Abstract
Microbial fermentation has been widely used to improve the quality and functional composition of food and edibles; however, the approach has rarely been applied to traditional Chinese medicines. In this study, to understand the effect of microbial fermentation on the active ingredients of traditional Chinese medicines, we used Bifidobacterium bifidum and Bacillus subtilis to ferment the traditional Chinese medicine, Cornus officinalis fruit (COF), and determined the levels of active ingredients using HPLC (high-performance liquid chromatography). According to the results, both B. subtilis and B. bifidum substantially increased the amount of gallic acid in the COF culture broth after fermentation; however, the two species of bacteria had no effect on the loganin content. Moreover, the B. subtilis fermentation reduced the contents of ursolic acid and oleanolic acid in the COF broth, whereas the B. bifidum fermentation did not. This study contributes to a better understanding of the mechanism by which microbial fermentation alters the active ingredient levels of traditional Chinese medicines, and suggests that fermentation may potentially improve their functional ingredients.
1. Introduction
Microbial fermentation can increase or decrease certain ingredient contents in foods, fodders, and traditional medicines. Lactobacillus, which is one of the most studied microorganisms, produces various bioactive peptides with antioxidant, opioid antagonist, antiallergic, and hypotensive effects during the fermentation process [1,2]. The consumption of microbial-fermented foods also reduces the symptoms of lactose malabsorption and helps to eliminate Helicobacter pylori [2]. Microbial fermentation can reduce the antinutrients in pig feed and improve the feed absorption and intestinal flora [3]. In fermented food or feed, the vitamin A and carotenoids content, and tyrosinase inhibitory and antioxidant activities, are noticeably increased [4,5]. Consequently, fermentation is a promising method to ameliorate the quality ingredients of foods, fodders, and traditional herbs. In addition, microorganisms in human or other mammalian guts have different enzyme profiles that are involved in the metabolic pathways of carbohydrates, proteins, plant polyphenols, and vitamins, enhancing the efficiency of the absorption and utilization of these food components [6,7]. Microorganisms in the gastrointestinal tract were able to enhance the total release of phenolic compounds and flavonoids from Moringa leaves, thereby increasing the antioxidant activity when they were digested [8]. Fermented feeds can raise the productivity of pigs by improving their intestinal microflora to increase the utilization of the feed [9]. In vitro studies on the effects of the probiotic fermentation of food and traditional medicine ingredients may elucidate these changes in those components and ingredients within animal and human digestive systems.
Cornus officinalis fruit (COF), which is also called “Shan Zhu Yu”, is a commonly used Chinese herbal medicine for certain kidney and liver diseases [10]. COF is rich in active ingredients, such as ursolic acid, oleanolic acid, and loganin. These active compounds have different therapeutic effects, including antibacterial [11,12], anti-inflammatory [13,14], and anticancer [15,16,17] activities. Thus, increasing the active ingredient contents can improve the therapeutic effectiveness of herbal medicines. Su and Wang found that, with the increase in a wine yeast addition, the degradation rate of the polysaccharides in a COF fermentation broth was accelerated, and the tannin content was increased; however, there was no substantial effect on the loganin content [18]. Xu et al. report that an increase in yeast inoculation accelerated the polysaccharide degradation rate in COF, and that the fermentation reduced the dissolution resistance of ursolic acid but did not affect its level [19]. Park and Lee [20] demonstrated that a fermented COF extract promoted hair growth better than unfermented COF. However, we do not know what happens to the active compositions of COF during the fermentation process. Therefore, comparing the active ingredient contents of fermented COF with those of unfermented COF is a crucial step toward in elucidating the mechanism that underlies the improvement in the therapeutic effects of fermented COF via fermentation, as well as what happens to COF after it is fermented by the gut microbiota inside human or animal bodies.
Ursolic acid and oleanolic acid are effective at protecting against chemically induced liver damage, inflammation, tumor suppression, and the induction of apoptosis [21,22]. Loganin improves diabetic nephropathy [23] and drug-induced memory impairment [24], and it inhibits ex vivo inflammatory responses [25] and hydrogen peroxide-induced apoptosis [26]. Gallic acid has preventive and therapeutic activities in gastrointestinal, neuropsychological, obesity-related, metabolic, and cardiovascular diseases [27,28]. Ursolic acid, oleanolic acid, loganin, and gallic acid are the main bioactive components of Cornus officinalis fruit [29,30,31,32]. In this study, we fermented COF with B. subtilis and B. bifidum, and we determined the loganin, ursolic acid, oleanolic acid, and gallic acid contents of the COF culture broth at various fermentation times using high-performance liquid chromatography (HPLC). We demonstrated the microbial fermentation effects on the active ingredients of Cornus officinalis, providing a better understanding of the mechanism by which microbial fermentation changes the bioactive component contents of traditional Chinese herbs.
