Valorization of Hemp-Based Packaging Waste with One-Pot Ionic Liquid Technology
Abstract
1. Introduction
2. Results and Discussion
2.1. Biomass Composition
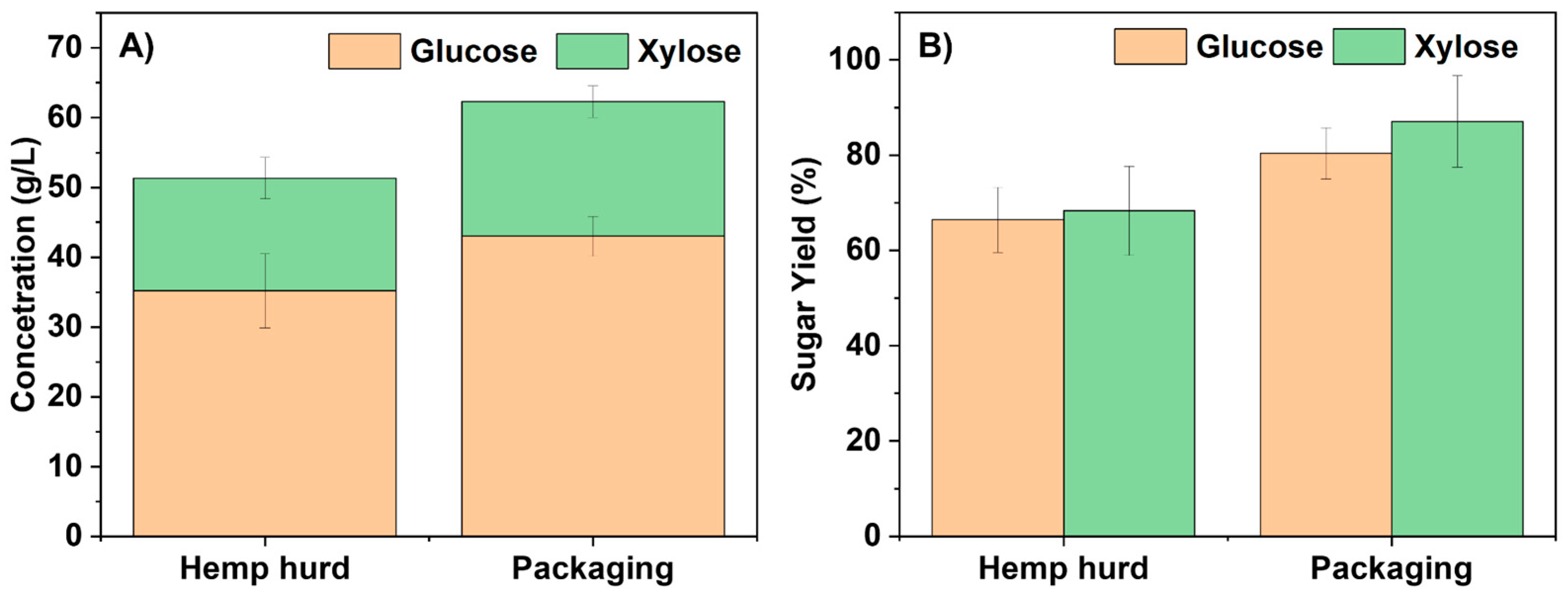
2.2. Hydrolysate Generation Using a One-Pot Ionic Liquid Process
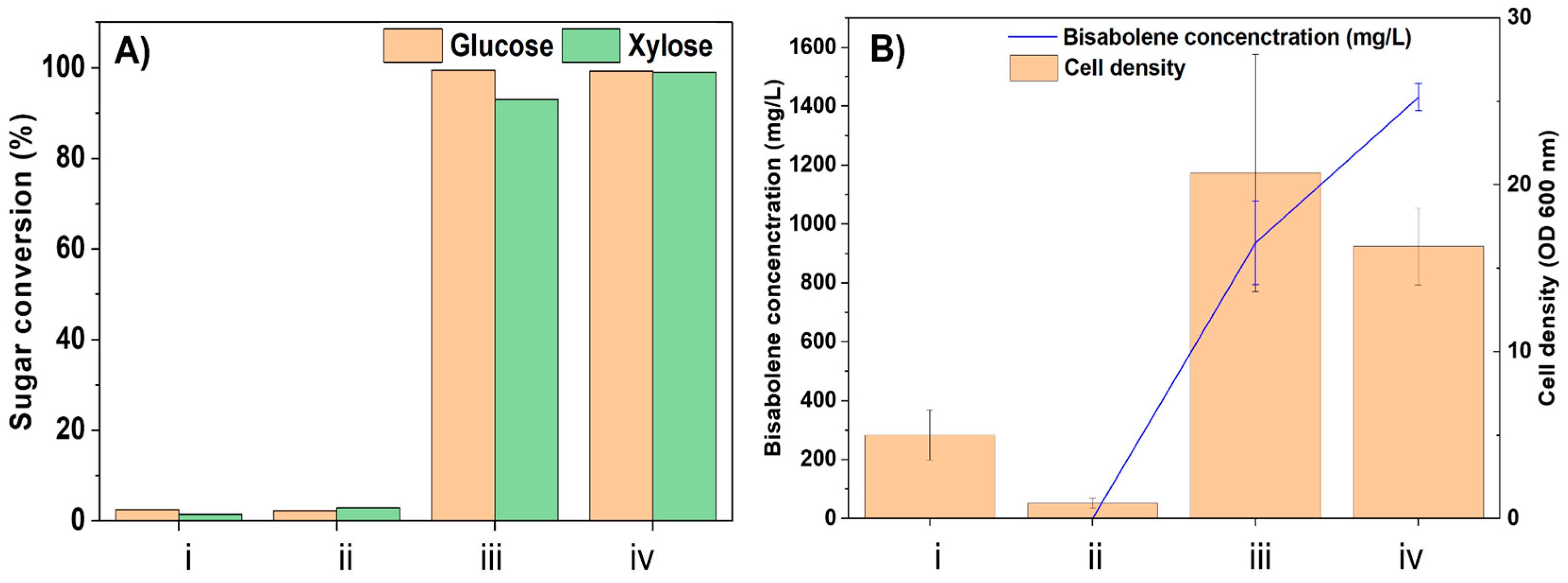
2.3. Biocompatibility of Hydrolysates
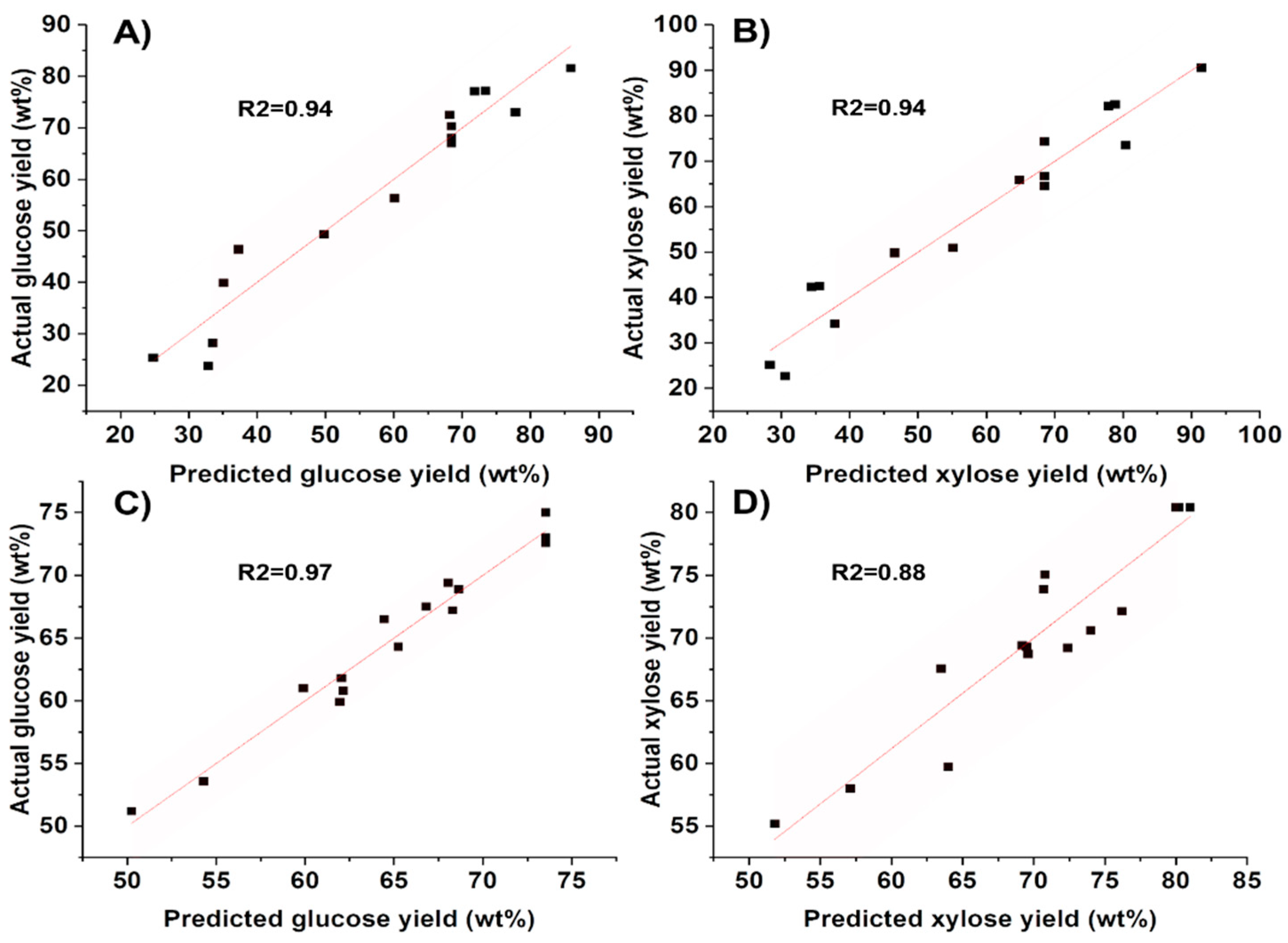
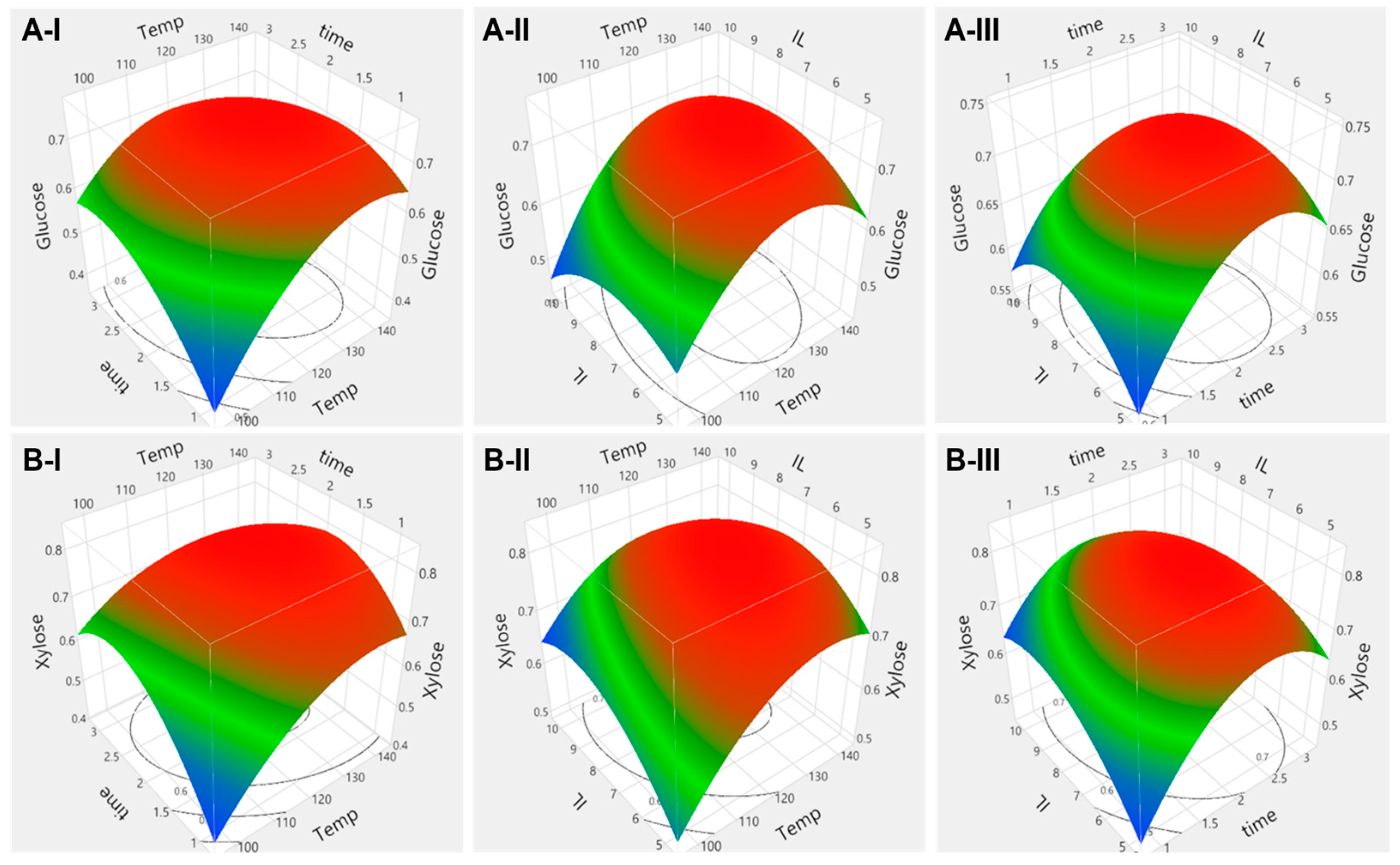
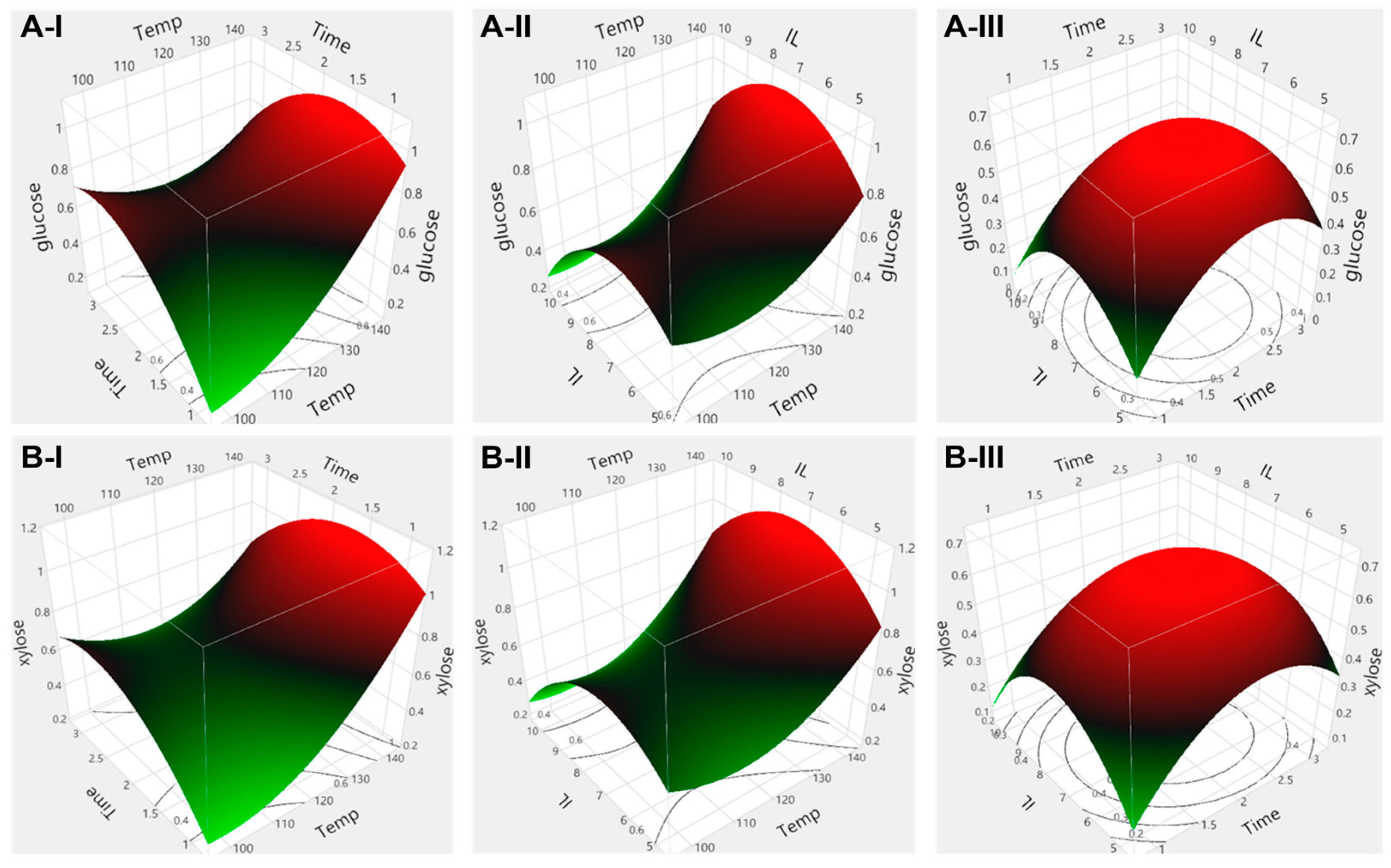
2.4. Effect of Process Parameters on Sugar Yield and Optimization of Pretreatment Conditions
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Compositional Analysis
3.3. Biomass Pretreatment and Enzymatic Hydrolysis
3.4. Process Optimization
3.5. Yeast Strain and Cultivation Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, J.; Won, W.; Capareda, S.C. The economical production of functionalized Ashe juniper derived-biochar with high hazardous dye removal efficiency. Ind. Crops Prod. 2019, 137, 672–680. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.M.; Sels, B.F.; Wang, F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2021, 14, 262–292. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Comparative sugar recovery data from laboratory scale application of leading pretreatment technologies to corn stover. Bioresour. Technol. 2005, 96, 2026–2032. [Google Scholar] [CrossRef]
