Abstract
A series of five esters of lasalocid with neopentyl alcohol (LasNeo), geraniol (LasGeran), 2-ethylhexanol (LasEtHex), eicosanol (LasEico) and vanillyl alcohol (LasVanil) were synthesized and studied by NMR, FT-IR and ESI-MS. Then, their complexes with lithium, sodium and potassium cations were obtained and examined using FT-IR. The analysis of the products confirmed the synthesis of new esters with good yields. The newly obtained compounds, as well as their complexes with monovalent cations, were proved to be stabilized by a strong system of intramolecular hydrogen bonds. The PM6 semiempirical calculations provided information on the heat of formation (HOF) and permitted the making of visual representations of the structures of the newly synthesized esters and their complexes with the investigated cations. All the computational outcomes were consistent with the spectroscopic data.
1. Introduction
Polyether antibiotics constitute a very interesting group of substances, showing a broad spectrum of biological activities, including antibacterial, antiprotozoal and antiviral, as well as anti-inflammatory and anticancer ones [1,2,3,4,5,6]. For many years, some of these compounds, such as lasalocid, salinomycin, monensin and semduramycin, have been used in agriculture due to their strong activity against parasites of the genus Eimeria, which causes coccidiosis in birds. Therefore, the introduction of ionophore antibiotics has contributed to better disease control and improved health levels in industrial poultry farming [7].
The molecules of ion carrier ionophores have a non-cyclic structure, but thanks to the presence of a carboxyl group at one end and hydroxyl groups at the other, hydrogen bonds can be formed inside these molecules to form pseudocyclic structures stabilized by these bonds. Ether oxygen atoms are directed towards the interior of the molecule, creating a hydrophilic cavity in which the cation may be complexed [8,9,10,11,12,13]. Certain polyether ionophores are capable of transporting only monovalent cations, e.g., monensin A, while others may also transport divalent cations, e.g., lasalocid acid [14]. In solutions, depending on the solvent and the ion, lasalocid acid can form complexes with different stoichiometries: 1:1, 2:1 and 2:2 [15].
Nowadays, some ionophore antibiotics, like salinomycin, have aroused much interest because of their potent activity against cancer cells, including cancer stem cells [16,17]. For the first time, salinomycin was identified as an effective tumor-targeting agent by Gupta and co-workers [18]. In the following years, salinomycin has been proved to be effective against colon, prostate and gastric cancers and lung adenocarcinoma [19]. Moreover, it has been shown that semisynthetic derivatives of salinomycin have biological activity comparable to that of the unmodified ionophore [20,21,22].
Of note, in 2021, Esben B. Svenningsen and co-workers confirmed the broad-spectrum of antiviral activities of polyether ionophores, including salinomycin, monensin and lasalocid, against the SARS-CoV-2 pandemic [23].
Increasing interest in ionophore antibiotics and the discovery of their new applications have stimulated the search for their new derivatives showing biological activity. These new derivatives should show physicochemical properties at least in one aspect superior to those of the original structure. In this study, our aim was to improve the hydrophobicity of the ionophore molecule. To achieve this, we decided to obtain esters of lasalocid acid with selected alcohols. The newly obtained derivatives were expected to increase its solubility in biological membranes and improve ion transportation, which may be the subject of future research.
2. Results and Discussion
2.1. ESI Mass Spectrometry
The mass spectra of LasNeo, LasGeran, LasEtHex, LasEico and LasVanil are shown in Figure 1. The MS spectra were recorded using the technique of electrospray ionization (ESI) at a very low cone voltage (10 V) so that clear molecular peaks corresponding to the formed complexes could be observed (Table 1).
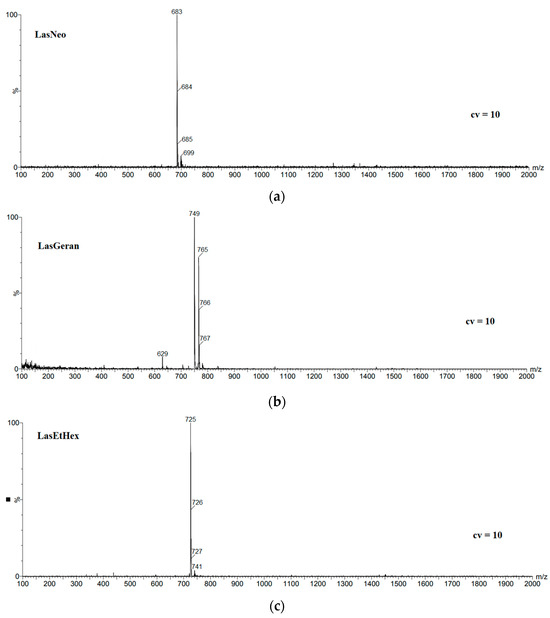
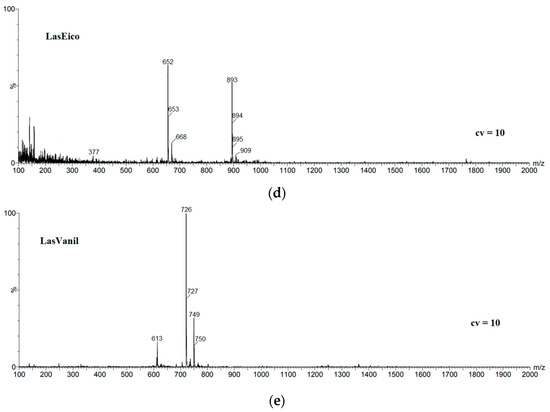
Figure 1.
