Abstract
Glucosinolates (GSLs) are a unique class of thioglucosides that evolved as defense mechanisms in the 16 families of the Brassicales order and present molecular tags which can be placed in a robust phylogenetic framework through investigations into their evolution and diversity. The GSL profiles of three Resedaceae species, Reseda alba, R. lutea, and R. phyteuma, were examined qualitatively and quantitatively with respect to their desulfo-counterparts utilizing UHPLC-DAD-MS/MS. In addition, NMR analysis of isolated 2-hydroxy-2-methylpropyl desulfoGSL (d31) was performed. Three Phe-derived GSLs were found in R. lutea, including glucotropaeolin (11) (0.6–106.69 mol g−1 DW), 2-(α-L-ramnopyranosyloxy)benzyl GSL (109) (8.10–57.89 μmol g−1 DW), glucolepigramin (22) (8.66 μmol g−1 DW in flower), and Trp-derived glucobrassicin (43) (0.76–5.92 μmol g−1 DW). The Phe-derived GSLs 109 (50.79–164.37 μmol g−1 DW), gluconasturtiin (105) (1.97 μmol g−1 DW), and 11 (tr), as well as the Trp-derived GSL glucobrassicin (43) (3.13–11.26 μmol g−1 DW), were all present in R. phyteuma. R. alba also contained Phe-derived 105 (0.10–107.77 μmol g−1 DW), followed by Trp-derived 43 (0.85–3.50 μmol g−1 DW) and neoglucobrassicin (47) (0.23–2.74 μmol g−1 DW). However, regarding the GSLs in R. alba, which originated from Leu biosynthesis, 31 was the major GSL (6.48 to 52.72 μmol g−1 DW) and isobutyl GSL (62) was the minor GSL (0.13 to 1.13 μmol g−1 DW). The discovered Reseda profiles, along with new evidence provided by GSL characterizations, were studied in the context of the current knowledge on GLSs in the Resedaceae family. With the exception of R. alba, the aliphatic GSLs of which were outliers among the Resedaceae species studied, this family typically contains GSLs derived primarily from Trp and Phe biosynthesis, which modifications resulted in GSLs unique to this family, implying presence of the specific genes. responsible for this diversification.
1. Introduction
There are ca. 96 species in the family Resedaceae Martinov, which are distributed predominantly in the Northern Hemisphere and a few Southern African countries [1,2]. Phylogenetic analysis of internal transcribed spacers of the nuclear ribosomal DNA and plastid trnL-trnF sequences of 66 species from all genera of the Resedaceae confirmed its traditional subdivision into three tribes: two monophyletic genera (Caylusea and Sesamoides) and one natural group (core Reseda), which includes four genera (Ochradenus, Oligomeris, Randonia, and Reseda). Four out of six taxonomic sections within Reseda (Leucoreseda, Luteola, Glaucoreseda, and Phyteuma) are monophyletic in origin [3]. Crown-group Resedacae, which includes Reseda genus, (represented by 68 species), has been dated to (13.5-)12.6, 10.5(−8.7) million years ago (Ma) [1,2,4]. Many species of Reseda are restricted to the Mediterranean Basin, while four species, R. alba, R. lutea, R. luteola, and R. phyteuma, are distributed worldwide [3]. There are five spp. of Reseda genus (mignonettes) known to be wild-growing in Croatia, i.e., Reseda alba L. (white mignonette), R. lutea L. (yellow or wild or cutleaf mignonette), R. luteola L. (dyer’s rocket; weld), R. phyteuma L. (garden mignonette, common mignonette), and the critically endangered R. inodora Rchb. [5].
Decisive species identifications, the availability of trustworthy phylogenies, and conclusive chemical analyses are conditions of utmost relevance in exploring the evolution of any class of metabolites [6]. It is generally accepted that genes for secondary metabolites, including GSLs, are inherited due to the evolutionary advantages they impart to the plant, especially for defense against abiotic stress, plant pathogens, parasites, and herbivores [7,8]. There are more than 130 distinct GSLs produced by Brassicales species, while the structures of only 90 glucosinolates (GSLs) have been confirmed using MS and NMR to date [9]. Structural variation in intact GSLs is achieved through the use of different amino acid precursors, including methionine (Met), alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), glutamic acid (Glu), tyrosine (Tyr), phenylalanine (Phe), and tryptopane (Trp), and the sequential modification of side chains. The genetic mechanisms governing GSL biosythesis in the model plant Arabidopsis thaliana, and to some extent in Brassica spp., are well understood and are useful for investigating the underlying mechanisms of GSL production at a very fundamental level. Novel GSLs and the genes that encode the proteins that control their biosynthesis pathways outside of these plant species could significantly improve our understanding of the phytochemistry, evolution, and natural history of Brassicales [7].
The progenitors of Brassica (mustards and cabbage) and similar plants evolved GSLs as a chemical defense over 90 Ma [10]. When they first emerged 92 Ma ago, Brassicales could only produce GSLs from phenylalanine and branched-chain amino acids. Indolic GSLs, which are produced from the amino acid tryptophan, first occurred 77.5 Ma after the At-β whole-genome duplication event (95% highest posterior density, HPD, 42–112 Ma). A second significant phase of escalation took place when the ancestors of the plant lineages Capparaceae and Cleomaceae produced a new set of GSLs derived from methionine, another novel substrate. The final escalation event appeared 32 Ma (95% HPD 17–46 Ma) with the evolution of Brassicaceae (the mustard family), which contains the greatest diversity of GSLs within Brassicales [10].