2. Results
2.1. Effects of B. subtilis and B. bifidum Fermentation on Ursolic Acid and Oleanolic Acid Contents
After the fermentation process, B. bifidum and B. subtilis had obviously different effects on the ursolic and oleanolic acid contents in the COF culture broth. Compared with those of unfermented COF, the ursolic and oleanolic acid contents of the COF culture broth fermented with B. subtilis were significantly (p < 0.05) decreased at different COF concentrations (Figure 1 and Figure 2) (Tables S1 and S2), which suggests that the bacteria can break down these compounds. Generally, the amounts of both active ingredients most rapidly decreased during the 12–24 h fermentation period; however, the contents exhibited no obvious changes during the 30–42 h fermentation period (Figure 1 and Figure 2), which indicates that the breakdown of the ursolic and oleanolic acid by B. subtilis was active in the early stage of fermentation, but became inactive in the later stage. In contrast, the B. bifidum fermentation had almost no effect on the ursolic and oleanolic acid contents in the COF culture broth. During the 6–42 h fermentation period, the contents of the two active compounds had no obvious alterations (Figure 1 and Figure 2), which suggests that the B. bifidum fermentation did not degrade the ursolic and oleanolic acid.
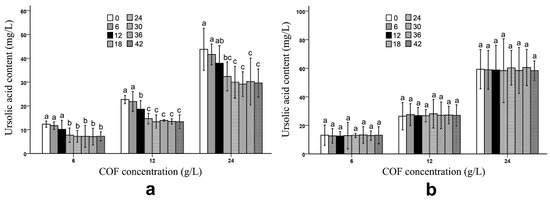
Figure 1.
Ursolic acid content in Cornus officinalis fruit (COF) culture broth at different concentrations and fermentation times of Bacillus subtilis and Bifidobacterium bifidum. (a) Ursolic acid content in COF culture broth at different concentrations and fermentation duration of B. subtilis. (b) Ursolic acid content in COF culture broth at different concentrations and fermentation duration of B. bifidum. Data are the means ± SD from three independent tests. The different letters (a, ab, b, bc, and c) on the error bars indicate significant differences at p < 0.05 between the ursolic acid contents of different fermentation durations on the same COF concentration and strain. The significant differences (p < 0.05) of the mean values were detected by T-test.
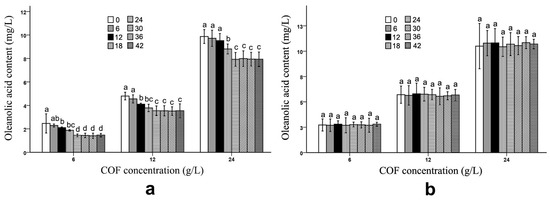
Figure 2.
Oleanolic acid content in Cornus officinalis fruit (COF) culture broth at different concentrations and fermentation times of Bacillus subtilis and Bifidobacterium bifidum. (a) Oleanolic acid content in COF culture broth at different concentrations and fermentation duration of B. subtilis. (b) Oleanolic acid content in COF culture broth at different concentrations and fermentation duration of B. bifidum. Data are the means ± SD from three independent tests. Different letters (a, ab, b, bc, c, and d) on the error bars indicate significant differences at p < 0.05 between the oleanolic acid contents of different fermentation durations on the same COF concentration and strain. The significant differences (p < 0.05) of the mean values were detected by T-test.
2.2. Effects of B. subtilis and B. bifidum Fermentation on the Loganin Content
The B. subtilis and B. bifidum fermentation did not have any obvious effects on the loganin amount in the COF culture broth. As listed in Figure 3 and Table S3, although the loganin content showed some alteration in the B. subtilis culture broth with the different COF concentrations, the variation was not obvious, which implies that neither bacteria decomposed the loganin during the fermentation process.
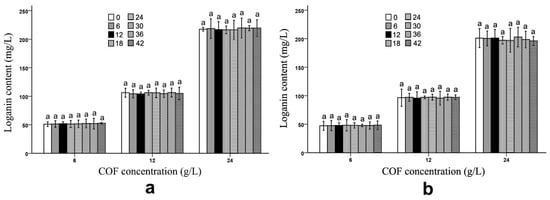
Figure 3.
Loganin content in Cornus officinalis fruit (COF) culture broth at different concentrations and fermentation times of Bacillus subtilis and Bifidobacterium bifidum. (a) Loganin content in COF culture broth at different concentrations and fermentation duration of B. subtilis. (b) Loganin content in COF culture broth at different concentrations and fermentation duration of B. bifidum. Data are the means ± SD from three independent tests. Letters (a) on the error bars indicate significant differences at p < 0.05 between the loganin contents of different fermentation durations on the same COF concentration and strain. The significant differences (p < 0.05) of the mean values were detected by T-test.
2.3. Effects of B. subtilis and B. bifidum Fermentation on Gallic Acid Content
During the fermentation, both B. subtilis and B. bifidum were able to increase the gallic acid content in the COF culture broth. In Figure 4 and Table S4, we can see that the gallic acid content in the COF culture broth fermented with B. subtilis was significantly (p < 0.05) promoted during the 6–18 h fermentation period at various COF concentration levels; however, there were no significant changes in the content during the 24–42 h fermentation period. Similarly, a significant (p < 0.05) increase in the gallic acid content in the COF culture broth fermented with B. bifidum generally occurred during the 6–18 h fermentation period; however, the content had no obvious alterations during the other fermentation periods (Figure 4), which suggests that the increases in the gallic acid caused by the two bacteria were active in the early stages of the fermentation, but became inactive in the later stages. The increase in the gallic acid level implied that it may be achieved by the degradation of certain compounds via B. subtilis or B. bifidum fermentation.