ESI mass spectra of lasalocid esters, (a) LasNeo, (b) LasGeran, (c) LasEtHex, (d) LasEico, (e) LasVanil.

Table 1.
The main peaks in the ESI mass spectra of the lasalocid complexes at cone voltage 10 V. Signal values assigned to ester complexes with sodium cation are bolded.
In the spectrum shown in Figure 1a, the most intensive signal at m/z = 683 is assigned to the LasNeo complex with sodium cations. Accordingly, weaker signals at m/z = 684 and m/z = 685 are assigned to isotopic peaks originating from the ester molecule incorporated with 13C carbon atoms. Also, very weak signals at m/z = 699 assigned to the LasNeo complex with the K+ cation can be observed.
However, in the mass spectrum of geraniol lasalocid ester (Figure 1b), the strongest signal originating from the LasGeran complex with the sodium cation appears at m/z = 749, and a slightly weaker signal at m/z = 765 is assigned to the LasGeran complex with the K+ cation, while the weaker signals at m/z = 766 and m/z = 767 are assigned to the isotopic peaks coming from the ester molecule incorporated with 13C carbon atoms. A very weak signal at m/z = 629 is attributed to the complex of lasalocid acid with the potassium cation, which is probably a fragment ion.
In the LasEtHex mass spectrum (Figure 1c), the strongest signal at m/z = 725 was assigned to the LasEtHex complex with the Na+ cation. Thus, the weaker signals at m/z = 726 and m/z = 727 are the isotopic peaks originating from the ester molecule incorporated with 13C carbon atoms. Additionally, a very weak signal visible at m/z = 741 is assigned to the LasEtHex complex with the potassium cation.
In the mass spectrum of LasEico (Figure 1d), the strongest signal at m/z = 893 is assigned to the LasEico complex with Na+ cations. Therefore, the weaker signals at m/z = 894 and m/z = 895 are assigned to an ion containing 13C carbon atoms incorporated into the ester molecule. Moreover, a very weak signal appeared at m/z = 909, assigned to the LasEico complex with the potassium cation. The signal at m/z = 652 of a similar intensity to that at m/z = 893 suggests the probable formation of a fragment ion with the Las-C4H9 + Na+ structure. The weaker signal at m/z = 653 is the isotopic peak originating from the ester molecule incorporated with 13C carbon atoms. There is also a very weak signal at m/z = 668 assigned to the Las-C4H9 + K+ complex. Another very weak signal at m/z = 377 is consistent with the structure proposed by Lopes et al. [24]. Probably, the ion at m/z = 377 formed as a result of the internal rearrangement of protons, assisted by sodium.
In the mass spectrum of vanillyl lasalocid ester (Figure 1e), the most intensive signal at m/z = 726 comes from the LasVanil ester, while the weaker signal at m/z = 727 is the isotopic peak that originates from the ester molecule incorporated with 13C carbon atoms. The low-intensity signal at m/z = 749 is assigned to the LasVanil complex with the Na+ cation, while the weaker signal at m/z = 750 is also the isotopic peak from an ion containing the ester molecule incorporated with 13C carbon atoms. A very weak signal at m/z = 613 is assigned to the complex of lasalocid acid with the sodium cation, which is probably a fragment ion.
The data obtained through mass spectrometry definitively confirmed the identity of the synthesized chemical compounds. It is important to highlight that the analyzed samples were prepared without incorporating specific sodium and potassium salts. The complex cations that were observed to form likely originated from the glassware used at the synthesis and purification stages. The observation of their formation demonstrates the remarkable capability of the derived lasalocid derivatives to form complexes with monovalent metal cations.
2.2. NMR Measurements
The Δ1H and Δ13C NMR data of the esters with neopentyl alcohol, geraniol, 2-ethylenohexanol, eicosanol and vanillyl alcohol in chloroform are collected in Table 2. The numbering of atoms in the molecules is given in Figure 2. The tables with the detailed chemical shifts of lasalocid derivatives are included in Supplementary Materials (Tables S1–S5). Table 2 has been reduced to ensure better readability.

Table 2.
Δ1H NMR and Δ13C NMR chemical shifts (ppm) of LasNeo, LasGeran, LasEtHex, Las Eico and LasVanil in chloroform.