- Nitsos, C.; Lazaridis, P.; Mach-Aigner, A.; Matis, K.; Triantafyllidis, K. Increasing the efficiency of lignocellulosic biomass enzymatic hydrolysis: Hydrothermal pretreatment, extraction of surface lignin, wet milling and production of cellulolytic enzymes. ChemSusChem 2019, 12, 1179–1195. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Li, C.; Knierim, B.; Manisseri, C.; Arora, R.; Scheller, H.V.; Auer, M.; Vogel, K.P.; Simmons, B.A.; Singh, S. Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour. Technol. 2010, 101, 4900–4906. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Liggenstoffer, A.S.; Youssef, N.H.; Wilkins, M.R.; Elshahed, M.S. Evaluating the utility of hydrothermolysis pretreatment approaches in enhancing lignocellulosic biomass degradation by the anaerobic fungus Orpinomyces sp. strain C1A. J. Microbiol. Methods 2014, 104, 43–48. [Google Scholar] [CrossRef]
- Liu, K.; Atiyeh, H.K.; Pardo-Planas, O.; Ezeji, T.C.; Ujor, V.; Overton, J.C.; Berning, K.; Wilkins, M.R.; Tanner, R.S. Butanol production from hydrothermolysis-pretreated switchgrass: Quantification of inhibitors and detoxification of hydrolyzate. Bioresour. Technol. 2015, 189, 292–301. [Google Scholar] [CrossRef]
- Hastrup, A.C.S.; Howell, C.; Larsen, F.H.; Sathitsuksanoh, N.; Goodell, B.; Jellison, J. Differences in crystalline cellulose modification due to degradation by brown and white rot fungi. Fungal Biol. 2012, 116, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Parthasarathi, R.; Socha, A.M.; Shi, J.; Zhang, S.; Stavila, V.; Sale, K.L.; Simmons, B.A.; Singh, S. Understanding pretreatment efficacy of four cholinium and imidazolium ionic liquids by chemistry and computation. Green Chem. 2014, 16, 2546–2557. [Google Scholar] [CrossRef]