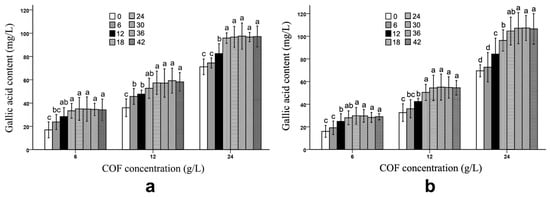
Figure 4.
Gallic acid content in Cornus officinalis fruit (COF) culture broth at different concentrations and fermentation times of Bacillus subtilis and Bifidobacterium bifidum. (a) Gallic acid content in COF culture broth at different concentrations and fermentation duration of B. subtilis. (b) Gallic acid content in COF culture broth at different concentrations and fermentation duration of B. bifidum. Data are the means ± SD from three independent tests. Different letters (a, ab, b, bc, c, and d) on the error bars indicate significant differences at p < 0.05 between the gallic acid contents of different fermentation durations on the same COF concentration and strain. The significant differences (p < 0.05) of the mean values were detected by T-test.
3. Discussion
In this study, both B. subtilis and B. bifidum increased the gallic acid level in the COF culture broth (Figure 4) (Table 1). Researchers have demonstrated that wine yeast fermentation increases the tannin content in COF culture broth [18], and that fermentation can increase the bioactive components of traditional herbal medicines. The antioxidant activities of traditional herbs can be substantially enhanced via microbial fermentation [5,8,33]. Wen et al. demonstrated that Aspergillus oryzae NCH 42 can improve the functional components, such as the total phenol content and antioxidant activity and content, of the traditional Chinese medicines Trichosanthes kirilowii Maxim, Salvia miltiorrhiza Bge, Magnolia officinalis, and Glycyrrhizae radix [34]. Green tea and Houttuynia cordata leaves fermented with Lactobacillus paracasei, subsp. paracasei NTU 101, contained higher levels of epigallocatechin gallate, epigallocatechin, and chlorogenic acid than with no fermentation [35]. According to the results, microbial fermentation can promote the bioactive component contents of traditional herbs.

Table 1.
Alteration of four active ingredients in COF after fermentation with B. subtilis and B. bifidum.
In our study, we demonstrated that the B. subtilis fermentation reduced the ursolic and oleanolic acid levels in the COF culture broth (Figure 1 and Figure 2) (Table 1). Yeast fermentation can degrade the polysaccharides in COF fermentation broth [18,19]. Under optimized conditions, the tannin contents of traditional herbal Xaun Mugua fruits fermented with lactic acid bacteria were reduced by 78% compared with those of unfermented Xaun Mugua fruits [36]. The contents of hexanoic acid, octanoic acid, and butanoic acid were substantially decreased in noni juice fermented with Acetobacter sp. [37]. Through fermentation, the levels of antinutrients, toxic substances, and digestive enzyme inhibitors, in food are substantially reduced [38,39]. When traditional herbal medicines are fermented with microorganisms, the toxic components in them are decomposed, and their side effects are alleviated [40,41,42]. Fermentation reduces the levels of some components in food and traditional herbs.
The fermentation of Acetobacter sp. had no significant effect on most of the active ingredients in noni juice, although it substantially reduced the hexanoic, octanoic, and butanoic acid contents in the fruit [37]. In a traditionally fermented Chinese medicine, Massa Medicata Fermentata (MMF), the caffeic acid content did not change compared with that of unfermented MMF [43]. Yeast fermentation could not change the brucine and ursolic acid contents in COF [18,19]. In the current study, the B. bifidum fermentation did not alter the ursolic acid, oleanolic acid and loganin levels in the COF (Figure 1, Figure 2 and Figure 3) (Table 1), and the B. subtilis fermentation did not change the loganin levels in this fruit (Figure 3) (Table 1). Therefore, during microbial fermentation, not all of the fermented herbal component contents are affected.
Gallic acid is a potential precursor molecule for drug development [44], and its alkyl ester derivatives have anticancer and antioxidant abilities, neuroprotective functions, and induce the apoptosis of cancer cells [45,46,47]. In our study, both B. subtilis and B. bifidum could substantially increase the gallic acid levels of the COF culture broth. Therefore, before gallic acid is extracted from Cornus officinalis fruits as the lead molecule for drug development, the fermentation of these fruits using B. subtilis and B. bifidum may be one method of improving the gallic acid yield. Gallic acid also has potential preventive and therapeutic effects in oxidative-stress-related diseases, such as neurodegenerative disorders, cancer, cardiovascular diseases, and aging [48,49]. When COF is used as a functional food to prevent these diseases, B. subtilis- and B. bifidum-fermented COF have better effects than unfermented COF.
