A Review on the Sources, Structures, and Pharmacological Activities of Lucidenic Acids
Abstract
1. Introduction
2. Sources and Contents
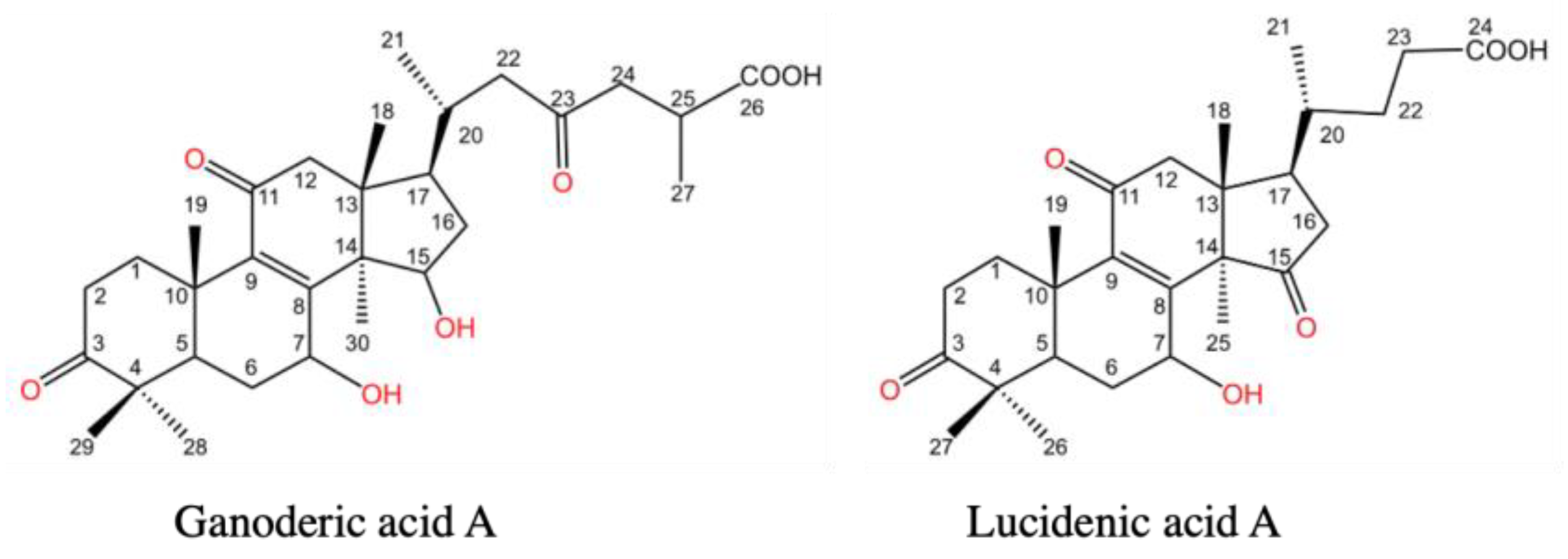
3. Chemical Structures of Lucidenic Acids
4. Potential Pharmacological Effects of Lucidenic Acids
4.1. Anti-Cancer Effect
4.2. Anti-Inflammatory Effect
4.3. Antioxidant Effect
4.4. Anti-Viral Effect
4.5. Neuroprotective Effect
4.6. Anti-Hyperlipidemic Effect
4.7. Anti-Hypercholesterolemic Effect
4.8. Anti-Hyperglycemic Effect
4.9. Other Pharmacological Effects
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hamid, K.; Alqahtani, A.; Kim, M.; Cho, J.; Cui, P.H.; Li, C.G.; Groundwater, P.W.; Li, G.Q. Tetracyclic triterpenoids in herbal medicines and their activities in diabetes and its complications. Curr. Top. Med. Chem. 2015, 15, 2406–2430. [Google Scholar] [CrossRef]
- Ren, Y.; Kinghorn, A.D. Natural product triterpenoids and their semi-synthetic derivatives with potential anticancer activity. Planta Med. 2019, 85, 802–814. [Google Scholar] [CrossRef]
- Madasu, C.; Xu, Y.; Wijeratne, E.M.K.; Liu, M.X.; Molnár, I.; Gunatilaka, A.A.L. Semi-synthesis and cytotoxicity evaluation of pyrimidine, thiazole, and indole analogues of argentatins A–C from guayule (Parthenium argentatum) resin. Med. Chem. Res. 2022, 31, 1088–1098. [Google Scholar] [CrossRef]
- Xu, Y.M.; Madasu, C.; Liu, M.X.; Wijeratne, E.M.K.; Dierig, D.; White, B.; Molnár, I.; Gunatilaka, A.A.L. Cycloartane- and lanostane-type triterpenoids from the resin of Parthenium argentatum AZ-2, a byproduct of Guayule rubber production. ACS Omega 2021, 6, 15486–15498. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yan, S.; Zhang, Z.; Gong, W.; Zhu, Z.; Zhou, Y.; Yan, L.; Hu, Z.; Ai, L.; Peng, Y. Mapping the metabolic signatures of fermentation broth, mycelium, fruiting body and spores powder from Ganoderma lucidum by untargeted metabolomics. LWT 2020, 129, 109494. [Google Scholar] [CrossRef]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from Ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef]
- Kubota, T.; Asaka, Y.; Miura, I.; Mori, H. Structures of Ganoderic acid A and B, two new lanostane type bitter triterpenes from Ganoderma lucidum (FR.) KARST. Helv. Chim. Acta 1982, 65, 611–619. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.; Sharma, A.; Tuli, H.S.; Batra, P.; Beniwal, V.; Gupta, G.K.; Sharma, A.K. Bioactive metabolites of Ganoderma lucidum: Factors, mechanism and broad spectrum therapeutic potential. J. Herb. Med. 2019, 17, 100268. [Google Scholar] [CrossRef]
- Sato, N.; Zhang, Q.; Ma, C.M.; Hattori, M. Anti-human immunodeficiency virus-1 protease activity of new lanostane-type triterpenoids from Ganoderma sinense. Chem. Pharm. Bull. 2009, 57, 1076–1080. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Tsilingiris, D.; Christodoulatos, G.S.; Karampela, I.; Dalamaga, M. Anti-viral treatment for SARS-CoV-2 infection: A race against time amidst the ongoing pandemic. Metab. Open 2021, 10, 100096. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-L.; Yu, Y.S.; Yen, G.C. Lucidenic acid B induces apoptosis in human leukemia cells via a mitochondria-mediated pathway. J. Agric. Food Chem. 2008, 56, 3973–3980. [Google Scholar] [CrossRef]
- Weng, C.J.; Chau, C.F.; Hsieh, Y.S.; Yang, S.F.; Yen, G.C. Lucidenic acid inhibits PMA-induced invasion of human hepatoma cells through inactivating MAPK/ERK signal transduction pathway and reducing binding activities of NF-κB and AP-1. Carcinogenesis 2008, 29, 147–156. [Google Scholar] [CrossRef]