- Das, L.; Achinivu, E.C.; Barcelos, C.A.; Sundstrom, E.; Amer, B.; Baidoo, E.E.K.; Simmons, B.A.; Sun, N.; Gladden, J.M. Deconstruction of woody biomass via protic and aprotic ionic liquid pretreatment for ethanol production. ACS Sustain. Chem. Eng. 2021, 9, 4422–4432. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.; Konda, N.V.S.N.M.; Shi, J.; Dutta, T.; Scown, C.D.; Simmons, B.A.; Singh, S. Transforming biomass conversion with ionic liquids: Process intensification and the development of a high-gravity, one-pot process for the production of cellulosic ethanol. Energy Environ. Sci. 2016, 9, 1042–1049. [Google Scholar] [CrossRef]
- Shi, J.; Gladden, J.M.; Sathitsuksanoh, N.; Kambam, P.; Sandoval, L.; Mitra, D.; Zhang, S.; George, A.; Singer, S.W.; Simmons, B.A.; et al. One-pot ionic liquid pretreatment and saccharification of switchgrass. Green Chem. 2013, 15, 2579. [Google Scholar] [CrossRef]
- Das, L.; Geiselman, G.M.; Rodriguez, A.; Magurudeniya, H.D.; Kirby, J.; Simmons, B.A.; Gladden, J.M. Seawater-based one-pot ionic liquid pretreatment of sorghum for jet fuel production. Bioresour. Technol. Rep. 2020, 13, 100622. [Google Scholar] [CrossRef]