The initial decomposition stages of cyclic and aromatic compounds by bacteria and fungi require molecular oxygen for the oxidation of the compounds; therefore, aerobic conditions contribute to their breakdown [50,51,52]. In the current study, the pentacyclic triterpenoids ursolic acid and oleanolic acid from COF could be decomposed by B. subtilis under aerobic conditions; however, they could not be degraded by B. bifidum under anaerobic conditions, which suggests that oxygen may play a key role in the breakdown, by B. subtilis, of ursolic and oleanolic acids. Researchers have reported the involvement of different microorganisms in the degradation of the tannins in plant material to gallic acid during fermentation [53,54,55,56]. COF contains about 0.75% total tannins [57]. In this study, both B. subtilis and B. bifidum promoted the gallic acid levels in the COF culture broth after fermentation. The increased amount of gallic acid during fermentation may have been due to the degradation of tannin by these two bacteria.
In the present study, we investigated the effects of aerobic fermentation with B. subtilis and anaerobic fermentation by B. bifidum on the four active ingredients levels of COF, and we explored the mechanisms by which the bacterial fermentations influenced the active ingredients of the herbal medicine. The findings of this research contribute to a better understanding of the mechanisms by which microbial fermentation improves the active ingredients of traditional Chinese medicines. However, considerable work remains. We require investigations into the effects that these two bacteria have on the other components of COF, as well as on the active ingredients of other traditional Chinese medicines. In addition, we require studies on the molecular mechanisms by which these bacteria improve the active ingredients and disease-preventive effects of traditional herbal medicines.
4. Materials and Methods
4.1. Material Collection and Pretreatment
The material collection and pretreatment were performed according to the Pharmacopoeia of the People’s Republic of China (2015) and Song et al. (2018) [32,58]. In July 2020, fresh COF was harvested from a Cornus officinalis cultivation base of the Zhongjing Wanxi Pharmaceutical Co., Ltd. (Nanyang, China) and the kernels of the fruit were removed. The resultant flesh was mixed with rice wine at a ratio of 3:1 by mass, and kneaded. After rubbing, the flesh was steamed for 3 h, and then dried at 60 °C in an oven for 72 h. The dried flesh was ground into <3 mm particles. The powder was then prepared for the subsequent fermentation and extraction.
4.2. Reagents
The ursolic acid and loganin standards were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Both the oleanolic acid and gallic acid standards were obtained from Sangon Biotech Co., Ltd. (Shanghai, China). The HPLC-grade methanol, acetonitrile, formic acid, and water were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). The medicines and consumables for the fermentation were purchased from the Shanghai Yuanye Biotech Co., Ltd. (Shanghai, China). The B. subtilis and B. bifidum strains were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Except for the liquid chromatography reagents, all of the other experimental chemicals were of analytical grade, and double-distilled water was applied.
4.3. COF Fermentation
The COF fermentation with B. bifidum was modified with reference to [59]. The COF fermentation was slightly modified with B. subtilis according to [60].
The COF was fermented not only with B. bifidum, which is an anaerobic bacterium, under anaerobic conditions, but also with B. subtilis, which is an aerobic bacterium, under aerobic conditions. Then, 180 mL of liquid media of the B. bifidum or B. subtilis was added to 500 mL flasks, and then mixed with 0, 1.2, 2.4, or 4.8 g of dried COF powder. In addition to water, the components of the B. bifidum medium were as follows: peptone: 10.0 g/L; beef extract powder: 8.0 g/L; yeast extract: 4.0 g/L; glucose: 20.0 g/L; sorbitan monooleate: 1 mL/L; dipotassium hydrogen phosphate: 2.0 g/L; sodium acetate: 5.0 g/L; triammonium citrate: 2.0 g/L; manganese sulfate heptahydrate: 0.2 g/L. One liter of the B. subtilis medium contained the following: water: 1000 mL; glucose: 20 g; peptone: 15 g; sodium chloride: 5 g; beef extract: 0.5 g. The pH values of the media mixed with COF powder were adjusted to 7.0 by adding sodium hydroxide or hydrochloric acid solution, which made up to 200 mL with the corresponding liquid medium. Afterwards, the flasks that contained the media were sterilized in an autoclave at 121 °C for 30 min. In a sterile environment, each flask containing the B. bifidum medium was incubated with 3 mL of 1 × 107 cfu/mL B. bifidum culture, and all the flasks containing the B. subtilis medium were incubated with 2 mL of 2 × 107 cfu/mL B. subtilis culture. The COF was fermented with B. bifidum at 38 °C in an anaerobic incubator (Defendor AMW1000; HUA YUE Enterprise holdings LTD, Guangzhou, China) for different durations: 0, 6, 12, 18, 24, 30, 36, or 42 h. The COF with B. subtilis was fermented at a speed of 120 rpm in a thermostatic shaker (HY-150; Wuhan Huicheng Biological Technology Co., Ltd., Wuhan, China) at 37 °C for different durations: 0, 6, 12, 18, 24, 30, 36, or 42 h. The samples without any COF were used as negative controls. The fermentation, with samples in triplicate, was then performed. When the specific fermentation times were reached, the fermentation processes of the samples were ended in a 0–4 °C environment. Then, the culture broths were centrifuged at 7500 rpm at 4 °C for 30 min, and 120 mL of the supernatant was aspirated, aliquoted into 40 mL portions, and placed in a 4 °C refrigerator for the subsequent assays.