- Ćilerdžić, J.L.; Sofrenić, I.V.; Tešević, V.V.; Brčeski, I.D.; Duletić-Laušević, S.N.; Vukojević, J.B.; Stajić, M.M. Neuroprotective potential and chemical profile of alternatively cultivated Ganoderma lucidum basidiocarps. Chem. Biodivers. 2018, 15, e1800036. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Y.; Zhang, X.; Xu, J.; Wang, H.; Zhao, L.; Wang, Y. Screening immunoactive compounds of Ganoderma lucidum spores by mass spectrometry molecular networking combined with in vivo zebrafish assays. Front. Pharmacol. 2020, 11, 287. [Google Scholar] [CrossRef]
- Lee, I.; Ahn, B.; Choi, J.; Hattori, M.; Min, B.; Bae, K. Selective cholinesterase inhibition by lanostane triterpenes from fruiting bodies of Ganoderma lucidum. Bioorg. Med. Chem. Lett. 2011, 21, 6603–6607. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chang, Q.; Wong, L.K.; Chong, F.S.; Li, R.C. Triterpene antioxidants from Ganoderma lucidum. Phytother. Res. 1999, 13, 529–531. [Google Scholar] [CrossRef]
- Lin, D.; Yu-Rong, L.; Jun, Y.; Xiao-Hong, F.; Wei, D. Studies on chemical constituents of triterpenoids from Ganoderma sinense. Nat. Prod. Res. Dev. 2018, 30, 1669. [Google Scholar]
- Welti, S.; Moreau, P.A.; Decock, C.; Danel, C.; Duhal, N.; Favel, A.; Courtecuisse, R. Oxygenated lanostane-type triterpenes profiling in laccate Ganoderma chemotaxonomy. Mycol. Prog. 2015, 14, 45. [Google Scholar] [CrossRef]
- Weng, C.J.; Fang, P.S.; Chen, D.H.; Chen, K.D.; Yen, G.C. Anti-invasive effect of a rare mushroom, Ganoderma colossum, on human hepatoma cells. J. Agric. Food Chem. 2010, 58, 7657–7663. [Google Scholar] [CrossRef]
- Liu, L.Y. Studies on the Chemical Constituents and Bioactivities of the Fruiting Bodies of Ganoderma Theaecolum, Ganoderma Sessile and Ganoderma Mastoporum (in Chinese). Ph.D. Thesis, Peking Union Medical College, Beijing, China, 2017. [Google Scholar]
- Liu, C.; Pu, Q.; Wang, H.; Chen, R. Chemical constituents from fruiting bodies of Ganoderma tsugae (Ⅱ) (in Chinese). Chin. Trad. Herb. Drugs 2007, 38, 1610–1612. [Google Scholar]
- Trigos, Á.; Suárez Medellín, J. Biologically active metabolites of the genus Ganoderma: Three decades of myco-chemistry research. Rev. Mex. Micol. 2011, 34, 63–83. [Google Scholar]
- Ha, D.T.; Loan, L.T.; Hung, T.M.; Han, L.V.N.; Khoi, N.M.; Dung, L.V.; Min, B.S.; Nguyen, N.P.D. An improved HPLC-DAD method for quantitative comparisons of triterpenes in Ganoderma lucidum and its five related species originating from Vietnam. Molecules 2015, 20, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Luo, Y.; Huang, S.Z.; Guo, Z.K.; Dai, H.F.; Zhao, Y.X. Lanostane triterpenoids with cytotoxic activities from the fruiting bodies of Ganoderma hainanense. J. Asian Nat. Prod. Res. 2013, 15, 1214–1219. [Google Scholar] [CrossRef]
- Xiong, Q.; Sun, C.; Shi, H.; Cai, S.; Xie, H.; Liu, F.; Zhu, J. Analysis of related metabolites affecting taste values in rice under different nitrogen fertilizer amounts and planting densities. Foods 2022, 11, 1508. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Li, R.; Wu, X.; Zheng, C.; Shiu, P.H.T.; Chan, J.C.K.; Rangsinth, P.; Liu, C.; Leung, S.W.S.; et al. Protective effects of Amauroderma rugosum on doxorubicin-induced cardiotoxicity through suppressing oxidative stress, mitochondrial dysfunction, apoptosis, and activating Akt/mTOR and Nrf2/HO-1 signaling pathways. Oxid. Med. Cell. Longev. 2022, 2022, 9266178. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.K.; Dash, U.C.; Kanhar, S.; Mahapatra, A.K. In vitro biological assessment of Homalium zeylanicum and isolation of lucidenic acid A triterpenoid. Toxicol. Rep. 2017, 4, 274–281. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, X.; Sun, Y.; Li, Y.; Wang, Y.; Jiang, Y.; Shao, H.; Yong, B.; Li, H.; Tao, X. Comparative Metabolomic Profiling of Compatible and Incompatible Interactions between Potato and Phytophthora infestans. Front. Microbiol. 2022, 13, 57160. [Google Scholar] [CrossRef]
