Extract-Loaded Chitosan Microspheres in Ethanol-Induced Ulcer in Rat Model. Evid. Based Complement. Altern. Med. 2022, 2022, 4907585. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sarker, M.T.; Alam Tumpa, M.A.; Yamin, M.; Islam, T.; Park, M.N.; Islam, M.R.; Rauf, A.; Sharma, R.; Cavalu, S.; et al. Exploring the Recent Trends in Perturbing the Cellular Signaling Pathways in Cancer by Natural Products. Front. Pharmacol. 2022, 13, 3609. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Emran, T. Bin Impact of Nutrition in Brain Function and Development: Potential Brain Foods. Int. J. Surg. 2022, 106, 106908. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Akash, S.; Harun-Or-Rashid, M.; Ray, T.K.; Rahaman, M.S.; Islam, M.; Anika, F.; Hosain, M.K.; Aovi, F.I.; et al. Recent Advancements of Nanoparticles Application in Cancer and Neurodegenerative Disorders: At a Glance. Biomed. Pharmacother. 2022, 153, 113305. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Mim, S.A.; Sultana, N.; Chellappan, D.K.; Dua, K.; Kamal, M.A.; Sharma, R.; Emran, T. Bin Insights into the Promising Prospect of G Protein and GPCR-Mediated Signaling in Neuropathophysiology and Its Therapeutic Regulation. Oxid. Med. Cell. Longev. 2022, 2022, 8425640. [Google Scholar] [CrossRef] [PubMed]
- Soong, T.R.; Dinulescu, D.M.; Xian, W.; Crum, C.P. Frontiers in the Pathology and Pathogenesis of Ovarian Cancer: Cancer Precursors and “Precursor Escape”. Hematol. Oncol. Clin. N. Am. 2018, 32, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, J.; Gronwald, J.; McCuaig, J.M.; Karlan, B.Y.; Eisen, A.; Tung, N.; Bordeleau, L.; Senter, L.; Eng, C.; Couch, F.; et al. Breastfeeding and the Risk of Epithelial Ovarian Cancer among Women with a BRCA1 or BRCA2 Mutation. Gynecol. Oncol. 2020, 159, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.M.; Wang, Y.; Wang, T.L. The Origin of Ovarian Cancer Species and Precancerous Landscape. Am. J. Pathol. 2021, 191, 26–39. [Google Scholar] [CrossRef]
- Yang, D.H.; Smith, E.R.; Cohen, C.; Wu, H.; Patriotis, C.; Godwin, A.K.; Hamilton, T.C.; Xu, X.X. Molecular Events Associated with Dysplastic Morphologic Transformation and Initiation of Ovarian Tumorigenicity. Cancer 2002, 94, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Hasegawa, K.; Matsushita, H.; Fujieda, N.; Sato, S.; Miyagi, E.; Kakimi, K.; Fujiwara, K. Expression of Multiple Immune Checkpoint Molecules on t Cells in Malignant Ascites from Epithelial Ovarian Carcinoma. Oncol. Lett. 2018, 15, 6457–6468. [Google Scholar] [CrossRef] [PubMed]
- Crum, C.P.; Drapkin, R.; Kindelberger, D.; Medeiros, F.; Miron, A.; Lee, Y. Lessons from BRCA: The Tubal Fimbria Emerges as an Origin for Pelvic Serous Cancer. Clin. Med. Res. 2007, 5, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Roh, M.H.; Yassin, Y.; Miron, A.; Mehra, K.K.; Mehrad, M.; Monte, N.M.; Mutter, G.L.; Nucci, M.R.; Ning, G.; McKeon, F.D.; et al. High-Grade Fimbrial-Ovarian Carcinomas Are Unified by Altered P53, PTEN and PAX2 Expression. Mod. Pathol. 2010, 23, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Kindelberger, D.W.; Lee, Y.; Miron, A.; Hirsch, M.S.; Feltmate, C.; Medeiros, F.; Callahan, M.J.; Garner, E.O.; Gordon, R.W.; Birch, C.; et al. Intraepithelial Carcinoma of the Fimbria and Pelvic Serous Carcinoma: Evidence for a Causal Relationship. Am. J. Surg. Pathol. 2007, 31, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Chivukula, M.; Niemeier, L.A.; Edwards, R.; Nikiforova, M.; Mantha, G.; McManus, K.; Carter, G. Carcinomas of Distal Fallopian Tube and Their Association with Tubal Intraepithelial Carcinoma: Do They Share a Common “Precursor” Lesion? Loss of Heterozygosity and Immunohistochemical Analysis Using PAX 2, WT-1, and P53 Markers. ISRN Obstet. Gynecol. 2011, 2011, 858647. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.A.; Rouzbahman, M.; Pizer, E.S.; Pintilie, M.; Begley, H. Candidate Serous Cancer Precursors in Fallopian Tube Epithelium of BRCA1/2 Mutation Carriers. Mod. Pathol. 2009, 22, 1133–1138. [Google Scholar] [CrossRef]
- Hunn, J.; Rodriguez, G.C. Ovarian Cancer: Etiology, Risk Factors, and Epidemiology. Clin. Obstet. Gynecol. 2012, 55, 3–23. [Google Scholar] [CrossRef]
- Koh, S.C.L.; Chan, Y.H.; Lutan, D.; Marpuang, J.; Ketut, S.; Budiana, N.G.; Saleh, A.Z.; Aziz, M.F.; Winarto, H.; Pradjatmo, H.; et al. Combined Panel of Serum Human Tissue Kallikreins and CA-125 for the Detection of Epithelial Ovarian Cancer. J. Gynecol. Oncol. 2012, 23, 175–181. [Google Scholar] [CrossRef]
- Budiana, I.N.G.; Angelina, M.; Pemayun, T.G.A. Ovarian Cancer: Pathogenesis and Current Recommendations for Prophylactic Surgery. J. Turk. Ger. Gynecol. Assoc. 2019, 20, 47–54. [Google Scholar] [CrossRef]
- Worley, M.J.; Welch, W.R.; Berkowitz, R.S.; Ng, S.W. Endometriosis-Associated Ovarian Cancer: A Review of Pathogenesis. Int. J. Mol. Sci. 2013, 14, 5367–5379. [Google Scholar] [CrossRef]
- Mai, P.L.; Loud, J.T.; Greene, M.H. A Major Step Forward for BRCA1/2-Related Cancer Risk Management. J. Clin. Oncol. 2014, 32, 1531–1533. [Google Scholar] [CrossRef]
- Akash, S.; Kumer, A.; Rahman, M.M.; Emran, T.B.; Sharma, R.; Singla, R.K.; Alhumaydhi, F.A.; Khandaker, M.U.; Park, M.N.; Idris, A.M.; et al. Development of New Bioactive Molecules to Treat Breast and Lung Cancer with Natural Myricetin and Its Derivatives: A Computational and SAR Approach. Front. Cell. Infect. Microbiol. 2022, 12, 1400. [Google Scholar] [CrossRef] [PubMed]
- Akash, S.; Rahman, M.M.; Islam, M.R.; Sharma, R. Emerging Global Concern of Langya Henipavirus: Pathogenicity, Virulence, Genomic Features, and Future Perspectives. J. Med. Virol. 2023, 95, e28127. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Singla, R.K.; Narwal, S.; Tanushree; Kumar, N.; Mominur Rahman, M. Medicinal Plants Used as an Alternative to Treat Gingivitis and Periodontitis. Evid. Based Complement. Altern. Med. 2022, 2022, 2327641. [Google Scholar] [CrossRef]
- Kazi, M.A.; Sahito, R.; Abbas, Q.; Ullah, S.; Majid, A.; Phull, A.R.; Rahman, M.M.; Kim, S.J. The Inhibitory Effect of Polyphenon 60 from Green Tea on Melanin and Tyrosinase in Zebrafish and A375 Human Melanoma Cells. Evid. Based Complement. Altern. Med. 2022, 2022, 7739023. [Google Scholar] [CrossRef]
- Rhaman, M.; Islam, R.; Akash, S.; Mim, M.; Noor, A.; Nepovimova, E.; Valis, M.; Kuca, K.; Sharma, R. Exploring the Role of Nanomedicines for the Therapeutic Approach of Central Nervous System Dysfunction: At a Glance. Front. Cell Dev. Biol. 2022, 10, 1780. [Google Scholar] [CrossRef]
