Abstract
This work reports the synthesis of CuxSny alloy aerogels for electrochemical CO2 reduction catalysts. An in situ reduction and the subsequent freeze-drying process can successfully give CnxSny aerogels with tuneable Sn contents, and such aerogels are composed of three-dimensional architectures made from inter-connected fine nanoparticles with pores as the channels. Density functional theory (DFT) calculations show that the introduction of Sn in Cu aerogels inhibits H2 evolution reaction (HER) activity, while the accelerated CO desorption on the catalyst surface is found at the same time. The porous structure of aerogel also favors exposing more active sites. Counting these together, with the optimized composition of Cu95Sn5 aerogel, the high selectivity of CO can be achieved with a faradaic efficiency of over 90% in a wide potential range (−0.7 V to −1.0 V vs. RHE).
1. Introduction
Electrochemical CO2 reduction reaction (CO2RR) can convert CO2 to useful fuels and chemicals, and the efficiency of such a reaction highly depends on the electrocatalysts [1,2,3,4]. Despite the different types of new catalysts reported recently [5,6,7,8,9], copper (Cu) still holds its advantages of low cost, being earth-abundant, and the capability to convert CO2 to a series of chemicals, including CO, hydrocarbons, and other value-added liquid alcohol and acid [10]. Further improving the selectivity to a specific product, minimizing the overpotential, and suppressing the main competing H2 evolution reaction (HER) are the key to developing Cu-based electrocatalysts [11], and this has been accomplished by several methods, such as adopting oxide-derived Cu [12], building Cu-oxide heterojuctions [13,14,15], constructing Cu complexes [16], and alloying Cu with other elements [17].
Alloying or constructing bimetallic compounds can alter the local geometric and/or electronic structures and bring new active metal sites [18,19,20]. Both theoretical calculations and experimental works have shown that the selectivity towards CO can be greatly improved in a Cu alloy or bimetallic electrocatalyst, due to the change in binding strength between the reaction intermediates and the catalyst surface. In the literature, CuIn [21], CuAg [22], CuPd [23], and CuSn alloys [24,25] have been extensively studied. Among the possible elements, Tin (Sn) is one of the effective candidates due to its unique activities and low cost [26]. Porous Cu-Sn electrodes are favorable to producing formate during CO2RR [27]. A similar phenomenon has been observed in electrodeposited Cu-Sn alloy on carbon fibers [25]. In CuSn systems, previous studies showed that controlling the geometry or the composition can improve the catalytic performance for CO2 reduction. For example, monodisperse Cu core/SnO2 shell nanoparticles prepared by the seed-mediated method have shown a faradaic efficiency (FE) of CO reaching 93% at −0.7 V vs. RHE [28]. Takanabe et al. reported a Cu-Sn bimetallic catalyst by electrodeposition Sn species on the surface of oxide-derived Cu, which can selectively produce CO (CO faradaic efficiency > 90%) over a wide potential range [29]. However, such electrodes often suffer from a low total current density compared with their pristine Cu counterparts.
Further optimizing such electrodes could be realized by adopting porous structures to offer more active sites while the selectivity may be retained. Aerogels that have intrinsic features, such as large surface area, high porosity, and abundant active sites, would fulfill such requirements, especially for catalytic reactions [30,31,32,33,34]. Eychmüller’s pioneer work has proven the feasibility of preparing various alloy hydrogels in aqueous solution, and the yielded aerogels have often shown superior activity compared to their nanoparticle analogs because of the special properties of nanomaterials which are incorporated and enlarged on a macroscopic scale [35]. Apart from the well-studied noble metal alloy aerogels, Cu-based aerogel has also attracted a great deal of interest. A series of MCu (M = Pd, Pt, and Au) alloy aerogels can serve as unsupported electrocatalysts, providing enhanced oxygen reduction reaction catalytic activities and high stabilities [36]. Therefore, suitable Cu-based aerogels could be a promising candidate to drive CO2RR.
Inspired by the above discussion, in this study, we present the work to employ CuxSny alloy aerogels as electrocatalysts for electrochemical CO2RR. By using the well-established simple co-reduction and gelation process, CuxSny hydrogel can readily form. The synthesis relies on the rapid reduction of Cu and Sn cations in a liquid medium and the populated nanoparticles form a porous gel structure. After freeze-drying, porous CuxSny alloy aerogels with large surface area and pore volume are obtained. Such an aerogel preserves the well-known HER suppression effect brought by the Sn additive and the open structure for exposing electrochemical active sites. As a result, a robust and highly efficient CuxSny electrocatalyst that can selectively reduce CO2 to CO is developed. Potentially with the newly emerged new preparation methods for fine metal nanoparticles, such as microfluid assistant synthesis [37,38], it would be feasible to explore more possible applications of such metal aerogels.