- Nishitoba, T.; Sato, H.; Kasai, T.; Kawagishi, H.; Sakamura, S. New bitter C27 and C30 terpenoids from the fungus Ganoderma lucidum (Reishi). Agric. Biol. Chem. 1985, 49, 1793–1798. [Google Scholar]
- Pavlik, M.; Zhou, S.; Zhang, J.; Tang, Q.; Feng, N.; Kurjak, D.; Pavlík, M., Jr.; Kunca, A. Comparative analysis of triterpene composition between Ganoderma lingzhi from China and G. lucidum from Slovakia under different growing conditions. Int. J. Med. Mushrooms 2020, 22, 793–802. [Google Scholar] [CrossRef]
- Pecić, S.; Nikićević, N.; Veljović, M.; Jardanin, M.; Tešević, V.; Belović, M.; Nikšić, M. The influence of extraction parameters on physicochemical properties of special grain brandies with Ganoderma lucidum. Chem. Ind. Chem. Eng. Q. 2016, 22, 181–189. [Google Scholar] [CrossRef]
- Nishitoba, T.; Sato, H.; Shirasu, S.; Sakamura, S. Novel triterpenoids from the mycelial mat at the previous stage of fruiting of Ganoderma lucidum. Agric. Biol. Chem. 1987, 51, 619–622. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Ganoderma–a therapeutic fungal biofactory. Phytochemistry 2006, 67, 1985–2001. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Liu, S.; Xie, F.; Zhao, L.; Wu, X. Enhanced production of polysaccharides and triterpenoids in Ganoderma lucidum fruit bodies on induction with signal transduction during the fruiting stage. PLoS ONE 2018, 13, e0196287. [Google Scholar] [CrossRef]
- Nishitoba, T.; Sato, S.; Sakamura, S. New terpenoids from Ganoderma lucidum and their bitterness. Agric. Biol Chem. 1985, 49, 1547–1549. [Google Scholar] [CrossRef]
- Wu, T.S.; Shi, L.S.; Kuo, S.C.; Cherng, C.Y.; Tung, S.F.; Teng, C.M. Platelet aggregation inhibitor from Ganoderma lucium. J. Chin. Chem. Soc. 1997, 44, 157–161. [Google Scholar] [CrossRef]
- Li, L.; Guo, H.J.; Zhu, L.Y.; Zheng, L.; Liu, X. A supercritical-CO2 extract of Ganoderma lucidum spores inhibits cholangiocarcinoma cell migration by reversing the epithelial–mesenchymal transition. Phytomedicine 2016, 23, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.J.; Zheng, H.X.; Hong, Z.P.; Wang, H.B.; Wang, Y.; Li, M.Y.; Li, Z.H. Antitumor effects of different Ganoderma lucidum spore powder in cell-and zebrafish-based bioassays. J. Integr. Med. 2021, 19, 177–184. [Google Scholar] [CrossRef]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef]
- Kikuchi, T.; Matsuda, S.; Kadota, S.; Murai, Y.; Ogita, Z. Ganoderic acid D, E, F, and H and lucidenic acid D, E, and F, new triterpenoids from Ganoderma lucidum. Chem. Pharm. Bull. 1985, 33, 2624–2627. [Google Scholar] [CrossRef]
- Kikuchi, T.; Kanomi, S.; Murai, Y.; Kadota, S.; Tsubono, K.; Ogita, Z.I. Constituents of the fungus Ganoderma lucidum (FR.) KARST. II.: Structures of ganoderic acids F, G, and H, lucidenic acids D2 and E2, and related compounds. Chem. Pharm. Bull. 1986, 34, 4018–4029. [Google Scholar] [CrossRef]
- Nishitoba, T.; Sato, H.; Sakamura, S. New terpenoids, ganolucidic acid D, ganoderic acid L, lucidone C and lucidenic acid G, from the fungus Ganoderma lucidum. Agric. Biol. Chem. 1986, 50, 809–811. [Google Scholar] [CrossRef]
- Chen, B.; Tian, J.; Zhang, J.; Wang, K.; Liu, L.; Yang, B.; Bao, L.; Liu, H. Triterpenes and meroterpenes from Ganoderma lucidum with inhibitory activity against HMGs reductase, aldose reductase and α-glucosidase. Fitoterapia 2017, 120, 6–16. [Google Scholar] [CrossRef]
- Nishitoba, T.; Sato, H.; Sakamura, S. Triterpenoids from the fungus Ganoderma lucidum. Phytochemistry 1987, 26, 1777–1784. [Google Scholar] [CrossRef]
- Min, B.S.; Gao, J.J.; Hattori, M.; Lee, H.K.; Kim, Y.H. Anticomplement activity of terpenoids from the spores of Ganoderma lucidum. Planta Med. 2001, 67, 811–814. [Google Scholar] [CrossRef]