- Shohag, S.; Akhter, S.; Islam, S.; Sarker, T.; Sifat, M.K.; Rahman, M.M.; Islam, M.R.; Sharma, R. Perspectives on the Molecular Mediators of Oxidative Stress and Antioxidant Strategies in the Context of Neuroprotection and Neurolongevity: An Extensive Review. Oxid. Med. Cell. Longev. 2022, 2022, 7743705. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Yamin, M.; Islam, M.M.; Sarker, M.T.; Meem, A.F.K.; Akter, A.; Emran, T.B.; Cavalu, S.; Sharma, R. Emerging Role of Neuron-Glia in Neurological Disorders: At a Glance. Oxid. Med. Cell. Longev. 2022, 2022, 3201644. [Google Scholar] [CrossRef]
- Rauf, A.; Rahman, M.M. Potential Therapeutics against Neurological Disorders: Natural Products-Based Drugs. Front. Pharmacol. 2022, 13, 3178. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Rahman, F.; Rahaman, M.S.; Khan, M.S.; Abrar, S.; Ray, T.K.; Uddin, M.B.; Kali, M.S.K.; Dua, K.; et al. Emerging Promise of Computational Techniques in Anti-Cancer Research: At a Glance. Bioengineering 2022, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.F.; Jabbarzadeh, E. The Use of Natural Products to Target Cancer Stem Cells. Am. J. Cancer Res. 2017, 7, 1588–1605. [Google Scholar]
- Walcher, L.; Kistenmacher, A.-K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.-R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells—Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Telang, N. Stem Cell Models for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 7055. [Google Scholar] [CrossRef]
- Jia, D.; Nagaoka, Y.; Katsumata, M.; Orsulic, S. Inflammation Is a Key Contributor to Ovarian Cancer Cell Seeding. Sci. Rep. 2018, 8, 12394. [Google Scholar] [CrossRef] [PubMed]
- Yamulla, R.J.; Nalubola, S.; Flesken-Nikitin, A.; Nikitin, A.Y.; Schimenti, J.C. Most Commonly Mutated Genes in High-Grade Serous Ovarian Carcinoma Are Nonessential for Ovarian Surface Epithelial Stem Cell Transformation. Cell Rep. 2020, 32, 108086. [Google Scholar] [CrossRef]
- Saed, G.M.; Diamond, M.P.; Fletcher, N.M. Updates of the Role of Oxidative Stress in the Pathogenesis of Ovarian Cancer. Gynecol. Oncol. 2017, 145, 595–602. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Wang, Y.; Zhao, X.; Qi, X. Hormone Therapy for Ovarian Cancer: Emphasis on Mechanisms and Applications (Review). Oncol. Rep. 2021, 46, 223. [Google Scholar] [CrossRef] [PubMed]
- Risch, H.A. Hormonal Etiology of Epithelial Ovarian Cancer, with a Hypothesis Concerning the Role of Androgens and Progesterone. J. Natl. Cancer Inst. 1998, 90, 1774–1786. [Google Scholar] [CrossRef]
- Browning, L.; Patel, M.R.; Horvath, E.B.; Tawara, K.; Jorcyk, C.L. IL-6 and Ovarian Cancer: Inflammatory Cytokines in Promotion of Metastasis. Cancer Manag. Res. 2018, 10, 6685–6693. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.C.; Kim, K.M.; Lee, K.S.; Namkoong, S.; Lee, S.J.; Han, J.A.; Jeoung, D.; Ha, K.S.; Kwon, Y.G.; Kim, Y.M. Serum Bioactive Lysophospholipids Prevent TRAIL-Induced Apoptosis via PI3K/Akt-Dependent CFLIP Expression and Bad Phosphorylation. Cell Death Differ. 2004, 11, 1287–1298. [Google Scholar] [CrossRef]
- Giuntoli, R.L.; Webb, T.J.; Zoso, A.; Rogers, O.; Diaz-Montes, T.P.; Bristow, R.E.; Oelke, M. Ovarian Cancer-Associated Ascites Demonstrates Altered Immune Environment-2009. Anticancer Res. 2009, 29, 2875–2884. [Google Scholar] [PubMed]
- Bast, R.C.; Hennessy, B.; Mills, G.B. The Biology of Ovarian Cancer: New Opportunities for Translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef]
- Chou, C.H.; Wei, L.H.; Kuo, M.L.; Huang, Y.J.; Lai, K.P.; Chen, C.A.; Hsieh, C.Y. Up-Regulation of Interleukin-6 in Human Ovarian Cancer Cell via a Gi/PI3K-Akt/NF-ΚB Pathway by Lysophosphatidic Acid, an Ovarian Cancer-Activating Factor. Carcinogenesis 2005, 26, 45–52. [Google Scholar] [CrossRef]
- Mackay, H.J.; Twelves, C.J. Targeting the Protein Kinase C Family: Are We There Yet? Nat. Rev. Cancer 2007, 7, 554–562. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; Murali Mohan, G.; Shailender, G.; Malla, R.R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Cancer Prevention and Cancer Promotion. Adv. Pharmacol. Sci. 2019, 2019, 3418975. [Google Scholar] [CrossRef]
- Chen, S.N.; Chang, R.; Lin, L.T.; Chern, C.U.; Tsai, H.W.; Wen, Z.H.; Li, Y.H.; Li, C.J.; Tsui, K.H. MicroRNA in Ovarian Cancer: Biology, Pathogenesis, and Therapeutic Opportunities. Int. J. Environ. Res. Public Health 2019, 16, 1510. [Google Scholar] [CrossRef]
- Serhan, K.; Gartung, A.; Panigrahy, D. Drawing a Link between the Thromboxane A2 Pathway and the Role of Platelets and Tumor Cells in Ovarian Cancer. Prostaglandins Other Lipid Mediat. 2018, 137, 40–45. [Google Scholar] [CrossRef]
- Rajagopal, C.; Lankadasari, M.B.; Aranjani, J.M.; Harikumar, K.B. Targeting Oncogenic Transcription Factors by Polyphenols: A Novel Approach for Cancer Therapy. Pharmacol. Res. 2018, 130, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Senthil, K.; Aranganathan, S.; Nalini, N. Evidence of Oxidative Stress in the Circulation of Ovarian Cancer Patients. Clin. Chim. Acta 2004, 339, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, N.M.; Jiang, Z.; Ali-Fehmi, R.; Levin, N.K.; Belotte, J.; Tainsky, M.A.; Diamond, M.P.; Abu-Soud, H.M.; Saed, G.M. Myeloperoxidase and Free Iron Levels: Potential Biomarkers for Early Detection and Prognosis of Ovarian Cancer. Cancer Biomark. 2012, 10, 267–275. [Google Scholar] [CrossRef]
- Jiang, Z.; Fletcher, N.M.; Ali-Fehmi, R.; Diamond, M.P.; Abu-Soud, H.M.; Munkarah, A.R.; Saed, G.M. Modulation of Redox Signaling Promotes Apoptosis in Epithelial Ovarian Cancer Cells. Gynecol. Oncol. 2011, 122, 418–423. [Google Scholar] [CrossRef]
- Benhar, M. Roles of Mammalian Glutathione Peroxidase and Thioredoxin Reductase Enzymes in the Cellular Response to Nitrosative Stress. Free Radic. Biol. Med. 2018, 127, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Tong, D.C.; Pils, D.; Heinze, G.; Braicu, I.; Sehouli, J.; Reinthaller, A.; Schuster, E.; Wolf, A.; Watrowski, R.; Maki, R.A.; et al. Association of Myeloperoxidase with Ovarian Cancer. Tumor Biol. 2014, 35, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Saed, G.M.; Ali-Fehmi, R.; Jiang, Z.L.; Fletcher, N.M.; Diamond, M.P.; Abu-Soud, H.M.; Munkarah, A.R. Myeloperoxidase Serves as a Redox Switch That Regulates Apoptosis in Epithelial Ovarian Cancer. Gynecol. Oncol. 2010, 116, 276–281. [Google Scholar] [CrossRef]