2. Experimental
2.1. Synthesis of CuxSny Aerogels
The preparation of the aerogel includes the preparation of the hydrogel and the freeze-drying of the hydrogel to the aerogel. The CuxSny hydrogels were synthesized by simply mixing CuCl2 (analytical grade, Sinopharm chemical reagent) and SnCl4 (analytical grade, Sinopharm chemical reagent) with NaBH4 (analytical grade, Sinopharm chemical reagent) in an aqueous solution, which has been reported for preparing various noble metal hydrogels [39,40]. Taking the preparation of the Cu95Sn5 hydrogel as an example, 0.756 mmol CuCl2·2H2O and 0.084 mmol SnCl4·5H2O were completely dissolved in 70 mL H2O and stirred at 60 °C for 10 min, and a green solution was obtained. After that, 7 mL of 2 M NaBH4 aqueous solution was quickly injected into the above solution under stirring. The color of the mixture turned black immediately. The obtained suspension was further kept at 60 °C for 3 h and a black monolithic hydrogel gradually emerged at the bottom of the container. The hydrogel was then collected with caution and washed with H2O several times to remove the soluble impurities and by-products. The hydrogel monolithic was loaded into a freeze dryer (FD-1A-80, Boyikang, Beijing, China) at 195 K and then vacuumed for 48 h to give Cu95Sn5 aerogel. Furthermore, the molar ratio of CuCl2 and SnCl4 can be adjusted for the preparation of other CuxSny aerogels with different compositions. By the identical protocol, Cu97Sn3, Cu95Sn5, and Cu90Sn10 aerogels were prepared (the detail can be seen in the supporting information Table S1). Pristine Cu and Sn aerogels were also prepared as control samples.
2.2. Materials Characterization
X-ray diffraction (XRD) patterns of the samples were collected on SHIMADZU XRD-6000. The morphology was observed by using HITACHI S-4800 scanning electron microscopy (SEM) and JEOL-2100F transmission electron microscopy (TEM). X-ray photoelectron spectroscopy (XPS) data were obtained on Thermo Scientific K-Alpha using monochromatic Al Kα radiation. The nitrogen adsorption/desorption isotherms of the samples were performed using Micromeritics ASAP2460. The exact content of the chemical element was obtained with an inductively coupled plasma optical emission spectrometer (ICP-OES, Agilent 720ES). Temperature-programmed desorption mass spectrometry (TPD-MS) of CO on the samples was performed on AutoChem1 II 2920.
2.3. Electrochemical Measurements
In a typical experiment, 5 mg of aerogel was dispersed in a solvent of 400 μL ethanol, 100 μL deionized water, and 20 μL 5 wt% Nafion solution (Sigma-Aldrich) to form the ink. Then, 100 μL ink was dropped on carbon paper (1 cm × 1 cm) and dried in a vacuum oven as the working electrode. Ag/AgCl and Pt foil were used as the reference electrode and counter electrode, respectively. A 0.1 M KHCO3 solution was used as the electrolyte and purged with CO2 gas (20 mL min−1) to ensure sufficient CO2 transport to the electrode surface during the testing.
The electrochemical CO2 reduction was conducted on an IVIUM VERTEX electrochemical workstation using a typical gas-tight H-type cell, separated with a Nafion 117 proton exchange membrane. The electrolyte was constantly stirred during the testing. The linear sweep voltammetry (LSV) measurement was performed from 0 V to −1.1 V vs. RHE at 10 mV s−1. Potentiostatic electrochemical reduction of CO2 was investigated at different potentials. The gas products were quantified with a gas chromatograph (GC, Fuly GC9720Plus) equipped with a thermal conductivity detector (TCD) and a flame ionization detector (FID). The GC was calibrated by the standard gas before each measurement. The detailed calculation of the Faradaic efficiencies of gaseous products can be found in the supporting information.
3. Results and Discussion
Pristine Cu, Sn, and CuxSny alloy aerogels (including Cu97Sn3, Cu95Sn5, and Cu90Sn10) are obtained via the in situ reduction of the metal cations and freeze-drying. The detailed chemicals can be found in Table S1. Figure 1a gives the XRD (X-ray diffraction) patterns of the corresponding samples. The diffraction peaks of the pristine Cu and Sn samples can well match with the standard Cu (JCPDS#00-004-0836, cubic) and Sn (JCPDS#00-004-0673, tetragonal) crystals. This proves that NaBH4 can effectively reduce the high valence Cu and Sn cations to zero valence and form crystalized metal species. CuxSny aerogels have similar diffraction peaks to that of pristine Cu aerogel despite their different compositions, and no other notable oxide impurities or peaks to Sn can be found. The observed diffraction peaks can be assigned to (111), (200), and (220) planes of Cu. Similar patterns have been found in Cu-Sn alloy samples in the literature [41,42], suggesting that the CnSn alloy nanowires and Cu-Sn foam have an identical set of patterns to the standard Cu. A closer view of the (111) peak of CuxSny series samples in Figure 1b reveals that the peak shifts to a lower angle, which is a sign of the expanded crystalline lattice. Considering that the radius of Sn atoms (140 pm) is larger than that of Cu (128 pm), the crystalline phase of the CuxSny aerogel can be identified as a Cu-Sn alloy, and Sn atoms are implanted into the Cu crystalline lattice. According to the Bragg equation, the (111) plane spacing of the Cu, Cu97Sn3, Cu95Sn5, and Cu90Sn10 samples was calculated as 0.2087 nm, 0.2089 nm, 0.2090 nm, and 0.2090 nm, respectively, which was consistent with the peak shift. The broad diffraction feature also confirms the aerogels are composed of fine nanoparticles. Based on Sherrer’s equation, the calculated crystalline sizes in Cu, Cu97Sn3, Cu95Sn5, and Cu90Sn10 aerogels are ~17.3 nm, 13.1 nm, 11.8 nm, and 11.9 nm, respectively.
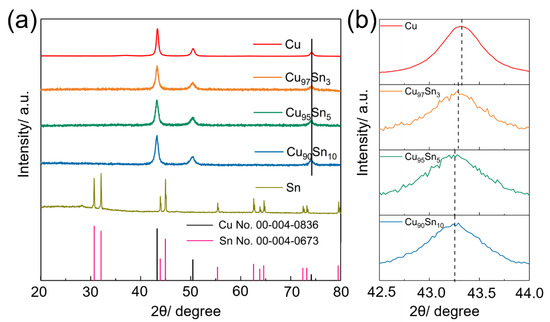
Figure 1.