- Wu, T.S.; Shi, L.S.; Kuo, S.C. Cytotoxicity of Ganoderma lucidum triterpenes. J. Nat. Prod. 2001, 64, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, X.; Yang, Q. Recent advances on triterpenes from Ganoderma mushroom. Food Rev. Int. 2006, 22, 259–273. [Google Scholar] [CrossRef]
- Nghien, N.X.; Thuy, N.T.B.; Luyen, N.T.; Thu, N.T.; Quan, N.D. Morphological Characteristics, Yield Performance, and Medicinal Value of Some Lingzhi Mushroom (Ganoderma lucidum) Strains Cultivated in Tam Dao, Vietnam. Vietn. J. Agr. Sci. 2019, 2, 321–331. [Google Scholar]
- Mizushina, Y.; Takahashi, N.; Hanashima, L.; Koshino, H.; Esumi, Y.; Uzawa, J.; Sugawara, F.; Sakaguchi, K. Lucidenic acid O and lactone, new terpene inhibitors of eukaryotic DNA polymerases from a basidiomycete, Ganoderma lucidum. Biorg. Med. Chem. 1999, 7, 2047–2052. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Akihisa, T.; Tokuda, H.; Ukiya, M.; Oshikubo, M.; Kimura, Y.; Asano, T.; Nomura, A.; Nishino, H. Lucidenic acids P and Q, methyl lucidenate P, and other triterpenoids from the fungus Ganoderma lucidum and their inhibitory effects on Epstein− Barr virus activation. J. Nat. Prod. 2003, 66, 1582–1585. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Han, F.; Luan, S.S.; Ai, R.; Zhang, P.; Li, H.; Chen, L.X. Triterpenoids from Ganoderma lucidum and their potential anti-inflammatory effects. J. Agric. Food Chem. 2019, 67, 5147–5158. [Google Scholar] [CrossRef]
- Cheng, C.R.; Yue, Q.X.; Wu, Z.Y.; Song, X.Y.; Tao, S.J.; Wu, X.H.; Xu, P.P.; Liu, X.; Guan, S.H.; Guo, D.A. Cytotoxic triterpenoids from Ganoderma lucidum. Phytochemistry 2010, 71, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Kondo, R.; Shimizu, K. Structure-activity relationships of lanostane-type triterpenoids from Ganoderma lingzhi as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 5900–6903. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.T.; Cuong, T.D.; Hung, T.M.; Kim, J.A.; Woo, M.H.; Choi, J.S.; Lee, J.H.; Min, B.S. Cytotoxic and anti-angiogenic effects of lanostane triterpenoids from Ganoderma lucidum. Phytochem. Lett. 2015, 12, 69–74. [Google Scholar]
- Lee, M.K.; Hung, T.M.; Cuong, T.D.; Na, M.; Kim, J.C.; Kim, E.J.; Park, H.S.; Choi, J.S.; Lee, I.; Bae, K. Ergosta-7, 22-diene-2β, 3α, 9α-triol from the fruit bodies of Ganoderma lucidum induces apoptosis in human myelocytic HL-60 cells. Phytother. Res. 2011, 25, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Cör, D.; Knez, Ž.; Knez Hrnčič, M. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Pathak, P.; Chaudhary, N.; Rathi, A.; Vyas, D. Recent Trends in Mushroom Biology. In Ganoderma lucidum: Cultivation and Production of Ganoderic and Lucidenic Acid; Global Books Organisation: Delhi, India, 2021; pp. 91–106. ISBN 9789383837991. [Google Scholar]
- Weng, C.J.; Chau, C.F.; Yen, G.C.; Liao, J.W.; Chen, D.H.; Chen, K.D. Inhibitory effects of Ganoderma lucidum on tumorigenesis and metastasis of human hepatoma cells in cells and animal models. J. Agric. Food Chem. 2009, 57, 5049–5057. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.X.; Xie, F.B.; Guan, S.H.; Ma, C.; Yang, M.; Jiang, B.H.; Liu, X.; Guo, D.A. Interaction of Ganoderma triterpenes with doxorubicin and proteomic characterization of the possible molecular targets of Ganoderma triterpenes. Cancer Sci. 2008, 99, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Nakamura, Y.; Tagata, M.; Tokuda, H.; Yasukawa, K.; Uchiyama, E.; Suzuki, T.; Kimura, Y. Anti-inflammatory and anti-tumor-promoting effects of triterpene acids and sterols from the fungus Ganoderma lucidum. Chem. Biodivers. 2007, 4, 224–231. [Google Scholar] [CrossRef]
- Xu, J.; Yang, W.; Pan, Y.; Xu, H.; He, L.; Zheng, B.; Xie, Y.; Wu, X. Lucidenic acid A inhibits the binding of hACE2 receptor with spike protein to prevent SARS-CoV-2 invasion. Food Chem. Toxicol. 2022, 169, 113438. [Google Scholar] [CrossRef]