- Kusriani, H.; Subarnas, A.; Diantini, A.; Iskandar, Y. Cytotoxicity of Quercetin and Quercetin-3-O-Rhamnoside of Etlingera Elatior (Jack) RM Sm. Leaves against HeLa Cervical Cancer Cells. J. App. Pharm. Sci. 2021, 11, 85–90. [Google Scholar]
- Rais, J.; Jafri, A.; Siddiqui, S.; Tripathi, M.; Arshad, M. Phytochemicals in the Treatment of Ovarian Cancer. Front. Biosci. 2017, 9, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Hosein Farzaei, M.; Bahramsoltani, R.; Rahimi, R. Phytochemicals as Adjunctive with Conventional Anticancer Therapies. Curr. Pharm. Des. 2016, 22, 4201–4218. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A Natural Compound for Ovarian Cancer Treatment. J. Ovarian Res. 2019, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and Cancer: New Insights into Its Therapeutic Effects on Ovarian Cancer Cells. Cell Biosci. 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Kubczak, M.; Szustka, A.; Rogalińska, M. Molecular Targets of Natural Compounds with Anti-Cancer Properties. Int. J. Mol. Sci. 2021, 22, 13659. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, T.; Wang, Y.; Jiang, Y.; Wang, Y. Mechanisms and Advances in Anti-Ovarian Cancer with Natural Plants Component. Molecules 2021, 26, 5949. [Google Scholar] [CrossRef]
- Pistollato, F.; Calderón Iglesias, R.; Ruiz, R.; Aparicio, S.; Crespo, J.; Dzul Lopez, L.; Giampieri, F.; Battino, M. The Use of Natural Compounds for the Targeting and Chemoprevention of Ovarian Cancer. Cancer Lett. 2017, 411, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ferrell, J.E. Apoptosis Propagates through the Cytoplasm as Trigger Waves. Science 2018, 361, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, S.; Maresca, M.; Yumashev, A.V.; Choopani, R.; Hajimehdipoor, H. The Most Competent Plant-Derived Natural Products for Targeting Apoptosis in Cancer Therapy. Biomolecules 2021, 11, 534. [Google Scholar] [CrossRef]
- Liu, C.; Zeng, Y.; Wen, Y.; Huang, X.; Liu, Y. Natural Products Modulate Cell Apoptosis: A Promising Way for the Treatment of Ulcerative Colitis. Front. Pharmacol. 2022, 13, 806148. [Google Scholar] [CrossRef] [PubMed]
- Taparia, S.S.; Khanna, A. Procyanidin-Rich Extract of Natural Cocoa Powder Causes ROS-Mediated Caspase-3 Dependent Apoptosis and Reduction of pro-MMP-2 in Epithelial Ovarian Carcinoma Cell Lines. Biomed. Pharmacother. 2016, 83, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shi, J.; Gao, H.; Li, Q. Zeylenone Inhibits Proliferation and Promotes Apoptosis in Ovarian Carcinoma Cells via Janus Kinase 2 / Signal Transducers and Activators of Transcription 3 Pathways. J. Obstet. Gynaecol. Res. 2018, 44, 1451–1457. [Google Scholar] [CrossRef]
- Lee, D.; Ko, H.; Kim, Y.J.; Kim, S.N.; Choi, K.C.; Yamabe, N.; Kim, K.H.; Kang, K.S.; Kim, H.Y.; Shibamoto, T. Inhibition of A2780 Human Ovarian Carcinoma Cell Proliferation by a Rubus Component, Sanguiin H-6. J. Agric. Food Chem. 2016, 64, 801–805. [Google Scholar] [CrossRef]
- Yoon, J.H.; Shin, J.W.; Pham, T.H.; Choi, Y.J.; Ryu, H.W.; Oh, S.R.; Oh, J.W.; Yoon, D.Y. Methyl Lucidone Induces Apoptosis and G2/M Phase Arrest via the PI3K/Akt/NF-ΚB Pathway in Ovarian Cancer Cells. Pharm. Biol. 2020, 58, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Kuan, C.-P.; Lin, J.-Y.; Lai, J.-S.; Ho, T.-F. Tanshinone IIA Facilitates TRAIL Sensitization by Up-Regulating DR5 through the ROS-JNK-CHOP Signaling Axis in Human Ovarian Carcinoma Cell Lines. Chem. Res. Toxicol. 2015, 28, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Roudbari, N.H.; Amidi, F.; Parivar, K. The Protective Effect of Sulforaphane against Oxidative Stress in Granulosa Cells of Patients with Polycystic Ovary Syndrome (PCOS) through Activation of AMPK/AKT/NRF2 Signaling Pathway. Reprod. Biol. 2021, 21, 100563. [Google Scholar] [CrossRef] [PubMed]
- Ribaudo, G.; Coghi, P.; Yang, L.J.; Ng, J.P.L.; Mastinu, A.; Memo, M.; Wong, V.K.W.; Gianoncelli, A. Computational and Experimental Insights on the Interaction of Artemisinin, Dihydroartemisinin and Chloroquine with SARS-CoV-2 Spike Protein Receptor-Binding Domain (RBD). Nat. Prod. Res. 2022, 36, 5358–5363. [Google Scholar] [CrossRef] [PubMed]
- Coghi, P.; Yang, L.J.; Ng, J.P.L.; Haynes, R.K.; Memo, M.; Gianoncelli, A.; Wong, V.K.W.; Ribaudo, G. A Drug Repurposing Approach for Antimalarials Interfering with SARS-CoV-2 Spike Protein Receptor Binding Domain (RBD) and Human Angiotensin-Converting Enzyme 2 (ACE2). Pharmaceuticals 2021, 14, 954. [Google Scholar] [CrossRef]
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia Annual. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Zhu, J.; Zheng, Y.; Zhang, H.; Sun, H. Dihydroartemisinin Induces Apoptosis and Inhibits Proliferation, Migration, and Invasion in Epithelial Ovarian Cancer via Inhibition of the Hedgehog Signaling Pathway. Cancer Med. 2018, 7, 5704–5715. [Google Scholar] [CrossRef]
- Zhang, H.; Jiao, Y.; Shi, C.; Song, X.; Chang, Y.; Ren, Y.; Shi, X. Berbamine Suppresses Cell Proliferation and Promotes Apoptosis in Ovarian Cancer Partially via the Inhibition of Wnt/β-Catenin Signaling. Acta Biochim. Biophys. Sin. 2018, 50, 532–539. [Google Scholar] [CrossRef]
- Wang, F.; Chang, Z.; Fan, Q.; Wang, L. Epigallocatechin-3-Gallate Inhibits the Proliferation and Migration of Human Ovarian Carcinoma Cells by Modulating P38 Kinase and Matrix Metalloproteinase-2. Mol. Med. Rep. 2014, 9, 1085–1089. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Hamad, M.N.; Jaafar, N.S. Phytochemical Investigation of Chenopodium Murale (Family: Chenopodiaceae) Cultivated in Iraq, Isolation and Identification of Scopoletin and Gallic Acid. Asian J. Pharm. Clin. Res. 2017, 10, 70–77. [Google Scholar] [CrossRef]
- Jeong, M.; Kim, H.M.; Kim, H.J.; Choi, J.-H.; Jang, D.S. Kudsuphilactone B, a Nortriterpenoid Isolated from Schisandra Chinensis Fruit, Induces Caspase-Dependent Apoptosis in Human Ovarian Cancer A2780 Cells. Arch. Pharmacal Res. 2017, 40, 500–508. [Google Scholar] [CrossRef]
