Organo-Montmorillonite Modified by Gemini Quaternary Ammonium Surfactants with Different Counterions for Adsorption toward Phenol
Abstract
1. Introduction
2. Results and Discussion
2.1. Surface Activity of Gemini Quaternary Ammonium Surfactants
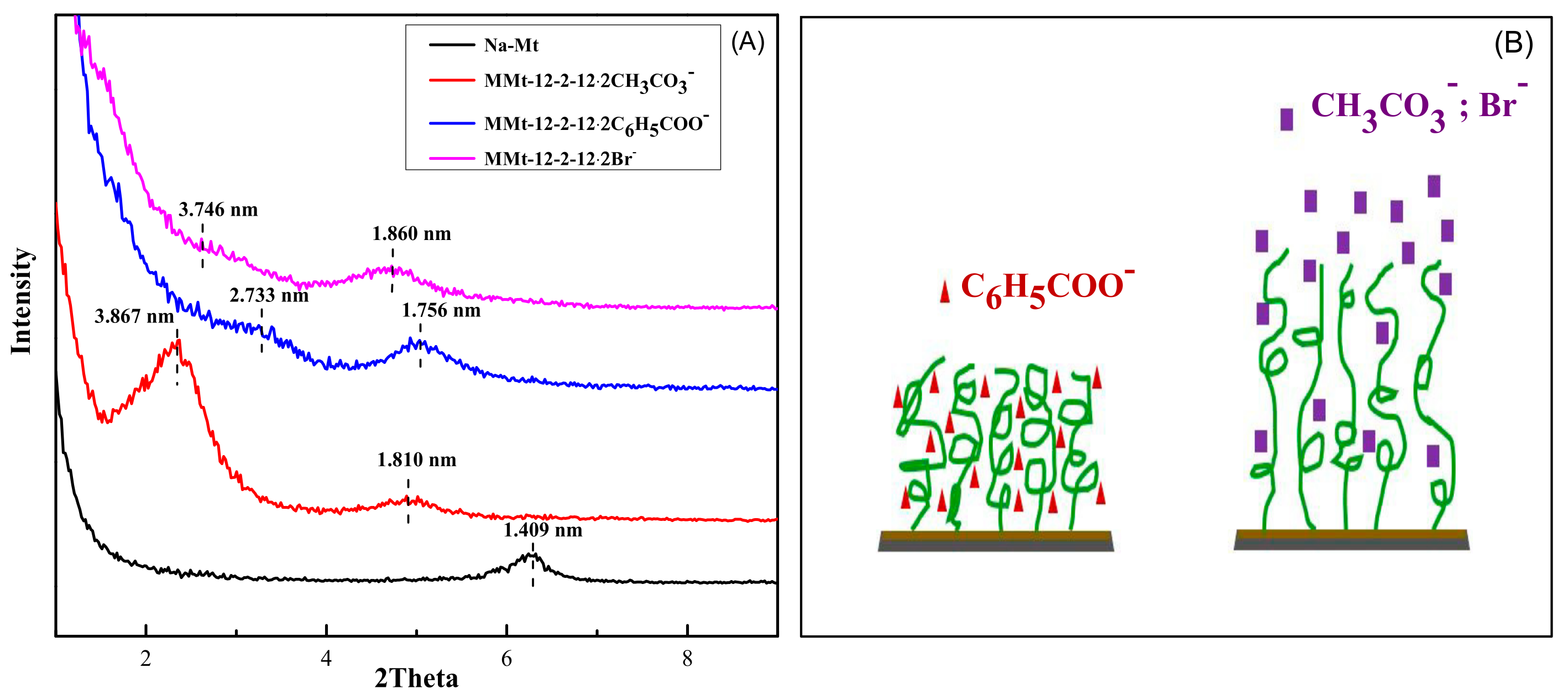
2.2. Analysis of X-ray Diffraction
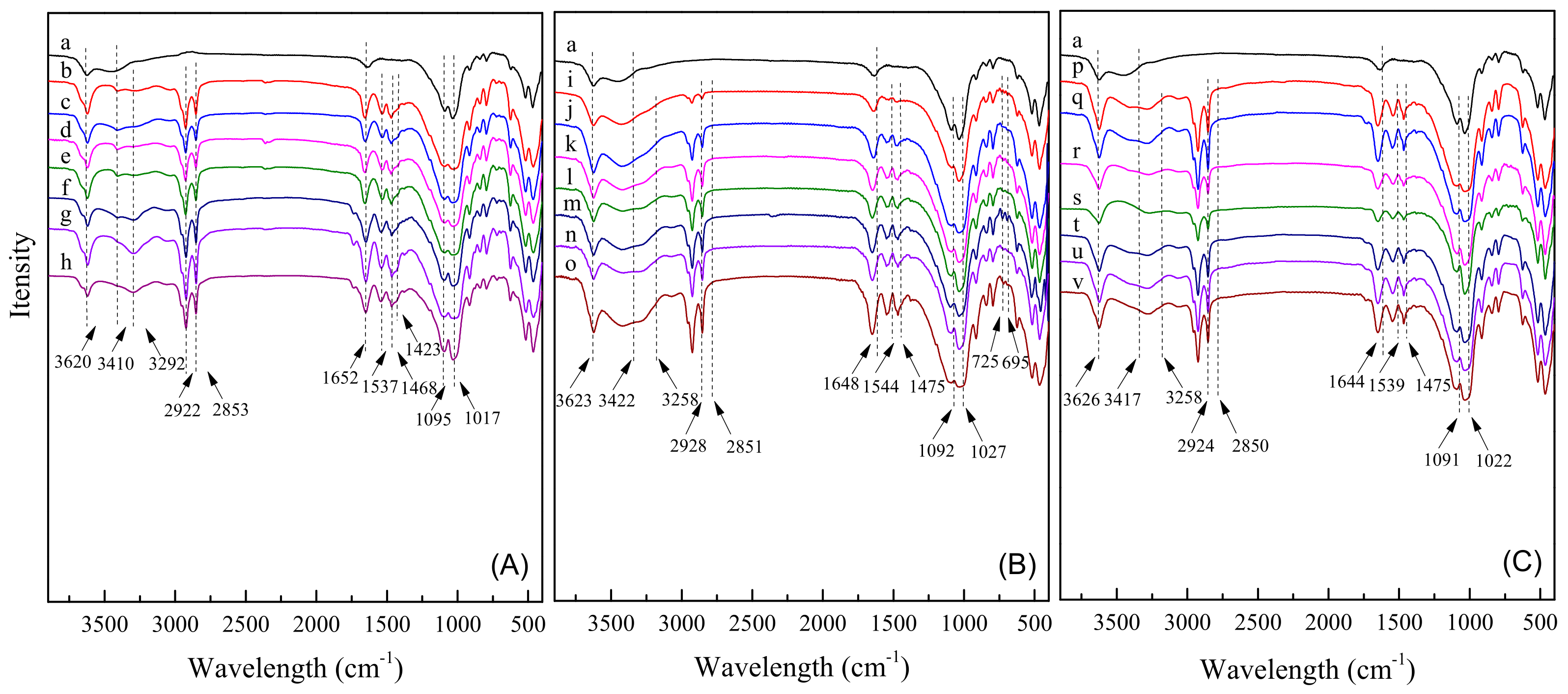
2.3. FT–IR Spectra Analysis
2.4. TG–DTG Analysis
2.5. SEM Analysis
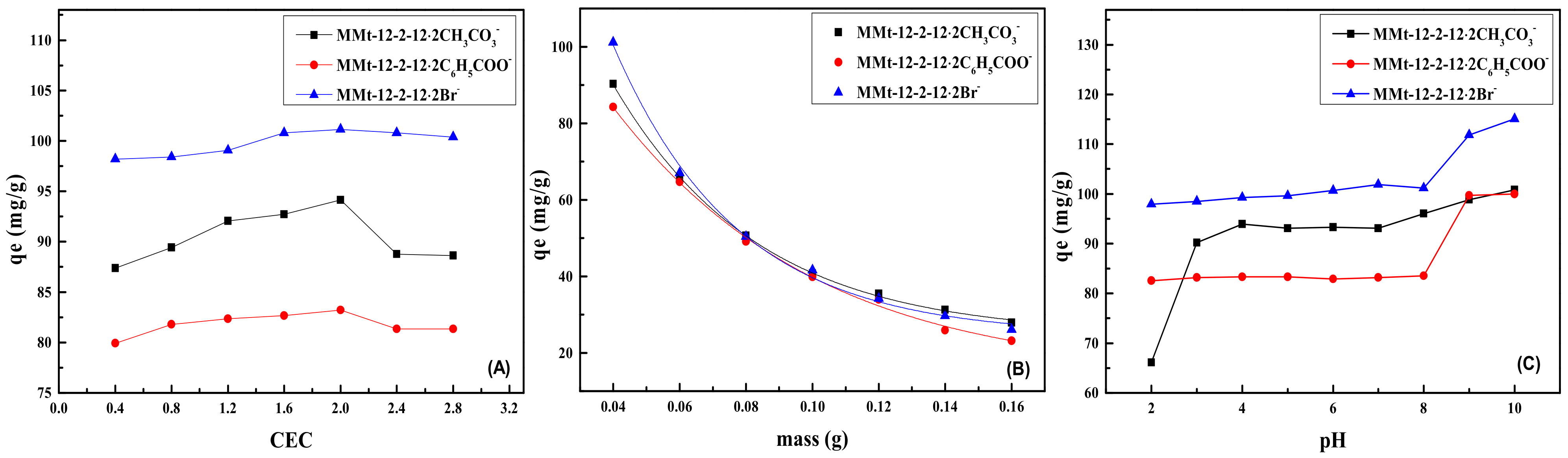
2.6. Effect of Surfactant Addition on Phenol Adsorption by MMt
2.7. Effect of Adsorbent Addition on Phenol Adsorption by MMt
2.8. Effect of pH on Phenol Adsorption by MMt
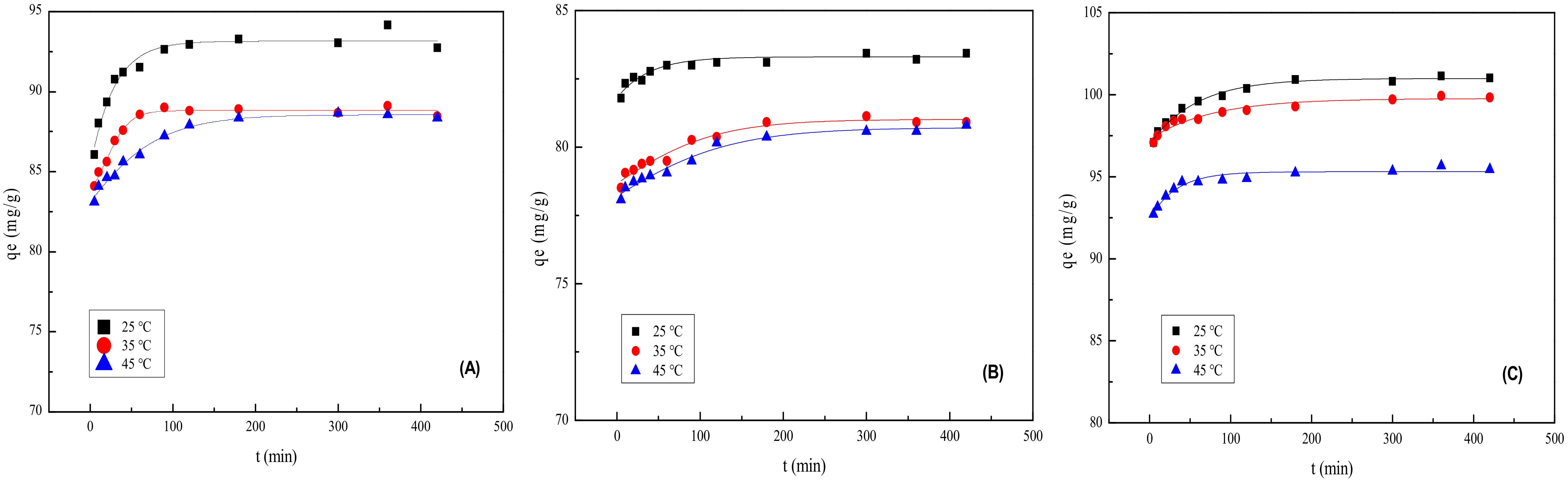
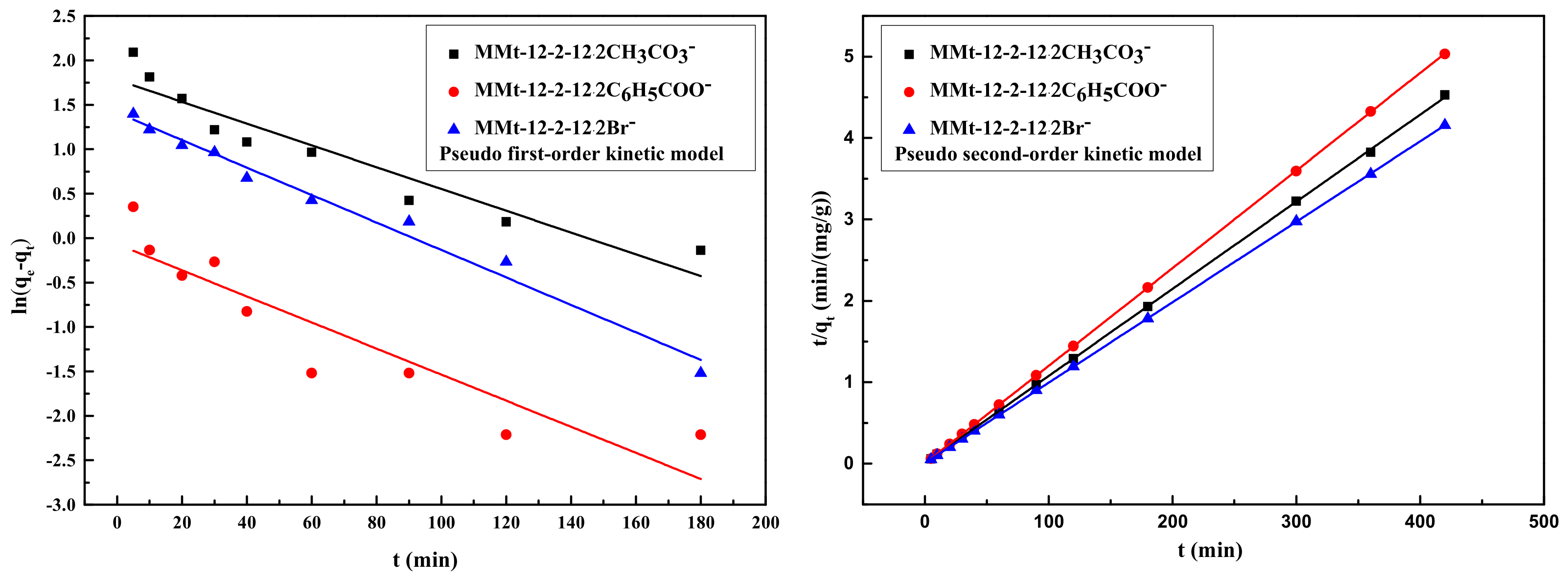
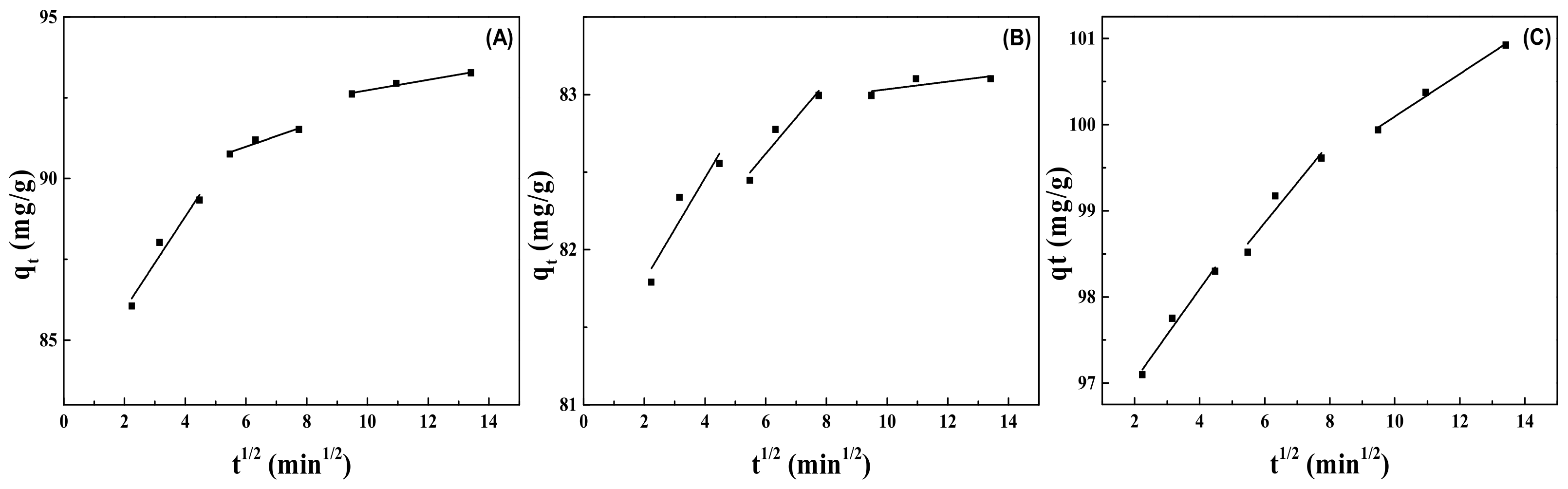
2.9. Adsorption Kinetics
2.10. Adsorption Equilibrium Isotherms
2.11. Thermodynamic Parameters
3. Materials and Methods
3.1. Materials
3.2. Surface Tension Measurements of Gemini Quaternary Ammonium Surfactants
3.3. Preparation of MMt
3.4. Characterization of MMt
3.5. Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mohammadi, K.A.; Sanaeepur, H.; Abbassian, K.; Najafi, A.; Mofarrah, E. Phenol Removal from Industrial Wastewaters: A Short Review. Desalin. Water Treat. 2015, 46, 2215–2234. [Google Scholar] [CrossRef]
- Tchieno, F.; Tonle, I.K. p-Nitrophenol determination and remediation: An overview. Rev. Anal. Chem. 2018, 37, 20170019. [Google Scholar] [CrossRef]
- Zheng, H.; Guo, W.; Li, S.; Chen, Y.; Chang, J.S. Adsorption of p-nitrophenols (PNP) on microalgal biochar: Analysis of high adsorption capacity and mechanism. Bioresour. Technol. 2017, 244, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Duan, R. Simulation and assessment of a water pollution accident caused by phenol leakage. Water Policy 2021, 23, 750–764. [Google Scholar] [CrossRef]
- Xu, Y.; Khan, M.A.; Wang, F.; Xia, M.; Lei, W. Novel multi amine-containing Gemini surfactant modified montmorillonite as adsorbents for removal of phenols. Appl. Clay Sci. 2018, 162, 204–213. [Google Scholar] [CrossRef]