- Rigual, V.; Papa, G.; Rodriguez, A.; Wehrs, M.; Kim, K.H.; Oliet, M.; Alonso, M.V.; Gladden, J.M.; Mukhopadhyay, A.; Simmons, B.A.; et al. Evaluating protic ionic liquid for woody biomass one-pot pretreatment + saccharification, followed by Rhodosporidium toruloides cultivation. ACS Sustain. Chem. Eng. 2019, 8, 782–791. [Google Scholar] [CrossRef]
- Das, L.; Li, W.; Dodge, L.A.; Stevens, J.C.; Williams, D.W.; Hu, H.; Li, C.; Ray, A.E.; Shi, J. Comparative evaluation of industrial hemp cultivars: Agronomical practices, feedstock characterization, and potential for biofuels and bioproducts. ACS Sustain. Chem. Eng. 2020, 8, 6200–6210. [Google Scholar] [CrossRef]
- Cranshaw, W.; Schreiner, M.; Britt, K.; Kuhar, T.P.; McPartland, J.; Grant, J. Developing insect pest management systems for hemp in the United States: A work in progress. J. Integr. Pest Manag. 2019, 10, 26. [Google Scholar] [CrossRef]
- Shahzad, A. Impact and fatigue properties of hemp–glass fiber hybrid biocomposites. J. Reinf. Plast. Compos. 2011, 30, 1389–1398. [Google Scholar] [CrossRef]
- González-García, S.; Hospido, A.; Feijoo, G.; Moreira, M.T. Life cycle assessment of raw materials for non-wood pulp mills: Hemp and flax. Resour. Conserv. Recycl. 2010, 54, 923–930. [Google Scholar] [CrossRef]
- Murugu Nachippan, N.; Alphonse, M.; Bupesh Raja, V.K.; Shasidhar, S.; Varun Teja, G.; Harinath Reddy, R. Experimental investigation of hemp fiber hybrid composite material for automotive application. Mater. Today Proc. 2021, 44, 3666–3672. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Luo, X.; Li, Y.; Shah, A. Comparative study of changes in composition and structure during sequential fungal pretreatment of non-sterile lignocellulosic feedstocks. Ind. Crops Prod. 2019, 133, 383–394. [Google Scholar] [CrossRef]