- Vergara, D.; Simeone, P.; Toraldo, D.; Del Boccio, P.; Vergaro, V.; Leporatti, S.; Pieragostino, D.; Tinelli, A.; De Domenico, S.; Alberti, S.; et al. Resveratrol Downregulates Akt/GSK and ERK Signalling Pathways in OVCAR-3 Ovarian Cancer Cells. Mol. Biosyst. 2012, 8, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-A.; Kim, B.; Dhanasekaran, D.N.; Tsang, B.K.; Song, Y.S. Curcumin Induces Apoptosis by Inhibiting Sarco/Endoplasmic Reticulum Ca2+ ATPase Activity in Ovarian Cancer Cells. Cancer Lett. 2016, 371, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, E.; Amawi, H.; Hussein, N.; Karthikeyan, C.; Fetcenko, A.; Narayana Moorthy, N.S.H.; Trivedi, P.; Tiwari, A.K. Design and Discovery of Silybin Analogues as Antiproliferative Compounds Using a Ring Disjunctive—Based, Natural Product Lead Optimization Approach. Eur. J. Med. Chem. 2017, 133, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Liu, Y.; Gao, H.; Xiao, J.; So, K.F. The Anti-Oxidant and Antitumor Properties of Plant Polysaccharides. Am. J. Chin. Med. 2016, 44, 463–488. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. IJMS 2017, 18, 96. [Google Scholar] [CrossRef]
- Ribaudo, G.; Bortoli, M.; Pavan, C.; Zagotto, G.; Orian, L. Antioxidant Potential of Psychotropic Drugs: From Clinical Evidence to In Vitro and In Vivo Assessment and toward a New Challenge for in Silico Molecular Design. Antioxidants 2020, 9, 714. [Google Scholar] [CrossRef]
- Kwon, Y. Food-Derived Polyphenols Inhibit the Growth of Ovarian Cancer Cells Irrespective of Their Ability to Induce Antioxidant Responses. Heliyon 2018, 4, e00753. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. An Insight into the Health-Promoting Effects of Taxifolin (Dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef]
- Ribaudo, G.; Coghi, P.; Zanforlin, E.; Law, B.Y.K.; Wu, Y.Y.J.; Han, Y.; Qiu, A.C.; Qu, Y.Q.; Zagotto, G.; Wong, V.K.W. Semi-Synthetic Isoflavones as BACE-1 Inhibitors against Alzheimer’s Disease. Bioorg. Chem. 2019, 87, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Bazer, F.W.; Lim, W.; Song, G. The O-Methylated Isoflavone, Formononetin, Inhibits Human Ovarian Cancer Cell Proliferation by Sub G0/G1 Cell Phase Arrest through PI3K/AKT and ERK1/2 Inactivation. J. Cell. Biochem. 2018, 119, 7377–7387. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Lu, Y.; Qiu, S.; Fan, Z. Overcoming Cisplatin Resistance of Ovarian Cancer Cells by Targeting HIF-1-Regulated Cancer Metabolism. Cancer Lett. 2016, 373, 36–44. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Jeong, W.; Bazer, F.W.; Lee, D.; Song, G. Sideroxylin (Callistemon lanceolatus) Suppressed Cell Proliferation and Increased Apoptosis in Ovarian Cancer Cells Accompanied by Mitochondrial Dysfunction, the Generation of Reactive Oxygen Species, and an Increase of Lipid Peroxidation. J. Cell. Physiol. 2018, 233, 8597–8604. [Google Scholar] [CrossRef]
- Ribaudo, G.; Zanforlin, E.; Canton, M.; Bova, S.; Zagotto, G. Preliminary Studies of Berberine and Its Semi-Synthetic Derivatives as a Promising Class of Multi-Target Anti-Parkinson Agents. Nat. Prod. Res. 2018, 32, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Xu, G.; Zhang, C.; Li, B.; Qin, J.; Hao, X.; Liu, Q.; Zhang, X.; Liu, J.; Wei, J.; et al. Berberine Induces Oxidative Dna Damage and Impairs Homologous Recombination Repair in Ovarian Cancer Cells to Confer Increased Sensitivity to Parp Inhibition. Cell Death Dis. 2017, 8, e3070. [Google Scholar] [CrossRef] [PubMed]
- Kakar, S.S.; Jala, V.R.; Fong, M.Y. Synergistic Cytotoxic Action of Cisplatin and Withaferin A on Ovarian Cancer Cell Lines. Biochem. Biophys. Res. Commun. 2012, 423, 819–825. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Z.; Wang, Z.; Liu, G.; Liu, Y.; Wang, H. Astragalus Polysaccharides Inhibit Ovarian Cancer Cell Growth via MicroRNA-27a/FBXW7 Signaling Pathway. Biosci. Rep. 2020, 40, BSR20193396. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Cobucci, R.N.O.; Jatobá, C.A.N.; de Medeiros Fernandes, T.A.A.; de Azevedo, J.W.V.; de Araújo, J.M.G. The Role of the Mediators of Inflammation in Cancer Development. Pathol. Oncol. Res. 2015, 21, 527–534. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, K.; Han, J.Y.; Lim, J.M.; Song, Y.S. Modulation of Inflammatory Signaling Pathways by Phytochemicals in Ovarian Cancer. Genes Nutr. 2011, 6, 109–115. [Google Scholar] [CrossRef]
- Yin, J.; Yu, C.; Yang, Z.; He, J.L.; Chen, W.J.; Liu, H.Z.; Li, W.M.; Liu, H.T.; Wang, Y.X. Tetramethylpyrazine Inhibits Migration of SKOV3 Human Ovarian Carcinoma Cells and Decreases the Expression of Interleukin-8 via the ERK1/2, P38 and AP-1 Signaling Pathways. Oncol. Rep. 2011, 26, 671–679. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Y.; Wei, X.; Zhao, Z.; Xue, J.; Liu, P. Emodin Inhibits the Epithelial to Mesenchymal Transition of Epithelial Ovarian Cancer Cells via ILK/GSK-3 β /Slug Signaling Pathway. BioMed Res. Int. 2016, 2016, 6253280. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Wei, C.; Rankin, G.O.; Rojanasakul, Y.; Ren, N.; Ye, X.; Chen, Y.C. Dietary Compound Proanthocyanidins from Chinese Bayberry (Myrica Rubra Sieb. et Zucc.) Leaves Inhibit Angiogenesis and Regulate Cell Cycle of Cisplatin-Resistant Ovarian Cancer Cells via Targeting Akt Pathway. J. Funct. Foods 2018, 40, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, Y.-Y.; Wang, X.-X.; Wang, H.-P.; Chen, H.; Wu, Y.-C.; Wang, L.; Pu, X.; Yue, G.-Z.; Zhang, L. Tanshinone IIA Suppresses Ovarian Cancer Growth through Inhibiting Malignant Properties and Angiogenesis. Ann. Transl. Med. 2020, 8, 1295. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Koklesova, L.; Samec, M.; Varghese, E.; Abotaleb, M.; Samuel, S.M.; Smejkal, K.; Biringer, K.; Petras, M.; Blahutova, D.; et al. Implications of Flavonoids as Potential Modulators of Cancer Neovascularity. J. Cancer Res. Clin. Oncol. 2020, 146, 3079–3096. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, H.; Wan, H.; Zou, X.; Ma, X.; Gao, G. Harmine Suppresses the Proliferation and Migration of Human Ovarian Cancer Cells through Inhibiting ERK/CREB Pathway. Oncol. Rep. 2017, 38, 2927–2934. [Google Scholar] [CrossRef]
- Kim, K.K.; Singh, A.P.; Singh, R.K.; DeMartino, A.; Brard, L.; Vorsa, N.; Lange, T.S.; Moore, R.G. Anti-Angiogenic Activity of Cranberry Proanthocyanidins and Cytotoxic Properties in Ovarian Cancer Cells. Int. J. Oncol. 2012, 40, 227–235. [Google Scholar] [CrossRef]
- Dias, A.S.; Helguero, L.; Almeida, C.R.; Duarte, I.F. Natural Compounds as Metabolic Modulators of the Tumor Microenvironment. Molecules 2021, 26, 3494. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Li, C.; Li, T.; Huang, Y. Remodeling Tumor Microenvironment with Natural Products to Overcome Drug Resistance. Front. Immunol. 2022, 13, 1051998. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.A.; Hart, P.C.; Kordylewicz, K.; Lal, M.; Shen, M.; Kara, B.; Chen, Y.-J.; Grassl, N.; Alharbi, Y.; Pattnaik, B.R.; et al. The Natural Product β-Escin Targets Cancer and Stromal Cells of the Tumor Microenvironment to Inhibit Ovarian Cancer Metastasis. Cancers 2021, 13, 3931. [Google Scholar] [CrossRef]