- Divya, M.; Aparna, C.; Mayank, R.; Mp, S. In-silico insights to identify the bioactive compounds of edible mushrooms as potential MMP9 inhibitor for Hepatitis-B. Res. J. Biotechnol. 2021, 16, 2. [Google Scholar]
- Miao, H.; Li, M.H.; Zhang, X.; Yuan, S.J.; Ho, C.C.; Zhao, Y.Y. The antihyperlipidemic effect of Fu-Ling-Pi is associated with abnormal fatty acid metabolism as assessed by UPLC-HDMS-based lipidomics. RSC Adv. 2015, 5, 64208–64219. [Google Scholar] [CrossRef]
- Shen, C.Y.; Xu, P.H.; Shen, B.D.; Min, H.Y.; Li, X.R.; Han, J.; Yuan, H.L. Nanogel for dermal application of the triterpenoids isolated from Ganoderma lucidum (GLT) for frostbite treatment. Drug Deliv. 2016, 23, 610–618. [Google Scholar] [CrossRef]
- Dudhgaonkar, S.; Thyagarajan, A.; Sliva, D. Suppression of the inflammatory response by triterpenes isolated from the mushroom Ganoderma lucidum. Int. Immunopharmacol. 2009, 9, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fu, D.; Chen, G.; Guo, M. Comparative and chemometric analysis of correlations between the chemical fingerprints and anti-proliferative activities of ganoderic acids from three Ganoderma species. Phytochem. Anal. 2019, 30, 474–480. [Google Scholar] [CrossRef]
- Grienke, U.; Kaserer, T.; Pfluger, F.; Mair, C.E.; Langer, T.; Schuster, D.; Rollinger, J.M. Accessing biological actions of Ganoderma secondary metabolites by in silico profiling. Phytochemistry 2015, 114, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, H.; Youn, U.; Kim, J.; Min, B.; Jung, H.; Na, M.; Hattori, M.; Bae, K. Effect of lanostane triterpenes from the fruiting bodies of Ganoderma lucidum on adipocyte differentiation in 3T3-L1 cells. Planta Med. 2010, 76, 1558–1563. [Google Scholar] [CrossRef]
- Lee, I.; Seo, J.; Kim, J.; Kim, H.; Youn, U.; Lee, J.; Jung, H.; Na, M.; Hattori, M.; Min, B. Lanostane triterpenes from the fruiting bodies of Ganoderma lucidum and their inhibitory effects on adipocyte differentiation in 3T3-L1 Cells. J. Nat. Prod. 2010, 73, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, J.; Ryoo, I.; Kim, Y.; Choo, S.; Yoo, I.; Min, B.; Na, M.; Hattori, M.; Bae, K. Lanostane triterpenes from Ganoderma lucidum suppress the adipogenesis in 3T3-L1 cells through down-regulation of SREBP-1c. Bioorg. Med. Chem. Lett. 2010, 20, 5577–5581. [Google Scholar] [CrossRef]
- Weng, C.J.; Chau, C.F.; Chen, K.D.; Chen, D.H.; Yen, G.C. The anti-invasive effect of lucidenic acids isolated from a new Ganoderma lucidum strain. Mol. Nutr. Food Res. 2007, 51, 1472–1477. [Google Scholar] [CrossRef]
- Raghavan, V.; Manasa, D. Identification and Analysis of Disease Target Network of Human MicroRNA and Predicting Promising Leads for ZNF439, a Potential Target for Breast Cancer. Int. J. Biosci. 2012, 2, 358. [Google Scholar] [CrossRef]
- Borah, D.; Gogoi, D.; Yadav, R. Computer aided screening, docking and ADME study of mushroom derived compounds as Mdm2 inhibitor, a novel approach. Natl. Acad. Sci. Lett. 2015, 38, 469–473. [Google Scholar] [CrossRef]
- Sillapapongwarakorn, S.; Yanarojana, S.; Pinthong, D.; Thithapandha, A.; Ungwitayatorn, J.; Supavilai, P. Molecular docking based screening of triterpenoids as potential G-quadruplex stabilizing ligands with anti-cancer activity. Bioinformation 2017, 13, 284. [Google Scholar] [CrossRef]
- Khelifa, S. Low Molecular Weight Compounds from Mushrooms as Potential Bcl-2 Inhibitors: Docking and Virtual Screening Studies. Master’s Thesis, Escola Superior Agrária, Bragança, Portugal, 2016. [Google Scholar]
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on the Public Health Response to Dementia; World Health Organization: Geneva, Switzerland, 2021; ISBN 978–92–4-003324–5.