(a) XRD patterns of Cu, Cu97Sn3, Cu95Sn5, Cu90Sn10, and Sn aerogels, and (b) the closer view of (111) peak.
The chemical states of the elements in the aerogels were further characterized by XPS (X-ray photoelectron spectroscopy) measurements. Figures S1 and S2 give detailed scans of Cu 2p and Sn 3d core levels of the aerogels. Generally, the Cu 2p of the aerogels can be well fitted by two pairs of peaks, which correspond to Cu+/Cu0 (2p3/2 at 932.63 eV and 2p1/2 at 952.43 eV, separation 19.8 eV) and Cu2+ (2p3/2 at 934.83 eV and 2p1/2 at 954.83 eV, separation 20.0 eV) [28,43]. The component of Cu+/Cu0 is dominated in the spectra and matched with XRD. The Sn 3d spectra show the Sn 3d5/2 maximum at 486.4 eV, which is related to the presence of Sn (IV), together with a small shoulder at 485.9 eV due to Sn (II) [24,28,42]. The component of Cu2+ and Sn (IV) and Sn (II) may mainly result from the surface oxidation of the aerogels upon exposure to air [42]. The overall Cu:Sn composition determined by inductively coupled plasma atomic emission spectrometry (ICP-AES) is ~97:3, 95:5, and 90:10 for Cu97Sn3, Cu95Sn5, and Cu90Sn10 aerogels, respectively, as shown in the supporting information Table S1. It should be pointed out that the Sn content in the final aerogel is always lower than that in the precursors, which may be due to the different hydrolysis rates of Cu and Sn cations.
The freeze-drying process of the hydrogel ensures the porous structure of the aerogels. The morphology of the Cu and CuxSny aerogels are characterized by SEM (scanning electron microscope) and TEM (transmission electron microscope), and the results are displayed in Figure 2. The appearance of the pristine Cu aerogel (Figure 2ai) shows a typical 3D porous structure composed of fine nanoparticles, which is a characteristic morphology for aerogels and is similar to that of the PtxPdy aerogel [44] and Pd aerogel [35] reported in the literature. The TEM image in Figure 2aii further revealed the microstructure of the interconnected nanoparticles with plenty of voids between them. The average size of the nanoparticles is around 20 nm, which is close to the value calculated from XRD. HRTEM image indicates that an individual nanoparticle is highly crystallized, and a lattice fringe of 0.21 nm can be seen, which corresponds to the (111) interplane distance of Cu (Figure 2aiii). This point is further confirmed by the selected area electron diffraction (SAED) pattern in Figure 2aiv since clear diffraction dots are presented. With the incorporation of Sn, there is no obvious change in the microstructure as shown in Figure 2bi–biv,ci–civ,di–div, and a porous nanoparticle skeleton is always seen. This indicates that the reduction and gelation processes were not significantly affected by the composition of the precursor. The EDX mappings of CuxSny aerogels show a uniform distribution of Sn and Cu elements within the aerogel framework, further indicating an alloy has formed.
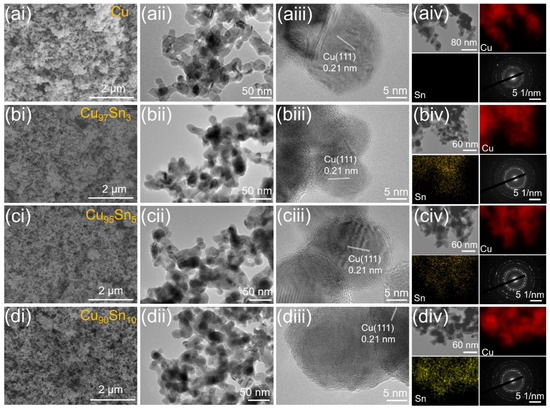
Figure 2.
SEM, TEM, HRTEM, EDX mapping images and SAED patterns of Cu (ai–aiv), Cu97Sn3 (bi–biv), Cu95Sn5 (ci–civ), and Cu90Sn10 (di–div) aerogels.
The surface area and the porosity of the aerogels samples are obtained by N2 physisorption experiments at 77 K. Figure S3 displays the isothermal adsorption/desorption curves, and the inset is the pore size distribution plots which are assessed by Barrett–Joyner–Halenda (BJH) method. The surface areas estimated from Brunauer–Emmett–Teller (BET) theory are 23.1 m2 g−1, 29.5 m2 g−1, 32.2 m2 g−1, and 29.7 m2 g−1 for the Cu, Cu97Sn3, Cu95Sn5, and Cu90Sn10 aerogels, respectively. The values are within the range of the reported MCu (M= Pd, Pt, and Au) alloy aerogels prepared by a similar method [36]. All the aerogels show a wide range of pores in the mesopores ranges. At high relative pressure (P/P0), no plateau appears in the adsorption isotherm, which implies the presence of macropores at the same time [35]. From SEM and TEM images, it is also clear that there are mesopores and macropores in the aerogels. The pore volumes are 0.11 cm3 g−1, 0.20 cm3 g−1, 0.21 cm3 g−1, and 0.22 cm3 g−1 for the Cu, Cu97Sn3, Cu95Sn5, and Cu90Sn10 aerogels, respectively. Interestingly, the pore size distributions of the Cu aerogel and CuxSny aerogels are different. The majority of the pores in the Cu aerogel are below 10 nm, while there are larger pores (proved by the elevated tails in the pore size distribution plots above 10 nm) presented in CnxSny aerogels. Potentially, these pores can act as the microchannels for reactant diffusion and product releasing in the electrode.