To date, some important studies on the morphology, anatomy, palynology, cytogenetics, pharmacology, and phytochemistry of the family Resedaceae have been conducted [3,4,10]. For this study, three Reseda species—R. alba, R. lutea, and R. phyteuma—were collected. R. alba is a well-liked ornamental plant on account of its spike-like racemes of fragrant white flowers, although in some parts of Italy and Greece its young leaves and flowering branches have been traditionally used as wild vegetables [11]. Since the first millennium BC, R. lutea leaves and flowers have been used to manufacture a yellow dye known as “weld,” primarily in the form of the flavonoid luteolin, even though a related plant, R. luteola, has more frequently been used for that purpose [12]. R. phyteuma, having a taste similar to cabbage, is used as a potherb in Greece [13]. Pharmacological studies of extracts from the investigated Reseda species have revealed great biological potential, including cytotoxic, analgesic, anti-inflammatory, antibacterial, and antioxidant activities [14,15,16,17,18,19,20,21]. Resedaceae plants are known to contain GSLs in their tissues, just like all the other families in the order Brassicales. GSLs, through their breakdown products isothiocyanates, are extremely harmful to most insects, and give mustards their pungent flavors, appreciated by humans, and have been investigated for their diversified and generally marked bioactivites, especially anticancer activities [22,23]. Table 1 displays the distribution of GSLs in plants of the Resedaceae family that have been the subject of research to date.

Table 1.
Distribution of glucosinolates in plants belonging to the Resedaceae family investigated to date.
Qualitative analyses of GSLs in Resedaceae published up to 2001 were reviewed by Fahey et al. [24]. Bennett et al. (2004) reported a quantitative analysis of arylaliphatic and indolic GSLs in the seeds of Reseda luteola and R. odorata, while the O-glycosylated GSL, 2-(α-L-rhamnopyranosyloxy)benzyl GSL (109) was found only in R. lutea (25.0–50.0 µmol g−1 dry weight, DW). Outside of the genus Reseda, GSL 109 was found in the plant Ochradenus baccatus, with 7.0 µmol g−1 DW in the root [36]. Two arylaliphatic GSLs, glucobarbarin (40S) and epiglucobarbarin (40R), were identified in R. luteola, with contents ranging from 0.1 to 10.0 and 25.0 to 50.0 µmol g−1 DW, respectively. In the same work, in the seeds of Caylusea abyssinica, in addition to the same arylaliphatic GSLs, gluconasturtiin (105) and 40R were found to have the highest contents (10.0–25.0 µmol g−1 DW) [25]. The presence of 40S, a characteristic GSL in the genus Barbarea (Brassicaceae), was confirmed also in R. luteola, despite the great evolutionary distance between them. Agerbirk et al. (2021) investigated the same plant and determined the presence of 40R, which in the seeds accounted for 5% of the total amount of enantiomeric glucobarbarins, i.e., 1% in the leaf. In addition, the analysis also revealed significant levels of the apparent hydroxybutyl GSL, which could be either Met-derived 4-hydroxybutyl GSL ([26]) (unknown from basal families at the time) or Leu-derived 2-hydroxy-2-methylpropyl GSL (glucoconringiin, 31) [17]. GSL 40S was identified as the main GSL in the leaf of R. luteola and accounted for over 90% of the total GSLs from the leaf surface (0.5 µmol g−1 of fresh plant material) [22,32]. The indole GSL glucobrassicin (43) was found in the seeds of R. odorata in the range of 10.0–25.0 µmol g−1 DW [25].
GSLs identified in R. alba included plant aliphatic GSLs, not common in the Rese-daceae family, i.e., hydroxyaliphatic GSL glucoconringin (31) and (2S)-2-hydroxybut-3-enyl GSL (epiprogoitrin), as well as two arylaliphatic GSLs, glucosinalbin (23) and 105, and indole GSLs 43 and 47 [24,26,27,28].
2-(α-l-Arabinopyranosyloxy)-2-phenylethyl GSL was the first identified extraglycosylated GSL containing arabinose as an additional carbohydrate moiety. It was isolated from the plant Sesamoides interrupta and was also found in a plant of another genus, R. phyteuma [35]. Recently, another arabinosylated GSL, 2′′-O-(α-l-arabinopyranosyloxy)benzyl GSL (158), was found in the roots of the desert plant Ochradenus baccatus (4.1 µmol g−1 DW), representing an additional genus in the Resedaceae family in which these specific GSLs were identified [36].
The purpose of this work was to identify and quantify GSLs in different plant parts of three Reseda species that are wild-growing in Croatia utilizing their desulfo-counterparts using UHPLC-DAD-MS/MS in order to understand the Resedaceae family’s biosynthetic potential. Additionally, 2-hydroxy-2-methylpropyl GSL (glucoconringiin, 31), which was thoroughly characterized by means of MS2 and NMR, as a desulfated derivative, was isolated and purified from flowers, which were found to be the best source for this uncommon GSL. By examining different plant parts, the existing GSL profiles of R. alba, R. lutea, and R. phyteuma were expanded. Finally, the revised GSL profiles were evaluated in terms of their biosynthetic characteristics and evolution.
2. Results and Discussion
In this study, three Reseda plant species wild-growing in Croatia, R. alba, R. lutea, and R. phyteuma, were investigated. According to UHPLC-DAD-MS/MS analysis, R. lutea and R. phyteuma showed comparable GSL profiles, while that of R. alba was completely different (Figures S1–S3 and Table 2). The MS2 spectra are given in Figures S4A,B and S5A–D.

Table 2.
Quantitative analysis of GSLs in individual plant organs of researched plants of the genus Reseda.
Table 2.