- Salvachúa, D.; Katahira, R.; Cleveland, N.S.; Khanna, P.; Resch, M.G.; Black, B.A.; Purvine, S.O.; Zink, E.M.; Prieto, A.; Martínez, M.J.; et al. Lignin depolymerization by fungal secretomes and a microbial sink. Green Chem. 2016, 18, 6046–6062. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Eudes, A.; George, A.; Mukerjee, P.; Kim, J.S.; Pollet, B.; Benke, P.I.; Yang, F.; Mitra, P.; Sun, L.; Cetinkol, O.P.; et al. Biosynthesis and incorporation of side-chain-truncated lignin monomers to reduce lignin polymerization and enhance saccharification. Plant Biotechnol. J. 2012, 10, 609–620. [Google Scholar] [CrossRef]
- Wu, W.; Dutta, T.; Varman, A.M.; Eudes, A.; Manalansan, B.; Loqué, D.; Singh, S. Lignin valorization: Two hybrid biochemical routes for the conversion of polymeric lignin into value-added chemicals. Sci. Rep. 2017, 7, 8420. [Google Scholar] [CrossRef]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Frederix, M.; Mingardon, F.; Hu, M.; Sun, N.; Pray, T.; Singh, S.; Simmons, B.A.; Keasling, J.D.; Mukhopadhyay, A. Development of an E. coli strain for one-pot biofuel production from ionic liquid pretreated cellulose and switchgrass. Green Chem. 2016, 18, 4189–4197. [Google Scholar] [CrossRef]