- Wei, J.C.; Wang, Y.X.; Dai, R.; Tian, X.G.; Sun, C.P.; Ma, X.C.; Jia, J.M.; Zhang, B.J.; Huo, X.K.; Wang, C. C27-Nor lanostane triterpenoids of the fungus Ganoderma lucidum and their inhibitory effects on acetylcholinesteras. Phytochem. Lett. 2017, 20, 263–268. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef]
- Combs, A.P. Recent advances in the discovery of competitive protein tyrosine phosphatase 1B inhibitors for the treatment of diabetes, obesity, and cancer. J. Med. Chem. 2010, 53, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, L.; Wu, Y.; Huang, L.; Xu, X.; Lin, R. Study on Quality Control of Compound Anoectochilus roxburghii (Wall.) Lindl. by Liquid Chromatography–Tandem Mass Spectrometry. Molecules 2022, 27, 4130. [Google Scholar] [CrossRef]
- Cao, F.R.; Xiao, B.X.; Wang, L.S.; Tao, X.; Yan, M.Z.; Pan, R.L.; Liao, Y.H.; Liu, X.M.; Chang, Q. Plasma and brain pharmacokinetics of ganoderic acid A in rats determined by a developed UFLC-MS/MS method. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1052, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Guo, J.B.; Liu, B.Y.; Lu, J.Q.; Chen, M.; Liu, B.; Bai, W.D.; Rao, P.F.; Ni, L.; Lv, X.C. Ganoderic acid A from Ganoderma lucidum ameliorates lipid metabolism and alters gut microbiota composition in hyperlipidemic mice fed a high-fat diet. Food Funct. 2020, 11, 6818–6833. [Google Scholar] [CrossRef]
- Ren, L. Protective effect of ganoderic acid against the streptozotocin induced diabetes, inflammation, hyperlipidemia and microbiota imbalance in diabetic rats. Saudi J. Biol. Sci. 2019, 26, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Yang, H.S.; Jo, J.H.; Lee, S.C.; Park, T.Y.; Choi, B.S.; Seo, K.S.; Huh, C.K. Anti-Amnesic Effect of Fermented Ganoderma lucidum Water Extracts by Lactic Acid Bacteria on Scopolamine-Induced Memory Impairment in Rats. Prev. Nutr. Food Sci. 2015, 20, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Qi, H.; Tang, W.; Zhang, C.; Liu, Z.; Liu, Y.; Wei, X.; Kong, Z.; Jia, S.; et al. Probiotic fermentation of Ganoderma lucidum fruiting body extracts promoted its immunostimulatory activity in mice with dexamethasone-induced immunosuppression. Biomed. Pharmacother. 2021, 141, 111909. [Google Scholar] [CrossRef] [PubMed]
| Serial Number | Lucidenic Acid Type | Molecular Formula | Species | Extraction Method | Amount | References |
|---|---|---|---|---|---|---|
| 1 | Lucidenic acid A | C27H38O6 | Ganoderma lucidum (fruiting bodies) | 100% Ethanol | 2.8 mg/g dry weight | [30] |
| Ganoderma lucidum (fruiting bodies) | 95% Ethanol | 1.53–1.74 mg/g dry weight | [34] | |||
| Ganoderma lucidum (fruiting bodies) | 45% Grain alcohol and chloroform | 1.226–2.497 mg/g in lyophilized sample | [29,31,35] | |||
| Ganoderma lucidum (fruiting bodies) | Water (soaked in 100% ethanol overnight prior to extraction) | 0.4 mg/g dry weight | [36] | |||
| Ganoderma lucidum (fruiting bodies) | Water | 51 μg/g dry weight | [26] | |||
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| Ganoderma lucidum (spores) | Supercritical fluid carbon dioxide | 0.3 mg/g in extract | [37] | |||
| Wall-removed Ganoderma lucidum (spores) | Water, alcohol, or a combination of the two | 0.05% | [38] | |||
| Ganoderma hainanense (fruiting bodies) | 95% Ethanol | * | [6,24] | |||
| Ganoderma sinense (fruiting bodies) | 95% Ethanol | * | [17] | |||
| Ganoderma curtisii (fruiting bodies) | Methanol | * | [18] | |||
| Ganoderma colossum (fruiting bodies) | 100% Ethanol | 16 μg/mL in extract | [19] | |||
| Ganoderma sessile (fruiting bodies) | 80% Ethanol | * | [20] | |||
| Amauroderma rugosum (fruiting bodies) | Water | 15.69 μg/g dry weight | [26] | |||
| Homalium zeylanicum (barks) | 70% Hydro-alcohol | * | [27] | |||
| 2 | Lucidenic acid B | C27H38O7 | Ganoderma lucidum (fruiting bodies) | Chloroform | * | [6,35,39] |
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| Ganoderma lucidum (spores) | Supercritical fluid carbon dioxide | 72 ± 0.95 μg/g in extract | [37] | |||
| 3 | Lucidenic acid C | C27H40O7 | Ganoderma lucidum (fruiting bodies) | Chloroform | * | [6,35,39] |
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| Ganoderma colossum (fruiting bodies) | 100% Ethanol | 6.7 μg/mL in extract | [19] | |||
| Ganoderma sessile (fruiting bodies) | 80% Ethanol | * | [20] | |||
| Ganoderma tsugae (fruiting bodies) | 95% Ethanol | * | [21] | |||
| 4 | Lucidenic acid D1 | C27H34O7 | Ganoderma lucidum (fruiting bodies) | Chloroform | * | [6,35] |