The electrocatalytic activity of the Cu and CuxSny aerogels is investigated by controlled-potential electrolysis in the CO2-saturated 0.1 M KHCO3 electrolyte. The linear sweep voltammetry (LSV) curves of Cu and CuxSny aerogels are shown in Figure 3a. The applied potential was swept at a rate of 10 mV s−1. A first glance at the curves indicates that the total current densities at different potentials did not show a significant difference among the samples. The curves all have a small current density from 0.0 to −0.5 V vs. RHE, and it increases dramatically afterward, indicating an electrochemical reduction reaction occurring. The current densities above −0.6 V vs. RHE almost overlapped in Cu and CuxSny series aerogels, while a small difference is found when the potential is swept beyond −0.6 V. The final current densities at −1.1 V vs. RHE are −14.0, −12.0, −13.1, and −13.4 mA cm−2 for the Cu, Cu97Sn3, Cu95Sn5, and Cu90Sn10 aerogel samples, respectively. This indicates that alloying Sn into Cu aerogel did not significantly deteriorate the electron-transfer rates of Cu. This point is different from the literature, in which it is found that adding Sn to Cu would often lower the overall current density. For instance, the current density of a Cu-Sn bimetallic catalyst reduces significantly compared to that of the pristine Cu, and similar behavior was observed in the CuSn nanowires catalyst [29,43]. The products of different aerogel electrodes show huge differences. Figure 3b gives the Faradaic efficiencies (FE) of the pristine Cu and CuxSny aerogels electrodes from −0.6 V to −1.1 V vs. RHE. For pristine Cu aerogel electrodes, it always exhibits a low FE of CO and a much higher FE of H2. Within the potential range, the FE of CO remains at a constant value of about 20%, while the remaining product is dominated by H2. The above results are consistent with the literature that HER is the main competing reaction to CO2RR on the Cu electrode, and a high FE of H2 (>80%) is usually seen [45]. In CuxSny series aerogels, even a small amount of Sn can significantly elevate the FE of CO2RR, and CO became the main product. The FE of CO for the Cu97Sn3 aerogel was 80% at −0.7 V vs. RHE, while this value can reach 90% in the Cu95Sn5 aerogel, and such FE of CO can maintain above 90% in a broad potential range from −0.7 V to −1.0 V vs. RHE. The highest CO FE was 93% at −0.9 V vs. RHE in the Cu95Sn5 aerogel. Further increasing Sn content in the aerogel or sweeping the potential to a more negative range causes the decrease in CO FE. For instance, the FE of CO in the Cu90Sn10 aerogel was 86% at −0.9 V vs. RHE. At −1.1 V vs. RHE, FE of CO in all CuxSny aerogels dropped to below 90% (Cu97Sn3: 78%; Cu95Sn5: 86%, and Cu90Sn10: 68%). Additionally, a trace amount of CH4 is detected as the by-product. The sum of the faradaic efficiency of these gas-phase products at −0.9 V vs. RHE is close to 100%, indicating no other main species are produced. At a more negative potential, the total faradaic efficiency of the gas phase product is below 90%, indicating the possible liquid products. The partial current density of H2 and CO can be calculated based on the chronoamperometry curves and the results are shown in Figure 3c and Figure S4. In line with the trend of Faraday efficiency, with Sn additives, the H2 partial current densities dropped dramatically, and the CO partial current densities raised notably compared with those in the pristine Cu. The H2 partial current density for the Cu aerogel is 9.07 mA cm−2 at −0.9 V vs. RHE, and it decreased to 0.54 mA cm−2 in the Cu97Sn3 aerogel. As for the Cu95Sn5 and Cu90Sn10 aerogels, the H2 current density is similar and lowers to ~0.29 mA cm−2. The CO partial current density for the pristine Cu aerogel is 2.33 mA cm−2 at −0.9 V vs. RHE, and it increases almost 3-fold in the Cu97Sn3 aerogel (6.18 mA cm−2) and further to 6.58 mA cm−2 in the Cu95Sn5 aerogel. However, when the Sn content increases to Cu90Sn10, the current density of CO decreases to 4.33 mA cm−2. Therefore, among the above materials, the Cu95Sn5 aerogel shows the lowest HER activity and the highest CO production rate, which results in it having the highest selectivity for CO. A comparison of CO partial current of the Cu95Sn5 aerogel and other Cu-Sn bimetallic [29,42], Cu-In bimetallic [21], and Cu-Ag [46] bimetallic catalysts can be found in Table S2, which highlights its high activity. The durability of the Cu95Sn5 aerogel was further verified by constant potential electrolysis at −0.9 V vs. RHE for 20 h and the I-t curve is shown in Figure 3d. The current density did not show a notable decay during the long-term stability testing, and the FE of CO remained above 90% during the long-term testing. The TEM images (Figure S5) before and after electrolysis show that the microstructure of the Cu95Sn5 aerogel is still composed of nanoparticles, although the porosity was reduced in the tested sample.
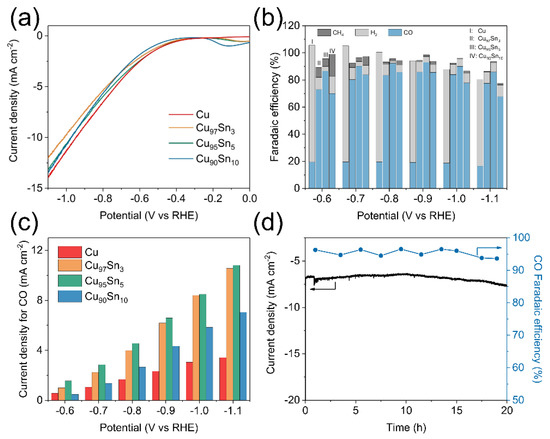
Figure 3.