Quantitative analysis of GSLs in individual plant organs of researched plants of the genus Reseda.
| No. * | Identified Glucosinolate | tR (min) | [M + Na]+ | Plant Tissue (μmol g−1 DW) | |||
|---|---|---|---|---|---|---|---|
| Reseda alba | Flower | Leaf | Stem | Root | |||
| Leu-derived | |||||||
| 31 | Glucoconringiin | 1.64 | 334 | 52.72 ± 2.22 | 6.48 ± 0.51 | 25.29 ± 1.13 | 19.70 ± 1.89 |
| 62 | Isobutyl GSL | 5.30 | 318 | 1.00 ± 0.24 | 0.13 ± 0.04 | 0.13 ± 0.05 | 1.13 ± 0.22 |
| Phe-derived | |||||||
| 105 | Gluconasturtiin | 7.93 | 366 | n.d. | 0.10 ± 0.03 | 1.20 ± 0.37 | 107.77 ± 2.83 |
| Trp-derived | |||||||
| 43 | Glucobrassicin | 7.21 | 391 | 1.64 ± 0.32 | 3.50 ± 0.11 | 0.85 ± 0.10 | 1.69 ± 0.67 |
| 47 | Neoglucobrassicin | 9.34 | 421 | 0.32 ± 0.08 | 0.23 ± 0.02 | 0.55 ± 0.11 | 2.74 ± 0.38 |
| Reseda lutea | Flower | Leaf | Stem | Root | |||
| Phe-derived | |||||||
| 22 | Glucolepigramin | 5.22 | 368 | 8.66 ± 1.00 | n.d. | n.d. | n.d. |
| 11 | Glucotropaeolin | 6.51 | 352 | 1.64 ± 0.76 | 0.6 ± 0.09 | 5.67 ± 0.75 | 106.69 ± 3.04 |
| 109 | 2-(α-L-Ramnopyranosyloxy)-benzyl GSL | 6.78 | 514 | 57.89 ± 3.19 | 20.50 ± 1.50 | 14.86 ± 1.86 | 8.10 ± 1.06 |
| Trp-derived | |||||||
| 43 | Glucobrassicin | 7.21 | 391 | 5.92 ± 0.34 | 0.76 ± 0.15 | 2.45 ± 0.25 | 3.26 ± 0.17 |
| Reseda phyteuma | Flower | Leaf | Stem | Siliquae | |||
| Phe-derived | |||||||
| 11 | Glucotropaeolin | 6.51 | 352 | n.d. | n.d. | tr | n.d. |
| 109 | 2-(α-L-Ramnopyranosyloxy)-benzyl GSL | 6.78 | 514 | 150.84 ± 3.52 | 164.37 ± 3.72 | 50.79 ± 1.23 | 123.93 ± 2.64 |
| 105 | Gluconasturtiin | 7.93 | 366 | n.d. | n.d. | 1.97 ± 0.21 | n.d. |
| Trp-derived | |||||||
| 43 | Glucobrassicin | 7.21 | 391 | 8.47 ± 0.18 | 4.79 ± 0.73 | 11.26 ± 0.77 | 3.13 ± 0.12 |
* No.—Numbering system is related to the glucosinolate numbers given in the review paper by Blažević et al. [9]. The structures are shown in Figure 1. All chromatograms are given in Figures S1–S3, while MS2 spectra are given in Figure S4A,B. [M + Na]+—sodium adduct of desulfoglucosinolate; tR—retention time at the UHPLC-DAD-MS/MS conditions reported here; GSL—glucosinolate; tr—traces; n.d.—not detected; DW—dry weight of plant material. Data are expressed as mean values ± standard errors (n = 3).
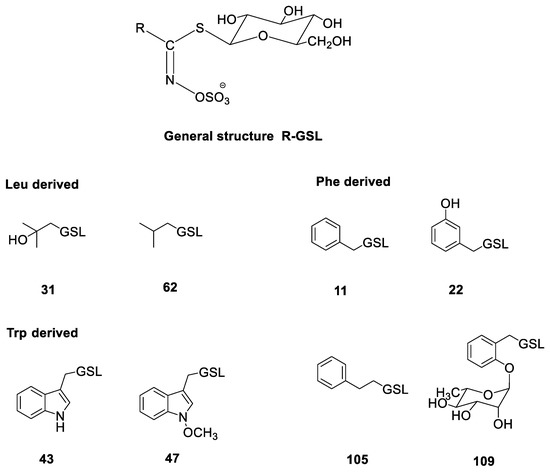
Figure 1.
Structures of the GSLs identified in the investigated Reseda sp. (cf. Table 2): benzyl GSL (glucotropaeolin, 11); 3-hydroxybenzyl GSL (glucolepigramin, 22); 2-hydroxy-2-methylpropyl GSL (glucoconringiin, 31); indol-3-ylmethyl GSL (glucobrassicin, 43); N-methoxyindol-3-ylmethyl GSL (neoglucobrassicin, 47); 2-phenylethyl GSL (gluconasturtiin, 105); 2-(α-L-ramnopyranosyloxy)benzyl GSL (109). Numbering system is related to the GSL numbers given in the review paper by Blažević et al. [9].
Arylaliphatic, indolic, and O-glycosylated GSLs were identified in R. lutea. All plant tissues contained GSLs 11, 43, and 109. In addition, it was observed that as the content of 11 decreased, the content of 109 increased in each individual tissue. The highest concentration of 11 was found in the root, with 106.69 µmol g−1 DW, while the highest concentration of 109 was found in the flower, with 57.89 µmol g−1 DW. GSL 109 is an isomer of 4-(α-L-ramnopyranosyloxy)benzyl GSL (glucomoringin, 110), which is a hallmark of another family, Moringaceae. In addition to having different retention times, d109 and d110 vary in their MS2 spectra [6]. Figure S4B shows the MS2 spectra of d109 at collision energies of 20 and 30 V. Typical thioglucosidic bond fragmentation results in [anhydroglucose + Na]+ at m/z 185 (type a) and [M-162 + Na]+ at m/z 219 (type b), while the type c fragment results from loss of an anhydroglucose, [M-162 + Na]+ [37]. Fragments from the elimination of anhydrorhamnose, anhydroglucose, and thioglucose (fragment h) were observed, with the characteristic fragment m/z 334 resulting from the loss of glucose (m/z 180). Glucolepigramin (22) was identified only in the flower (8.66 µmol g−1 DW) using desulfoglucolepigramin as a standard isolated from Lepidium graminifolium [38], corroborating a recent discovery [29].