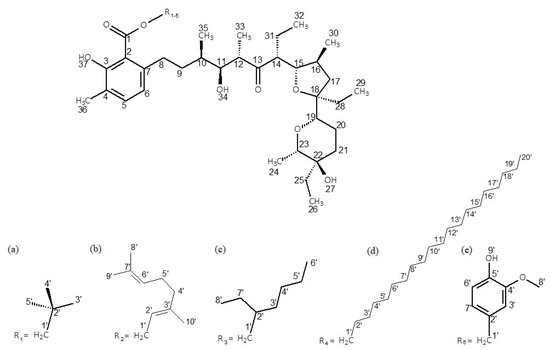
Figure 2.
Chemical structures of the studied esters: (a) LasNeo, (b) LasGeran, (c) LasEtHex, (d) LasEico, and (e) LasVanil.
In the 1H NMR spectrum, the signals of protons from the OH groups of lasalocid derivatives are found at 11.20, 3.40 and 2.40 ppm. The signal at 11.20 ppm is assigned to the O37H proton of the phenolic group, involved in the middle of a strong intramolecular hydrogen bond. The positions of proton signals from the remaining hydroxyl (OH) groups indicate their participation in relatively weak intramolecular hydrogen bonds. In the 1H NMR spectrum, the signals from the protons at C8 are split, which implies an inhibition of rotation of the salicylic part of the molecule. Additionally, the decoupling of the signals assigned to C17H, C20H and C30H indicates a constriction of the movement of the chain itself. Most probably, this constriction is induced by the formation of a “head-to-tail” hydrogen bond and additional intramolecular hydrogen bonds that stabilize the overall conformation of the molecule. This has been verified through semiempirical and DFT calculations.
The analysis of the 1H NMR and 13C NMR spectra of LasNeo reveals significant chemical shifts in the C1′H signals by 0.50 and 5.59 ppm, which proves the formation of an ester bond with lasalocid, as these are the atoms located directly next to it. We can draw similar conclusions based on the data presented in the tables in Supplementary Materials. The chemical shifts of C1’H are clearly visible, and the chemical shifts of C1’H in the 1H NMR spectra of the other investigated molecules are similar, which proves the formation of the corresponding esters.
In the 1H NMR spectrum of LasVanil, we observe a clear shift in the signal assigned to O37H from the phenolic group towards stronger fields by 0.39 ppm, which indicates the weakening of this hydrogen bond.
A comparable alteration in the chemical shift is noted in the signal attributed to C11H, indicating a modification in the chemical surroundings, likely brought about by a shift in the configuration of the neighboring hydrogen bonds. The signal from C31H was also averaged, underscoring the heightened flexibility of the polyether chain. The signals originating from the protons C9H, C17H and C20H are split, similarly to those in the LasH spectrum, indicating that the rotation of the salicylic part is blocked. The locking of rotation can also be deduced from analysis of the other spectra.
2.3. FT-IR Measurements
The FT-IR spectra of the new lasalocid esters with neopentyl alcohol (LasNeo) (Figure 3), geraniol (LasGeran) (Supplementary Materials, Figure S6), 2-ethylenohexanol (LasEtHex) (Supplementary Materials, Figure S7), eicosanol (LasEico) (Supplementary Materials, Figure S8) and vanillyl alcohol (LasVanil) (Figure 4) and their 1:1 complexes with monovalent cations in the mid-infrared region are presented in Figure 3 and Figure 4, respectively.
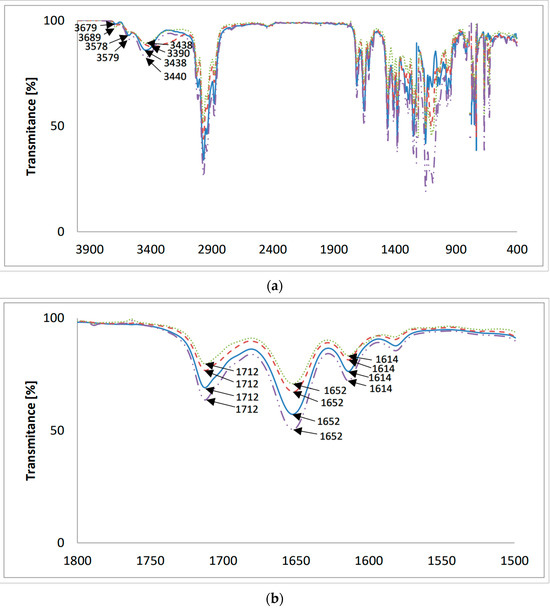
Figure 3.
The FT-IR spectra of (—) LasNeo and its 1:1 complexes with cations: (– –) Li+; (⋯) Na+; (-··-) K+; (a) 4000–400 cm−1; (b) 1800–1500 cm−1.