- Awad, A.M.; Shaikh, S.; Jalab, R.; Gulied, M.H.; Adham, S. Adsorption of Organic Pollutants by Natural and Modified Clays: A Comprehensive Review. Sep. Purif. Technol. 2019, 228, 115719–115758. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, C.; Hu, X.; Wang, Y.; Xu, R.; Xia, C.; Zhang, H.; Song, Z. Catalytic wet peroxide oxidation of 4-chlorophenol over Al-Fe-, Al-Cu-, and Al-Fe-Cu-pillared clays: Sensitivity, kinetics and mechanism. Appl. Clay Sci. 2014, 95, 275–283. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.; Zhao, Y.; Xie, M. Cationic surfactant modified attapulgite for removal of phenol from wastewater. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 641, 128479. [Google Scholar] [CrossRef]
- Mohamad Said, K.A.; Ismail, A.F.; Karim, Z.A.; Abdullah, M.S.; Hafeez, A. A review of technologies for the phenolic compounds recovery and phenol removal from wastewater. Process. Saf. Environ. Protect. 2021, 151, 257–289. [Google Scholar] [CrossRef]
- Eryilmaz, C.; Genc, A. Review of Treatment Technologies for the Removal of Phenol from Wastewaters. J. Water Chem. Techno. 2021, 43, 145–154. [Google Scholar] [CrossRef]
- Ortiz-Martinez, K.; Reddy, P.; Cabrera-Lafaurie, W.A.; Roman, F.R.; Hernandez-Maldonado, A.J. Single and multi-component adsorptive removal of bisphenol A and 2,4-dichlorophenol from aqueous solutions with transition metal modified inorganic-organic pillared clay composites: Effect of pH and presence of humic acid. J. Hazard. Mater. 2016, 312, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Mousavia, S.M.; Babapoorb, A.; Hashemia, S.A.; Medic, B. Adsorption and Removal Characterization of Nitrobenzene by Graphene Oxide Coated by Polythiophene Nanoparticle. Phys. Chem. Res. 2020, 8, 225–240. [Google Scholar]