| 5 | Lucidenic acid D2 | C29H38O8 | Ganoderma lucidum (fruiting bodies) | 45% Grain alcohol and chloroform | 1.538–2.227 mg/g in lyophilized sample | [31,35,40] |
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| Ganoderma sinense (fruiting bodies) | Chloroform | * | [6,9] | |||
| Potato leaf | Methanol: Water (4:1, v/v) | * | [28] | |||
| 6 | Lucidenic acid E1 | C27H38O7 | Ganoderma lucidum (fruiting bodies) | Chloroform | * | [35] |
| 7 | Lucidenic acid E2 | C29H40O8 | Ganoderma lucidum (fruiting bodies) | Methanol | 0.319–1.766 mg/g dry weight (wild samples); 0.258–0.481 mg/g dry weight (cultivated samples) | [23,39,40] |
| Ganoderma lucidum (fruiting bodies) | 45% Grain alcohol | 2.246–3.306 mg/g in lyophilized sample | [31] | |||
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| Ganoderma australe (fruiting bodies) | Methanol | 121.65 ± 4.50 μg/g dry weight | [23,39,40] | |||
| Ganoderma colossum (fruiting bodies) | Methanol | 201.92 ± 2.45 μg/g dry weight | [23,39,40] | |||
| 8 | Lucidenic acid F | C27H36O6 | Ganoderma lucidum (fruiting bodies) | Ether | * | [6,39,40,41] |
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| Ganoderma curtisii (fruiting bodies) | Methanol | * | [18] | |||
| Potato leaf | Methanol: water (4:1, v/v) | * | [28] | |||
| metabolites of rice | Methanol: water (4:1, v/v) | * | [25] | |||
| 9 | Lucidenic acid G | C27H40O7 | Ganoderma lucidum (fruiting bodies) | Ethanol | * | [6,42] |
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| 10 | Lucidenic acid H | C27H40O7 | Ganoderma lucidum (fruiting bodies) | Ethanol and crystallized from fraction CHCl3-MeOH, 9:1 | * | [43,44] |
| 11 | Lucidenic acid I | C27H38O7 | Ganoderma lucidum (fruiting bodies) | Ethanol and crystallized from fraction CHCl3-MeOH, 9:1 | * | [6,44] |
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| 12 | Lucidenic acid J | C27H38O8 | Ganoderma lucidum (fruiting bodies) | Ethanol and crystallized from fraction CHCl3-MeOH, 9:1 | * | [6,44] |
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| 13 | Lucidenic acid K | C27H40O7 | Ganoderma lucidum (fruiting bodies) | 100% Ethanol | * | [6,44] |
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| 14 | Lucidenic acid L | C27H38O7 | Ganoderma lucidum (fruiting bodies) | 100% Ethanol | * | [6,44] |
| 15 | Lucidenic acid M | C27H42O6 | Ganoderma lucidum (fruiting bodies) | 100% Ethanol | * | [6,44] |
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| 16 | Lucidenic acid N (lucidenic acid SP1, LM1) | C27H40O6 | Ganoderma lucidum (fruiting bodies) | Methanol | 257.80–884.05 μg/g dry weight (wild samples); 52.53–139.08 μg/g dry weight (cultivated samples) | [23,39,45,46,47] |
| Ganoderma lucidum (fruiting bodies) | 45% Grain alcohol | 0.866–2.004 mg/g in lyophilized sample | [31] | |||
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| Ganoderma lucidum (spores) | Supercritical fluid carbon dioxide | 161 ± 2.21 μg/g in extract | [37] | |||
| Ganoderma lucidum (mycelia) | 96% Ethanol | 0.23–0.33 mg/g dry weight | [48] | |||
| Ganoderma curtisii (fruiting bodies) | Methanol | * | [18] | |||
| Ganoderma sessile (fruiting bodies) | 80% Ethanol | * | [20] | |||
| Ganoderma tsugae (fruiting bodies) | 95% Ethanol | * | [21] | |||
| Ganoderma subresinosum (fruiting bodies) | Methanol | 57.50 ± 0.65 μg/g dry weight | [23,39,45,46,47] | |||
| Ganoderma colossum (fruiting bodies) | Methanol | 207.73 ± 2.05 μg/g dry weight | [23,39,45,46,47] | |||
| Ganoderma australe (fruiting bodies) | Methanol | 63.13 ± 1.45 μg/g dry weight | [23,39,45,46,47] | |||
| Ganoderma hainanense (fruiting bodies) | 95% Ethanol | * | [24] | |||
| 17 | Lucidenic acid O | C27H40O7 | Ganoderma lucidum (fruiting bodies) | Acetone | * | [6,49] |
| 18 | Lucidenic acid P | C29H42O8 | Ganoderma lucidum (fruiting bodies) | Methanol | * | [6,50] |
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| 19 | Lucidenic acid Q | C27H40O6 | Ganoderma lucidum (fruiting bodies) | Ethyl acetate | * | [43] |
| Ganoderma lucidum (spores) | Methanol | * | [14] | |||
| 20 | Lucidenic acid R | C29H40O9 | Ganoderma lucidum (fruiting bodies) | 80% Ethanol | * | [51] |
| Basic Chemical Structure | 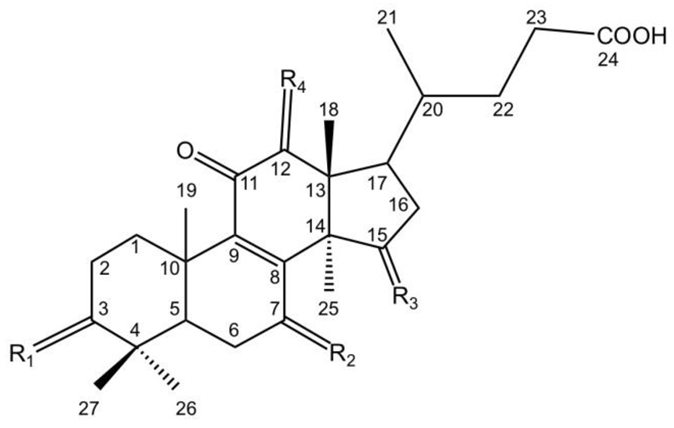 | ||||
|---|---|---|---|---|---|
| Lucidenic Acid Type | R1 | R2 | R3 | R4 | References |
| Lucidenic acid A | R1 = O | R2 = -OH | R3 = O | R4 = H | [29] |
| Lucidenic acid B | R1 = O | R2 = -OH | R3 = O | R4 = -OH | [29] |