Electrochemical CO2 reductions in 0.1 M KHCO3. (a) LSV curves for Cu, Cu97Sn3, Cu95Sn5, Cu90Sn10 aerogels. (b) Faradaic efficiency for Cu, Cu97Sn3, Cu95Sn5, Cu90Sn10 aerogels. (c) CO partial current density for Cu, Cu97Sn3, Cu95Sn5, Cu90Sn10 aerogels. (d) Current density and faradaic efficiency of long-term electrolysis over Cu95Sn5 aerogel electrodes at −0.9 V vs. RHE.
The above results confirm that the Cu95Sn5 alloy aerogel possesses high activity and high selectivity to CO. The activity is featured by the higher CO partial current density while the selectivity is highlighted by the high CO FE. The former can be partially ascribed to the highly porous unsupported network, which may favor the exposure of electrochemically active sites and a high reactant flux. Such a strategy has been well-proven in other bimetallic aerogels as an efficient electrocatalyst [39,47]. The latter has been reported in the previous literature since high selectivity to CO or HCOOH is found in bimetallic Cu-based nanostructures, such as Cu-Sn [24,29,41], Cu-Pd [23], and Cu-In [21]. The CO partial current at −0.9V vs. RHE (6.58 mA ccm−2) and the Faradaic efficiency are comparable to this work. A straightforward explanation for such a phenomenon is the suppression of the HER side reaction. For instance, Sarfraz’s work reported that depositing an additional layer of Sn on the Cu electrode can significantly increase CO FE from 63% to more than 90% at −0.6 V vs. RHE [29]. However, a great decrease in the total current (−1.0 mA cm−2 for Cu-Sn and −2.1 mA cm−2 for Cu) was observed at the same time, which indicates that the overall activity of the electrode decreased. Such increased CO FE can then be understood by the combination of the decreased total current (as a denominator) and the barely unchanged CO current (as a numerator). Similar results were also observed by Zeng et al. in the Cu-Sn foam electrode, that the total current decreased by almost half compared to that of pristine Cu foam [42]. Here, the LSV curves had different features. The overall current density did not show a great difference, even at −0.8 V vs. RHE when a big selectivity deviation is observed. HER is undoubtedly suppressed here, since the H2 current is reduced, but solely suppressed HER may not be enough to explain such an observation and it is speculated that the improvement of the intrinsic activity towards CO formation would be another reason.
To prove this point, a calculation based on density function theory (DFT) was performed to see how the active site is altered between the Cu and CuxSny alloy aerogels, which makes the electrode highly selective toward CO [48,49,50,51,52]. The calculation aims to verify the suppressed HER on the CuxSny aerogels and focuses on a Cu cluster with 100 Cu atoms. An Sn atom can be placed on the face or inside of this cluster [29]. The effect of replacing a Cu atom with one Sn atom was first simulated to gain a reasonable configuration. Figure S6 shows the model of one Sn atom replacing one Cu atom on the (111) facet and in the inner structure, and the formation energy on the surface is −1.54 eV and for the inner structure is 0.40 eV, which indicates that the Sn atom preferentially replaces a Cu atom on the (111) plane. Then, the adsorption energy of key intermediates on the (100) and (111) facets of Cu is investigated to gain information on the possible effect of nearby Sn on the surface. In the case of H2 production, H* would be the relevant intermediate and the limiting step is suggested to be the formation of H* [41]. As shown in Figure 4a,b, Figures S7 and S8, H* can be easily adsorbed on surfaces (111) and (100) of Cu due to the more negative free energies of −0.87 eV and −0.32 eV, respectively. After Sn is introduced, such free energies become more positive—0.13 eV and −0.04 eV for Cu97Sn3 (111) and (100), 0.24 eV and 0.01 eV for Cu95Sn5 (111) and (100), and −0.43 eV and −0.016 eV for Cu90Sn10 (111) and (100), respectively. This indicates that an external energy barrier needs to be overcome to ensure effective H* adsorption on the CuxSny aerogel surfaces [24]. Therefore, the suppressed HER is evident, and the free energy of H* adsorption on the Cu95Sn5 aerogel is the most positive among these samples, which is in line with its lowest H2 partial current.
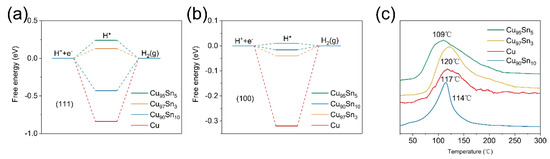
Figure 4.
(a) Free energies of H* adsorbed on the (111) facet. (b) Free energies of H adsorbed on the (100) facet. (c) TPD-MS spectra of CO adsorbed on the Cu, Cu97Sn5, Cu95Sn5, Cu90Sn10 aerogels.