O-Rhamnosylated 109 and indole 43 GSLs were identified in all parts of the R. phyteuma plant. The content of 109 was significantly higher than in R. lutea, up to 164.37 µmol g−1 DW in the flower, which is why this plant species represents a good source of this GSL. Arylaliphatic 11 and 105 were identified only in the stem and at very low concentrations.
R. alba had a markedly different GSL profile compared to the other two species of this family that have been studied. The dominant desulfoglucosinolate (dGSL) signal at tR = 1.64 min and m/z 334 was assumed to be 2-hydroxy-2-methylpropyl GSL (glucoconringiin, 31) based on a previous report by Olsen and Sorensen (1979) [27]. The MS2 spectrum (Figure S4A) revealed characteristic fragments, the origins of which were previously explained (a, b, and c fragments), and a fragment specific to hydroxylated aliphatic dGSLs that originated from water loss (type f, m/z 316). As no standard was available, the GSL was isolated from the flower due to its high amount (52.72 µmol g−1 DW), and NMR spectra of its desulfo-counterpart were recorded (Figure 2 and Figure S5A–D). The H-1/H-2 and H-5/H-6a/H-6b spin systems’ coupling constants correspond to a thioglucosyl moiety. Additionally, two distinct doublets were found between two H-8 protons (2J = 15.2 Hz) at 2.83 and 2.74 ppm, respectively. Furthermore, two singlets at 1.35 and 1.33 ppm, respectively, denote two methyl groups (H-10a and H-10b). Finally, according to 13C NMR, the same methyl groups are represented by two peaks at 28.5 and 27.9 ppm, and the precise quaternary carbon that those methyl groups are next to may be seen at 71.1 ppm.
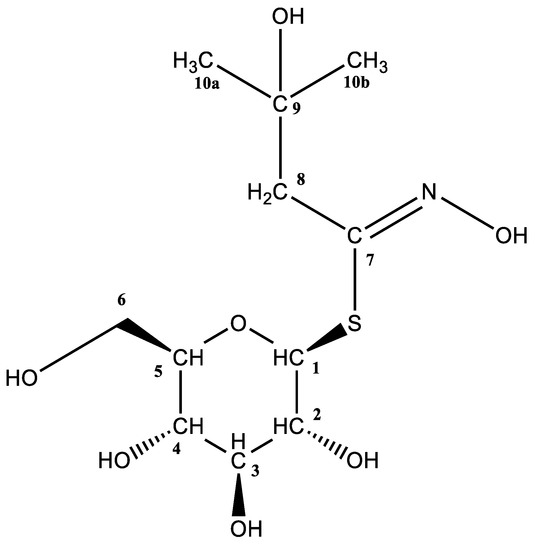
Figure 2.
Structure of 2-hydroxy-2-methylpropyl desulfoglucosinolate (desulfoglucoconringiin, d31).
The m/z 318 signal in MS2 (Figure S4A) may correspond to two isomers, desulfoglucocochlearin (d61) and isobutyl dGSL (d62). This GSL was previously identified in Sisymbrium officinale at the same retention time (tR = 5.30 min), which is consistent with this study [37].
In evolutionary terms, Brassicales were initially only able to synthesize GSLs from Phe and branched-chain amino acids, after which the indolic GSLs, which are produced from the amino acid Trp, started to appear [10]. As can be seen from Table 1 and from the results obtained in this study, the GSLs of Resedaceae are mostly biosynthesized from Phe/Tyr and Trp. The only indole-type GSLs appear to be 43 and 47. The distribution of side-chain-modified Trp-derived GSLs in basal families is poorly understood, with Salvadoraceae and Tovariaceae as exceptions, giving some reason to believe that N-methoxylation is rather ancient [6,39]. In the case of Phe GSLs, biosynthetic diversification occurred during evolution. Glucotropaeolin (11) (R. lutea and R. phyteuma) originated directly from Phe biosynthesis, which can be further hydroxylated to produce 22 (R. lutea). The GSL 109, a Phe-derived GSL additionally glycosylated in the ortho position and with a rhamnose moiety, as a tag for the Resedaceae family, was found in high amounts in R. lutea and R. phyteuma. Furthermore, another Phe-derived GSL with an arabinose moiety was recently reported in Ochradenus baccatus, implying that plants in the Resedaceae have specific genes responsible for the glycosylation of the benzylic ring in the ortho position, which needs to be investigated further. Additionally, other GSLs are biosynthesized after the elongation of Phe into homoPhe, which is a precursor of gluconasturtiin (105) found in high amounts in the root of R. alba (107.77 µmol g−1 DW) and only in traces in the stem of R. phyteuma. This GSL, after hydroxylation, can produce epiglucobarbarin (40R) and glucobarbarin (40S), which were not detected in our study, although they were previously reported elsewhere. Further modification of these GSLs can produce 2-(α-L-arabinopyranosyloxy)-2-phenylethyl GSL (4), another exotic GSL, previously reported in R. phyteuma and Sesamoides interrupta, originating from homoPhe biosynthesis (Table 1).
R. alba seems to diverge from the other investigated species in Resedaceae with respect to GSL chemistry, as aliphatic GSLs have additionally been detected only in R. luteola [6]. Isobutyl GSL (62), found in all plant parts, is biosynthesized from Leu, which is a precursor of glucocorningiin (31), and was found in all investigated plant parts. This GSL is found in numerous plant families, including most members of the Brassicaceae (such as Cochlearia spp., Conringia orientalis, Arabis procurrens, Draba aizoides, etc.), but also the Akaniaceae (Bretschneidera sinensis), the Limnanthaceae (Limnanthes spp.), and the Tropaeolaceae (Tropaeolum peregrinum) [24,25,40]. In terms of side-chain modification, β-hydroxylation of aliphatic GSLs is considered to be ancient [39].