- Yaegashi, J.; Kirby, J.; Ito, M.; Sun, J.; Dutta, T.; Mirsiaghi, M.; Sundstrom, E.R.; Rodriguez, A.; Baidoo, E.; Tanjore, D.; et al. Rhodosporidium toruloides: A new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts. Biotechnol. Biofuels 2017, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.; Geiselman, G.M.; Yaegashi, J.; Kim, J.; Zhuang, X.; Tran-Gyamfi, M.B.; Prahl, J.-P.; Sundstrom, E.R.; Gao, Y.; Munoz, N.; et al. Further engineering of R. toruloides for the production of terpenes from lignocellulosic biomass. Biotechnol. Biofuels 2021, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, G.; Wijffels, R.H.; Marzocchella, A.; Russo, M.E. Bioreactor and bioprocess design issues in enzymatic hydrolysis of lignocellulosic biomass. Catalysts 2021, 11, 680. [Google Scholar] [CrossRef]
- Li, C.; Tanjore, D.; He, W.; Wong, J.; Gardner, J.L.; Sale, K.L.; Simmons, B.A.; Singh, S. Scale-up and evaluation of high solid ionic liquid pretreatment and enzymatic hydrolysis of switchgrass. Biotechnol. Biofuels 2013, 6, 154. [Google Scholar] [CrossRef]
- Mohamed, E.T.; Wang, S.; Lennen, R.M.; Herrgård, M.J.; Simmons, B.A.; Singer, S.W.; Feist, A.M. Generation of a platform strain for ionic liquid tolerance using adaptive laboratory evolution. Microb. Cell Fact. 2017, 16, 204. [Google Scholar] [CrossRef]
- Chan, K.S.; Greaves, S.J.; Rahardja, S. Techniques for addressing saddle points in the response surface methodology (RSM). IEEE Access 2019, 7, 85613–85621. [Google Scholar] [CrossRef]