4.4. Extraction and Determination of Ursolic Acid and Oleanolic Acid
Next, 40 mL of the resultant fermentation broth supernatant was evaporated under reduced pressure in a rotary evaporator, and concentrated to form a paste residue. The residue was extracted twice with 50 mL of petroleum ether. Next, the residue was added to 40 mL of 95% alcohol, extracted using ultrasound at 60 °C for 90 min, and then concentrated into a paste residue under a rotary evaporator. The resulting residue was dissolved in water, and the solution was extracted three times with chloroform. Afterwards, 20 mL of supernatant was obtained, filtered through a 0.45 μm filter, and evaporated in a rotary vacuum evaporator. The resultant residue was dissolved in 20 mL of methanol. A 20 μL sample was injected into an HPLC system (LC-2010HT; Shimadzu Corporation, Kyoto, Japan). The ursolic acid and oleanolic acid assays were performed at 218 nm using an ODS-2 C18 analytical column (Thermo Scientific, MA, USA) and a mobile phase comprising methanol, water, glacial acetic acid, and triethylamine at a ratio of 90:9.9:0.06:0.04 (v/v). The flow rate was 0.5 mL/min, and the column temperature was 30 °C. The ursolic acid and oleanolic acid of the samples were identified by comparing the retention times of the peaks in the samples with those of standard ursolic acid and oleanolic acid (Figure S1a). The ursolic acid and oleanolic acid contents in the test samples were estimated by measuring and analyzing the peak areas of the samples based on the ursolic acid and oleanolic acid standards. The unfermented COF and control samples were treated in the same manner. The determination of the ursolic acid and oleanolic acid was conducted according to Tian et al. [61].
4.5. Extraction and Determination of Loganin
Next, 40 mL of the culture broth supernatant was evaporated in a rotary evaporator. The resulting residue was extracted twice with 50 mL of petroleum ether, and then dissolved in 40 mL of 80% methanol. The solution was heated under reflux for 1 h and then left to cool. Afterwards, the amount of solution lost was supplemented during the reflux with 80% methanol, and filtered through a 0.45 μm filter. The resulting solution was used as the sample to be tested. A 20 μL sample was used for the high-performance liquid chromatography (LC-2010HT; Shimadzu Corporation, Kyoto, Japan), which was run at 240 nm using an ODS2C18 analytical column and a mobile phase formed of methanol and water at a ratio of 75:25 (v/v). The column temperature was 35 °C. The loganin in the samples was identified by comparing the retention times of the peaks in the samples with those of standard loganin. The loganin content in the test samples was estimated by measuring and analyzing the peak areas of the sample and standard loganin (Figure S1c). The loganin contents in the unfermented COF and control samples were determined in the same manner as described in [32].
4.6. Extraction and Determination of Gallic Acid
Next, 40 mL of the broth supernatant was evaporated using a rotary evaporator, and then the paste residue was washed with 50 mL of petroleum ether to remove the lipid compounds. The resultant residue was dissolved in 40 mL of methanol, and the solution was filtered through a 0.45 μm filter. The solution was used as an assayed sample. A 20 μL sample was injected into the HPLC system (LC-2010HT; Shimadzu Corporation, Kyoto, Japan). The determination of the gallic acid in the test samples was performed at 30 °C using an ODS-2 C18 analytical column, with a mobile phase comprising a methanol and phosphoric acid solution (pH = 3) at a ratio of 95:5 (v/v). The column temperature was set at 30 °C, and the flow rate was 0.5 mL per minute. Gallic acid at 272 nm was detected. The gallic acid in the culture broth was identified by comparing the retention times of the peaks in the samples with those of standard gallic acid (Figure S1b). The quantitation of the gallic acid samples was performed by measuring and analyzing the peak areas of the sample and standard gallic acid. The identification and quantitation of the gallic acid of the unfermented COF and control samples were performed in the same manner. The gallic acid content was determined according to the method described in [62].