3. Materials and Methods
3.1. Materials and Reagents
All plant samples were collected from plants wild-growing in Croatia in April 2021. Reseda alba L., R. lutea L., and R. phyteuma L. samples were collected in Split (43°30’7″ N, 16°29′8″ E), Split (43°30’31″ N, 16°23’30″ E), and Tisno (43°48’34″ N, 15°37’45″ E), respectively. The specimen vouchers were stored under numbers ZOKRA1, ZOKRL1, and ZOKRF1. Sinigrin, DEAE-Sephadex A-25 (GE Healthcare), and sulfatase (type H-1 from Helix pomatia) were purchased from Sigma-Aldrich (St. Louis, MO, USA); glucotropaeolin (11), glucobrassicin (43), N-methoxyglucobrassicin (47), and gluconasturtiin (105) were obtained from Phytoplan Diehm & Neuberger GmbH (Heidelberg, Germany); while glucolepigramin (22) was previously isolated from L. graminifolium [38]. All other chemicals and reagents were of analytical grade.
Commercial sulfatase requires additional purification steps. Ultrapure water (30 mL) and 10 kU of aryl sulfatase were mixed with absolute ethanol (30 mL). The mixture was centrifuged for 20 min at room temperature at 2650× g. The supernatant was mixed with ethanol (90 mL). The mixture was further centrifuged for 15 min at room temperature at 1030× g, after which supernatants were removed and discarded. The combined pellets were dissolved in ultrapure water (25 mL) and thoroughly vortexed, dispensed into 1 mL tubes, and frozen (−20 °C).
3.2. Isolation and Chemical Analysis
3.2.1. Isolation of Desulfoglucosinolates
GSLs were extracted from different plant parts, as previously reported [37]. To inactivate the endogenous myrosinase, the plant material was ground into a fine powder, and 100 mg was extracted for 5 min at 80 °C in 2 mL MeOH/H2O (70:30 v/v). Each extract was loaded onto a mini-column containing 0.5 mL of DEAE-Sephadex A-25 anion-exchange resin and conditioned with 25 mM acetate buffer (pH 5.6). To achieve the best desulfation conditions, buffer solution was added to the column after it had been washed with 70% MeOH and 1 mL of ultrapure water. Purified sulfatase at an amount of 20 µL (0.35 U/mL) was placed into each mini-column and allowed to stand for 18 h at room temperature. The dGSLs were then eluted with 1.5 mL of ultrapure H2O, lyophilized, and diluted to 1 mL. The samples were kept at −20 °C until they underwent UHPLC-DAD-MS/MS analysis.
3.2.2. UHPLC-DAD-MS/MS Analysis
UHPLC-DAD-MS/MS (Ultimate 3000RS with a TSQ Quantis MS/MS detector, Thermo Fisher Scientific, MA, USA) and a Hypersil GOLD C18 column (3.0 µm, 3.0 × 100 mm, Thermo Fisher Scientific, MA, USA) were used for the analysis. A gradient consisting of solvent A (50 μM NaCl in H2O) and solvent B (acetonitrile:H2O 30:70 v/v) was applied at a flow rate of 0.5 mL/min as follows: 0.14 min 96% A and 4% B; 7.84 min 14% A and 86% B; 8.96 min 14% A and 86% B; 9.52 min 5% A and 95% B; 13.16 min 5% A and 95% B; 13.44 min 96% A and 4% B; 15.68 min 96% A and 4% B. The injection volume was 5 µL, and the column temperature was maintained at 25 °C. The electrospray interface was an H-ESI source operating at 350 °C with 3.5 kV of capillary voltage. The ion-transfer tube was set at 325 °C. The system was operated in the positive ion mode with a mass range of m/z 150–800, a scan rate of 1000 (Da/sec), and a resolution of 0.4 (FWHM). Nitrogen was used as: sheath gas set at 5.58 L/min, aux gas at 7.97 L/min, and sweep gas at 1.5 L/min. MS2 parameters included Q1 resolution 0.4 (FWHM), Q3 resolution 0.4 (FWHM), and CID Gas 1.5 (mTorr). MS2 analysis of each visually detected peak was performed with a systematic search for m/z values of dGSL Na+ adducts, along with characteristic MS2 fragments (described in the Supplementary Materials). The signals were recorded at 227 nm with a DAD detector. Peaks of GSLs were quantified from UV peak areas using a calibration curve of pure desulfosinigrin solution with a concentration range from 0.14 to 1.4 mM (R2 = 0.98, y = 0.019x+0.997) and response proportionality factors (RPFs) for each individual dGSL. The following RPF values were used to quantify dGSLs: 0.29 for 43; 0.20 for 47; 0.95 for 11 and 105 [41]; and an arbitrary RPF of 1.0 for aliphatic GSLs 22, 31, 62, and 109 [42].