- Parvin, N.; Babapoor, A.; Nematollahzadeh, A.; Mousavia, S.M. Removal of phenol and β-naphthol from aqueous solution by decorated graphene oxide with magnetic iron for modified polyrhodanine as nanocomposite adsorbents: Kinetic, equilibrium and thermodynamic studies. React. Funct. Polym. 2020, 156, 104718. [Google Scholar] [CrossRef]
- Esmaeili, H.; Mousavi, S.M.; Hashemi, S.A.; Chiang, W.H.; Abnavi, S.A. Application of biosurfactants on the removal of oil from emulsion. Carbon Lett. 2021, 31, 851–862. [Google Scholar] [CrossRef]
- Zadeh, B.S.; Esmaeili, H.; Foroutan, R.; Mousavi, S.M.; Hashemi, S.A. Removal of cd2+ from aqueous solution using eucalyptus sawdust as a bio-adsorbent: Kinetic and equilibrium studies. J. Environ. Treat. Tech. 2020, 8, 112–118. [Google Scholar]
- Hashemi, S.A.; Mousavi, S.M.; Ramakrishna, S. Effective removal of mercury, arsenic and lead from aqueous media using Polyaniline-Fe3O4- silver diethyldithiocarbamate nanostructures. J. Clean Prod. 2019, 239, 118023. [Google Scholar] [CrossRef]
- Nematollahzadeh, A.; Babapoor, A.; Mousavi, S.M.; Nuri, A. Nitrobenzene adsorption from aqueous solution onto polythiophene-modified magnetite nanoparticles. Mater. Chem. Phys. 2021, 262, 124266. [Google Scholar] [CrossRef]
- Flores, F.M.; Undabeytia, T.; Morillo, E.; Torres Sánchez, R.M. Technological applications of organo-montmorillonites in the removal of pyrimethanil from water: Adsorption/desorption and flocculation studies. Environ. Sci. Pollut. Res. 2017, 24, 14463–14476. [Google Scholar] [CrossRef]
- Taiga, T.; Yoshiumi, K.; Masashi, S.; Yasumasa, T.; Choji, F.; Yasuhisa, M. An easy and effective method for the intercalation of hydrophobic natural dye into organo-montmorillonite for improved photostability. J. Phys. Chem. Solids. 2018, 116, 168–173. [Google Scholar]
- Issabayeva, G.; Hang, S.Y.; Wong, M.C.; Aroua, M.K. A review on the adsorption of phenols from wastewater onto diverse groups of adsorbents. Rev. Chem. Eng. 2018, 34, 855–873. [Google Scholar] [CrossRef]