- Di, W.; Zhang, L.; Yi, H.; Han, X.; Zhang, Y.; Xin, L. Exopolysaccharides Produced by Lactobacillus Strains Suppress HT-29 Cell Growth via Induction of G0/G1 Cell Cycle Arrest and Apoptosis. Oncol. Lett. 2018, 16, 3577–3586. [Google Scholar] [CrossRef]
- Ren, L.; Cao, Q.X.; Zhai, F.R.; Yang, S.Q.; Zhang, H.X. Asiatic Acid Exerts Anticancer Potential in Human Ovarian Cancer Cells via Suppression of PI3K/Akt/MTOR Signalling. Pharm. Biol. 2016, 54, 2377–2382. [Google Scholar] [CrossRef]
- Yu, S.; Yan, H.; Zhang, L.; Shan, M.; Chen, P.; Ding, A.; Li, S.F.Y. A Review on the Phytochemistry, Pharmacology, and Pharmacokinetics of Amentoflavone, a Naturally-Occurring Biflavonoid. Molecules 2017, 22, 299. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Wei, C.; Rankin, G.O.; Ye, X.; Chen, Y.C. Dietary Compound Proanthocyanidins from Chinese Bayberry (Myrica Rubra Sieb. et Zucc.) Leaves Attenuate Chemotherapy-Resistant Ovarian Cancer Stem Cell Traits via Targeting the Wnt/β-Catenin Signaling Pathway and Inducing G1 Cell Cycle Arrest. Food Funct. 2018, 9, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Fadaeinasab, M.; Mohan, S.; Hashim, N.M.; Othman, R.; Karimian, H.; Iman, V.; Ramli, N.; Ali, H.M.; Majid, N.A. Pulchrin A, a New Natural Coumarin Derivative of Enicosanthellum Pulchrum, Induces Apoptosis in Ovarian Cancer Cells via Intrinsic Pathway. PLoS ONE 2016, 11, e0154023. [Google Scholar] [CrossRef] [PubMed]
- Kavandi, L.; Lee, L.R.; Bokhari, A.A.; Pirog, J.E.; Jiang, Y.; Ahmad, K.A.; Syed, V. The Chinese Herbs Scutellaria Baicalensis and Fritillaria Cirrhosa Target NFκB to Inhibit Proliferation of Ovarian and Endometrial Cancer Cells. Mol. Carcinog. 2015, 54, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica Charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Huang, T.C.; Shieh, T.M.; Wu, C.H.; Lin, L.C.; Hsia, S.M. Isoliquiritigenin Induces Autophagy and Inhibits Ovarian Cancer Cell Growth. Int. J. Mol. Sci. 2017, 18, 2025. [Google Scholar] [CrossRef]
- Zhan, L.; Zhang, Y.; Wang, W.; Song, E.; Fan, Y.; Li, J.; Wei, B. Autophagy as an Emerging Therapy Target for Ovarian Carcinoma. Oncotarget 2016, 7, 83476–83487. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Peng, Y.; Wang, J.; Gu, A.X.; Li, Q.; Mao, D.W.; Guo, L.Y. Effect of Autophagy on the Resveratrol-Induced Apoptosis of Ovarian Cancer SKOV3 Cells. J. Cell. Biochem. 2019, 120, 7788–7793. [Google Scholar] [CrossRef]
- Fong, M.Y.; Jin, S.; Rane, M.; Singh, R.K.; Gupta, R.; Kakar, S.S. Withaferin a Synergizes the Therapeutic Effect of Doxorubicin through ROS-Mediated Autophagy in Ovarian Cancer. PLoS ONE 2012, 7, e42265. [Google Scholar] [CrossRef]
- Che, X.; Yan, H.; Sun, H.; Dongol, S.; Wang, Y.; Lv, Q.; Jiang, J. Grifolin Induces Autophagic Cell Death by Inhibiting the Akt/MTOR/S6K Pathway in Human Ovarian Cancer Cells. Oncol. Rep. 2016, 36, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Baehrecke, E.H. Autophagy, Cell Death, and Cancer. Mol. Cell. Oncol. 2015, 2, e985913. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, Y.-Y.; Chen, H.; Wu, Y.-C.; Zhang, L. Tanshinone I Attenuates the Malignant Biological Properties of Ovarian Cancer by Inducing Apoptosis and Autophagy via the Inactivation of PI3K/AKT/MTOR Pathway. Cell Prolif. 2020, 53, e12739. [Google Scholar] [CrossRef] [PubMed]
- Gossner, G.; Choi, M.; Tan, L.; Fogoros, S.; Griffith, K.A.; Kuenker, M.; Liu, J.R. Genistein-Induced Apoptosis and Autophagocytosis in Ovarian Cancer Cells. Gynecol. Oncol. 2007, 105, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Engelke, L.H.; Hamacher, A.; Proksch, P.; Kassack, M.U. Ellagic Acid and Resveratrol Prevent the Development of Cisplatin Resistance in the Epithelial Ovarian Cancer Cell Line A2780. J. Cancer 2016, 7, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Pastorek, M.; Simko, V.; Takacova, M.; Barathova, M.; Bartosova, M.; Hunakova, L.; Sedlakova, O.; Hudecova, S.; Krizanova, O.; Dequiedt, F.; et al. Sulforaphane Reduces Molecular Response to Hypoxia in Ovarian Tumor Cells Independently of Their Resistance to Chemotherapy. Int. J. Oncol. 2015, 47, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, S.M.; Kim, J.H. Unripe Rubus Coreanus Miquel Suppresses Migration and Invasion of Human Prostate Cancer Cells by Reducing Matrix Metalloproteinase Expression. Biosci. Biotechnol. Biochem. 2014, 78, 1402–1411. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, H.S.; Cho, S.G.; Shin, Y.C.; Ko, S.G. Rubus Coreanus Miquel Extract Causes Apoptosis of Doxorubicin-Resistant NCI/ADR-RES Ovarian Cancer Cells via JNK Phosphorylation. Mol. Med. Rep. 2016, 13, 4065–4072. [Google Scholar] [CrossRef]
- Uche, F.I.; McCullagh, J.; Claridge, T.W.D.; Richardson, A.; Li, W.-W. Synthesis of (Aminoalkyl)Cycleanine Analogues: Cytotoxicity, Cellular Uptake, and Apoptosis Induction in Ovarian Cancer Cells. Bioorg. Med. Chem. Lett. 2018, 28, 1652–1656. [Google Scholar] [CrossRef]
- Chen, H.; Landen, C.N.; Li, Y.; Alvarez, R.D.; Tollefsbol, T.O. Epigallocatechin Gallate and Sulforaphane Combination Treatment Induce Apoptosis in Paclitaxel-Resistant Ovarian Cancer Cells through HTERT and Bcl-2 down-Regulation. Exp. Cell Res. 2013, 319, 697–706. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.; Guo, H.; Liu, Y.; Hu, P.; Yang, X.; Li, X.; Ge, S.; Velu, S.E.; Nadkarni, D.H.; et al. Experimental Therapy of Ovarian Cancer with Synthetic Makaluvamine Analog: In Vitro and In Vivo Anticancer Activity and Molecular Mechanisms of Action. PLoS ONE 2011, 6, e20729. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, C.; Zhang, J.; Jiang, Q.; Wang, Z.; Nie, S.; Gao, Z.; Li, G.; Fang, H.; Ren, S.; et al. Discovery of Semisynthetic Celastrol Derivatives Exhibiting Potent Anti-Ovarian Cancer Stem Cell Activity and STAT3 Inhibition. Chem. Biol. Interact. 2022, 366, 110172. [Google Scholar] [CrossRef]
- Beretta, G.L.; Ribaudo, G.; Menegazzo, I.; Supino, R.; Capranico, G.; Zunino, F.; Zagotto, G. Synthesis and Evaluation of New Naphthalene and Naphthoquinone Derivatives as Anticancer Agents: Naphthalene and Naphthoquinone Derivatives as Anticancer Agents. Arch. Pharm. Chem. Life Sci. 2017, 350, e1600286. [Google Scholar] [CrossRef]