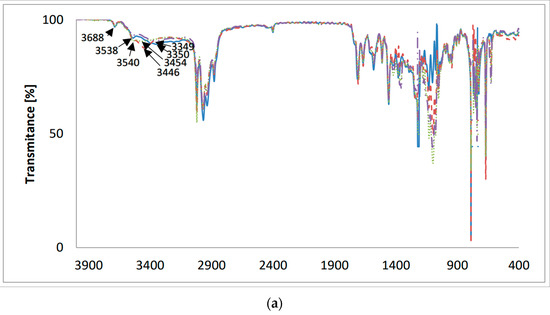
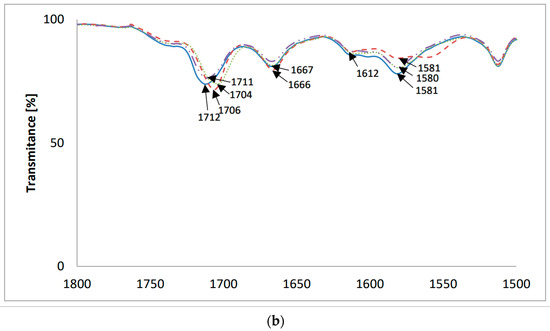
Figure 4.
The FT-IR spectra of (—) LasVanil and its 1:1 complex with cations: (– –) Li+; (⋯) Na+; (-··-) K+; (a) 4000–400 cm−1; (b) 1800–1500 cm−1.
A comparison of the absorption maxima of the individual bands in the FT-IR spectra of lasalocid esters and their 1:1 complexes with the following cations, Li+, Na+ and K+, is presented in Table 3.

Table 3.
Maxima of the absorption bands observed in the FT-IR spectra of LasNeo, LasGeran, LasEtHex, LasEico, LasVanil and their complexes.
The band assigned to the ν(O-H) stretching vibrations of the hydroxyl groups in the spectrum of LasNeo (Figure 3a) appears at 3438 cm−1. The analogous bands in the spectra of LasNeo complexes are in the same positions or are slightly shifted to higher wavenumbers, such as 3438 cm−1 for LasNeo-Na+ and 3440 cm−1 for LasNeo-K+. The largest shift in the signal coming from an OH group was observed in the spectrum of the LasNeo-Li+ complex, moving by 48 cm−1 towards the lower wavenumbers, which indicates the strengthening of hydrogen bonds formed by OH groups in these complexes relative to the strength of the hydrogen bonds in the LasNeo ester.
In the FT-IR spectrum of LasNeo (Figure 3b), the absorption maximum of the ν(C=O) carbonyl band is at 1712 cm−1, and that of the ν(C=O) carboxyl band is at 1652 cm−1. However, for lasalocid complexes with Li+, Na+ and K+ cations, there are no changes in the absorption maximum of the band assigned to the vibrations of the ν(C=O) of the carbonyl group (1712 cm−1) or the band ν(C=O) of the carboxyl group (1652 cm−1).
For the LasGeran, LasEtHex and LasEico esters and their complexes with the same cations (Supplementary Materials), we have a more or less similar situation, except for the LasEico complex with the lithium cation.
In the FT-IR spectrum of LasVanil (Figure 4), the band assigned to the ν(O-H) stretching vibrations of the hydroxyl groups appears at 3350 cm−1, but in the spectra of the LasVanil-Li+ and LasVanil-Na+ complexes, the bands corresponding to the ν(O-H) stretching vibrations of hydroxyl groups are shifted towards higher wavenumbers, which indicates the weakening of the hydrogen bonds formed by OH groups relative to those in the LasVanil ester. The largest shift in the signal coming from the OH group by 104 cm−1 was observed in the spectrum of the LasVanil-Na+ complex.
Figure 4b shows the same spectra on an extended scale in the range of 1800–1500 cm−1. In the spectrum of LasVanil, the maximum of the ν(C=O) stretching vibration of the ketone group is observed at 1712 cm−1, whereas in the spectra of the complexes with lithium or sodium, it is very slightly shifted to 1706 cm−1 and 1704 cm−1, respectively, indicating no or weak interactions of this ketone group with the cations studied. In the spectrum of the potassium cation complex, a shift in the absorption maximum towards lower wavenumbers only by 1 cm−1 was noted.
2.4. PM6 and DFT Study
The enthalpies of the formations of LasNeo, LasGeran, LasEtHex, LasEico, LasVanil and the associated species—both complexed and uncomplexed with different monovalent cations—are compiled in Table 4. These findings indicate that the formation of complexes between the investigated esters and cations is thermodynamically advantageous.

Table 4.
Heat of formation (HOF, kJ/mol) of LasNeo, LasGeran, LasEtHex, LasEico and LasVanil and their complexes with various monovalent cations calculated by PM6 method.
A lower ΔHOF value means a higher energy gain is obtained from cation complexation. According to the heat of formation values, the complexation of sodium cation is most preferred, while that of lithium cation is slightly less preferred, which is true for all the compounds studied. The heat of the formation of the complex with the potassium cation is more than half less than that of the complex formation with the sodium cation, which indicates that all esters will preferentially form complexes with Na+. This is related to the size of the cavity formed in the ester molecule. Clearly, the ligand structure exhibits a pseudocyclic nature, and the potential size of the cavity is not theoretically constrained. However, intramolecular hydrogen bonds play a crucial role in stabilizing the overall structure.