- Feng, J.J.; Yan, Z.H.; Song, J.M.; He, J.C.; Zhao, G.; Fan, H.M. Study on the structure-activity relationship between the molecular structure of sulfate Gemini surfactant and surface activity, thermodynamic properties and foam properties. Chem. Eng. Sci. 2021, 245, 116857–116871. [Google Scholar] [CrossRef]
- Lyu, W.F.; Zhou, Z.H.; Huang, J.; Yan, K. Study on adsorption behavior of a new type Gemini surfactant onto quartz surface by a molecular dynamics method. Nano 2022, 17, 2150151. [Google Scholar] [CrossRef]
- Yang, S.; Gao, M.; Luo, Z.; Yang, Q. The characterization of organo-montmorillonite modified with a novel aromatic-containing gemini surfactant and its comparative adsorption for 2-naphthol and phenol. Chem. Eng. J. 2015, 268, 125–134. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, M.; Gu, Z.; Luo, Z.; Ye, Y.; Lu, L. Compatibility analysis of municipal solid waste incineration residues and clay for producing lightweight aggregates. J. Hazard. Mater. 2014, 267, 71–80. [Google Scholar] [CrossRef]
- Taleb, K.; Pillin, I.; Grohens, Y.; Saidi-Besbes, S. Gemini surfactant modified clays: Effect of surfactant loading and spacer length. Appl. Clay Sci. 2018, 161, 48–56. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, M.L.; Luo, Z.X.; Yang, S.F. Enhanced removal of bisphenol A from aqueous solution by organo-montmorillonites modified with novel Gemini pyridinium surfactants containing long alkyl chain. Chem. Eng. J. 2016, 285, 27–38. [Google Scholar] [CrossRef]
- Ren, H.; Tian, S.; Zhu, M.; Zhao, Y.; Li, K.; Ma, Q.; Ding, S.; Gao, J.; Miao, Z. Modification of montmorillonite by Gemini surfactants with different chain lengths and its adsorption behavior for methyl orange. Appl. Clay Sci. 2018, 151, 29–36. [Google Scholar] [CrossRef]
- Srinivasarao, K.; Prabhu, S.M.; Luo, W.; Sasaki, K. Enhanced adsorption of perchlorate by gemini surfactant-modified montmorillonite: Synthesis, characterization and their adsorption mechanism. Appl. Clay Sci. 2018, 163, 46–55. [Google Scholar] [CrossRef]