- He, Y.-C.; Liu, F.; Gong, L.; Di, J.-H.; Ding, Y.; Ma, C.-L.; Zhang, D.-P.; Tao, Z.-C.; Wang, C.; Yang, B. Enzymatic in situ saccharification of chestnut shell with high ionic liquid-tolerant cellulases from Galactomyces sp. CCZU11-1 in a biocompatible ionic liquid-cellulase media. Bioresour. Technol. 2016, 201, 133–139. [Google Scholar] [CrossRef]
- Magurudeniya, H.D.; Baral, N.R.; Rodriguez, A.; Scown, C.D.; Dahlberg, J.; Putnam, D.; George, A.; Simmons, B.A.; Gladden, J.M. Use of ensiled biomass sorghum increases ionic liquid pretreatment efficiency and reduces biofuel production cost and carbon footprint. Green Chem. 2021, 23, 3127–3140. [Google Scholar] [CrossRef]
- Fu, D.; Mazza, G. Optimization of processing conditions for the pretreatment of wheat straw using aqueous ionic liquid. Bioresour. Technol. 2011, 102, 8003–8010. [Google Scholar] [CrossRef]
- Rodriguez, A.; Salvachúa, D.; Katahira, R.; Black, B.A.; Cleveland, N.S.; Reed, M.; Smith, H.; Baidoo, E.E.K.; Keasling, J.D.; Simmons, B.A.; et al. Base-Catalyzed Depolymerization of Solid Lignin-Rich Streams Enables Microbial Conversion. ACS Sustain. Chem. Eng. 2017, 5, 8171–8180. [Google Scholar] [CrossRef]
- Benz, G.T. Agitator Design Technology for Biofuels and Renewable Chemicals; Wiley: Hoboken, NJ, USA, 2022; ISBN 9781119815495. [Google Scholar]
- NREL. NREL: Preparation of Samples for Compositional Analysis. Laboratory Analytical Procedure; NREL: Golden, CO, USA, 2008. [Google Scholar]