Figure 5 and Figure 6 present the structures of LasNeo and LasNeo–Li+ (Figure 5) and LasVanil and LasVanil–Li+ (Figure 6) calculated by the DFT methods.
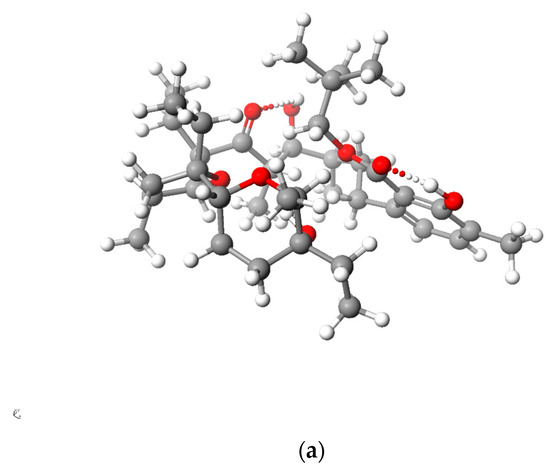
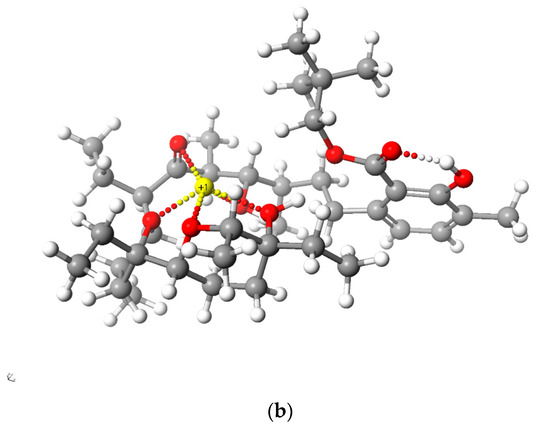
Figure 5.
Calculated (DFT) structure of LasNeo (a) and its 1:1 complex Li+ cation (b). Carbon atoms are marked in dark gray, hydrogen in light gray, oxygen in red and lithium in yellow.
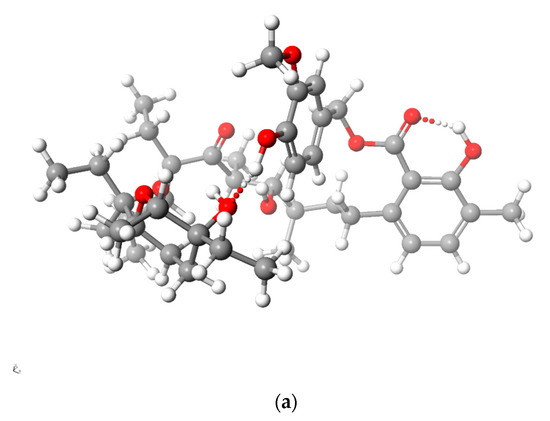
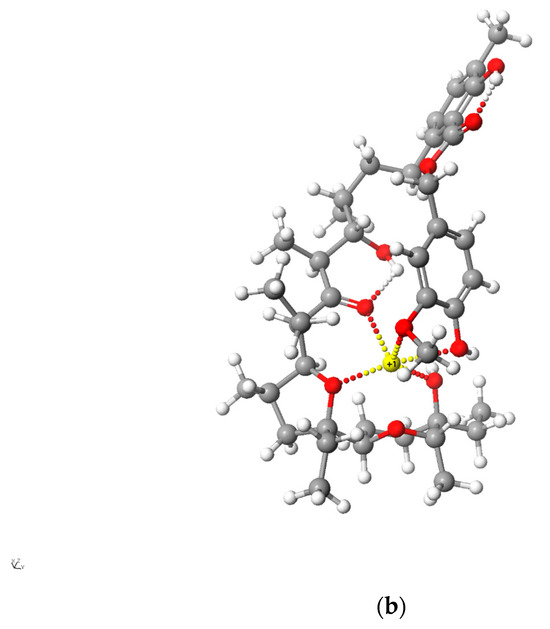
Figure 6.
Calculated (DFT) structure of LasVanil (a) and its 1:1 complex with Li+ cation (b). Carbon atoms are marked in dark gray, hydrogen in light gray, oxygen in red and lithium in yellow.
For all the esters, robust intramolecular hydrogen bonds persisted, even following the cation complexation, which is in agreement with the findings from the spectroscopic analysis. However, the configuration of these bonds and the molecular conformation undergo certain alterations, as indicated by the analysis of the 1H NMR spectra. For instance, these alterations are evident from the disappearance of the splitting in the signal assigned to the C31H proton.