- Yang, S.F.; Gao, M.L.; Luo, Z.X. Adsorption of 2-Naphthol on the organo-montmorillonites modified by Gemini surfactants with different spacers. Chem. Eng. J. 2014, 256, 39–50. [Google Scholar] [CrossRef]
- Shen, T.; Gao, M.L. Gemini surfactant modified organo-clays for removal of organic pollutants from water: A review. Chem. Eng. J. 2019, 375, 121910–121917. [Google Scholar] [CrossRef]
- Wang, C.Y.; Morgner, H. The dependence of surface tension on surface properties of ionic surfactant solution and the effects of counterions therein. Phys. Chem. Chem. Phys. 2014, 16, 23386–23393. [Google Scholar] [CrossRef] [PubMed]
- Li, P.P.; Khan, M.A.; Xia, M.Z.; Lei, W.; Zhu, S.D.; Wang, F.Y. Efficient preparation and molecular dynamic (MD) simulations of Gemini surfactant modified layered montmorillonite to potentially remove emerging organic contaminants from wastewater. Ceram. Int. 2019, 45, 10782–10791. [Google Scholar] [CrossRef]
- Willott, J.D.; Murdoch, T.J. Anion-specific effects on the behavior of pH-sensitive polybasic brushes. Langmuir 2015, 31, 3707–3717. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Wu, P.; Yang, L.; Huang, Z.; Zhu, N.; Hu, Z. Efficient removal of cesium from aqueous solution with vermiculite of enhanced adsorption property through surface modification by ethylamine. J. Colloid Interface Sci. 2014, 428, 295–301. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.I. Effect of thermally stable oligomerically modified clay on the properties of aramid-based nanocomposite materials. J. Mater. Res. 2008, 23, 3330–3338. [Google Scholar] [CrossRef]
- Yu, M.M.; Gao, M.L.; Shen, T. Organo-vermiculites modified by low-dosage Gemini surfactants with different spacers for adsorption toward p-nitrophenol. Colloid Surf. A-Physicochem. Eng. Asp. 2018, 553, 601–611. [Google Scholar] [CrossRef]