3.2.3. NMR Mesurements
NMR spectra were recorded using a Bruker AV600 spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) with a 5 mm diameter probe and z-gradient accessories at 25 °C. The 1H (zg30) and 13C{1H} (zgpg30) NMR spectra were recorded at 600.130 and 150.903 MHz, respectively. The chemical shifts (δ/ppm) of the 1H spectra were referenced to the D2O signal (1H: δ = 4.80 ppm), and those of the 13C spectra were referenced to 1,4 dioxane d8 (13C: δ = 66.66 ppm), which was used as an external reference. The 1H spectra were recorded with the following parameters: sweep width of 20.0 ppm, transmitter frequency offset of 9.0 ppm, FID resolution of 0.37 Hz, relaxation delay of 1 s, acquisition time of 1.36 s, and 64 scans. The 13C spectra were acquired using the following parameter values: sweep width of 240.0 ppm, transmitter frequency offset of 100.0 ppm, FID resolution of 0.55 Hz, relaxation delay of 1 s, acquisition time of 0.91 s, and 128 scans during 64 loop counts. The assignment of 1H and 13C signals in the NMR spectra was confirmed by cross peaks in the 1H-1H COSY (correlation spectroscopy) and 1H-13C HSQC (heteronuclear single-quantum coherence) 2D spectra. The COSY (cosygpqf) with a standard π/2 pulse sequence was measured with 2048 points in dimension F2 and 512 increments in dimension F1. The latter was subsequently zero-filled to 1024 points. The increments were obtained with 4 scans each, a 16.00 ppm spectral width, and a relaxation delay of 1.0 s. The FID resolution was 4.69 Hz/point and 37.51 Hz/point in the F2 and F1 dimensions, respectively. HSQC spectra (hsqcedetgppsisp.2) were recorded with 2048 points in the F2 dimension and 256 increments in the F1 dimension, subsequently zero-filled to 1024 points. For each increment, 32 scans were collected using a relaxation delay of 1.0 s. Spectral widths were 15.00 ppm (F2) and 180.0 ppm (F1), with corresponding resolutions of 8.78 and 212.22 Hz/point in the F2 and F1 dimensions, respectively.
2-Hydroxy-2-methylpropyl desulfoglucosinolate (desulfoglucoconringiin, d31): 1H NMR (600 MHz, D2O) δ (ppm) = 5.20 (d, 3J = 9.9 Hz, 1H, H-1), 3.93 (dd, 2J6a-6b = 12.6 Hz, 3J6a-5 = 2.2 Hz, 1H, H-6a), 3.73 (dd, 2J6b-6a = 12.6 Hz, 3J6b-5 = 6.0 Hz, 1H, H-6b), 3.61–3.55 (m, 2H, H-3, H-5), 3.50–3.44 (m, 2H, H-2, H-4), 2.83 (d, 2J = 15.2 Hz, 1H, H-8a), 2.74 (d, 2J = 15.2 Hz, 1H, H-8b), 1.35 (s, 3H, H-10a), 1.33 (s, 3H, H-10b); 13C NMR (151 MHz, D2O) δ (ppm) = 152.7 (C=N), 81.9 (C-1), 80.0 (C-5), 77.1 (C-3), 72.3 (C-2), 71.1 (Cq), 69.3 (C-4), 60.7 (C-6), 43.7 (C-8), 28.5 (C-10a), 27.9 (C10-b).
4. Conclusions
The investigation of GSL profiles of Reseda spp., as well as a review of the literature and an experimental study of other species, enabled a biosynthetic characterization of the Resedaceae family. Advances in our understanding of GSL biosynthesis and its evolution in Resedaceae species would benefit further from molecular genetic investigations. The existence of specific extraglycosylated GSLs, i.e., 2′′-O-(α-L-arabinopyranosyloxy)benzyl GSL (158) and 2-(α-L-arabinopyranosyloxy)-2-phenylethyl GSL (4) bearing arabinose and 2-(α-L-ramnopyranosyloxy)benzyl GSL (109) bearing rhamnose, suggests that specific genes are responsible for the evolution of these GSLs. However, more species should be investigated in order to chemically relate this family to other families that contain GSLs as chemical tags, as our and previous studies on R. alba suggest diversity in the genus itself. Appropriate analytical methods allowed the identification of previously unknown Leu-derived isobutyl GSLs. This study encourages further research into the relationship between GSLs as chemical tags of 16 families and corresponding phylogenetic investigations of aspects of their evolution.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules28041753/s1, Figure S1: Chromatogram of desulfoglucosinolates obtained from the different plant parts of Reseda alba (flower, leaf, stem, and root): d31—desulfo-2-hydroxy-2-methylpropyl dGSL (desulfoglucoconringiin), d43—desulfoindol-3-ylmethyl dGSL (desulfoglucobrassicin), d47—desulfo-N-methoxyindol-3-ylmethyl dGSL (desulfoneoglucobrassicin), d62—desulfoisobutyl dGSL, d105—desulfo-2-phenylethyl dGSL (desulfogluconasturtiin). Figure S2: Chromatogram of desulfoglucosinolates obtained from the different plant parts of Reseda lutea (stem, flower, leaf, and root): d11—desulfobenzyl dGSL (desulfoglucotropaeolin), d22—desulfo-3-hydroxybenzyl dGSL (desulfoglucolepigramin), d43—desulfoindol-3-ylmethyl dGSL (desulfoglucobrassicin), d109—desulfo-2-(α-L-rhamnopyranosyloxy)benzyl dGSL. Figure S3: Chromatogram of desulfoglucosinolates obtained from the different plant parts of Reseda phyteuma (flower, leaf, stem, and siliquae): d11—desulfobenzyl dGSL (desulfoglucotropaeolin), d43—desulfoindol-3-ylmethyl desulfoGSL (desulfoglucobrassicin), d105—desulfo-2-phenylethyl dGSL (desulfogluconasturtiin), d109—desulfo-2-(α-L-rhamnopyranosyloxy)benzyl dGSL. Figure S4: (A). MS2 spectra at 15V of detected desulfoglucosinolates. (B). MS2 spectra of d109 at 20V and 30 V. Figure S5: (A). 1H NMR spectrum of desulfo-2-hydroxy-2-methylpropyl dGSL (desulfoglucoconringiin). (B). 13C NMR spectrum of desulfo-2-hydroxy-2-methylpropyl dGSL (desulfoglucoconringiin). (C). COSY spectrum of desulfo-2-hydroxy-2-methylpropyl dGSL (desulfoglucoconringiin). (D). HSQC spectrum of desulfo-2-hydroxy-2-methylpropyl dGSL (desulfoglucoconringiin).