Besides, it is often proposed that the stepwise reactions for the reduction of CO2 to CO in an aqueous solution can be summarized as [19,53]:
CO2(g) + * + H+(aq) + e− → COOH*
COOH* + e− + H+(aq) → CO* + H2O(l)
and CO* → CO(g) + *
In the third step, CO is released from the surface and is usually inhibited by strong CO binding on the catalyst, which can be considered one of the rate-determining steps [23]. In this case, the desorption capability of CO is important. The desorption behavior of CO on the CuxSny and Cu aerogels was then studied by TPD-MS (temperature-programmed desorption mass spectroscopy), which can give hints as to the binding strength of CO on the catalyst surface. Figure 4c shows the TPD profiles of CO on different metal aerogels. For all samples, a broad desorption peak is presented. The desorption peak temperature of CO for the Cu aerogel is located at 117 °C, while in the Cu95Sn5 aerogel, it is the lowest (109 °C). Thus, it can be concluded that alloying Sn in Cu aerogels leads to a decrease in the adsorption affinity of CO intermediates during CO2 reduction compared to monometallic Cu aerogels. The relatively weak binding to CO on CuxSny can accelerate CO production because CO can be easily released from the surface before it is further reduced to products, such as alcohols and hydrocarbons. With more Sn presented in the Cu90Sn10 aerogel, the desorption peak temperature of CO becomes higher to 114 °C. Therefore, the Cu95Sn5 aerogel shows the highest CO selectivity due to its promotion of CO production and inhibition of HER.
4. Conclusions
In conclusion, the CuxSny alloy aerogels were prepared by the simple co-reduction method. The synthesis is accomplished by the rapid reduction of the metal cations with the aid of NaBH4. The composition can be tuned by adjusting the precursor. The electrocatalytic reduction of CO2 was investigated on a series of CuxSny aerogels. The Cu95Sn5 aerogel electrode is a very efficient and stable electrocatalyst and the faradaic efficiency of CO production can reach 93% with a current density of 6.58 mA cm−2 at −0.9 V vs. RHE. The excellent catalytic performance of the Cu95Sn5 aerogel is mainly from the inhibition of HER and the promotion of CO2 conversion to CO simultaneously.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031033/s1, including additional characterization results of the samples.
Author Contributions
Conceptualization, Y.P. and M.Z.; methodology, Y.P.; software, Z.H. and H.T.; validation, Y.P. and M.W.; formal analysis, Y.P. and M.Z.; investigation, Y.P. and M.Z.; resources, M.Z.; data curation, Y.P. and M.Z.; writing—original draft preparation, Y.P.; writing—review and editing, Z.Y. and M.Z.; visualization, Z.H.; supervision, M.Z.; project administration, M.Z.; funding acquisition, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by Zhejiang Provincial Natural Science Foundation under Grant No. LY19E020014, and NSFC (Grant No. 21303162 and Grant No. 11604295).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available upon reasonable request to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the alloy aerogels samples are available from the authors.
References
- Lin, S.; Diercks, C.S.; Zhang, Y.-B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Jing, J.; Wu, Y.; Wu, Z.; Guo, X.; Materna, K.L.; Liu, W.; Batista, V.S.; Brudvig, G.W.; Wang, H. Electrochemical CO2 Reduction to Hydrocarbons on a Heterogeneous Molecular Cu Catalyst in Aqueous Solution. J. Am. Chem. Soc. 2016, 138, 8076–8079. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pang, Y.; Zhang, B.; De Luna, P.; Voznyy, O.; Xu, J.; Zheng, X.; Dinh, C.T.; Fan, F.; Cao, C.; et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 2016, 537, 382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef]
- Kibria, M.G.; Dinh, C.-T.; Seifitokaldani, A.; De Luna, P.; Burdyny, T.; Quintero-Bermudez, R.; Ross, M.B.; Bushuyev, O.S.; de Arguer, F.P.G.; Yang, P.; et al. A Surface Reconstruction Route to High Productivity and Selectivity in CO2 Electroreduction toward C2+ Hydrocarbons. Adv. Mater. 2018, 30, 1804867. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Zhou, Z.; Cai, F.; Zhao, X.; Huang, W.; Li, Y.; Zhu, J.; Liu, P.; Yang, F. Enhancing CO2 electroreduction with the metal–oxide interface. J. Am. Chem. Soc. 2017, 139, 5652–5655. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Xue, M.; Williams, T.; Zhang, Y.; MacFarlane, D.R.; Zhang, J. Towards a better Sn: Efficient electrocatalytic reduction of CO2 to formate by Sn/SnS2 derived from SnS2 nanosheets. Nano Energy 2017, 31, 270–277. [Google Scholar] [CrossRef]
- Dae-Hyun, N.; Bushuyev, O.S.; Li, J.; De Luna, P.; Seifitokaldani, A.; Cao-Thang, D.; de Arquer, F.P.G.; Wang, Y.; Liang, Z.; Proppe, A.H.; et al. Metal-Organic Frameworks Mediate Cu Coordination for Selective CO2 Electroreduction. J. Am. Chem. Soc. 2018, 140, 11378–11386. [Google Scholar]