- Cao, G.; Gao, M.; Shen, T.; Zhao, B.; Zeng, H. Comparison between Asymmetric and Symmetric Gemini Surfactant-Modified Novel Organo-vermiculites for Removal of Phenols. Ind. Eng. Chem. Res. 2019, 58, 12927–12938. [Google Scholar] [CrossRef]
- Luo, W.H.; Ouyang, J.P.; Antwi, P.; Meng, Z.Q.; Huang, W.W. Microwave/ultrasound-assisted modification of montmorillonite by conventional and Gemini alkyl quaternary ammonium salts for adsorption of chromate and phenol: Structure-function relationship. Sci. Total Environ. 2019, 655, 1104–1112. [Google Scholar] [CrossRef]
- Li, X.J.; Wang, Z.M.; Ning, J.K.; Gao, M.M.; Jiang, W.B.; Zhou, Z.D.; Li, G.Y. Preparation and characterization of a novel polyethyleneimine cation-modified persimmon tannin bioadsorbent for anionic dye adsorption. J. Environ. Manag. 2018, 217, 305–314. [Google Scholar] [CrossRef]
- Sarkar, B.; Xi, Y.; Megharaj, M.; Krishnamurti, G.S.; Rajarathnam, D.; Naidu, R. Remediation of hexavalent chromium through adsorption by bentonite based Arquad 2HT-75 organoclays. J. Hazard. Mater. 2010, 183, 87–97. [Google Scholar] [CrossRef]
- Xue, G.H.; Gao, M.L.; Gu, Z.; Luo, Z.X.; Hu, Z.C. The removal of p-nitro phenol from aqueous solutions by adsorption using Gemini surfactants modified montmorillonites. Chem. Eng. J. 2013, 218, 223–231. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, M.L.; Zang, W.L. Comparative study of 2,4,6-trichlorophenol adsorption by montmorillonites functionalized with surfactants differing in the number of head group and alkyl chain. Colloid Surf. A-Physicochem. Eng. Asp. 2017, 520, 805–816. [Google Scholar] [CrossRef]
- Tang, D.Y.; Zheng, Z.; Lin, K.; Luan, J.F.; Zhang, J.B. Adsorption of p-nitrophenol from aqueous solutions onto activated carbon fiber. J. Hazard. Mater. 2007, 143, 49–56. [Google Scholar] [CrossRef]