3. Materials and Methods
3.1. Preparation of Lasalocid Acid
Lasalocid acid was prepared from the animal feed additive (Avatec), which contains lasalocid sodium salts. Preliminarily, 250 g of the feed additive was pre-extracted in 2 L of hexane with Soxhlet apparatus for 12 h to remove dyes and other impurities. Then, relevant extraction in 2 L of methylene chloride with Soxhlet apparatus for the next 12 h was performed to obtain the sodium salt of lasalocid.
Lasalocid acid was obtained from lasalocid sodium salt by extraction with H2SO4 (pH 1.5) in CH2Cl2 as described previously [25].
3.2. Preparation of the Esters—LasNeo, Las Geran, LasEtHex, LasEico and LasVanil
Lasalocid (0.005 mol) as well as neopentyl alcohol (or geraniol, 2-ethylenohexanol, eicosanol and vanillyl alcohol) (0.005 mol) were dissolved in 50 mL of dichloromethane. The reaction mixture was stirred vigorously for 30 min. Subsequently, 0.005 moles of 1,3-dicyclohexylcarbodiimide (DCC) was introduced to the reaction mixture, which was left stirring overnight at room temperature. The resulting dicyclohexylurea precipitate was separated through filtration, and the remaining solution was concentrated under reduced pressure. The purification process consisted of passing the obtained product through a silica gel column using a CombiFlash NEXGEN 300+ system (Teledyne ISCO) (0 → 30% CH2Cl2/acetone), which gave us the product as an oil.
After purification, the relevant esters were obtained with the following yields: LasNeo—31.62%; LasGeran—38.46%; LasEtHex—52.76%; LasEico—46.26%; LasVanil—35.22%.
3.3. Preparation of Complexes
We have synthesized the relevant complexes with the use of: LiClO4, NaClO4 and KClO4 (Sigma-Aldrich, St. Louis, MI, USA). The solutions were obtained by dissolving the salt and the lasalocid ester in acetonitrile at the ratio 1:1. Acetonitrile was of spectroscopic grade. All the preparations and transfers of solutions were carried out in a carefully dried glovebox.
3.4. Elementary Analysis
Elementary analysis was carried out on Vario EL III GmbH equipped with a standard CHN detector. The measurements were repeated triplicate for each ester. For the derivatives: LasNeo–(C39H64O8) (calculated: C 70.80% H 9.77%, found C 70.91% H 9.65%); LasGeran–(C44H70O8) (calculated: C 72.71% H 9.72%, found C 72.78% H 9.81%); LasEtHex–(C42H70O8) (calculated: C 71.70% H 10.06%, found C 71.57% H 10.12%); LasEico–(C54H94O8) (calculated: C 74.50% H 10.92%, found C 74.63% H 11.07%); LasVanil–(C42H62O10) (calculated: C 69.33% H 8.61%, found C 69.11% H 8.40%).
3.5. ESI MS Measurements
The electrospray ionization (ESI) mass spectra were recorded with a Waters/Micromass ZQ mass spectrometer. The measurements were performed for the solutions of LasNeo, LasGeran, LasEtHex, LasEico and LasVanil (5 × 10−4 mol dm3). The samples were prepared in dry acetonitrile and were infused into the ESI source using a Harvard pump at a flow rate of 20 mL min−1. Standard ESI mass spectra were recorded at the cone voltages of 10 and 30 V. The source temperature was 120 °C, and the desolvation temperature was 300 °C. Nebulization and desolvation were achieved using nitrogen as the working gas at the flow rates of 100 and 300 dm3 h−1, respectively. The mass spectra were obtained using the positive ion detection mode, maintaining unit mass resolution with a step size of 1 m/z unit. The ESI experiments covered a mass range from m/z = 100 to m/z = 1300.
3.6. NMR Measurements
Nuclear Magnetic Resonance (NMR) spectra were recorded using a BRUKER Avance III HD (Bruker, Billerica, MA, USA) magnetic resonance spectrometer, operating at 400.2 MHz for 1H NMR and 100.6 MHz for 13C NMR. The 1H NMR spectra are presented with chemical shifts relative to Tetramethylsilane (TMS), utilizing the respective residual solvent peaks as the internal standards (CDCl3 δ 7.26 ppm). Similarly, the 13C NMR spectra are expressed in chemical shifts relative to TMS, with the internal standard being the respective residual solvent peak (CDCl3 δ 77.16 ppm). Line broadening parameters were set at 0.5 or 1.0 Hz, and the error in chemical shift values was 0.01 ppm. The assignments of 1H and 13C NMR signals were accomplished independently for each species on the basis of one- or two-dimensional spectra (COSY, HMQC).
3.7. FT-IR Measurement
The infrared spectra in the mid infrared region were recorded in a chloroform solution. The mass of each sample was 10 mg. The FT-IR spectra were obtained using a Bruker IFS 66/s FT-IR spectrophotometer (Bruker, Billerica, MA, USA) equipped with an MCT detector (125 scans, resolution 2 cm−1).