- NREL. NREL: Determination of Extractives in Biomass: Laboratory Analytical Procedure; NREL: Golden, CO, USA, 2005. [Google Scholar]
| Hemp Hurd (wt%) | Packaging Material (wt%) | |
|---|---|---|
| Extractives | 8.3 ± 3.2 | 14.7 ± 1.1 |
| Glucan | 30.3 ± 0.9 | 28.6 ± 0.1 |
| Xylan | 13.5 ± 0.5 | 11.9 ± 0.1 |
| Klason lignin | 22.4 ± 0.7 | 22.4 ± 3.0 |
| Ash | 0.6 ± 0.4 | 0.6 ± 0.3 |
| Variables | Factor Code | Level of Factor | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Temperature (°C) | X1 | 100 | 120 | 140 |
| Time (h) | X2 | 1 | 2 | 3 |
| Ionic liquid loading (%) | X3 | 5 | 7.5 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Rodriguez, A.; Simmons, B.A.; Gladden, J.M. Valorization of Hemp-Based Packaging Waste with One-Pot Ionic Liquid Technology. Molecules 2023, 28, 1427. https://doi.org/10.3390/molecules28031427
Choi J, Rodriguez A, Simmons BA, Gladden JM. Valorization of Hemp-Based Packaging Waste with One-Pot Ionic Liquid Technology. Molecules. 2023; 28(3):1427. https://doi.org/10.3390/molecules28031427
Chicago/Turabian StyleChoi, Julius, Alberto Rodriguez, Blake A. Simmons, and John M. Gladden. 2023. "Valorization of Hemp-Based Packaging Waste with One-Pot Ionic Liquid Technology" Molecules 28, no. 3: 1427. https://doi.org/10.3390/molecules28031427
APA StyleChoi, J., Rodriguez, A., Simmons, B. A., & Gladden, J. M. (2023). Valorization of Hemp-Based Packaging Waste with One-Pot Ionic Liquid Technology. Molecules, 28(3), 1427. https://doi.org/10.3390/molecules28031427