- Amrine, C.S.M.; Huntsman, A.C.; Doyle, M.G.; Burdette, J.E.; Pearce, C.J.; Fuchs, J.R.; Oberlies, N.H. Semisynthetic Derivatives of the Verticillin Class of Natural Products through Acylation of the C11 Hydroxy Group. ACS Med. Chem. Lett. 2021, 12, 625–630. [Google Scholar] [CrossRef]
- Prasad, S.; Aggarwal, B.B. Turmeric, the Golden Spice. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Zhou, H.; Beevers, C.S.; Huang, S. The Targets of Curcumin. Curr. Drug Targets 2012, 12, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Dobbin, Z.C.; Landen, C.N. The Importance of the PI3K/AKT/MTOR Pathway in the Progression of Ovarian Cancer. Int. J. Mol. Sci. 2013, 14, 8213–8227. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wan, Y.; Liu, Y.; Yang, J.; Li, L.; Zhang, W. Curcumin Induced Apoptosis via PI3K/Akt-Signalling Pathways in SKOV3 Cells. Pharm. Biol. 2016, 54, 2026–2032. [Google Scholar] [CrossRef]
- Watson, J.L.; Greenshields, A.; Hill, R.; Hilchie, A.; Lee, P.W.; Giacomantonio, C.A.; Hoskin, D.W. Curcumin-Induced Apoptosis in Ovarian Carcinoma Cells Is P53-Independent and Involves P38 Mitogen-Activated Protein Kinase Activation and Downregulation of Bcl-2 and Survivin Expression and Akt Signaling. Mol. Carcinog. 2010, 49, 13–24. [Google Scholar] [CrossRef]
- Bowman, T.; Garcia, R.; Turkson, J.; Jove, R. STATs in Oncogenesis. Oncogene 2000, 19, 2474–2488. [Google Scholar] [CrossRef]
- Saydmohammed, M.; Joseph, D.; Syed, V. Curcumin Suppresses Constitutive Activation of STAT-3 by up-Regulating Protein Inhibitor of Activated STAT-3 (PIAS-3) in Ovarian and Endometrial Cancer Cells. J. Cell. Biochem. 2010, 110, 447–456. [Google Scholar] [CrossRef]
- Seo, J.H.; Jeong, K.J.; Oh, W.J.; Sul, H.J.; Sohn, J.S.; Kim, Y.K.; Cho, D.Y.; Kang, J.K.; Park, C.G.; Lee, H.Y. Lysophosphatidic Acid Induces STAT3 Phosphorylation and Ovarian Cancer Cell Motility: Their Inhibition by Curcumin. Cancer Lett. 2010, 288, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kinose, Y.; Sawada, K.; Nakamura, K.; Kimura, T. The Role of MicroRNAs in Ovarian Cancer. BioMed Res. Int. 2014, 2014, 249393. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Sha, X. Demethoxycurcumin Inhibited Human Epithelia Ovarian Cancer Cells’ Growth via up-Regulating MiR-551a. Tumor Biol. 2017, 39, 1010428317694302. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y.; Kadomatsu, K.; Muramatsu, T.; Matsuo, S.; Itoh, H.; Nakazawa, K.; Kubota, S. Antisense Oligodeoxynucleotide Targeted to Midkine, a Heparin-Binding Growth Factor, Suppresses Tumorigenicity of Mouse Rectal Carcinoma Cells. Cancer Res. 2001, 61, 8486–8491. [Google Scholar] [PubMed]
- Zhao, S.F.; Zhang, X.; Zhang, X.J.; Shi, X.Q.; Yu, Z.J.; Kan, Q.C. Induction of MicroRNA-9 Mediates Cytotoxicity of Curcumin against SKOV3 Ovarian Cancer Cells. Asian Pac. J. Cancer Prev. 2014, 15, 3363–3368. [Google Scholar] [CrossRef]
- Al-Alem, L.; Curry, T.E., Jr. Ovarian Cancer: Involvement of the Matrix Metalloproteinases. Reproduction 2015, 150, R55. [Google Scholar] [CrossRef]
- Lv, J.; Shao, Q.; Wang, H.; Shi, H.; Wang, T.; Gao, W.; Song, B.; Zheng, G.; Kong, B.; Qu, X. Effects and Mechanisms of Curcumin and Basil Polysaccharide on the Invasion of SKOV3 Cells and Dendritic Cells. Mol. Med. Rep. 2013, 8, 1580–1586. [Google Scholar] [CrossRef]
- Pei, H.; Yang, Y.; Cui, L.; Yang, J.; Li, X.; Yang, Y.; Duan, H. Bisdemethoxycurcumin Inhibits Ovarian Cancer via Reducing Oxidative Stress Mediated MMPs Expressions. Sci. Rep. 2016, 6, 28773. [Google Scholar] [CrossRef]
- Slack-Davis, J.K.; Atkins, K.A.; Harrer, C.; Daniel Hershey, E.; Conaway, M. Vascular Cell Adhesion Molecule-1 Is a Regulator of Ovarian Cancer Peritoneal Metastasis. Cancer Res. 2009, 69, 1469–1476. [Google Scholar] [CrossRef]
- Daleprane, J.B.; Abdalla, D.S. Emerging Roles of Propolis: Antioxidant, Cardioprotective, and Antiangiogenic Actions. Evid.-Based Complement. Altern. Med. 2013, 2013, 175135. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The in Vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Pavan, V.; Mucignat-Caretta, C.; Redaelli, M.; Ribaudo, G.; Zagotto, G. The Old Made New: Natural Compounds against Erectile Dysfunction: Natural Compounds against Erectile Dysfunction. Arch. Pharm. Chem. Life Sci. 2015, 348, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, W.; He, G.; Kuick, R.D.; Gossner, G.; Kueck, A.S.; Wahl, H.; Opipari, A.W.; Liu, J.R. Resveratrol Inhibits Ovarian Tumor Growth in an in Vivo Mouse Model. Cancer 2016, 122, 722–729. [Google Scholar] [CrossRef]
- Gwak, H.; Kim, S.; Dhanasekaran, D.N.; Song, Y.S. Resveratrol Triggers ER Stress-Mediated Apoptosis by Disrupting N-Linked Glycosylation of Proteins in Ovarian Cancer Cells. Cancer Lett. 2016, 371, 347–353. [Google Scholar] [CrossRef]
- Majewska, E.; Szeliga, M. AKT/GSK3β Signaling in Glioblastoma. Neurochem. Res. 2017, 42, 918–924. [Google Scholar] [CrossRef]
- Tino, A.B.; Chitcholtan, K.; Sykes, P.H.; Garrill, A. Resveratrol and Acetyl-Resveratrol Modulate Activity of VEGF and IL-8 in Ovarian Cancer Cell Aggregates via Attenuation of the NF-ΚB Protein. J. Ovarian Res. 2016, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Mikuła-Pietrasik, J.; Sosińska, P.; Książek, K. Resveratrol Inhibits Ovarian Cancer Cell Adhesion to Peritoneal Mesothelium in Vitro by Modulating the Production of A5β1 Integrins and Hyaluronic Acid. Gynecol. Oncol. 2014, 134, 624–630. [Google Scholar] [CrossRef]
- Park, S.Y.; Jeong, K.J.; Lee, J.; Yoon, D.S.; Choi, W.S.; Kim, Y.K.; Han, J.W.; Kim, Y.M.; Kim, B.K.; Lee, H.Y. Hypoxia Enhances LPA-Induced HIF-1α and VEGF Expression: Their Inhibition by Resveratrol. Cancer Lett. 2007, 258, 63–69. [Google Scholar] [CrossRef]
- Sopo, M.; Anttila, M.; Hämäläinen, K.; Kivelä, A.; Ylä-Herttuala, S.; Kosma, V.M.; Keski-Nisula, L.; Sallinen, H. Expression Profiles of VEGF-A, VEGF-D and VEGFR1 Are Higher in Distant Metastases than in Matched Primary High Grade Epithelial Ovarian Cancer. BMC Cancer 2019, 19, 584. [Google Scholar] [CrossRef]
- Nag, S.A.; Qin, J.J.; Wang, W.; Wang, M.H.; Wang, H.; Zhang, R. Ginsenosides as Anticancer Agents: In Vitro and in Vivo Activities, Structure-Activity Relationships, and Molecular Mechanisms of Action. Front. Pharmacol. 2012, 3, 25. [Google Scholar] [CrossRef]