4.7. Statistical Analysis
All the data for normality and constant variance were tested prior to the statistical analysis. One-way analysis of variance (ANOVA) and least significant difference (LSD) multiple comparison tests were used to detect the differences between the groups of active compound contents. The resulting values were expressed as means ± standard errors. The mean differences were considered to be statistically significant at p < 0.05. The statistical analysis was performed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
5. Conclusions
Microbial fermentation improves not only the nutritional compositions of food and feedstuff, but also the bioactive compositions of traditional herbal medicines. Aerobic B. subtilis fermentation and anaerobic B. bifidum fermentation increased the gallic acid levels in COF; however, they had no effect on the loganin content. B. subtilis reduced the levels of ursolic acid and oleanolic acid; however, B. bifidum did not. In addition, the degradation of the ursolic and oleanolic acids by B. subtilis may have been related to the aerobic conditions, and the increases in gallic acid by B. subtilis and B. bifidum may have been the result of tannin degradation. These findings contribute to a better understanding of the mechanisms by which microbial fermentation improves the bioactive components of traditional herbal medicines, and they also imply that microbial fermentation is a potential approach to improving the active ingredients and disease prevention effects of traditional herbal medicines.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031032/s1, Figure S1: High-performance liquid chromatography for the active compounds in C. officinalis fruit culture broth and the corresponding standar; Table S1: Ursolic acid contents in Cornus officinalis fruit (COF) culture broth at different concentrations and fermentation times; Table S2: Oleanolic acid contents in Cornus officinalis fruit (COF) culture broth at different concentrations and fermentation times; Table S3: Loganin content in Cornus officinalis fruit (COF) culture broth at different concentrations and fermentation times; Table S4: Gallic acid contents in Cornus officinalis fruit (COF) culture broth at different concentrations and fermentation times.
Author Contributions
Conceptualization, X.Z. and Y.Z.; methodology, X.Z. and Y.Z.; software, L.D.; validation, L.D. and G.X.; formal analysis, L.D.; investigation, L.D.; resources, X.Z.; data curation, X.Z.; writing—original draft preparation, Y.Z.; writing—review and editing, X.Z.; supervision, X.Z.; project administration, X.Z.; funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We would like to thank Jinhua Li for his hospitability and the sample collection.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds and Cornus officinalis fruits are available from the authors.
References
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Płacheta, B.; Motyl, I.; Berłowska, J.; Mroczyńska-Florczak, M. The use of fermented plant biomass in pigs feeding. Sustainability 2022, 14, 14595. [Google Scholar] [CrossRef]
- Kiczorowski, P.; Kiczorowska, B.; Samolińska, W.; Szmigielski, M.; Winiarska-Mieczan, A. Effect of fermentation of chosen vegetables on the nutrient, mineral, and biocomponent profile in human and animal nutrition. Sci. Rep. 2022, 12, 13422. [Google Scholar] [CrossRef]
- Lang, B.; Zhao, Y.; Yang, R.; Liu, A.; Ranjitkar, S.; Yang, L. Antioxidant and tyrosinase inhibitory activities of traditional fermented Rosa from Dali Bai communities, Northwest Yunnan, China. Sci. Rep. 2021, 11, 22700. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- Dou, Z.; Chen, C.; Fu, X. Bioaccessibility, antioxidant activity and modulation effect on gut microbiota of bioactive compounds from Moringa oleifera Lam. leaves during digestion and fermentation in vitro. Food Funct. 2019, 10, 5070–5079. [Google Scholar] [CrossRef]
- Wang, C.; Shi, C.; Zhang, Y.; Song, D.; Lu, Z.; Wang, Y. Microbiota in fermented feed and swine gut. Appl. Microbiol. Biotechnol. 2018, 102, 2941–2948. [Google Scholar] [CrossRef]
- Ye, X.S.; Hao, J.; Zhang, J.L.; Pang, X.B.; Zhang, L.; Qiao, H.Y.; Pan, X.G.; Zhang, J.; Liu, S.N.; Zhang, W.K.; et al. Study on chemical constituents of Cornus officinalis fruit. Chin. J. Chin. Mat. Med. 2016, 41, 4605–4609. [Google Scholar]
- Mau, J.L.; Chen, C.P.; Hsieh, P.C. Antimicrobial effect of extracts from Chinese chive, cinnamon, and corni fructus. J. Agric. Food Chem. 2001, 49, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Ruan, Q. Study on separation technology and antibacterial activity of glycosides from Cornus officinalis fruits. Food Sci. 2008, 29, 353–357. [Google Scholar]