- Ouachtak, H.; Guerdaoui, A.E.; Haounati, R.; Akhouairi, S.; Haouti, R.E.; Hafid, N.; Addi, A.A.; Šljukić, B.; Santos, D.M.F.S.; Taha, M.L. Highly efficient and fast batch adsorption of orange G dye from polluted water using superb organo-montmorillonite: Experimental study and molecular dynamics investigation. J. Mol. Liq. 2021, 335, 116560. [Google Scholar] [CrossRef]
- Haounati, R.; Ouachtak, H.; Haouti, R.E.; Akhouairi, S.; Largo, F.; Akbal, F.; Benlhachemi, A.; Jada, A.; Addi, A.A. laboration and properties of a new SDS/CTAB@Montmorillonite organo-clay compositeas a superb adsorbent for the removal of malachite green from aqueous solutions. Sep. Purif. Technol. 2021, 255, 117335. [Google Scholar] [CrossRef]
- Yang, W.G.; Cao, Y.P.; Wang, Y.K.; Ju, H.B.; Jiang, Y.J.; Geng, T. Effects of unsaturated double bonds on adsorption and aggregation behaviors of amide-based cationic Gemini surfactants. Colloid Surf. A-Physicochem. Eng. Asp. 2021, 611, 125778–125788. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Hu, S.Q.; Wang, Y.; Sana, U.; Hu, J.; Liu, H.Q.; Xu, B.C. The surface adsorption, aggregate structure and antibacterial activity of Gemini quaternary ammonium surfactants with carboxylic counterions. R. Soc. Open Sci. 2019, 6, 190378–190393. [Google Scholar] [CrossRef]
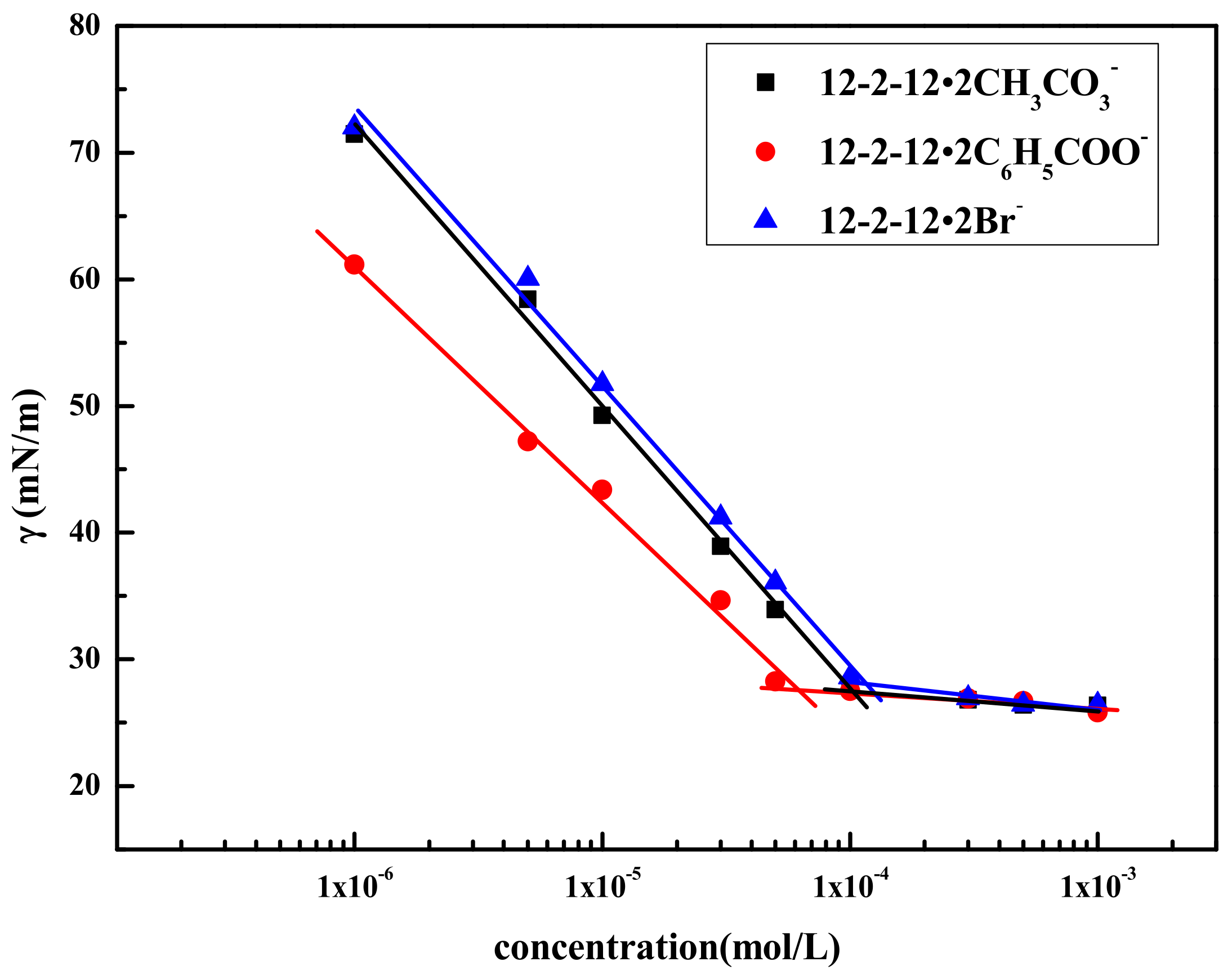



| Compound | CMC | γCMC | pC20 | |
|---|---|---|---|---|
| (mol·L−1) | (mN·m−1) | (mol·L−1) | (KJ·mol−1) | |
| 12–2–12·2CH3CO3− | 1.04 × 10−4 | 27.65 | 3.983 | −32.69 |
| 12–2–12·2C6H5COO− | 6.20 × 10−5 | 27.52 | 4.208 | −38.49 |
| 12–2–12·2Br− | 1.19 × 10−4 | 27.93 | 3.924 | −38.49 |
| MMt | qe exp (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| k1 | qe cal | R2 | k2 | qe cal | R2 | ||
| MMt–12–2–12·2CH3CO3− | 94.140 | 0.01226 | 5.923 | 0.90843 | 0.01438 | 93.458 | 0.99992 |
| MMt–12–2–12·2C6H5COO− | 83.212 | 0.01467 | 0.933 | 0.84495 | 0.04016 | 83.403 | 1 |
| MMt–12–2–12·2Br− | 101.139 | 0.01546 | 4.102 | 0.98400 | 0.01417 | 101.215 | 0.99999 |
| MMt | Intra-Particle Diffusion Model | ||
|---|---|---|---|
| kp | C | R2 | |
| MMt–12–2–12·2CH3CO3− | 0.32625 | 89.02926 | 0.94919 |