Author Contributions
A.Đ.: Formal analysis, Data curation, Writing—review and editing; J.T.: Data curation, Writing—review and editing; I.B.: Conceptualization, Supervision, Data curation, Writing—original draft, Writing—review and editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been fully supported by the Croatian Science Foundation (Grant IP-2016-06-1316).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We appreciate Mirko Ruščić’s help in botanically identifying the plants under investigation as well as the scientific research equipment provided by the EU grant “Functional integration of the University of Split, PMF-ST, PFST, and KTFST through the development of the scientific and research infra-structure” (KK.01.1.1.02.0018). NMR spectra were recorded at the NMR Center, Ruđer Bošković Institute.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Stevens, P.F. Angiosperm Phylogeny Website. Version 14 July 2017. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 23 December 2022).
- Çilden, E.; Yıldırımlı, Ş. The impact of seed micromorphology in the subgeneric classification of the genus Reseda L. (Resedaceae) in Turkey. Microsc. Res. Tech. 2021, 84, 1992–2003. [Google Scholar] [CrossRef]
- Martín-Bravo, S.; Meimberg, H.; Luceño, M.; Märkl, W.; Valcárcel, V.; Bräuchler, C.; Vargas, P.; Heubl, G. Molecular systematics and biogeography of Resedaceae based on ITS and trnL-F sequences. Mol. Phylogenet. Evol. 2007, 44, 1105–1120. [Google Scholar] [CrossRef]
- Martín-Bravo, S.; Vargas, P.; Luceño, M. Is oligomeris (Resedaceae) indigenous to North America? Molecular evidence for a natural colonization from the Old World. Am. J. Bot. 2009, 96, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, T. (Ed.) Flora Croatica Database; Faculty of Science, University of Zagreb: Zagreb, Croatia, 2005; Available online: http://hirc.botanic.hr/fcd (accessed on 18 December 2022).
- Agerbirk, N.; Hansen, C.C.; Olsen, C.E.; Kiefer, C.; Hauser, T.P.; Christensen, S.; Jensen, K.R.; Ørgaard, M.; Pattison, D.I.; Lange, C.B.A.; et al. Glucosinolate profiles and phylogeny in Barbarea compared to other tribe Cardamineae (Brassicaceae) and Reseda (Resedaceae), based on a library of ion trap HPLC-MS/MS data of reference desulfoglucosinolates. Phytochemistry 2021, 185, 112658. [Google Scholar] [CrossRef]
- Bell, L. The biosynthesis of glucosinolates: Insights, inconsistencies, and unknowns. Ann. Plant Rev. Online 2019, 2, 969–1000. [Google Scholar] [CrossRef]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health—Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Edger, P.P.; Heidel-Fischer, H.M.; Bekaert, M.; Rota, J.; Glöckner, G.; Platts, A.E.; Heckel, D.G.; Der, J.P.; Wafula, E.K.; Tang, M.; et al. The butterfly plant arms-race escalated by gene and genome duplications. Proc. Natl. Acad. Sci. USA 2015, 112, 8362–8383. [Google Scholar] [CrossRef]
- Leonti, M.; Nebel, S.; Rivera, D.; Heinrich, M. Wild gathered food plants in the European Mediterranean: A comparative analysis. Econ. Bot. 2006, 60, 130–142. [Google Scholar] [CrossRef]
- Gilbert, K.G.; Cooke, D.T. Dyes from plants: Past usage, present understanding and potential. Plant Growth Regul. 2001, 34, 57–69. [Google Scholar] [CrossRef]
- Abdallah, M.S.; De Witt, H.D.C. The Resedaceae—A taxonomical revision of the family (final instalment). Meded. Landbouwhogeschoo Wagening. 1978, 14, 308. Available online: https://edepot.wur.nl/287583 (accessed on 18 December 2022).
- Radulović, N.S.; Zlatković, D.B.; Ilić-Tomić, T.; Senerović, L.; Nikodinovic-Runic, J. Cytotoxic effect of Reseda lutea L.: A case of forgotten remedy. J. Ethnopharmacol. 2014, 153, 125–132. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening seeds of Scottish plants for antibacterial activity. J. Ethnopharmacol. 2002, 83, 73–77. [Google Scholar] [CrossRef]
- Benmerache, A.; Berrehal, D.; Khalfallah, A.; Kabouche, A.; Semra, Z.; Kabouche, Z. Antioxidant, antibacterial activities and flavonoids of Reseda phyteuma L. Der Pharm. Lett. 2012, 4, 1863–1867. [Google Scholar]
- Susplugas, P.; Mongold, J.J.; Taillade, C.; Serrano, J.J. Anti-inflammatory and analgesic activity of Reseda phyteuma. Plantes Med. Phytother. 1993, 26, 375–382. [Google Scholar]
- Moghaddam, N.S.; Eryılmaz, M.; Altanlar, N.; Yıldırım, O. Antimicrobial screening of some selected Turkish medicinal plants. Pak. J. Pharm. Sci. 2019, 32, 947–951. [Google Scholar] [PubMed]
- Asadi-Samani, M.; Khaledi, M.; Khaledi, F.; Samarghandian, S.; Gholipour, A. Phytochemical properties and antibacterial effects of Salvia multicaulis Vahl., Euphorbia microsciadia Boiss., and Reseda lutea on Staphylococcus aureus and Acinetobacter baumanii. Jundishapur J. Nat. Pharm. Prod. 2019, 14, e63640. [Google Scholar] [CrossRef]
- Badridze, G.; Kacharava, N.; Chkhubianishvili, E.; Rapava, L.; Kikvidze, M.; Chigladze, L.; Chanishvili, S. Content of antioxidants in leaves of some plants of Tbilisi environs. Bull. Georg. Natl. Acad. Sci. 2013, 7, 105–111. [Google Scholar]