- Sung, Y.-H.; Chang, H.-K.; Kim, S.-E.; Kim, Y.-M.; Seo, J.-H.; Shin, M.-C.; Shin, M.-S.; Yi, J.-W.; Shin, N.-H.; Kim, H.; et al. Anti-inflammatory and analgesic effects of the aqueous extract of corni fructus in murine RAW 264.7 macrophage cells. J. Med. Food 2009, 12, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Chi, H.; Kodama, H.; Chen, G. Anti-inflammatory effect of three iridoids in human neutrophils. Nat. Prod. Res. 2013, 27, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Chiang, L.C.; Hsu, F.F.; Lin, C.C. Chemoprevention against hepatocellular carcinoma of Cornus officinalis in vitro. Am. J. Chin. Med. 2004, 32, 717–725. [Google Scholar] [CrossRef]
- Telang, N.T.; Li, G.; Sepkovic, D.W.; Bradlow, H.L.; Wong, G.Y.C. Anti-proliferative effects of Chinese herb Cornus officinalis in a cell culture model for estrogen receptor-positive clinical breast cancer. Mol. Med. Rep. 2012, 5, 22–28. [Google Scholar] [CrossRef][Green Version]
- Zou, P.; Zhao, C.; Li, P.; Huang, H. Study on the anti-tumor effect of polysaccharides from Cornus officinalis and its immunologic mechanism. Chin. J. Hosp. Pharm. 2012, 01, 20–22. [Google Scholar]
- Su, Z.; Wang, B. Preliminary research of fermentation conditions and determination of loganin for Cornus Officinalis health wine. J. Taiyuan Univ. Sci. Technol. 2008, 01, 70–74. [Google Scholar]
- Xu, B.T.; He, G.Q.; Li, X.H.; Yu, H.N.; Shen, S.R. Extraction of ursolic acid in Cornus officinalis by fermentation combined with ultrasonic-assisted technique. J. Zhejiang Univ. (Agriculture and Life Sciences) 2009, 35, 272–277. [Google Scholar]
- Park, J.S.; Lee, J.S. The promoting effect of Cornus officinalis fermented with Lactobacillus rhamnosus on hair growth. Korean J. Pharm. 2011, 42, 260–264. [Google Scholar]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Liu, J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.L.; Zhang, S.P.; Hou, J.; Zhu, H.B. Effect of loganin on experimental diabetic nephropathy. Phytomedicine 2012, 19, 217–222. [Google Scholar] [CrossRef]
- Kwon, S.H.; Kim, H.C.; Lee, S.Y.; Jang, C.G. Loganin improves learning and memory impairments induced by scopolamine in mice. Eur. J. Pharmacol. 2009, 619, 44–49. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Shi, L.; Zhao, C.; Shen, B.; Tian, Y.; Feng, H. Loganin inhibits the inflammatory response in mouse 3T3L1 adipocytes and mouse model. Int. Immunopharmacol. 2016, 36, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Kim, J.A.; Hong, S.I.; Jung, Y.H.; Kim, H.C.; Lee, S.Y.; Jang, C.G. Loganin protects against hydrogen peroxide-induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem. Int. 2011, 58, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Bishayee, A. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225. [Google Scholar]
- Hsu, C.L.; Yen, G.C. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br. J. Nutr. 2007, 98, 727–735. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, D.; Shu, Q.; Zhou, L. Determination of eight major components in differnt processed productions of corni fructus. Mod. Chin. Med. 2021, 23, 1444–1450. [Google Scholar]
- Li, Q.; Hu, S.; Huang, L.; Zhang, J.; Cao, G. Evaluating the therapeutic mechanisms of selected active compounds in Cornus officinalis and Paeonia lactiflora in rheumatoid arthritis via network pharmacology analysis. Front. Pharmacol. 2021, 12, 648037. [Google Scholar] [CrossRef]
- Hwangbo, H.; Jeung, J.S.; Kim, M.Y.; Ji, S.Y.; Yoon, S.; Kim, T.H.; Choi, Y.H. A study on antioxidant and anti-inflammatory effects based on analysis of functional components of Cornus officinalis Siebold & Zucc. J. Life Sci. 2021, 31, 287–297. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 1st ed.; China Medical Science Press: Beijing, China, 2015; Volume 1, pp. 27–28. [Google Scholar]
- Hussain, A.; Bose, S.; Wang, J.H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Wen, Y.L.; Yan, L.P.; Chen, C.S. Effects of fermentation treatment on antioxidant and antimicrobial activities of four common Chinese herbal medicinal residues by Aspergillus oryzae. J. Food Drug Anal. 2013, 21, 219–226. [Google Scholar] [CrossRef]
- Wang, L.C.; Pan, T.M.; Tsai, T.Y. Lactic acid bacteria-fermented product of green tea and Houttuynia cordata leaves exerts anti-adipogenic and anti-obesity effects. J. Food Drug Anal. 2018, 26, 973–984. [Google Scholar] [CrossRef]
- Shang, Y.F.; Cao, H.; Ma, Y.L.; Zhang, C.; Ma, F.; Wang, C.X.; Wei, Z.J. Effect of lactic acid bacteria fermentation on tannins removal in Xuan Mugua fruits. Food Chem. 2019, 274, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hong, Q.; Yu, C.; Wang, R.; Li, C.; Liu, S. Acetobacter sp. improves the undesirable odors of fermented noni (Morinda citrifolia L.) juice. Food Chem. 2023, 401, 134126. [Google Scholar] [CrossRef] [PubMed]
- Anal, A.K. Quality Ingredients and safety concerns for traditional fermented foods and beverages from Asia: A review. Fermentation 2019, 5, 8. [Google Scholar] [CrossRef]