3.8. Theoretical Calculations
Scigress FJ2.6 (EU 3.1.9) software from Fujitsu, Tokyo, Japan, was employed for the PM6 semiempirical calculations [26]. In all instances, full geometry optimization was conducted without applying symmetry constraints. The DFT calculations were executed using the GAUSSIAN 16 package [27], and the geometries were optimized using Becke’s three-parameter hybrid method with the Lee, Yang and Parr correlation function (B3LYP), along with a 6-311G(d) basis set.
4. Conclusions
Lasalocid comprises in its molecular scaffold both the lipophilic as well as the hydrophilic counterparts, which permit the complexation of metal cations.
In order to search for new, alternative lasalocid derivatives showing a superior hydrophobicity, we carried out esterification with the compounds of different structures: neopentyl alcohol, geraniol, 2-ethylenohexanol, eicosanol and vanillyl alcohol. The identity of pure LasNeo, LasGeran, LasEtHex, LasEico and LasVanil ester molecules was confirmed (after purification using flash chromatography). All the new lasalocid derivatives and their complexes maintained the properties of the original ionophore, and they effectively complexed the monovalent cations. On the basis of quantum chemical calculations (PM6), we can conclude that the order of preference for complexing cations is as follows: Na+ > Li+ > K+.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules28248085/s1, Figure S1: Structure of neopenthyl lasalocid ester, Figure S2: Structure of geraniol lasalocid ester, Figure S3: Structure of 2-ethylhexanol lasalocid ester, Figure S4: Structure of eicosanol lasalocid ester, Figure S5: Structure of vanillyl lasalocid ester, Figure S6: The FT-IR spectra of (—) LasGeran and its 1:1 complexes with cations: (– –) Li+; (⋯) Na+; (-··-) K+; (a) 4000–400 cm−1; (b) 1800–1500 cm−1, Figure S7: The FT-IR spectra of (—) LasEtHex and its 1:1 complexes with cations: (– –) Li+; (⋯) Na+; (-··-) K+; (a) 4000–400 cm−1; (b) 1800–1500 cm−1, Figure S8: The FT-IR spectra of (—) LasEico and its 1:1 complexes with cations: (– –) Li+; (⋯) Na+; (-··-) K+; (a) 4000–400 cm−1; (b) 1800–1500 cm−1, Table S1: 1H NMR and 13C NMR chemical shifts (ppm) of LasNeopent in chloroform, Table S2: 1H NMR and 13C NMR chemical shifts (ppm) of LasGeran in chloroform, Table S3: 1H NMR and 13C NMR chemical shifts (ppm) of LasEtHex in chloroform, Table S4: 1H NMR and 13C NMR chemical shifts (ppm) of LasEico in chloroform, Table S5: 1H NMR and 13C NMR chemical shifts (ppm) of LasVanillyl in chloroform.
Author Contributions
Conceptualization, R.P. and M.P.; methodology, R.P. and M.P.; formal analysis, R.P.; investigation, M.P.; resources, R.P. and M.P.; data curation, M.P.; writing—original draft preparation, R.P. and M.P.; writing—review and editing, R.P. and M.P.; visualization, R.P. and M.P.; supervision, R.P. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by grant no. POWR.03.02.00-1026/16 and co-financed by the European Union through the European Social Fund under the Operation Program Knowledge Education Development.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kevin, D.A., II; Meujo, D.A.F.; Hamann, M.T. Polyether ionophores: Broad spectrum and promising biologically active molecules for the control of drug-resistant bacteria and parasites. Expert Opin. Drug. Discov. 2009, 4, 109–146. [Google Scholar] [CrossRef] [PubMed]
- Pankiewicz, R.; Schroeder, G.; Przybylski, P.; Brzezinski, B.; Bartl, F. Lasalocid polyoxaalkyl esters complexes with Li+, Na+, K+, Rb+ and Cs+ cations studied by ESI MS and semiempirical methods. J. Mol. Struct. 2004, 688, 171–176. [Google Scholar] [CrossRef]
- Huczyński, A. Polyether ionophores—Promising bioactive molecules for cancer therapy. Bioorg. Med. Chem. Lett. 2012, 22, 7002–7010. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Liu, Y.; Li, J.; Huang, J.H.; Hu, X.; Wu, E. Salinomycin as a potent anticancer stem cell agent: State of the art and future directions. Med. Res. Rev. 2022, 42, 1037–1063. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.; Brzezinski, B. Structures and properties of naturalny occyrring polyether antibiotics. BioMed. Res. Int. 2013, 2013, 162513. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, R.; Willingham, M.; Pastan, I. Monensin blocks endocytosis of vesicular stomatitis virus. Bioch. Biophys. Res. Commun. 1981, 102, 992–998. [Google Scholar] [CrossRef]