| MMt–12–2–12·2C6H5COO− | 0.23190 | 81.22691 | 0.93390 |
| MMt–12–2–12·2Br− | 0.46000 | 96.07646 | 0.93390 |
| MMt | Freundlich Adsorption Isotherm | Langmuir Adsorption Isotherm | ||||
|---|---|---|---|---|---|---|
| kF | N | R2 | kL | qmax | R2 | |
| MMt–12–2–12·2CH3CO3− | 2.236 | 1.212 | 0.996 | 0.00431 | 331.126 | 0.829 |
| MMt–12–2–12·2C6H5COO− | 0.623 | 0.940 | 0.997 | 0.00123 | 598.802 | 0.670 |
| MMt–12–2–12·2Br− | 1.581 | 1.040 | 0.969 | 0.00162 | 909.091 | 0.143 |
| MMT | C0 Mg·L−1 | ΔH° kJ·mol−1 | ΔS° J·mol−1·K−1 | ΔG° (KJ·mol−1) | R2 | ||
|---|---|---|---|---|---|---|---|
| 298K | 308K | 318K | |||||
| MMt–12–2–12·2CH3CO3− | 100 | −3.380 | −8.122 | −0.959 | −0.878 | −0.797 | 0.847 |
| MMt–12–2–12·2C6H5COO | 100 | −1.967 | −4.819 | −0.531 | −0.483 | −0.435 | 0.904 |
| MMt–12–2–12·2Br− | 100 | −3.736 | −8.105 | −1.321 | −1.240 | −1.159 | 0.934 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, R.; Mo, Y.; Fu, D.; Liu, H.; Xu, B. Organo-Montmorillonite Modified by Gemini Quaternary Ammonium Surfactants with Different Counterions for Adsorption toward Phenol. Molecules 2023, 28, 2021. https://doi.org/10.3390/molecules28052021
Wei R, Mo Y, Fu D, Liu H, Xu B. Organo-Montmorillonite Modified by Gemini Quaternary Ammonium Surfactants with Different Counterions for Adsorption toward Phenol. Molecules. 2023; 28(5):2021. https://doi.org/10.3390/molecules28052021
Chicago/Turabian StyleWei, Ran, Yuanhua Mo, Duojiao Fu, Hongqin Liu, and Baocai Xu. 2023. "Organo-Montmorillonite Modified by Gemini Quaternary Ammonium Surfactants with Different Counterions for Adsorption toward Phenol" Molecules 28, no. 5: 2021. https://doi.org/10.3390/molecules28052021
APA StyleWei, R., Mo, Y., Fu, D., Liu, H., & Xu, B. (2023). Organo-Montmorillonite Modified by Gemini Quaternary Ammonium Surfactants with Different Counterions for Adsorption toward Phenol. Molecules, 28(5), 2021. https://doi.org/10.3390/molecules28052021