- Reddy, N.R.; Pierson, M.D. Reduction in antinutritional and toxic components in plant foods by fermentation. Food Res. Int. 1994, 27, 281–290. [Google Scholar] [CrossRef]
- Cao, G.; Ma, F.; Xu, J.; Zhang, Y. Microbial community succession and toxic alkaloids change during fermentation of Huafeng Dan Yaomu. Lett. Appl. Microbiol. 2020, 70, 318–325. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Fan, W.; Jiang, Y.; Zhang, C.; Li, J.; Wu, C. The application of fermentation technology in traditional Chinese medicine: A review. Am. J. Chin. Med. 2020, 48, 899–921. [Google Scholar] [CrossRef]
- Fu, F.Q.; Xu, M.; Wei, Z.; Li, W. Biostudy on traditional Chinese medicine Massa Medicata Fermentata. ACS Omega 2020, 5, 10987–10994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, S.; Zhang, X.; Meng, N.; Chai, X.; Wang, Y. Fermentation characteristics and the dynamic trend of chemical components during fermentation of Massa Medicata Fermentata. Arab. J. Chem. 2022, 15, 103472. [Google Scholar] [CrossRef]
- Nayeem, N.; Asdaq, S.M.B.; Salem, H.; AHEl-Alfqy, S. Gallic acid: A promising lead molecule for drug development. J. Appl. Pharm. 2016, 8, 1000213. [Google Scholar] [CrossRef]
- Locatelli, C.; Filippin, M.F.B.; Creczynski, P.T.B. Alkyl esters of gallic acid as anticancer agents: A review. Eur. J. Med. Chem. 2013, 60, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Dwibedy, P.; Dey, G.R.; Naik, D.B.; Kishore, K.; Moorthy, P.N. Pulseradiolysis studies on redox reaction of gallic acid: One electron oxidation of gallic acid by hallic acid OH adduct. Phys. Chem. Chem. Phys. 1999, 1, 1915–1918. [Google Scholar] [CrossRef]
- Saeki, K.; Yuo, A.; Isemura, M.; Abe, I.; Seki, T.; Noguchi, H. Apoptosis inducing activity of lipid derivatives of gallic acid. Biol. Pharm. Bull. 2000, 23, 1391–1394. [Google Scholar] [CrossRef]
- Karamaæ, M.; Kosiñska, A.; Pegg, R.B. Comparison of radical-scavenging activities of selected phenolic acids. Pol. J. Food Nutr. Sci. 2005, 14, 165–170. [Google Scholar]
- Kaur, S.; Michael, H.; Arora, S.; Harkonen, P.L.; Kumar, S. The in vitro cytotoxic and apoptotic activity of Triphala—An Indian herbal drug. J. Ethnopharmacol. 2005, 97, 15–20. [Google Scholar] [CrossRef]
- Leahy, J.G.; Colwell, R.R. Microbial degradation of hydrocarbons in the environment. Microbiol. Rev. 1990, 54, 305–315. [Google Scholar] [CrossRef]
- Cerniglia, C.E. Microbial transformation of aromatic hydrocarbons. In Petroleum Microbiology; Atlas, R.M., Ed.; Macmillan Publishing Co.: New York, NY, USA, 1984; pp. 99–128. [Google Scholar]
- Perry, J.J. Microbial metabolism of cyclic alkanes. In Petroleum Microbiology; Atlas, R.M., Ed.; Macmillan Publishing Co.: New York, NY, USA, 1984; pp. 61–98. [Google Scholar]
- Deschamps, A.M.; Lebeault, J.M. Production of gallic acid from tara tannin by bacterial strains. Biotechnol. Lett. 1984, 6, 237–242. [Google Scholar] [CrossRef]
- Banerjee, R.; Mukherjee, G.; Patra, K.C. Microbial transformation of tannin-rich substrate to gallic acid through co-culture method. Bioresour. Technol. 2005, 96, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, G.; Banerjee, R. Biosynthesis of tannase and gallic acid from tannin rich substrates by Rhizopus oryzae and Aspergillus foetidus. J. Basic Microb. 2004, 44, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, B.; Patil, S. A new approach to microbial production of gallic acid. Braz. J. Microbiol. 2008, 39, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, G.; Gao, J.; Yan, Y.; Wei, Y.; Chen, Y. Dynamic changes of tannins during fruit development of Cornus officinalis. Acta Bot. Boreal. Occident. Sin. 2021, 41, 1834–1842. [Google Scholar]
- Song, Y.; Wang, Z.; Li, J.; Guo, T.; Wang, T.; Zhu, Y. Optimization of preparing procedure of wined Cornus officinalis fruit by overall desirability. J. Chin. Med. Mat. 2018, 41, 325–329. [Google Scholar]
- Wang, G.H.; Chen, C.Y.; Lin, C.P.; Huang, C.L.; Lin, C.H.; Cheng, C.Y.; Chung, Y.C. Tyrosinase inhibitory and antioxidant activities of three Bifidobacterium bifidum-fermented herb extracts. Ind. Crops Prod. 2016, 89, 376–382. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Y.; Qin, Y.; Tang, S.; Huang, P.; Zhang, L. Bacillus subtilis liquid fermentation and degradation of tea saponin. Chin. J. Environ. Eng. 2016, 10, 2023–2030. [Google Scholar]
- Tian, S.; Shi, Y.; Yu, Q.; Upur, H. Determination of oleanolic acid and ursolic acid contents in Ziziphora clinopodioides Lam. by HPLC method. Pharmacogn. Mag. 2010, 6, 116–119. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Determination of hamamelitannin, catechins and gallic acid in witch hazel bark, twig and leaf by HPLC. J. Pharm. Biomed. Anal. 2003, 33, 539–544. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).