- Ahuja, A.; Kim, J.H.; Kim, J.-H.; Yi, Y.-S.; Cho, J.Y. Functional Role of Ginseng-Derived Compounds in Cancer. J. Ginseng Res. 2018, 42, 248–254. [Google Scholar] [CrossRef]
- Li, J.; Liu, T.; Zhao, L.; Chen, W.; Hou, H.; Ye, Z.; Li, X. Ginsenoside 20(S)-Rg3 Inhibits the Warburg Effect through STAT3 Pathways in Ovarian Cancer Cells. Int. J. Oncol. 2015, 46, 775–781. [Google Scholar] [CrossRef]
- Lu, J.; Wang, L.; Chen, W.; Wang, Y.; Zhen, S.; Chen, H.; Cheng, J.; Zhou, Y.; Li, X.; Zhao, L. MiR-603 Targeted Hexokinase-2 to Inhibit the Malignancy of Ovarian Cancer Cells. Arch. Biochem. Biophys. 2019, 661, 1–9. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.-X.; Ai, B.; Pan, H.-F.; Zhang, D.; Jiang, Y.; Hu, L.-H.; Sun, L.-L.; Chen, Z.-S.; Lin, L.-Z. Ginsenoside Rg3 Promotes Cell Growth Through Activation of MTORC1. Front. Cell Dev. Biol. 2021, 9, 730309. [Google Scholar] [CrossRef]
- Li, J.; Xi, W.; Li, X.; Sun, H.; Li, Y. Advances in Inhibition of Protein-Protein Interactions Targeting Hypoxia-Inducible Factor-1 for Cancer Therapy. Bioorg. Med. Chem. 2019, 27, 1145–1158. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, L.; Hou, H.; Ding, L.; Chen, W.; Li, X. Ginsenoside 20(S)-Rg3 Suppresses Ovarian Cancer Migration via Hypoxia-Inducible Factor 1 Alpha and Nuclear Factor-Kappa B Signals. Tumor Biol. 2017, 39, 1010428317692225. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, T.; Teng, Y.; Chen, W.; Zhao, L.; Li, X. Ginsenoside Rb1 Inhibits Hypoxia-Induced Epithelial-Mesenchymal Transition in Ovarian Cancer Cells by Regulating MicroRNA-25. Exp. Ther. Med. 2017, 14, 2895–2902. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. Ovarian Cancer Development and Metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Ribaudo, G.; Oselladore, E.; Ongaro, A.; Zagotto, G.; Memo, M.; Gianoncelli, A. Enhanced G-Quadruplex Selectivity of Flavonoid Glycoside Rutin over Quercetin. Nat. Prod. Res. 2020, 36, 3469–3473. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, I.; Lamotte-Brasseur, J.; Chessa, J.P.; Ntarima, P.; Claeyssens, M.; Devreese, B.; Marino, G.; Gerday, C. Xylanase from the Psychrophilic Yeast Cryptococcus Adeliae. Extremophiles 2000, 4, 137–144. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, W.; Yang, Z.Y.; Zhou, X.S.; Gong, C.; Zhang, T.R.; Wei, X.; Ma, D.; Ye, F.; Gao, Q.L. Quercetin Induces Protective Autophagy and Apoptosis through ER Stress via the P-STAT3/Bcl-2 Axis in Ovarian Cancer. Apoptosis 2017, 22, 544–557. [Google Scholar] [CrossRef]
- Driak, D.; Dvorska, M.; Bolehovska, P.; Svandova, I.; Novotny, J.; Halaska, M. Bad and Bid—Potential Background Players in Preneoplastic to Neoplastic Shift in Human Endometrium. Neo 2014, 61, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Kemp, K.; Griffiths, J.; Campbell, S.; Lovell, K. An Exploration of the Follow-up up Needs of Patients with Inflammatory Bowel Disease. J. Crohn’s Colitis 2013, 7, e386–e395. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, B.; Wei, X.; Men, K.; Zheng, F.; Zhou, Y.; Zheng, Y.; Gou, M.; Huang, M.; Guo, G.; et al. Anticancer Effect and Mechanism of Polymer Micelle-Encapsulated Quercetin on Ovarian Cancer. Nanoscale 2012, 4, 7021–7030. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Yang, Z.; Zhang, L.; Wang, Y.; Gong, W.; Liu, Y. Quercetin Suppresses DNA Double-Strand Break Repair and Enhances the Radiosensitivity of Human Ovarian Cancer Cells via P53-Dependent Endoplasmic Reticulum Stress Pathway. OncoTargets Ther. 2018, 11, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Y.; Liao, J.; Gong, C.; Sun, C.; Zhou, X.; Wei, X.; Zhang, T.; Gao, Q.; Ma, D.; et al. Quercetin Induces Endoplasmic Reticulum Stress to Enhance CDDP Cytotoxicity in Ovarian Cancer: Involvement of STAT3 Signaling. FEBS J. 2015, 282, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Xie, Y.; Huang, Y.; Wu, Q.; Zhang, H.C.; Xiong, S.; Liu, Y.; Chen, L.; Wei, Y.; Zhao, X.; et al. Induction of Apoptosis and Inhibition of Angiogenesis by PEGylated Liposomal Quercetin in Both Cisplatin-Sensitive and Cisplatin-Resistant Ovarian Cancers. J. Biomed. Nanotechnol. 2013, 9, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, D.; Ragazzi, E.; Vianello, C.; Caparrotta, L.; Montopoli, M. Effect of Quercetin on Cell Cycle and Cyclin Expression in Ovarian Carcinoma and Osteosarcoma Cell Lines. Nat. Prod. Commun. 2015, 10, 1365–1368. [Google Scholar] [CrossRef]
- Rashidi, Z.; Khosravizadeh, Z.; Talebi, A.; Khodamoradi, K.; Ebrahimi, R.; Amidi, F. Overview of Biological Effects of Quercetin on Ovary. Phytother. Res. 2021, 35, 33–49. [Google Scholar] [CrossRef]
- Dhanaraj, T.; Mohan, M.; Arunakaran, J. Quercetin Attenuates Metastatic Ability of Human Metastatic Ovarian Cancer Cells via Modulating Multiple Signaling Molecules Involved in Cell Survival, Proliferation, Migration and Adhesion. Arch. Biochem. Biophys. 2021, 701, 108795. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Afroze, S.H.; Mitsunaga, T.; McCormick, T.C.; Kuehl, T.J.; Zawieja, D.C.; Uddin, M.N. 3,4′,7-O-Trimethylquercetin Inhibits Invasion and Migration of Ovarian Cancer Cells. Anticancer Res. 2017, 37, 2823–2829. [Google Scholar] [CrossRef]
- Li, N.; Sun, C.; Zhou, B.; Xing, H.; Ma, D.; Chen, G.; Weng, D. Low Concentration of Quercetin Antagonizes the Cytotoxic Effects of Anti-Neoplastic Drugs in Ovarian Cancer. PLoS ONE 2014, 9, e100314. [Google Scholar] [CrossRef]
- Scambia, G.; Ranelletti, F.O.; Benedetti Panici, P.; Bonanno, G.; De Vincenzo, R.; Piantelli, M.; Mancuso, S. Synergistic Antiproliferative Activity of Quercetin and Cisplatin on Ovarian Cancer Cell Growth. Anti-Cancer Drugs 1990, 1, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, H.; Karki, N.; Pal, M.; Basak, S.; Verma, R.K.; Bal, R.; Kandpal, N.D.; Bisht, G.; Sahoo, N.G. Functionalized Graphene Oxide as a Nanocarrier for Dual Drug Delivery Applications: The Synergistic Effect of Quercetin and Gefitinib against Ovarian Cancer Cells. Colloids Surf. B Biointerfaces 2019, 178, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Fatease, A.A.; Shah, V.; Nguyen, D.X.; Cote, B.; LeBlanc, N.; Rao, D.A.; Alani, A.W.G. Chemosensitization and Mitigation of Adriamycin-Induced Cardiotoxicity Using Combinational Polymeric Micelles for Co-Delivery of Quercetin/Resveratrol and Resveratrol/Curcumin in Ovarian Cancer. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 39–48. [Google Scholar] [CrossRef]