| Lucidenic acid C | R1 = -OH | R2 = -OH | R3 = O | R4 = -OH | [29] |
| Lucidenic acid D1 | R1 = O | R2 = O | R3 = O | R4 = O | [35] |
| Lucidenic acid D2 | R1 = O | R2 = O | R3 = O | R4 = OCOCH3 | [40] |
| Lucidenic acid E1 | R1 = O | R2 = -OH | R3 = O | R2 = -OH | [35] |
| Lucidenic acid E2 | R1 = -OH | R2 = O | R3 = O | R4 = OCOCH3 | [40] |
| Lucidenic acid F | R1 = O | R2 = O | R3 = O | R4 = H | [40] |
| Lucidenic acid K | R1 = O | R2 = O | R3 = O | R4 = -OH | [44] |
| Lucidenic acid L | R1 = -OH | R2 = O | R3 = O | R4 = -OH | [44] |
| Lucidenic acid M | R1 = -OH | R2 = -OH | R3 = -OH | R4 = H | [44] |
| Lucidenic acid N | R1 = -OH | R2 = -OH | R3 = O | R4 = H | [46] |
| Lucidenic acid P | R1 = -OH | R2 = -OH | R3 = O | R4 = OCOCH3 | [50] |
| Lucidenic acid Q | R1 = O | R2 = -OH | R3 = -OH | R4 = H | [43] |
| Basic Chemical Structure |  | ||||
|---|---|---|---|---|---|
| Lucidenic Acid Type | R1 | R2 | R3 | R4 | References |
| Lucidenic acid G | R1 = O | R2 = -OH | R3 = -OH | R4 = H | [42] |
| Lucidenic acid H | R1 = OH | R2 = -OH | R3 = O | R4 = H | [44] |
| Lucidenic acid I | R1 = -OH | R2 = O | R4 = O | R4 = H | [44] |
| Lucidenic acid J | R1 = -OH | R2 = O | R3 = O | R4 = -H | [44] |
| Lucidenic acid O | R1 = -OH | R2 = -OH | R3 = -OH | R4 = -OH | [49] |
| Lucidenic acid R | R1 = -OH | R2 = O | R3 = O | R4 = OCOCH3 | [51] |
| Lucidenic Acids and Derivatives | Potential Pharmacological Effects | References |
|---|---|---|
| Lucidenic acid A | Anti-cancer | [11,46,54,55,56,57,58,59] |
| Anti-inflammatory | [27,50,60] | |
| Anti-viral | [50,60,61,62] | |
| Neuroprotective | [15] | |
| Anti-hyperlipidemic | [63] | |
| Treatment of frostbite | [64] | |
| Lucidenic acid B | Anti-cancer | [11,55,57,58] |
| Anti-inflammatory | [65] | |
| Antioxidant | [16] | |
| Anti-viral | [62] | |
| Lucidenic acid C | Anti-cancer | [11,43,55,56,57,58] |
| Anti-viral | [50,60,62] | |
| Lucidenic acid D1 | Anti-cancer | [12,66] |
| Anti-inflammatory | [65] | |
| Lucidenic acid D2 | Anti-inflammatory | [60,65] |
| Anti-viral | [50,60] | |
| Lucidenic acid E1 | Anti-inflammatory | [65] |
| Lucidenic acid E2 | Anti-cancer | [59] |
| Anti-inflammation | [60] | |
| Anti-hypercholesterolemia | [67] | |
| Anti-hyperglycemic | [16] | |
| Anti-viral | [50,60] | |
| Lucidenic acid F | Anti-viral | [50,60] |
| Lucidenic acid H | Treatment of frostbite | [64] |
| Lucidenic acid I | Immunomodulatory | [14] |
| Lucidenic acid L | Anti-inflammation | [65] |
| Lucidenic acid N | Anti-cancer | [11,46,55,56,57,58,59] |
| Anti-viral | [62] | |
| Neuroprotective | [15] | |
| Anti-hyperlipidemic | [68,69] | |
| Lucidenic acid O | Anti-viral | [49] |
| Lucidenic acid P | Anti-inflammatory | [60] |
| Anti-viral | [50,60] | |
| Lucidenic acid Q | Anti-hyperglycemic | [16] |
| Lucidenic acid R | Anti-inflammatory | [51] |
| Methyl lucidenate A, | Anti-viral | [50,60] |
| Methyl lucidenic E2 | Neuroprotective | [15] |
| Anti-hyperlipidemic | [69] | |
| Anti-viral | [50,60] | |
| Immunomodulatory | [14] | |
| Methyl lucidenate F | Anti-hyperlipidemic | [69] |
| Butyl lucidenate N | Anti-hyperlipidemic | [70] |
| 20(21)-Dehydrolucidenic acid N | Ant-viral | [9] |
| Immunomodulatory | [14] | |
| 20-Hydroxylucidenic acid N | Anti-viral | [9,50,60] |
| Methyl lucidenate Q | Anti-viral | [50,60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, C.; Rangsinth, P.; Shiu, P.H.T.; Wang, W.; Li, R.; Li, J.; Kwan, Y.-W.; Leung, G.P.H. A Review on the Sources, Structures, and Pharmacological Activities of Lucidenic Acids. Molecules 2023, 28, 1756. https://doi.org/10.3390/molecules28041756
Zheng C, Rangsinth P, Shiu PHT, Wang W, Li R, Li J, Kwan Y-W, Leung GPH. A Review on the Sources, Structures, and Pharmacological Activities of Lucidenic Acids. Molecules. 2023; 28(4):1756. https://doi.org/10.3390/molecules28041756
Chicago/Turabian StyleZheng, Chengwen, Panthakarn Rangsinth, Polly H. T. Shiu, Wen Wang, Renkai Li, Jingjing Li, Yiu-Wa Kwan, and George P. H. Leung. 2023. "A Review on the Sources, Structures, and Pharmacological Activities of Lucidenic Acids" Molecules 28, no. 4: 1756. https://doi.org/10.3390/molecules28041756
APA StyleZheng, C., Rangsinth, P., Shiu, P. H. T., Wang, W., Li, R., Li, J., Kwan, Y.-W., & Leung, G. P. H. (2023). A Review on the Sources, Structures, and Pharmacological Activities of Lucidenic Acids. Molecules, 28(4), 1756. https://doi.org/10.3390/molecules28041756