- Russel, J.B. A proposed mechanism of monensin action in inhibiting ruminal bacterial growth: Effects on ion flux and protonmotive force. J. Anim. Sci. 1987, 64, 1519–1525. [Google Scholar] [CrossRef]
- Ferdani, R.; Gokel, G.W. Ionophores. In Encyclopedia of Supramolecular Chemistry; Atwood, J.L., Steed, J.W., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 760–766. [Google Scholar]
- Fong, C.W. Physiology of ionophore transport of potassium and sodium ions across cell membranes: Valinomycin and 18-crown-6 ether. Int. J. Comput. Biol. Drug Des. 2016, 9, 228. [Google Scholar] [CrossRef]
- Dorkov, P.; Pantcheva, I.N.; Sheldrick, W.S.; Mayer-Figge, H.; Petrova, R.; Mitewa, M. Synthesis, structure and antimicrobial activity of manganese(II) and cobalt(II) complexes of the polyether ionophore antibiotic Sodium Monensin A. J. Inorg. Biochem. 2008, 102, 26–32. [Google Scholar] [CrossRef]
- Pankiewicz, R.; Schroeder, G.; Brzezinski, B.; Bartl, F. Spectroscopic and PM5 semiempirical study of New Lasalocid 5-hydroxypentyl Ester and its complexes with monovalent cations. J. Mol. Struct. 2004, 699, 53–64. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, N.; Deng, Y.; Guan, Y.; Xiao, H.; Nitka, T.A.; Yang, H.; Yadav, A.; Vukovic, L.; Mathews, I.I.; et al. Triepoxide formation by a flavin-dependent monooxygenase in monensin biosynthesis. Nat. Commun. 2023, 14, 6273. [Google Scholar] [CrossRef] [PubMed]
- Pankiewicz, R.; Schroeder, G.; Brzezinski, B. Spectroscopic, spectrometric and PM5 semiempirical investigation of new lasalocid 8–hydroxy–3,6–dioxaoctyl ester and its complexes with monovalent cations. J. Mol. Struct. 2005, 733, 217–229. [Google Scholar] [CrossRef]
- Westley, J.W. (Ed.) Chemical transformations of polyether antibiotics. In Polyether Antibiotics: Naturally Occurring Acid Ionophores; Chemistry, Marcel Dekker, Inc.: New York, NY, USA, 1983; Volume 2, pp. 51–87. [Google Scholar]
- Schroeder, G.; Gierczyk, B.; Brzezinski, B.; Różalski, B.; Bartl, F.; Zundel, G.; Sośnicki, J.; Grech, E. 23Na NMR and FT-IR studies of sodium complexes with the ionophore lasalocid in solution. J. Mol. Struct. 2000, 516, 91–98. [Google Scholar] [CrossRef]
- Fuchs, D.; Heinold, A.; Opelz, G.; Daniel, V.; Naujokat, C. Salinomycin induces apoptosis and overcomes apoptosis resistance in human cancer cells. Biochem. Biophys. Res. Commun. 2009, 390, 743–749. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, J.; Li, B.; Xia, J.; Wu, H.; Wang, L.; Hao, J.; Zhou, Q.; Wu, S. Synthesis and biological activity evaluation of 20-epi-salinomycin and its 20-O-acyl derivatives. RSC Adv. 2006, 6, 41885–41890. [Google Scholar] [CrossRef]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, C.; Steinhart, R. Salinomycin as a drug for targeting human cancer stem cells. J. Biomed. Biotechnol 2012, 2012, 950658. [Google Scholar] [CrossRef]
- Versini, A.; Saier, L.; Sindikubwabo, F.; Müller, S.; Cañeque, T.; Rodriguez, R. Chemical biology of salinomycin. Tetrahedron 2018, 74, 5585–5614. [Google Scholar] [CrossRef]
- Antoszczak, M. A comprehensive review of salinomycin derivatives as potent anticancer and anti-CSCs agents. Eur. J. Med. Chem. 2019, 166, 48–64. [Google Scholar] [CrossRef]
- Antoszczak, M.; Huczyński, A. Salimocycin and its derivatives—A new class of multiple-targeted “magic bullets”. Eur. J. Med. Chem. 2019, 176, 208–277. [Google Scholar] [CrossRef]
- Svenningsen, E.B.; Thyrsted, J.; Blay-Cadanet, J.; Liu, H.; Lin, S.; Moyano-Villameriel, J.; Olagnier, D.; Idorn, M.; Paludan, S.R.; Holm, C.K.; et al. Ionophore antibiotic X-206 is a potent inhibitor of SARS-CoV-2 infection in vitro. Antivir. Res. 2021, 185, 104988. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.P.; Gates, P.J.; Wilkinsc, J.P.G.; Stauntona, J. Fragmentation studies on lasalocid acid by accurate mass electrospray mass spectrometry. Analyst 2002, 127, 1224–1227. [Google Scholar] [CrossRef] [PubMed]
- Papsdorf, M.; Pankiewicz, R. New hydrophilic derivatives of lasalocid and their complexes with selected metal cations. Molecules 2023, 28, 5114. [Google Scholar] [CrossRef] [PubMed]
- Fujitsu Limited. MO-G Version 1.2A; Fujitsu Limited: Tokyo, Japan, 2013. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).