- Xu, G.; Li, B.; Wang, T.; Wan, J.; Zhang, Y.; Huang, J.; Shen, Y. Enhancing the Anti-Ovarian Cancer Activity of Quercetin Using a Self-Assembling Micelle and Thermosensitive Hydrogel Drug Delivery System. RSC Adv. 2018, 8, 21229–21242. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, C.P.; Cui, B.; Zhang, H.; Panagal, M.; Paramasivam, S.; Chinnaiyan, U.; Jeyaraman, S.; Murugesan, K.; Rostagno, M.; Sekar, V.; et al. Anti-Ovarian Cancer Potential of Phytocompound and Extract from South African Medicinal Plants and Their Role in the Development of Chemotherapeutic Agents. Am. J. Cancer Res. 2021, 11, 1828–1844. [Google Scholar]
- Löcken, H.; Clamor, C.; Müller, K. Napabucasin and Related Heterocycle-Fused Naphthoquinones as STAT3 Inhibitors with Antiproliferative Activity against Cancer Cells. J. Nat. Prod. 2018, 81, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Shchekotikhin, A.E.; Nadysev, G.Y.; Tikhomirov, A.S.; Dezhenkova, L.G. Semi-Synthetic Derivatives of Heliomycin with an Antiproliferative Potency. PRA 2018, 13, 469–472. [Google Scholar] [CrossRef]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; deTakats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I Clinical Trial of the Flavonoid Quercetin: Pharmacokinetics and Evidence for in Vivo Tyrosine Kinase Inhibition. Clin. Cancer Res. 1996, 2, 659–668. [Google Scholar]
- Mei, J.-Y.; Zhang, M.-J.; Wang, Y.-Y.; Liu, Y.-H. The Positive Clinical Therapeutically Effects of Escin on Advanced Thyroid Cancer. Cancer Med. 2017, 6, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Budisan, L.; Gulei, D.; Zanoaga, O.M.; Irimie, A.I.; Sergiu, C.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. Dietary Intervention by Phytochemicals and Their Role in Modulating Coding and Non-Coding Genes in Cancer. Int. J. Mol. Sci. 2017, 18, 1178. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.B.; Tian, Q.; Zhang, J.F.; Xiang, Y. Antitumor Effects and Molecular Mechanisms of Action of Natural Products in Ovarian Cancer (Review). Oncol. Lett. 2020, 20, 141. [Google Scholar] [CrossRef] [PubMed]


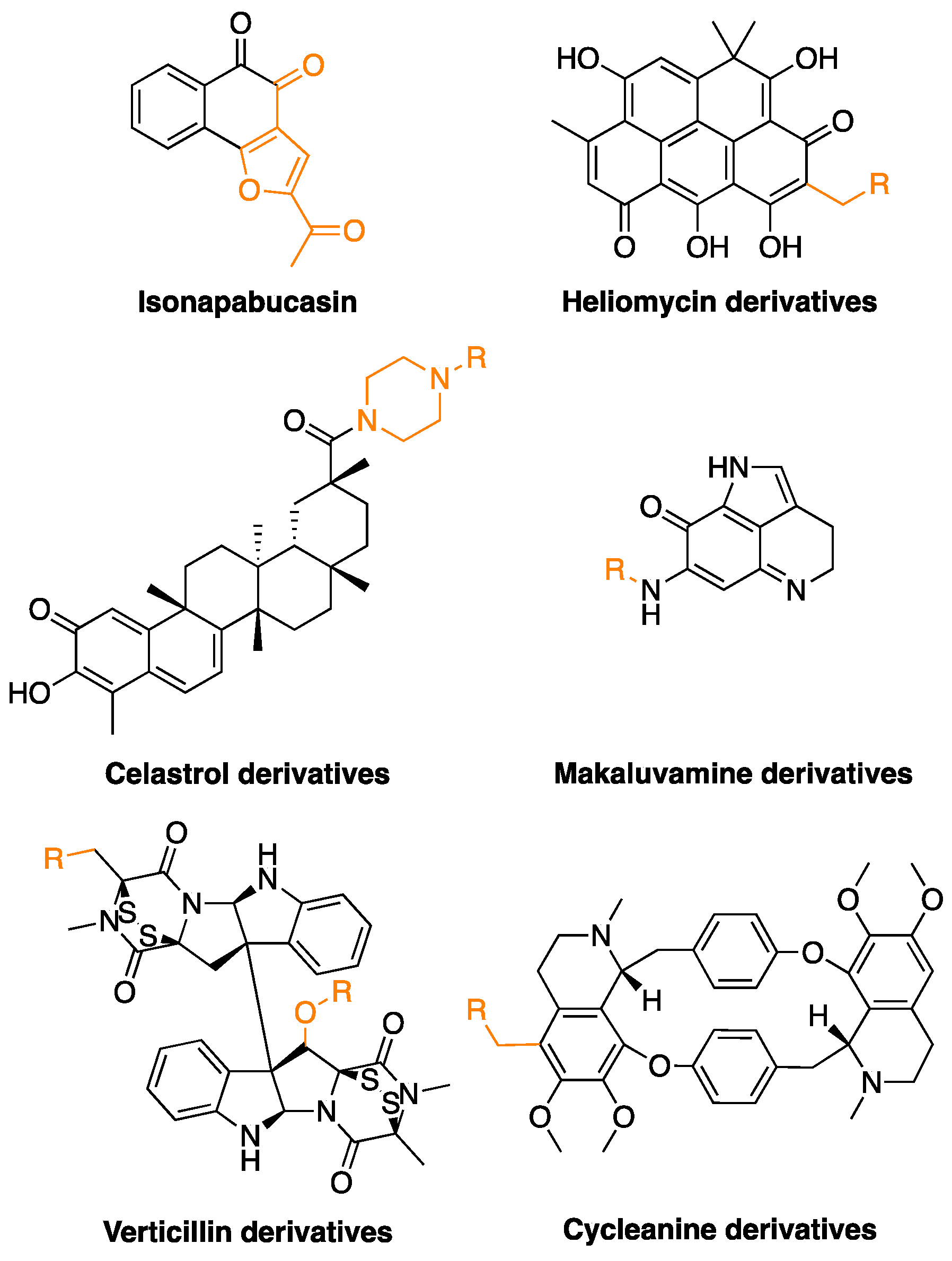
| Compound | Source | Chemical Structure of the Representative Component | Classification | Model | Mechanism of Action | References |
|---|---|---|---|---|---|---|
| Aminoalkyl derivatives of cycleanine | Triclisia subcordata | 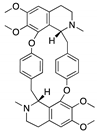 (Cycleanine) | Bisbenzylisoquinoline macrocyclic alkaloid | Cell lines | activation of caspases 3/7, cleavage of PARP | [149] |
| Berberine | European barberry, goldenseal, goldthread, Oregon grape, phellodendron, and tree turmeric |  | Alkaloid | A2780, HEY, HO8910 | Triggering oxidative DNA damage, targeting of cancer stem cells | [52,115] |
| Epigallocatechin gallate (EGCG) | Green tea |  | Flavonoid | SKOV3-ip1, SKOV3TR-ip2 | Reduction of hTERT and Bcl-2, alteration of the metabolism of stromal cells | [99,127,150] |
| FBA-TPQ (derivative of makaluvamines) | Zyzzya sponges | 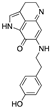 (Makaluvamine scaffold) | Pyrroloiminoquinone alkaloid | in vitro and in vivo (xenograft) | ROS species, p53-MDM2 and PI3K-Akt pathways | [151] |
| Phloretin | Apple tree leaves | 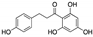 | Dihydrochalcone | in vitro | Alteration of the metabolism of stromal cells | [127] |
| Semi-synthetic derivatives of celastrol | Tripterygium species | 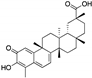 (Celastrol) | Nortriterpen quinone | in vitro | STAT-3 pathway, induction of apoptosis, reduction of cell migration | [152] |
| Shikonin | Alkanna tinctoria | 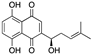 | Naphthoquinone | A278 cells, in vitro | Alteration of the metabolism of stromal cells | [127,153] |
| Tanshinones | Salvia miltiorrhiza | 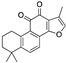 (Tanshinone IIA) | Terpenoid/Abietane | A-549, TOV-21G | Growth capacity is inhibited by reducing cell viability, alteration of the microenvironment | [92,123,128,143] |
| Verticillin H esters | Fungi | 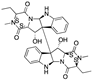 | Verticillins | OVCAR-3 | Reduced cell proliferation | [154] |
| β-escin | horse chestnut seed |  | Pentacyclic triterpenoid saponin | in vitro and in vivo | Alteration of the microenvironment | [129] |
| Compound/Formulation | Ovarian Cancer Model | Type of Study | Major Findings and Mechanisms | Reference |
|---|---|---|---|---|
| Graphene oxide polyvinylpyrrolidone-quercetin-gefitinib (GO-PVP-QSR-GEF) | Ovarian cancer cells | In vitro | Synergistic cytotoxic effect | [205] |
| Micellar(nanostructures) resveratrol (R):quercetin (Q) (mRQ) | Xenograft model | In vivo | Improvement of the efficacy of adriamycin | [206] |
| Quercetin | - | In vitro | Human telomeric G-quadruplex stabilization | [190] |
| Quercetin | Ovarian cancer cells | In vitro | Attenuation of metastatic ability | [201] |
| Quercetin micelle and thermosensitive hydrogel drug delivery system | SKOV-3 cells and animal model | In vitro and in vivo | Enhanced cytotoxicity | [207] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.R.; Rahman, M.M.; Dhar, P.S.; Nowrin, F.T.; Sultana, N.; Akter, M.; Rauf, A.; Khalil, A.A.; Gianoncelli, A.; Ribaudo, G. The Role of Natural and Semi-Synthetic Compounds in Ovarian Cancer: Updates on Mechanisms of Action, Current Trends and Perspectives. Molecules 2023, 28, 2070. https://doi.org/10.3390/molecules28052070
Islam MR, Rahman MM, Dhar PS, Nowrin FT, Sultana N, Akter M, Rauf A, Khalil AA, Gianoncelli A, Ribaudo G. The Role of Natural and Semi-Synthetic Compounds in Ovarian Cancer: Updates on Mechanisms of Action, Current Trends and Perspectives. Molecules. 2023; 28(5):2070. https://doi.org/10.3390/molecules28052070
Chicago/Turabian StyleIslam, Md. Rezaul, Md. Mominur Rahman, Puja Sutro Dhar, Feana Tasmim Nowrin, Nasrin Sultana, Muniya Akter, Abdur Rauf, Anees Ahmed Khalil, Alessandra Gianoncelli, and Giovanni Ribaudo. 2023. "The Role of Natural and Semi-Synthetic Compounds in Ovarian Cancer: Updates on Mechanisms of Action, Current Trends and Perspectives" Molecules 28, no. 5: 2070. https://doi.org/10.3390/molecules28052070
APA StyleIslam, M. R., Rahman, M. M., Dhar, P. S., Nowrin, F. T., Sultana, N., Akter, M., Rauf, A., Khalil, A. A., Gianoncelli, A., & Ribaudo, G. (2023). The Role of Natural and Semi-Synthetic Compounds in Ovarian Cancer: Updates on Mechanisms of Action, Current Trends and Perspectives. Molecules, 28(5), 2070. https://doi.org/10.3390/molecules28052070