- Cui, X.; Pan, Z.; Zhang, L.; Peng, H.; Zheng, G. Selective Etching of Nitrogen-Doped Carbon by Steam for Enhanced Electrochemical CO2 Reduction. Adv. Energy Mater. 2017, 7, 1701456. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic Conversion of Carbon Dioxide to Methane and Methanol on Transition Metal Surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazabal, G.O.; Perez-Ramirez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.; Lee, J. Electrocatalytic Production of C3-C4 Compounds by Conversion of CO2 on a Chloride-Induced Bi-Phasic Cu2O-Cu Catalyst. Angew. Chem.-Int. Ed. 2015, 54, 14701–14705. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Liu, Q.; Wang, J.; Wu, X.; Li, X.; Yang, Y.; Yan, J.; Wu, A.; Wu, H.B. Grain refining enables mixed Cu+/Cu0 states for CO2 electroreduction to C2+ products at high current density. Appl. Catal. B 2023, 324, 122272. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Wang, J.; Meng, D.; Shu, Y.; Lv, X.; Zhao, B.; Yang, H.; Cheng, T.; Gao, Q.; et al. Enhanced electroreduction of CO2 to C2+ products on heterostructured Cu/oxide electrodes. Chem 2022, 8, 2148–2162. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Wang, J.; Lv, X.; Wu, H.B. CeO2-modified Cu electrode for efficient CO2 electroreduction to multi-carbon products. J. CO2 Util. 2021, 54, 101741. [Google Scholar] [CrossRef]
- Angamuthu, R.; Byers, P.; Lutz, M.; Spek, A.L.; Bouwman, E. Electrocatalytic CO2 Conversion to Oxalate by a Copper Complex. Science 2010, 327, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.L.; Hahn, C.; Jaramillo, T.F.; Bell, A.T. Electrochemical CO2 Reduction over Compressively Strained CuAg Surface Alloys with Enhanced Multi-Carbon Oxygenate Selectivity. J. Am. Chem. Soc. 2017, 139, 15848–15857. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-K.; Shin, H.; Goddard, W.A., III.; Hwang, Y.J.; Min, B.K.; Kim, H. Embedding Covalency into Metal Catalysts for Efficient Electrochemical Conversion of CO2. J. Am. Chem. Soc. 2014, 136, 11355–11361. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.A.; Varley, J.B.; Peterson, A.A.; Norskov, J.K. Understanding Trends in the Electrocatalytic Activity of Metals and Enzymes for CO2 Reduction to CO. J. Phys. Chem. Lett. 2013, 4, 388–392. [Google Scholar] [CrossRef]
- Kim, C.; Dionigi, F.; Beermann, V.; Wang, X.; Moeller, T.; Strasser, P. Alloy Nanocatalysts for the Electrochemical Oxygen Reduction (ORR) and the Direct Electrochemical Carbon Dioxide Reduction Reaction (CO2RR). Adv. Mater. 2019, 31, 1805617. [Google Scholar] [CrossRef]
- Rasul, S.; Anjum, D.H.; Jedidi, A.; Minenkov, Y.; Cavallo, L.; Takanabe, K. A Highly Selective Copper-Indium Bimetallic Electrocatalyst for the Electrochemical Reduction of Aqueous CO2 to CO. Angew. Chem.-Int. Ed. 2015, 54, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.-K.; Ahn, S.H. Electrochemical preparation of Ag/Cu and Au/Cu foams for electrochemical conversion of CO2 to CO. J. Ind. Eng. Chem. 2017, 54, 218–225. [Google Scholar] [CrossRef]
- Yin, Z.; Gao, D.; Yao, S.; Zhao, B.; Cai, F.; Lin, L.; Tang, P.; Zhai, P.; Wang, G.; Ma, D.; et al. Highly selective palladium-copper bimetallic electrocatalysts for the electrochemical reduction of CO2 to CO. Nano Energy 2016, 27, 35–43. [Google Scholar] [CrossRef]
- Xiong, W.; Yang, J.; Shuai, L.; Hou, Y.; Qiu, M.; Li, X.; Leung, M.K. CuSn Alloy Nanoparticles on Nitrogen-Doped Graphene for Electrocatalytic CO2 Reduction. ChemElectroChem 2019, 6, 5951–5957. [Google Scholar] [CrossRef]
- Morimoto, M.; Takatsuji, Y.; Yamasaki, R.; Hashimoto, H.; Nakata, I.; Sakakura, T.; Haruyama, T. Electrodeposited Cu-Sn Alloy for Electrochemical CO2 Reduction to CO/HCOO. Electrocatalysis 2018, 9, 323–332. [Google Scholar] [CrossRef]
- Del Castillo, A.; Alvarez-Guerra, M.; Solla-Gullon, J.; Saez, A.; Montiel, V.; Irabien, A. Electrocatalytic reduction of CO2 to formate using particulate Sn electrodes: Effect of metal loading and particle size. Appl. Energy 2015, 157, 165–173. [Google Scholar] [CrossRef]
- Li, D.; Huang, L.; Tian, Y.; Liu, T.; Zhen, L.; Feng, Y. Facile synthesis of porous Cu-Sn alloy electrode with prior selectivity of formate in a wide potential range for CO2 electrochemical reduction. Appl. Catal. B Environ. 2021, 292, 120119. [Google Scholar] [CrossRef]
- Li, Q.; Fu, J.; Zhu, W.; Chen, Z.; Shen, B.; Wu, L.; Xi, Z.; Wang, T.; Lu, G.; Zhu, J.-j.; et al. Tuning Sn-Catalysis for Electrochemical Reduction of CO2 to CO via the Core/Shell Cu/SnO2 Structure. J. Am. Chem. Soc. 2017, 139, 4290–4293. [Google Scholar] [CrossRef]
- Sarfraz, S.; Garcia-Esparza, A.T.; Jedidi, A.; Cavallo, L.; Takanabe, K. Cu-Sn Bimetallic Catalyst for Selective Aqueous Electroreduction of CO2 to CO. ACS Catal. 2016, 6, 2842–2851. [Google Scholar] [CrossRef]