- Abdalrahman, K.S.; Güneş, M.G.; Shomali, N.; Işgör, B.S.; Yildirim, Ö. Screening effects of methanol extracts of Diplotaxis tenuifolia and Reseda lutea on enzymatic antioxidant defense systems and aldose reductase activity. Turk. J. Pharm. Sci. 2018, 15, 97–102. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Rollin, P. Glucosinolates: Novel sources and biological potential. In Glucosinolates; Mérillon, J.-M., Ramawat, G.K., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–60. [Google Scholar]
- Burčul, F.; Generalić Mekinić, I.; Radan, M.; Rollin, P.; Blažević, I. Isothiocyanates: Cholinesterase inhibiting, antioxidant, and anti-inflammatory activity. J. Enzyme Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Mellon, F.A.; Kroon, P.A. Screening crucifer seeds as sources of specific intact glucosinolates using ion-pair high-performance liquid chromatography negative ion electrospray mass spectrometry. J. Agric. Food Chem. 2004, 52, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Gmelin, R.; Kjær, A. 2-Hydroxy-2-methylpropyl glucosinolate in Reseda alba. Phytochemistry 1970, 9, 599–600. [Google Scholar] [CrossRef]
- Olsen, O.; Sorensen, H. Isolation of glucosinolates and the identification of O-(α-L-rhamnopyranosyloxy)benzylglucosinolate from Reseda odorata. Phytochemistry 1979, 18, 1547–1552. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Bennett, R.N.; Kiddle, G.; Ihmig, S.; Ruppel, M.; Hilgenberg, W. The host range of Plasmodiophora brassicae and its relationship to endogenous glucosinolate content. New Phytol. 1999, 141, 443–458. [Google Scholar] [CrossRef]
- Pagnotta, E.; Montaut, S.; Matteo, R.; Rollin, P.; Nuzillard, J.-M.; Lazzeri, L.; Bagatta, M. Glucosinolates in Reseda lutea L.: Distribution in plant tissues during flowering time. Biochem. Syst. Ecol. 2020, 90, 104043. [Google Scholar] [CrossRef]
- Mithen, R.; Bennett, R.; Marquez, J. Glucosinolate biochemical diversity and innovation in the Brassicales. Phytochemistry 2010, 71, 2074–2086. [Google Scholar] [CrossRef] [PubMed]
- Kjær, A.; Gmelin, R. An isothiocyanate glucoside (glucobarbarin) of Reseda luteola L. Acta Chem. Scand. 1958, 12, 1693–1694. [Google Scholar] [CrossRef]
- Griffiths, D.W.; Deighton, N.; Birch, A.N.; Patrian, B.; Baur, R.; Städler, E. Identification of glucosinolates on the leaf surface of plants from the Cruciferae and other closely related species. Phytochemistry 2001, 57, 693–700. [Google Scholar] [CrossRef]
- Agerbirk, N.; Matthes, A.; Erthmann, P.Ø.; Ugolini, L.; Cinti, S.; Lazaridi, E.; Nuzillard, J.-M.; Müller, C.; Bak, S.; Rollin, P.; et al. Glucosinolate turnover in Brassicales species to an oxazolidin-2-one, formed via the 2-thione and without formation of thioamide. Phytochemistry 2018, 153, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Olsen, O.; Rasmussen, K.W.; Sørensen, H. Glucosinolates in Sesamoides canescens and S. pygmaea: Identification of 2-(α-L-arabinopyranosyloxy)-2-phenylethylglucosinolate. Phytochemistry 1981, 20, 1857–1861. [Google Scholar] [CrossRef]
- Olsen, O.; Sorensen, H. Glucosinolates and amines in Reseda media. Phytochemistry 1980, 19, 1783–1787. [Google Scholar] [CrossRef]
- Trabelcy, B.; Chinkov, N.; Samuni-Blank, M.; Merav, M.; Izhaki, I.; Carmeli, S.; Gerchman, Y. Investigation of glucosinolates in the desert plant Ochradenus baccatus (Brassicales: Resedaceae). Unveiling glucoochradenin, a new arabinosylated glucosinolate. Phytochemistry 2021, 187, 112760. [Google Scholar] [CrossRef] [PubMed]
- Đulović, A.; Popović, M.; Burčul, F.; Čikeš Čulić, V.; Marijan, S.; Ruščić, M.; Anđelković, N.; Blažević, I. Glucosinolates of Sisymbrium officinale and S. orientale. Molecules 2022, 27, 8431. [Google Scholar] [CrossRef]
- Montaut, S.; Read, S.; Blažević, I.; Nuzillard, J.-M.; Harakat, D.; Rollin, P. Glucosinolates of Lepidium graminifolium L. (Brassicaceae) from Croatia. Nat. Prod. Res. 2021, 35, 494–498. [Google Scholar] [CrossRef]
- Agerbirk, N.; Hansen, C.C.; Kiefer, C.; Hauser, T.P.; Ørgaard, M.; Lange, C.B.A.; Cipollini, D.; Koch, M.A. Comparison of glucosinolate diversity in the crucifer tribe Cardamineae and the remaining order Brassicales highlights repetitive evolutionary loss and gain of biosynthetic steps. Phytochemistry 2021, 185, 112668. [Google Scholar] [CrossRef] [PubMed]
- Agerbirk, N.; Pattison, D.I.; Mandáková, T.; Lysak, M.A.; Montaut, S.; Staerk, D. Ancient biosyntheses in an oil crop: Glucosinolate profiles in Limnanthes alba and its relatives (Limnanthaceae, Brassicales). J. Agric. Food Chem. 2022, 70, 1134–1147. [Google Scholar] [CrossRef]
- Wathelet, J.-P.; Iori, R.; Leoni, O.; Quinsac, A.; Palmieri, S.; Rollin, P. Guidelines for glucosinolate analysis in green tissues used for biofumigation. Agroindustria 2004, 3, 257–344. [Google Scholar]
- European Community. Oil seeds–determination of glucosinolates High Performance Liquid Chromatography. Off. J. Eur. Communities 1990, 170, 28. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