- Gao, Q.; Shi, Z.; Xue, K.; Ye, Z.; Hong, Z.; Yu, X.; Zhi, M. Cobalt sulfide aerogel prepared by anion exchange method with enhanced pseudocapacitive and water oxidation performances. Nanotechnology 2018, 29, 215601. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, X.; Shi, Z.; Ye, Z.; Wang, W.; Zhang, N.; Hong, Z.; Zhi, M. Synthesis of porous NiCo2S4 aerogel for supercapacitor electrode and oxygen evolution reaction electrocatalyst. Chem. Eng. J. 2018, 331, 185–193. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Z.; Zhi, M.; Hong, Z. Synthesis of CO2Ni/reduced graphene oxide composite aerogels as efficient hydrogen evolution catalysts. J. Sol-Gel Sci. Technol. 2022, 103, 515–525. [Google Scholar] [CrossRef]
- Wu, M.; Ouyang, C.; Ye, Z.; Li, S.; Hong, Z.; Zhi, M. Ag–CeO2 Composite Aerogels as Photocatalysts for CO2 Reduction. ACS Appl. Energy Mater. 2022, 5, 7335–7345. [Google Scholar] [CrossRef]
- Zhi, M.; Tang, H.; Wu, M.; Ouyang, C.; Hong, Z.; Wu, N. Synthesis and Photocatalysis of Metal Oxide Aerogels: A Review. Energy Fuels 2022, 36, 11359–11379. [Google Scholar] [CrossRef]
- Liu, W.; Herrmann, A.-K.; Geiger, D.; Borchardt, L.; Simon, F.; Kaskel, S.; Gaponik, N.; Eychmueller, A. High-Performance Electrocatalysis on Palladium Aerogels. Angew. Chem.-Int. Ed. 2012, 51, 5743–5747. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Fu, S.; Song, J.; Xia, H.; Du, D.; Lin, Y. Efficient synthesis of MCu (M = Pd, Pt, and Au) aerogels with accelerated gelation kinetics and their high electrocatalytic activity. Adv. Mater. 2016, 28, 8779–8783. [Google Scholar] [CrossRef]
- Ye, Z.; Jia, X.; Lou, M.; Huang, H.; Lu, P.; Ye, G.; Gong, X.; Zhu, Y.; Yan, B. Surface-enhanced Raman scattering substrates prepared by controlled synthesis of gold nanobipyramids in microchannels. Microfluid. Nanofluid. 2022, 26, 28. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, K.; Lou, M.; Jia, X.; Xu, F.; Ye, G. Consecutive synthesis of gold nanobipyramids with controllable morphologies using a microfluidic platform. Microfluid. Nanofluid. 2020, 24, 38. [Google Scholar] [CrossRef]
- Liu, W.; Herrmann, A.-K.; Bigall, N.C.; Rodriguez, P.; Wen, D.; Oezaslan, M.; Schmidt, T.J.; Gaponik, N.; Eychmüller, A. Noble Metal Aerogels—Synthesis, Characterization, and Application as Electrocatalysts. Acc. Chem. Res. 2015, 48, 154–162. [Google Scholar] [CrossRef]
- Bigall, N.C.; Herrmann, A.K.; Vogel, M.; Rose, M.; Simon, P.; Carrillo-Cabrera, W.; Dorfs, D.; Kaskel, S.; Gaponik, N.; Eychmüller, A. Hydrogels and aerogels from noble metal nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 9731–9734. [Google Scholar] [CrossRef]
- Zheng, X.; Ji, Y.; Tang, J.; Wang, J.; Liu, B.; Steinrück, H.-G.; Lim, K.; Li, Y.; Toney, M.F.; Chan, K. Theory-guided Sn/Cu alloying for efficient CO2 electroreduction at low overpotentials. Nat. Catal. 2019, 2, 55–61. [Google Scholar] [CrossRef]
- Zeng, J.; Bejtka, K.; Ju, W.; Castellino, M.; Chiodoni, A.; Sacco, A.; Farkhondehfal, M.A.; Hernandez, S.; Rentsch, D.; Battaglia, C.; et al. Advanced Cu-Sn foam for selectively converting CO2 to CO in aqueous solution. Appl. Catal. B-Environ. 2018, 236, 475–482. [Google Scholar] [CrossRef]
- Wang, J.; Ji, Y.; Shao, Q.; Yin, R.; Guo, J.; Li, Y.; Huang, X. Phase and structure modulating of bimetallic CuSn nanowires boosts electrocatalytic conversion of CO2. Nano Energy 2019, 59, 138–145. [Google Scholar] [CrossRef]
- Liu, W.; Rodriguez, P.; Borchardt, L.; Foelske, A.; Yuan, J.; Herrmann, A.K.; Geiger, D.; Zheng, Z.; Kaskel, S.; Gaponik, N. Bimetallic Aerogels: High-Performance Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 9849–9852. [Google Scholar] [CrossRef]
- Raciti, D.; Livi, K.J.; Wang, C. Highly dense Cu nanowires for low-overpotential CO2 reduction. Nano Lett. 2015, 15, 6829–6835. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gong, S.; Liu, J.; Ge, Y.; Wang, J.; Lv, X. Ag-Cu aerogel for electrochemical CO2 conversion to CO. J. Colloid Interface Sci. 2021, 595, 159–167. [Google Scholar] [CrossRef]
- Dubale, A.A.; Zheng, Y.; Wang, H.; Hübner, R.; Li, Y.; Yang, J.; Zhang, J.; Sethi, N.K.; He, L.; Zheng, Z.; et al. High-Performance Bismuth-Doped Nickel Aerogel Electrocatalyst for the Methanol Oxidation Reaction. Angew. Chem. Int. Ed. 2020, 59, 13891–13899. [Google Scholar] [CrossRef]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An electronic structure and molecular dynamics software package—Quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Goedecker, S.; Teter, M.; Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 1996, 54, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Michalsky, R.; Metin, O.N.; Lv, H.; Guo, S.; Wright, C.J.; Sun, X.; Peterson, A.A.; Sun, S. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).